Abstract
Coronavirus disease-2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), poses an enormous challenge to the medical system, especially the lack of safe and effective COVID-19 treatment methods, forcing people to look for drugs that may have therapeutic effects as soon as possible. Some old drugs have shown clinical benefits after a few small clinical trials that attracted great attention. Clinically, however, many drugs, including those currently used in COVID-19, such as chloroquine, hydroxychloroquine, azithromycin, and lopinavir/ritonavir, may cause cardiotoxicity by acting on cardiac potassium channels, especially hERG channel through their off-target effects. The blocking of the hERG channel prolongs QT intervals on electrocardiograms; thus, it might induce severe ventricular arrhythmias and even sudden cardiac death. Therefore, while focusing on the efficacy of COVID-19 drugs, the fact that they block hERG channels to cause arrhythmias cannot be ignored. To develop safer and more effective drugs, it is necessary to understand the interactions between drugs and the hERG channel and the molecular mechanism behind this high affinity. In this review, we focus on the biochemical and molecular mechanistic aspects of drug-related blockade of the hERG channel to provide insights into QT prolongation caused by off-label use of related drugs in COVID-19, and hope to weigh the risks and benefits when using these drugs.
Keywords: COVID-19, Drugs, hERG channel, QT interval, Long QT syndrome (LQTS)
Graphical abstract
1. Introduction
By the end of October 2020, in the nearly one year since the end of 2019, coronavirus disease-2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spread throughout the world, with more than 46 million infections and over 1.1 million deaths, making it a terrifying destroyer of human life recently. Effective and safe treatments are urgently required in the COVID-19 pandemic, with scientists striving for rapid research and application of drugs, and at least 12 potential COVID-19 treatments are now being tested. This research is a race against COVID-19 (Kupferschmidt and Cohen, 2020). However, although a series of drugs approved for other indications and a variety of research drugs are being studied in hundreds of clinical trials (available at ClinicalTrials.gov) around the world, there is currently no Food and Drug Administration (FDA)-approved COVID-19 drug (Jan et al., 2020; Panel, 2020). In the treatment guidelines issued on April 21, the National Institutes of Health (NIH) pointed out that, at present, there are no drugs proven to be safe and effective in the treatment of COVID-19 (Panel, 2020).
Early on in the pandemic, after small clinical randomized trials, some compounds were shown to be beneficial to patients, such as antimalarial drugs (Mercuro et al., 2020; Saleh et al., 2020; Wang et al., 2020; Yao et al., 2020) and antiviral drugs (Cao et al., 2020a; Hung et al., 2020). However, the evidence of effective treatment with the use of these drugs is still insufficient, and the advocation of some of these drugs, such as chloroquine (CQ), by some public figures may hinder research on new potential therapeutic drugs (Ledford, 2020). More recently, a large-scale multicenter clinical randomized controlled trial (RCT) conducted in the UK has been shown that the 28-day mortality rates of hospitalized patients with COVID-19 in the hydroxychloroquine (HCQ) treatment group were higher than those in the usual-care group (59.6% vs. 62.9%) (Horby et al., 2020). This result reminds us to reconsider a question: Is it appropriate to use any drug on COVID-19 patients before large-scale RCTs are completed? In addition, a noticeable problem is that these drugs may increase the risk of QT prolongation and ventricular arrhythmias (Giudicessi et al., 2020; Naksuk et al., 2020). A statement from the Canadian Cardiovascular Society (CCS) recommended that the use of unnecessary drugs be discontinued, especially CQ, HCQ, azithromycin, and lopinavir/ritonavir, and baseline electrocardiograms (ECGs) should be performed in high-risk patients (Sapp et al., 2020). Indeed, the use of the drugs mentioned above alone or in combination against COVID-19 has a risk of prolonging the QT interval, and even CQ and HCQ have been listed as drugs that can cause direct myocardial toxicity. More importantly, patients with potential congenital arrhythmias, particularly long QT syndrome (LQTS), are at greater risk of fatal arrhythmias such as Torsades de Pointes (TdP) while receiving the above drugs at present (Giudicessi et al., 2020; Kannankeril et al., 2010).
