Abstract
Background
Previous case-control studies have reported a strong association between statin use and lower cancer risk. It is unclear whether this association reflects a benefit of statins or is the result of design decisions that cannot be mapped to a (hypothetical) target trial (that would answer the question of interest).
Methods
We outlined the protocol of a target trial to estimate the effect of statins on colorectal cancer incidence among adults with low-density lipoprotein (LDL) cholesterol below 5 mmol/L. We then emulated the target trial using linked electronic health records of 752 469 eligible UK adults (CALIBER 1999–2016) under both a cohort design and a case-control sampling of the cohort. We used pooled logistic regression to estimate intention-to-treat and per-protocol effects of statins on colorectal cancer, with adjustment for baseline and time-varying risk factors via inverse-probability weighting. Finally, we compared our case-control effect estimates with those obtained using previous case-control procedures.
Results
Over the 6-year follow-up, 3596 individuals developed colorectal cancer. Estimated intention-to-treat and per-protocol hazard ratios were 1.00 (95% confidence interval [CI]: 0.87, 1.16) and 0.90 (95% CI: 0.71, 1.12), respectively. As expected, adequate case-control sampling yielded the same estimates. By contrast, previous case-control analytical approaches yielded estimates that appeared strongly protective (odds ratio 0.57, 95% CI: 0.36, 0.91, for ≥5 vs. <5 years of statin use).
Conclusions
Our study demonstrates how to explicitly emulate a target trial using case-control data to reduce discrepancies between observational and randomized trial evidence. This approach may inform future case-control analyses for comparative effectiveness research.
Keywords: Case-control, causal inference, comparative effectiveness, electronic health records, target trial
Key Messages
Previous case-control studies have reported a strong association between statin use and lower cancer risk; it is unclear whether this reflects a benefit of statins or is the result of design decisions that cannot be mapped to a (hypothetical) target trial (that would answer the question of interest).
A target trial can be emulated using case-control data by (i) specifying the protocol of the target trial that would have answered the causal question of interest, (ii) defining the observational cohort study that explicitly emulates this target trial, and (iii) sampling cases and controls from that cohort.
This approach reduces bias in the effect estimates derived from case-control studies and minimizes discrepancies between observational and randomized trial evidence.
Case-control analyses that deviate from this approach may lead to severe bias, particularly on the multiplicative scale.
Introduction
Many important clinical decisions must be made in the absence of evidence from randomized trials, which may be impractical or too lengthy to provide a timely answer. In these cases, we resort to analyses of observational data to emulate the target trial that we would have liked to conduct and provide the best available evidence to inform decision making.1,2
The target trial approach has mostly been applied to cohort (follow-up) studies, but it can be readily extended to case-control studies when (i) the goal is to estimate relative (not absolute) risks or rates, and (ii) information on treatments or confounders is not available for the entire cohort but can be obtained for a smaller subset of cases and controls.3 It is well known that an analysis of the entire cohort and an analysis of the case-control data (which is just an efficient sampling from the underlying cohort) are expected to yield identical results.4 However, for these estimates to be equivalent (and meaningful), both the cohort analysis and the case-control analysis must estimate the same quantities as the target trial. For example, if adjustment for time-varying confounding or selection bias due to loss to follow-up is required to emulate the target trial in the cohort, then such adjustment is also required to emulate the target trial using the case-control data.
Therefore, like any study that attempts to emulate a target trial, case-control designs generally require an explicit definition of the start of follow-up (time zero) as well as data on time-varying treatments and time-varying confounders from the start of follow-up. Deviations from the target trial framework may lead to bias in case-control studies as in cohort studies.
Consider the example of statins and cancer. Several case-control studies have reported a strong association between statin use and lower cancer risk.5–10 For example, a case-control study reported a substantially lower risk of colorectal cancer among long-term statin users compared with shorter-term and non-users.6 The magnitude of this protective estimate is implausible, and it is not compatible with the estimates from meta-analyses of randomized trials (odds ratio for colon cancer 0.95, 95% confidence interval [CI]: 0.73, 1.25).11,12 This lower risk is also unlikely to be entirely explained by confounding, because the indications for statins (e.g. elevated low-density lipoprotein [LDL] cholesterol) are not such strong drivers of colorectal cancer risk.
Here we estimate the effect of statins on colorectal cancer using observational data from electronic health records. We use a case-control design rather than, as we did previously,13 a cohort design, and we add linkage of electronic health records from primary care, hospital and death registries. To describe how a target trial can be emulated using case-control data, we first specify the protocol of the (hypothetical) target trial that would have answered the causal question of interest, then define the observational cohort study that explicitly emulates this target trial, and finally sample cases and controls from that cohort. We show that a case-control design that deviates from the target trial may lead to implausible estimates similar to those previously reported.
