Abstract
Prescription opioid abuse is a serious public health issue. Recently, we showed that female and male Sprague-Dawley rats acquire conditioned place preference (CPP) to the mu opioid receptor agonist oxycodone. Anatomical analysis of the hippocampus from these rats unveiled sex differences in the opioid system in a way that would support excitation and opiate associative learning processes, especially in females. In this study, we examined the expression and protein densities of opioid, plasticity, stress and related kinase and signaling molecules in the hippocampus of female and male rats following oxycodone CPP. Oxycodone CPP females have: a) increases in ARC (activity regulated cytoskeletal-associated protein)-immunoreactivity (ir) in CA3 pyramidal cells; b) decreases in Npy (neuropeptide Y) gene expression in the medial hippocampus but higher numbers of NPY-containing hilar interneurons compared to males; c) increases in Crhr2 (corticotropin releasing factor receptor 2) expression in CA2/3; d) increases in Akt1 (AKT serine/threonine kinase 1) expression in medial hippocampus; and e) decreases in phosphorylated MAPK (mitogen activated protein kinase)-ir in CA1 and dentate gyrus. Oxycodone CPP males have: a) increases in Bdnf (brain derived-neurotrophic factor) expression, which is known to be produced in granule cells, relative to females; b) elevated Mapk1 expression and pMAPK-ir in the dentate hilus which harbors newly generated granule cells; and c) increases in CRHR1-ir in CA3 pyramidal cell soma. These sex-specific changes in plasticity, stress and kinase markers in hippocampal circuitry parallel previously observed sex differences in the opioid system after oxycodone CPP.
Keywords: Activity regulated cytoskeletal-associated protein, AKT serine/threonine kinase, Corticotropin releasing hormone receptors, Mitogen activated protein kinase, Neuropeptide Y, Opioid addiction
INTRODUCTION
Prescription opioid abuse has increased dramatically over the past two decades and has become a serious public health issue (CDC, 2015). Notably, drug overdose rates involving synthetic opioids and heroin have dramatically increased in women (VanHouten et al., 2019). The influence of sex in opioid addiction processes has been challenging to elucidate, as it is difficult to separate the role of genes, environment, and multidrug use in humans. However, the finding that women may have altered sensitivity to morphine over the menstrual cycle (Ribeiro-Dasilva et al., 2011) suggests that ovarian hormones could be involved in addictive processes. Indeed, rodent studies have indicated that the hormonal milieu likely plays a part in opioid addiction (Becker et al., 2017). For example, fluctuations in circulating estrogen levels over the estrous cycle in rats have been reported to alter the patterns of heroin self-administration (Lacy et al., 2016).
Neural circuits involved in associative memory formation and the encoding of motivational incentives are critically involved in the pathway to drug addiction for both sexes (Koob and Volkow, 2010). In rodents, opioid signaling in the CA3 region of the hippocampus is implicated in associative learning and spatial memory (Meilandt et al., 2004; Kesner and Warthen, 2010). Additionally, a type of opioid-mediated long-term potentiation (LTP) has been demonstrated in mossy fiber-CA3 synapses from proestrus (high estrogen) female rats, but not diestrus (low estrogen) female or male rats (Harte-Hargrove et al., 2015), suggesting that opioid associative learning processes could be heightened in females in particular hormonal states.
Recently, we have shown that both female and male Sprague-Dawley rats acquire conditioned place preference (CPP) to the mu opioid receptor (MOR) agonist oxycodone (Ryan et al., 2018). Notably, anatomical analysis of the hippocampus from these rats unveiled sex differences in the opioid system in a way that would bolster excitation and opiate associative learning processes to a greater degree in females than males (Ryan et al., 2018). In particular, delta opioid receptors (DOR) redistributed within mossy fiber-CA3 synapses in both oxycodone-injected (Oxy) females and males in a manner similar to that demonstrated to be important for opioid-mediated LTP in proestrus females (Harte-Hargrove et al., 2015; Ryan et al., 2018). Additionally, MORs and DORs rearranged in hilar interneurons in Oxy-females in a configuration that could enhance granule cell disinhibition using two different circuits:
1) plasmalemmal associated MORs increased in parvalbumin (PARV)-labeled dendrites (Ryan et al., 2018) which are known to inhibit granule cell soma (Drake et al., 2007); 2) plasmalemmal associated DORs increased on GABAergic interneuron dendrites (Ryan et al., 2018) which are known to contain neuropeptide Y (NPY) and are important for promoting lateral perforant pathway (lpp) LTP (Sperk et al., 2007). However, whether these changes in hippocampal opioid receptor trafficking also are accompanied by changes in gene expression is unknown.
Thus, the goal of this study was to investigate the effect of oxycodone CPP in female and male rats on opioid peptides and receptor gene expression as well as synaptic plasticity, stress and related signaling molecules. Genes selected for analysis were the same as those in our recent study in which we demonstrated sex differences in their expression following chronic stress (Randesi et al., 2018). To elucidate these findings, we examined the immunocytochemical distributions of the relevant proteins in the hippocampus from a separate cohort of rats that had been given oxycodone in a CPP paradigm.
EXPERIMENTAL PROCEDURES
Animals
A total of 48 adult Sprague-Dawley female (~225-250 g upon arrival) and male (~275-325 g upon arrival) rats were used in this study (RGD Cat# 734476, RRID:RGD_734476; Charles River Laboratories, Wilmington, PA). To maintain rigor and reproducibility and minimize experimental variability due to differences in the vendor and institute environment as well as experimenter handling, molecular experiments were performed on a single cohort of 24 female and male rats obtained from the vendor at the same time. Similarly, anatomical experiments were performed on a single cohort of 24 female and male rats that were used in our previous study (Ryan et al., 2018). Rats were provided ad libitum food (PicoLab Rodent diet 20; LabDiet, St. Louis, MO) and water in a room with a 12:12 light/dark cycle (lights on at 0600) where they were single-housed in R20 rat cages (10.5 in × 19 in × 8 in; Ancare, Bellmore, NY). Animal procedures were approved by the Institutional Animal Care and Use Committees at the Rockefeller University and Weill Cornell Medicine and were compliant with the 2011 8th Edition of the NIH guidelines for the Care and Use of Laboratory Animals.
Handling and Estrous Cycle Determination
As rodent cortisol levels can be significantly altered following handling (Deutsch-Feldman et al., 2015; Collins et al., 2016), rats were gently handled (~3-5 minutes) once daily for a week before the initiation of behavioral experiments to reduce corticosterone concentrations in adult male rats (Deutsch-Feldman et al., 2015). For each experimental cohort, the same investigators throughout the studies handled the rats. As cortisol levels vary throughout the day, behavioral experiments were performed between 9:00 am and 1:00 pm.
Previous studies have shown that associative memory behaviors including cocaine CPP will be extinguished following regular estrous cycle monitoring of rodents using swabbing or vaginal lavage (Walker et al., 2001; Walker et al., 2002; Van Kempen et al., 2014). Moreover, significant side preference in the CPP apparatus can be induced following regular estrous cycling monitoring with vaginal lavage in the absence of CPP (Walker et al., 2002). Given the drawbacks of combining CPP behavior with estrous cycling and difficulty in reproducing this exact process in males, estrous cycle stage using vaginal smear cytology (Turner and Bagnara, 1971) was only assessed on the last experimental day at the time of euthansia. As in our prior studies (Milner et al., 2013; Pierce et al., 2014; Mazid et al., 2016), estrous cycles were classified as proestrus (0.5-1 day long, elevated estrogen levels), estrus (2-2.5 days long, declining estrogen levels), or diestrus (2-2.5 days long, low estrogen and progestin levels). All but two female rats were in estrus in the cohort used for the anatomical studies (Ryan et al., 2018) and in the cohort used for the mRNA studies.
Oxycodone CPP
Behavioral experiments were staggered over a period of 4 days between 9:00 am and 1:00 pm for all rats. The CPP apparatus (Med Associates Inc., Fairfax, VT) consisted of three compartments (lateral black, lateral white and a central gray) separated by removable doors. During the test, locomotor activity and time spent in each compartment were monitored using infrared photobeams. A dose of 3 mg/kg (i.p.) oxycodone (Sigma-Aldrich #O1378) was used because it has been shown to induce 90-100% CPP in rats in prior studies (Olmstead and Burns, 2005; Ryan et al., 2018). Previous oxycodone self-administration studies in Sprague-Dawley rats using higher doses and longer duration of oxycodone than the present experiment showed that the females exhibited normal estrous cycles (Mavrikaki et al., 2017), suggesting no impairment by the drug on normal cycling.
A 14-day CPP protocol was employed: 1) preconditioning (Day 1): the rats were allowed to freely explore the entire apparatus for 30 minutes, after removing the compartment doors. In the cohort of rats used for the molecular studies, individual rats had a compartmental bias following preconditioning. In the cohort of rats used for the anatomical studies, all females preferred the black compartment and all males preferred the white compartment (Ryan et al., 2018). Thus, for both cohorts we used a biased CPP design in which rat were injected with oxycodone on the less-preferred side of the apparatus. 2) CPP training (Days 2-9): After adding the doors to the apparatus to separate compartments, rats were subjected to four training sessions, each two days long. On the first day of each session, rats were injected with oxycodone (3 mg/kg, i.p.) and put in the less preferred compartment for 30 minutes [a time span within the 3-5 hour half-life of oxycodone (Ordonez Gallego et al., 2007)]. On the second day of each session the rats were injected with saline and placed in the opposite side from which they were injected with oxycodone. In each session, the control rats were injected with saline in both compartments. 3) CPP test (Day 14; four days after the last session): After removing the compartment doors, the rats were put in the central gray compartment of the apparatus and their behavior was recorded for 30 minutes. For each rat, preference score was calculated by first dividing the time spent in the oxy-paired chamber over the time spent in both the oxy- and saline-paired chambers both during preconditioning and on the final test day. We subtracted pre-test preference score from the post-test preference score, yielding a measure of change in preference score. As previously reported for the cohort of rats used for the anatomical studies (Ryan et al., 2018), one female and one male rat did not acquire oxycodone CPP. Thus, these rats were excluded from the present studies.
RNA Isolation and cDNA Synthesis
Procedures for preparing RNA were similar to previous studies (Zhang et al., 2016). Collection of the brains for molecular studies was staggered over a period of 4 days and occurred between 9:00 am and 1:00 pm. Rats were deeply anesthetized with CO2, decapitated with a guillotine and their brains were extracted from the skull. The analysis was limited to the dorsal hippocampus so as to correlate with prior studies showing changes in opioid, plasticity and stress markers (McEwen and Milner, 2017). A 4 mm thick coronal section of the dorsal hippocampus was dissected from each rat using a rat brain matrix (ASI Instruments, Warren MI). The hippocampal slab was further divided into medial [(CA1 and dentate gyrus (DG)] and lateral (CA2/CA3) parts which were homogenized in 700 μl of Qiazol (Qiagen, Inc., Germantown, MD) and immediately frozen and stored at −80°C until the RNA was prepared.
Total RNA was isolated from homogenates using the miRNeasy Kit (Qiagen Inc.), as per the manufacturer’s recommendations. An Agilent 2100 Bioanalyzer (Agilent Technologies, San Francisco, CA) was used to determine the quantity and quality of RNA from each sample. All RNA samples were of high quality with an RNA Integrity Number (RIN) ranging from 8.0–9.0. Complementary DNA (cDNA) was prepared from 0.5 μg of RNA using the RT2 First Strand Kit (Qiagen Inc.). Random hexamers and oligo-dT primers were used in the RT reaction to produce cDNA in an unbiased manner. The RT reaction contained an external RNA control to monitor the efficiency and test for enzyme inhibitors that may contaminate the RNA samples during subsequent gene expression analysis.
RT2 Profiler Array
Gene expression levels were measured using RT2SYBR® Green qPCR Mastermix and a custom RT2 PCR Array (both from Qiagen, Inc.) according to the manufacturer’s instructions. The expression array contained 23 genes of interest (Table 1) and five reference genes. The genes of interest were chosen because prior studies showed them to be important for opioid, plasticity and stress responses in the rat hippocampus or because prior studies had shown them to be down-regulated following chronic stress in male rats (Randesi et al., 2018) or upregulated after oxycodone self-administration in mice (Zhang et al. 2014). The genes of interest were: opioid peptides and receptors (N = 7), synaptic plasticity-related (N = 5), stress-related (N = 6), and kinase or signaling molecules (N = 5). An ABI PRISM™ 7900HT Sequence Detection System (Applied Biosystems, Inc., Foster City, CA) was used to run the qPCR reactions. One control female was eliminated from the analysis as the values for the housekeeping genes were outliers compared to the other female and male rats. Threshold cycle (Ct) values were employed to calculate the mean (± SEM) normalized gene expression levels.
Table 1.
Genes selected for analysis.
| Group | Symbol | Gene Name |
|---|---|---|
| Opioid | Oprd1 | opioid receptor, delta |
| Oprk1 | opioid receptor, kappa | |
| Oprl1 | nociceptin receptor | |
| Oprm1 | opioid receptor, mu | |
| Pdyn | prodynorphin | |
| Penk | proenkephalin | |
| Pomc | proopiomelanocortin | |
| Plasticity | Arc | activity regulated cytoskeleton-associated protein |
| Bdnf | brain-derived neurotrophic factor | |
| Cdh2 | calcium dependent adhesion transmembrane protein | |
| Npy | neuropeptide Y | |
| Ntrk2 | neurotrophic tyrosine kinase, receptor, type 2 | |
| Stress | Avpr1a | Avp receptor 1a |
| Crh | corticotropin releasing hormone | |
| Crhr1 | corticotropin releasing hormone receptor 1 | |
| Crhr2 | corticotropin releasing hormone receptor 2 | |
| Fkbp5 | FK506 binding protein 5 | |
| Nr3c1 | glucocorticoid receptor | |
| Kinases & Signaling | Akt1 | AKT serine/threonine kinase 1 |
| Arrb1 | arrestin, beta 1 | |
| Arrb2 | arrestin, beta 2 | |
| Mapk1 | mitogen activated protein kinase 1 | |
| Pim1 | Pim1 proto-oncogene, serine/threonine kinase |
Immunocytochemistry Procedures
Section Preparation
Collection of the rat brains for the anatomical studies occurred between 9:00 am and 1:00 pm over a span of 4 days (Ryan et al., 2018). Rats were deeply anesthetized with a mixture of xylazine (10 mg/kg) and ketamine (100 mg/kg) and perfused sequentially through the ascending aorta with: 1) 10-15 ml normal (0.9%) saline, 2) 50 ml of 2% paraformaldehyde (PFA) and 3.75% acrolein in 0.1 M phosphate buffer (PB; pH = 7.4), and 3) 200 ml of 2% PFA in PB. After removing brains from the skull, they were cut into 5 mm coronal blocks using a rat brain mold (RBM-4000C; ASI Instruments), and post-fixed in 2% PFA in PB for 30 minutes prior to transferring into PB. Hippocampal sections (40 μm thick) were cut with a vibratome (VT1000S, Leica Microsystems, Buffalo Grove, IL) and collected into 24-well plates containing PB. Sections were stored in a cryoprotectant solution (30% ethylene glycol and 30% sucrose in PB) at −20°C. Sections were selected from the dorsal hippocampus [−3.5 to −4.2 mm from Bregma (Swanson, 1992)] for each immunocytochemical experiment. To ensure identical labeling conditions between experimental groups, the sections were coded with hole punches and processed together in single containers through all immunocytochemistry procedures (Pierce et al., 1999). This method has been shown to reliably yield equal penetration of antibodies in tissues for both cell counts and densitometry measurements (Milner, 2011). Sections were incubated in 1% sodium borohydride in PB for 30 minutes to neutralize reactive aldehydes (Milner, 2011) then rinsed 8-10 times in PB until gaseous bubbles disappeared.