It is well known that many factors participate in prolonging the QT interval, and these factors are mainly divided into congenital LQTS (cLQTS), caused by genetic mutation, and acquired LQTS (aLQTS), mainly caused by off-target drug effects (also called drug-induced LQTS (diLQTS)), which can both cause TdP (Cubeddu, 2016; Schwartz and Woosley, 2016). Importantly, the hERG (Kv11.1) channel is the main target of drugs in diLQTS (Kannankeril et al., 2010; van Noord et al., 2010). Many drugs, including antibiotics, antivirals, antifungals, antimalarials, and antidepressants, have been withdrawn from the clinic because of their severe potential arrhythmia targeting the hERG channel (Cubeddu, 2016; Mladěnka et al., 2018). The potential for hERG channel involvement in drug-associated arrhythmia is so strong that we need to pay more attention to the biological characteristics of the hERG channel itself to explain and understand the high affinity of drugs for COVID-19 for the hERG channel (Butler et al., 2019). An understanding of the molecular basis for hERG channel interactions with drugs is required to produce safer drugs for the treatment of diseases such as COVID-19.
2. The hERG channel
2.1. The significance of the hERG channel
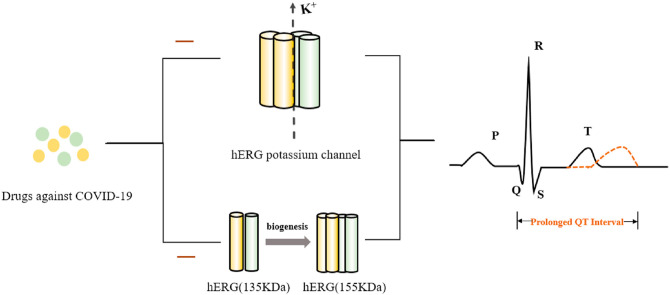
The hERG channel is also known as the Kv11.1 channel or ERG1 potassium channel. The hERG gene encodes the pore-forming alpha subunit of a fast component of the delayed rectifier potassium channel (IKr), which plays a fundamental role in the three-phase repolarization of the ventricular action potential, i.e., the repolarization of cardiac myocytes (Vandenberg et al., 2012). hERG channel dysfunction contributes to a partial or complete reduction in the IKr current, resulting in prolonged action potential duration (APD) (Smith et al., 2016), manifested as a prolonged QT interval on an ECG (Fig. 1 B), and if the extension exceeds the normal range (440 ms in men, 460 ms in women), LQTS results (Schwartz and Ackerman, 2013; van Noord et al., 2010). Clinically, LQTS caused by hERG deficiency is called type 2 LQTS (LQT2), which is the second most common subtype of LQTS (Kapplinger et al., 2009).
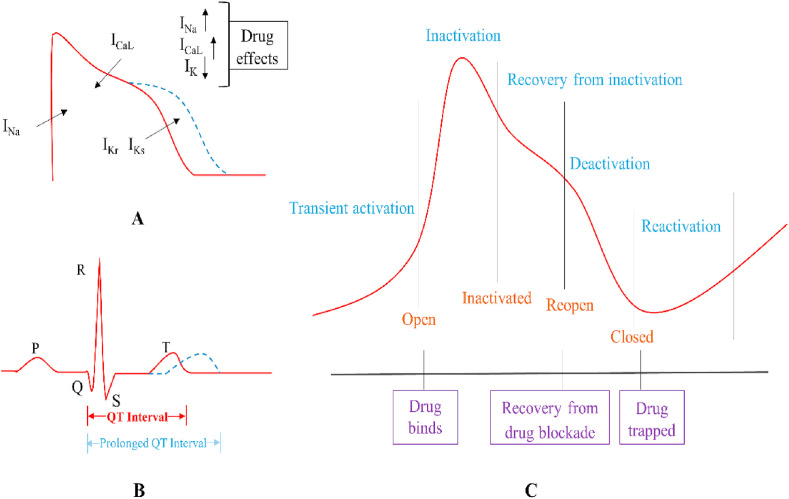
Fig. 1.
