Abstract
Background
Although live attenuated monovalent human rotavirus vaccine (Rotarix) efficacy has been characterized through randomized studies, its effectiveness, especially in non-clinical settings, is less clear. In this study, we estimate the impact of childhood Rotarix® vaccination on community rotavirus prevalence.
Methods
We analyse 10 years of serial population-based diarrhoea case-control study, which also included testing for rotavirus infection (n = 3430), and 29 months of all-cause diarrhoea active surveillance from a child cohort (n = 376) from rural Ecuador during a period in which Rotarix vaccination was introduced. We use weighted logistic regression from the case-control data to assess changes in community rotavirus prevalence (both symptomatic and asymptomatic) and all-cause diarrhoea after the vaccine was introduced. We also assess changes in all-cause diarrhoea rates in the child cohort (born 2008–13) using Cox regression, comparing time to first all-cause diarrhoea case by vaccine status.
Results
Overall, vaccine introduction among age-eligible children was associated with a 82.9% reduction [95% confidence interval (CI): 49.4%, 94.2%] in prevalence of rotavirus in participants without diarrhoea symptoms and a 46.0% reduction (95% CI: 6.2%, 68.9%) in prevalence of rotavirus infection among participants experiencing diarrhoea. Whereas all age groups benefited, this reduction was strongest among the youngest age groups. For young children, prevalence of symptomatic diarrhoea also decreased in the post-vaccine period in both the case-control study (reduction in prevalence for children <1 year of age = 69.3%, 95% CI: 8.7%, 89.7%) and the cohort study (reduction in hazard for receipt of two Rotarix doses among children aged 0.5-2 years = 57.1%, 95% CI: 16.6, 77.9%).
Conclusions
Rotarix vaccination may suppress transmission, including asymptomatic transmission, in low- and middle-income settings. It was highly effective among children in a rural community setting and provides population-level benefits through indirect protection among adults.
Keywords: Rotarix, rotavirus, vaccine effectiveness, Ecuador, rural
Key Messages
We found that older children and adults, who were too old to be vaccinated, were protected against rotavirus infection, providing evidence for indirect effects of Rotarix® vaccination.
Young children were protected against both rotavirus infection and all-cause diarrhoea.
Due to indirect effects, the total impact of vaccination and its cost effectiveness may be higher than previously expected for low-resource settings, where the burden of rotavirus diarrhoea is high among all age groups.
Introduction
Rotavirus is a major cause of severe diarrhoea worldwide, particularly in young children.1–3 In 2006, the Rotarix® and Rotateq® vaccines were approved with the objective of reducing severe rotavirus infections.3,4 Whereas both vaccines have shown similar effectiveness, Rotarix is used in Ecuador and in most lower-and-middle-income countries (LMICs) because of its better cost-effectiveness, lower dose requirements and thermostability.5–7 In general, Rotarix vaccination is thought to be most effective against severe rotavirus infection and decreases the burden of severe diarrhoea in populations where it is introduced.8–14 However, most studies on rotavirus vaccine performance have come from clinical populations or focus on children; data on how the vaccine performs against asymptomatic infections for older individuals are lacking.5 Moreover, many of the previous studies have focused on large cities;8–11 data on how the vaccine performs in rural settings are needed.5 Here we examine Rotarix effectiveness on rotavirus infections (including asymptomatic infections) and all-cause diarrhoea in a rural community setting in Ecuador.
In addition to demonstrating efficacy against severe rotavirus among young children (who may not yet have acquired natural immunity against rotavirus infection), some studies have also shown reductions in rotavirus infections in unvaccinated populations,15–17 suggesting that rotavirus vaccination may reduce the transmission rate. However, most evidence of this effect comes from high-income countries.15,16 There is a need for data to determine if vaccination also reduces transmission rates in LMICs, which could increase the impact of vaccination in such settings.5,17 It may also be easier to interrupt transmission in rural regions of LMICs because some are partially protected from illness due to their relative isolation.18 In this context, rotavirus transmission patterns can be driven by periodic reintroductions by older children and adults, who are not age-eligible to be vaccinated.19 Therefore, indirect protection might help maintain vaccine effects over time by reducing community susceptibility to periodic reintroduction.
Our objective was to determine if Rotarix implementation reduced rotavirus infection, including asymptomatic infection, and all-cause diarrhoea in a rural region of Ecuador with high levels of endemic diarrhoea. We used these results to quantify: (i) the indirect effect of vaccination on un-vaccinated populations; and (ii) the impact of vaccination on asymptomatic rotavirus infection.
Methods
Data
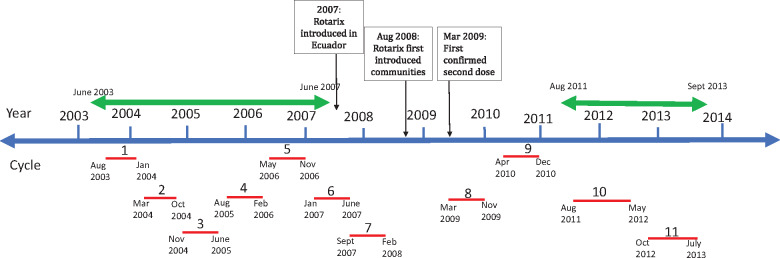
This analysis used data collected for a study on diarrhoeal disease in rural Ecuador over a 10-year period (from 2003 to 2013, Figure 1).18 This included: yearly census information, active all-cause diarrhoea surveillance and periodic case-control data. All protocols were approved by institutional review boards at the University of Michigan and Universidad San Francisco de Quito. An overview of the data used is shown in Table 1, and more details about the analysis are provided below.