Methods
Target trial specification
We specified the protocol of a target trial to estimate the effect of statins on colorectal cancer incidence among adults with LDL cholesterol below 5 mmol/L.13 Table 1 summarizes the key protocol components (see also Supplementary Appendix 1, available as Supplementary data at IJE online). Briefly, the eligibility criteria include age ≥30, no history of cancer, no statin contraindication, no statin prescription within the past year and LDL cholesterol <5 mmol/L. The treatment strategies to be compared are initiation of any statin therapy at baseline and continuation over follow-up until the development of a contraindication (hepatic impairment or myopathy) and no initiation of statin therapy over follow-up unless there is an indication (LDL cholesterol ≥5 mmol/L). Participants are followed for up to 6 years or until colorectal cancer diagnosis.
Table 1.
Specification and emulation of a target trial of statin therapy and colorectal cancer risk using observational data from linked electronic health records accessed through the CALIBER resource
| Target trial emulation |
|||
|---|---|---|---|
| Protocol | Target trial specification | Cohort analysis | Case-control analysis |
| Eligibility criteria |
|
Same as for the target trial We defined hepatic impairment as a code for hepatic failure or ALT ≥120 IU/L and myopathy as codes for its symptoms: muscle aches, pain or weakness We required information on laboratory values measured during the past year and lifestyle factors during the past 4 years |
Same as for the cohort analysis We performed incidence density sampling of the eligible individuals, selecting 1000 controls per 1 colorectal cancer case |
| Treatment strategies |
(i) Initiation of any statin therapy at baseline and continuation over follow-up until the development of a contraindication (hepatic impairment or myopathy) (ii) No initiation of statin therapy over follow-up until the development of an indication (LDL cholesterol ≥5 mmol/L) Treatment is considered continuous if there is a gap of <30 days between successive prescriptions. When clinically warranted during the follow-up, patients and their physicians will decide whether to start stop or switch therapy. Participants must have a primary care consultation at least once every 4 years to assess prognostic factors associated with adherence and loss to follow-up |
Same as for the target trial We defined the date of medication initiation to be the first date of a prescription We calculated discontinuation dates using the daily dose and quantity of pills in the prescription |
Same as for the cohort analysis |
| Treatment assignment | Individuals are randomly assigned to a strategy at baseline, and individuals and their treating physicians will be aware of the assigned treatment strategy | We classified individuals according to the strategy that their data were compatible with at baseline and attempted to emulate randomization by adjusting for baseline confounders | Same as for the cohort analysis |
| Outcomes | Colorectal cancer |
Same as for the target trial Colorectal cancer diagnoses were recorded as Read codes and ICD-10 codes |
Same as for the cohort analysis |
| Follow-up | Starts at baseline and ends at the month of colorectal cancer diagnosis, death, loss to follow-up (transfer out of the practice or incomplete follow-up [4 years after the last recorded prognostic factors]), 6 years after baseline or administrative end of follow-up (end of practice data collection or February 2016), whichever happens first | Same as for the target trial | Same as for the cohort analysis |
| Causal contrasts | Intention-to-treat effect and per-protocol effect | Observational analogue of intention-to-treat and per-protocol effect | Same as for the cohort analysis |
| Statistical analysis |
Intention-to-treat analysis: apply inverse-probability weights to adjust for pre- and post-baseline prognostic factors associated with loss to follow-up Per-protocol analysis: censor individuals if and when they deviate from their assigned treatment strategy and apply inverse-probability weights to adjust for pre- and post-baseline prognostic factors associated with adherence and loss to follow-up14 |
Same as for the target trial with adjustment for baseline confounders | Same as for the cohort analysis |
ALT, alanine transaminase; CPRD, Clinical Practice Research Database; LDL, low-density lipoprotein.
Target trial emulation
We explicitly emulated this target trial under both a cohort design and a case-control sampling of the cohort, using observational data from the Clinical Practice Research Datalink, Hospital Episode Statistics and Office of National Statistics: population-based datasets comprised of longitudinal UK electronic health records from primary care, hospital and death registries, accessed through the CALIBER resource (see also Supplementary Appendix 1).15,16
Cohort analysis
We mirrored each protocol component as closely as possible, with several modifications to accommodate our use of observational data (Table 1). For example, to assess baseline confounders, we required information on laboratory values measured during the past year and lifestyle factors during the past 4 years. We classified individuals into two groups according to their prescription records at baseline. We assumed these groups were exchangeable at baseline conditional on the covariates in Table 2. The analysis proceeded as for the target trial, with adjustment for these baseline covariates in an attempt to emulate randomization (see also Supplementary Appendix 2, available as Supplementary data at IJE online).
