Abstract
Pacific island countries and territories (PICTs) face the challenge of a growing cancer burden. In response to these challenges, examples of innovative practice in cancer planning, prevention, and treatment in the region are emerging, including regionalisation and coalition building in the US-affiliated Pacific nations, a point-of-care test and treat programme for cervical cancer control in Papua New Guinea, improving the management of children with cancer in the Pacific, and surgical workforce development in the region. For each innovation, key factors leading to its success have been identified that could allow the implementation of these new developments in other PICTs or regions outside of the Pacific islands. These factors include the strengthening of partnerships within and between countries, regional collaboration within the Pacific islands (eg, the US-affiliated Pacific nations) and with other regional groupings of small island nations (eg, the Caribbean islands), a local commitment to the idea of change, and the development of PICT-specific programmes.
Introduction
The first paper in this Series by Sarfati and colleagues1 describes the unique and complex challenges relating to cancer control and prevention in the Pacific island countries and territories (PICTs), including geographical isolation, small capacities (in terms of both people skills and infrastructure) and populations, shortage of health-care workers, and under-resourced health services. Patients with cancer in the Paific islands, many with highly preventable and treatable cancers, often present late and face the reality of a few treatment options and palliative care services.
Although a comprehensive approach to cancer prevention and control in the PICTs remains the aim of this Series paper, we also provide examples of innovation and good practice that have, or are likely to result in, improved cancer prevention and control outcomes in the region. The four operational examples described herein have been selected to represent a range of cancer-control strategies, from policy development to treatment, which can be initiated by funders (including the major donor countries [eg, Australia and New Zealand] and development partners [eg, WHO and UNICEF], governments, or the private sector and were developed in response to locally identified priorities, challenges, and needs (table 1). We conclude by describing key success factors that link all of the examples together, so that these approaches can be considered for possible implementation in other countries or regions.
Table 1:
Examples of innovative practices within Pacific island countries and territories to improve health-care delivery and services
| Problem | Solution | Key processes | Outcomes | |
|---|---|---|---|---|
| Regionalisation and coalition building: the US-affiliated Pacific islands and territories | High cancer incidence, small populations, small health-care capacities, small number of resources | Regionalisation, collaboration, and sharing of resources | Key principles and committed champions; linking diverse jurisdictions and comprehensive cancer planning; needs assessment and development of partnerships with funders and academic institutions; capacity building | Effective cancer control planning; efficient use of resources; coordinated and shared expertise; effective leverage of resources; development of specific strategies and policies resulting in action (eg, cancer surveillance, cervical cancer control, cancer treatment) |
| Improving the management of children with cancer from selected Pacific island countries and territories: the Pacific Child Cancer Project | Poor access to care and late presentations of children with cancer to health-care facilities | Partnerships between oncology specialists in high-income countries and health-care professionals in Pacific island countries and territories | Building capacity and developing infrastructure; development of local models of care and ownership; improved availability of essential cytotoxic drugs; visiting overseas specialists; clinical and political champions | Development of triage criteria for children with cancer; development of context-specific treatment protocols and supportive care guidelines; increased number of patients treated on-island; increased support to on-island clinicians |
| Point-of-care test and treat: developing innovative approaches to cervical cancer control (in Papua New Guinea) | High burden of cervical cancer and no effective cervical screening programmes | Research-informed cervical screening and treatment at point-of-care in routine clinical settings in Papua New Guinea | Research collaboration and funding between Papua New Guinea and Australian researchers | Provides evidence supporting the practicality, feasibility, acceptability, and effectiveness of a model of cervical cancer screening with self-collected specimens at point-of-care for HPV-DNA in a challenging small island nation setting, with a larger, confirmatory study underway |
| Improvement and support of surgical services in the Pacific island countries and territories | Shortage of surgeons to treat patients with cancer | Collaboration and support by professional organisations of regional training institutions and developing capacity of individual surgeons | Royal Australasian College of Surgeons assisted capacity building by supporting the two regional training institutions; visiting surgeons; a formalised relationship between the Australasian College of Surgeons and the Pacific island countries and territories trained surgeons | Postgraduate surgical training from the Master of Medicine programme, Surgery, at the universities in Papua New Guinea and Fiji; a substantial increase in numbers of surgeons throughout the Pacific island countries and territories; increased number of surgeons trained in the Pacific island countries and territories by local surgeons; a lessening of the so-called brain drain to high-income countries |
Regionalisation and coalition building: the US-affiliated Pacific islands (USAPI) and territories
USAPI consist of three US territories, American Samoa, Northern Mariana Islands, and Guam, and three independent countries in free association with the USA, the Federated States of Micronesia (Yap, Pohnpei, Kosrae, and Chuuk States), the Marshall Islands, and Palau (figure 1).2,3 Like many other small island nations, USAPIs still face major challenges in achieving effective cancer control policies and interventions because of severe ongoing resource limitations; restricted human resources and skills, policies and systems, and infrastructure; geographical isolation; adoption of developed lifestyles and diets; and an increase in non-communicable diseases.4,5 As a result of increases in cancer incidence and delayed diagnoses, and because no reliable cancer data were available, a concerted, synergistic and regionalised approach was initiated in 1997 to strengthen the governance and management of cancer control and prevention by a strategic coalition of health leaders from USAPI.4,5
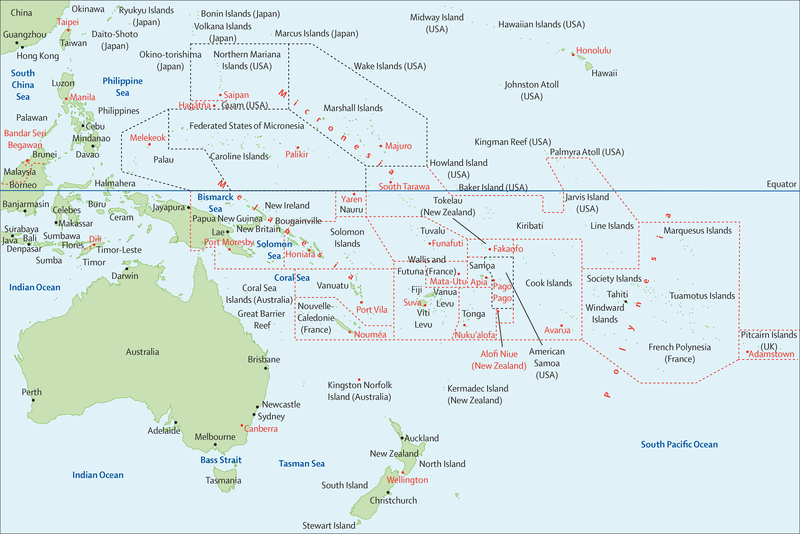
Figure 1: Map of the Pacific islands countries and territories.