Figure 1. Diagram of data collected during the study period (2003–13). Timing of case-control data collection cycles (1–11) is shown below the timeline with corresponding months for each cycle. Double headed arrows above the timeline show the timing of all-cause diarrhea surveillance. The boxes with arrows illustrate the timing of Rotarix vaccine introduction.
Table 1.
Summary information of the two analytical approaches used in this analysis, one based on a case control study of diarrhoea, which also measured rotavirus infection among both diarrhoea cases and controls, and the other active surveillance for all-cause diarrhoea. We restricted the case-control analysis to communities who had been included in the case-control study for the full 10-year period and who had vaccine data available (14 communities). For the cohort analysis, all communities with vaccine records with data from 2011 to 2013 were eligible for inclusion (total of 21 communities).
| Case control study | Child cohort study | |
|---|---|---|
| Outcome(s) | All-cause diarrhoea | All-cause diarrhoea |
| Rotavirus infection | ||
| Exposure | Pre/post vaccine introduction | Individual vaccination status (0, 1 or 2 doses) |
| Community-level vaccine coverage (proportion, 0 to 1) | ||
| Data collection mode | Diarrhoea cases and randomly selected controls | Active surveillance |
| Years of data included | 10 years | 2 years |
| Age groups (years) | <1, 1-5, >5, combined | 0.5-2, 2-5 |
| Number of communities | 14 | 21 |
| Number of individuals | 3430 | 376 |
| Regression tool | Weighted logistic regressiona | Cox regression |
| Effect measure | Odds ratio (OR) | Hazard ratio (HR) |
Weighted by inverse probability of sampling.
Outcome variables
Our outcome variables come from two datasets. Approximately once every 9 months from 2003 to 2013, staff visited each community and conducted a 2-week, prospective, population-based, case-control study for symptomatic diarrhoea. Here cases and controls were tested for the presence of rotavirus. From the case-control data we have three outcome variables: all-cause diarrhoea, defined as cases in the case-control study; symptomatic rotavirus infections, defined as cases testing positive for rotavirus; and asymptomatic rotavirus infections, defined as controls without diarrhoea symptoms who tested positive for rotavirus. In addition, between August 2011 and December 2013 we collected all-cause diarrhoeal surveillance data from all households every 2 weeks. This surveillance did not include any testing for rotavirus status.
Each case-control study cycle occurred over an approximate 9-month study period and included communities sampled in the main rainy (December-May) and dry (June-November) seasons.21 The order of sampling for each community rotated over time, such that data were available from both seasons for all communities (see Figure 1). In total, there were 11 cycles of case-control sampling across 15 communities. During each 14-day period, cases of diarrhoea were identified prospectively. A case in the case-control study was defined by having three or more loose stools in the past 24 h, irrespective of the pathogen causing that diarrhoea. For the first seven study cycles, we collected one household control and two community controls per case (both time-matched to cases such that a control could later become a case). For cycles 8-11, a random sample of 10% of the community population was sampled as controls at baseline. We obtained stool samples from each case and control and these samples were tested for rotavirus (using the EIA kit, RIDA Quick Rotavirus; R-Biopharm, Darmstadt, Germany).
Exposure variables
We collected vaccine records for children born in study villages between 2008 and 2013. Rotarix was introduced in Ecuador in 2007 and was introduced into the study region in late August of 2008. Children began receiving their second doses of Rotarix in early 2009 (during cycle 8, see Figure 1).20 Using birth date, community of residence and name, we linked vaccination records to individuals in our larger study (see Supplementary Section S1 for more details, available as Supplementary data at IJE online). The two primary exposure variables used in the case-control analysis were: (ii) a binary variable comparing cycles before the vaccine was introduced with those after the vaccine was introduced; and (ii) community-level vaccine coverage for each cycle, which is a measure of the proportion of children who received two doses of Rotarix. Vaccine coverage should provide more statistical power than a pre/post analysis, but were missing vaccine coverage data for about half the children in our study region, making absolute coverage estimates uncertain. For this reason, we report the results using both exposure variables, but focus our discussion on the pre/post analysis. We considered cycles 1-7 to be the pre-vaccine period and cycles 9-11 to be the post-vaccine period. Case-control data from the study cycle during which the vaccine was introduced (cycle 8) were excluded from both the pre/post and vaccine coverage case-control analyses.
For the cohort analysis, we use individual Rotarix vaccination status (zero, one or two doses). Because we only had vaccine records for 74 individuals in the cohort study in later cycles, we did not assess the impact of individual vaccine status for children on rotavirus infection in the case-control study.
To estimate community-level vaccine coverage, we used the number of children receiving their second dose of rotavirus vaccine by the start of a given case-control cycle (meaning that doses were administered during the previous cycle) divided by the number of children who were eligible to receive their second dose in that cycle, based on their age. We also considered alternative methods for calculating coverage (see Supplementary Section S2 for more details, available as Supplementary data at IJE online).
Case-control analysis
For the case-control analysis, only communities that were followed for the full 10-year period and for which vaccine data were available were included (14 communities, 3430 individuals). We compared study cycles before and after the vaccine was introduced for both all-cause diarrhoea and rotavirus infection for the population overall as well as for three age groups separately: <1, 1-5 and ≥5 years. We also estimated the association between vaccine coverage of two doses of Rotarix and both outcomes.
