Abstract
Multiple myeloma (MM) is a disease in which abnormal plasma cells proliferate and secrete monoclonal immunoglobulin in the bone marrow. The main characteristic of plasma cells is the expression of the cell surface antigen syndecan-1 (CD138). However, the expression of CD138 is limited to terminally differentiated plasma cells during B cell development. A small subpopulation (2~5%) of human MM cells that lack CD138 expression has been shown to possess enormous proliferation potential in vitro experiment and in animal models, and they also can differentiate into CD138+ plasma cells. Thus, this small subset of MM cells was regarded as myeloma cancer stem cell (MCSC). However, its characteristics associated with the pathogenesis of MM remain unclear. In this study, we analyzed the gene expression data of CD138 cell lines downloaded from Gene Expression Omnibus (GEO) database. Limma package in RStudio was used to identify differentially expressed genes (DEGs). Genes enrichment and protein-protein interaction (PPI) network analysis were performed on DAVID and STRING databases. Furthermore, overall survival (OS) analysis in MM patient was utilized to screen out the hub-genes closely associate with the MM pathogenesis process. Hub-genes expression validation and receiver operating characteristic curve (ROC) analysis was performed in different stages of plasma cell disorder diseases. Finally, we verified these findings in MM patient samples. Through integrated bioinformatics analysis of MM CD138- and CD138+ cell lines, we found that CDC7, CDK1, and CHK1 are highly expressed in CD138- MM cells. These genes are crucial in the G2/M phase of the cell cycle pathway, which is closely related to the malignant proliferation in various tumor cells. Of note, we found that patients with high expression of CDC7, CDK1, and CHK1 had shorter overall survival time. The expression of CHK1 was significantly increased in MM cells compared with normal plasma cell (NPC) and MGUS. More importantly, we further clarified that the expression of CHK1 in release/refraction MM (R/R MM) has obviously increased compared with new diagnosed MM (ND MM).
Keywords: multiple myeloma, CD138, CHK1, cancer stem cell, bioinformatics
INTRODUCTION
Multiple myeloma (MM) is an incurable malignant plasma cell disease and is the second most common hematology malignant tumor [1]. Numerous advances are available for the treatment of MM, such as immunomodulators drugs (IMiDs), proteasome inhibitors (PI), monoclonal antibodies, autologous stem-cell transplantation (ASCT), and chimeric antigen receptor T(CAR-T) cell therapy, [2–4]. The utilization of these therapies has dramatically improved the overall survival of MM patients. However, almost all of the patients ultimately relapse and drug resistance [5]. To understand this issue, a great many works have been devoted to explore the oncogenesis of MM [6].
MM cells have obvious genetic heterogeneity. Only a small number of myeloma cells have the ability of pathogenicity and tumorigenic. These cells are called multiple myeloma "stem cells" [7]. It is currently believed that these “stem cells” should responsible for the initiation, maintenance, and relapse of MM. The character of plasma cells is the expression of syndecan-1 (CD138), but the expression is highly specific both in MM cells and plasma cells of patient specimens. Moreover, during normal B cell development, CD138 is only expressed on end-stage differentiated normal plasma cells, but not in highly proliferating plasma cells and all early stages B cell [8, 9]. Previous studies have detected that, when compared to CD138- MM plasma cells, the proliferation of CD138+ plasma cells are inefficient. Furthermore, CD138- MM plasma cells have the ability of carcinogenic and drug resistance, meanwhile these CD138- plasma cells can differentiate into CD138+ plasma cells [7, 10]. However, some scholars have different opinions, H. Svachova et al. showed that CD138+ MM plasma cells express Nestin protein, which is the hallmark of cancer stem cell. Subsequently, they proved that the expression of Nestin in CD138+ cells throughout multistep pathogenesis of MM [11, 12]. Therefore, a better understanding of the distinction between CD138- and CD138+ MM plasma cellular characterization will help to develop new targets and strategies for the prognosis and treatment of MM.
In this study, though integrated bioinformatics we found the differential expression of genes in CD138- and CD138+ MM cell lines. Furthermore, we validated our findings in MM patient samples which provided new therapeutic targets to MM.
RESULTS
Screening of DEGs in CD138- and CD138+ cell lines
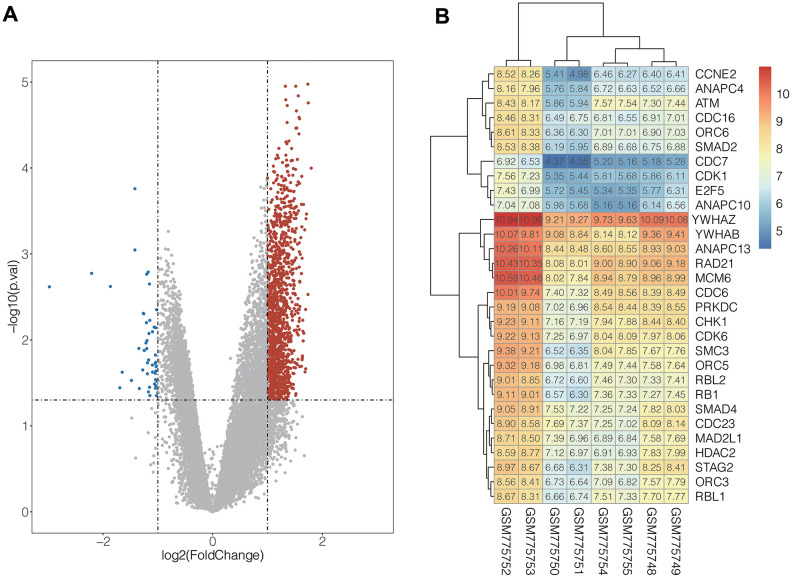
In the present study, integrated bioinformatics analysis was performed on the GSE31305 dataset, which contains CD138- and CD138+ human MM cell lines. After systematic data standardization, 18892 DEGs were identified which included 8608 up-regulated genes and 10284 down-regulated genes. Among these DEGs, 1318 up-regulated and 48 down-regulated genes were significant (| log2 FC |> 1 and p <0.05) (Figure 1A).
