Abstract
In the context of the coronavirus disease 2019 (COVID-19) pandemic, environmental surveillance for the detection of SARS-CoV-2 has become increasingly important. Studies have demonstrated that the SARS-CoV-2 RNA is present in the feces of infected individuals; further, its presence in wastewater has been reported. However, an optimized method for its detection in sewage has not yet been adequately investigated. Therefore, in this study, the efficient detection of SARS-CoV-2 RNA in the solid fraction of wastewater was investigated using two quantitative PCR assays. In particular, wastewater samples were collected from a manhole located in the commercial district of a metropolitan region in Japan, where COVID-19 is highly prevalent, and two wastewater treatment plants (WWTPs). The samples were concentrated using four separate methods, namely, electronegative membrane adsorption, polyethylene glycol precipitation, ultrafiltration, and solid precipitation. Each method revealed a significant concentration of pepper mild mottle virus (PMMoV) RNA, which is an indicator virus for wastewater. As expected, non-enveloped PMMoV RNA was enriched in the supernatant fraction such that relatively low concentrations were detected in the solid fraction of the wastewater samples. In contrast, higher SARS-CoV-2 RNA concentrations were consistently detected in the solid fractions compared with the supernatant fractions based on the other methods that were investigated in this study. Spearman's correlation tests showed that the SARS-CoV-2 RNA concentrations in wastewater samples from the WWTP were significantly correlated with the number of COVID-19 cases recorded during the data collection period. These results demonstrate that viral recovery from the solid fraction is an effective method for SARS-CoV-2 RNA surveillance in an aqueous environment.
Keywords: SARS-CoV-2, COVID-19, Wastewater, Sewage, Environmental surveillance
Graphical abstract
1. Introduction
The coronavirus disease 2019 (COVID-19), which is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become an ongoing global pandemic. Presymptomatic and asymptomatic infections are frequently observed in COVID-19 infection cases, making patient-based surveillance difficult. Symptoms are mainly respiratory diseases; however, it has been reported that some patients manifest diarrhea and nausea (Wang et al. 2020), suggesting gastrointestinal infection caused by SARS-CoV-2. It has also been reported that SARS-CoV-2-infected enterocytes from the small intestine, along with the virus, are shed through feces (Wolfel et al. 2020; Xu et al. 2020; Zhang et al. 2020). Significant copies of SARS-CoV-2 RNA have been detected in the feces of both symptomatic and asymptomatic individuals (Mizumoto et al. 2020; Nishiura et al. 2020; Treibel et al. 2020), even after recovery from respiratory symptoms (Y. Wu et al. 2020; Zheng et al. 2020). Therefore, wastewater-based epidemiology (WBE) is considered an effective approach for monitoring the prevalence of SARS-CoV-2 (Kitajima et al. 2020). The presence of SARS-CoV-2 RNA has also been detected in wastewater in Australia, Italy, Spain, the Netherlands, and the USA (Ahmed et al. 2020a; La Rosa et al. 2020b; Medema et al. 2020; Peccia et al. 2020; Randazzo et al. 2020; Rimoldi et al. 2020; F. Wu et al. 2020). Some of the wastewater samples showed a correlation between the SARS-CoV-2 RNA concentrations in the sewage and the number of COVID-19 clinical cases (Ahmed et al. 2020a; Medema et al. 2020; Peccia et al. 2020). In Japan, SARS-CoV-2 RNA was first reported in the aqueous environment in Yamanashi Prefecture, although the detected copy number was very low since the sampling area had a low prevalence of COVID-19 (Haramoto et al. 2020). Environmental surveillance is widely utilized in the monitoring of enteric viruses, such as norovirus and poliovirus. These non-enveloped viruses are also excreted through feces and their presence in wastewater in high concentrations has been reported (Haramoto et al. 2018). However, SARS-CoV-2 RNA concentrations in wastewater are considered to be less than those of enteric viruses by a factor of 10 to 100 (Hata and Honda 2020). Several methods for the recovery of viruses from wastewater have been developed for non-enveloped viruses, while optimized methods for enveloped viruses, such as coronaviruses, have not yet been sufficiently investigated (La Rosa et al. 2020a). Although enveloped viruses, including SARS-CoV-2, can be detected using methods that are normally used for the detection of non-enveloped viruses, reliable methods for the efficient quantification of SARS-CoV-2 in wastewater are required (Carducci et al. 2020; Rusinol et al. 2020). Recently, the presence of SARS-CoV-2 RNA in the solid fraction of wastewater in addition to the supernatant fraction that is typically used in poliovirus environmental surveillance has been reported (Yokohama City Institute of Public Health and National Institute of Infectious Diseases 2020). In this study, 32 wastewater samples collected from one manhole and two wastewater treatment plants (WWTPs) in a metropolitan region in Japan from June 9 to August 19, 2020, were analyzed, and four RNA recovery methods were compared based on two quantitative PCR assays for SARS-CoV-2 RNA detection. The results suggest that the solid fraction obtained after the initial centrifugation of the collected wastewater samples contains higher SARS-CoV-2 RNA concentrations than the supernatant fraction.