Antibody Characterization
Phosphorylated Akt (serine/threonine kinase 1; pAKT):
A rabbit polyclonal antibody against pAKT (Thr308; Cell Signaling Technology Cat# 9275, RRID:AB_329828) was used. The antibody does not detect non-phosphorylated AKT or phosphorylated AKT at other sites and it does not cross-react with related family members such as PKC or p70 S6 kinase (Cell Signaling manufacturer’s specification sheet). This antibody has been used in prior light and electron microscopic (EM) studies on acrolein/PFA perfusion-fixed hippocampal brain sections (Znamensky et al., 2003).
Activity-regulated cytoskeleton-associated protein (ARC):
A goat anti-ARC antibody (Santa Cruz Biotechnology Cat# sc-6381, RRID:AB_634090) was used in this study. The antibody was raised against the C-terminus of ARC of rat origin. On Western blot, ARC protein detection is absent in homozygous mice lacking Arc (Wang et al., 2006).
Corticotropin releasing hormone receptor 1 (CRHR1):
A goat polyclonal antibody raised against the 425-444 amino acid sequence of the carboxyterminus of the CRH receptor precursor of human origin, which is identical to the corresponding sequence in the rat (Santa Cruz Biotechnology Cat# sc-1757, RRID:AB_673600) was used in the EM study. Several lines of evidence indicate that the antibody preferentially recognizes CRHR1 and not CRHR2 [reviewed in Bangasser et al. (2010), supplementary material]. This antibody recognizes 1 band at ~80kD on Western blots of mouse hypothalamus; this band is absent on Western blots from CRHR1 knockout mice (Chen et al., 2000). Labeling for the CRHR1 antibody is absent on acrolein/PFA fixed sections from the dorsal raphe of CRHR1 knockout mice (Waselus et al., 2009). Additionally, no labeling is seen in immunoblots and tissue sections following preadsorption with the antigenic peptide (Chen et al., 2000; Sauvage and Steckler, 2001; Reyes et al., 2006; Bangasser et al., 2010). This antibody was used in our prior EM studies (Williams et al., 2011a; Williams and Milner, 2011; McAlinn et al., 2018).
Mitogen-activated protein kinase (MAPK):
A rabbit anti-p44/42 MAPK antibody (Erk1 / Erk2; Cell Signaling Technology Cat# 9102, RRID:AB_330744) was used. In Western blots, this antibody detects endogenous levels of total p44/42 MAPK protein and does not recognize either JNK/SAPK or p38 MAP kinase (manufacturer’s data sheet).
Phosphorylated MAPK (pMAPK):
A rabbit anti-phospho-p44/42 MAPK antibody (Thr202/Try204; Cell Signaling Technology; Cat# 9101) was used. On Western blots, this antibody detects endogenous levels of p44 and p42 MAPK when phosphorylated either individually or dually at Thr202 and Tyr204 of Erk1 (Thr185 and Tyr187 of Erk2; manufacturer’s data sheet). This antibody does not cross-react with the corresponding phosphorylated residues of p38 MAP kinase or JNK/SAPK and does not cross-react with non-phosphorylated Erk1/2 (manufacturer’s data sheet).
Neuropeptide Y (NPY):
A rabbit polyclonal antibody against porcine NPY (Peninsula Laboratories Cat# T-4070.0050 San Carlos, CA; RRID:AB_518504) was used. This antibody recognizes a 10−8 M concentration of NPY and closely related peptides YY and pancreatic polypeptide at 10−5 M concentrations on immunodot blots (Milner & Veznedaroglu 1992). This antibody has been employed in prior light and EM studies (Ledoux et al. 2009; Milner & Veznedaroglu 1992; Rogers et al. 2016).
Light Microscopic Immunocytochemistry
Experimental procedure:
Hippocampal tissue sections were processed using formerly described methods (Milner, 2011) to assess changes in the density of ARC, pAKT, MAPK, pMAPK and NPY-immunoreactivities. In brief, tissue sections were rinsed in 0.1 M Tris-buffered saline (TS; pH = 7.6) and blocked in 0.5% bovine serum albumin (BSA) in TS for 30 minutes. Sections were incubated in either goat anti-ARC (1:800 dilution), rabbit anti-pAKT (1:800 dilution), rabbit anti-MAPK (1:500 dilution), rabbit anti-pMAPK (1:500 dilution) or rabbit anti-NPY (1:15,000 dilution) antibody in 0.1% BSA and TS for 24 hours at room temperature (25°C) followed by an additional incubation at 4°C for 24 hours (ARC and pAKT), 3 days (NPY) or 4 days (MAPK and pMAPK). Triton X-100 (0.25%) was added to the NPY diluent. Sections then were incubated in a 1:400 dilution of either biotinylated goat anti-rabbit immunoglobulin (IgG; pAKT, MAPK, pMAPK and NPY), or donkey anti-goat IgG (ARC; all from Jackson Labs.) for 30 minutes followed by TS rinses. Next, sections were incubated in avidin-biotin complex (ABC; at half the recommended dilution; Vectastain elite kit, Vector Laboratories, Burlingame, CA) for 30 minutes. After washing in TS, tissue sections were reacted in 3,3’-diaminobenzidine (DAB; Sigma-Aldrich, St. Louis, MO) and 3% H2O2 in TS for 4-7 minutes. Tissue sections were rinsed in PB and then were mounted from 0.05 Μ PB on 1% gelatin-coated glass slides, dehydrated through an ascending series of ethanols, and coverslipped from xylene with DPX mounting media (Sigma-Aldrich, Cat# 06522).
Analysis 1: pAKT, ARC, MAPK and pMAPK levels:
Densitometric quantification was performed using methods described in prior studies (Williams et al., 2011b; Pierce et al., 2014). All slides first were coded to ensure a blind analysis. Hippocampal images were captured at 10× on a Nikon Eclipse 80i microscope using a Micropublisher 5.0 digital camera (Q-imaging, BC, Canada) and IP Lab software (Scanalytics IPLab, RRID:SCR_002775). ImageJ64 (ImageJ, RRID:SCR_003070) software was used to measure average pixel density within hippocampal regions of interest (ROI). Pixel density was determined in four ROIs within the CA1 region: stratum oriens (SO), pyramidal cell layer (PCL), stratum radiatum (SR) or stratum lacunosum moleculare (SLM), four ROIs within the CA3 region: SO, PCL, stratum lucidum (SLu) or SR, or three ROIs within the DG: dorsal and ventral blades of the granule cell layer (gel) or central hilus. To control for variations in overall illumination levels and background staining, the pixel density of an unlabeled tissue region (e.g. the corpus callosum) was subtracted from ROI pixel density measurements. Prior studies verified the accuracy of this technique through a strong linear correlation between average pixel density and neutral density values of gelatin filters with defined transmittances ranging from 1 to 80% (Auchus and Pickel, 1992; Pierce et al., 1999; Pierce et al., 2014).
Analysis 2: NPY cell counts:
The number of NPY cells was determined in the DG hilus using previously described methods (Milner et al., 1997; Williams et al 2011b). NPY-labeled cells containing a nucleus were counted within a 200-μm2 rectangle in the CA3b (SO, PCL, SLu, SR combined) and CA1 (SO, PCL, SR combined) regions. The number of NPY-labeled cells in the DG hilus was determined by counting all cells lying within the gel and the CA3 PCL layers and dividing by the area of the hilus, measured using ImageJ64 software. In addition, NPY-labeled cells were counted in 200 μm2 subregions of the hilus [dorsal blade (db), central hilus (cen), and crest (cr)].
Electron microscopic immunocytochemistry
Hippocampal sections were labeled for CRHR1 with immunogold using previously described methods (Milner, 2011). After blocking with 0.5% BSA in TS for 30 minutes, sections were rinsed in TS and incubated in goat anti-CRHR1 (1:100 dilution) in 0.1% BSA in TS for 72 hours at 4°C. After rinsing in TS, sections were placed in washing buffer [with 2% gelatin and 0.1% BSA in 0.1 M phosphate-buffered saline (PBS)], and incubated overnight at 4°C in a 1:50 dilution of donkey anti-goat IgG conjugated to 1 nm gold particles [Cat# 25800, Electron Microscopy Sciences (EMS), RRID: AB_2631210] in 0.08% BSA and 0.01% gelatin. Sections were rinsed in PBS, post-fixed in 2% glutaraldehyde/PBS (10 min), rinsed in PBS and then placed in 0.2 M sodium citrate buffer (pH 7.4). The conjugated gold particles were enhanced for 6.5 minutes in silver solution (SEKL15 Silver enhancement kit, Prod No. 15718 Ted Pella Inc.).
Sections were fixed in 2% osmium tetroxide for 1 hour, dehydrated in increasing ethanol concentrations to propylene oxide and embedded in EMBed 812 (EMS) between two sheets of Aclar plastic (Honeywell, Pottsville, PA). Three rats from each experimental group (Sal- and Oxy-females, and Sal- and Oxy-males) were chosen randomly for EM analysis (N = 12).
Ultrathin sections (70-nm thick) from the CA3b region were cut on a Leica UCT ultratome and collected on 400 mesh thin-bar copper grids (T400-Cu, EMS). The grids were counterstained with Uranyless (EMS Cat# 22409) and Lead citrate (EMS Cat# 22410).
Electron microscopic localization of CRHR1
For the EM analysis of CRHR1, only tissues from females in estrus were used. People who were blinded to experimental conditions performed all data collection and analyses. Sections were analyzed on a Tecnai Biotwin transmission electron microscope (FEI, Hillsboro, OR) equipped with an Advanced Microscopy Techniques digital camera (Danvers, MA). Ultrathin sections were photographed from the plastic-tissue interface where immunolabeling access is optimal (Milner, 2011). Images of CA3 SR were collected at a magnification of 13,500x. Dendritic profiles were usually postsynaptic to axon terminal profiles and contained regular microtubular arrays and mitochondria (Peters, 1991).
Silver intensified immunogold (SIG) labeling for CRHR1 appeared as black electron-dense particles with varied sizes. To insure consistency in SIG labeling between blocks, samples were selected proximal to the plastic-tissue interface [i.e., the tissue surface; Milner (2011)].
For the CA3 PCL analysis, photographs were taken of 10 random CRHR1-SIG labeled soma with clearly distinguishable nuclei from each animal. For the analysis of CA3 SR, photographs of 50 random CRHR1-SIG labeled dendrites were taken from each animal. The area of each labeled soma and dendrite was measured using Microcomputer Imaging Device software (MCID Analysis, RRID:SCR_014278). Final cytoplasmic area of the soma was determined by subtracting total area from nuclear area. The number of SIG particles per unit area was then determined.
Figure Preparation
Adjustments were applied uniformly to the images; no feature within an image was introduced, removed, moved, obscured, or enhanced. Adjustments to brightness, sharpness, and contrast for light micrographs were made in Microsoft PowerPoint 2010. Electron micrographs were adjusted for image size, levels and sharpness in Adobe Photoshop 9.0 (Adobe Photoshop, RRID:SCR_014199) and then were imported into Microsoft PowerPoint 2010 for final layout, labeling and final adjustments to brightness and contrast. Graphs were made using Prism 7 software (Graphpad Prism, RRID:SCR_002798).
Statistical analysis
Data are expressed as mean ± SEM. Statistical analyses were conducted on JMP 12 Pro software (JMP, RRID:SCR_014242). Significance levels were set to an alpha < 0.05.
Behavior:
CPP data was analyzed using a two-way analysis of variance (ANOVA) followed by Tukey’s HSD post-hoc analysis on the change in preference for the oxy-paired chamber following the pre-test and the CPP paradigm post-test.
Molecular studies:
As described in prior studies (Randesi et al., 2018), a two-way ANOVA with sex and condition (saline vs. oxycodone CPP) as main effects, as well as their interaction was determined. For genes that produced significant ANOVA results (p < 0.05), Tukey-Kramer HSD post-hoc pairwise comparisons among the four studied groups were conducted. Post-hoc p-values < 0.05 were reported as nominally significant and p-values <0.1 were reported for exploratory purposes.
Light microscopic immunocytochemistry studies:
Comparisons of optical density for ARC, pAKT, pMAPK, and MAPK, as well as NPY cell counts were conducted using a Student’s t-test.
Electron microscopic immunocytochemistry study:
Main effect comparisons in CRHR1-SIG particle distributions were determined through a two-way ANOVA followed by Tukey’s HSD post-hoc analysis for specific effects.
