ABSTRACT
Susac’s syndrome is a rare immune-mediated endotheliopathy that mainly affects young women. It is characterised by the presence of encephalopathy, retinal vaso-occlusive disease and hearing loss. Diagnosis is based on the clinical presentation, brain magnetic resonance imaging, retinal fluorescein angiography, and audiometry. Treatment consists of immunosuppressive therapy. This review focuses on recent developments in the diagnosis and management of the condition.
KEYWORDS: Susac’s syndrome, encephalopathy, retinal occlusions, hearing loss
Introduction
Susac’s syndrome (SS) is a rare disorder characterised by a classic triad of hearing loss, encephalopathy, and branch retinal artery occlusions (BRAO). The syndrome was first described in 1979 by J.O. Susac.1
It mainly affects young women, with a female/male ratio of 3:1. Due to the rarity of this condition, the true incidence and prevalence are unknown, with only a few hundred cases described in the literature.2
Pathogenesis
SS is currently considered to be an autoimmune endotheliopathy that causes damage to the microvasculature of the brain, inner ear, and retina. Susac et al. proposed, in 2007, a role for anti-endothelial cell antibodies (AECA) in the pathogenesis of SS, supporting the autoimmune basis for this condition.3 Since then, some studies have also reported AECAs in association with SS patients.1,4,5 Recently, in a cohort of SS patients studied by Jarius et al., 30% of patients were positive for serum AECA.6 AECAs are neither specific nor pathognomonic of SS. It is also still unknown whether they have a pathogenic role in the disease or if they are secondary to the disease’s endothelial damage. Further studies are needed to establish the impact of such antibodies in the pathogenesis and diagnosis of the condition. Currently, routine screening of these antibodies is not recommended.6,7
The disease’s natural history and findings strongly support an autoimmune basis, including the higher prevalence in young women. Some authors have described occurrences or relapses of the syndrome in the context of pregnancy or the postpartum period.8–10 The role of pregnancy and the postpartum period is poorly defined in SS; however, hormonal changes and immuno-modulation during pregnancy can be responsible for exacerbations or relapses of autoimmune conditions.10
Clinical presentation
Although SS is characterised by the presence of a clinical trial, the full triad rarely manifests at first presentation, which can make the diagnosis challenging.8 Dorr et al., in a review of all published cases of SS, reported that only 13% of patients fulfilled the clinical triad at disease onset.2 At the onset, central nervous system (CNS) symptoms are the most frequent, followed by visual and lastly hearing disturbances.11
The syndrome is commonly categorised into two clinical subsets: one characterised by severe neurological involvement; and the other by ophthalmological involvement with recurrent episodes of BRAO and milder, or even absent, neurological findings.12,13
Rennebohm et al. suggested stratification of the syndrome into three major clinical courses: monocyclic – fluctuating disease that is self-limited over a maximum period of 2 years; polycyclic – a recurrent disease for over a two-year period; and a chronic-continuous form.12
Neurological findings
Encephalopathy symptoms are varied and include memory loss, psychiatric disturbances, cranial nerve disorders, seizures, and dementia.2,10,14-16 Headache, although non-specific, is the most frequent symptom, affecting up to 80% of patients.2 Headache can present as migrainous-like or oppressive, with varying degrees of intensity, and can be prodromal, preceding the onset of new symptoms for about 6 months.1,17