A prolonged action potential duration (APD) by increased inward current and weakened outward currents induced by drugs (A) and manifested on an electrocardiogram (ECG) (B). The hERG channel undergoes a transient activation process of channel opening, followed by rapid inactivation and then recovery from inactivation. At this time, the channel opens again, followed by a slow deactivation process to close the channel. The opening of the channel is necessary for drug binding, and when the channel is closed, the drug is trapped due to the blocking effect and is released when the channel reopens (C).
The electrical activity of the heart is mediated by the regulation of the channels in which ions flow into and out of cardiomyocytes. Theoretically, ion currents that constitute the ventricular action potential include inward and outward currents, and the balance of outward and inward currents is the key to the formation of normal APD. Na+ and L-type Ca2+ currents (INa and ICaL, respectively) are the most important inward currents in cardiomyocytes, and the K+ current (IK) is the major outward current. Increased inward current or a weakening of outward currents due to drug effects can result in a prolongation of APD, leading to a prolonged QT interval (Fig. 1A) (van Noord et al., 2010). Excessive prolongation can facilitate the production of early after depolarization (EAD) in three-phase repolarization that will trigger TdP and even ventricular fibrillation if EAD reaches its threshold (Albert and Schuller, 2014).
A decrease in outward potassium currents causes a longer repolarization time due to the blocking of the hERG channel, the effect of which is consistent with strengthening sodium or calcium currents. Therefore, the effect of drug inhibition on the hERG channel appears to be corrected by drugs that block INa and ICaL. A classic example is amiodarone, a widely used class III antiarrhythmic drug that inhibits multiple ion channels to balance the blocking of IKr; therefore, amiodarone leads to rare malignant arrhythmic events generated by potassium channel blockage. However, cardiac and non-cardiac QT-prolonging drugs often easily target the hERG channel but do not involve in other ion channels to cause arrhythmias. Thus, drugs against COVID-19 with single-ion-channel effects that act on the hERG channel are inevitably potentially arrhythmogenic. Many studies have focused on revealing the biological characteristics of hERG channels to explain possible mechanisms of drug action that involve various drug effects not only on the structure but also on the gating kinetics of these channels.
2.2. hERG channel structure and gating kinetics
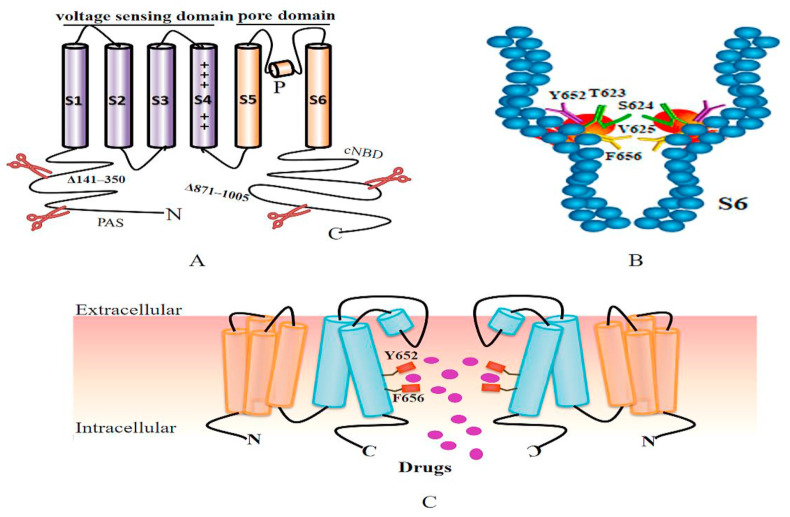
The hERG channel shares structural homology with other voltage-gated potassium channels (Whicher and MacKinnon, 2016). The hERG channel is a tetramer, and four hERG subunits are arranged in a ring-shaped manner rather linearly arranged on the membrane (Vandenberg et al., 2012). Each subunit has six transmembrane fragments (S1–S6), an N-terminal Per-Arnt-Sim (PAS) domain, and a C-terminal cyclic nucleotide-binding domain (cNBD) (Barros et al., 2020; Shi et al., 2020; Warmke and Ganetzky, 1994). S1–S4 function