Figure 1.
(A) Volcanic map of DEGs distribution. The abscissa of volcano map is the logarithmic value of the fold change (FC) of each sample, and the ordinate is the logarithm of 10 corresponding to the P value of the corresponding sample Negative values, the red and blue dots respectively represent genes that are up-regulated and down-regulated, and the grey dots represent genes that are not significantly different. (B) The expression heat map of the genes in cell cycle pathway. Red represents high gene expression and blue represents low gene expression.
Functional analysis of differential genes
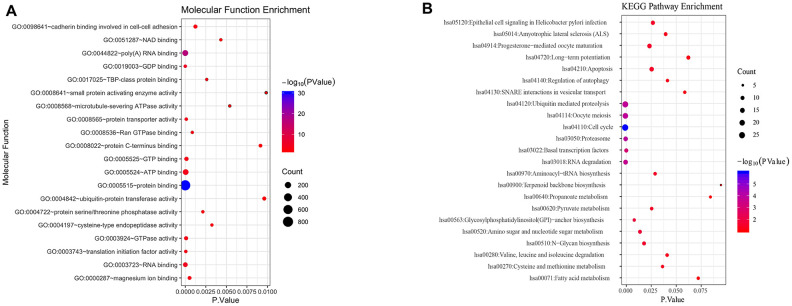
To understand biological function roles of the above identified DEGs, the online biological tools DAVID database and KEGG database were used for enrichment analysis. Top enriched GO terms and KEGG pathways were listed in Table 1 and Table 2 (Supplementary Table 2). In molecular function analysis (Figure 2A), differential genes are mainly concentrated in protein binding. In KEGG analysis (Figure 2B), it was found that differential genes are mainly involved in cell cycle pathways. The volcano map of all differentially expressed genes in cell cycle pathway is shown in Figure 1B (Supplementary Table 1).
Table 1. List of top enriched gene ontology terms of up-regulated and down-regulated DEGs.
| Direction | Category | Term | Description | Count | PValue |
| Upregulated | BP | GO:0007062 | sister chromatid cohesion | 35 | 9.29E-15 |
| Upregulated | BP | GO:0007067 | mitotic nuclear division | 53 | 1.05E-12 |
| Upregulated | BP | GO:0051301 | cell division | 65 | 1.86E-12 |
| Upregulated | BP | GO:0015031 | protein transport | 69 | 6.70E-12 |
| Upregulated | BP | GO:0051436 | negative regulation of ubiquitin-protein ligase activity involved in mitotic cell cycle | 22 | 1.13E-08 |
| Upregulated | MF | GO:0005515 | protein binding | 808 | 6.68E-31 |
| Upregulated | MF | GO:0044822 | poly(A) RNA binding | 158 | 1.85E-17 |
| Upregulated | MF | GO:0019003 | GDP binding | 16 | 3.02E-06 |
| Upregulated | MF | GO:0003723 | RNA binding | 67 | 1.16E-05 |
| Upregulated | MF | GO:0005524 | ATP binding | 145 | 5.32E-05 |
| Upregulated | CC | GO:0005829 | cytosol | 407 | 6.62E-36 |
| Upregulated | CC | GO:0005654 | nucleoplasm | 326 | 4.82E-24 |
| Upregulated | CC | GO:0005739 | mitochondrion | 159 | 6.60E-12 |
| Upregulated | CC | GO:0005783 | endoplasmic reticulum | 112 | 9.03E-12 |
| Upregulated | CC | GO:0016020 | membrane | 231 | 3.10E-11 |
| Downregulated | BP | GO:0050911 | detection of chemical stimulus involved in sensory perception of smell | 9 | 2.29E-07 |
| Downregulated | MF | GO:0004984 | olfactory receptor activity | 9 | 1.25E-07 |
| Downregulated | MF | GO:0004930 | G-protein coupled receptor activity | 9 | 5.39E-06 |
| Downregulated | CC | GO:0045095 | keratin filament | 6 | 6.82E-07 |
Table 2. List of top enriched KEGG terms of up-regulated and down-regulated DEGs.
| Direction | Category | Term | Description | Count | PValue |
| Upregulated | KEGG | hsa04110 | cell cycle | 30 | 1.01E-06 |
| Upregulated | KEGG | hsa04141 | Protein processing in endoplasmic reticulum | 34 | 1.15E-04 |
| Upregulated | KEGG | hsa03013 | RNA transport | 30 | 1.38E-04 |
| Upregulated | KEGG | hsa03050 | proteasome | 13 | 1.57E-04 |
| Upregulated | KEGG | hsa04114 | oocyte meiosis | 22 | 2.07E-04 |
| Downregulated | KEGG | hsa04740 | olfactory transduction | 8 | 1.29E-06 |
Figure 2.
Functional analysis of differential genes. (A) Molecular function analysis of differential genes. (B) KEGG signal pathway of differential genes.