We used individual-level logistic regression models, weighted by inverse probability of sampling, to compare the odds of rotavirus infection over time, using a pre/post analysis to compare odds before and after the vaccine was introduced. We also conducted a similar analysis, using community coverage of two doses of Rotarix attained during each case-control study cycle as our exposure of interest (comparing 100% coverage vs 0% coverage, the range of coverage estimates observed after vaccine introduction). Our weighting method accounted for differences in selection probability between household and community controls (see Supplementary Section S3 for details, available as Supplementary data at IJE online). Because the method of control selection varied over time, we re-ran all models excluding data from household controls to see if inclusion of this group early on might have biased our results, and the results were similar. We did not have adequate power to analyse vaccine coverage using quantiles; although coverage ranged from 0% to 100%, nearly half of the community/cycle observations after vaccine introduction had 100% coverage. We conceptualised our data for this analysis as repeated cross-sectional surveys that provided an unbiased estimate of: (i) overall rotavirus infection prevalence, using both symptomatic and asymptomatic infections—data from both cases and controls were weighted by inverse probability of sampling (see Supplementary Section S3 for weighting details);(ii2) subclinical rotavirus infection prevalence, estimated among controls; and (iii) rotavirus infection prevalence among persons with diarrhoea, estimated among cases within the community at each time point. Because previous studies have shown the strongest protection among children aged under 1 year,5 we conducted this analysis separately by age group (<1 year, 1-5 years and ≥5 years) and for all age groups combined. We calculated overall percent reduction in all-cause diarrhoea and rotavirus infection for all age groups separately and for the population overall (Reduction = (1 − OR) × 100).
Cohort analysis
We created a dynamic cohort of children, enrolling all children with vaccine records born between August 2008 and September 2013. Since our surveillance did not start until August 2011 (Figure 1), children born between March 2011 and September 2013 entered the cohort at 6 months of age to ensure they were vaccinated (6 months was the latest age Rotarix was administered according to local policy). Children born between August 2008 and February 2011 entered the cohort at the start of active surveillance. Children born between 2008 and 2011 were, therefore, older upon cohort entry (although they were still vaccinated before 6 months of age). All children were followed until their first diarrhoea episode, until 5 years of age or until the end of the active surveillance period, whichever came first.
To account for differences in age upon cohort entry, we conducted a stratified analysis based on baseline age using two age groups: 6 months to 2 years and 2 years to 5 years of age. We initially planned to use 1 year of age as a cut point, but only 62 children entered the cohort before 1 year of age, and only two of those children had received zero doses of vaccine. We chose to use 2 years as a cut point instead, which corresponds to the age range commonly used in other vaccine effectiveness studies.5
Based on census records, 819 children born in the 21 study communities between 2008 and 2013 were included in the analysis with surveillance data. Vaccine records and covariate data were available for 376 of these children (46%). Because we were concerned about the potential for selection bias, we compared children with and without vaccine records to assess whether these groups were comparable. Some children may not have vaccine records because they were not vaccinated, some of these records may have been lost and many childrenmay have been vaccinated elsewhere, particularly in Borbón, the nearest city in the region and location of the region’s primary health centre. Given that travel patterns in our study region are positively associated with socioeconomic status among adults,19 parents of children without vaccine records, who had higher education levels, may have brought their children to Borbón to receive vaccines. Not all children had dates associated with their vaccine records, but children generally received both doses by 6 months of age. For communities included in the case-control analysis, we had vaccine coverage data for 43.9% of eligible children in cycle 8, 57.6% of eligible children in cycle 9 and 78.6% of eligible children in cycle 10 (see Supplementary Section S1 for vaccine availability by community).
We compared the time to first all-cause diarrhoea case by vaccination status (2 doses, 1 dose, reference group = 0 doses) for these 376 children, using Cox regression to estimate the direct effect of vaccination [using the hazard ratio (HR)].
Summary of effect measures of interest
We used these data to calculate: (i) the overall effect of Rotarix coverage on rotavirus infection for all age groups; (ii) the indirect effect of Rotarix coverage on rotavirus infection among older age groups, who were not vaccinated; and (iii3) the effect of rotavirus vaccination on all-cause diarrhoea.
The indirect and overall effects were estimated using vaccine period (pre- or post-vaccine introduction) as the exposure variable and rotavirus status (based on the case-control study) as the outcome variable using weighted logistic regression (weighted by inverse probability of sampling). We also estimated these variables using vaccine coverage as our exposure of interest. Vaccine coverage was categorical taking one of 154 coverage values depending on the community (14 in total) and cycle (11 in total) that the outcome variable was recorded. Vaccine coverage was set to zero for all data collection cycles before the vaccine introduction and was then set to the coverage level estimated for each community and data collection cycle thereafter. Individual vaccine status was not included in either of these models. The overall effect models used data from the entire population, including young children in cycles after vaccine introduction who may have been vaccinated. Indirect effects models were restricted to older age groups, none of whom could have been vaccinated; see Table 1 for more details.
The effect on all-cause diarrhoea was measured for both the case-control study and the cohort study. For the case-control study, similar models were run for all-cause diarrhoea outcome (case status in the case-control study). For the cohort, the effect on all-cause diarrhoea was estimated by comparing the time to first all-cause diarrhoea disease episode captured using the active surveillance data (2008–13, all years post-vaccine introduction) in children who received two doses of the rotavirus vaccine, with those who did not, using Cox regression.
Sensitivity analysis
In the main analysis tables we assume that the coverage for those without vaccine records is the same as for those with vaccine records. However, the number of vaccine records used to estimate coverage in the case-control study was small for some communities and some study cycles. We therefore did sensitivity analyses to assess whether or not our results were robust to different coverage assumptions among those without records. In particular, we assumed vaccine coverage among children without records was 0%, 25%, 50%, 75% and 100% (see Supplementary Section S2 for more details). We also considered the potential for chance confounding by season to have biased the results, by explicitly adjusting for the season of case-control sampling (rainy vs dry, see Supplementary Section S4 for details, available as Supplementary data at IJE online).