as a voltage-sensing domain (VSD), and S5–S6 coupled with the intermediate pore loops of the four subunits constitutes the ion permeation pore-gate domain (PD) (Fig. 2 A) (Barros et al., 2020; Wang et al., 2011). Recently, a new hERG channel structure obtained from cryo-electron microscopy (cryo-EM), named hERGT, was discovered, with deletions in most of the expected unstructured cytoplasmic regions (Δ141–350 at the N-terminus and Δ871–1005 at the C-terminus) for preventing structural aggregation (Fig. 2A) (Wang and MacKinnon, 2017). hERGT, with a resolution of up to 3.8 Å, retains gating properties similar to those of full-length channels because it does not change the major function of the hERG channel already recognized. hERGT provides new insights into the molecular basis of hERG channel blockade by high-affinity drugs, the binding of hERG channel activators, and unique hERG channel gating kinetics by demonstrating the importance of the special structure of hERG channels for the binding of compounds (Butler et al., 2019; Vandenberg et al., 2017).
Fig. 2.
hERGT structure and key drug-binding sites. The cryo-EM structure of hERGT (i.e., the hERG channel with deletions in cytoplasmic regions 141–350 at the N-terminus and 871–1005 at the C-terminus) (A). The positions of key amino acids of drug-binding sites in S6 obtained from hERGT are highlighted and arranged on the surface of hydrophobic pouches protruding from the central cavity (B). Four hERG channel subunits are coupled on the cell membrane to form a pore region (only two are shown) where the drug enters when the channel opens and binds to key drug-binding residues, including Y652 and F656 (C).
The blockade of the hERG channel by drugs is associated with hERG channel conformational change. In the early stage of the action potential, the hERG channel slowly opens but rapidly inactivates. As the depolarization voltage continues to decrease, the hERG channel gradually recovers from the inactivation (Witchel et al., 2001). Due to recovery from inactivation, hERG currents improve during repolarization followed by a much slower deactivation (Barros et al., 2012; Vandenberg et al., 2017). The altering rate of inactivation and deactivation reflects the potential potency of drug blockade. Similarly, drugs show different affinities for the channel in its different conformational states (Fig. 1C). More recently, a selectivity filter at the hERG channel was reported as a pharmacological master mechanism to open potassium channels, offering a substantial boost in the design of channel activators to treat aLQTS or diLQTS (Schewe et al., 2019).
3. The pharmacology of the hERG channel
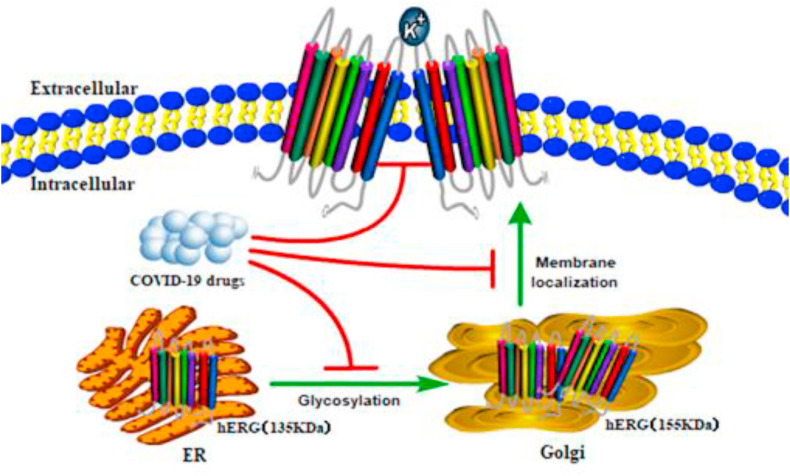
IKr is the target of class III antiarrhythmic drugs, such as dofetilide, amiodarone, and D-sotalol. These drugs and non-cardiac drugs, such as those for COVID-19, produce voltage- and dose-dependent hERG channel blockade due to direct blocking of ion pathways once the drugs enter cells (van Noord et al., 2010; Wang and MacKinnon, 2017). In addition to the direct blocking effect, the disruption of the biogenesis of the hERG channel is also a significant reason for the decrease in the IKr current (Fig. 3 ).