RESULTS
Female and male rats both acquire oxycodone CPP
Two-way ANOVA showed a significant main effect of condition (saline vs. oxycodone CPP) for the cohort of female and male rats used in the molecular study [F(3,18) = 28.7; p < 0.0001)]. Neither a significant main effect of sex nor a significant interaction between sex and condition was observed. Post-hoc analysis showed that Oxy-females and Oxy-males had a greater percent change in preference for the oxycodone-paired side of the box compared to their saline-injected (Sal) counterparts (females: p = 0.032; males; p = 0.001; Fig. 1). There was no difference in preference scores between Oxy-males and Oxy-females (p = 0.796). On testing day, neither Sal-female nor Sal-male rats had a preference for either side of the box (p = 0.946). As reported in our previous study, the Oxy-injected rats used for the present anatomical studies also showed a significant preference for the Oxy-paired chamber and Sal-injected rats showed no preference for either compartment (Ryan et al., 2018). On the day of euthanasia, all females used in the molecular cohort were in the estrus phase of the estrous cycle.
Fig. 1. Both female and male rats used for molecular studies exhibit oxycodone conditioned place preference (CPP).
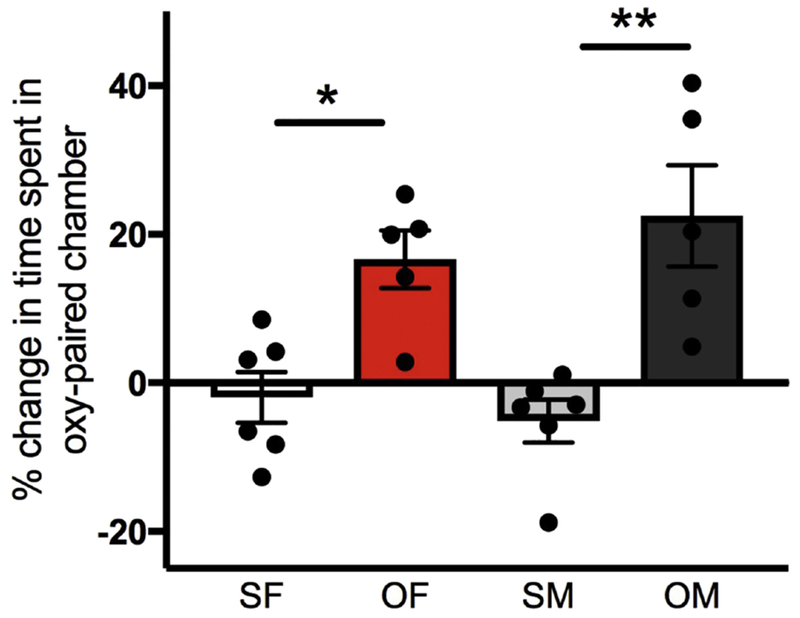
During the preconditioning phase, individual rats had a compartmental bias. Thus, oxycodone was administered on the less-preferred side for the animal. Preference score is the amount of time spent in the Oxy-paired chamber over the sum of time spent in Oxy- and Saline-paired chambers, calculated as a percent. The percent change in preference score, shown in the graph, is the pre-test preference score (of the side that would later be paired with oxycodone) subtracted from the post-test preference score of the Oxy-paired side. Saline-injected rats showed no significant change in preference score by sex. However, preference was significantly greater for Oxy-injected rats compared to their Saline-injected counterparts. SF, Sal-female; OF, Oxy-female; SM, Sal-male; OM, Oxy-male; * p < 0.05, ** p < 0.01.
Molecular studies – general considerations
Sex differences between Sal- and Oxy-CPP rats were found in plasticity and stress genes as well as their associated signaling molecules. A schematic defining the medial and lateral hippocampal samples is shown in figure 2.
Fig. 2. Schematic of hippocampal dissection used for molecular studies.
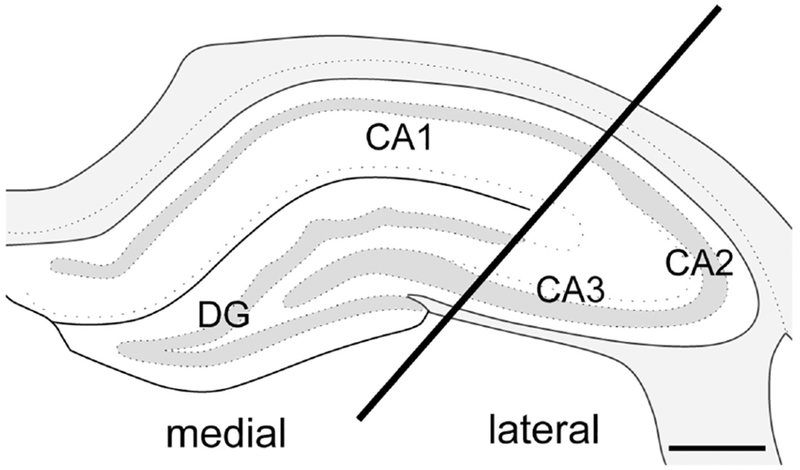
For the molecular studies, coronal dorsal hippocampal samples (~4 mm thick) were isolated and the block was separated into a medial sample primarily containing the CA1 and dentate gyrus and a lateral sample primarily containing the CA2 and CA3. Bar = 0.5 mm. Adapted from (Swanson, 1992).
Plasticity genes and related proteins
Arc and ARC:
Two-way ANOVA demonstrated a significant sex × condition (saline vs. oxycodone CPP) interaction for Arc mRNA expression in the lateral hippocampus [F(1,20) = 4.68, p = 0.043], Post-hoc analysis showed Sal-females had significantly greater Arc mRNA expression than Sal-males (p = 0.048) and that Oxy-males had significantly greater Arc mRNA expression than Sal-males (p = 0.049; Fig. 3A). Despite no significant main effect of Arc mRNA expression in the medial hippocampus, post-hoc analysis revealed Oxy-males had a trend toward more Arc expression compared to Sal-males (p = 0.087; Fig. 3B).
Fig. 3. Sex differences in Arc mRNA expression and ARC protein densities following oxycodone CPP.
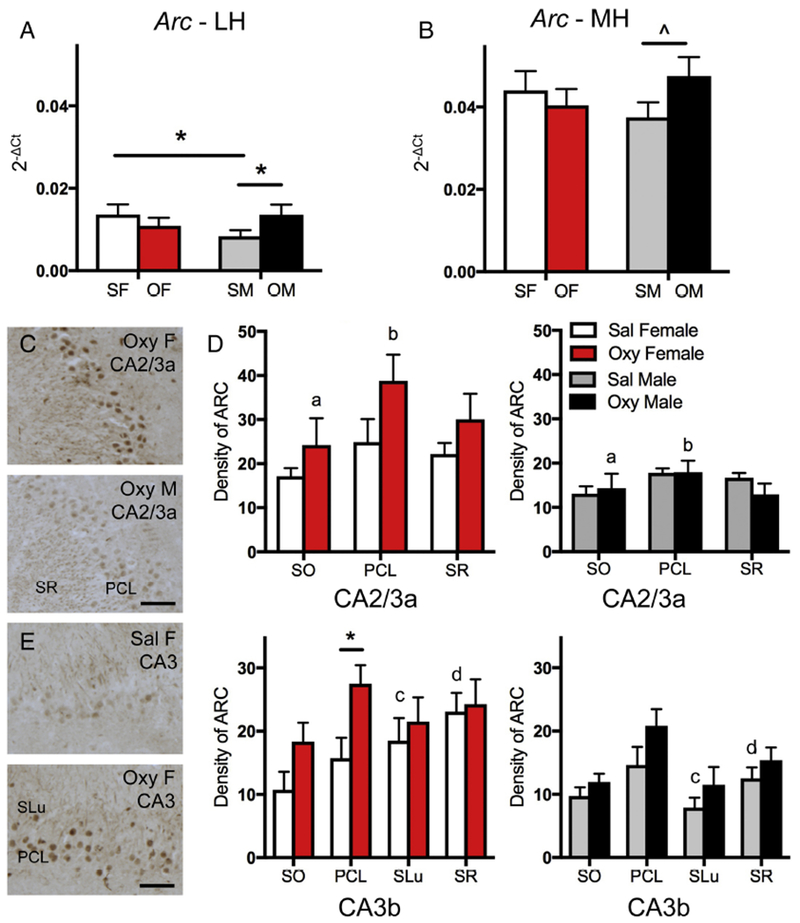
A. In the lateral hippocampus (LH), Sal-females (SF) had significantly more Arc mRNA expression than Sal-males (SM). Moreover, Sal-males had significantly less Arc mRNA expression than Oxy-males (OM). B. In the medial hippocampus (MH), Sal-males had a trend towards less Arc mRNA expression than Oxy-males. OF, Oxy-females. C. Representative light microscopic micrographs show higher densities of ARC-ir in the CA2/3a pyramidal cell layer (PCL) in an Oxy-female compared to an Oxy-male. D. In CA2/3a, the density of ARC-ir was significantly greater in stratum oriens (SO) and the PCL of Oxy-females compared to Oxy-males. SR, stratum radiatum. E. Representative light microscopic micrographs show lower densities of ARC-ir in the CA3b pyramidal cell layer (PCL) from a Sal-female compared to an Oxy-female. F. In CA3b, the density of ARC-ir was significantly greater in stratum lucidum (SLu) and SR of Sal-females compared to Sal-males. The density of ARC-ir was significantly greater in Oxy-females compared to Sal-females in the PCL. * p < 0.05, ^ p = 0.087, ap = 0.042, bp = 0.022, cp = 0.018, dp = 0.010.
Analysis of ARC-immunoreactivity (ir) in the hippocampal subregions contained in the lateral hippocampal sample revealed regional sex differences in ARC protein. Consistent with previous studies (Vazdarjanova et al., 2006), ARC-ir was detected within pyramidal cells and in processes within other hippocampal strata (for example, see Fig. 3E). In CA2/CA3a, there was no significant difference between the density of ARC-ir in any lamina in Sal-females compared to Sal-males (Fig. 3D). However, Oxy-females showed a greater density of ARC-ir than Oxy-males in both the SO [t(7) = −2.49; p = 0.042] and PCL [t(7) = −2.92; p = 0.022] of CA2/CA3a (Fig. 3C,D). In CA3b, Sal-females demonstrated a greater density of ARC-ir in SLu [t(9) = −2.89; p = 0.018] and SR [t(9) = −3.25; p = 0.01] compared to Sal-males. Further, Oxy-females demonstrated a greater density of ARC-ir in the CA3 PCL compared to Sal-females [t(8) = −2.70; p = 0.027; Fig. 3E,F]. In the PCL, ARC-ir was mostly increased within nuclei (Fig. 3E).
Bdnf:
Two-way ANOVA showed a significant sex × condition (saline vs. oxycodone CPP) interaction for Bdnf mRNA expression [F(1,19) = 5.55, p = 0.029] in the medial hippocampus. Post-hoc analysis showed Oxy-females had significantly less Bdnf mRNA expression than Oxy-males (p = 0.010; Fig. 4). Since previous studies have shown that brain derived neurotrophic factor (BDNF)-labeling is primarily detected in mossy fiber terminals by light microscopy [see review by Gray et al. (2013)], and a specific commercial antibody for BDNF could not be located, we did not analyze BDNF-ir further.
Fig. 4. Sex differences in Bdnf mRNA expression following oxycodone CPP.
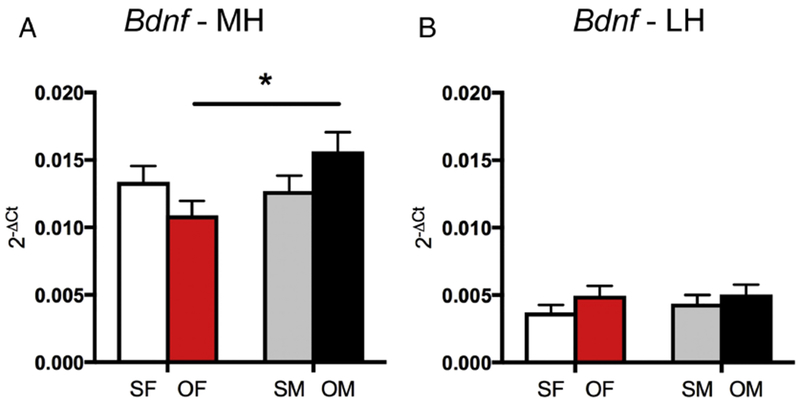
A. In the medial hippocampus (MH), Oxy-females (OF) had significantly less Bdnf mRNA expression than Oxy-males (OM). B. In the lateral hippocampus (LH) there are no differences in Bdnf mRNA expression between the groups. *p < 0.05.
Npy and NPY:
Two way ANOVA showed a significant sex × condition (saline vs. oxycodone CPP) interaction for Npy mRNA expression in the medial hippocampus [F(1,19) = 4.75, p = 0.042]. Post-hoc analysis of the medial hippocampus revealed Sal-females had significantly more Npy mRNA expression than Oxy-females (p = 0.028) while Oxy-males showed a trend toward more Npy mRNA expression compared to Oxy-females (p = 0.053; Fig. 5A). No significant differences in Npy mRNA expression were found between any group in the lateral hippocampus (Fig. 5B).
Fig. 5. Sex differences in Npy mRNA expression and NPY protein densities following oxycodone CPP.