Comparing analyses
To assess the internal consistency of our two analyses, which used different communities and different study designs, we conducted a comparative analysis. Because controls were time-matched to cases, the odds ratio (OR) from the case-control study approximates an incidence rate ratio (IRR). Therefore, to compare estimates obtained for the two samples, we ran Poisson models for the cohort analysis with the count of all-cause diarrhoea episodes as the outcome (to directly estimate the IRR). We then compared the rate ratio from this Poisson model with the OR quantifying the association between vaccine coverage and all-cause diarrhoea among children from the case-control analysis; see Supplementary Section S5 for details, available as Supplementary data at IJE online.
All statistical analyses were conducted in R (version 3.4). Weighted regression analyses used the package ‘survey,=’, clustered logistic regression used the package ‘gee’ and Cox regression analyses used the ‘OIsurv’ function from the package ‘stats’.
Results
Descriptive statistics
Vaccine coverage
Out of 819 children in the cohort, 443 were missing vaccine records (54%), suggesting the importance of comparing children with and without vaccine records (Table 2). Children with vaccine records available had a higher illness rate than children without vaccine records (18 vs 14.3 cases per 1000 person-weeks) and a higher proportion of them became sick during the surveillance period (36% vs 27%).
Table 2.
Surveillance data descriptive statistics comparing children with vaccine records with those without. Continuous variables are shown as mean (standard deviation), categorical variables and risks are shown as % (n), and rates are shown as rates (number of events).
| No vaccine record | Vaccine record |
||||
|---|---|---|---|---|---|
| Total | Zero doses | One dose | Two doses | ||
| (N = 443) | (N = 376) | (N = 47) | (N = 58) | (N = 271) | |
| Remoteness (%) | |||||
| Close | 46.7 (207) | 48.7 (183) | 59.6 (28) | 27.6 (16) | 51.3 (139) |
| Medium | 6.6 (29) | 8.5 (32) | 10.6 (5) | 6.9 (4) | 8.5 (23) |
| Far | 46.7 (207) | 42.8 (161) | 29.8 (14) | 65.5 (38) | 40.2 (109) |
| Age (years) | |||||
| Cohort entry | 1.49 (1.00) | 1.41 (0.92) | 1.66 (1.01) | 1.58 (1.05) | 1.33 (0.86) |
| First diarrhoeal case | 1.89 (1.04) | 1.79 (0.96) | 1.92 (0.87) | 1.61 (0.86) | 1.80 (1.00) |
| BCG vaccination (%) | N/A | 94.1 (353) | 74.5 (35) | 94.8 (55) | 98.5 (266) |
| Male (%) | 48.7 (182) | 47.6 (156) | 63.2 (24) | 43.2 (19) | 45.9 (113) |
| Household size (total N) | 6.36 (3.28) | 6.61 (3.57) | 6.89 (4.51) | 6.93 (3.38) | 6.51 (3.45) |
| Household size (children) | 3.16 (1.75) | 3.41 (1.89) | 3.31 (2.29) | 3.68 (1.81) | 3.38 (1.85) |
| Highest household education (yrs) | 8.79 (3.56) | 8.29 (3.27) | 8.56 (2.81) | 8.26 (3.60) | 8.26 (3.28) |
| Overall illness rate (per 1000 person-weeks) | |||||
| Diarrhoea | 14.28 (246) | 18.0 (271)* | 17.8 (36) | 19.9 (49) | 17.6 (186) |
| Non-diarrhoea | 3.7 (61) | 5.71 (86)* | 4.94 (10) | 8.10 (20) | 5.29 (56) |
| Any | 18.4 (307) | 23.7 (357)* | 22.7 (46) | 28.0 (69) | 22.9 (242) |
| Person-weeks | 16672 | 15071 | 2025 | 2468 | 10578 |
| Overall illness risk (%) | |||||
| Diarrhoea | 27.4 (121) | 36.3 (137)* | 38.3 (18) | 37.9 (22) | 35.7 (92) |
| Non-diarrhoea | 6.1 (27) | 8.8 (33) | 6.4 (3) | 13.8 (8) | 8.1 (22) |
| Any | 31.4 (139) | 41.1 (155)* | 38.3 (18) | 43.1 (25) | 41.2 (112) |
| Length diarrhoea episode (days) | 2.61 (2.02) | 2.47 (1.98) | 2.13 (1.95) | 2.26 (1.51) | 2.61 (2.05) |
| Length first diarrhoea episode (days) | 3.40 (1.84) | 3.20 (1.79) | 2.74 (1.63) | 3.18 (1.82) | 3.29 (1.82) |
Significant difference between children with and without vaccine records (comparing columns 1 and 2).
Overall, 271 of the 376 children with vaccine records had received two doses of Rotarix (72.1%) (Table 2). This coverage level was relatively constant over time (see Supplementary data, available as Supplementary data at IJE online). At the community level, coverage estimates varied ranging from 0% to 100%, with higher vaccine coverage in less remote villages.