Table 2.
Baseline characteristics of eligible individuals in the cohort analysis and selected individuals in the case-control analysis when emulating a target trial of statin therapy and colorectal cancer risk, CALIBER, 1999–2015a
| Cohort analysis |
Case-control analysis |
|||
|---|---|---|---|---|
| Characteristic, mean (SD) or % | Initiators(n = 25 032) | Non-initiators(n = 727 437) | Cases(n = 3596) | Controls(n = 3 596 000) |
| Age (years) | 62.7 (11.6) | 55.9 (13.7) | 68.5 (10.7) | 56.7 (13.4) |
| Female, % | 42 | 53 | 43 | 52 |
| Body mass index (kg/m2) | 28.8 (5.6) | 28.0 (5.7) | 27.8 (5.1) | 28.2 (5.7) |
| Smoking status, % | ||||
| Never | 43 | 54 | 49 | 53 |
| Former | 32 | 27 | 37 | 28 |
| Current | 25 | 19 | 14 | 19 |
| Low-density lipoprotein cholesterol (mmol/L) | 3.7 (0.9) | 3.3 (0.8) | 3.3 (0.8) | 3.3 (0.8) |
| High-density lipoprotein cholesterol (mmol/L) | 1.4 (0.4) | 1.5 (0.4) | 1.4 (0.4) | 1.4 (0.4) |
| Coronary heart disease, % | 9 | 2 | 5 | 3 |
| Hypertension, % | 27 | 17 | 24 | 19 |
| Cerebrovascular disease, % | 2 | 1 | 1 | 1 |
| Other cardiovascular diseaseb, % | 16 | 14 | 19 | 14 |
| Diabetes, % | 18 | 5 | 9 | 7 |
| Antihypertensive usec, % | 54 | 30 | 50 | 34 |
| Aspirin use, % | 29 | 7 | 17 | 9 |
| Hormone replacement therapy, % of women | 3 | 4 | 2 | 4 |
| Oral contraceptive use, % of women | 4 | 7 | 2 | 7 |
| Referrals in the past 3 months, ≥2, % | 4 | 2 | 3 | 2 |
SD, standard deviation.
Baseline ranges from January 1999 to February 2015.
Includes acute rheumatic fever, chronic rheumatic heart disease, pulmonary heart disease and other circulatory disease.
Includes all primary care prescriptions from British National Formulary chapters: 2.2.1 thiazides and related diuretics, 2.2.3 potassium-sparing diuretics and aldosterone antagonists, 2.2.4 potassium-sparing diuretics with other diuretics, 2.4 beta-adrenoceptor blocking drugs, 2.5 hypertension and heart failure, 2.6.2 calcium-channel blockers.
Case-control analysis
We sampled cases and controls from the assembled cohort of eligible individuals via incidence density sampling.17 Cases were all individuals diagnosed with colorectal cancer over the study period. Controls were individuals who were alive, under follow-up and free of colorectal cancer at the time of selection. To reduce differences due to random variability when comparing the cohort and case-control estimates, we randomly selected 1000 controls per case (case-control studies are typically based on a much lower number of controls). The analysis of the case-control data proceeded as for the cohort analysis (see also Supplementary Appendix 3, available as Supplementary data at IJE online). The odds ratio from the case-control data is an unbiased estimator of the rate ratio obtained from the full cohort.4 Therefore, if the cohort analysis correctly estimates the hazard ratios from the target trial in Table 1, then the case-control analysis does too.
Deviations from the target trial
In separate analyses, we applied the analytical approach of a previous case-control study to our data to demonstrate how deviations from the target trial framework lead to bias. The previous study reported an odds ratio of 0.53 (95% CI: 0.38, 0.74) when comparing colorectal cancer cases and controls in terms of their statin use: ≥5 vs. <5 years.6 To assess statin use and potential confounders, eligible cases and controls were interviewed in person by the research team. This study deviated from its corresponding target trial in several ways.
First, the analysis was restricted to eligible cases and controls who could be interviewed, that is, individuals had to remain alive and under follow-up for a period after being selected for the study. The length of the period between selection and interview is unknown, but the authors reported that 19.4% of eligible cases could not be located or approached because they had died or been lost to follow-up.6 In our study, a similar 18.7% loss of eligible cases would require a 3-month period between selection and interview. This 3-month survival requirement does not exist in the target trial.