Fig. 3.
Mechanisms of COVID-19 drugs blocking the hERG channel in cells. After COVID-19 drugs enter a cell, in addition to producing direct channel-blocking effect, they also interfere with the glycosylation process (135 kDa–155 kDa), which affects channel maturation from the endoplasmic reticulum (ER) to the Golgi and thus hinders the membrane localization of mature proteins.
3.1. Structural basis of drug binding
Many studies have reported the importance of multiple aromatic residues for drug blockade based on differences in the homology among Kv channels via the mutagenesis of individual residues in the S6 helix (Han et al., 2011, 2015; Jie et al., 2017; Rajamani et al., 2006; Sănchez-Chapula et al., 2003; Sánchez-Chapula et al., 2002; Takemasa et al., 2008). Among them, two aromatic residues of S6, Tyr652 and Phe 656, are particularly significant (Fig. 2C). Mutations at these residues can attenuate the drug blocking effect, confirming their high affinity for drug binding. For example, when used in the treatment of COVID-19, CQ-generated channel blockade can be restored by mutation at Tyr652, showing that these key sites participate in channel blockade (Sánchez-Chapula et al., 2002). It is believed that the Y652A and F656A mutations decrease the inhibitory effect by 17 times and 75 times, respectively (Helliwell et al., 2018). In addition to the two key residues above, other residues at the bottom of the pore helix (e.g., Thr623, Ser 624, Val 625 and Phe 557 in the S5 helix) also contribute to drug binding equally as potently as Y652 (Mitcheson et al., 2000; Saxena et al., 2016).
The structure of hERGT retains all the functions essential for drug binding, which illustrates why these special amino acids of drug binding are so important (Wang and MacKinnon, 2017). The positions of these amino acids in the sequence and the molecular structure are highlighted, and they are arranged on the surface of elongated, relatively hydrophobic pouches protruding from the central cavity (Fig. 2B) (Wang and MacKinnon, 2017). These pockets provide potential interaction sites for hERG blockers (Butler et al., 2019). Furthermore, the narrow cavity leads to a more negative electrostatic potential, making it easy for positively charged drugs to bind (Wang and MacKinnon, 2017). To elucidate the chemical basis of drug binding, higher resolution structures will be needed in combination with molecular dynamics simulations. These simulations could accurately identify where and how drugs bind to the hERG channel and why they bind differently to distinct conformational states of the channel.
3.2. The disruption of hERG channel trafficking
As indicated earlier, drugs binding to the pore region of the hERG channel exert a direct blocking effect that disrupts the conduction of ions through the pore. Many different-structured drug groups targeting the hERG channel cause QT prolongation by disrupting the trafficking of protein (Dennis et al., 2007; Kuryshev et al., 2005; Nogawa and Kawai, 2014). These drugs include arsenic (Ficker et al., 2004), pentamidine (Kuryshev et al., 2005), fluconazole (Han et al., 2011) ketoconazole (Takemasa et al., 2008), fluoxetine (Hancox and Mitcheson, 2006), cardiac glycosides (Wang et al., 2007), rosuvastatin (Feng et al., 2019), thioridazine (Liu et al., 2020) and so on. Arsenic trioxide (As2O3) is the first example of a drug that produces hERG channel liability through the inhibition of channel protein trafficking by disrupting hERG-chaperone complexes to reveal a new significant mechanism in decreased IKr current (Ficker et al., 2004). Up to 40% of all hERG channel blockers exert combined hERG channel blockade and trafficking inhibition (Dennis et al., 2011). As an increasing number of drugs target the hERG channel, more drug studies have confirmed that the above two mechanisms can work together and are probably mediated by different mechanisms (Han et al., 2011; Hancox and Mitcheson, 2006; Rajamani et al., 2006; Takemasa et al., 2008), making the interfering effect on the hERG channel more complicated.