Reproduced from 123RF Rainer Lesniewski.3 The dashed lines are an approximation of the territorial waters of the Pacific islands and territories. Countries and territories within the black area are part of the US-affiliated Pacific islands and territories area; those countries or terrorities within the red dashed lines are outside of US-affiliated Pacific islands and territories.
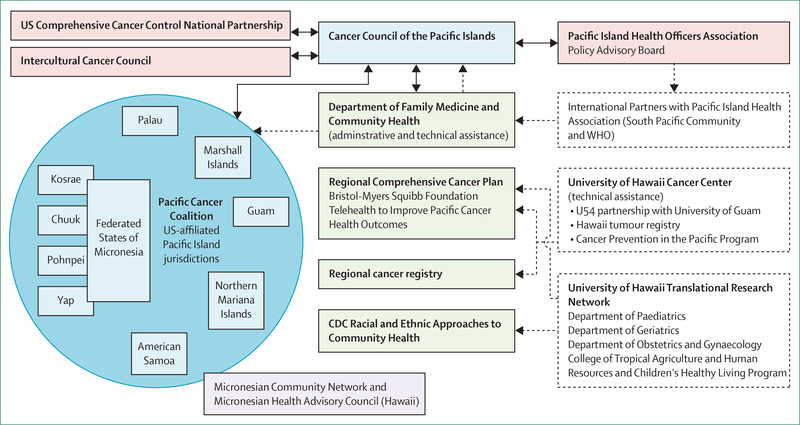
The Cancer Council of the Pacific Islands was formed in 2002, with funding from the US National Cancer Institute and National Institutes of Health. It aimed to better characterise the cancer burden and health systems in the USAPI. The formation of the Council led to the development of the Pacific Regional Comprehensive Cancer Control Programme and Partners (PRCP), which had representatives from each USAPI jurisdiction.6,7 PRCP has evolved since 2002 and now includes a number of enabling partners from USAPI (figure 2).8
Figure 2: US Pacific Comprehensive Cancer Control Programme and Partners.
Reproduced from Palafox and colleagues (CC BY 4.0).6 CDC=US Center for Disease Control and Prevention.
Comprehensive cancer control, within a formal structure and programme, was introduced to USAPI in 2003, with the formation of local coalitions and substantial health-care workforce development. The US Center for Disease Control and Prevention (CDC) has provided funding to USAPI since its inception in 2003. In 2005, an assessment was made to determine the feasibility of a regional cancer registry programme that would provide centralised administration, training, and support, but also ensure control and regulation of each individual jurisdiction’s data.
In 2006, the first USAPI 5-year comprehensive cancer control plan was formulated,9 acknowledging the varying contexts of cultures, available medical resources, funds to improve infrastructure, skilled personnel, and health-care coverage across all USAPIs. Part of the plan was for each USAPI government to form functional multisector, multilevel cancer coalitions to develop and implement a country-specific comprehensive cancer control plan. The country-specific plans complemented the USAPI-wide comprehensive cancer control plan by obtaining resources from across the entire PRCP (including Hawaii, USAPI, and US national [eg, the CDC] and international partners [eg, WHO]) to provide training and technical assistance for health-care staff, policy, health systems, and cancer surveillance development to six USAPI jurisdictions: the US territories (American Samoa, Northern Mariana Islands, and Guam); and the three independent countries in free association with the USA (Federated States of Micronesia, Marshall Islands, and Palau).
One of the major successes is the CDC-funded PRCP regional central cancer registry. It has systematically collected data since 2007, which has informed strategies on practice approach and policy decisions. For example, high numbers of patients with late-stage cervical cancer in the Marshall Islands and the Federated States of Micronesia resulted in a change in cervical screening methods, which changed from cervical smears to visual inspection with acetic acid. In addition, registry data informed programme-delivery policies in cancer risk reduction, including tobacco control, obesity reduction, healthy eating, increasing physical activity, and improved screening programmes for the cervix, breast, and colon. The data obtained have been used to drive the necessary policy and health system changes to address the large disparities in prevention, screening, and early detection across the USAPI. Local and regional comprehensive cancer control plans were developed in close collaboration with non-communicable disease programmes. The plans have leveraged substantial resources since their implementation to strengthen local health systems, policies, and public awareness of cancer and related risk factors. An adaptable USAPI palliative cancer curriculum was introduced, which led to the adoption of various palliative care and home-based care methods in each jurisdiction of USAPI.
The PRCP has been effective at improving cancer care for USAPI through a systematic approach that engaged committed cancer and local experts, increased participation of indigenous people in the programme, and improved equipment and medicine procurement efficiencies through economies of scale that use organisational and partnership strengths.
Leadership and advocacy from the PRCP allowed USAPI to speak with one voice. Additional advocacy from development partners continues to be fundamental to improving multilateral, regional cancer prevention and control initiatives. In addition, the PRCP was guided by the principle of sustainability—in other words, by building country-specific and PICT-wide workforce capacity by collaborating with centres of excellence, such as the John Burns School of Medicine and the University of Hawaii Cancer Center, University of Hawaii, Honolulu, HI, USA and the University of Guam, Mangilao, Guam.10 The transferability and feasibility of the collaborative regional model is dependent on strong political will, committed leadership, and advocacy by health experts, being inclusive of diversity of context, and are guided by principles of partnership, cost-effectiveness, addressing health inequities, building workforce capacity, and ensuring sustainability.11
Improving the management of cancers among children in selected PICTs: the Pacific child cancer project
Children with cancer in many PICTs have poorer outcomes than those in high-income Pacific Rim countries; additionally, only a few PICTs have the medical resources and infrastructure needed to address the cancer burden. There are little published data on childhood cancer in the Pacific;12 estimates of the potential child cancer burden in the region range from approximately one new case biennially in the Tuvalu, Tokelau, and Niue to 45–50 new cases in Fiji annually (table 2).13 Various PICTs have different processes for the management of childhood cancer; in this Series paper, we highlight programmes in four PICTs (Fiji, Tonga, Samoa, and Vanuatu).
