Sexual reproduction and related processes play a somewhat limited but important role in generating genetic diversity in Candida species and other fungal pathogens. These processes are also thought to be an important contributor to the evolution of pathogenicity and drug resistance. Candida auris is a recently emerged, human-pathogenic yeast causing nosocomial outbreaks all over the globe [1]. It can cause serious blood stream infections with the complication that isolates are typically resistant to the available antifungal therapies; mortality rates are approximately 60% [2]. Genetic diversity is likely a major driver of its pathogenesis and virulence features. Here, we discuss which mechanisms could be behind the genetic diversity observed between C. auris isolates. Specifically, our review examines the evidence around sexual reproduction in this fungus.
How do fungal pathogens create genetic diversity?
Fungal pathogens are able to create genetic diversity in multiple ways. Some have true meiotic cycles that generate diversity via homologous recombination, while others have evolved mechanisms of producing diverse offspring that do not depend on meiosis.
Candida albicans has a parasexual cycle, where fusion (mating) of 2 diploid cells is followed by concerted chromosome loss, rather than meiosis, to result in viable, but often aneuploid, progeny. Parasex generates genetic diversity and enables adaptation to stressful environments [3–5]. Although meiosis has not been observed in C. albicans, a complete meiotic cycle has been identified in the distantly related Candida (Clavispora) lusitaniae, a haploid yeast that can form spores through mating and meiosis [6]. C. lusitaniae often produces aneuploid progeny during meiosis, which most likely confer a selective advantage [6]. The pathogenic basidiomycete Cryptococcus neoformans is also capable of generating genetic diversity via chromosome copy number variations and ploidy changes, as unisexual meiosis (see below) often results in aneuploid and diploid spores [7]. Chromosome copy number variation (aneuploidies) are a means of creating diversity, as has been found in many fungal species [3,6,8]. Aneuploidies can arise by parasexual, asexual, and sexual mechanisms [7,9]. Importantly, aneuploidies can confer resistance to antifungal drugs by altering gene dosage, e.g., copy number variations (of the left arm) of chromosome 5 in C. albicans confer resistance to fluconazole [10]. The higher dosage of 2 genes on chromosome 5, ERG11 and TAC1, contributes to an increase in production of the azole drug target Erg11, and higher drug efflux activity via increased expression of Tac1-regulated efflux pumps; notably some, but not all, copies of TAC1 also were mutant expressing a hyperactive allele [10].
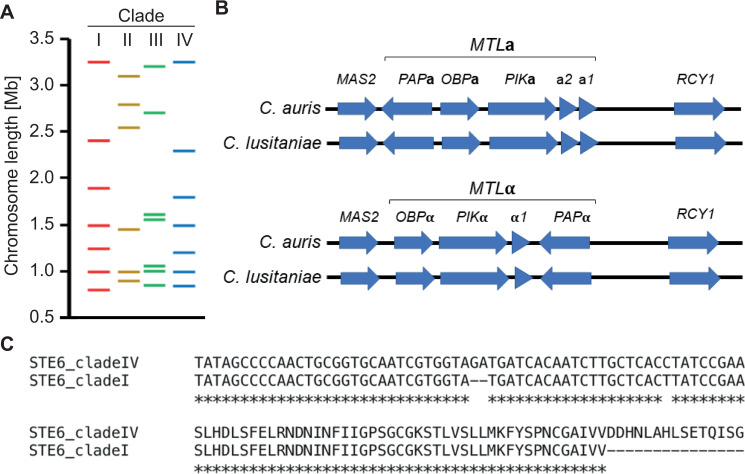
Karyotype variability, including chromosome rearrangements, is common in fungi and could be a basis for genetic diversity leading to phenotypes with enhanced fitness. A wide range of species, including Malassezia spp., Fusarium spp., and Candida glabrata, have highly variable karyotypes that are apparently well tolerated [11–13]. Genetically identical C. auris isolates from a hospital outbreak had very similar karyotypes (except for chromosomes bearing the rRNA gene arrays which showed some size differences), suggesting that genome rearrangements do not play a major role in quickly establishing genetic variability within individual outbreaks [14]. However, passaging C. auris through several rounds of various stresses generated massive karyotype changes [14]. Moreover, the variation in karyotype between C. auris isolates from different clades would indicate that genome rearrangements are indeed a potential mechanism to generate variation (Fig 1A), as has been described for other Candida species. For example, studies in C. albicans have shown that chromosome rearrangements occurring after 1 passage through a mouse model are able to generate genetic and phenotypic diversity [15]. Similarly, chromosome rearrangements have also been identified in C. glabrata from sequential blood stream isolates [16].