PPI network analysis and hub-gene selection
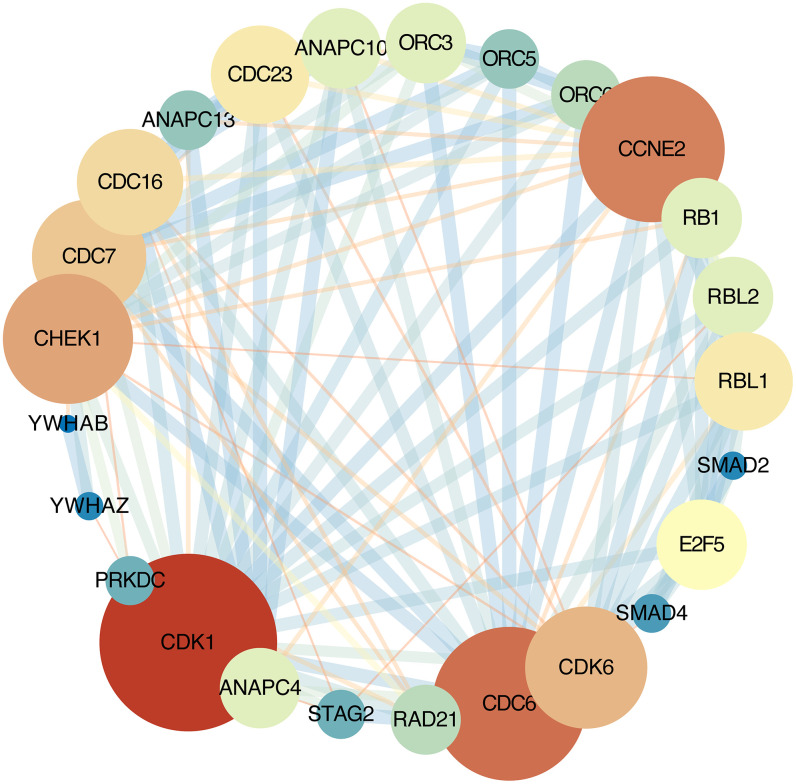
The STRING online database was used analyze the DEGs contained in the cell cycle pathway. This pathway contains 30 nodes and 172 edges, average node degree:11.5. Network nodes represent genes and edges represent protein-protein associations. Cytoscape software were used to visualization [13] (Figure 3).
Figure 3.
Protein-protein interaction network. Each node represents a gene; Node size represents the degree value; Edge size represents the combined score; Low value to blue and high value to red.
Overall survival analysis
GSE24080 was selected for overall survival analysis by using survival package in Rstudio, 313 MM patient samples with clinical and overall survival information were divided into high expression group and low expression group according to the average expression of each gene. The result suggested that the expression of cell division cycle 7 (CDC7), cyclin-dependent kinase 1 (CDK1), and checkpoint kinase 1 (CHK1) genes among cell cycle pathway were closely related with the overall survival time of patients, and the OS of the low-expression group was significantly prolonged than that of the high-expression group (CDC7, P = 0.032, CDK1, P = 0.003; CHK1, P = 0.011) (Figure 4A–4C). However, due to the lack of risk stratification of MM patients in the GSE24080 dataset, the relationship between these three genes and MM risk stratification cannot be calculated.
Figure 4.
Kaplan-Meier survival curves of MM patient. (A) Kaplan-Meier analysis for overall survival of CDK1; (B) Kaplan-Meier analysis for overall survival of CDC7;(C) Kaplan-Meier analysis for overall survival of CHK1.
Expression of hub-genes in different plasma cell diseases
The GSE47552 dataset was selected to verify the expression of CDC7, CDK1, and CHK1 at different stages of plasma cell diseases. The results showed that there was no significant difference between them when compared MM with NPC and MGUS, but the expression of CHK1 has statistically significant difference compared MM with NPC and MGUS (Figure 5A–5C).
Figure 5.
Expression validation of the hub genes in NPC (5), MGUS (20) and MM (41). (A) expression of CDC7; (B) expression of CDK1; (C) expression of CHK1.
Receiver operating characteristic curve analysis
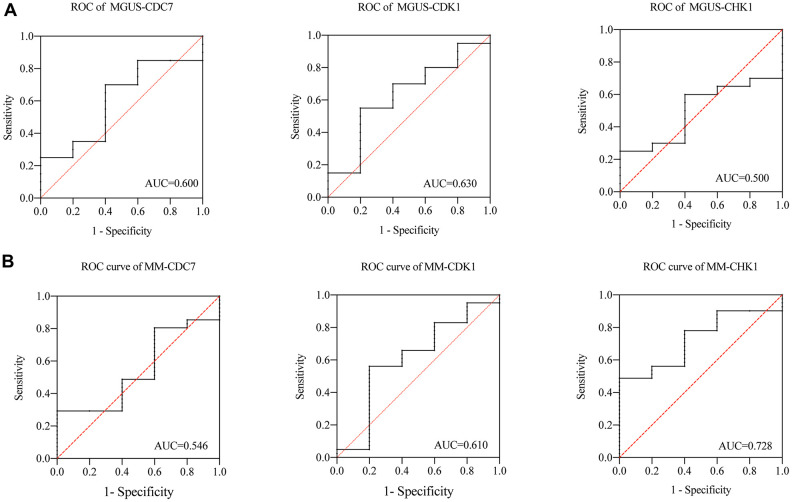
we subsequently analyzed the ROC of MGUS and MM. As shown in the Figure 6, in the MGUS, the area under the curve (AUC) of CDC7, CDK1, and CHK1 are 0.600, 0.630, and 0.500 (Figure 6A). In MM, the AUC of CDC7, CDK1, and CHK1 are 0.546, 0.610, and 0.728 (Figure 6B). That means the diagnostic value of CDC7 and CDK1 in MGUS and MM is not significantly different, while CHK1 has an apparent difference between MGUS and MM.