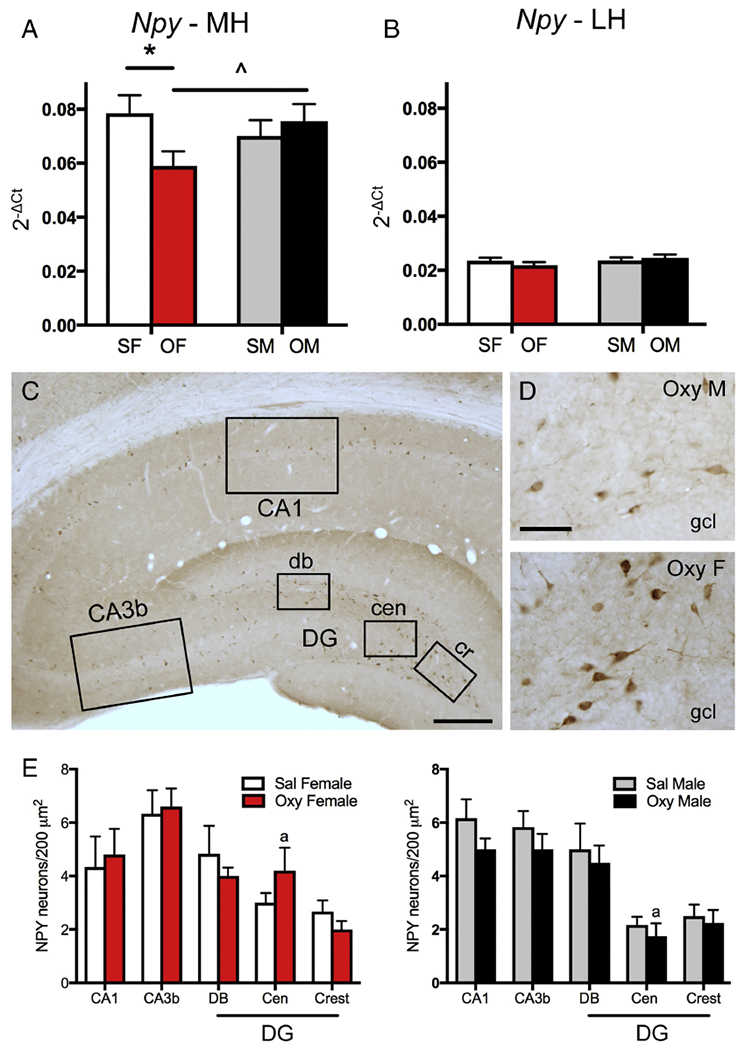
A. In the medial hippocampus (MH), Sal-females (SF) had significantly greater Npy expression than Oxy-females (OF). Moreover, Oxy-females had a trend towards less Npy expression compared to Oxy-males. B. In the lateral hippocampus (LH), there were no differences in Npy expression between any groups. C. Low magnification photomicrograph of the rat dorsal hippocampus shows NPY-labeled cells scattered throughout the hippocampus. Boxed areas show regions sampled for quantitative analysis: CA1, CA3b and the dorsal blade (db), central hilus (cen) and crest (cr) of the dentate gyrus (DG). Bar = 500 μm. D. Representative light microscope micrographs show fewer NPY-labeled neurons in the central hilus of the dentate gyrus of an Oxy-male compared to an Oxy-female. gel, granule cell layer Bar = 50 μm. E. There was a trend for more NPY-labeled cells in the central hilus of Oxy-females compared to Oxy-males. * p < 0.05, ap = 0.055.
Consistent with previous descriptions (Milner et al. 1997; Williams et al. 2011b), NPY is detected within scattered interneurons in the rat dorsal hippocampus. The numbers of NPY-labeled neurons were determined in the CA1 region and DG (both contained in the medial hippocampal sample) and CA3b (contained in the lateral hippocampal sample; Fig. 5C). There were no significant differences between Sal-female and Sal-male rats in any of these hippocampal regions (Fig. 5E). However, in contrast to Npy expression, Oxy-females trended toward a greater number of NPY-labeled neurons in the central hilus of the DG compared to Oxy-males [t(7) = −2.31; p = 0.055; Fig. 5D,E].
Stress genes and proteins
Crhr1, Crhr2 and CRHR1:
Two-way ANOVA showed a significant sex × condition (saline vs. oxycodone CPP) interaction on Crhr1 mRNA expression in the lateral hippocampus [F(1,20) =4.64, p = 0.044]. Post-hoc analysis showed that the Crhr1 mRNA expression of the Oxy-females was significantly greater than the Oxy-males (p = 0.032) and showed a trend for higher Crhr1 mRNA expression than the Sal-females (p = 0.081; Fig. 6A). Also in the lateral hippocampal region, two-way ANOVA showed that there was a significant main effect of condition (saline vs. oxycodone CPP) on Crhr2 expression [F(1,20) = 4.83, p = 0.03993]. Post-hoc analysis revealed that Oxy-females had significantly more Crhr2 mRNA expression than Sal-females (p = 0.023; Fig. 6B). Further, Sal-males had significantly greater Crhr2 mRNA expression than Sal-females (p = 0.040; Fig. 6B).
Fig 6. Sex differences in Crhr1 and Crhr2 mRNA expression and CRHR1 protein densities following oxycodone CPP.
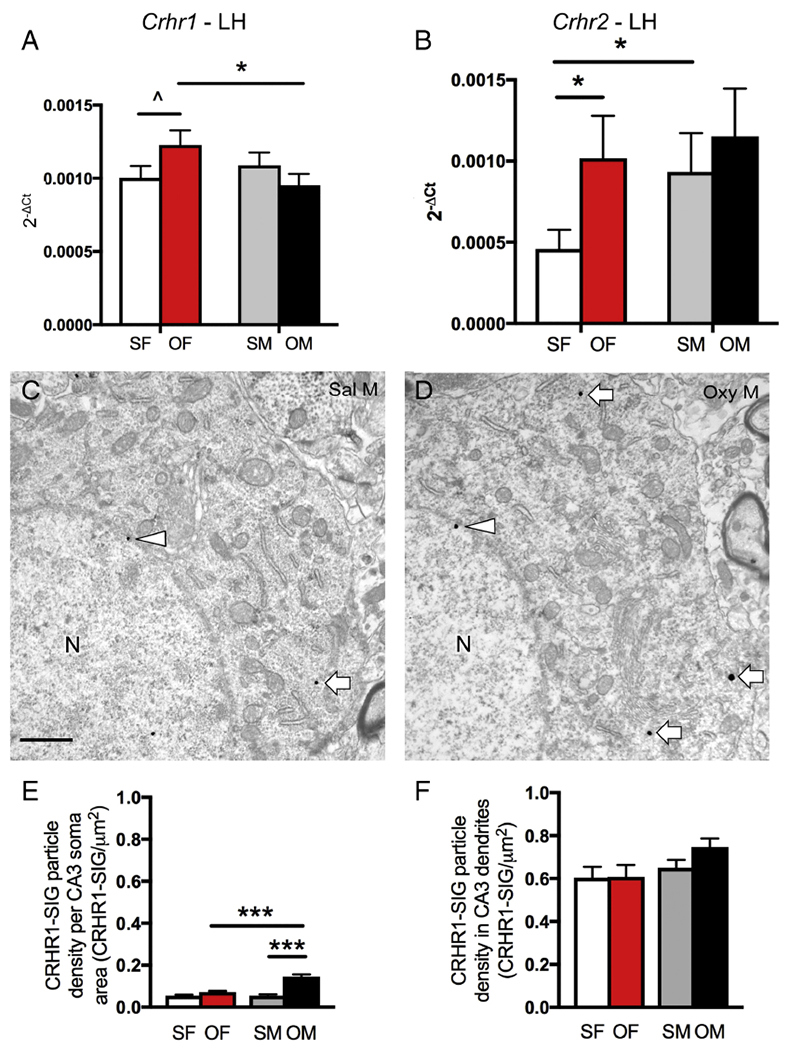
A. In the lateral hippocampus (LH), Sal-females (SF) had a trend towards less Crhr1 mRNA expression than Oxy-females (OF). Oxy-females had significantly more Crhr1 mRNA expression than Oxy-males (OM). B. In the lateral hippocampus, Sal-females had significantly less Crhr2 mRNA expression than Sal-males (SM) and Oxy-females. C & D. Representative electron micrographs show that fewer CRHR1-SIG particles (arrows) are found in the cytoplasm of soma from a Sal-male compared to an Oxy-male. In both micrographs, a CRHR1-SIG particle is found on the nuclear membrane (arrowhead). N, nucleus. Bar = 500 nm. E. In CA3, significantly more CRHR1-SIG particles are found in the Oxy-males compared to the Sal-males and Oxy-females. F. The density of CRHR1-SIG particles in CA3 dendrites is not different between the 4 groups. * p < 0.05, Λ p = 0.081.
Although CRHR1-ir is not detectable at the light level. our previous studies have demonstrated CRHR1-ir within CA3 pyramidal cells (McAlinn et al., 2018). Thus the CA3 region was further analyzed by electron microscopy. Consistent with our earlier studies, CRHR1-SIG particles were detected within the cytoplasm of pyramidal cell somata (Fig. 6C,D) and dendrites (not shown). Two-way ANOVA of the CRHR1-SIG particles within CA3 pyramidal cell somata showed significant main effects of sex [F(1,119) = 24.7; p< 0.0001], condition (saline vs. oxycodone CPP) [F(1,119) = 56.6; p< 0.0001], and the interaction between sex and condition [F(1,119) = 25.1; p< 0.0001]. Post-hoc analysis revealed significantly more CRHR1-SIG particles in pyramidal cell somata from Oxy-males compared to both Oxy-females and Sal-males (p < 0.0001 for both pairs; Fig. 6E). However, there were no differences between any groups in the number of CRHR1-SIG particles in CA3 dendrites (Fig. 6F).
Plasticity- and stress-related kinases and signaling genes and proteins
Akt1 and pAKT:
Two-way ANOVA showed a significant sex × condition (saline vs. oxycodone CPP) interaction on Akt1 mRNA expression [F(1,19) = 10.92, p = 0.004] in the medial hippocampus. Post-hoc analysis revealed that Oxy-females had significantly greater Akt1 mRNA expression than both Sal-females (p = 0.050) and Oxy-males (p = 0.003; Fig. 7A). In addition, Sal-males had significantly more Akt1 mRNA expression than Oxy-males (p = 0.018; Fig. 7A).
Fig. 7. Sex differences in Akt1 mRNA expression and pAKT protein densities following oxycodone CPP.
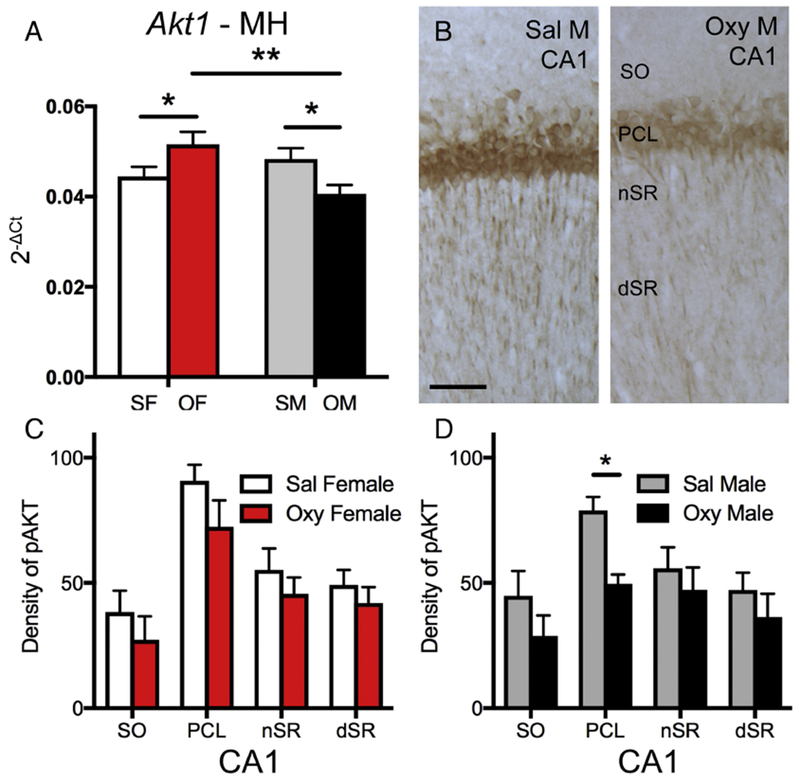
A. In the medial hippocampus (MH), Akt1 mRNA expression is significantly higher in Oxy-females (OF) compared to Sal-females (SF) and Oxy-males (OM). Moreover, Akt1 mRNA expression is significantly higher in Sal-males (SM) compared to Oxy-males. B. Representative light microscope micrographs show greater pAKT-ir in the CA1 pyramidal cell layer (PCL) of a Sal-male compared to an Oxy-male. SO, stratum oriens; nSR and dSR, near and distal stratum radiatum, respectively. C & D. There were no differences in pAKT-ir between Oxy- and Sal-females. Sal-males had significantly more pAKT-ir in the PCL compared to Oxy-males. * p < 0.05, ** p < 0.01.
Hippocampal subregions contained in the medial hippocampus were further analyzed for pAKT-ir. Consistent with earlier studies (Znamensky et al., 2003), pAKT-ir is found primarily in CA1 pyramidal cells and, to a lesser extent, in dendrites within SO and SR (Fig. 7B). Almost no pAKT-ir is detected in the DG (not shown). No differences were found in the densities of pAKT-ir between Sal-females and Sal-males in any of the CA1 lamina (Fig. 7C,D). However, Sal-males showed a significantly greater density of pAKT-ir in the CA1 PCL compared to that of Oxy-males [t(9) = 4.18; p = 0.0012; Fig. 7D].
Mapk1, MAPK and pMAPK:
Two-way ANOVA showed a significant main effect of sex on Mapk1 mRNA expression in the medial hippocampus [F(1,19) = 5.28, p = 0.0331]. Post-hoc analysis revealed that Oxy-females had significantly less Mapk1 mRNA expression than Oxy-males (p = 0.0105; Fig. 8A).
Fig. 8. Sex differences in Mapk1 expression and MAPK and pMAPK protein densities following oxycodone CPP.