Histopathological examination of brain biopsies demonstrates arteriolar wall proliferation and lymphocytic infiltration, evidencing a micro-angiopathic process as the cause for brain micro-infarcts.1,4
Ophthalmological findings
BRAOs are the classical retinal finding in SS. Visual loss resulting from the occlusions can present as altitudinal defects or central/paracentral scotomas; however, when the occlusions are located in the peripheral retina, patients may be asymptomatic.13,18 Blurred vision and photopsias are also frequently reported. BRAOs can be bilateral and can involve multiple arterioles.2,19 Affected areas of retina may suffer from ischaemia. Rarely, neo-vascularisation and vitreous haemorrhage may develop.13
Aside from BRAOs, Gass plaques are commonly found in SS patients. These plaques consist of extravasation of lipids from vessels and they appear as yellow refractile lesions resembling emboli. Unlike emboli, Gass plaques are located far from arteriolar bifurcations and BRAOs.20,21 Gass plaques are commonly found in acute disease stages, however they fluctuate with disease activity and may even disappear with treatment or with inactive disease.7,21 Although characteristic of SS, Gass plaques can also be found in other retinal diseases.13
Egan et al. reported the presence of arterio-arterial collaterals as a newly discovered ophthalmological finding in SS. These collaterals are located away from the optic disc and develop later in the course of disease.22
Vestibular-cochlear findings
Sensorineural hearing loss is the main feature of auditory involvement in SS and is caused by inner ear vasculopathy. Hearing loss is typically abrupt and unilateral, but contralateral hearing loss may follow, leading to total deafness.11,23 The hearing loss is frequently accompanied by tinnitus and vertigo, reflecting some degree of vestibulocochlear dysfunction. Unlike other symptoms and deficits, hearing loss in SS is often irreversible, with frequent need for hearing devices or cochlear implants.9,24,25
Diagnosis
Diagnosis of SS is based on the clinical presentation and findings from retinal fluorescein angiography (FA), brain magnetic resonance imaging (MRI) and audiometry. A high index of suspicion is necessary, considering that some patients may never develop the classic clinical presentation, which can be a major cause of misdiagnosis.1,2
When the full clinical triad is present the diagnosis of complete SS is straightforward. The occurrence of only two manifestations of the triad suggests the diagnosis of incomplete SS.8
The main differential diagnosis of SS includes multiple sclerosis (MS) and acute disseminated encephalomyelitis.26
The white matter of the brain is most frequently affected, but the leptomeninges, grey matter, cerebellum and thalamus can also be affected. “Snowball” lesions of the corpus callosum on MRI are pathognomonic and represent multifocal callosal micro-infarcts (Figure 1); these lesions can evolve into callosal “holes”, usually called “punched out” lesions (Figure 2).2,27,28 Other MRI signs of SS include leptomeningeal enhancement, deep grey matter, or cortical involvement and micro-infarcts of the internal capsule, which constitute the “string of pearls” sign.12,20,27
Figure 1.
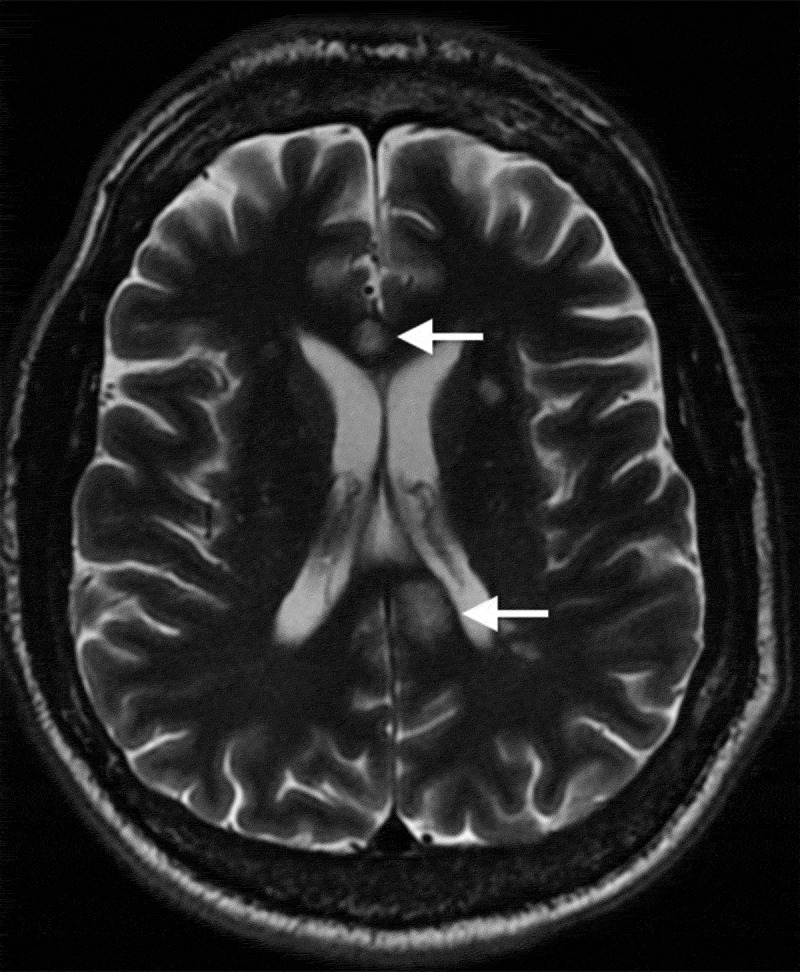
Axial T2-weighted magnetic resonance imaging demonstrating typical central callosal lesions (white arrows)
Figure 2.