Table 2:
Estimation of new childhood cancer cases annually for selected Pacific island countries and territories
| Estimated total population (2016) | Estimated population youngerthan 14 years old | Estimated new child cancer cases (0–14 years) per year | |
|---|---|---|---|
| Papua New Guinea | 10 000 000 | 4 000 000 | 450–475 |
| Fiji | 880 400 | 240 200 | 47.5 |
| Solomon Islands | 651 700 | 267300 | 26.8 |
| Vanuatu | 289700 | 108 800 | 11.8 |
| Samoa | 194000 | 75 100 | 10.4 |
| Tonga | 100 600 | 35 500 | 5.7 |
| Kiribati | 114 395 | 38 432 | 5.5 |
| Cook Islands | 15 200 | 4100 | 1.8 |
| Tuvalu | 10 100 | 3200 | 0.56 |
| Tokelau | 1400 | 400 | 0.06 |
| Niue | 1600 | 400 | 0.06 |
In the early 2000s, all of the PICTs (except for the Soloman Islands) would, funding permitting, refer childhood cancer cases to New Zealand and Australia. However, major problems occurred as a result of incorrect triaging, late presentations, and high of mortality. Because no in-country training on expected toxicities and complications had been done these issues often led to the death of patients from intercurrent infections upon their return home.14 Before 2017, almost all children in Vanuatu died of cancer because no treatment was available within the country and patients could not be referred to hospitals in other countries.
In response to this substantial unmet need, a Pacific working group was established as part of the New Zealand Paediatric Oncology Steering Group in 2006, which transitioned to the New Zealand National Child Cancer Network in 2011. Partnerships between New Zealand paediatric oncologists and Pacific island clinicians were formed to review the outcomes of children referred from the Pacific islands, to investigate whether twinning with PICTs was feasible and to initiate cooperative agreements with PICTs that regarded childhood cancer as a priority health area. New Zealand Aid provided the initial funding for visits to Fiji, Tonga, Samoa, and Vanuatu, where selected members of the working group, accompanied by two Pacific advisers (working with New Zealand aid), held discussions with the respective ministries of health and health-care professionals to determine whether curative therapy for childhood cancer was a priority; to determine whether an improved paediatric infrastructure existed; whether there were sufficient childhood patient numbers to develop expertise at the paediatric centre; and whether any funding mechanisms for off-island referrals for treatment existed.
From 2007, Tonga, Fiji, and Samoa (and Vanuatu from 2017) regarded childhood cancer as a health priority and welcomed the opportunity to develop a care programme with New Zealand. In 2007, Tonga, Fiji, and Samoa had varied needs, capabilities, and priorities, and each country required a different set of solutions, approaches, and speeds of implementation. Triage criteria were established listing so-called good risk cancers, in which most or all treatments can be or are provided on-island by a paediatrician and trained chemotherapy nurses.15 Developing childhood cancer treatment regimens adapted to local conditions provided an opportunity to treat as many children as possible with the available resources while also working to improve services and supportive care.16 Pacific treatment protocols were developed for a range of childhood cancers, including acute lymphoblastic leukaemia, Wilms’ tumour, and Hodgkin lymphoma, accompanied by a suite of supportive care guidelines.15,17 Regular training and upskilling of staff has allowed more children to be treated closer to home.
Children with cancer can expect positive outcomes of up to 50% when treated with low-cost therapies in all PICTs, and the provision of these basic services can be organised and supported through a twinning arrangement with well resourced countries.16,18 Professional partnerships were successfully established between tertiary health-care centres in New Zealand (the Starship Blood and Cancer Centre, Auckland, New Zealand with Tonga, Samoa, Vanuatu, Cook Islands, Tokelau, and Niue and the Children’s Haematology Oncology Centre, Christchurch, New Zealand with Fiji). Fiji’s two paediatric centres manage 45–50 new childhood cancer cases annually using the Pacific protocols19 as per the triage criteria with remote support from Christchurch, New Zealand, by weekly video and teleconferencing and at least one annual visit by the Christchurch team to Fiji.
Despite the multiple challenges faced by PICTs, this innovative approach to planning, delivering, and sustainably maintaining a paediatric oncology service in these countries has had key successes. First, there has been an increase in the number of successful diagnoses of childhood cancer cases and an increase in the number of patients who have successfully completed therapy. Second, the improved care of children with cancer has also had positive effects on the care of other sick children. Third, good practice has been observed with the growth of specialist paediatric surgical services and in the increasing number of cancer societies and organisations in Fiji, Tonga, and Samoa actively supporting children and their families with travel to the hospital and financial assistance for food, clothing, and medications.
Programmes have worked well when PICT has identified clinical and political champions who have shown substantial commitment to the strategies. and ensured PICT alignment with the health plan. A focus on increasing the number and skills of health-care professionals and the capability of PICT health-care services to detect and manage children with cancer requires ongoing training and education (eg, credentialling [a process that most health professionals in New Zealand need to complete which is part of a competency assessment to ensure quality and safety] in paediatric oncology for PICT nurses), ensuring that PICT teams are aware of the expected toxicities and complications associated with cancer treatment as treatment protocols are intensified, that maintaining expertise with core clinicians and a small number of patients is required, and checking that follow-up is done. Each country needs to work with their ministry of health to ensure ongoing funding is allocated for children with cancer including the ability to travel overseas for treatment if necessary. The Pacific Child Cancer Registry is in development, which will address the need for data in this area; additionally, assistance in expanding local availability of essential cytotoxic agents is being addressed globally.20–22 A viable palliative care service for children with cancer who are not deemed good risk (ie, cancers associated with poor outcomes) is needed for each PICT, ensuring those seeking traditional therapies can still be managed within the region’s health system. As health-care expertise and capacity improve in each PICT, the intensity of protocols can be increased; increasing the number of treatable cancers will result in more children with cancer accessing treatment and improving survival. Additional countries can be added to this model, and similar models are likely to be feasible in other small island nations.