Second, cases and controls were classified based on their observed cumulative duration of statin therapy through the time of diagnosis (selection) for cases but through the time of interview (post-selection) for controls. Compared with the target trial, this approach corresponds to neither the intention-to-treat analysis (which assigns individuals to a treatment strategy based on baseline information only) nor the per-protocol analysis (which assigns individuals to a treatment strategy based on baseline information and then censors them at deviation from the baseline assignment). Further, this case-control study used a longer period of potential statin use for controls (baseline to interview) than for cases (baseline to diagnosis).
Third, the analysis adjusted for covariates assessed at the time of interview. From a target trial perspective, this is equivalent to adjusting for variables measured at or after the end of follow-up. By contrast, a correct intention-to-treat analysis adjusts for baseline confounders and a correct per-protocol analysis adjusts for baseline and post-baseline (time-varying) confounders during the follow-up. Because this case-control study ignored time-varying confounders, the analysis did not need inverse-probability weighting.
Fourth, the study included cases and controls who were using statins before baseline (prevalent users) and used pre-baseline statin therapy to quantify total duration of use. These individuals would not be eligible for the target trial.
To assess the cumulative impact of these deviations from the target trial on the estimates, we sequentially implemented them in our own case-control analysis. First, we restricted our case-control analysis to individuals alive and under follow-up 3 months after selection. We also implemented an equivalent cohort analysis that excludes all monthly records within 3 months of death or censoring. As a sensitivity analysis, we examined a 6-month (rather than 3-month) survival requirement.
Second, we classified cases and controls by their cumulative duration of statin use (≥5 vs. <5 years) after baseline through selection for cases and through selection + 3 months for controls. Again, we implemented an equivalent cohort analysis that (i) excludes all monthly records within 3 months of death or censoring and (ii) assesses cumulative statin use through the current month for event person-months and through the current month + 3 months for non-event person-months.
Third, we adjusted for covariates measured at the time of selection, instead of at baseline or later, by including them in the pooled logistic model. We were unable to use pre-baseline statin therapy to quantify total duration of use because we lacked complete pre-baseline histories for some individuals in the cohort.
Statins and all-cause mortality
To show the generality of our approach, we repeated these analyses for statin therapy and all-cause mortality. We selected all-cause mortality as an alternative outcome because the magnitude of the intention-to-treat effect of statins on all-cause mortality is known from randomized trials (risk ratio 0.86, 95% CI: 0.80, 0.93) and can be used as a benchmark.18 We emulated a target trial using the same data, with additional eligibility criteria of no cardiovascular disease at baseline and an increased cardiovascular risk (defined as LDL cholesterol ≥3.4 mmol/L) and with up to 10 years of follow-up. Here, replicating a 3-month survival requirement after selection only resulted in a loss of controls, not cases.
All analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
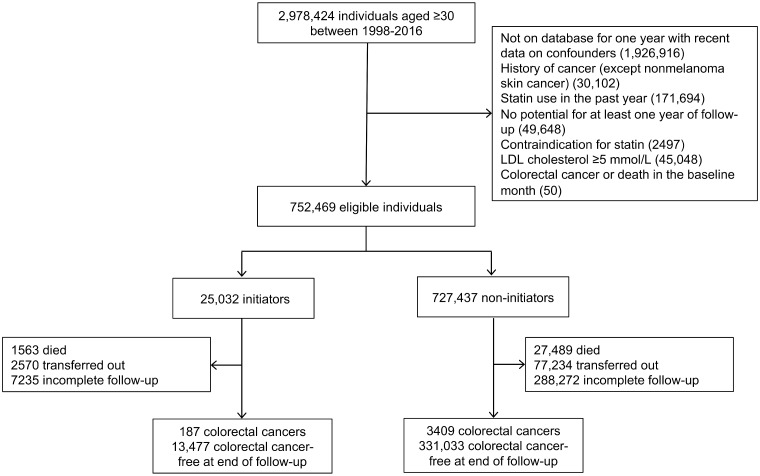
Figure 1 shows a flowchart of participant selection, and Table 2 shows baseline characteristics of the 752 469 eligible individuals in the cohort analysis and the 3596 cases and 3 596 000 controls in the case-control analysis. Compared with statin non-initiators at baseline, statin initiators were, on average, older and had higher LDL cholesterol and body mass index (BMI) and included a higher proportion of men, current smokers, antihypertensive and aspirin users and individuals with cardiovascular disease and diabetes. Compared with controls, cases were, on average, older and included a higher proportion of men, former smokers, antihypertensive and aspirin users and individuals with cardiovascular disease and diabetes.