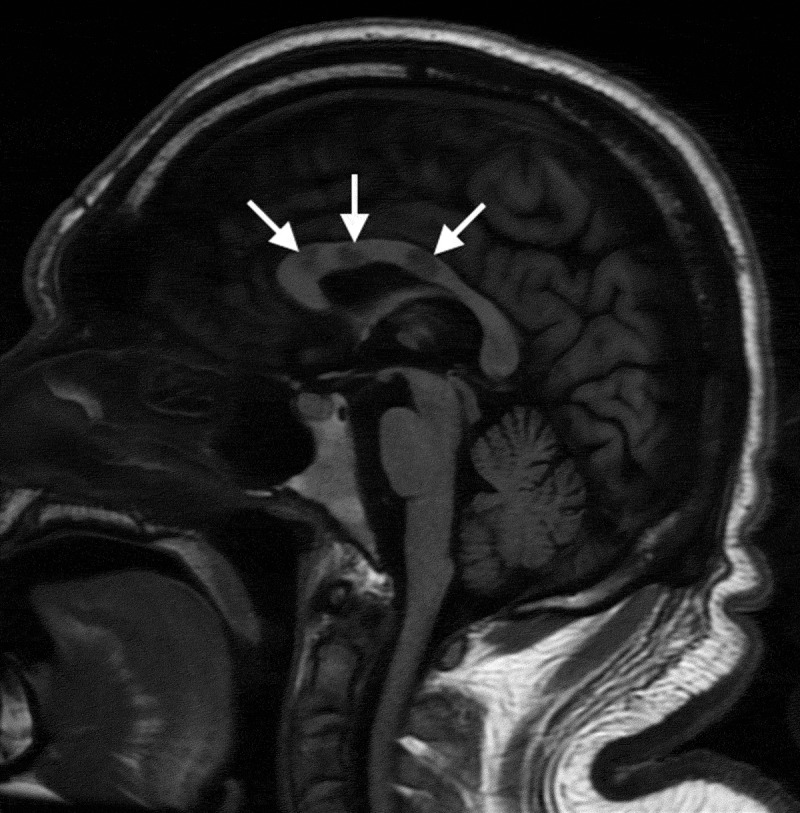
Sagittal T1-weighted magnetic resonance image demonstrating central callosal punched-out lesions (white arrows)
Lumbar puncture can reveal elevated protein levels in the cerebrospinal fluid with lymphocytic pleocytosis.3,28 Oligoclonal bands are usually absent, unlike in MS.2,4
BRAOs can be identified on ophthalmoscopy but FA is extremely useful, aside from detection of BRAOs, for monitoring disease control and treatment response.29 In a comprehensive review of all SS reported cases, FA showed BRAOs in 217 of 219 patients (99%).2 Arterial wall hyperfluorescence (AWH) is the typical angiographic sign (Figures 3 and Figures 4).20 AWH results from vascular leakage from damaged vessels. It indicates disease activity and can be considered pathognomonic for retinal involvement in SS.11,12,20 Changes in the vasculature in SS are limited to the retinal vessels with the choroidal vessels being spared. Indocyanine angiography is usually normal.13
Figure 3.