Point-of-care test and treat: developing innovative approaches to cervical cancer control in Papua New Guinea
Of the 266 000 deaths from cervical cancer worldwide in 2012, 87% occurred in low-income countries, including PICT countries. The Polynesian (American Samoa, Cook Islands, French Polynesia, Niue, Pitcairn Islands, Samoa, Tokelau, Tonga, Tuvalu, and Wallis and Futuna) and Micronesian (Federated States of Micronesia, Guam, Kiribati, Marshall Islands, Nauru, Northern Mariana Islands, and Palau) PICTs have an estimated age-standardised cervical cancer incidence of ten patients per 100 000 population, whereas Melanesian PICTs (Fiji, Papua New Guinea, New Caledonia, Solomon Islands, and Vanuatu) have a 3-times greater incidence, at 33 patients per 100 000—the second highest incidence in the world.23 Because many PICTs do not have cancer registries, incidence and mortality rates from cervical cancer remain as estimates: Estimated age-standardised rates of cervical cancer range from an incidence of 16·5 in New Caledonia to 29·1 in Papua New Guinea per 100 000 women and, with mortality ranging from 8·2 in New Caledonia to 19·8 in Papua New Guinea per 100 000 women. Corresponding figures in Australia and New Zealand are 6·0 (incidence) and 1·7 (mortality) per 100 000 women.23,24 To place incidence and mortality from cervical cancer into context, women in Melanesia are 13-times more likely to die of cervical cancer than women in Australia or New Zealand.23 High-risk human papillomavirus (HPV) types 16 and 18 are found in 77% of women with cervical cancers in Fiji, 83% in Papua New Guinea,25,26 and 56% of women with high-grade smears in Vanuatu.27 National HPV vaccination programmes have started in several PICTs in the region (New Caledonia, Cook Islands, Federated States of Micronesia, Fiji, Guam, Kiribati, Wallis and Futuna, Marshall Islands, Northern Mariana Islands, and Palau); however, only Fiji and Cook Islands have a large population coverage. The mainstay of cervical screening in the region has been Papanicolaou (Pap) cytology and visual inspection of the cervix with acetic acid, but neither strategy has been successfully implemented at a national or regional scale.28
Research partnerships have identified an innovative solution for cervical screening in Papua New Guinea that might be suitable for other settings in the Pacific. Papua New Guinea is the most populous PICT with a population of at least 8 million across 600 islands, with 800 distinct languages. More than 80% of residents live in rural and remote areas, and over half of the country is inaccessible by road. Low population-level coverage, poor clinical recall in a Pap test screening programme,29 and disappointing overall performance of Pap testing (caused by low laboratory capacity, delays in processing, and missed diagnosis, among other factors)30 led to the search for new cervical screening strategies.
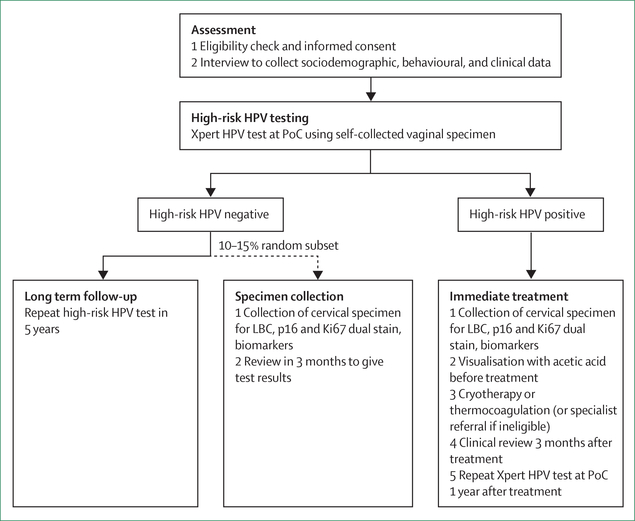
In a study of 1005 women attending screening services at two clinical centres Mount Hagen and Goroka Papua New Guinea, led by the Papua New Guinea Institute of Medical Research (Goroka, Papua New Guinea) and the Kirby Institute, University of New South Wales (Kensington, NSW, Australia), women provided self-collected vaginal specimens for HPV DNA testing at the point-of-care on the portable GeneXpert platform (Xpert HPV Test; Cepheid, Sunnyvale, CA, USA).31 Self-collected specimens were found to have excellent performance compared with clinician-collected cervical specimens for the detection of oncogenic high-risk HPV types32 and for detecting underlying high-grade squamous intraepithelial lesions (HSIL) or worse (HSIL+).33 The study also investigated alternative clinical screening algorithms (figure 3) for future large-scale evaluation and found that the point-of-care HPV testing alone had superior performance for the detection of underlying disease than does either visual inspection of the cervix with acetic acid alone or as a composite algorithm that included point-of-care HPV testing followed by visual inspection of the cervix with acetic acid. The sensitivity for detection of high-grade squamous intraepithelial lesions or worse was 92% for point-of-care HPV testing, 47% for visual inspection of the cervix with acetic acid, and 42% for the composite algorithm.34,35 A further study of 3400 women is underway to confirm these findings and to evaluate the cost-effectiveness, health system implementation requirements, and acceptability (from client and provider perspectives of point-of-care HPV testing with self-collected specimens followed by same-day ablative cervical thermocoagulation for those who test positive.36 By November, 2018, more than 1000 women were screened as part of this study: 12·5% of women screened were HPV-positive, of whom around 93% received same-day cervical ablation with the portable, battery-operated WISAP C3 device (WISAP Medical Technologies, Munich, Germany). The remaining 6–7% of women who tested positive for HPV were referred to a local specialist for review because of the identification of lesions that were suspected to be cervical cancer following pelvic examination.
Figure 3: Cervical screening management algorithm for Papua New Guinea.
PoC=point of care. HPV=human palliomavirus. LBC=liquid-based cytology.
The findings of this study are expected to inform future scale-up of a HPV-based test and treatment strategy in Papua New Guinea and within other high-burden, low-income settings. Innovation through research has continued with the development of the cervical cancer prevention in the Pacific partnership, which involves institutions in Papua New Guinea, Australia, New Zealand, and the USA who are cooperating with the Samoan Government and other PICTs on how to introduce and scale-up the use of point-of-care HPV-based testing and treatment strategies. Political commitment and sustainable funding for all components of cervical cancer control and prevention strategies will be crucial to the successful implementation of these programmes.