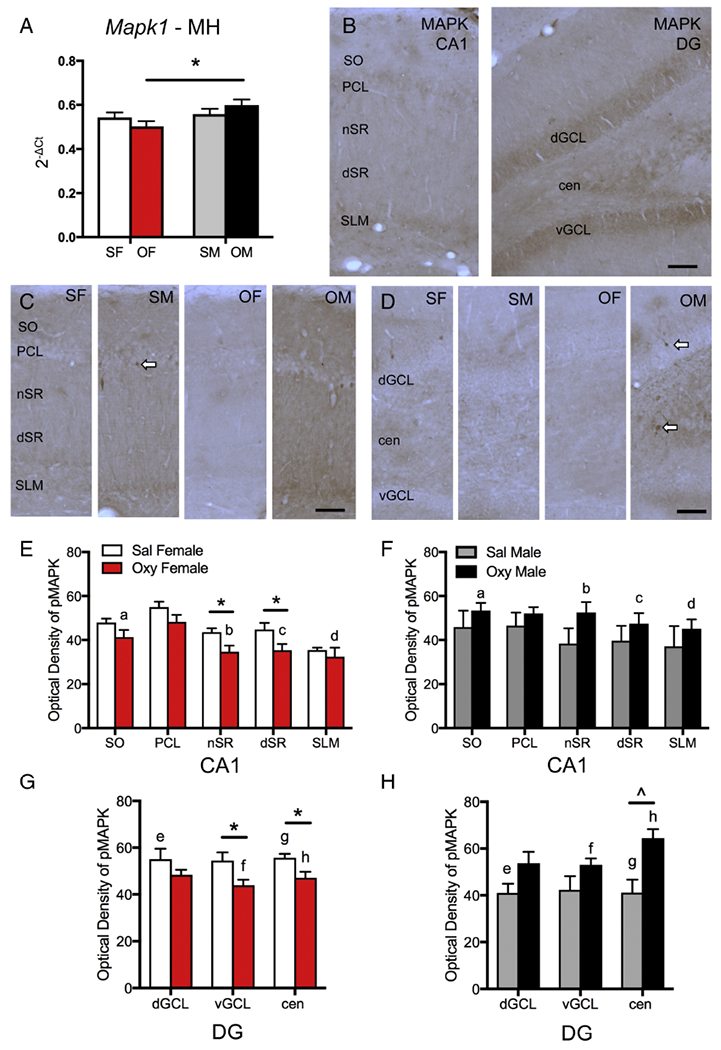
A. In the medial hippocampus (MH), Mapk1 expression is significantly lower in Oxy-females (OF) compared to Oxy-males (OM). B. In CA1 (left), MAPK-ir is densest in the pyramidal cell layer (PCL) and stratum lacunosum-moleculare (SLM) but is also found throughout the other lamina (SO, nSR, dSR). In DG (right), MAPK-ir is dense in both the dorsal and ventral granule cell layers (dGCL, vGCL) and in the central hilus (cen). C. Representative light microscope photographs show distribution of pMAPK-ir in the CA1 region. D. Representative light microscope photographs show distribution of pMAPK-ir in the dentate gyrus. SF, Sal-female; SM, Sal-male. Arrows indicate examples of pMAPK-labeled cells. Bars = 25 μm. E & F. In CA1, the density of pMAPK-ir significantly decreased in the nSR and dSR of Oxy-females compared to Sal-females. Moreover, Oxy-females had significantly lower densities of pMAPK-ir in SO and nSR and a trend towards lower density of pMAK-ir in dSR and SLM compared to Oxy-males. G & H. In the dentate gyrus, Sal-females had significantly higher densities of pMAPK-ir the dGCL and central hilus compared to Sal-males. Sal-females had significantly higher densities of pMAPK-ir than Oxy-females in the vGCL and in the central hilus. Sal-males had a trend towards lower density of pMAPK-ir than Oxy-males in the central hilus. Oxy-females compared to Oxy-males had significantly lower densities of pMAPK-ir in the vGCL and central hilus. *p < 0.05, ap = 0.034, bp = 0.013, cp = 0.061, dp = 0.063, ep = 0.040; fp = 0.030, gp = 0.041; hp = 0.006.
The density of MAPK- and pMAPK-labeling next was analyzed in hippocampal subregions contained in the medial hippocampus. MAPK-ir was densest in CA1 pyramidal cells and DG granule cells; moreover, lightly-labeled MAPK-ir dendrites were found in the CA1 SR (Fig. 8B). No differences between saline- and oxycodone-injected rats were found in the density of MAPK-ir in either the CA1 or DG (not shown).
As previously described (Crews and Vetreno, 2011), punctate immunolabeling for pMAPK in CA1 was densest in lamina containing pyramidal cell dendrites (Fig. 8C). Occasionally, darkly pMAPK-labeled cells were observed scattered throughout the CA1 PCL (Fig. 8C). In the DG, pMAPK-ir was found in scattered cells within the GCL and in punctate processes scattered throughout the hilus (Fig. 8D). No differences in the density of pMAPK-ir between Sal-females and Sal-males were observed in any region of the CA1 (Fig. 8E,F). However, Sal-females showed a significantly greater density of pMAPK in the dorsal GCL [t(10) = −2.35, p = 0.04] and the central hilus [t(10) = −2.53, p = 0.030] of the DG compared to Sal-males (Fig. 8G,H).
In Sal-females compared to Oxy-females, the density of pMAPK-ir was significantly greater in the near and distal regions of SR [nSR: t(9) = 2.81, p = 0.020; dSR: t(9) = 2.28, p = 0.020] in CA1 as well as the central hilus [t(10) = 2.91, p = 0.017] and ventral GCL [t(9) = 2.42, p = 0.038] of the DG (Fig. 8E,G). Conversely, Sal-males trended toward lower densities of pMAPK-ir in the central hilus [t(10) = −2.10, p = 0.0626] compared to Oxy-males (Fig. 8H). In Oxy-males compared to other groups, scattered pMAPK-labeled cells resembling interneurons were more noticeable near the CA1 pyramidal cell layer (Fig. 8C) and in the central hilus (Fig. 8D).
In the CA1, Oxy-females showed significantly lower density of pMAPK-ir in the SO [t(8) = 2.55, p = 0.034] and nSR [t(8) = 3.20, p = 0.013] when compared to Oxy-males (Fig. 8E,F). Additionally, Oxy-females trended towards a lower density of pMAPK-ir in dSR [t(8) = 2.18, p = 0.061] and SLM [t(8) = 2.15, p = 0.063] when compared to Oxy-males (Fig. 8E,F). In the DG, Oxy-females, compared to Oxy-males showed significantly lower density of pMAPK-ir in the ventral GCL [t(7) = 2.51, p = 0.041] and the central hilus [t(8) = 3.74, p = 0.006; Fig. 8G,H].
DISCUSSION
The present study demonstrates that oxycodone CPP results in regional sex-dependent changes in plasticity (Arc, Bdnfand Npy), stress (Crhr1 and Crhr2), and related kinase (Akt1 and Mapk1) gene expression in the hippocampus. Similarly, there were sex-dependent differences in the related proteins (ARC, NPY, CRHR1, pAKT, pMAPK) following oxycodone CPP (Fig. 9). These changes could be involved in the previously observed sex differences in the opioid system after oxycodone CPP (Ryan et al., 2018). Moreover, these changes could contribute to our understanding of previous findings in chronically stressed males, who, unlike chronically stressed females, did not acquire CPP and demonstrated diminished plasticity in the hippocampal opioid system (Reich et al., 2019).
Fig. 9. Summary of sex differences in plasticity, stress markers and related kinase and signaling molecules following oxycodone CPP.
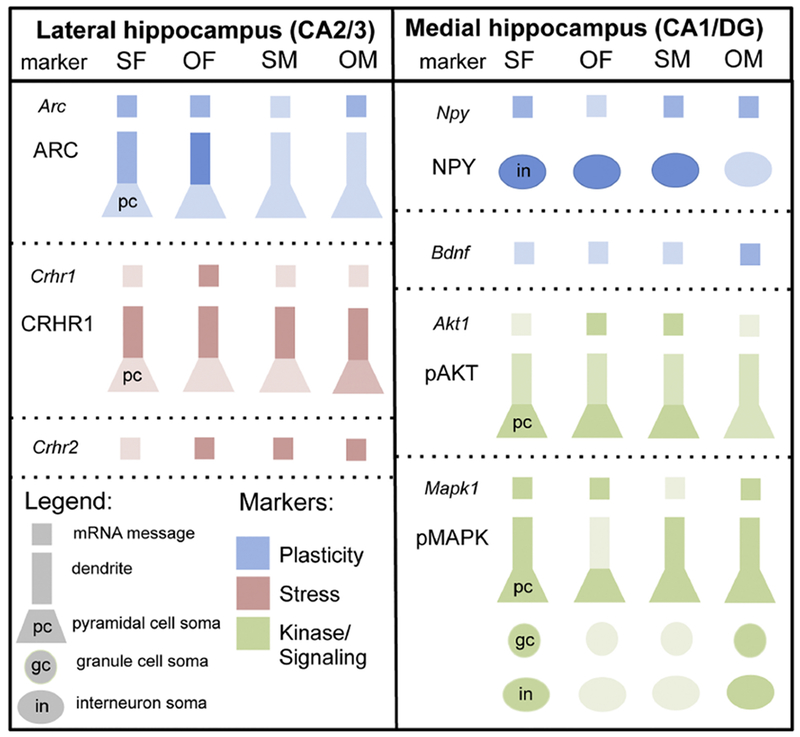
Relative levels of RNA expression and protein densities are indicated by the differences in the shading. Pyramidal cells include dendrites and soma. gc, granule cell; in, interneuron; pc, pyramidal cell.
Arc expression increases in males while ARC protein levels increase in females following oxycodone CPP
Arc is an immediate early gene (IEG) targeted to synapses to regulate protein synthesis-dependent forms of synaptic plasticity [reviewed in Bramham et al. (2010)]. Arc is particularly important in LTP learning processes [reviewed in Plath et al. (2006)]. In the present study, Sal-females compared to Sal-males had elevated expression of Arc in the lateral hippocampus as well as ARC-immunolabeling in the dendrites of CA3 pyramidal cells in SLu and SR (Fig. 10A). In contrast, naïve control female rats compared to control male rats showed less Arc expression in our previous study with CIS but no behavioral apparatus (Randesi et al., 2018). The present study’s finding suggests that daily exploration of the enriched environment of the CPP behavioral apparatus may activate Arc expression in females. In support, exposure to environmental enrichment results in an upregulation of Arc in the CA1, CA2, and CA3 of the hippocampus, and, to a lesser extent, the DG in male rats (Pinaud et al., 2001). Moreover, estrogen levels are known to influence Arc expression in the hypothalamus (Christensen et al., 2015) suggesting that estrogens may be involved in the observed sex difference in Arc expression.
Fig. 10. Plasticity, stress and kinase gene and protein densities are altered differentially within hippocampal circuits in females and males both in saline rats and following oxycodone CPP.
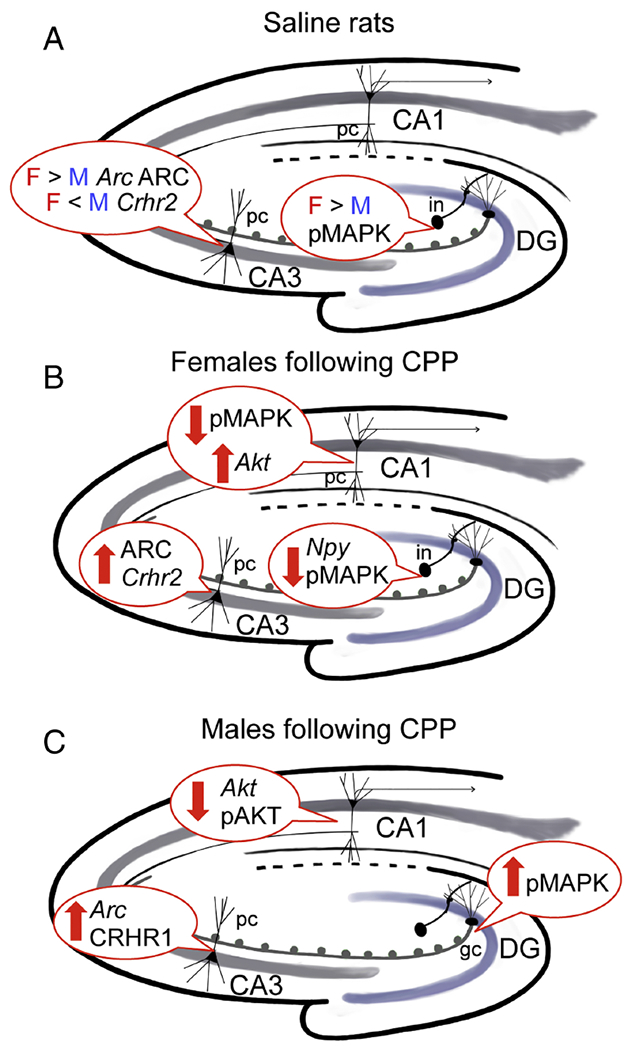
A. In CA3, saline rats have: 1) greater ARC protein density and Arc expression in Sal-females compared to Sal-males; 2) greater Crhr2 expression in Sal-males compared to Sal-females. In DG, Sal-female rats have greater pAKT density compared to Sal-males. B. After CPP, Oxy-females have: 1) increases in plasticity protein markers for ARC in CA3 pyramidal cells (pc) and NPY in hilar interneurons (in) but decreases in Npy mRNA expression in the medial hippocampus; 2) increases in the mRNA expression for the stress marker Crhr2 in the CA2/3 region; and 3) increases in Akt1 mRNA expression and pAKT-ir density in CA3 pyramidal cells and decreases in pMAPK-ir density in CA1 pyramidal cells. C. After CPP, Oxy-males have: 1) increases in the mRNA expression of Bdnf which is known to be produced in granule cells (Scharfman and MacLusky, 2014); 2) elevations in pMAPK-ir densities in the dentate hilus which harbors newly generated granule cells; and 3) increases in Arc mRNA expression and CRHR1-SIG particles in the soma of CA3 pyramidal cells.
Following CPP, Arc mRNA expression increased in the Oxy-males relative to the Sal-males in both the lateral and medial hippocampal samples so that they were equivalent to the Sal- and Oxy-females (Fig. 10C). Moreover, ARC-ir is elevated in the nuclei of pyramidal cells in Oxy-females (Fig. 10B). Euthanasia occurred within 15 minutes following the behavior, which is within the normal timeframe of Arc IEG expression (Wallace et al., 1998; Guzowski et al., 1999). Opioid receptors can activate intracellular pathways involving IEGs like Arc (Ziolkowska et al., 2005). Our previous studies demonstrated that oxycodone CPP increases DORs in pyramidal dendritic spines as well as near the plasma membrane in pyramidal cell dendrites in females (Ryan et al., 2018). Thus, these findings suggest that CA3 pyramidal cells may be more sensitive to opioids and may contribute to increases in ARC-ir seen in the females following oxycodone CPP.
Bdnf expression is increased in males compared to females following oxycodone CPP
BDNF is important for synaptogenesis and has a critical role in LTP (Cunha et al., 2010). Surprisingly, we found that Sal-females and Sal-males had no difference in Bdnf mRNA expression in the medial hippocampus (Fig. 9), where Bdnf is primarily expressed in granule cells (Choy et al., 2008). This finding was unexpected as in situ hybridization studies have shown that Bdnf mRNA expression decreases following ovariectomy and increases with addition of estradiol (Berchtold et al., 2001). Moreover, BDNF protein levels are elevated in the granule cell mossy fibers in high estrogen females (both proestrus and estrus) relative to low estrogen-state females and males [reviewed in Scharfman and MacLusky (2014)]. Although the reason for this discrepancy is unclear, it is possible that other cell types, in addition to granule cells, may contribute. Following CPP, Bdnf expression in the medial hippocampal sample is increased in Oxy-males compared to Oxy-females. The increase of Bdnf mRNA expression in the Oxy-males is consistent with the finding that intracerebroventricular injections of enkephalin increase Bdnf mRNA expression in the granule cell layer of male rats (Zhang et al., 2006). Exogenous BDNF also increases pre-proEnk expression in the hippocampus (Croll et al., 1994), suggesting that these two systems are complementary. Therefore, the increase in Bdnf mRNA expression in Oxy-males is consistent with our findings that in Oxy-males, LEnk-ir increases in CA3b, where mossy fibers terminate (Ryan et al., 2018).