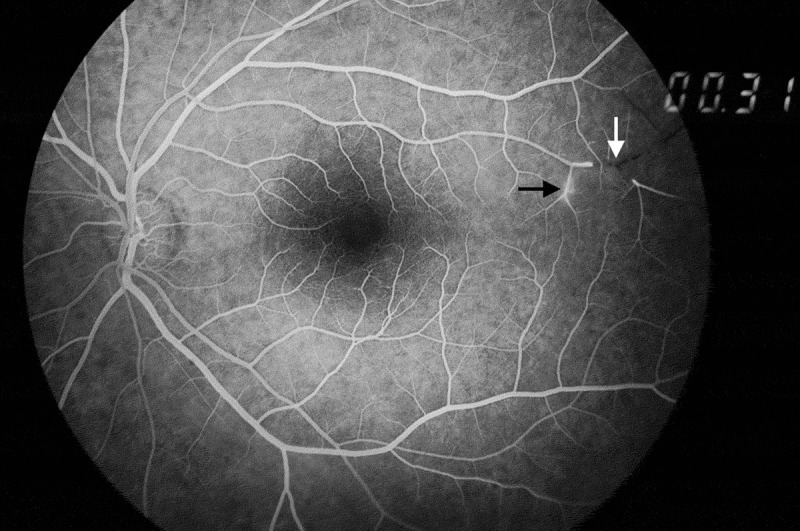
Arterial wall hyperfluorescence (black arrow) and a BRAO (white arrow) on fluorescein angiography
Figure 4.
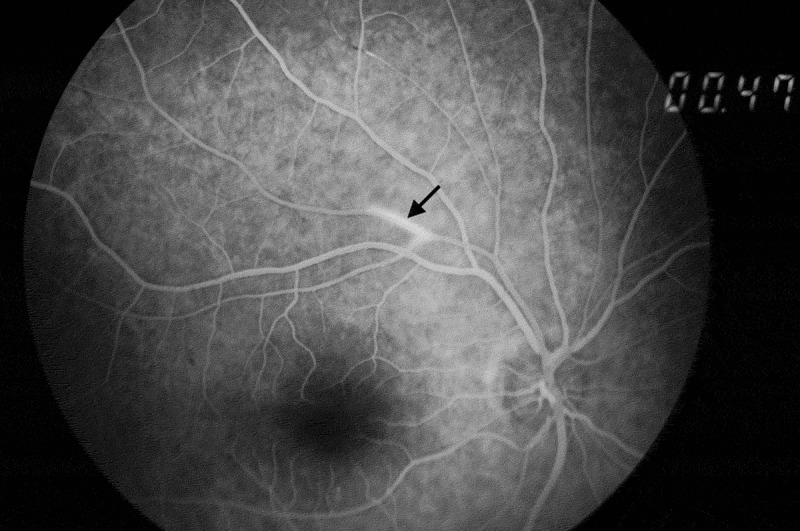
Typical arterial wall hyperfluorescence remote from a BRAO (black arrow) on fluorescein angiography
Recently, some authors have suggested that central callosal lesions on MRI and AWH on FA can be considered as independent diagnostic criteria for definite SS. Therefore, in patients lacking the clinical triad, imaging findings of central callosal lesions on MRI or AWH on FA (in arterioles remote from any BRAO) are enough for a definite diagnosis of SS, obviating the need for further investigation.7
Optical coherence tomography (OCT) performed in SS patients reveals patchy atrophy of inner retina layers and normal external retinal layers, supporting the idea that the syndrome causes a retinal vasculopathy.30 New imaging techniques, such as optical coherence tomography angiography (OCTA) can provide a better understanding of the involvement of the retinal vasculature in SS. In a case report by Azevedo et al., OCTA showed superficial and deep retinal vascular non-perfusion without chorio-capillary vasculature changes in a SS patient. The areas affected on OCTA corresponded to points of low sensitivity on microperimetry.31 OCT and OCTA may show benefit in monitoring disease activity and in differentiating SS from other ophthalmological or neurological entities, such as MS.20,30
Audiometric data is useful at documenting the sensorineural hearing loss and usually demonstrate loss of low and middle frequencies.24
Treatment
Most knowledge on treatment of SS comes from anecdotal reports and clinical experience, with standardised treatment and guidelines still lacking. Aggressive and early immunosuppressive treatment is the modality of choice in all patients with studies showing good results with recovery or stabilisation of disease.