Figure 6.
(A) ROC of CDC7, CDK1 and CHK1 in MGUS, (B) ROC of CDC7, CDK1 and CHK1 in MM.
The expression of CHK1 in MM patients
To verify our findings, we tested the MM patients mRNA expression of CHK1 and found that there was no statistical difference between HC and ND MM, but there are significant statistical differences when compared R/R MM with HC (P=0.0009) and ND MM (P=0.0267, Figure 7A). Then we measured the protein expression of CHK1 between ND MM and R/R MM. The results showed that the expression of CHK1 in the R/R MM was obviously increased than that of ND MM (P<0.0001, Figure 7B, 7C).
Figure 7.
Verification of CHK1 expression in HC, ND MM, and R/R MM samples. (A) mRNA expression of CHK1 in HC, NDMM and R/R MM samples were analyzed using qRT-PCR. The mRNA expression level of CHK1 was showed by relative expression level. (B) Western blot analysis of the protein expression of CHK1 in ND MM, and R/R MM samples. (C) Results of western blot analysis showing a drastic increase in R/R MM. P< 0.05 was considered statistically significant (*p<0.05, **P<0.01, ***P<0.001, ****P<0.0001).
DISCUSSION
Multiple myeloma (MM) is characterized by proliferation of plasma cells, which secret a great many of monoclonal immunoglobulins [14]. The expression of cell surface antigen CD138 is the feature of plasma cells. However, CD138 is expressed in the terminal differentiation stage of plasma cells, but lost in the early stages of B cell and highly clonal plasma cells [15, 16]. Furthermore, recently study by Akhmetzyanova et al. shows that CD138+ cells are mainly related to the proliferation and enhance the sensitivity of IL-6 receptor, and CD138- myeloma cells are mainly involved in the migration process. The expression of CD138 in myeloma can be shed and recycled according to the different serum nutritional environment, so as to facilitate the adaptation of myeloma cells to the environment during the initiation and migration process [17]. Thus, it is necessary to further study the difference between CD138+ and CD138- MM cells. CD138- cells account for a small proportion of MM cells, and their characteristics of tumor stem cells in MM have been reported in previous study. Reghunathan et al found that CD138- cells have many common characteristics with normal hematology stem cells, including self-renewal, increase the activation of aldehyde dehydrogenase 1 (ALDH1) and differentiated into CD138+ plasma cells [18]. Such a small number cells owning the characteristics of tumor stem cells were also found in many other hematology malignity diseases, such as chronic myelogenous leukemia (CML), acute myeloid leukemia (AML), and acute lymphocytic leukemia (ALL) [19–22]. Therefore, studying the differences in gene expression and biological functions of CD138- and CD138+ MM cell lines can help us further understand the progress of MM.
In order to identify the characteristics of CD138- MM malignant profiling cells, integrated bioinformatics analysis has been performed with the GEO datasets, GSE24080 dataset, containing CD138+ and CD138- cell lines. First of all, based on the gene expression profiles of the above datasets, 3756 significantly DEGs were identified, then GO and KEGG enrichment analysis found that these DEGs were mainly concentrated in the cell cycle related pathways. Subsequently, we analyzed biological functional and PPI of the DEGs in the cell cycle. Through overall survival analysis, three hub-genes in cell cycle pathways were filtered out, including CDC7, CDK1, and CHK1. The analysis of OS of above hub-genes in MM patients showed that patients with high expression of CDC7, CDK1 and CHK1 had a poor overall survival time.
Because of MGUS can progress to MM, and there are overlaps in cancer related genes between MGUS and MM [23]. In order to further explore the relationship among different states of plasma cells disorder, the expression of CDC7, CDK1 and CHK1 was verified in the gene expression dataset of NPC, MGUS, and MM. The results showed that the expression of CHK1 in MM patients increased compared with NPC and MGUS but p = 0.084, which may be due to insufficient sample size in GSE47552. We will further expand the sample size for verification in the following experiments. Subsequently, the ROC analysis of CDC7, CDK1, and CHK1 in MGUS and MM were conducted. The result suggested that CHK1 could act as a reliable prognosis indicator in MM. In order to verify these findings from datasets, we validated our results in MM patient samples, and the results showed that the expression of CHK1 in mRNA and protein was obviously increased in R/R MM than ND MM.
Through the above analysis, it was found that the high expression of CHK1 in CD138- cells may play an essential role in the pathogenesis and maintenance of MM. CHK1 protein kinase regulates the G2/M phase transition in the cell cycle pathway, which is significant in gene replication and transcription. The transmission of genetic information is critical for cell survival, not only does it require the accurate transmission of genetic information, but it also needs to respond and repair in time when DNA is damaged [24]. Genomic instability is a common feature of cancer cells and contributes to the accumulation of oncogenic mutations [25]. DNA damage response (DDR) is a crucial factor in the development and treatment of various cancers. DNA damage causes cell cycle delay, mainly in G1/S and G2/M transitions, and causes a decrease in the rate of DNA synthesis [25]. Cell responses to DNA damage are mainly coordinated by two different protein kinase signal transducers Ataxia Telangiectasia Mutated (ATM) and Ataxia Telangiectasia and Rad3-Related kinase (ATR). ATM-CHK2 pathway primarily responds to DNA double-strand breaks (DSBs), whereas the ATR-Chk1 pathway recognizes extensive DNA abnormalities such as single-stranded DNA (ssDNA), DSBs end resection, DNA replication inhibition, and inter-strand DNA crosslinking [26]. In ATR-CHK1 signaling pathway, ATR activates CHK1 by phosphorylation, and the activation of CHK1 further phosphorylates downstream Cdc25A, limits its ability to drive progression during the S phase. CHK1 also can causes Cdc25B/C phosphorylation, to degrade or release the nucleus, thereby preventing the activation of CDK1 and CDK2, inactivating CDK2 and CDK1 causes G1/S and G2/M phase cell cycle arrest (Figure 8) [26–28]. Cells undergo DNA repair and maintain genome integrity and stable promote cell survival. Similar to the ATR, ATM controls the phosphorylation of p53, BRCA1, and CHK2, which play a crucial role in the ATM-CHK2 pathway [29–31]. CHK1 and CDK1 are hub genes in the ATR-CHK1 pathway. Consistent with our findings, the expression of these two genes in both CD138 cell lines and patient specimens are statistically significant, which further confirmed that this pathway was closely related to the initiation and maintenance of MM.