Figure 1.
Flowchart for selection of eligible individuals from CALIBER when emulating a target trial of statin therapy and colorectal cancer risk, 1999–2016
Table 3 shows estimated 6-year risk differences and hazard ratios when emulating a target trial of statin therapy and colorectal cancer. In the full cohort, the estimated 6-year risk differences were 0% (95% CI: -0.1%, 0.2%) in the intention-to-treat analysis and -0.1% (95% CI: -0.2%, 0.1%) in the per-protocol analysis, and the estimated hazard ratios were 1.00 (95% CI: 0.87, 1.16) in the intention-to-treat analysis and 0.90 (95% CI: 0.71, 1.12) in the per-protocol analysis. The odds ratios from the case-control sample were identical to the hazard ratios from the cohort. Estimated hazard ratios were identical when additionally adjusting for cancer screening in the past year (data not tabled). Estimated hazard ratios were similar when only adjusting for age (intention-to-treat hazard ratio 1.03, 95% CI: 0.89, 1.19; per-protocol hazard ratio 0.97, 95% CI: 0.80, 1.20) (data not tabled).
Table 3.
Estimated risk of colorectal cancer comparing statin therapy with no statin therapy, CALIBER, 1999–2016
| Case-control analysis |
Cohort analysis |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 6-year risk (%) |
|||||||||
| Cases | Odds ratio | 95% CI | Hazard ratio | 95% CI | Initiator | Non-initiatora | Risk difference (%) | 95% CI | |
| Emulating a target trialb | |||||||||
| Intention-to-treatc | 3596 | 1.00 | 0.86, 1.16 | 1.00 | 0.87, 1.16 | 0.8 | 0.8 | 0 | −0.1, 0.2 |
| Per-protocold | 2735 | 0.90 | 0.71, 1.15 | 0.90 | 0.71, 1.12 | 0.8 | 0.9 | −0.1 | −0.2, 0.1 |
| Replicating the approach of a previous case-control studye | |||||||||
| Imposing a 3-month survival requirement from the time of selectionf | 2924 | 1.02 | 0.86, 1.20 | 1.02 | 0.86, 1.20 | 0.8 | 0.7 | 0.1 | −0.1, 0.2 |
| + Comparing ≥5 vs. <5 years of statin useg | 2924 | 0.55 | 0.35, 0.87 | 0.55 | 0.35, 0.87 | — | — | — | — |
| + Adjusting for covariates instead measured at the time of selection | 2924 | 0.57 | 0.36, 0.91 | 0.57 | 0.36, 0.91 | — | — | — | — |
Refers to statin use for <5 years when replicating the previous case-control approach.
Estimates from weighted pooled logistic regression models adjusted for age, sex, BMI, smoking status, LDL cholesterol, HDL cholesterol, months since last measure of LDL cholesterol, months since last measure of HDL cholesterol, coronary heart disease, hypertension, cerebrovascular disease, other cardiovascular disease, diabetes, antihypertensive use, aspirin use, hormone replacement therapy, oral contraceptive use, number of referrals in the past 3 months. The number of cases is lower in the per-protocol analysis because of the censoring under this approach (see also Supplementary Appendix 1, available as Supplementary data at IJE online).
Comparing statin initiation vs. no initiation at baseline.
Comparing statin initiation at baseline and continuation over follow-up unless contraindicated with no statin initiation over follow-up unless indicated.
Estimates from unweighted pooled logistic regression models adjusted for the covariates above, assessed at baseline.
Comparing treatment initiation vs. no initiation at baseline. In the case-control sample, the analysis was restricted to individuals alive and under follow-up 3 months after selection. In the full cohort, the analysis excluded monthly records within 3 months of death or censoring.
In the case-control sample: (i) the analysis was restricted to individuals alive and under follow-up 3 months after selection, and (ii) cumulative statin use after baseline was assessed through the time of selection (diagnosis) for cases and through the time of selection + 3 months for controls. In the full cohort: (i) the analysis excluded monthly records within 3 months of death or censoring, and (ii) cumulative statin use was assessed through the current month for event person-months and through the current month + 3 months for non-event person months.