The duration and type of treatment depend on the chronicity and severity of disease, but also depend on the clinical presentation. Patients with encephalopathy usually require a more aggressive and longer course of treatment than patients with predominant ophthalmological involvement. Rennebohm et al. recently suggested a new treatment algorithm for SS based on the clinical presentation and disease severity.32
First-line therapy consists of high-dose intravenous corticosteroids followed by high-dose oral corticosteroids with slow tapering. Use of intravitreal corticosteroids has been reported to lead to resolution of a patient’s symptoms.33
Intravenous immunoglobulins are also a mainstay of treatment, used with concomitant corticosteroids, and has shown great efficacy in acute and prolonged disease.34 Other agents that can be used include mycophenolate mofetil, rituximab, cyclophosphamide, and azathioprine.7,12 Different agents can be combined, in order to achieve better results or tolerability.
Anti-platelet and anticoagulant agents have been implemented as an adjuvant treatment with limited benefit. These agents should be considered in the presence of pro-coagulant risk factors.4,34 Plasma exchange can be used as an adjuvant or alternative therapy in corticosteroid-resistant patients.35
Specific treatment for complications or sequelae include cochlear implants and hearing aids for hearing loss and hyperbaric oxygen therapy for retinal occlusions.25,34
Therapy should be monitored with clinical evaluation and regular MRIs and retinal FAs. Change of therapy should be considered in the presence of new symptoms or lesions.7
Prognosis
SS is considered to be, in most cases, a self-limiting disease. Less frequently it can present with a polycyclic or chronic-continuous course. The prognosis is good with early treatment, despite the initial presentation. While encephalopathy and visual disorders can resolve or remit with treatment, hearing loss is usually permanent.9,11 Delays in diagnosis and treatment can lead to important sequelae, with up to 50% of patients developing cognitive impairment in such circumstances.4,28
Regardless of the favourable prognosis, recurrences of the disease have been reported, some several years after the initial diagnosis and others in association with pregnancy.9,36 Lifelong monitoring for disease recurrence is required for these patients.
Declaration of interest statement
The authors report no conflict of interest.
References
- 1.Kleffner I, Duning T, Lohmann H, et al. A brief review of Susac syndrome. J Neurol Sci. 2012;322(1–2):35–40. doi: 10.1016/j.jns.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 2.Dorr J, Krautwald S, Wildemann B, et al. Characteristics of Susac syndrome: a review of all reported cases. Nat Rev Neurol. 2013;9(6):307–316. doi: 10.1038/nrneurol.2013.82. [DOI] [PubMed] [Google Scholar]
- 3.Susac JO, Egan RA, Rennebohm RM, Lubow M.. Susac’s syndrome: 1975-2005 microangiopathy/autoimmune endotheliopathy. J Neurol Sci. 2007;257(1–2):270–272. doi: 10.1016/j.jns.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Carrasco M, Mendoza-Pinto C, Cervera R. Diagnosis and classification of Susac syndrome. Autoimmun Rev. 2014;13(4–5):347–350. doi: 10.1016/j.autrev.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 5.Jarius S, Neumayer B, Wandinger KP, Hartmann M, Wildemann B. Anti-endothelial serum antibodies in a patient with Susac’s syndrome. J Neurol Sci. 2009;285(1–2):259–261. doi: 10.1016/j.jns.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Jarius S, Kleffner I, Dorr JM, et al. Clinical, paraclinical and serological findings in Susac syndrome: an international multicenter study. J Neuroinflammation. 2014;11:46. doi: 10.1186/1742-2094-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egan RA. Diagnostic criteria and treatment algorithm for susac syndrome. J Neuroophthalmol. 2019;39(1):60–67. doi: 10.1097/WNO.0000000000000677. [DOI] [PubMed] [Google Scholar]