Improvement and support of surgical services in the Pacific
The Pacific has a shortage of medical staff across all PICTs due to a small number of training opportunities and scholarships to study abroad in the two established Pacific island medical schools (the University of Papua New Guinea, Port Moresby, Papua New Guinea and the Fiji National University, Suva, Fiji); additionally, a large number of the health-care professionals who are trained on the islands move to Australia or New Zealand. The shortage of surgeons reflects the small number of postgraduate training opportunities and the inadequate funding of positions and infrastructure. Training of surgeons to subspecialise in cancer surgery is unlikely to be feasible or a priority for PICTs in the short-term, but general surgeons are expected to operate on a range of cancers with minimal training or supervision. In many PICTs, cancer treatment rarely extends beyond surgery, with few islands having chemotherapy or radiotherapy facilities, as discussed by Sarfati and colleagues.1
Before 1995, the absence of surgeons in the PICTs has meant there has been a heavy reliance on a largely transient, often fragmented, expatriate health-care professional and visiting specialists.37 In 1995, a push led by the Royal Australasian College of Surgeons attempted to address these issues. Australia Government aid funding was secured to engage PICT ministries of health, clinical champions, and academic institutions to cooperate and find ways to improve the delivery of surgical services and build capacity in Papua New Guinea and the rest of the PICTs. The Pacific Islands Project also began in 1995, aiming to streamline visiting specialist services, support surgical training, and provide professional support for surgeons in PICTs.38
At the request of PICT ministries of health for improved training capacity for local surgeons, postgraduate specialist training programmes were expanded at the University of Papua New Guinea and Fiji School of Medicine. First introduced at the University of Papua New Guinea in 1974, the Master of Medicine programme was a 4-year course strategically developed to train surgeons to be capable of performing surgery in all settings (including rural areas and remote islands). From 1994, the University of Papua New Guinea increased the capacity of the course and began to offer additional subspecialisation training in orthopaedics, urology, head and neck surgery, paediatric surgery, and neurosurgery. In 1997, the Fiji School of Medicine (now known as Fiji National University) developed a similar surgical training programme.37,39 The need to support trainee surgeons throughout their training, including overseas placement, was recognised and close communications between the respective ministries of health and other agencies (including the Pacific Community) was developed for strategic input and for the targeted employment of the surgeons when returning to their home country. The overseas placements in New Zealand and Australia were aligned to the area of interest for the trainee and their respective country, although not all PICT physicians could pass the registration requirements of the host country.
The postgraduate surgical training programmes offered in Papua New Guinea and Fiji have been highly successful in increasing and retaining a large number of surgeons working in PICTs.38,40 For example, the number of surgeons in Papua New Guinea has increased from 12 in 199041 to 82 working in the country in 2018, meaning that general surgeons can be found at all major hospitals, which is the only medical specialty to achieve this.37,42 Similarly, Fiji has graduated at least 35 Pacific surgeons with postgraduate qualifications between 1998 and mid-2018.40 Surgical training in both Papua New Guinea and Fiji is increasingly being taught by Pacific surgeons, reducing the reliance on external assistance;37,40 additionally, local training has stalled the migration of PICT surgeons to developed countries,38,40 with the Fiji National University retaining 82% of students and the University of Papua New Guinea retaining more than 95% of its graduates.42 Training opportunities and clear progression pathways driven by successful surgical advocacy by the ministries of health and medical councils has promoted the success and retainment of trainee surgeons.41 In addition, with support from the Pacific Island Surgeons Association, the Royal Australasian College of Surgeons, and aid from the Australian and New Zealand Governments, avenues for research, teaching, and continuous professional development have contributed to the ongoing improvement of surgical services in the region.43
This growing contingent of surgeons remaining in PICT (table 3) are increasingly responsible for providing specialist care, including managing complex cancer cases in their own and neighbouring countries.37,39 For example, two paediatric surgeons provide care for childhood cancers in Fiji and its neighbours: Kiribati, Tuvalu, and Vanuatu, services which previously would have been provided by foreign partners at a greater cost. Similarly, two Fijian urologists work in Fiji in cooperation with urological societies in New Zealand and Australia. With support from the Gastroenterological Society of Australia, many PICTs now have the skills and equipment to diagnose and provide surveillance for gastric and colon cancers.44
Table 3:
Surgical workforce capacity for selected Pacific island countries and territories at the end of 2018
| Papua New Guinea | Fiji | Solomon Islands | Vanuatu | Samoa | Kiribati | Tonga | Cook Islands | Tuvalu | |
|---|---|---|---|---|---|---|---|---|---|
| Population (estimated 2018) | 8 558 800 | 888 400 | 682 500 | 304 500 | 196 700 | 120 100 | 100 300 | 15 200 | 10 200 |
| General surgery | 69 | 5 | 3 | 3 | 5 | 1 | 3 | 1 | 1 |
| Obstetrics and gynaecology | 44 | 20 | 4 | 4 | 3 | 1 | 4 | 1 | 0 |
| Orthopaedics | 7 | 4 | 2 | 1 | 1 | NA | 0 | NA | NA |
| Urology | 3 | 2 | 1 | 0 | NA | NA | NA | NA | NA |
| Neurosurgery | 1 | 1 | 0 | 0 | NA | NA | NA | NA | NA |
| Plastics | 2 | 1 | 0 | 0 | NA | NA | NA | NA | NA |
| Paediatric | 4 | 1 | 1 | 1 | NA | NA | NA | NA | NA |
| Ear, nose, and throat | 10 | NA | NA | NA | NA | NA | NA | NA | NA |
| Total number of health-care professionals per Pacific Island Country or Territory | 140 | 34 | 11 | 9 | 9 | 2 | 7 | 2 | 1 |
Updated data from those initially published by Guest and colleagues.45The data were updated by emailing all heads of departments and key clinicians and administrators in the selected Pacific island nations to report on the number of specialists currently working on the island or territory, whether they were trained in the region through the Master of Medicine (for surgery) programme or outside it (eg, in Australia and New Zealand). NA=not applicable.