We then replicated the approach of the previous case-control study in our data (Table 3). The estimated odds ratio for colorectal cancer was 0.55 (95% CI: 0.35, 0.87) when we imposed the 3-month survival requirement and assessed cumulative statin use through the time of selection (diagnosis) for cases and through the time of selection + 3 months for controls. When imposing this survival requirement and instead assessing statin use through the time of selection for both cases and controls, the odds ratio estimate was 0.84 (95% CI: 0.53, 1.34) (data not tabled). Estimates were similar when adjusting for covariates measured at the time of selection instead of at baseline. Cohort analyses mimicking these decisions returned the same estimates. A 6-month survival requirement yielded stronger inverse associations (Supplementary Table S2, available as Supplementary data at IJE online).
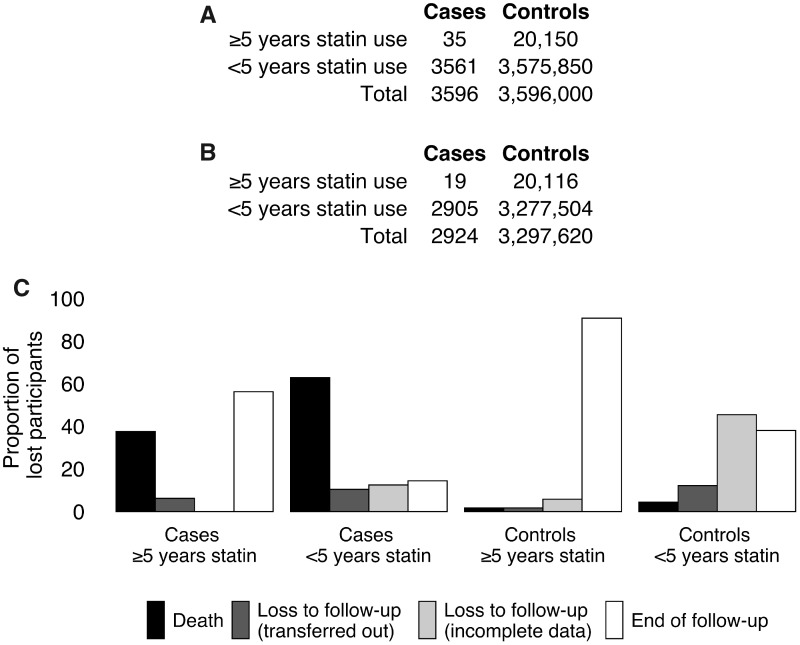
Imposing the 3-month survival requirement resulted in a loss of 672 eligible cases (18.7%), including 418 who died (11.6%) (similar to the proportions reported in the published study: 19.4% and 8.6%, respectively), and a loss of 298 380 eligible controls (8.3%), including 13 047 who died (0.4%) (Figure 2). Among individuals who remained alive and under follow-up 3 months after selection, a slightly lower proportion of cases was classified as having ≥5 years of statin use compared with the distribution of exposure at the time of selection (0.6% vs. 1.0%). In addition, 6847 surviving controls were re-classified as having ≥5 years of statin use when statin use was assessed through selection + 3 months. These small shifts in absolute proportions (slight depletion of cases and enrichment of controls for ≥5 years of statin use) are responsible for the large shifts on the odds ratio (multiplicative) scale.
Figure 2.
Distribution of statin exposure among cases and controls under no survival requirement (A) and a 3-month survival requirement (B) from the time of selection, and proportions of individuals lost to various causes (C). In addition, 6847 surviving controls who were classified as having <5 years of statin use under no survival requirement were re-classified as having ≥5 years of statin use under the 3-month survival requirement
Statins and all-cause mortality
When emulating a target trial of statins and all-cause mortality, we estimated an intention-to-treat hazard ratio of 0.87 (95% CI: 0.79, 0.95) which is close to the estimate from a meta-analysis of randomized trials (risk ratio 0.86, 95% CI: 0.80, 0.93) (Supplementary Table S3, available as Supplementary data at IJE online).18 This estimate progressively decreased when we applied the analytical flaws described above (Supplementary Table S3).
Discussion
After emulating a target trial using the electronic health records of 752 469 adults with up to 6 years of follow-up, we found little evidence that the risk of colorectal cancer differs between statin users and nonusers. This finding is consistent with meta-analyses of randomized trials.11,12 As expected, adequate case-control sampling returned the same estimates as the cohort analysis. By contrast, after replicating the analytical approach of a previous case-control study in our data, we found implausibly protective estimates similar to those previously reported.
Case-control studies may have a role to play when conducting causal inference research based on health care databases. While such databases provide access to the underlying cohort that gives rise to cases and controls, they may not contain high-quality information on treatment or confounders needed to answer certain causal questions.3 In these settings, case-control studies allow us to focus limited resources on collecting this information for random samples of cases and controls.