2. Materials and methods
2.1. Collection of wastewater samples
A total of 32 grab wastewater samples (500 mL each) were collected weekly from a manhole (Manhole A, n = 10) and two wastewater treatment plants (WWTP B, n = 11 and WWTP C, n = 11) in a metropolitan region in Japan from June 9 to August 19, 2020, except for the sample that was collected during the 10th week from Manhole A, which is located in a commercial district characterized by a high prevalence of COVID-19 and upstream of WWTP B. The samples from the WWTPs were collected from the influent, which was considered raw wastewater. All the samples were collected in sterile plastic bottles and immediately transported to the laboratory. During transportation, the samples were kept frozen at −20 °C until analysis within 10 days after collection.
2.2. Virus recovery methods and RNA extraction
Virus recovery from the collected wastewater samples was performed using four methods, namely, electronegative membrane adsorption, polyethylene glycol (PEG) precipitation, ultrafiltration, and solid precipitation. First, 400 mL of each sample was centrifuged at 3000 rpm (1840 ×g) for 30 min. Details regarding the separation of the supernatant and sediment in each method are as follows. 1) After prefilter treatment using a filter with a pore size of 1.0 μm, 300 mL of the resulting supernatant was concentrated 100-fold using the electronegative membrane adsorption method (Ozawa et al. 2019). The pH of the samples was adjusted to 3.5 using 0.5 N HCl, after which the samples were passed through an electronegative membrane (0.45 μM pore size). The adsorbed viruses were eluted with 3% beef extract solution. Further, using one-third of the total 3-mL elution, RNA extraction was performed using the QIAamp UltraSens Virus Kit (Qiagen, Hilden, Germany) as per the manufacturer's instructions. 2) Regarding PEG precipitation, 30 mL of the supernatant was used (Ahmed et al. 2020b; F. Wu et al. 2020). PEG 8000 and NaCl were added to the supernatant (final concentrations were 10% and 1 M, respectively) and incubated at 4 °C overnight with gentle agitation. After centrifugation at 10,000 ×g for 30 min, the PEG precipitant was dissolved in 500 μL of PBS. Using 300 μL of this solution, RNA was extracted using the QIAamp Viral RNA kit (Qiagen) as per the manufacturer's instructions. 3) To realize ultrafiltration, 24 mL of supernatant from the initial centrifugation was concentrated via ultrafiltration using Amicon Ultra-15 (molecular weight cutoff 30 kDa; Merck Millipore Ltd. Sydney, Australia) (Ahmed et al. 2020b). The samples were then centrifuged at 4700 ×g for 10 min to concentrate them to approximately 200 μL. RNA was then extracted from the concentrated samples using a RNeasy Microbiome kit (Qiagen) as per the manufacturer's instructions. 4) Regarding solid precipitation, the sediment from the initial centrifugation containing both solid and semisolid materials was used as the solid fraction. Approximately 4 mL of the solid fraction was obtained from 400 mL of the initial sewage sample and used to perform the RNA extraction using the RNeasy PowerSoil kit (Qiagen) according to the manufacturer's instructions.
2.3. Performance of RT-qPCR assays
To quantify SARS-CoV-2 RNA, two RT-qPCR assays, NIID_2019-nCoV_N (hereafter, NIID_N2) (Shirato et al. 2020), and a combination of CDC 2019-nCoV_N1 and CDC 2019-nCoV_N2 (CDC_N1N2) (Centers for Disease Control and Prevention 2020), were performed. Primers and probes, with sequences that are listed in Table S1, were purchased from Takara Bio (Kusatsu, Japan). Reaction mixtures were prepared using the One Step PrimeScript III RT-qPCR Mix (Takara Bio) for the NIID_N2 assay, and the SARS-CoV-2 Direct Detection RT-qPCR Kit (Takara Bio) for the CDC_N1N2 assay. Thermal cycling was performed with an ABI 7500 Fast thermal cycler (Thermo Fisher Scientific, MA, USA), and the thermal cycling conditions for both RT-qPCR assays were as follows: initial incubation at 50 °C for 30 min and initial denaturation at 95 °C for 5 min, followed by 45 cycles of denaturation at 95 °C for 15 s, and the primer annealing and extension reaction at 60 °C for 60 s.
The previously isolated strain, JPN/TY/WK-521 (provided by Dr. Nagata, National Institute of Infectious Diseases) (Matsuyama et al. 2020), was used to compare the two RT-qPCR assays. RNA was extracted from the stocked isolate using the QIAamp Virus RNA kit (Qiagen) and analyzed via RT-qPCR assays using NIID_N2 and CDC_N1N2 sets.
2.4. Quality control
Pepper mild mottle virus (PMMoV) RNAs in wastewater samples were quantified via RT-qPCR using One Step PrimeScript™ III RT-qPCR Mix (Takara Bio) as the internal control (Kitajima et al. 2018). The corresponding primer and probe sequences are listed in Table S1 (Haramoto et al. 2013; Zhang et al. 2006). The thermal cycling conditions for the PMMoV RT-qPCR assay were as follows: initial incubation at 50 °C for 30 min and initial denaturation at 95 °C for 30 s, followed by 45 cycles of denaturation at 95 °C for 5 s, and primer annealing and extension reaction at 60 °C for 60 s. Given that PMMoV is a non-enveloped virus unlike SARS-CoV-2, the results of the quantification were not used to calculate the efficiency of virus recovery and RT-qPCR, but to check substantial loss of SARS-CoV-2 detection.