Time trends in all-cause diarrhoea and rotavirus infection (outcomes)
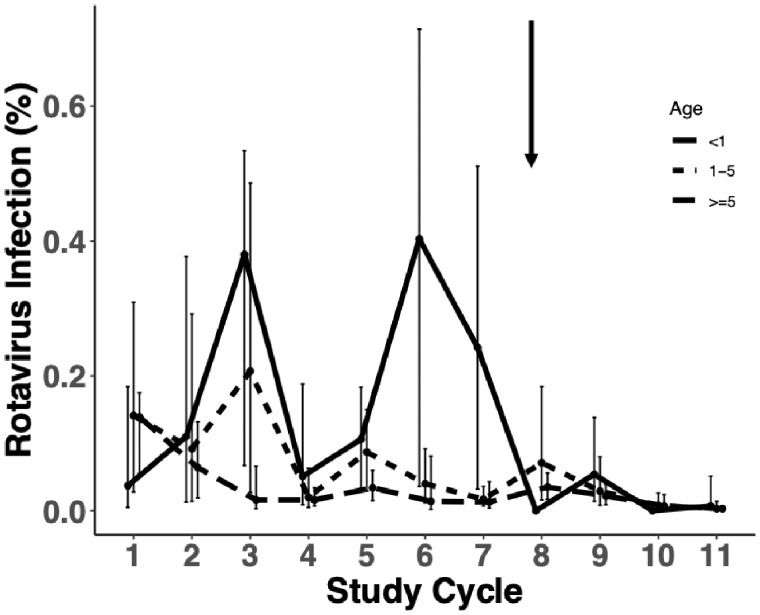
Whereas older children and adults had the lowest risk of rotavirus infection (Figure 2), they explain a substantial proportion of the symptomatic infections due to their higher proportion in the population, ranging 20–60% of the total infections, depending on the cycle. In this older age group, rotavirus was also a causative diarrhoeal pathogen based on an analysis of the case-control data (see Supplementary Section S6, available as Supplementary data at IJE online). The total prevalence of rotavirus infection and all-cause diarrhoea decreased after the vaccine was introduced, with young children <1 year of age showing strong reductions beginning in cycle 8 when the vaccine was first introduced and older age groups showing this benefit one cycle later (Figure 2). Specifically for young children, 24.2% (95% CI: 3.2%, 51.1%) of the children were positive for rotavirus in the cycle preceding vaccine introduction; in cycle 8, when Rotarix was first introduced, there were no cases of rotavirus infection detected (estimated prevalence = 0%) and thereafter estimated prevalence remained at or below 6% for all remaining study cycles. In contrast, older age groups had slightly higher prevalence in cycle 8 compared with previous cycles (percent increase of 5.4% for children aged 1-5 and 2.2% for older children and adults) but thereafter their risk of infection also fell and was sustained at a lower level. There were no discernible trends for all-cause diarrhoea. Graphs of rotavirus infection (symptomatic and asymptomatic) and all-cause diarrhoea over time are shown in Supplementary Figures S3 and S4, available as Supplementary data at IJE online. We did not evaluate time trends for the cohort data because we only had 2 years of surveillance data.
Figure 2.
Based on the 14 communities from the case-control study, percentage of population infected with rotavirus by age group. Children <1 year of age are shown in red, children between 1 and 5 years of age are shown in light blue, and older children (≥5 years) and adults are shown in gold. The arrow shows the timing of Rotarix introduction, between cycles 7 and 8.
Pre- and post-vaccine comparison
In the case-control study, the prevalence of rotavirus infection in the six communities declined after vaccine introduction (3.8% to 1.0%; Table 3). Over time, the prevalence of all-cause diarrhoea also declined (3.1% to 1.8%). The socioeconomic status of these communities improved, as indicated by smaller household size and higher average household education.
Table 3.
Characteristics of the 14 study communities included in the case-control analysis before (cycles 1–7) and after (cycles 9–11) the vaccine was introduced. All statistics are weighted by inverse probability of sampling, and the total sample size (N) is the total population size of the six communities included in the analysis. Continuous variables are shown as mean (standard error) and categorical variables are shown as % (n).
| Case-control analysis |
||
|---|---|---|
| Pre-vaccine | Post-vaccine | |
| (N = 23 972) | (N = 10 831) | |
| Symptomatic for diarrhoea (%) | 3.1 (745) | 1.8 (199) |
| Rotavirus infection (%) | 3.8 (915) | 1.0 (114) |
| Male (%) | 52.2 (12 520) | 51.3 (5561) |
| Age (%) years | ||
| <1 | 3.3 (785) | 4.9 (527) |
| 1-5 | 12.1 (2899) | 13.8 (1498) |
| >5 | 84.6 (20 289) | 81.3 (8807) |
| Household size (n) | 6.82 (0.10) | 6.29 (0.14) |
| Highest household education (yrs) | 7.29 (0.11) | 8.34 (0.21) |
| Remoteness (%) | ||
| Close | 43.6 (10 446) | 50.9 (5512) |
| Medium | 12.7 (3048) | 14.1 (1526) |
| Far | 43.7 (10 479) | 35.0 (3792) |
Overall effect on rotavirus infection
Community-level prevalence of rotavirus infection was lower after vaccine introduction, with cases and controls in the post-vaccine period having 0.266 times the odds of rotavirus infection compared with the pre-vaccine period (Table 4), corresponding to a reduction in infection of 73.4% (95% CI: 50.0%, 85.8%) (Table 5). The reduction in asymptomatic infection in the post-vaccine period was 79.0% (95% CI: 52.7%,90.7%) and 56.0% (95% CI: 21.3%, 75.5%) for symptomatic infection (Table 5). Results were similar using the vaccine coverage version of the analysis but the point estimates were stronger (Table 5).
Table 4.