Case-control analyses may seem simple: we compare the treatment status of cases with non-cases. However, a failure to anchor this to an underlying cohort study that explicitly emulates a target trial contributes to two common misconceptions about case-control analyses: (i) that they are immune to many of the biases that afflict cohort analyses, such as time-varying confounding and selection bias due to loss to follow-up and the inclusion of prevalent users, and (ii) that they do not require complete treatment and confounder history for cases and controls. While critics of case-control designs within existing databases have largely focused on design flaws leading to confounding bias,19 our evaluation showed that other deviations from a target trial in case-control analyses lead to the same biases that affect cohort analyses.
Two deviations from the target trial appeared to drive the biased estimates in this particular application: (i) the requirement for cases and controls to survive for 3 additional months and (ii) the assessment of treatment duration over a longer time period for controls compared with cases. Together, these decisions led to small shifts in treatment classification that depleted cases and enriched controls for ≥5 years of statin use. Importantly, we found that effect estimates on the multiplicative scale, which are generally all that we can obtain from case-control studies, may be particularly susceptible to these biases.
Other deviations from the target trial may, in general, matter. First, comparing cumulative duration of treatment above vs. below a certain threshold (e.g. ≥5 vs. <5 years) does not capture information on the precise timing, duration, or reasons for switching treatment, which may be important for risk. In our analysis, the estimated odds ratio comparing ≥5 vs. <5 years of statin use (0.95, 95% CI: 0.67, 1.34; with no survival requirement, data not tabled) was similar to the intention-to-treat hazard ratio (1.00, 95% CI: 0.86, 1.16), possibly because treatment had no effect on the outcome in this particular application. Second, adjustment for variables measured at (or after) the time of selection will not appropriately adjust for confounding and may induce selection bias. In our analysis, this had little impact possibly because, as suggested by the similarity between age- and fully-adjusted estimates, the adjustment variables were not strong predictors of the outcome no matter when they were measured. Third, failure to adjust for loss to follow-up may result in selection bias if remaining uncensored depends on treatment history and risk factors. In our analysis, estimates were similar when additionally applying inverse-probability weights for censoring due to loss to follow-up. Last, including prevalent users at baseline may contribute to selection bias due to the selection of individuals who received pre-baseline treatment for some time and remained at risk and under follow-up at baseline. We were unable to explore this deviation in our data. Our approach of explicitly specifying the protocol of the target trial and its observational emulation naturally leads to analytical approaches that prevent these biases.
Our study has several additional strengths. The volume and variety of data in the electronic health records allowed us to evaluate statins and colorectal cancer in a population-based sample with adjustment for many potential confounders. Our analytical approach allowed us to estimate both relative and absolute risks under sustained strategies that realistically depend on dynamic clinical features. Last, our analyses of all-cause mortality support that the target trial approach can reproduce effect estimates from trials and that the analytical flaws described above will result in bias for this alternative outcome.
Nevertheless, we were limited by our reliance on diagnosis codes and prescription records, which may contribute to measurement error and residual confounding. However, previous validation studies have confirmed a high proportion of recorded cancers (95%) and other diagnoses in this database.20,21
In summary, our findings suggest that flaws in case-control analyses can be mapped to decisions in a cohort analysis which would lead to bias, particularly on the multiplicative scale. Explicitly mapping case-control sampling to the target trial helped us to reduce bias. Our approach may help to inform the design and analysis of any case-control study where the goal is to assess the benefit-risk of medical treatments.
Data availability
This study is based in part on data from the Clinical Practice Research Datalink obtained under license from the UK Medicines and Healthcare Products Regulatory Agency. The data are provided by patients and collected by the UK National Health Service as part of their care and support. This study is also based in part on data from the Hospital Episode Statistics and Office of National Statistics, re-used with permission of The Health & Social Care Information Centre. The study was approved by the Medicines and Healthcare Products Regulatory Agency Independent Scientific Advisory Committee (protocol 16_221), under Section 251 (National Health Service Social Care Act 2006). The interpretation and conclusions contained in this study are those of the authors alone.
Supplementary data
Supplementary data are available at IJE online.
Funding
This work was supported by National Institutes of Health grants K99 CA248335 (B.A.D.) and P01 CA134294.
Conflict of interest
None declared.