Relationships with multiple organisations and individuals have been necessary for the improvement of surgeon retention over the past 24 years to be possible, most notably with the Royal Australasian College of Surgeons, the Governments of Australia and New Zealand, and many local and international clinical champions. More than 500 specialist teams have visited PICTs as part of the Pacific Islands Project, delivering or augmenting health-care services.38 The visiting teams provide an opportunity for local clinicians to develop and practice skills, share knowledge, attend teaching sessions, and develop close supportive relationships with international surgical teams and college associations. PICTs also benefit from infrastructure and supplies left behind by visiting teams. PICT surgeons are increasingly leading visiting surgical teams both in-country and overseas to neighbouring island nations.38 This approach is one that could be built on to develop the capacity of other specialised health-care workers crucial in cancer control, such as pathologists.
Although surgical services continue to improve, many challenges remain, and much work is still needed. Guided by the Lancet Commission for Global Surgery45 target of at least 20 surgeons, anaesthetists, and obstetricians per 100 000 population, over 1000 new surgeons need to be trained to provide an adequate Pacific surgical workforce by 2030.42,46 For many PICTs, achieving the baseline numbers of surgeons for best practice remains a distant goal,45,47 and comprehensive cancer care remains limited by a shortage of resources and supporting infrastructure.42
Success factors
Despite the multiple challenges faced by PICTs, we have described four examples of concerted efforts that have strengthened PICT-specific health-care capacity and improved the infrastructure to enable PICTs to work towards reducing their cancer burden. All of the initiatives discussed share key success factors, which we outline here as a complementary to the recommendations for the Pacific region that was synthesised in the first paper of the Series by Sarfati and colleagues.1
Building and strengthening partnerships to develop sustainable cancer initiatives and grow local health capacity
Partnerships between local organisations and various development partners, including funding bodies, academic institutions, regional organisations, and Pacific Rim centres of excellence have been crucial in advancing cancer control efforts in the region. Because of their small population size and narrow economic base, PICTs are reliant on financial and technical support provided by their development partners. However, effective, sustainable change is unlikely unless the explicit goals of any potential partnerships include improving local capacity, capability, and infrastructure, and produce sustainable positive outcomes. Each of the four examples provided here clearly shows this principle.
Regional collaborative approach to cancer control
For individual small PICTs with a small number of resources for cancer control, sharing infrastructure and expertise holds major potential advantages for improving cancer care and prevention. Sarfati and colleagues1 call on PICTs to consider a regional or sub collaborative approach to cancer control. The regional cancer coalition of USAPI jurisdictions and partners provides a globally unique example of collaborative cancer control planning across a group of countries that others can learn from. In addition, PICTs banding together have the potential to form an effective lobby for a PICT-wide cancer control agenda, such as effective cancer prevention, bulk procurement of essential cancer medications, or regional cancer research.
Local commitment to change
In each of the examples provided here, local champions that have shown remarkable commitment and leadership have advocated and campaigned for improved cancer care and prevention within the region, with an explicit focus on gaining political support. This support is essential to ensure sustainable solutions that are effectively implemented within PICTs.
Adapting frameworks, policies, and guidelines for PICTs
Frameworks that succeed in PICTs are those that have been developed for the unique local context of each island or area, considering cultural, health system, demographic, and economic factors. For instance, PICT treatment protocols used to treat cancer in children might prompt the adaption of best practice guidelines for adults; moreover, the model for training and retaining surgeons in Fiji and Papua New Guinea could guide training programmes for other specialties such as pathology. PICT clinicians are generally adept at delivering care in resource-constrained settings and are in a good position to advocate for guidelines that will succeed in their respective Pacific island.
Conclusion
Many of the lessons learnt from the initiatives examined in this Series paper are applicable across the cancer care continuum and can provide a framework for ongoing initiatives and collaborations that aim for future innovative good practices to improve cancer outcomes in PICTs, which may then serve as a model for other small island nations globally. PICT leaders must prioritise the cancer control agenda and continue to seek capacity building strategies that leverage resources through regional partnerships and help develop cancer registration, improve screening and preventive practices, deliver better care to more individuals, and undertake more PICT-focused cancer research.
Search strategy and selection criteria.
We searched PubMed and the grey literature using the search terms “Pacific”, “cancer”, “small island developing states”, “SIDS”, and “cervical cancer”, “screening”, “childhood “OR “children” OR “paediatric cancers”, “surgery”, “surgeons”, and “workforce” for papers and reports published between Jan 1, 2000, up until June 29, 2019. Only papers and reports published in English were reviewed. We included references on the basis of originality and relevance to the broad scope of this Series paper. We also liaised with a number of key informants throughout the region who assisted with information on relevant literature and local practice.
Acknowledgments
Declaration of interests
NP and LB-L were supported by current Centers for Disease Control and Prevention Cooperative Agreement numbers 17NU58DP006312 (Pacific Regional Central Cancer Registry 2017-2022), NU58DP006335 (American Samoa CCC 2017-2022), NU58DP006348 (CNMI CCC 2017-2022), NU58DP006269 (Guam CCC 2017-2022), NU58DP006303 (FSM CCC 2017-2022), NU58DP006336 (RMI CCC 2017-2022), NU58DP006289 (Palau CCC 2017-2022), NU58DP005810 (REACH 2014-2018), and the National Cancer Institute 2U54CA143727. The contribution of NP and LB-L is the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention, the National Cancer Institute, or the Department of Health and Human Services. All other authors have no competing interests.
Footnotes
See Online for a French translation of the abstract of this paper
This is the second in a Series of five papers about cancer control in small island nations
Contributor Information
Alec Ekeroma, National University of Samoa, Le Papaigalagala Campus, To’omatagi, Samoa; Department of Obstetrics and Gynaecology, University of Otago, Wellington, Wellington, New Zealand.
Rachel Dyer, Department of Public Health, University of Otago, Wellington, Wellington, New Zealand.
Neal Palafox, Pacific Regional Cancer Programs, Department of Family Medicine and Community Health, John A Burns School of Medicine, University of Hawaii at Manoa, Honolulu, HA, USA; Population Sciences in the Pacific Program (Cancer Prevention in the Pacific), University of Hawaii Cancer Center, Honolulu, HA, USA.
Kiki Maoate, Pacific Islands Programme, Royal Australasian College of Surgeons, Melbourne, VIC, Australia; Department of Paediatric Surgery, Christchurch Public Hospital, Christchurch, New Zealand.
Jane Skeen, Starship Blood and Cancer Centre, Starship Children’s Health, Auckland, New Zealand.
Sunia Foliaki, Centre for Public Health Research, Massey University-Wellington Campus, Wellington, New Zealand.