Logistic regression of rotavirus infection (combined symptomatic and asymptomatic). All cells represent OR (95% CI). Reference groups for categorical variables are noted above. OR for the post-vaccine period compares the post-vaccine period with the pre-vaccine period which represents the overall effect (also shown in Table 5, 1st column, rows for the total population). Regression results are adjusted for whether or not infection was symptomatic, gender, age, household size, highest household education, and remoteness.
| Case-control analysis of rotavirus infection | |
|---|---|
| OR (95% CI) | |
| Symptomatic for diarrhoea | 9.25 (5.10, 16.8) |
| Male | 1.39 (0.762, 2.52) |
| Age, years | |
| <1 | 2.37 (0.814, 6.90) |
| 1-5 | 0.980 (0.405, 2.73) |
| >5 | Ref |
| Household size | 0.981 (0.889, 1.08) |
| Highest household education | 0.959 (0.877, 1.05) |
| Remoteness | |
| Close | Ref |
| Medium | 0.591 (0.292, 1.20) |
| Far | 0.358 (0.173, 0.742) |
| Post-vaccine period (compared with pre-vaccine period) | 0.266 (0.142, 0.500) |
Table 5.
Overall effect of vaccination (% reduction) by age group, VE = (1 − OR) × 100 comparing: pre-vaccine case control cycles (cycles 1–7) vs post-vaccine case control cycles (cycles 9–11; pre/post analysis); and 100% vaccine coverage with 0% vaccine coverage (vaccine coverage models). All models are adjusted for remoteness, gender, household size and highest household education. Models for any rotavirus infection (column 2) are also adjusted for whether or not the infection was symptomatic. Models for the total population (row 4) are adjusted for age.
| Rotavirus infection risk |
All-cause diarrhoea | |||
|---|---|---|---|---|
| Age group | Any | Symptomatic | Asymptomatic | |
| Pre-/post analysis | ||||
| <1 | 88.9% | 72.6% | 100% (N/A)* | 69.1% |
| (29.4%, 98.3%) | (0.6%, 92.5%) | (23.1%, 87.6%) | ||
| 1-5 | 84.7% | 57.3% | 100% (N/A)* | 54.5% |
| (38.9%, 96.2%) | (-13.2%, 83.9%) | (18.0%, 74.7%) | ||
| >5 | 68.7% | 48.3% | 70.3% | 17.4% |
| (34.1%, 85.1%) | (-5.3%, 74.6%) | (-5.3%, 87.0%) | (-16.9%, 41.6%) | |
| Total population | 73.4% | 56.0% | 79.0% | 44.1% |
| (50.0%, 85.8%) | (21.3%, 75.5%) | (52.7%, 90.7%) | (20.6%, 60.7%) | |
| Vaccine coverage | ||||
| <1 | 92.6% | 69.1% | 100% (N/A)* | 67.4% |
| (-19.4%, 99.5%) | (-68.6%, 94.4%) | (-4.6%, 89.8%) | ||
| 1-5 | 92.5% | 71.1% | 100% (N/A)* | 58.2% |
| (53.9%, 98.8%) | (10.6%, 90.7%) | (13.2%, 79.8%) | ||
| >5 | 81.8% | 57.8% | 83.5% | 9.3% |
| (53.1%, 92.9%) | (5.8%, 81.1%) | (-5.3%, 94.5%) | (-38.1%, 40.4%) | |
| Total population | 84.5% | 62.4% | 89.6% | 39.9% |
| (64.4%, 93.2%) | (25.4%, 81.0%) | (66.3%, 96.8%) | (9.2%, 60.2%) | |
Indirect effect among the unvaccinated age group
The indirect effect, measured among people too old to have been vaccinated (>5 years), is significant, with an infection reduction of 68.7% (95% CI: 34.1% to 85.1%) comparing the post-vaccine period with the pre-vaccine period (Table 5).
Effect on all-cause diarrhoea
Among children (aged <1 year) in our case-control study, the prevalence of all-cause diarrhoea was significantly reduced in the post-vaccine period (69.1% , 95% CI: 23.1%, 87.6%), but not among older children (Table 5). Similarly in the cohort study, the reductions in all-cause diarrhoea were only present in the youngest age group. Among young children in our cohort study (aged 6 months-2 years), receiving two doses of the rotavirus vaccine was associated with a decreased hazard of all-cause diarrhoea [hazard ratio (HR) = 0.429, 95% CI: 0.221, 0.834; see Supplementary Section S8, available as Supplementary data at IJE online). Among older children born closer to the time of vaccine introduction (aged 2-5 years) and who may have experienced multiple infections by the start of surveillance, the sum of two doses of vaccine was also associated with a decreased hazard, but this association did not reach significance (HR = 0.565, 95% CI: 0.121, 2.63). Children who received one dose of rotavirus also tended to have a decreased hazard of diarrhoea compared with unvaccinated children during the first 2 years of life, but this association was not significant (HR = 0.577, 95% CI: 0.256, 1.30), which likely reflects the small sample size of this group.
Sensitivity analysis
The effect estimates were extremely similar before and after adjustment for season, so we did adjust our final models for season (see Supplementary Section S4 for details).
Comparing analyses
Comparing the impact of vaccination on the rate of all-cause diarrhoea, the point estimates were both significant, but stronger for the case-control study (see Supplementary Section S5, among children <2 yesar, 39.8% reduction for the rate of all-cause diarrhoea from the cohort compared with 51.9% for the case- control study).