Supplementary Material
References
- 1. Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol 2016;183:758–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hernán MA, Sauer BC, Hernandez-Diaz S, Platt R, Shrier I. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J Clin Epidemiol 2016;79:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schneeweiss S, Suissa S. Discussion of Schuemie et al: “A plea to stop using the case-control design in retrospective database studies”. Stat Med 2019;38:4209–12. [DOI] [PubMed] [Google Scholar]
- 4. Miettinen O. Estimability and estimation in case-referent studies. Am J Epidemiol 1976;103:226–35. [DOI] [PubMed] [Google Scholar]
- 5. Graaf MR, Beiderbeck AB, Egberts AC, Richel DJ, Guchelaar HJ. The risk of cancer in users of statins. J Clin Oncol 2004;22:2388–94. [DOI] [PubMed] [Google Scholar]
- 6. Poynter JN, Gruber SB, Higgins PD et al. Statins and the risk of colorectal cancer. N Engl J Med 2005;352:2184–92. [DOI] [PubMed] [Google Scholar]
- 7. Khurana V, Bejjanki HR, Caldito G, Owens MW. Statins reduce the risk of lung cancer in humans: a large case-control study of US veterans. Chest 2007;131:1282–88. [DOI] [PubMed] [Google Scholar]
- 8. Shannon J, Tewoderos S, Garzotto M et al. Statins and prostate cancer risk: a case-control study. Am J Epidemiol 2005;162:318–25. [DOI] [PubMed] [Google Scholar]
- 9. Hoffmeister M, Chang-Claude J, Brenner H. Individual and joint use of statins and low-dose aspirin and risk of colorectal cancer: a population-based case-control study. Int J Cancer 2007;121:1325–30. [DOI] [PubMed] [Google Scholar]
- 10. Boudreau DM, Gardner JS, Malone KE, Heckbert SR, Blough DK, Daling JR. The association between 3-hydroxy-3-methylglutaryl conenzyme A inhibitor use and breast carcinoma risk among postmenopausal women: a case-control study. Cancer 2004;100:2308–16. [DOI] [PubMed] [Google Scholar]
- 11. Dale KM, Coleman CI, Henyan NN, Kluger J, White CM. Statins and cancer risk: a meta-analysis. JAMA 2006;295:74–80. [DOI] [PubMed] [Google Scholar]
- 12. Emberson JR, Kearney PM, Blackwell L et al. ; Cholesterol Treatment Trialists Collaboration. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One 2012;7:e29849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dickerman BA, García-Albéniz X, Logan RW, Denaxas S, Hernán MA. Avoidable flaws in observational analyses: an application to statins and cancer. Nat Med 2019;25:1601–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hernán MA, Lanoy E, Costagliola D, Robins JM. Comparison of dynamic treatment regimes via inverse probability weighting. Basic Clin Pharmacol Toxicol 2006;98:237–42. [DOI] [PubMed] [Google Scholar]
- 15. Denaxas SC, George J, Herrett E et al. Data Resource Profile: Cardiovascular disease research using linked bespoke studies and electronic health records (CALIBER). Int J Epidemiol 2012;41:1625–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Denaxas S, Gonzalez-Izquierdo A, Direk K et al. UK phenomics platform for developing and validating electronic health record phenotypes: CALIBER. J Am Med Inform Assoc 2019;26:1545–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. , Rothman KJ, Greenland SL, Lash TL, Case-control studies In: Modern Epidemiology. 3rd edn Philadelphia, PA: Lippincott Williams & Wilkins, 2008. [Google Scholar]
- 18. Chou R, Dana T, Blazina I, Daeges M, Jeanne TL. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016;316:2008–24. [DOI] [PubMed] [Google Scholar]
- 19. Schuemie MJ, Ryan PB, Man KKC, Wong ICK, Suchard MA, Hripcsak G. A plea to stop using the case-control design in retrospective database studies. Stat Med 2019;38:4199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Margulis AV, Fortuny J, Kaye JA et al. Validation of cancer cases using primary care, cancer registry, and hospitalization data in the United Kingdom. Epidemiology 2018;29:308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 2010;69:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study is based in part on data from the Clinical Practice Research Datalink obtained under license from the UK Medicines and Healthcare Products Regulatory Agency. The data are provided by patients and collected by the UK National Health Service as part of their care and support. This study is also based in part on data from the Hospital Episode Statistics and Office of National Statistics, re-used with permission of The Health & Social Care Information Centre. The study was approved by the Medicines and Healthcare Products Regulatory Agency Independent Scientific Advisory Committee (protocol 16_221), under Section 251 (National Health Service Social Care Act 2006). The interpretation and conclusions contained in this study are those of the authors alone.