All the RT-qPCR assays for SARS-CoV-2 and PMMoV were performed in duplicates and included negative and positive standard controls. To obtain the standard curves for both assays, a 10-fold dilution series of standard RNAs was prepared; 5 × 100–5 × 102 and 5 × 104–5 × 106 gene copies/reaction for SARS-CoV-2 and PMMoV, respectively. The limit of quantification value for SARS-CoV-2 was set as five gene copies/reaction (Fig. 1 ), and reactions containing less than four gene copies were occasionally amplified in only one of the duplicates, which was regarded as negative. To avoid contamination, RNA extraction and RT-qPCR preparation were performed in separate laboratories, and the RT-qPCR mixtures were prepared on a clean bench, except for the addition of the template.
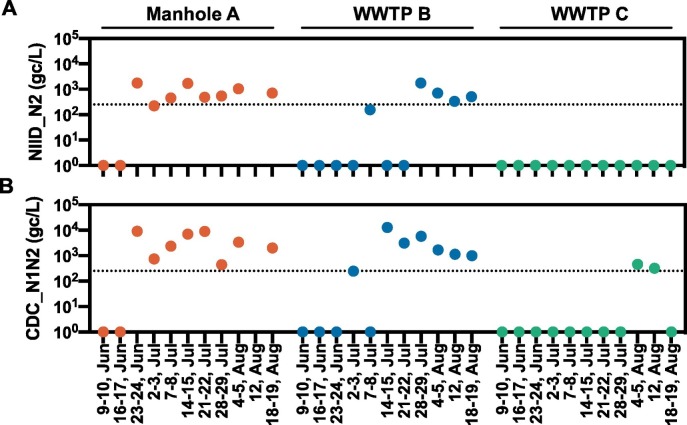
Fig. 1.
Quantification of SARS-CoV-2 RNA in the solid fraction of the wastewater samples. Samples were collected weekly from Manhole A (red), WWTP B (blue), and WWTP C (green). Two different RT-qPCR assays, (A) NIID_N2 and (B) CDC_N1N2, were performed. Values are presented as the number of SARS-CoV-2 RNA gene copies per litter of wastewater (gc/L). Dots represent weekly collected samples and dotted lines represent the limit of quantification. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.5. RT-PCR and Sanger sequencing
The RNAs in the sewage samples were reverse-transcribed with SuperScript IV (Thermo Fisher Scientific) as per the manufacturer's instructions, and the resulting cDNAs were amplified via nested PCR using the EmeraldAmp PCR Master Mix (Takara Bio). The first and second PCR conditions were the same as follows: initial denaturation at 94 °C for 1 min, followed by 40 cycles of denaturation at 94 °C for 30 s, primer annealing at 56 °C for 30 s, and the extension reaction at 68 °C for 60 s. PCR products were analyzed via direct sequencing using the Sanger method. The primer sequences are listed in Table S1 (Shirato et al. 2020).
2.6. Statistical analysis
The total number of COVID-19 cases, based on confirmation date and onset date, was obtained from the official information portal of the metropolitan government (Tokyo Metropolitan Government 2020) and seven-day averages were calculated. A Spearman's rank correlation test was performed using GraphPad Prism8. Significant difference from zero was defined as p < 0.05.
3. Results
3.1. Detection of SARS-CoV-2 RNA in sewage from different sampling locations and by different methods
From a total of 32 wastewater samples, SARS-CoV-2 RNAs were recovered using four methods and quantified using two RT-qPCR assays (Table 1). Null or low SARS-CoV-2 RNA concentrations were detected in the samples that were extracted using electronegative membrane adsorption, PEG precipitation, and ultrafiltration. In contrast, viral RNAs with concentrations in the range 1.6 × 102–1.3 × 104 gene copies/L were efficiently and consistently detected in the solid fractions. The RT-qPCR results corresponding to the solid fractions obtained using the two NIID_N2 and CDC_N1N2 assay sets are summarized in Fig. 1. Both sets showed similar trends in terms of positivity; however, the CDC_N1N2 assay showed higher copy numbers compared with the NIID_N2 assay in almost all the positive samples. No SARS-CoV-2 RNA was detected within the first two weeks of the sample collection period (June 9–17) at any of the three collection sites. The first detection, which was made within the third week, on June 23, corresponded to a sample from Manhole A. Viral RNAs were continuously detected in samples from Manhole A and WWTP B after the third and sixth weeks, respectively. Notably, WWTP B is downstream of Manhole A and is located in a high COVID-19 prevalence area. Conversely, WWTP C is located in a low COVID 19 prevalence area, and samples from this WWTP rarely showed the presence of SARS-CoV-2 RNA. Additionally, to validate the presence of SARS-CoV-2 RNA in the solid fraction, sequencing analyses were performed using RNA samples that showed high concentrations. Amplified fragments from the RT-PCR were analyzed by performing Sager sequencing, and the SARS-CoV-2 sequences of the ORF1a and S protein regions were found to be 99–100% identical to the earlier isolated strain isolated (Fig. S1). The low mutation frequency could possibly be attributed to the high fidelity of SARS-CoV-2 RNA polymerase.