Discussion
Rotarix has strong effectiveness on both rotavirus infection and all-cause diarrhoea in a rural, non-clinical population in Ecuador. These associations are strongest among young children who are generally at highest risk of severe disease. However, we also found indirect effects on rotavirus infection among older children and adults who could not have been vaccinated. This is most likely due to suppression of overall transmission among all age groups and highlights the key role of young children in transmission to older age groups.22,23
Specifically, children <1 year old tended to have the strongest reductions in both rotavirus infection and all-cause diarrhoea. We estimate higher direct effectiveness for all-cause diarrhoea (57.1%) among young children compared with the 39-42% efficacy reported previously against hospitalization for all-cause diarrhoea,9,24 This difference may partially be explained by the fact that we report hazard ratios, whereas others in the literature reported rate ratios. Previous work has shown that relying on rates trends can cause downward bias in estimates of vaccine efficacy.25 The point estimate for our Poisson regression sensitivity analysis, which more directly approximates the rate ratio, is closer to the findings from these previous studies (39.8%). Although we did not collect data on the severity of diarrhoeal disease episodes for either the active surveillance or the case-control study, this difference in point estimate may also be due to the fact that our study focused on self-reported cases of diarrhoea, which are likely to be milder on average than in other studies that have focused on medically attended diarrhoea. The smaller and non-significant effect size for vaccine status on all-cause diarrhoea among older children in the cohort is likely partially attributable to naturally-acquired immunity during the years between vaccination and cohort entry.26,27
All age groups were protected from rotavirus infection, including the oldest, unvaccinated age group (68.7% reduction in rotavirus infection in the post-vaccine period compared with the pre-vaccine period). This result implies that Rotarix vaccination suppresses not only severe disease (seen in the younger children) but also overall transmission (including the less severe cases seen in the older children and adults) and helps explain why we and others have observed indirect effects of vaccination in older age groups.16,17,23
We find that this indirect effects benefit is attainable in low-resource settings where other studies have estimated indirect benefits to be weaker and not statistically significant.16 Our findings extend recent work by Baker et al. who also found indirect effects on rotavirus hospitalization in the USA among older, unvaccinated age groups. Notably, we find a similar benefit at similar, modest coverage levels (72% in our study compared with 73% in the USA), and also find that this benefit is achievable in non-clinical populations.23 This suppression of overall infection also suggests that previous studies, which focused on severe rotavirus infection in a clinical setting, may have underestimated the overall impact of vaccination on rotavirus infection.
Strengths and limitations
Due to our retrospective study design, we had high levels of missing data for the cohort analysis, decreasing our sample size. However, given that our main findings for the case-control study were robust to a variety of different coverage assumptions (see Supplementary Section 2) and also exhibited strong differences between the pre- and post-vaccine periods, our results strongly suggest that rotavirus vaccination has important overall effects in reducing rotavirus transmission.
As with any non-randomized study, our results are also potentially subject to bias from secular trends and unmeasured confounding. We have previously shown how many community characteristics changed over our study period, including socioeconomic status and mobility/migration, with impacts on rotavirus infection over the same period.19 Whereas such secular trends could have biased the pre/post comparison, the similarity between the vaccine coverage and pre/post model results suggests that these changes are unlikely to be completely due to secular trends.
Because the case-control study was focused on symptomatic diarrhoea, our confidence intervals for asymptomatic infection are wide and we cannot determine with certainty relative effectiveness on asymptomatic vs symptomatic infection. Although uncertain, the higher point estimate against asymptomatic infection in this study may result from dose-dependent effects of vaccination, where the vaccine has higher protection against lower dose exposures. Additionally, the method used to detect rotavirus infections is somewhat less sensitive than real-time polymerase chain reaction (RT-PCR) and may have failed to detect asymptomatic rotavirus infections with lower shedding rates.28 For this reason, our overall statistical power may have been reduced for asymptomatic infections in general and we may also have overestimated the effect of vaccination on asymptomatic infections with lower shedding rates. However, the asymptomatic infections with sufficient shedding rates to be detected by our methodology are probably those that are most relevant for secondary transmission. Despite these limitations, our results strongly suggest that Rotarix vaccination is protective against milder rotavirus infections, including asymptomatic infection, in addition to its effects on severe rotavirus. At a minimum, our results suggest that vaccination reduces shedding to below to the detection limit for the EIA assay, which would be expected to dampen overall transmission.
Conclusions
Rotarix vaccination has substantially reduced both rotavirus infection and all-cause diarrhoea in a rural region of Ecuador, despite significant challenges to vaccination. Much of this effect is driven by suppression of overall rotavirus transmission at a population level, including among older age groups who were too old to be vaccinated. The impact of rotavirus vaccination may be higher than previously estimated, given its effect on milder diarrhoeal disease and asymptomatic infections.
Supplementary data
Supplementary data are available at IJE online.
Supplementary Material
Acknowledgements
We would like to thank the Ecuador Ministry of Health for providing vaccine data and the EcoDess field team for their valuable contribution collecting the data. We would like to thank Benjamin Arnold for helpful comments.
Funding
This work was supported by the Models of Infectious Disease Agent Study (MIDAS) programme within the National Institute of General Medical Sciences [U01 GM110712] and National Institute of Allergy and Infectious Diseases [R01 AI050038].
Conflicts of interest
None declared.