Fig 1. Chromosomal and genetic features of C. auris related to sexual reproduction.
(A) Length and number of chromosomes of 1 isolate from each of the 4 main C. auris clades as measured by pulsed-field gel electrophoresis (strains representing clades are: clade I, UACa1/470026; clade II, UACa18/B11220; clade III, UACa20/B11221; clade IV, UACa22/B11244) [14]. (B) The mating type locus regions MTLa and MTLα are conserved between C. auris and C. lusitaniae [26]. (C) ClustalΩ (https://www.ebi.ac.uk/Tools/msa/clustalo/) [38] alignments of the STE6 nucleotide sequences (top) from a clade I and a clade IV isolate, showing the 2-nucleotide deletion in clade I; and of the translated sequences (bottom) showing the premature stop codon in the clade I isolate at position 421 generated by the 2-nt deletion [36].
The C. auris clades differ from each other genetically by thousands of single nucleotide polymorphisms (SNPs), yet within each clade, independent clonal expansions typically take place within an outbreak [2]. This population structure, characterised by distinct and highly variable clades that are distributed worldwide and clonal expansions of a single genotype within individual outbreaks, is puzzling and suggests that the clades emerged independently. The C. auris clades also differ in genome organisation as structural rearrangements have been identified between the clades [17]. The origins of the variability seen within C. auris are not yet known. Importantly, determining the origins of genetic diversity in this dangerous human pathogen will potentially elucidate evolutionary mechanisms behind its virulence and antifungal drug resistance. This raises the question whether this is potentially related to (para)sexual outcrossing of strains to generate new genotypes.
Does C. auris have a complete mating type locus?
Mating types are the sex-determining genetic loci of fungi. Most fungi, including the Candida clade, have a mating type locus (MAT) or mating type-like locus (MTL) that occurs in 2 idiomorphs, MATa and MATα (or MTLa and MTLα). Generally, mating is only possible between cells of opposite mating types. However, there are exceptions to this rule.
The MTL loci in most diploid C. albicans isolates are heterozygous (a/α); therefore, these isolates were thought to never sexually reproduce as phenotypic switching from a ‘white’ to the mating-competent ‘opaque’ form is blocked in these isolates. Usually, only isolates that are homozygous at the MTL locus are able to switch into the opaque form [18], although MTL-heterozygous isolates can switch under certain conditions [19]. It was later discovered that the rare isolates that were homozygous at the MTL locus could form cell fusion products with isolates homozygous for the opposite mating type [18,20]. After mating, the resulting tetraploid C. albicans fusion products undergo concerted chromosome loss instead of meiosis to generate progeny often harbouring complex aneuploidies [5]. Parasex in C. albicans has the capability to produce progeny that have enhanced virulence and, in some cases, increased resistance to fluconazole, making this a clinically relevant process [4].
Cryptococcus neoformans was described as capable of forming basidia (the generative cell type of Basidiomycetes) more than 40 years ago [21]. However, almost all Cryptococcus neoformans isolates are MATα (>99%); its mating type locus covers >100 kb of sequence, making it one of the largest in the fungal kingdom. Importantly, unisexual reproduction (a.k.a. haploid fruiting) between 2 MATα isolates apparently plays a major role, indicating that an unequal distribution of mating type idiomorphs in a population or species does not preclude sexuality [7,22]. It is speculated that unisexual reproduction in Cryptococcus neoformans benefits the species as it prevents deleterious mutations from accumulating and can also yield progeny with enhanced fitness [8,23]. Unisexual mating can also occur in C. albicans and Candida tropicalis, but only in the presence of the opposite mating pheromone [24,25].
Investigation into the MTL loci of C. lusitaniae and C. auris revealed a highly conserved gene order, orientation, and synteny between these 2 closely related species (Fig 1B) [26,27]. Genome annotations have identified both mating types in C. auris and they appear to be clade-specific. So far, all sequenced clade I and clade IV isolates are MTLa and all clade II and III isolates are MTLα [26]. Isolates of opposite mating types are yet to be found within the same clade. However, occasionally C. auris strains with opposite mating types are found in the same location, namely Canada, Kenya, the United Kingdom, and the United States of America [28,29]. The latter finding suggests that there could be a clinically relevant danger of sexual interaction producing a super-resistant or super-virulent strain.