- 8.Vishnevskia-Dai V, Chapman J, Sheinfeld R, et al. Susac syndrome: clinical characteristics, clinical classification, and long-term prognosis. Medicine (Baltimore). 2016;95(43):e5223. doi: 10.1097/MD.0000000000005223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aubart-Cohen F, Klein I, Alexandra JF, et al. Long-term outcome in Susac syndrome. Medicine (Baltimore). 2007;86(2):93–102. doi: 10.1097/MD.0b013e3180404c99. [DOI] [PubMed] [Google Scholar]
- 10.Ioannides ZA, Airey C, Fagermo N, Blum S, McCombe PA, Henderson RD. Susac syndrome and multifocal motor neuropathy first manifesting in pregnancy. Aust N Z J Obstet Gynaecol. 2013;53(3):314–317. doi: 10.1111/ajo.12069. [DOI] [PubMed] [Google Scholar]
- 11.Greco A, De Virgilio A, Gallo A, et al. Susac’s syndrome–pathogenesis, clinical variants and treatment approaches. Autoimmun Rev. 2014;13(8):814–821. doi: 10.1016/j.autrev.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Rennebohm R, Susac JO, Egan RA, Daroff RB. Susac’s syndrome–update. J Neurol Sci. 2010;299(1–2):86–91. doi: 10.1016/j.jns.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 13.Heng LZ, Bailey C, Lee R, Dick AD, Ross A. A review and update on the ophthalmic implications of Susac syndrome. Surv Ophthalmol. 2019;64(4):477–485. doi: 10.1016/j.survophthal.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Adelpoor M, Farahvash MS, Fard MA, Nikdel M, Kiarudi MY. Susac’s syndrome in a 27-year-old female. Middle East Afr J Ophthalmol. 2011;18(4):320–322. doi: 10.4103/0974-9233.90137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Do T FC H, Susac Syndrome: EF. Report of Four Cases and Review of the Literature. Am J Neuroradiol. 2004;25:382–388. [PMC free article] [PubMed] [Google Scholar]
- 16.Marrodan M, Correale J, Alessandro L, et al. Susac Syndrome: A differential diagnosis of white matter lesions. Mult Scler Relat Disord. 2017;15:42–46. doi: 10.1016/j.msard.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Obelieniene D, Macaityte R, Balnyte R, Pestininkaite R, Gleizniene R, Balciuniene J. Characteristics of headache in relation to the manifestation of Susac syndrome. Medicina (Kaunas). 2017;53(6):420–425. doi: 10.1016/j.medici.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Khan IJ, Allroggen H, Pagliarini S. Susac’s syndrome: the value of fundus fluorescein angiography. BMJ Case Rep. 2014. Oct 3;2014. doi: 10.1136/bcr-2014-206546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boukouvala S, Jacob S, Lane M, Denniston AK, Burdon MA. Detection of branch retinal artery occlusions in Susac’s syndrome. BMC Res Notes. 2014;7:56. doi: 10.1186/1756-0500-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorr J, Ringelstein M, Duning T, Kleffner I. Update on Susac syndrome: new insights in brain and retinal imaging and treatment options. J Alzheimers Dis. 2014;42(Suppl 3):S99–S108. doi: 10.3233/JAD-132519. [DOI] [PubMed] [Google Scholar]