Figure 8.
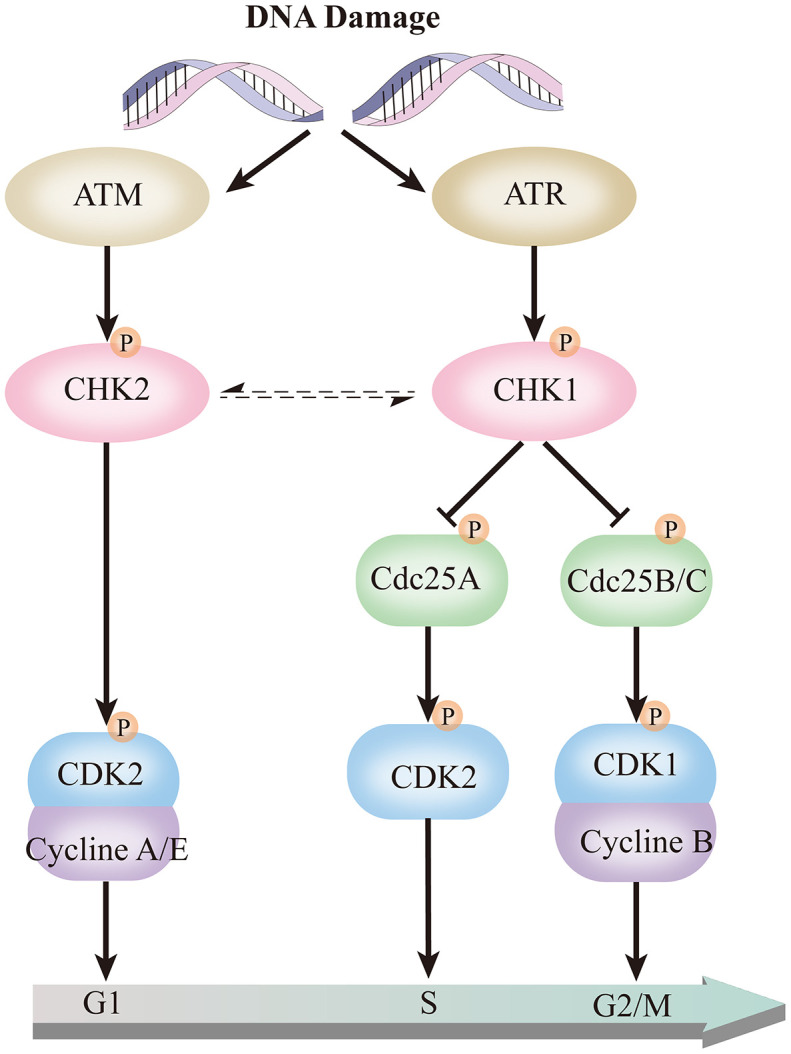
ATR-CHK1 and ATM-CHK2 in the cell cycle pathway.
CHK1 protein kinase can mediate cellular DNA damage response and repair. On the one hand, for normal cells, it does reduce the risk of malignant cell proliferation caused by DNA mutations. On the other hand, for tumor cells, CHK1 protein kinase also can repair DNA damage caused by various anticancer therapies [32]. High expression of CHK1 has been detected in numerous human tumors. Including breast cancer, colorectal cancer, cervical cancer, etc. [33–35]. It was also found that the higher expression of CHK1 is closely related to the relapse and drug resistance in tumors [36] and targeting CHK1 with specific inhibitor has been shown pro-apoptotic effect on CD138+ myeloma cells, but not unapparent in CD138- myeloma cells [37]. In short, the high expression of CHK1 makes tumor cells highly resistant to DNA damage induced by chemotherapy drugs and the severe tumor microenvironment. Eventually, these tumor cells dominate the body, creating clonal tumors that are more malignant and resistant.
Collectively, we identified a dramatical distinction gene expression between CD138- and CD138+ plasma cells. These DEGs are mainly concentrated in the ATR-CHK1 cell cycle pathway, which is closely related to the clonal proliferation characteristics of CD138- MM plasma cells. Multiple myeloma cells expressed high levels of CHK1, which correlated to overall survival time in MM patient. These findings provide clues for subsequent research, and also provide new targets for MM prognosis and treatment.