Andrew J Vallely, Papua New Guinea Institute of Medical Research, Goroka, Papua New Guinea; Kirby Institute, University of New South Wales, Sydney, NSW, Australia.
James Fong, Obstetrics and Gynaecology Unit, Colonial War Memorial Hospital, Ministry of Health, Suva, Fiji; Fiji National University, Suva, Fiji.
Merilyn Hibma, Cervical Cancer Prevention in the Pacific Alliance, Dunedin, New Zealand; Department of Pathology, University of Otago, Dunedin, New Zealand.
Glen Mola, Department of Obstetrics, Gynaecology and Reproductive Health, Port Moresby General Hospital, Port Moresby, Papua New Guinea; School of Medicine and Health Sciences, University of Papua New Guinea, Boroko, Papua New Guinea.
Martina Reichhardt, Cancer Council of the Pacific Islands, Yap State Department of Health Services, Yap State, Federated States of Micronesia.
Livinston Taulung, Cancer Council of the Pacific Islands, Kosrae State Department of Health Services, Kosrae State, Federated States of Micronesia.
George Aho, Department of Paediatrics, Vaiola Hospital, Nuku’alofa Tonga.
Toakase Fakakovikaetau, Department of Paediatrics, Vanuatu Hospital, Port Vila, Vanuatu.
David Watters, Deakin University and Barwon Health, University Hospital Geelong, Geelong, VIC, Australia.
Pamela J Toliman, Papua New Guinea Institute of Medical Research, Goroka, Papua New Guinea; Kirby Institute, University of New South Wales, Sydney, NSW, Australia.
Lee Buenconsejo-Lum, Pacific Regional Cancer Programs, Department of Family Medicine and Community Health, John A Burns School of Medicine, University of Hawaii at Manoa, Honolulu, HA, USA.
Diana Sarfati, Department of Public Health, University of Otago, Wellington, Wellington, New Zealand.
References
- 1.Sarfati D, Dyer R, Sam FA-L, et al. Cancer control in the Pacific: big challenges facing small island states. Lancet Oncol 2019; published online Aug 5 10.1016/S1470-2045(19)30400-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ASTHO. ASTHO US-Affiliated Pacific Islands (USAPI) profile report. 2014. http://www.astho.org/Profile/Volume-Three/US-Affiliated-Pacific-Islands/ (accessed April 7, 2019).
- 3.Lesniewski R Map of Oceania. https://www.123rf.com/portfolio/lesniewski/2.html?mediapopup=31061240 (accessed July 23, 2019).
- 4.Kessaram T, Mckenzie J, Girin N, et al. Noncommunicable diseases and risk factors in adult populations of several Pacific Islands: results from the WHO STEPwise approach to surveillance. Aust N Z J Public Health 2015; 39: 336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin HR, Shin A, Woo H, et al. Prevention of infection-related cancers in the WHO Western Pacific Region. Jpn J Clin Oncol 2016; 46: 13–22. [DOI] [PubMed] [Google Scholar]
- 6.Palafox NA, Tsark JU. Cancer in the US Associated Pacific Islands (UASPI): history and participatory development. Pac Health Dialog 2004; 11: 8–13. [PubMed] [Google Scholar]
- 7.Foliaki S, Bates C, Tukana I, Palafox NA. Cancer control in the Pacific: a South Pacific collaborative approach. Cancer Epidemiol 2017; 50: 193–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palafox NA, Given L, Hohman K, et al. Comprehensive Cancer Control Planning in the Pacific: the cancer council of the Pacific islands a multi-national regional coalition. Cancer Causes Control 2018; 29: 1287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldie SJ, Diaz M, Kim SY, Levin CE, Van Minh H, Kim JJ. Mathematical models of cervical cancer prevention in the Asia Pacific region. Vaccine 2008; 26 (suppl 1): M17–29. [DOI] [PubMed] [Google Scholar]
- 10.Robinett H, Leon Guerrero R, Underwood R, Palafox N, Ward D. University of Guam/University of Hawaii Cancer Center Partnership: an 11-year partnership to advance cancer health equity in Pacific islanders. Cancer Epidemiol Biomarkers Prev 2015; 24: 8. [Google Scholar]
- 11.Palafox NA, Reichhardt M, Taitano JR, et al. A socio-ecological framework for cancer control in the Pacific: a community case study of the US affiliated Pacific Island jurisdictions 1997–2017. Front Public Health 2018; 6: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vathaire C, De Vathaire F, Vu B, Al E. Childhood malignancies in French Polynesia during the 1985–1995 period. Trop Med Int Health 2004; 9: 1005–11. [DOI] [PubMed] [Google Scholar]
- 13.National Child Cancer Network. The New Zealand Children’s Cancer Registry (NZCCR) Working Group. 2019. http://childcancernetwork.org.nz/new-zealand-childrens-cancer-registry-nzccr-working-group/ (accessed July 25, 2019).
- 14.Ridgway D, Skeen J, Mauger D, Becroft D. Childhood cancer among the Polynesian population. Cancer 1991; 68: 451–54. [DOI] [PubMed] [Google Scholar]
- 15.National Child Cancer Network. Pacific working group (portal). 2018. http://childcancernetwork.org.nz (accessed Nov 9, 2018).
- 16.International Society of Paediatric Oncology. Paediatric oncology in developing countries. 2018. https://siop-online.org/sp_cb/oceania/ (accessed April 13, 2019).
- 17.Starship Child Health. Starship clinical guidelines. 2018. https://www.starship.org.nz/guidelines/browse/?index=All&type=pacific_island_protocol&cat=child_cancer (accessed Nov 7, 2018).
- 18.Ribeiro R, Antillon F, Pedrosa F, Pui C. Global pediatric oncology: lessons from partnerships between high-income countries and low- to mid-income countries. J Clin Oncol 2016; 34: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Starship. Pacific island protocol: child cancer. 2019. https://www.starship.org.nz/guidelines/browse/?index=All&type=pacific_island_protocol&cat=child_cancer (accessed July 25 2019).