What is the evidence for sexuality in C. auris?
Genome sequencing data revealed that C. albicans has orthologs for most of the genes involved in mating and sporulation in Saccharomyces cerevisiae [30]. This raised the question whether C. albicans may be sexual and resulted in the discovery of parasex (see above). C. lusitaniae is able to carry out meiosis despite missing a full ‘meiosis toolkit’ [6,31]. Key meiotic genes are conserved between the species of the Candida haemulonii complex (C. haemulonii, Candida duobushaemulonii, Candida pseudohaemulonii, C. auris) and C. lusitaniae [26]. Thus, C. auris and its closest relatives should have sufficient mating and meiosis factors to support a sexual cycle. Indeed, a complete mating locus and both mating types exist in C. auris, strengthening the evidence that C. auris may be capable of mating and meiosis, or at least mating and concerted chromosome loss (parasex). So far, mating could not be observed in C. haemulonii and C. duobushaemulonii [32]. Intriguingly, an investigation into transporter family proteins in C. auris identified a mutation in STE6 in clade I isolates. Ste6 (Hst6 in C. albicans) is an ABC family transporter which is only expressed in MATa (MTLa) strains and exports the a-factor pheromone in S. cerevisiae and C. albicans [33–35]. In S. cerevisiae, the a-factor and its export via Ste6 is essential for mating [33]. The STE6 homolog in C. auris MTLa clade I isolates is missing 2 nucleotides at positions 3,309 and 3,310, while in MTLa clade IV isolates and MTL⍺ strains, this open reading frame is complete [36]. The 2 missing nucleotides result in a premature stop codon at AA421 of AA1,225 and therefore, a truncated and likely nonfunctional protein (Fig 1C). This would render clade I MTLa strains sterile due to an inability to export a-factor. We cannot exclude the possibility that C. auris, similar to C. albicans and C. tropicalis [24,25], could undergo unisexual reproduction with isolates from within the same clade.
Furthermore, for meiosis to produce viable progeny, pairing and recombination of homologous chromosomes is required [37]. Therefore, any karyotypical changes (chromosome rearrangements) between isolates will likely have a negative impact on the viability of any progeny. The karyotype differences between C. auris clades (Fig 1A) [14,26] make it unlikely that the extant clinical strains of C. auris successfully intermingle. However, these differences might not restrict parasexual mechanisms. To determine whether C. auris is sexual, it is of the utmost importance to identify its environmental reservoirs, where different MTL idiomorphs within a population might exist. It would appear that, based on current data, there is no threat to healthcare of C. auris mating and creating diversity in a clinical context.
Acknowledgments
We are grateful to Dr Gustavo Bravo Ruiz and Dr Delma Childers for critically reading the manuscript.
Funding Statement
We acknowledge funding from the Medical Research Council (MRC) Centre for Medical Mycology at the University of Exeter [grant number MR/P501955/1] (https://mrc.ukri.org/, https://www.exeter.ac.uk/medicalmycology/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rhodes J, Fisher MC. Global epidemiology of emerging Candida auris. Curr Opin Microbiol. 2019;52:84–9. 10.1016/j.mib.2019.05.008 [DOI] [PubMed] [Google Scholar]
- 2.Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis. 2017;64:134–40. 10.1093/cid/ciw691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hickman MA, Paulson C, Dudley A, Berman J. Parasexual ploidy reduction drives population heterogeneity through random and transient aneuploidy in Candida albicans. Genetics. 2015;200:781–94. 10.1534/genetics.115.178020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirakawa MP, Chyou DE, Huang D, Slan AR, Bennett RJ. Parasex generates phenotypic diversity de novo and impacts drug resistance and virulence in Candida albicans. Genetics. 2017;207:1195–211. 10.1534/genetics.117.300295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forche A, Alby K, Schaefer D, Johnson AD, Berman J, Bennett RJ. The parasexual cycle in Candida albicans provides an alternative pathway to meiosis for the formation of recombinant strains. PLoS Biol. 2008;6:e110 10.1371/journal.pbio.0060110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reedy JL, Floyd AM, Heitman J. Mechanistic plasticity of sexual reproduction and meiosis in the Candida pathogenic species complex. Curr Biol. 2009;19:891–9. 