Table 1.
Quantification of SARS-CoV-2 RNA in wastewater.
| Sampling site | Sampling date | Concentration method and RT-qPCR assay (gene copies/L) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Solid fraction |
Electronegative membrane |
PEG precipitation |
Ultrafiltration |
||||||
| NIID_N2 | CDC_N1N2 | NIID_N2 | CDC_N1N2 | NIID_N2 | CDC_N1N2 | NIID_N2 | CDC_N1N2 | ||
| Manhole A | 9-Jun-20 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | - | - |
| 16-Jun-20 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | - | - | |
| 23-Jun-20 | 1.8.E+03 | 9.2.E+03 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| 2-Jul-20 | 2.2.E+02 | 7.4.E+02 | n.d. | n.d. | - | - | - | - | |
| 7-Jul-20 | 4.6.E+02 | 2.4.E+03 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| 14-Jul-20 | 1.7.E+03 | 7.0.E+03 | n.d. | n.d. | 2.0.E+03 | 4.1.E+03 | - | - | |
| 21-Jul-20 | 4.9.E+02 | 9.0.E+03 | n.d. | n.d. | - | - | n.d. | n.d. | |
| 28-Jul-20 | 5.5.E+02 | 4.4.E+02 | n.d. | n.d. | - | - | n.d. | n.d. | |
| 4-Aug-20 | 1.0.E+03 | 3.4.E+03 | n.d. | n.d. | n.d. | n.d. | - | - | |
| 18-Aug-20 | 7.0.E+02 | 2.0.E+03 | n.d. | n.d. | - | - | n.d. | n.d. | |
| WWTP B | 10-Jun-20 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | - | - |
| 17-Jun-20 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | - | - | |
| 24-Jun-20 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | - | - | |
| 3-Jul-20 | n.d. | 2.5.E+02 | n.d. | n.d. | - | - | - | - | |
| 8-Jul-20 | 1.6.E+02 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| 15-Jul-20 | n.d. | 1.3.E+04 | n.d. | n.d. | n.d. | n.d. | - | - | |
| 22-Jul-20 | n.d. | 3.1.E+03 | n.d. | n.d. | - | - | n.d. | n.d. | |
| 29-Jul-20 | 1.8.E+03 | 5.7.E+03 | n.d. | n.d. | - | - | n.d. | n.d. | |
| 5-Aug-20 | 7.0.E+02 | 1.7.E+03 | n.d. | 3.9.E+02 | 1.7.E+03 | n.d. | n.d. | n.d. | |
| 12-Aug-20 | 3.4.E+02 | 1.1.E+03 | n.d. | n.d. | n.d. | 1.0.E+03 | n.d. | n.d. | |
| 19-Aug-20 | 5.1.E+02 | 1.0.E+03 | n.d. | 1.1.E+02 | - | - | n.d. | n.d. | |
| WWTP C | 10-Jun-20 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | - | - |
| 17-Jun-20 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | - | - | |
| 24-Jun-20 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | - | - | |
| 3-Jul-20 | n.d. | n.d. | n.d. | n.d. | - | - | - | - | |
| 8-Jul-20 | n.d. | n.d. | n.d. | n.d. | - | - | n.d. | n.d. | |
| 15-Jul-20 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | - | - | |
| 22-Jul-20 | n.d. | n.d. | n.d. | n.d. | - | - | n.d. | n.d. | |
| 29-Jul-20 | n.d. | n.d. | n.d. | n.d. | - | - | n.d. | n.d. | |
| 5-Aug-20 | n.d. | 4.6.E+02 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| 12-Aug-20 | n.d. | 3.2.E+02 | n.d. | n.d. | n.d. | n.d. | - | - | |
| 19-Aug-20 | n.d. | n.d. | n.d. | n.d. | - | - | n.d. | n.d. | |
n.d.: not detected.
-: not tested.
3.2. Internal control of wastewater samples
The plant virus, PMMoV, is highly abundant in wastewater (Kitajima et al. 2018). To evaluate the virus detection procedure, the PMMoV copy number was also analyzed as an internal control of the wastewater RT-qPCR assays (Haramoto et al. 2013; Kitajima et al. 2018; Rosario et al. 2009; Symonds et al. 2018; Zhang et al. 2006). The results demonstrated significant concentrations of PMMoV RNA in all the tested samples (Table S2 and Fig. 2), validating the virus recovery methods, with no detrimental effect that could be attributed to PCR inhibitors present in wastewater. PMMoV RNA concentrations showed similar trends for wastewater samples from WWTPs B and C. The supernatant fraction (from electronegative membrane adsorption, PEG precipitation, and ultrafiltration) of the samples from the two WWTPs showed much higher PMMoV RNA concentrations (8.2 × 106–3.1 × 108 gene copies/L) than the solid fractions (1.6 × 102–1.0 × 107 gene copies/L). Samples from Manhole A showed a PMMoV RNA concentrations that were one order of magnitude higher than those corresponding to the samples collected from the two WWTPs. This difference could be due to the manhole wastewater containing a higher ratio of human excreta than the influent wastewater of the WWTPs, which are located further downstream.
Fig. 2.