- 20.Denburg A, Arora B, Arora RS, et al. Access to essential medicines for children with cancer: a joint SIOP-CCI position statement. Lancet Oncol 2017; 18: 20–22. [DOI] [PubMed] [Google Scholar]
- 21.Robertson J, Magrini N, Barr R, Ondari C, Forte G. Medicines for cancers in children: the WHO model for selection of essential medicines. Pediatr Blood Cancer 2015; 62: 1689–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta S Expanding the WHO list of essential medicines for children: a call for further action. Pediatr Blood Cancer 2015; 62: 1685–86. [DOI] [PubMed] [Google Scholar]
- 23.WHO. GLOBOCAN 2018. International Agency for Research on Cancer (IARC) 2019. http://gco.iarc.fr/today (accessed July 23, 2019). [Google Scholar]
- 24.Obel J, Souares Y, Hoy D, et al. A systematic review of cervical cancer incidence and mortality in the Pacific Region. Asian Pac J Cancer Prev 2014; 15: 9433–37. [DOI] [PubMed] [Google Scholar]
- 25.Tabrizi SN, Law I, Buadromo E, et al. Human papillomavirus genotype prevalence in cervical biopsies from women diagnosed with cervical intraepithelial neoplasia or cervical cancer in Fiji. Sex Health 2011; 8: 338–42. [DOI] [PubMed] [Google Scholar]
- 26.Tabone T, Garland SM, Mola G, O’Connor M, Danielewski J, Tabrizi SN. Prevalence of human papillomavirus genotypes in women with cervical cancer in Papua New Guinea. Int J Gynaecol Obstet 2012; 117: 30–32. [DOI] [PubMed] [Google Scholar]
- 27.Aruhuri B, Tarivonda L, Tenet V, et al. Prevalence of cervical human papillomavirus (HPV) infection in Vanuatu. Cancer Prev Res 2012; 5: 746–53. [DOI] [PubMed] [Google Scholar]
- 28.Obel J, McKenzie J, Buenconsejo-Lum LE, et al. Mapping HPV vaccination and cervical cancer screening practice in the Pacific region-strengthening national and regional cervical cancer prevention. Asian Pac J Cancer Prev 2015; 16: 3435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vallely A, Mola GDL, Kaldor JM. Achieving control of cervical cancer in Papua New Guinea: what are the research and program priorities? PNG Med J 2011; 54: 83–90. [PubMed] [Google Scholar]
- 30.Vallely AJ, Toliman PJ, Ryan C, et al. Association between visual inspection of the cervix with acetic acid examination and high-risk human papillomavirus infection, Chlamydia trachomatis, Neisseria gonorrhoeae and Trichomonas vaginalis in Papua New Guinea. ANZJOG 2018; 58: 576–81. [DOI] [PubMed] [Google Scholar]
- 31.Cepheid. GeneXpert HPV. 2019. http://www.cepheid.com/en/cepheid-solutions/clinical-ivd-tests/sexual-health/xpert-hpv (accessed April 13, 2019).
- 32.Toliman P, Badman SG, Gabuzzi J, et al. Field evaluation of Xpert HPV point-of-care test for detection of human papillomavirus infection by use of self-collected vaginal and clinician-collected cervical specimens. J Clin Microb 2016; 54: 1734–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toliman PJ, Kaldor JM, Badman SG, et al. Evaluation of self-collected vaginal specimens for the detection of high-risk human papillomavirus infection and the prediction of high-grade cervical intraepithelial lesions in a high-burden, low-resource setting. Clin Microbiol Infect 2018; 25: 496–503. [DOI] [PubMed] [Google Scholar]
- 34.Toliman P, Kaldor J, Badman SG, et al. Performance of clinical screening algorithms comprising point-of-care HPV-DNA testing using self-collected vaginal specimens, and visual inspection of the cervix with acetic acid, for the detection of underlying high-grade squamous intraepithelial lesions. Papillomavirus Res 2018; 6: 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toliman PJ, Kaldor JM, Tabrizi SNVA. Innovative approaches to cervical cancer screening in low- and middle-income countries. Climacteric 2018; 21: 235–38. [DOI] [PubMed] [Google Scholar]
- 36.WISAP. WISAP C3 thermo coagulator. 2018. https://www.thermo-coagulation.com/c3-mobile-coagulator/ (accessed April 13, 2019).
- 37.Kevau I, Watters DA. Specialist surgical training in Papua New Guinea: the outcomes after 10 years. ANZ J Surg 2006; 76: 937–41. [DOI] [PubMed] [Google Scholar]
- 38.Watters D, Ewing H, McCaig E. Three phases of the Pacific Islands project (1995–2010). ANZ J Surg 2012; 82: 318–24. [DOI] [PubMed] [Google Scholar]
- 39.Kaptigau WM, Rosenfeld JV, Kevau I, Watters DA. The establishment and development of neurosurgery services in Papua New Guinea. World J Surg 2016; 40: 251–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cama J, Nagra S. Short history of the post-graduate training in Fiji—where to from here? Pac Health Dialog 2018; 21: 103–07. [Google Scholar]
- 41.Dare A, Lee K, Bleicher J, et al. Prioritizing surgical care on national health agendas: a qualitative case study of Papua New Guinea, Uganda, and Sierra Leone. PLoS Med 2016; 13: e1002023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watters DA, McCaig E, Nagra S, Kevau I. Surgical training programs in the Pacific, Papua New Guinea and Timor Leste. Br J Surg 2019; 106: e53–61. [DOI] [PubMed] [Google Scholar]
- 43.Natuzzi ES, Kushner A, Jagilly R, et al. Surgical care in the Solomon Islands: a road map for universal surgical care delivery. World J Surg 2011; 35: 1183–93. [DOI] [PubMed] [Google Scholar]
- 44.Martiniuk A, Jagilli R, Natuzzi E, et al. Cancer in the Solomon Islands. Cancer Epidemiol 2017; 50: 176–83. [DOI] [PubMed] [Google Scholar]
- 45.Meara JG, Leather AJM, Hagander L, et al. Global Surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Lancet 2015; 386: 569–624. [DOI] [PubMed] [Google Scholar]
- 46.Guest G, McLeod E, Perry W, et al. Collecting data for global surgical indicators: a collaborative approach in the Pacific Region. BMJ Glob Health 2017; 2: e000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Federspiel F, Mukhopadhyay S, Milsom P, Scott JW, Riesel JN, Meara JG. Global surgical and anaesthetic task shifting: a systematic literature review and survey. Lancet 2015; 385 (suppl 2): S46. [DOI] [PubMed] [Google Scholar]