10.1016/j.cub.2009.04.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ni M, Feretzaki M, Li W, Floyd-Averette A, Mieczkowski P, Dietrich FS, et al. Unisexual and heterosexual meiotic reproduction generate aneuploidy and phenotypic diversity de novo in the yeast Cryptococcus neoformans. PLoS Biol. 2013;11:e1001653 10.1371/journal.pbio.1001653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun S, Billmyre RB, Mieczkowski PA, Heitman J. Unisexual reproduction drives meiotic recombination and phenotypic and karyotypic plasticity in Cryptococcus neoformans. PLoS Genet. 2014;10:e1004849 10.1371/journal.pgen.1004849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett RJ, Forche A, Berman J. Rapid mechanisms for generating genome diversity: whole ploidy shifts, aneuploidy, and loss of heterozygosity. Cold Spring Harb Perspect Med. 2014;4:a019604 10.1101/cshperspect.a019604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selmecki A, Gerami-Nejad M, Paulson C, Forche A, Berman J. An isochromosome confers drug resistance in vivo by amplification of two genes, ERG11 and TAC1. Mol Microbiol. 2008;68:624–41. 10.1111/j.1365-2958.2008.06176.x [DOI] [PubMed] [Google Scholar]
- 11.Sankaranarayanan SR, Ianiri G, Coelho MA, Reza MH, Thimmappa BC, Ganguly P, et al. Loss of centromere function drives karyotype evolution in closely related Malassezia species. elife. 2020;9:e53944 10.7554/eLife.53944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waalwijk C, Taga M, Zheng S-L, Proctor RH, Vaughan MM, O’Donnell K. Karyotype evolution in Fusarium. IMA Fungus. 2018;9:13–26. 10.5598/imafungus.2018.09.01.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muller H, Thierry A, Coppée JY, Gouyette C, Hennequin C, Sismeiro O, et al. Genomic polymorphism in the population of Candida glabrata: gene copy-number variation and chromosomal translocations. Fungal Genet Biol. 2009;46:264–76. 10.1016/j.fgb.2008.11.006 [DOI] [PubMed] [Google Scholar]
- 14.Bravo Ruiz G, Ross ZK, Holmes E, Schelenz S, Gow NAR, Lorenz A. Rapid and extensive karyotype diversification in haploid clinical Candida auris isolates. Curr Genet. 2019;65:1217–28. 10.1007/s00294-019-00976-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forche A, Magee PT, Selmecki A, Berman J, May G. Evolution in Candida albicans populations during a single passage through a mouse host. Genetics. 2009;182:799–811. 10.1534/genetics.109.103325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shin JH, Myung JC, Jeong WS, Jung SI, Cho D, Seung JK, et al. Changes in karyotype and azole susceptibility of sequential bloodstream isolates from patients with Candida glabrata candidemia. J Clin Microbiol. 2007;45:2385–91. 10.1128/JCM.00381-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sekizuka T, Iguchi S, Umeyama T, Inamine Y, Makimura K, Kuroda M, et al. Clade II Candida auris possess genomic structural variations related to an ancestral strain. PLoS ONE. 2019;14:e0223433 10.1371/journal.pone.0223433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magee BB, Magee PT. Induction of mating in Candida albicans by construction of MTLa and MTLα strains. Science. 2000;289:310–3. 10.1126/science.289.5477.310 [DOI] [PubMed] [Google Scholar]
- 19.Xie J, Tao L, Nobile CJ, Tong Y, Guan G, Sun Y, et al. White-opaque awitching in natural MTLa/α isolates of Candida albicans: evolutionary implications for roles in host adaptation, pathogenesis, and sex. PLoS Biol. 2013;11:e1001525 10.1371/journal.pbio.1001525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hull CM, Raisner RM, Johnson AD. Evidence for mating of the “asexual” yeast Candida albicans in a mammalian host. Science. 2000;289:307–10. 10.1126/science.289.5477.307 [DOI] [PubMed] [Google Scholar]