- 21.Egan RA, Hills WL, Susac JO. Gass plaques and fluorescein leakage in Susac syndrome. J Neurol Sci. 2010;299(1–2):97–100. doi: 10.1016/j.jns.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 22.Egan RA, Jirawuthiworavong G, Lincoff NS, Chen JJ, Francis CE, Leavitt JA. Retinal arterio-arterial collaterals in susac syndrome. J Neuroophthalmol. 2018;38(4):459–461. doi: 10.1097/WNO.0000000000000627. [DOI] [PubMed] [Google Scholar]
- 23.Patel VA, Dunklebarger M, Zacharia TT, Isildak H. Otologic manifestations of Susac syndrome. Acta Otorhinolaryngol Ital. 2018;38(6):544–553. doi: 10.14639/0392-100X-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roeser MM, Driscoll CL, Shallop JK, Gifford RH, Kasperbauer JL, Gluth MB. Susac syndrome--a report of cochlear implantation and review of otologic manifestations in twenty-three patients. Otology&Neurology. 2008;30:34–40. doi: 10.1097/mao.0b013e31818b6ac2. [DOI] [PubMed] [Google Scholar]
- 25.Lavinsky L, Scarton F, Lavinsky-Wolff M, Lavinsky J, Motta LH. Successful cochlear implantation in a Susac syndrome patient. Braz J Otorhinolaryngol. 2012;78(6):123. doi: 10.5935/1808-8694.20120045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Susac JO. Susac’s Syndrome. Am J Neuroradiol. 2004;25:351–352. [PMC free article] [PubMed] [Google Scholar]
- 27.Susac JO, Murtagh FR, Egan RA, et al. MRI findings in Susac’s syndrome. Neurology. 2003;61(12):1783–1787. doi: 10.1212/01.WNL.0000103880.29693.48. [DOI] [PubMed] [Google Scholar]
- 28.Vodopivec I, Venna N, Rizzo JF 3rd, Prasad S. Clinical features, diagnostic findings, and treatment of Susac syndrome: a case series. J Neurol Sci. 2015;357(1–2):50–57. doi: 10.1016/j.jns.2015.06.063. [DOI] [PubMed] [Google Scholar]
- 29.Littlewood R, Mollan SP, Pepper IM, Hickman SJ. The utility of fundus fluorescein angiography in neuro-ophthalmology. Neuroophthalmology. 2019;43(4):217–234. doi: 10.1080/01658107.2019.1604764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.M Ap R, Kleffner I, Buhn B, et al. Retinal pathology in Susac syndrome detected by spectral-domain optical coherence tomography. Neurology. 2015. Aug 18;85:610–618. doi: 10.1212/WNL.0000000000001852. [DOI] [PubMed] [Google Scholar]
- 31.Azevedo AGB, Lima LH, Muller L, et al. Anatomical and functional correlation in Susac syndrome: multimodal imaging assessment. Int J Retina Vitreous. 2017;3:39. doi: 10.1186/s40942-017-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rennebohm RM, Asdaghi N, Srivastava S, Gertner E. Guidelines for treatment of Susac syndrome - An update. Int J Stroke. 2018. Jan 1;1747493017751737. doi: 10.1177/1747493017751737. [DOI] [PubMed] [Google Scholar]
- 33.Jb Mf Y, Pettito M, Arevalo JF. Intravitreal Triamcinolone in Susac Syndrome. Retinal Cases Brief Reports. 2017:236–239. [DOI] [PubMed] [Google Scholar]
- 34.Grygiel-Górniak B, Czaplicka E. Susac syndrome – clinical insight and strategies of therapy. Eur Rev Med Pharmacol Sci. 2015;19(9):1729–1735. [PubMed] [Google Scholar]
- 35.Mateen FJ, Zubkov AY, Muralidharan R, et al. Susac syndrome: clinical characteristics and treatment in 29 new cases. Eur J Neurol. 2012;19(6):800–811. doi: 10.1111/j.1468-1331.2011.03627.x. [DOI] [PubMed] [Google Scholar]
- 36.Petty GW, Matteson EL, Younge BR, McDonald TJ, Wood CP. Recurrence of Susac syndrome (retinocochleocerebral vasculopathy) after remission of 18 years. Mayo Clin Proc. 2001;76(9):958–960. doi: 10.1016/S0025-6196(11)62119-8. [DOI] [PubMed] [Google Scholar]