MATERIALS AND METHODS
Patients and samples
Bone marrow specimens were obtained from newly diagnosed MM (ND MM) and refractory/relapse MM (R/R MM) patients at the department of hematology, Second Affiliated Hospital of Xi’an Jiaotong University, from 2019 to 2020. This study was approved by the Medical Ethics Committee of the Second Affiliated Hospital of Xi’an Jiaotong University and written informed consent was obtained from all parents or guardians. The diagnosis, stage and risk status of MM were made in accordance with the National Comprehensive Cancer Network (NCCN) (2020 version 4) and mSMART 3.0. Heathy donor bone marrow mononuclear cells (BMNCs) samples were used as health control (HC). All samples were isolated using lymphocyte separation liquid to harvest total cellular RNA, then stored at -80°C.
Selection of GEO datasets and data processing
The gene expression profiles of CD138 cell lines were downloaded from the GEO database (http://www.ncbi.nlm.nih.gov/geo/). The microarray data with accession number GSE31305 was based on the GPL570 platform (Affymetrix Human Genome U133 Plus2.0 Array, Affymetrix, Santa Clara, CA, USA). GSE31305 dataset contains two kinds of human MM cell lines: RPMI-8226 and NCI-H929, both of them included CD138- and CD138+ MM cell lines and have two replicates. When multiple expression levels corresponded to one specific gene, take an average of all expression-levels for that gene.
Identification of DEGs
To investigate DEGs among CD138- and CD138+ cell lines, the Limma package [38] in RStudio (Version 1.2.1335) was used to identify differential expressed genes, |log2 FC (fold change) |> 1 and P <0.05 were used as the cut-off criteria.
GO and KEGG pathway enrichment analysis
To identify the significant biological roles of these DEGs, Gene ontology (GO) enrichment analysis was performed using DAVID Bioinformatics Resources 6.8 [39]. Pathway enrichment analysis was performed on the Kyoto Encyclopedia of Genes and Genomes (KEGG; http://www.Genome.Jp/keg), meaningful enrichment of biological process (BP), molecular function (MF), and cellular component (CC), and KEGG pathway was selected with a cut-off of false discovery rate (FDR) <0.05.
PPI network analysis
PPI network analysis of all the DEGs in cell cycle pathway on the STRING online database (http://string-db.org) [13]. The PPI correlation coefficient was imported into Cytoscape 3.5.1 for visualization [40]. Each node in Cytoscape represents a gene, the edges width between the nodes represent the interaction between them, and the degree represents the number of edges.
Overall survival analysis based on MM patient
GSE24080 dataset was selected as the validation dataset, which includes clinical and overall survival information of 313 (IgG) patients. The details of this dataset as below: a. total number: 313 newly diagnosed MM patients; b. gender (male/female): 195/118; c. median age: 57.75 (29.70~76.50). The expression level of each hub-gene was divided into high expression group and low expression group according to the mean value. Finally, GraphPad Prism 8 (version 8.2.1) was used to make the Kaplan-Meier Survival Curve. Log-Rank test was used to calculate the OS of above MM patients. The difference was statistically significant at P <0.05.
Hub-genes expression validation and ROC analysis
Due to GSE24080 dataset lack of NPC and MGUS patient specimen, GSE47552 dataset, 5 Normal Plasma Cells (NPC) as health control, 20 MGUS patients, and 41 MM patients, all patient samples are newly diagnosed without treatment, was used for the hub-genes expression validation and ROC analysis. The area under the curve (AUC) is a key indicator to reflect the accuracy and specificity of the diagnoses.
Validation of CHK1 expression by real-time quantitative reverse transcription polymerase chain reactions (qRT-PCR)
To measure the gene expression of CHK1, we detected the expression of CHK1 MM bone marrow mononuclear cells (BMNCs) samples using qRT-PCR, including 13 HC, 15 newly diagnosed MM, and 15 R/R MM samples. Total RNA was extracted from BMSCs samples of HC and MM using TRIzol reagent (Invitrogen, Germany) and stored at −80 °C until use. RNA purity and concentration were determined by Thermo Scientific Multiskan GO. RNA samples were reversely transcribed into cDNA using a Primescript RT master mix with Oligo dT primers and random primers in accordance with manufacturer's protocols (CWBIO). Then, the qRT-PCR was performed by SYBR Premix Ex Taq™ II (CWBIO) and StepOne Software v2.1 according to manufacturer's instructions. Primers of detected CHK1 and GAPDH were designed and synthesized by Tsingke (Shanghai, China). And the primer sequences were as shown in Table 3. 2-ΔΔCt value was used to reflect the expression level of CHK1.
Table 3. The primer sequences of CHK1 and GAPDH.
| Name | Forward primer (5′-3′) | Reverse primer (5′-3′) |
| CHK1 | ACCAGATGCTCAGAGATTCTTCCA | TGAGGTTATCCCTTTCATCCAACAG |
| GAPDH | ACAACTTTGGTATCGTGGAAGG | GCCATCACGCCACAGTTTC |
Western blotting analysis
To validate the protein expression of CHK1, immunoblot analysis was adopted to evaluate the differential expression of CHK1 in 3 ND MM and 3 R/R MM and every single sample repeat three times. BMNCs samples obtained from ND MM and R/R MM patients and immediately frozen in liquid nitrogen and stored at -80 °C. Then The samples were centrifuged at 12,000 rpm at 4 °C for 30 min. The supernatants were collected and protein amounts were quantified by BCA method. Lysates containing 20 μg of protein was boiled at 95 °C in SDS sample buffer for 10 min, electrophoresed on 12% SDS PAGE gels, and transferred to polyvinyldifluoridine membranes. Subsequently membranes blocked in 5% (w/v) skimmed milk solution for 2h at room temperature and incubated overnight at 4 °C with primary antibody. Anti-CHK1 mouse monoclonal antibody (diluted 1:1000, Cell Signaling Technology, USA) and anti-GAPDH rabbit monoclonal antibody (diluted 1:1000, Beyotime Biotechnology, China) were used. After three washings for 30 min in TBST buffer, membranes were incubated at room temperature for 1h, horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit IgG (diluted 1:1000; Beyotime Biotechnology, China) was used as a secondary antibody and washed three times for 30 min with TBST buffer. Then membranes were developed with ECL reagents and the chemiluminescence signal was imaged using a MiniChemiTM (Sagecreation, China). Immunoblots were quantified using quantity-one software (Bio-Rad, USA).