Npy expression and NPY cell numbers are differentially altered in females and males following oxycodone CPP
NPY is prominent in GABAergic interneurons and can regulate many aspects of plasticity in hippocampal circuits (Sperk et al., 2007). Consistent with previous studies in naïve rats (Williams et al., 2011b; Mazid et al., 2016), there were no sex differences in the number of NPY-immunolabeled neurons in the CA1, CA3 or DG. However, following CPP, the number of NPY-labeled neurons in the central hilus is greater in Oxy-females relative to Oxy-males (Fig. 9). NPY-containing GABAergic interneurons are known to colocalize DORs (Williams et al., 2011b). Our previous studies showed that DOR-labeled cells in the central hilus of females also increased following oxycodone CPP (Ryan et al., 2018). Our EM studies additionally showed that near plasmalemmal DORs increased in GABAergic hilar interneuron dendrites in females, but not males, following oxycodone CPP (Ryan et al., 2018). Increases in both NPY/DOR containing cells, as well as the pool of DORs readily available to be inserted into the plasma membrane (Boudin et al., 1998) following oxycodone CPP, could have functional consequences. Hilar NPY interneurons are known to project to granule cell dendrites where they converge with entorhinal afferents (Milner and Veznedaroglu, 1992; Sik et al., 1995; Williams et al., 2011b). Since NPY release is inhibited by DORs, activation of DORs on NPY-containing interneurons could promote lpp LTP (Sperk et al., 2007). As estradiol can potentiate NPY release (Ledoux et al., 2009), plasticity processes in high estrogen state females are likely to be further enhanced following oxycodone CPP.
Unlike NPY cell number, Npy mRNA expression decreased in Oxy-females (Fig. 10B). Several factors could have contributed to this finding. First, the microarray sampling from the medial hippocampus included both the CA1 and DG, meaning that detection of the differences in Npy mRNA expression within a subregion of the hilus would be beyond the sensitivity of the method. Second, as NPY was detected in a larger population of cells in the females following oxycodone CPP, it is possible that the individual cells actually express lower levels of Npy message. Third, the medial hippocampal sample included the CA1 region. Prior studies have shown that NPY-containing neurons in CA1 colocalize with MORs (Drake and Milner, 2002). In other brain regions, changes in the expression of MORs can alter the levels of Npy mRNA expression (Yoo et al., 2005). Thus, it is possible that NPY-containing cells in CA1 colocalize different amounts of MORs in female and male rats, thus differentially affecting Npy mRNA expression.
Crhr1 and Crhr2 expression and the subcellular distribution of CRHR1 are differentially altered in females and males after oxycodone CPP
Crhr1 and Crhr2 mRNA show distinct but overlapping distributions in the brain (Reul and Holsboer, 2002). The effects of CRH receptors on hippocampal-mediated neuroplasticity can vary depending on the duration of the stressor (Smith and Vale, 2006; Regev and Baram, 2014). Evidence from other brain areas shows that activation of CRH receptors can potentiate the acute effects of drugs of abuse and enhance neuroplasticity induced following drug withdrawal [reviewed in Haass-Koffler and Bartlett (2012)]. In agreement with our prior studies (McAlinn et al., 2018; Randesi et al., 2018), Sal-female and Sal-male rats had similar Crhr1 expression levels in the lateral hippocampal region and similar densities of CRHR1-SIG particles in CA3 neurons (Fig. 9). Following CPP, Crhr1 mRNA expression in the lateral hippocampal sample tended to increase in Oxy-females compared to Sal-females. However, no changes in CRHR1-SIG particle density within CA3 pyramidal cells were observed. In contrast, the expression of Crhr1 mRNA in the lateral hippocampus was not changed in the Oxy-males relative to the other groups (Fig. 9). However, the total number of CRHR1-SIG particles in CA3 soma increased in the Oxy-males compared to both the Sal-males and Oxy-females (Fig. 10C). This suggests that oxycodone CPP may upregulate Crhr1 in different types of cells in females (i.e., non-pyramidal cells) and males (i.e., pyramidal cells). It is also possible that changes in cortisol levels following the behavior contributed to the sex differences in Crhr1 and CRHR1-SIG particles after oxycodone CPP.
The present study demonstrated that Crhr2 mRNA expression is greater in Sal-males compared Sal-females (Fig. 10A). These findings are consistent with the observation that androgens increase Crhr2 mRNA levels in whole rat hippocampus and primary hippocampal neurons (Weiser et al., 2008). Following CPP, Crhr2 expression in the lateral hippocampus increased only in the Oxy-females (Fig. 10B). Previous studies have suggested that in regions of the rat brain, such as hippocampus, where Crhr1 and Crhr2 are abundant, the two Crh subtypes can respond to one another (Weiser et al., 2008), suggesting the possibility that Crhr2 increases in females to compensate for the increase in Crhr1.
Following CPP, Akt1 expression and pAKT densities are opposite of those observed for Mapk1 expression and pMAPK densities
Akt1, a signal transduction intermediate, plays a crucial role in cell growth and synaptic protein translation, including post-synaptic density (PSD)-95 (Brunet et al., 2001; Akama and McEwen, 2003; Chong et al., 2005). Consistent with our prior study (Randesi et al., 2018), there was no sex difference in the mRNA expression of Akt1 in the medial hippocampal sample between the saline rats. We also found that AKT protein levels in CA1 were similar in Sal-females and Sal-males. As our previous studies had demonstrated higher densities of pAKT-ir (Thr308) levels in naïve proestrus females compared to males (Znamensky et al., 2003; Yildirim et al., 2011), the different results between the present study and the Randesi et al. (2018) study could be due to the hormonal state (proestrus vs. estrus) and/or the added behavioral experience of the saline-injected rats.
Following oxycodone CPP, a sex difference emerged: the expression of Akt1 in the medial sample increased in the Oxy-females and decreased in the Oxy-males relative to their saline counterparts (Fig. 10B,C). Moreover, the densities of pAKT-ir in CA1 were unchanged in the Oxy-females but decreased in the pyramidal cell layer in the Oxy-males compared to the Sal-males (Fig. 10C). These findings suggest that oxycodone CPP maintains AKT-mediated signaling events in female rats but reduces them in male rats. These results differ from prior studies in male rats showing that morphine CPP results in increased pAKT (Ser 473) protein levels on Western blots in CA1 (Shen et al., 2016) and DG (Guo et al., 2016). However, differences between oxycodone and morphine, including their pharmacodynamics and signaling processes (Lemberg et al., 2006), post-receptor binding effects on drug internalization (Bolan et al., 2002; Arttamangkul et al., 2008) and bioavailability (Soderberg Lofdal et al., 2013) could have contributed to the divergent results in male rats. Additionally, the experimental design of the morphine CPP in these studies differed from that for oxycodone CPP in the present study: 4-5 days vs. 8 days of testing; two training sessions/day vs. one training session per day; and the test day immediately followed the training session vs. a 4 day gap between training and testing (Guo et al., 2016; Shen et al., 2016). Lastly, the morphine CPP studies did not assess gene expression and measured pAKT protein using an antibody raised to a different phosphorylated site.
MAPK is critical for integrating membrane receptor signals to the nucleus (Sananbenesi et al., 2003). Alterations in MAPK signaling can affect many plasticity processes (Wu et al., 2005; Zheng et al., 2005; Murphy and Blenis, 2006) including glutamatergic NMDA receptor phosphorylation and LTP in the hippocampus (Bi et al., 2000). There were no differences in the mRNA expression of Mapk1 in the medial hippocampal sample and in the densities of MAPK-ir in the CA1 or DG in Sal-females and Sal-males. However, the densities of pMAPK-ir in the granule cells and hilus were elevated in Sal-females compared to Sal-males (Fig. 10A). This finding is consistent with studies demonstrating that estrogens activate the MAPK pathway (Bi et al., 2000; Singh et al., 2000).
Following CPP, Mapk1 mRNA expression in the medial hippocampus was lower in Oxy-females compared to Oxy-males. Moreover, densities of pMAPK-ir decreased in Oxy-females and increased in Oxy-males in most lamina in the CA1 and in the DG, especially the central hilus (Fig. 10B,C). These findings are consistent with several reports that the opioid system and the MAPK pathways are functionally interrelated (Trapaidze et al., 2000; Persson et al., 2003).
In particular, stimulation of opioid receptors increases MAPK protein levels in vitro (Gutstein et al., 1997; Kramer and Simon, 2000) and this increase in MAPK can activate IEGs such as c-fos and c-jun (Shoda et al., 2001). Moreover, DOR and MOR antagonists reduce MAPK-mediated cell proliferation in hippocampal rat progenitor cell cultures (Persson et al., 2003). Thus, as the DG harbors adult-generated progenitor cells (Opendak and Gould, 2015), our findings suggest that oxycodone CPP has the opposite effect on adult neurogenesis in female and male rats. Several studies have shown that factors that increase adult neurogenesis also increase spatial learning (Lieberwirth et al., 2016).
Interestingly, we found that Akt and Mapk1 exhibited opposite gene expression patterns in the medial hippocampus following oxycodone CPP depending on sex. It is known that the Ras-PI3K-AKT and Raf-MAPK systems mediate one another and have been shown to control opposite functions (Rommel et al., 1999; Zimmermann and Moelling, 1999; Menges and McCance, 2008). These opposing interactions may occur by two possible pathways, either by AKT inhibiting its downstream target, Raf, leading to inhibition of MAPK (Rommel et al., 1999), or by the negative regulation of the AKT pathway by Raf-MAPK, leading to inhibition of AKT (Menges and McCance, 2008). This provides a potential explanation for the opposite gene expression patterns observed in this cohort.
Functional considerations
The present study demonstrates sex-specific changes in the expression and protein densities of plasticity, stress and related kinase markers in hippocampal circuitry following oxycodone CPP (Fig. 10). These changes parallel many of the sex differences in the levels of LEnk and the redistribution of opioid receptors we previously observed in the hippocampus following oxycodone CPP (Ryan et al., 2018). Specifically, elevations in ARC-ir in CA3 dendrites and in Crhr2 mRNA expression in the lateral hippocampal sample accompany the redistribution of DORs to the spines of CA3 dendrites in Oxy-females. Additionally, the number of NPY-containing neurons increases in the same population of interneurons that have elevated near-plasma membrane DORs following oxycodone CPP. In Oxy-males, the elevation of CRHR1-ir in the soma of CA3 pyramidal cells accompanies the DORs in the dendritic spines of these cells. Moreover, gene expression of Bdnf as well as levels of LEnk, both of which are contained in granule cells and their mossy fibers (Gray et al., 2013; Pierce et al., 2014), are elevated in Oxy-males. BDNF-stimulated LTP elevates Arc mRNA expression (Wibrand et al., 2006) and this is coupled with the activation of MAPK (Alder et al., 2002; Ying and Sanders, 2003).
Our studies suggest additional mechanisms by which oxycodone CPP could interact with the hippocampal opioid system differentially in female and male rats to further enhance plasticity and learning processes. First, in Oxy-females, Akt mRNA expression is elevated in the medial (CA1/DG) hippocampal sample. Within this sample, CA1 pyramidal cells are known to contain high levels of DORs and pAKT and that their levels are altered by estrogens (Znamensky et al., 2003; Williams et al., 2011b; Yildirim et al., 2011). In particular, pAKT-labeling is elevated on the plasma membrane and spines of CA1 pyramidal cells in high estrogen female rats compared to male rats (Znamensky et al., 2003; Yildirim et al., 2011). In CA1, estrogens promote CA1-mediated LTP and plasticity [reviewed Spencer et al. (2008)] and AKT acts downstream of glutamatergic NR2B-NMDA receptors to enhance the expression of morphine CPP (Shen et al., 2016). In contrast, Oxy-males show reduced Akt mRNA in the medial sample and pAKT-ir in CA1, suggesting that plasticity processes may also be reduced.
Second, in Oxy-males, enhanced expression of Mapk1 and pMAPK in the hilus would be predicted to enhance adult neurogenesis of granule cells (Persson et al., 2003) and thus would potentially introduce more opioid-producing granule cells into hippocampal circuits. Additionally, MAPK activation can enhance NMDA receptor function and LTP (Bi et al., 2000). In contrast, Oxy-females showed a reduction of pMAPK-labeled interneurons and, in comparison with Oxy-males, a greater number of NPY cells in the central hilus. Although whether pMAPK and NPY are contained in the same interneurons is unknown, these findings suggest that opioid-associated learning could activate inhibitory interneurons via different mechanisms. Future studies will seek to determine the phenotype of pMAPK-labeled neurons.
The observed changes in CRHR1 and pMAPK protein densities and Arc expression in the lateral hippocampus are notable because they may help explain the sex-dependent processes at the mossy fiber-CA3 synapses in CIS rats (Mazid et al., 2016; McAlinn et al., 2018; Randesi et al., 2018; Reich et al., 2019) and the inability to acquire oxycodone CPP in chronic immobilization stress (CIS) males (Reich et al., 2019). We posit that CIS “primes” the hippocampal opioid system in females for opioid-associated learning while “shutting down” synapses important for opioid-associative learning in CIS males. In particular, CIS reduces DORs in mossy fiber-CA3 synapses that we showed in prior studies are critical for opioid-mediated LTP (Harte-Hargrove et al., 2015). In CIS Oxy-males, not only do DORs in mossy fiber-CA3 synapses remain low, but also the levels of LEnk in the mossy fiber pathway remain unaltered (Reich et al., 2019). CIS elevates the number of CRHR1s on the plasma membrane of CA3 dendrites in male rats (McAlinn et al., 2018). This would render males more vulnerable to cyclic AMP-dependent hyperexcitability (Hollrigel et al., 1998; Elliott-Hunt et al., 2002), possibly contributing to the dendritic retraction and spine loss of CA3 dendrites in response to CIS (Wang et al., 2013; McEwen et al., 2015). Moreover, CIS inhibits adult neurogenesis in male rats (Holmes and Galea, 2002), likely decreasing MAPK, suggesting a possible mechanism for reduced output of granule cell mossy fiber-CA3 synapses.