Quantification of PMMoV RNA in wastewater. Samples were collected weekly from Manhole A (red), WWTP B (blue), and WWTP C (green). RNAs were recovered using four methods: electronegative membrane adsorption, PEG precipitation, ultrafiltration, and solid precipitation. Values are presented as the number of PMMoV RNA gene copies per liter of wastewater (gc/L). Dots represent weekly collected samples. Average and standard error of mean (SEM) are indicated using bars, and details are shown in Table S2. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. Sensitivities of the two RT-qPCR assays to the isolated strain and the wastewater sample
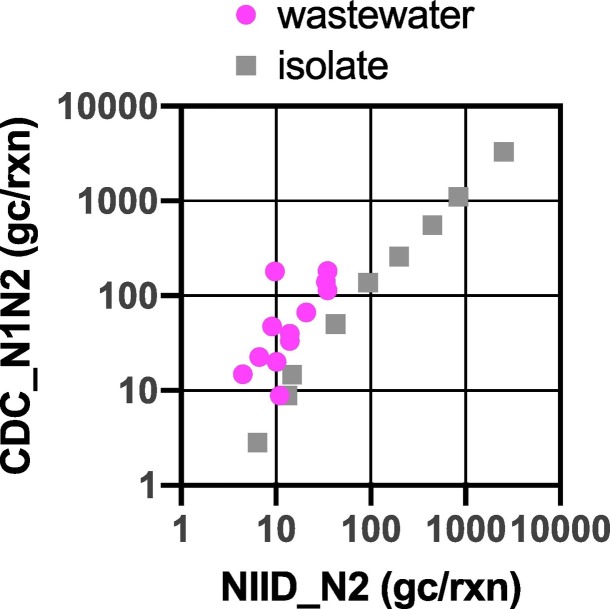
The results obtained showed that CDC_N1N2 always detected higher SARS-CoV-2 RNA concentrations in the sewage samples compared with NIID_N2 (Table 1 and Fig. 1). For comparison, serial dilutions of RNA extracted from the previously isolated strain, JPN/TY/WK-521, were quantified using the two RT-qPCR assays. The two assays showed similar sensitivities with respect to the isolated viral RNA, even at concentrations below 10 copies per reaction (Fig. 3 ). Unlike the isolated sample, the CDC_N1N2 assay quantified a higher copy number for SARS-CoV-2 RNA from the sewage samples.
Fig. 3.
Comparison of RT-qPCR assays (NIID_N2, & CDC_N1N2) based on copy number. Magenta circles represent the solid fractions of weekly collected samples from the three sample collection sites. Gray squares represent dilution series of RNA from SARS-CoV-2 isolate. Values are presented as the number of SARS-CoV-2 RNA gene copies per reaction of the RT-qPCR assays. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4. Association between SARS-CoV-2 RNA concentration and number of COVID-19 cases
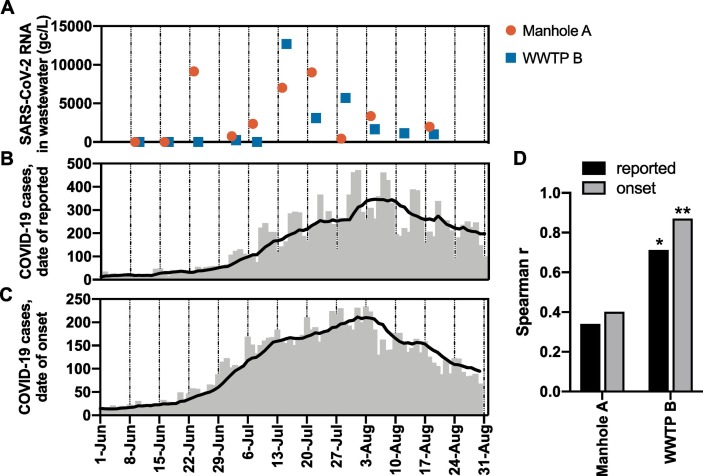
During the sample collection period, a “second wave” of the COVID-19 epidemic was witnessed in Japan from June 2020. Fig. 4 shows a summary of the number of COVID-19 cases based on confirmation date and onset date. In particular, as shown in Fig. 4B, the number of reported COVID-19 cases was less than 50 per day at the beginning of the sample collection period, i.e., June 9. The reported cases gradually increased by early August, with a peak of approximately 500 cases per day. Time-series of SARS-CoV-2 RNA concentrations in wastewater samples from Manhole A and WWTP B, in which the viral RNA was constantly detected, were plotted in parallel (Fig. 4A). Spearman's correlation analysis revealed a significant correlation between the number of COVID-19 cases and viral RNA concentration in WWTP B (Fig. 4D). The analysis indicated a higher correlation coefficient (r = 0.87; p < 0.001) between the viral RNA concentration in WWTP B and the number of new COVID 19 cases based on onset date rather than on the reported date (r = 0.71; p < 0.01).
Fig. 4.