Supplementary Material
ACKNOWLEDGMENTS
We gratefully thank professor Jinsong Hu from department of cell biology and genetics, Xi'an Jiaotong university health science center for valuable comments and critical revision of the manuscript. We thank all of the patients contributed to our study. We also express our appreciation to researchers who shared their data online.
Footnotes
AUTHOR CONTRIBUTIONS: DW and AH conceived and designed the study. DW, PZ, and FL collected and processed data. DW and YS prepared tables and figures. HC, YF, and FW helped to collect bone marrow samples from the patients. DW and FW drafted the manuscript. AH revised the manuscript. All authors critically reviewed content and approved the final version for publication.
CONFLICTS OF INTEREST: The authors declare that they have no conflicts of interest.
FUNDING: This work was supported by chimeric antigen receptor (CAR) modified T cells for multiple myeloma drug development and multi-center clinical research (2019ZX09301139).
REFERENCES
- 1.Kazandjian D. Multiple myeloma epidemiology and survival: a unique Malignancy. Semin Oncol. 2016; 43:676–81. 10.1053/j.seminoncol.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castillo JJ. Evolution of Therapy for Relapsed/Refractory Multiple Myeloma. J Natl Compr Canc Netw. 2019; 17:1433–1436. 10.6004/jnccn.2019.5035 [DOI] [PubMed] [Google Scholar]
- 3.Zhao WH, Liu J, Wang BY, Chen YX, Cao XM, Yang Y, Zhang YL, Wang FX, Zhang PY, Lei B, Gu LF, Wang JL, Yang N, et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J Hematol Oncol. 2018; 11:141. 10.1186/s13045-018-0681-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nijhof IS, van de Donk NW, Zweegman S, Lokhorst HM. Current and new therapeutic strategies for relapsed and refractory multiple myeloma: an update. Drugs. 2018; 78:19–37. 10.1007/s40265-017-0841-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harousseau JL, Attal M. How I treat first relapse of myeloma. Blood. 2017; 130:963–73. 10.1182/blood-2017-03-726703 [DOI] [PubMed] [Google Scholar]
- 6.Corre J, Munshi N, Avet-Loiseau H. Genetics of multiple myeloma: another heterogeneity level? Blood. 2015; 125:1870–76. 10.1182/blood-2014-10-567370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, Smith BD, Civin CI, Jones RJ. Characterization of clonogenic multiple myeloma cells. Blood. 2004; 103:2332–36. 10.1182/blood-2003-09-3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hosen N, Matsuoka Y, Kishida S, Nakata J, Mizutani Y, Hasegawa K, Mugitani A, Ichihara H, Aoyama Y, Nishida S, Tsuboi A, Fujiki F, Tatsumi N, et al. CD138-negative clonogenic cells are plasma cells but not B cells in some multiple myeloma patients. Leukemia. 2012; 26:2135–41. 10.1038/leu.2012.80 [DOI] [PubMed] [Google Scholar]
- 9.Chaidos A, Barnes CP, Cowan G, May PC, Melo V, Hatjiharissi E, Papaioannou M, Harrington H, Doolittle H, Terpos E, Dimopoulos M, Abdalla S, Yarranton H, et al. Clinical drug resistance linked to interconvertible phenotypic and functional states of tumor-propagating cells in multiple myeloma. Blood. 2013; 121:318–28. 10.1182/blood-2012-06-436220 [DOI] [PubMed] [Google Scholar]
- 10.Caligaris-Cappio F, Bergui L, Gregoretti MG, Gaidano G, Gaboli M, Schena M, Zallone AZ, Marchisio PC. ‘Role of bone marrow stromal cells in the growth of human multiple myeloma. Blood. 1991; 77:2688–93. [PubMed] [Google Scholar]
- 11.Svachova H, Pour L, Sana J, Kovarova L, Raja KR, Hajek R. Stem cell marker nestin is expressed in plasma cells of multiple myeloma patients. Leuk Res. 2011; 35:1008–13. 10.1016/j.leukres.2011.03.001 [DOI] [PubMed] [Google Scholar]
- 12.Svachova H, Kryukov F, Kryukova E, Sevcikova S, Nemec P, Greslikova H, Rihova L, Kubiczkova L, Hajek R. Nestin expression throughout multistep pathogenesis of multiple myeloma. Br J Haematol. 2014; 164:701–09. 10.1111/bjh.12689 [DOI] [PubMed] [Google Scholar]
- 13.Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, Jensen LJ, Mering CV. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019; 47:D607–13. 10.1093/nar/gky1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Röllig C, Knop S, Bornhäuser M. Multiple myeloma. Lancet. 2015; 385:2197–208. 10.1016/S0140-6736(14)60493-1 [DOI] [PubMed] [Google Scholar]