Finally, naïve male rats compared to naïve females have greater Arc expression in the lateral hippocampal sample but following CIS, Arc expression is significantly down-regulated in males but remains unaltered in females (Randesi et al., 2018). Other studies have demonstrated that stressors can disrupt Arc expression in the hippocampus (Kozlovsky et al., 2008; Biala et al., 2011). Thus, together with the present study, it is likely that Arc expression in the lateral hippocampus is particularly sensitive to the environment (e.g., stress) in males. The reduction of Arc expression in CA3 by CIS may contribute to the prevention of DOR redistribution at mossy fiber-CA3 synapses in CIS males following oxycodone CPP (Reich et al., 2019) and is possibly related to the dendritic retraction of CA3 neurons seen in males, but not females, following CIS [reviewed in McEwen et al. (2015)]. This change in Arc expression could negatively impact LTP processes in mossy fiber-CA3 synapses [reviewed in Plath et al. (2006)].
In conclusion, these sex-specific changes in plasticity, stress and kinase markers in hippocampal circuitry parallel previously observed sex differences in the opioid system after oxycodone CPP (Ryan et al., 2018). Moreover, the changes in stress-naïve males could contribute to the inability to acquire oxycodone CPP and the diminished plasticity in the hippocampal opioid system that we have recently observed in chronically stressed males (Reich et al., 2019).
Highlights.
Hippocampal gene expression and protein levels vary depending on sex after oxycodone (Oxy) conditioned place preference (CPP)
After Oxy CPP, Npy mRNA expression decreases but a subset of NPY-labeled cells increases in the dentate gyrus (DG) in females
In CA3, plasticity and stress markers differentially increase in Oxy CPP females (ARC, Crhr2) and males (Arc, CRHR1)
After Oxy CPP, signaling kinases (Akt1, pAKT, pMAPK) change in opposite directions in females and males in CA1 and DG
Differences in signaling, kinase, and stress markers suggest sex-specific hippocampal pathways for opioid-associated learning
Acknowledgements
Supported by NIH grants DA08259 (T.A.M., B.S.M.), HL098351 (T.A.M.), HL 136520 (T.A.M.), MH041256 (B.S.M.) and MH102065 (J.D.G.), and Hope for Depression Research grant (B.S.M.). We thank Megan Johnson for assistance with figure preparation.
ABBREVIATIONS
- ABC
avidin-biotin complex
- Akt
serine/threonine kinase 1
- Arc
activity-regulated cytoskeleton-associated protein
- Bdnf
brain-derived neurotrophic factor
- BSA
bovine serum albumin
- cen
central hilus
- CIS
chronic immobilization stress
- CPP
conditioned place preference
- cr
crest of hilus
- Crh
corticotropin releasing hormone
- CRHR
corticotropin releasing hormone receptor
- Ct
threshold cycle
- DAB
diaminobenzidine
- db
dorsal blade of hilus
- DG
dentate gyrus
- DOR
delta opioid receptor
- EM
electron microscopic
- gc
granule cell
- GCL
granule cell layer
- IEG
immediate early gene
- ir
immunoreactivity
- LEnk
Leucine-enkephalin
- lpp
lateral perforant pathway
- LTP
long-term potentiation
- Mapk
mitogen-activated protein kinase
- mf
mossy fiber
- ML
molecular layer
- MOR
mu opioid receptor
- NPY
neuropeptide Y
- Oxy
oxycodone CPP rat
- PFA
paraformaldehyde
- PB
phosphate buffer
- PBS
phosphate-buffered saline
- pc
pyramidal cell
- PCL
pyramidal cell layer
- RIN
RNA integrity number
- ROI
region of interest
- RT
reverse transcription
- Sal
saline CPP rat
- SIG
silver-intensified immunogold
- SLu
stratum lucidum
- SLM
stratum lacunosum-moleculare
- SO
stratum oriens
- SR
stratum radiatum
- TS
tris-buffered saline
All gene names are written in italics with only the first letter capitalized (e.g. Npy), while all protein names are written in uppercase (e.g. NPY).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Centers for Disease Control and Prevention (2015) Drug overdose deaths hit record numbers in 2014
- Akama KT, McEwen BS (2003) Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/protein kinase B pathway. J Neurosci 23:2333–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alder J, Thakker-Varia S, Black IB (2002) Transcriptional analysis in the brain: trophin-induced hippocampal synaptic plasticity. Neurochem Res 27:1079–1092. [DOI] [PubMed] [Google Scholar]
- Arttamangkul S, Quillinan N, Low MJ, von Zastrow M, Pintar J, Williams JT (2008) Differential activation and trafficking of micro-opioid receptors in brain slices. Mol Pharmacol 74:972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchus AP, Pickel VM (1992) Quantitative light microscopic demonstration of increased pallidal and striatal met5-enkephalin-like immunoreactivity in rats following chronic treatment with haloperidol but not with clozapine: implications for the pathogenesis of neuroleptic-induced movement disorders. Exp Neurol 117:17–27. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ (2010) Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry 15:877, 896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, McClellan ML, Reed BG (2017) Sex Differences, Gender, and Addiction. J Neurosci Res 95:136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold NC, Kesslak JP, Pike CJ, Adlard PA, Cotman CW (2001) Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. Eur J Neurosci 14:1992–2002. [DOI] [PubMed] [Google Scholar]
- Bi R, Broutman G, Foy MR, Thompson RF, Baudry M (2000) The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus. Proc Natl Acad Sci U S A 97:3602–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biala YN, Bogoch Y, Bejar C, Linial M, Weinstock M (2011) Prenatal stress diminishes gender differences in behavior and in expression of hippocampal synaptic genes and proteins in rats. Hippocampus 21:1114–1125. [DOI] [PubMed] [Google Scholar]
- Bolan EA, Tallarida RJ, Pasternak GW (2002) Synergy between mu opioid ligands: evidence for functional interactions among mu opioid receptor subtypes. J Pharmacol Exp Ther 303:557–562. [DOI] [PubMed] [Google Scholar]
- Boudin H, Pelaprat D, Rostene W, Pickel VM, Beaudet A (1998) Correlative ultrastructural distribution of neurotensin receptor proteins and binding sites in the rat substantia nigra. J Neurosci 18:8473–8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Alme MN, Bittins M, Kuipers SD, Nair RR, Pai B, Panja D, Schubert M, Soule J, Tiron A, Wibrand K (2010) The Arc of synaptic memory. Exp Brain Res 200:125–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Datta SR, Greenberg ME (2001) Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol 11:297–305. [DOI] [PubMed] [Google Scholar]
- Chen Y, Brunson KL, Muller MB, Cariaga W, Baram TZ (2000) Immunocytochemical distribution of corticotropin-releasing hormone receptor type-1 (CRF(1))-like immunoreactivity in the mouse brain: light microscopy analysis using an antibody directed against the C-terminus. J Comp Neurol 420:305–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K (2005) Activating Akt and the brain’s resources to drive cellular survival and prevent inflammatory injury. Histol Histopathol 20:299–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy KH, de Visser Y, Nichols NR, van den Buuse M (2008) Combined neonatal stress and young-adult glucocorticoid stimulation in rats reduce BDNF expression in hippocampus: effects on learning and memory. Hippocampus 18:655–667. [DOI] [PubMed] [Google Scholar]
- Christensen A, Dewing P, Micevych P (2015) Immediate early gene activity-regulated cytoskeletal-associated protein regulates estradiol-induced lordosis behavior in female rats. J Neurosci Res 93:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D, Reed B, Zhang Y, Kreek MJ (2016) Sex differences in responsiveness to the prescription opioid oxycodone in mice. Pharmacol Biochem Behav 148:99–105. [DOI] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP (2011) Addiction, adolescence, and innate immune gene induction. Front Psychiatry 2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croll SD, Wiegand SJ, Anderson KD, Lindsay RM, Nawa H (1994) Regulation of neuropeptides in adult rat forebrain by the neurotrophins BDNF and NGF. Eur J Neurosci 6:1343–1353. [DOI] [PubMed] [Google Scholar]
- Cunha C, Brambilla R, Thomas KL (2010) A simple role for BDNF in learning and memory? Front Mol Neurosci 3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch-Feldman M, Picetti R, Seip-Cammack K, Zhou Y, Kreek MJ (2015) Effects of handling and vehicle injections on adrenocorticotropic and corticosterone concentrations in Sprague-Dawley compared with Lewis rats. J Am Assoc Lab Anim Sci 54:35–39. [PMC free article] [PubMed] [Google Scholar]
- Drake CT, Milner TA (2002) Mu opioid receptors are in discrete hippocampal interneuron subpopulations. Hippocampus 12:119–136. [DOI] [PubMed] [Google Scholar]
- Drake CT, Chavkin C, Milner TA (2007) Opioid systems in the dentate gyrus. Prog Brain Res 163:245–263. [DOI] [PubMed] [Google Scholar]
- Elliott-Hunt CR, Kazlauskaite J, Wilde GJC, Grammatopoulos DK, Hillhouse EW (2002) Potential signalling pathways underlying corticotrophin-releasing hormone-mediated neuroprotection from excitotoxicity in rat hippocampus. Journal of Neurochemistry 80:416–425. [DOI] [PubMed] [Google Scholar]
- Gray JD, Milner TA, McEwen BS (2013) Dynamic plasticity: the role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience 239:214–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo SJ, Cui Y, Huang ZZ, Liu H, Zhang XQ, Jiang JX, Xin WJ (2016) Orexin A-mediated AKT signaling in the dentate gyrus contributes to the acquisition, expression and reinstatement of morphine-induced conditioned place preference. Addict Biol 21:547–559. [DOI] [PubMed] [Google Scholar]
- Gutstein MD, Howard B, Rubie BAElizabeth A, Mansour PA, Akil PH, Woodgett James R (1997) Opioid Effects on Mitogen-activated Protein Kinase Signaling Cascades Anesthesiology 87:1118–1126. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF (1999) Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci 2:1120–1124. [DOI] [PubMed] [Google Scholar]
- Haass-Koffler C, Bartlett S (2012) Stress and addiction: contribution of the corticotropin releasing factor (CRF) system in neuroplasticity. Frontiers in Molecular Neuroscience 5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte-Hargrove LC, Varga-Wesson A, Duffy AM, Milner TA, Scharfman HE (2015) Opioid Receptor-Dependent Sex Differences in Synaptic Plasticity in the Hippocampal Mossy Fiber Pathway of the Adult Rat. J Neurosci 35:1723–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollrigel GS, Chen K, Baram TZ, Soltesz I (1998) The pro-convulsant actions of corticotropin-releasing hormone in the hippocampus of infant rats. Neuroscience 84:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MM, Galea LA (2002) Defensive behavior and hippocampal cell proliferation: differential modulation by naltrexone during stress. Behav Neurosci 116:160–168. [PubMed] [Google Scholar]
- Kesner RP, Warthen DK (2010) Implications of CA3 NMDA and opiate receptors for spatial pattern completion in rats. Hippocampus 20:550–557. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlovsky N, Matar MA, Kaplan Z, Kotler M, Zohar J, Cohen H (2008) The immediate early gene Arc is associated with behavioral resilience to stress exposure in an animal model of posttraumatic stress disorder. Eur Neuropsychopharmacol 18:107–116. [DOI] [PubMed] [Google Scholar]
- Kramer HK, Simon EJ (2000) mu and delta-opioid receptor agonists induce mitogen-activated protein kinase (MAPK) activation in the absence of receptor internalization. Neuropharmacology 39:1707–1719. [DOI] [PubMed] [Google Scholar]
- Lacy RT, Strickland JC, Feinstein MA, Robinson AM, Smith MA (2016) The effects of sex, estrous cycle, and social contact on cocaine and heroin self-administration in rats. Psychopharmacology (Berl) 233:3201–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoux VA, Smejkalova T, May RM, Cooke BM, Woolley CS (2009) Estradiol facilitates the release of neuropeptide Y to suppress hippocampus-dependent seizures. J Neurosci 29:1457–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemberg KK, Kontinen VK, Siiskonen AO, Viljakka KM, Yli-Kauhaluoma JT, Korpi ER, Kalso EA (2006) Antinociception by spinal and systemic oxycodone: why does the route make a difference? In vitro and in vivo studies in rats. Anesthesiology 105:801–812. [DOI] [PubMed] [Google Scholar]
- Lieberwirth C, Pan Y, Liu Y, Zhang Z, Wang Z (2016) Hippocampal adult neurogenesis: Its regulation and potential role in spatial learning and memory. Brain Res 1644:127–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrikaki M, Pravetoni M, Page S, Potter D, Chartoff E (2017) Oxycodone self-administration in male and female rats. Psychopharmacology (Berl) 234:977–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazid S, Hall BS, Odell SC, Stafford K, Dyer AD, Van Kempen TA, Selegean J, McEwen BS, Waters EM, Milner TA (2016) Sex differences in subcellular distribution of delta opioid receptors in the rat hippocampus in response to acute and chronic stress. Neurobiol Stress 5:37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlinn HR, Reich B, Contoreggi NH, Kamakura RP, Dyer AG, McEwen BS, Waters EM, Milner TA (2018) Sex Differences in the Subcellular Distribution of Corticotropin-Releasing Factor Receptor 1 in the Rat Hippocampus following Chronic Immobilization Stress. Neuroscience 383:98–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, Nasca C (2015) Mechanisms of stress in the brain. Nat Neurosci 18:1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Milner TA (2017) Understanding the Broad Influence of Sex Hormones and Sex Differences in the Brain. J Neurosci Res 95:24–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meilandt WJ, Barea-Rodriguez E, Harvey SA, Martinez JL Jr. (2004) Role of hippocampal CA3 mu-opioid receptors in spatial learning and memory. J Neurosci 24:2953–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menges CW, McCance DJ (2008) Constitutive activation of the Raf-MAPK pathway causes negative feedback inhibition of Ras-PI3K-AKT and cellular arrest through the EphA2 receptor. Oncogene 27:2934–2940. [DOI] [PubMed] [Google Scholar]