Summary of COVID-19 cases and SARS-CoV-2 RNA concentrations in wastewater. (A) SARS-CoV-2 RNA concentrations in Manhole A and WWTP B. Daily new cases of COVID-19 based on (B) date reported and (C) date of onset in a metropolitan region in Japan. The red circles and blue squares indicate SARS-CoV-2 RNA concentrations in samples obtained from Manhole A and WWTP B, respectively. The gray bars and black lines represent new cases on each day and the seven-day average, respectively. (D) Correlation between COVID-19 cases (seven-day average) and SARS-CoV-2 RNA concentrations analyzed using Spearman's rank correlation test. *p < 0.05, **p < 0.005. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
In this study, four virus recovery methods for SARS-CoV-2 detection in wastewater samples were compared. The virus recovery/concentration method involving the use of electronegative membranes is a typical procedure that has been employed to realize national poliovirus surveillance in Japan. PEG precipitation and ultrafiltration are also effective in the realization of the surveillance of poliovirus as well as other enteroviruses (Kitajima et al. 2020). However, in this study, these three methods performed poorly in the detection of SARS-CoV-2 RNA in wastewater. In the electronegative membrane adsorption method, the sample pH was adjusted to 3.5; thus, it is possible that the low pH had an effect on the integrity of the enveloped viruses, resulting in a low recovery rate. In the PEG precipitation and ultrafiltration methods, the sample volume was limited. Therefore, it was difficult to detect viral RNA if the concentration is low. Notably, these three virus recovery methods commenced with the supernatant from the initial centrifugation. The data suggested that SARS-CoV-2 was adsorbed onto the solid fraction. Therefore, any method that commences with the supernatant will show lower SARS-CoV-2 RNA concentrations. A significant level of PMMoV RNA was detected in the supernatant samples obtained following these three methods, indicating that the lower SARS-CoV-2 concentration is not due to the failure of virus recovery or the presence of PCR inhibitory materials in the wastewater. In fact, the data collected indicated that the solid fraction contained lower PMMoV RNA and higher SARS-CoV-2 RNA. This could be explained by the absence of an envelope for PMMoV RNA and the presence of an envelope for SARS-CoV-2. In the first report on SARS-CoV-2 detection in wastewater in Italy, the addition of the solid fraction from the initial centrifugation process to the PEG precipitant resulted in the efficient detection of viral RNA (La Rosa et al. 2020b). Most recently, the detection of high SARS-CoV-2 RNA copy numbers in primary sludge from WWTPs have been reported, even though the results corresponding to the influent wastewater and sludge from the same WWTP were not compared (Peccia et al. 2020). Another coronavirus, namely, the mouse hepatitis virus, is reported to rapidly adsorb onto the solid fraction (Ye et al. 2016). Additionally, only 1% of the seeded SARS virus can be recovered using the electronegative adsorption method (Wang et al. 2005). These published studies support the idea that the solid fraction is an efficient resource for the detection of SARS-CoV-2 in wastewater.
The most sensitive primer/probe set for the detection of SARS-CoV-2 in wastewater has not yet been determined given that discrepancies between assays with different primer/probe sets have been observed in previous studies (Medema et al. 2020; Randazzo et al. 2020). The CDC_N1N2 assay that was employed in this study comprised two primer/probe sets, CDC 2019-nCoV_N1 and CDC 2019-nCoV_N2 (see Section 2, Materials and methods). The results indicated that this duplex qPCR assay showed a higher sensitivity in the detection of SARS-CoV-2 in sewage than the single set of NIID_2019-nCoV_N (NIID_N2) (Fig. 3). Such a difference was not observed in the serial dilutions of the RNA sample from the isolated strain, implying the possibility of RNA degradation in the wastewater sample. This possibility has also been suggested in another study (Rimoldi et al. 2020). Attempts to isolate SARS-CoV-2 from wastewater samples with even higher copy numbers were unsuccessful (data not shown). These observations suggest that SARS-CoV-2 in wastewater is degraded such that it becomes non-infectious. Additionally, the accumulation of SARS-CoV-2 in the solid fraction implies that activated sludge treatment efficiently removes SARS-CoV-2 from wastewater.
Samples from the manhole in the commercial district, with a high prevalence of infections, displayed higher sensitivity in the detection of SARS-CoV-2 RNA than the samples from the WWTPs (Fig. 1). The higher concentration of PMMoV RNA in the manhole samples indicated a higher ratio of human excreta relative to the samples obtained from the WWTP downstream (Fig. 2); this explains the higher sensitivity of these samples in the detection of SARS-CoV-2.
The increase in COVID-19 cases during the initial wave of infections in Japan was much slower than that in other countries in Europe as well as in the USA (Gloeckner et al., 2020), and a similar trend was observed during the second wave. Indeed, SARS-CoV-2 RNA in sewage was rarely detected in this study when PEG precipitation or ultrafiltration were used. This finding is consistent with the results reported for different areas in Italy or the Netherlands, with a higher prevalence of infection (La Rosa et al. 2020b; Medema et al. 2020). Utilizing the efficient detection in the solid fraction, the time-line of the increase in SARS-CoV-2 RNA concentration in wastewater could be summarized. These data indicated the existence of a correlation between SARS-CoV-2 RNA concentrations in sewage and the number of COVID-19 cases. Furthermore, Spearman correlation tests revealed a significant correlation between the SARS-CoV-2 RNA concentration in wastewater from WWTP B and the number of COVID-19 cases with respect to the onset date (Fig. 4). This result indicates that the method proposed in this study has potential applications in monitoring COVID-19 prevalence, even though absolute estimates of the prevalence based on SARS-CoV-2 RNA concentration are still difficult to realize. Therefore, further research is required to develop methods by which efficient viral RNA extraction from solid fractions can be realized and the development of sensitive primer/probe sets for qPCR multiplex assay is also necessary.