- 15.Kawano Y, Fujiwara S, Wada N, Izaki M, Yuki H, Okuno Y, Iyama K, Yamasaki H, Sakai A, Mitsuya H, Hata H. Multiple myeloma cells expressing low levels of CD138 have an immature phenotype and reduced sensitivity to lenalidomide. Int J Oncol. 2012; 41:876–84. 10.3892/ijo.2012.1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanderson RD, Lalor P, Bernfield M. B lymphocytes express and lose syndecan at specific stages of differentiation. Cell Regul. 1989; 1:27–35. 10.1091/mbc.1.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akhmetzyanova I, McCarron MJ, Parekh S, Chesi M, Bergsagel PL, Fooksman DR. Dynamic CD138 surface expression regulates switch between myeloma growth and dissemination. Leukemia. 2020; 34:245–56. 10.1038/s41375-019-0519-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reghunathan R, Bi C, Liu SC, Loong KT, Chung TH, Huang G, Chng WJ. Clonogenic multiple myeloma cells have shared stemness signature associated with patient survival. Oncotarget. 2013; 4:1230–40. 10.18632/oncotarget.1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham SM, Jørgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L, Holyoake TL. Primitive, quiescent, philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002; 99:319–25. 10.1182/blood.v99.1.319 [DOI] [PubMed] [Google Scholar]
- 20.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997; 3:730–37. 10.1038/nm0797-730 [DOI] [PubMed] [Google Scholar]
- 21.Blair A, Hogge DE, Sutherland HJ. Most acute myeloid leukemia progenitor cells with long-term proliferative ability in vitro and in vivo have the phenotype CD34(+)/CD71(-)/HLA-DR-. Blood. 1998; 92:4325–35. [PubMed] [Google Scholar]
- 22.Terpstra W, Prins A, Ploemacher RE, Wognum BW, Wagemaker G, Löwenberg B, Wielenga JJ. Long-term leukemia-initiating capacity of a CD34-subpopulation of acute myeloid leukemia. Blood. 1996; 87:2187–94. [PubMed] [Google Scholar]
- 23.Zingone A, Kuehl WM. Pathogenesis of monoclonal gammopathy of undetermined significance and progression to multiple myeloma. Semin Hematol. 2011; 48:4–12. 10.1053/j.seminhematol.2010.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou BB, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000; 408:433–39. 10.1038/35044005 [DOI] [PubMed] [Google Scholar]
- 25.Smith J, Tho LM, Xu N, Gillespie DA. The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv Cancer Res. 2010; 108:73–112. 10.1016/B978-0-12-380888-2.00003-0 [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Hunter T. Roles of Chk1 in cell biology and cancer therapy. Int J Cancer. 2014; 134:1013–23. 10.1002/ijc.28226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009; 461:1071–8. 10.1038/nature08467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartek J, Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 2003; 3:421–29. 10.1016/s1535-6108(03)00110-7 [DOI] [PubMed] [Google Scholar]
- 29.Ronco C, Martin AR, Demange L, Benhida R. ATM, ATR, CHK1, CHK2 and WEE1 inhibitors in cancer and cancer stem cells. Medchemcomm. 2016; 8:295–319. 10.1039/c6md00439c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maréchal A, Zou L. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb Perspect Biol. 2013; 5:a012716. 10.1101/cshperspect.a012716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sørensen CS, Syljuåsen RG, Falck J, Schroeder T, Rönnstrand L, Khanna KK, Zhou BB, Bartek J, Lukas J. Chk1 regulates the S phase checkpoint by coupling the physiological turnover and ionizing radiation-induced accelerated proteolysis of Cdc25A. Cancer Cell. 2003; 3:247–58. 10.1016/s1535-6108(03)00048-5 [DOI] [PubMed] [Google Scholar]
- 32.Lee JH, Choy ML, Ngo L, Venta-Perez G, Marks PA. Role of checkpoint kinase 1 (Chk1) in the mechanisms of resistance to histone deacetylase inhibitors. Proc Natl Acad Sci USA. 2011; 108:19629–34. 10.1073/pnas.1117544108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solyom S, Pylkäs K, Winqvist R. Screening for large genomic rearrangements of the BRIP1 and CHK1 genes in finnish breast cancer families. Fam Cancer. 2010; 9:537–40. 10.1007/s10689-010-9360-7 [DOI] [PubMed] [Google Scholar]
- 34.Madoz-Gúrpide J, Cañamero M, Sanchez L, Solano J, Alfonso P, Casal JI. A proteomics analysis of cell signaling alterations in colorectal cancer. Mol Cell Proteomics. 2007; 6:2150–64. 10.1074/mcp.M700006-MCP200 [DOI] [PubMed] [Google Scholar]
- 35.Xu J, Li Y, Wang F, Wang X, Cheng B, Ye F, Xie X, Zhou C, Lu W. Suppressed miR-424 expression via upregulation of target gene Chk1 contributes to the progression of cervical cancer. Oncogene. 2013; 32:976–87. 10.1038/onc.2012.121 [DOI] [PubMed] [Google Scholar]
- 36.Ashwell S, Zabludoff S. DNA damage detection and repair pathways—recent advances with inhibitors of checkpoint kinases in cancer therapy. Clin Cancer Res. 2008; 14:4032–37. 10.1158/1078-0432.CCR-07-5138 [DOI] [PubMed] [Google Scholar]
- 37.Pei XY, Dai Y, Youssefian LE, Chen S, Bodie WW, Takabatake Y, Felthousen J, Almenara JA, Kramer LB, Dent P, Grant S. Cytokinetically quiescent (G0/G1) human multiple myeloma cells are susceptible to simultaneous inhibition of Chk1 and MEK1/2. Blood. 2011; 118:5189–200. 10.1182/blood-2011-02-339432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015; 43:e47. 10.1093/nar/gkv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009; 4:44–57. 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- 40.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003; 13:2498–504. 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.