- 21.Erke KH. Light microscopy of basidia, basidiospores, and nuclei in spores and hyphae of Filobasidiella neoformans (Cryptococcus neoformans). J Bacteriol. 1976;128:445–55. 10.1128/JB.128.1.445-455.1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wickes BL, Mayorga ME, Edman U, Edman JC. Dimorphism and haploid fruiting in Cryptococcus neoformans: association with the alpha-mating type. Proc Natl Acad Sci U S A. 1996;93:7327–31. 10.1073/pnas.93.14.7327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roach KC, Heitman J. Unisexual reproduction reverses Muller’s ratchet. Genetics. 2014;198:1059–69. 10.1534/genetics.114.170472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du H, Zheng Q, Bing J, Bennett RJ, Huang G. A coupled process of same- and opposite-sex mating generates polyploidy and genetic diversity in Candida tropicalis. PLoS Genet. 2018;14:e1007377 10.1371/journal.pgen.1007377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alby K, Schaefer D, Bennett RJ. Homothallic and heterothallic mating in the opportunistic pathogen Candida albicans. Nature. 2009;460:890–3. 10.1038/nature08252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muñoz JF, Gade L, Chow NA, Loparev VN, Juieng P, Berkow EL, et al. Genomic insights into multidrug-resistance, mating and virulence in Candida auris and related emerging species. Nat Commun. 2018;9:5346 10.1038/s41467-018-07779-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatterjee S, Alampalli SV, Nageshan RK, Chettiar ST, Joshi S, Tatu US. Draft genome of a commonly misdiagnosed multidrug resistant pathogen Candida auris. BMC Genomics. 2015;16:686 10.1186/s12864-015-1863-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chow NAA, Muñoz JFF, Gade L, Berkow ELL, Li X, RMM W, et al. Tracing the evolutionary history and global expansion of Candida auris using population genomic analyses. MBio. 2020;11:e03364–19. 10.1128/mBio.03364-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borman AM, Szekely A, Johnson EM. Isolates of the emerging pathogen Candida auris present in the UK have several geographic origins. Med Mycol. 2017;55:563–7. 10.1093/mmy/myw147 [DOI] [PubMed] [Google Scholar]
- 30.Tzung K-W, Williams RM, Scherer S, Federspiel N, Jones T, Hansen N, et al. Genomic evidence for a complete sexual cycle in Candida albicans. Proc Natl Acad Sci U S A. 2001;98:3249–53. 10.1073/pnas.061628798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schurko AM, Logsdon JM. Using a meiosis detection toolkit to investigate ancient asexual “scandals” and the evolution of sex. BioEssays. 2008;30:579–89. 10.1002/bies.20764 [DOI] [PubMed] [Google Scholar]
- 32.Cendejas-Bueno E, Kolecka A, Alastruey-Izquierdo A, Theelen B, Groenewald M, Kostrzewa M, et al. Reclassification of the Candida haemulonii complex as Candida haemulonii (C haemulonii group I), C duobushaemulonii sp. nov. (C haemulonii group II), and C. haemulonii var. vulnera var. nov.: three multiresistant human pathogenic yeasts. J Clin Microbiol. 2012;50:3641–51. 10.1128/JCM.02248-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michaelis S, Herskowitz I. The a-factor pheromone of Saccharomyces cerevisiae is essential for mating. Mol Cell Biol. 1988;8:1309–18. 10.1128/mcb.8.3.1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magee BB, Legrand M, Alarco A-M, Raymond M, Magee PT. Many of the genes required for mating in Saccharomyces cerevisiae are also required for mating in Candida albicans. Mol Microbiol. 2002;46:1345–51. 10.1046/j.1365-2958.2002.03263.x [DOI] [PubMed] [Google Scholar]
- 35.Raymond M, Dignard D, Alarco A-M, Mainville N, Magee BB, Thomas DY. A Ste6p/P-glycoprotein homologue from the asexual yeast Candida albicans transports the a-factor mating pheromone in Saccharomyces cerevisiae. Mol Microbiol. 1998;27:587–98. 10.1046/j.1365-2958.1998.00704.x [DOI] [PubMed] [Google Scholar]
- 36.Wasi M, Kumar Khandelwal N, Moorhouse AJAJ, Nair R, Vishwakarma P, Bravo Ruiz G, et al. ABC transporter genes show upregulated expression in drug resistant clinical isolates of Candida auris: a genome-wide characterization of ATP-binding cassette (ABC) transporter genes. Front Microbiol. 2019;10:1445 10.3389/fmicb.2019.01445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunter N. Meiotic recombination: the essence of heredity. Cold Spring Harb Perspect Biol. 2015;7:a016618 10.1101/cshperspect.a016618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2014;7:539–9. 10.1038/msb.2011.75 [DOI] [PMC free article] [PubMed] [Google Scholar]