In Japan, the national environmental surveillance system was introduced for poliovirus in 2013 (World Health Organization 2013). To detect poliovirus, the supernatant from the initial centrifugation of wastewater is used. The two-phase method in the WHO protocol for the environmental surveillance of poliovirus was applied to the supernatant fraction (World Health Organization 2003). Therefore, the solid fraction can easily be used for COVID-19 surveillance after the sample for poliovirus surveillance has been collected. Thus, the combination of COVID-19 surveillance with the environmental surveillance of polio, which is already available, will be beneficial in terms of cost-effectiveness.
5. Conclusions
-
•
This study demonstrated the possibility of detecting SARS-CoV-2 RNA in wastewater samples from a manhole and WWTPs in a metropolitan region in Japan.
-
•
The results confirmed that manhole wastewater samples represent a more sensitive source for the surveillance of SARS-CoV-2 RNA than wastewater samples from WWTPs.
-
•
The solid fraction represents an effective source for the detection of SARS-CoV-2 RNA in wastewater compared with the use of the supernatant fraction. Thus, the adsorption of SARS-CoV-2 onto the solid fraction of wastewater is suggested.
-
•
The Taqman qPCR duplex assay (mixture of CDC 2019-nCoV_N1 and CDC 2019-nCoV_N2) showed higher detection sensitivity than the singleplex (NIID_2019-nCoV_N) assay, implying that SARS-CoV-2 tends to decay in wastewater.
-
•
Utilizing the efficient detection of SARS-CoV-2 RNA in the solid fraction using the duplex assay, a significant correlation was observed between the number of COVID-19 cases and viral RNA concentration in wastewater samples from WWTPs in areas with a high prevalence of the disease.
The following are the supplementary data related to this article.
Primers and probes used in study.
Quantification of PMMoV RNA in wastewater.
Sequencing analysis of PCR amplicon for sewage samples. The successfully amplified PCR products from the wastewater samples collected in this study and the previously isolated SARS-CoV-2 sequence (2019-nCoV/Japan/TY/WK-521/2020, Accession number: LC522975.1) are aligned. (A) Manhole A and (B) WWTP B. Sampling dates are indicated and the positions of sequencing primers are underlined.
CRediT authorship contribution statement
Kouichi Kitamura: Conceptualization, Investigation, Methodology, Formal analysis, Funding acquisition, Writing – original draft. Kenji Sadamasu: Investigation, Writing – review & editing. Masamichi Muramatsu: Project administration, Writing – review & editing. Hiromu Yoshida: Conceptualization, Investigation, Methodology, Formal analysis, Funding acquisition, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank T. Wakita and H. Shimizu from the National Institute of Infectious Diseases (NIID) for their critical evaluation of the manuscript, T. Osaka (NIID) for technical support, and N. Nagata (NIID) for providing the isolated SARS-CoV-2 strain. This study was partially supported by the Ministry of Health, Labour and Welfare of Japan [grant number 20HA2009] and the Japan Agency for Medical Research and Development [grant number JP20fk0108066].
Editor: Damia Barcelo
References
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Bivins A., Bibby K., Farkas K., Gathercole A. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci. Total Environ. 2020;739:139960. doi: 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carducci A., Federigi I., Liu D., Thompson J.R., Verani M. Making waves: coronavirus detection, presence and persistence in the water environment: state of the art and knowledge needs for public health. Water Res. 2020;179:115907. doi: 10.1016/j.watres.2020.115907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention U.S. Centers for Disease Control and Prevention; 2020. 2019-novel coronavirus (2019-nCoV) real-time rRT-PCR panel primers and probes. https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-panel-primer-probes.html
- Gloeckner S, Krause G, Hoehle M. 2020. 10.1101/2020.03.18.20037473. [DOI]
- Haramoto E., Kitajima M., Kishida N., Konno Y., Katayama H., Asami M. Occurrence of pepper mild mottle virus in drinking water sources in Japan. Appl. Environ. Microbiol. 2013;79:7413–7418. doi: 10.1128/AEM.02354-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E., Kitajima M., Hata A., Torrey J.R., Masago Y., Sano D. A review on recent progress in the detection methods and prevalence of human enteric viruses in water. Water Res. 2018;135:168–186. doi: 10.1016/j.watres.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;737:140405. doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A., Honda R. Potential sensitivity of wastewater monitoring for SARS-CoV-2: comparison with norovirus cases. Environ. Sci. Technol. 2020;54:6451–6452. doi: 10.1021/acs.est.0c02271. [DOI] [PubMed] [Google Scholar]
- Kitajima M., Sassi H.P., Torrey J.R. Pepper mild mottle virus as a water quality indicator. npj Clean Water. 2018:1. doi: 10.1038/s41545-018-0019-5. [DOI] [Google Scholar]
- Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton K.A. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci. Total Environ. 2020;739:139076. doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Bonadonna L., Lucentini L., Kenmoe S., Suffredini E. Coronavirus in water environments: occurrence, persistence and concentration methods - a scoping review. Water Res. 2020;179:115899. doi: 10.1016/j.watres.2020.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro G., Veneri C., Bonadonna L. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736:139652. doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S., Nao N., Shirato K., Kawase M., Saito S., Takayama I. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl. Acad. Sci. U. S. A. 2020;117:7001–7003. doi: 10.1073/pnas.2002589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ. Sci. Technol. Lett. 2020;7:511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura H., Kobayashi T., Miyama T., Suzuki A., Jung S.M., Hayashi K. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19) Int. J. Infect. Dis. 2020;94:154–155. doi: 10.1016/j.ijid.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa H., Yoshida H., Usuku S. Environmental surveillance can dynamically track ecological changes in enteroviruses. Appl. Environ. Microbiol. 2019;85 doi: 10.1128/AEM.01604-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peccia J., Zulli A., Brackney D.E., Grubaugh N.D., Kaplan E.H., Casanovas-Massana A. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nat. Biotechnol. 2020 doi: 10.1038/s41587-020-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simon P., Allende A., Sanchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181:115942. doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020;744:140911. doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario K., Symonds E.M., Sinigalliano C., Stewart J., Breitbart M. Pepper mild mottle virus as an indicator of fecal pollution. Appl. Environ. Microbiol. 2009;75:7261–7267. doi: 10.1128/AEM.00410-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusinol M., Martinez-Puchol S., Fores E., Itarte M., Girones R., Bofill-Mas S. Concentration methods for the quantification of coronavirus and other potentially pandemic enveloped virus from wastewater. Curr. Opin. Environ. Sci. Health. 2020;17:21–28. doi: 10.1016/j.coesh.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirato K., Nao N., Katano H., Takayama I., Saito S., Kato F. Development of genetic diagnostic methods for detection for novel coronavirus 2019(nCoV-2019) in Japan. Jpn. J. Infect. Dis. 2020;73:304–307. doi: 10.7883/yoken.JJID.2020.061. [DOI] [PubMed] [Google Scholar]
- Symonds E.M., Nguyen K.H., Harwood V.J., Breitbart M. Pepper mild mottle virus: a plant pathogen with a greater purpose in (waste)water treatment development and public health management. Water Res. 2018;144:1–12. doi: 10.1016/j.watres.2018.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokyo Metropolitan Government Updates on COVID-19 in Tokyo. 2020. https://stopcovid19.metro.tokyo.lg.jp
- Treibel T.A., Manisty C., Burton M., McKnight A., Lambourne J., Augusto J.B. COVID-19: PCR screening of asymptomatic health-care workers at London hospital. Lancet. 2020;395:1608–1610. doi: 10.1016/S0140-6736(20)31100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.W., Li J.S., Jin M., Zhen B., Kong Q.X., Song N. Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J. Virol. Methods. 2005;126:171–177. doi: 10.1016/j.jviromet.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Muller M.A. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- World Health Organization Guidelines for environmental surveillance of poliovirus circulation. 2003. https://apps.who.int/iris/handle/10665/67854
- World Health Organization Nineteenth Meeting of the Regional Commission for the Certification of Poliomyelitis Eradication in the Western Pacific Region, Manila, Philippines, 12–14 November 2013: meeting report. 2013. https://apps.who.int/iris/handle/10665/208784
- Wu F., Zhang J., Xiao A., Gu X., Lee W.L., Armas F. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. mSystems. 2020;5 doi: 10.1128/mSystems.00614-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Ellenberg R.M., Graham K.E., Wigginton K.R. Survivability, partitioning, and recovery of enveloped viruses in untreated municipal wastewater. Environ. Sci. Technol. 2016;50:5077–5085. doi: 10.1021/acs.est.6b00876. [DOI] [PubMed] [Google Scholar]
- Yokohama City Institute of Public Health and National Institute of Infectious Diseases Detection of SARS-CoV-2 in wastewater using poliovirus environmental surveillance system (in Japanese) Infect. Agents Surveill. Rep. 2020;41:122–123. https://www.niid.go.jp/niid/ja/diseases/ka/corona-virus/2019-ncov/2488-idsc/iasr-news/9714-485p02.html [Google Scholar]
- Zhang T., Breitbart M., Lee W.H., Run J.Q., Wei C.L., Soh S.W. RNA viral community in human feces: prevalence of plant pathogenic viruses. PLoS Biol. 2006;4 doi: 10.1371/journal.pbio.0040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Wang S., Xue Y. Fecal specimen diagnosis 2019 novel coronavirus-infected pneumonia. J. Med. Virol. 2020;92:680–682. doi: 10.1002/jmv.25742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S., Fan J., Yu F., Feng B., Lou B., Zou Q. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January–March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers and probes used in study.
Quantification of PMMoV RNA in wastewater.
Sequencing analysis of PCR amplicon for sewage samples. The successfully amplified PCR products from the wastewater samples collected in this study and the previously isolated SARS-CoV-2 sequence (2019-nCoV/Japan/TY/WK-521/2020, Accession number: LC522975.1) are aligned. (A) Manhole A and (B) WWTP B. Sampling dates are indicated and the positions of sequencing primers are underlined.