- Milner TA, Veznedaroglu E (1992) Ultrastructural localization of neuropeptide Y-like immunoreactivity in the rat hippocampal formation. Hippocampus 2:107–125. [DOI] [PubMed] [Google Scholar]
- Milner TA, Burstein SR, Marrone GF, Khalid S, Gonzalez AD, Williams TJ, Schierberl KC, Torres-Reveron A, Gonzales KL, McEwen BS, Waters EM (2013) Stress differentially alters mu opioid receptor density and trafficking in parvalbumin-containing interneurons in the female and male rat hippocampus. Synapse 67:757–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Waters EM, Robinson DC, Pierce JP (2011) Degenerating processes identified by electron microscopic immunocytochemical methods. Neurodegeneration, Methods and Protocols 23–59. [DOI] [PubMed] [Google Scholar]
- Murphy LO, Blenis J (2006) MAPK signal specificity: the right place at the right time. Trends Biochem Sci 31:268–275. [DOI] [PubMed] [Google Scholar]
- Olmstead MC, Burns LH (2005) Ultra-low-dose naltrexone suppresses rewarding effects of opiates and aversive effects of opiate withdrawal in rats. Psychopharmacology (Berl) 181:576–581. [DOI] [PubMed] [Google Scholar]
- Opendak M, Gould E (2015) Adult neurogenesis: a substrate for experience-dependent change. Trends Cogn Sci 19:151–161. [DOI] [PubMed] [Google Scholar]
- Ordonez Gallego A, Gonzalez Baron M, Espinosa Arranz E (2007) Oxycodone: a pharmacological and clinical review. Clin Transl Oncol 9:298–307. [DOI] [PubMed] [Google Scholar]
- Persson AI, Thorlin T, Bull C, Zarnegar P, Ekman R, Terenius L, Eriksson PS (2003) Mu- and delta-opioid receptor antagonists decrease proliferation and increase neurogenesis in cultures of rat adult hippocampal progenitors. Eur J Neurosci 17:1159–1172. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster H.d. (1991) The fine structure of the nervous system: neurons and their supporting cells. 3 ed., New York: Oxford University Press. [Google Scholar]
- Pierce JP, Kurucz OS, Milner TA (1999) Morphometry of a peptidergic transmitter system: Dynorphin B-like immunoreactivity in the rat hippocampal mossy fiber pathway before and after seizures. Hippocampus 9:255–276. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Kelter DT, McEwen BS, Waters EM, Milner TA (2014) Hippocampal mossy fiber leu-enkephalin immunoreactivity in female rats is significantly altered following both acute and chronic stress. J Chem Neuroanat 55:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinaud R, Penner MR, Robertson HA, Currie RW (2001) Upregulation of the immediate early gene arc in the brains of rats exposed to environmental enrichment: implications for molecular plasticity. Brain Res Mol Brain Res 91:50–56. [DOI] [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, Kobalz U, Stawrakakis A, Fernandez E, Waltereit R, Bick-Sander A, Therstappen E, Cooke SF, Blanquet V, Wurst W, Salmen B, Bosl MR, Lipp HP, Grant SG, Bliss TV, Wolfer DP, Kuhl D (2006) Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron 52:437–444. [DOI] [PubMed] [Google Scholar]
- Randesi M, Zhou Y, Mazid S, Odell SC, Gray JD, Correa da Rosa J, McEwen BS, Milner TA, Kreek MJ (2018) Sex differences after chronic stress in the expression of opioid- and neuroplasticity-related genes in the rat hippocampus. Neurobiology of Stress 8:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev L, Baram TZ (2014) Corticotropin Releasing Factor in Neuroplasticity. Front Neuroendocrinol 35:171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich B, Zhou Y, Goldstein E, Srivats SS, Contoreggi NH, Kogan JF, McEwen BS, Kreek MJ, Milner TA, Gray JD (2019) Chronic immobilization stress primes the hippocampal opioid system for oxycodone-associated learning in female but not male rats. Synapse e22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul JM, Holsboer F (2002) On the role of corticotropin-releasing hormone receptors in anxiety and depression. Dialogues Clin Neurosci 4:31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BAS, Fox K, Valentino RJ, Van Bockstaele EJ (2006) Agonist-induced internalization of corticotropin-releasing factor receptors in noradrenergic neurons of the rat locus coeruleus. European Journal of Neuroscience 23:2991–2998. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Dasilva MC, Shinal RM, Glover T, Williams RS, Staud R, Riley JL 3rd, Fillingim RB (2011) Evaluation of menstrual cycle effects on morphine and pentazocine analgesia. Pain 152:614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommel C, Clarke BA, Zimmermann S, Nunez L, Rossman R, Reid K, Moelling K, Yancopoulos GD, Glass DJ (1999) Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science 286:1738–1741. [DOI] [PubMed] [Google Scholar]
- Ryan JD, Zhou Y, Contoreggi NH, Bshesh FK, Gray JD, Kogan JF, Ben KT, McEwen BS, Jeanne Kreek M, Milner TA (2018) Sex differences in the rat hippocampal opioid system after oxycodone conditioned place preference. Neuroscience 393:236–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sananbenesi F, Fischer A, Schrick C, Spiess J, Radulovic J (2003) Mitogen-activated protein kinase signaling in the hippocampus and its modulation by corticotropin-releasing factor receptor 2: a possible link between stress and fear memory. J Neurosci 23:11436–11443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvage M, Steckler T (2001) Detection of corticotropin-releasing hormone receptor 1 immunoreactivity in cholinergic, dopaminergic and noradrenergic neurons of the murine basal forebrain and brainstem nuclei--potential implication for arousal and attention. Neuroscience 104:643–652. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ (2014) Sex differences in the neurobiology of epilepsy: a preclinical perspective. Neurobiol Dis 72 Pt B:180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F, Wang XW, Ge FF, Li YJ, Cui CL (2016) Essential role of the NO signaling pathway in the hippocampal CA1 in morphine-associated memory depends on glutaminergic receptors. Neuropharmacology 102:216–228. [DOI] [PubMed] [Google Scholar]
- Shoda T, Fukuda K, Uga H, Mima H, Morikawa H (2001) Activation of mu-opioid receptor induces expression of c-fos and junB via mitogen-activated protein kinase cascade. Anesthesiology 95:983–989. [DOI] [PubMed] [Google Scholar]
- Sik A, Penttonen M, Ylinen A, Buzsaki G (1995) Hippocampal CA1 interneurons: an in vivo intracellular labeling study. J Neurosci 15:6651–6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Setalo G, Guan X, Frail DE, Toran-Allerand CD (2000) Estrogen-Induced Activation of the Mitogen-Activated Protein Kinase Cascade in the Cerebral Cortex of Estrogen Receptor-α Knock-Out Mice. The Journal of Neuroscience 20:1694–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Vale WW (2006) The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. 8:383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderberg Lofdal KC, Andersson ML, Gustafsson LL (2013) Cytochrome P450-mediated changes in oxycodone pharmacokinetics/pharmacodynamics and their clinical implications. Drugs 73:533–543. [DOI] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS (2008) Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol 29:219–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperk G, Hamilton T, Colmers WF (2007) Neuropeptide Y in the dentate gyrus. Prog Brain Res 163:285–297. [DOI] [PubMed] [Google Scholar]
- Swanson LW (1992) Brain Maps: Structure of the Rat Brain. 1 ed, Amsterdam: Elsevier. [Google Scholar]
- Trapaidze N, Gomes I, Cvejic S, Bansinath M, Devi LA (2000) Opioid receptor endocytosis and activation of MAP kinase pathway. Brain Res Mol Brain Res 76:220–228. [DOI] [PubMed] [Google Scholar]
- Turner CD, Bagnara JT (1971) General Endocrinology. Philadelphia: W.B. Saunders. [Google Scholar]
- Van Kempen TA, Gorecka J, Gonzalez AD, Soeda F, Milner TA, Waters EM (2014) Characterization of Neural Estrogen Signaling and Neurotrophic Changes in the Accelerated Ovarian Failure Mouse Model of Menopause. Endocrinology 155:3610–3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanHouten JP, Rudd RA, Ballesteros MF, Mack KA (2019) Drug Overdose Deaths Among Women Aged 30-64 Years - United States, 1999-2017. MMWR Morb Mortal Wkly Rep 68:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazdarjanova A, Ramirez-Amaya V, Insel N, Plummer TK, Rosi S, Chowdhury S, Mikhael D, Worley PF, Guzowski JF, Barnes CA (2006) Spatial exploration induces ARC, a plasticity-related immediate-early gene, only in calcium/calmodulin-dependent protein kinase II-positive principal excitatory and inhibitory neurons of the rat forebrain. J Comp Neurol 498:317–329. [DOI] [PubMed] [Google Scholar]
- Walker QD, Cabassa J, Kaplan KA, Li ST, Haroon J, Spohr HA, Kuhn CM (2001) Sex differences in cocaine-stimulated motor behavior: disparate effects of gonadectomy. Neuropsychopharmacology 25:118–130. [DOI] [PubMed] [Google Scholar]
- Walker QD, Nelson CJ, Smith D, Kuhn CM (2002) Vaginal lavage attenuates cocaine-stimulated activity and establishes place preference in rats. Pharmacol Biochem Behav 73:743–752. [DOI] [PubMed] [Google Scholar]
- Wallace CS, Lyford GL, Worley PF, Steward O (1998) Differential intracellular sorting of immediate early gene mRNAs depends on signals in the mRNA sequence. J Neurosci 18:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KH, Majewska A, Schummers J, Farley B, Hu C, Sur M, Tonegawa S (2006) In vivo two-photon imaging reveals a role of arc in enhancing orientation specificity in visual cortex. Cell 126:389–402. [DOI] [PubMed] [Google Scholar]
- Wang X-D, Su Y-A, Wagner KV, Avrabos C, Scharf SH, Hartmann J, Wolf M, Liebl C, Kuhne C, Wurst W, Holsboer F, Eder M, Deussing JM, Muller MB, Schmidt MV (2013) Nectin-3 links CRHR1 signaling to stress-induced memory deficits and spine loss. Nature Neuroscience 16:706. [DOI] [PubMed] [Google Scholar]
- Waselus M, Nazzaro C, Valentino RJ, Van Bockstaele EJ (2009) Stress-induced redistribution of corticotropin-releasing factor receptor subtypes in the dorsal raphe nucleus. Biol Psychiatry 66:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser MJ, Goel N, Sandau US, Bale TL, Handa RJ (2008) Androgen regulation of corticotropin-releasing hormone receptor 2 (CRHR2) mRNA expression and receptor binding in the rat brain. Exp Neurol 214:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibrand K, Messaoudi E, Havik B, Steenslid V, Lovlie R, Steen VM, Bramham CR (2006) Identification of genes co-upregulated with Arc during BDNF-induced long-term potentiation in adult rat dentate gyrus in vivo. Eur J Neurosci 23:1501–1511. [DOI] [PubMed] [Google Scholar]
- Williams TJ, Akama KT, Knudsen MG, McEwen BS, Milner TA (2011a) Ovarian hormones influence corticotropin releasing factor receptor colocalization with delta opioid receptors in CA1 pyramidal cell dendrites. Exp Neurol 230:186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TJ, Milner TA (2011) Delta opioid receptors colocalize with corticotropin releasing factor in hippocampal interneurons. Neuroscience 179:9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TJ, Torres-Reveron A, Chapleau JD, Milner TA (2011b) Hormonal regulation of delta opioid receptor immunoreactivity in interneurons and pyramidal cells in the rat hippocampus. Neurobiol Learn Mem 95:206–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TW, Wang JM, Chen S, Brinton RD (2005) 17Beta-estradiol induced Ca2+ influx via L-type calcium channels activates the Src/ERK/cyclic-AMP response element binding protein signal pathway and BCL-2 expression in rat hippocampal neurons: a potential initiation mechanism for estrogen-induced neuroprotection. Neuroscience 135:59–72. [DOI] [PubMed] [Google Scholar]
- Yildirim M, Janssen WG, Lou WY, Akama KT, McEwen BS, Milner TA, Morrison JH (2011) Effects of estrogen and aging on the synaptic distribution of phosphorylated Akt-immunoreactivity in the CA1 region of the female rat hippocampus. Brain Res 1379:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying WZ, Sanders PW (2003) The interrelationship between TGF-beta1 and nitric oxide is altered in salt-sensitive hypertension. Am J Physiol Renal Physiol 285:F902–908. [DOI] [PubMed] [Google Scholar]
- Yoo JH, Cho JH, Lee SY, Loh HH, Ho IK, Jang CG (2005) A lack of mu-opioid receptors modulates the expressions of neuropeptide Y and substance P mRNA. Neurosci Lett 384:29–32. [DOI] [PubMed] [Google Scholar]
- Zhang H, Torregrossa MM, Jutkiewicz EM, Shi YG, Rice KC, Woods JH, Watson SJ, Ko MC (2006) Endogenous opioids upregulate brain-derived neurotrophic factor mRNA through delta- and micro-opioid receptors independent of antidepressant-like effects. Eur J Neurosci 23:984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Windisch K, Altschuler J, Rahm S, Butelman ER, Kreek MJ (2016) Adolescent oxycodone self administration alters subsequent oxycodone-induced conditioned place preference and anti-nociceptive effect in C57BL/6J mice in adulthood. Neuropharmacology 111:314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng FF, Wu RC, Smith CL, O’Malley BW (2005) Rapid estrogen-induced phosphorylation of the SRC-3 coactivator occurs in an extranuclear complex containing estrogen receptor. Mol Cell Biol 25:8273–8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann S, Moelling K (1999) Phosphorylation and regulation of Raf by Akt (protein kinase B). Science 286:1741–1744. [DOI] [PubMed] [Google Scholar]
- Ziolkowska B, Urbanski MJ, Wawrzczak-Bargiela A, Bilecki W, Przewlocki R (2005) Morphine activates Arc expression in the mouse striatum and in mouse neuroblastoma Neuro2A MOR1A cells expressing mu-opioid receptors. J Neurosci Res 82:563–570. [DOI] [PubMed] [Google Scholar]
- Znamensky V, Akama KT, McEwen BS, Milner TA (2003) Estrogen Levels Regulate the Subcellular Distribution of Phosphorylated Akt in Hippocampal CA1 Dendrites. The Journal of Neuroscience 23:2340–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]