References
- 1. Kotloff KL, Nataro JP, Blackwelder WC et al. Burden and aetiology of diarrheal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective case-control study. Lancet 2013;382:209–22. [DOI] [PubMed] [Google Scholar]
- 2. Platts-Mills JA, Liu J, Rogawski ET et al. Use of quantitative molecular diagnostic methods to assess the aetiology, burden, and clinical characteristics of diarrhea in low-resource settings: a reanalysis of the MAL-ED cohort study. Lancet Glob Health 2018;6:e1309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dennehy PH. Rotavirus vaccines: an overview. Clin Microbiol Rev 2008;21:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O’Ryan M, Linhares AC. Updates on Rotarix: an oral human rotavirus vaccine. Expert Rev Vaccines 2009;8:1627–41. [DOI] [PubMed] [Google Scholar]
- 5. Jonesteller CL, Burnett E, Yen C, Tate JE, Parashar UD. Effectiveness of rotavirus vaccination: a systematic review on the first decade of global postlicensure data, 2006-2016. Clin Infect Dis 2017;65:840–50. [DOI] [PubMed] [Google Scholar]
- 6. van Hoek AJ, Ngama M, Ismail A et al. A cost effectiveness and capacity analysis for the introduction of universal rotavirus vaccination in Kenya: comparison between Rotarix and RotaTeq vaccines. PLoS One 2012;7:e47511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lo Vecchio A, Liguoro I, Dias JA et al. Rotavirus immunization: global coverage and local barriers for implementation. Vaccine 2017;35:1637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Phua KB, Lim FS, Lau YL et al. Safety and efficacy of human rotavirus vaccine during the first 2 years of life in Asian infants: randomized, double-blind, controlled study. Vaccine 2009;27:5936–41. [DOI] [PubMed] [Google Scholar]
- 9. Linhares AC, Velázquez FR, Pérez-Schael I et al. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet 2008;371:1181–89. [DOI] [PubMed] [Google Scholar]
- 10. Justino MC, Linhares AC, Lanzieri TM et al. Effectiveness of the monovalent G1P[8] human rotavirus vaccine against hospitalization for severe G2P[4] rotavirus gastroenteritis in Belém, Brazil. Pediatr Infect Dis J 2011;30:396–401. [DOI] [PubMed] [Google Scholar]
- 11. Correia JB, Patel MM, Nakagomi O et al. Effectiveness of monovalent rotavirus vaccine (Rotarix) against severe diarrhea caused by serotypically unrelated G2P[4] strains in Brazil. J Infect Dis 2010;201:363–69. [DOI] [PubMed] [Google Scholar]
- 12. Patel MM, Steele D, Gentsch JR, Wecker J, Glass RI, Parashar UD. Real-world impact of vaccination. Pediatr Infect Dis J 2011;30:S1–S5. [DOI] [PubMed] [Google Scholar]
- 13. Tate JE, Cortese MM, Payne DC et al. Uptake, impact, and effectiveness of rotavirus vaccination in the United States: review of the first 3 years of postlicensure data. Pediatr Infect Dis J 2011;30:S56–S60. [DOI] [PubMed] [Google Scholar]
- 14. Yen C, Armero Guardado JA, Alberto P et al. Decline in rotavirus hospitalizations and health care visits for childhood diarrhea following vaccination in El Salvador. Pediatr Infect Dis J 2011;30:S6–S10. [DOI] [PubMed] [Google Scholar]
- 15. Payne DC, Staat MA, Edwards KM et al. Direct and indirect effects of rotavirus vaccination upon childhood hospitalizations in 3 US counties, 2006-2009. Clin Infect Dis 2011;53:245–53., [DOI] [PubMed] [Google Scholar]
- 16. Rosettie KL, Vos T, Mokdad AH et al. Indirect rotavirus vaccine effectiveness for the prevention of rotavirus hospitalization: a systematic review and meta-analysis. Am J Trop Med Hyg 2018;98:1197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bennett A, Bar-Zeev N, Cunliffe NA. Measuring indirect effects of rotavirus vaccine in low- income countries. Vaccine 2016;34: 4351–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eisenberg JNS, Cevallos W, Ponce K et al. Environmental change and infectious disease: how new roads affect the transmission of diarrheal pathogens in rural Ecuador. PNAS 2006;103:19460–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kraay ANM, Trostle J, Brouwer AF, Trujillo WC, Eisenberg J. Determinants of short-term movement in a developing region and implications for disease transmission. Epidemiology 2018;29:117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. Ecuador: WHO and UNICEF Estimates of Immunization Coverage: 2017 Revision. Geneva: WHO, 2018. [Google Scholar]
- 21. Bhavnani D, Goldstick JE, Cevallos W, Trueba G, Eisenberg J. Impact of rainfall on diarrheal disease risk associated with unimproved water and sanitation. Am J Trop Med Hyg 2014;90:705–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mossong J, Hens N, Jit M et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLOS Med 2008;5:e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baker JM, Tate JE, Steiner CA, Haber MJ, Parashar UD, Lopman BA. Longer-term direct and indirect effects of infant rotavirus vaccination across all ages in the United States in 2000-2013: analysis of a large hospital discharge data set. Clin Infect Dis 2019;68:976–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006;354:11–22. [DOI] [PubMed] [Google Scholar]
- 25. Lopman BA, Pitzer VE. Waxing understanding of waning immunity. J Infect Dis 2018;217: 851–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Farrington CP. The measurement and interpretation of age-specific vaccine efficacy. Int J Epidemiol 1992;21:1014–20. [DOI] [PubMed] [Google Scholar]
- 27. Rogawski ET, Platts-Mills JA, Colgate ER et al. Quantifying the impact of natural immunity on rotavirus vaccine efficacy estimates: a clinical trial in Dhaka, Bangladesh (PROVIDE) and a simulation study. J Infect Dis 2018;217:861–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tate JE, Mijatovic-Rustempasic S, Tam KA et al. Comparison of 2 assays for diagnosing rotavirus and evaluating vaccine effectiveness in children with gastroenteritis. Emerg Infect Dis 2013;19:1245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.