In general, numerous drugs reduce the generation of the mature form of the hERG channel (155 kDa) by disrupting the forward trafficking of the hERG protein from the endoplasmic reticulum (ER) to the Golgi (Fig. 3). This process is associated with molecular chaperones that help in protein folding and assembly. For instance, the inhibition of the chaperone protein Hsp 90 prevents maturation and promotes the proteasome degradation of hERG protein, thereby reducing the number of mature channels that can be integrated into the cell membrane (Cubeddu, 2016). Different maturation of the hERG channel protein can be estimated by comparing the expression levels of the two forms of this protein using Western blot. There is currently no definitive, relevant evidence to support the use of drugs that interfere with the maturation of the hERG protein in the treatment of COVID-19. The safe application of these drugs may require detailed information about blocking the intracellular mechanisms of the hERG protein.
3.3. Other mechanisms
Another issue worth considering is that many drugs may alter drug metabolism, which may further increase the plasma levels of drugs that extend the QT interval. Pharmacokinetic interactions usually involve agents metabolized by cytochrome P450 enzymes (van Noord et al., 2010). P450 (CYP) 3 A is the subfamily with the highest expression and includes the isoforms CYP3A4, CYP3A5, CYP3A7, and CYP3A43 (Eichelbaum and Burk, 2001). CYP3A4 is the isoform expressed in the liver and intestine (Eichelbaum and Burk, 2001), and it can oxidize a variety of drugs through many metabolic processes for detoxification (Dresser et al., 2000), and CYP3A4 is responsible for approximately 60% of the metabolism of currently known drugs (Feng et al., 2018; Zhou et al., 2005). Clinically important CYP3A4 inhibitors include antifungals (e.g., itraconazole and ketoconazole), macrolides (e.g., clarithromycin and erythromycin), antihypertensives (e.g., dihydralazine, verapamil, and diltiazem), and anti-HIV drugs (e.g., ritonavir and delavirdine) (Dresser et al., 2000; Zhou et al., 2005). These inhibitors can boost the plasma concentration of themselves or other drugs that directly act on the hERG channel and enhance cardiotoxicity (Feng et al., 2018; Zhi et al., 2015). Among these drugs, ritonavir in lopinavir/ritonavir, which has been shown to be effective for the treatment of COVID-19 as a strong CYP3A4 inhibitor, can increase the oral bioavailability of certain HIV protease inhibitors, such as lopinavir (Dresser et al., 2000).
4. Drugs used in COVID-19
According to the guidelines, there are no safe and effective drugs to kill SARS-CoV-2. The validity of the drugs currently in clinical use, including CQ, HCQ, azithromycin, lopinavir/ritonavir, etc. (Table 1 ) was obtained from small clinical trials. Most of these studies have a relatively small sample size, and some of them have opposite results. Therefore, when these drugs are prescribed, both the results of current trials and the patient's condition, as well as possible serious adverse reactions, should be considered, and careful assessments and choices should be made until a comprehensive and reliable clinical trial is completed.
Table 1.
Potential therapeutic drugs for COVID-19 and their adverse effect of QT prolongation.
| Drug name | Effects on COVID-19 | Assessments of the QT interval | Side effects on the hERG channel | References |
|---|---|---|---|---|
| CQ HCQ |
Reduced viral load and the inhibition of SARS-CoV-2 in vitro. | QT prolongation resulting in TdP. | Blocked IKr current. Slowed rate of deactivation and increased transport of the hERG protein by CQ. | (Borsini et al., 2012; Gautret et al., 2020; Saleh et al., 2020; Sánchez-Chapula et al., 2001, 2002; Yao et al., 2020) |
| Azithromycin | Enhanced HCQ potency of viral elimination. | QT prolongation and increased risk of TdP in combination with CQ or hydroxychloroquine. | Blocked IKr current under high plasma concentration. No evidence of interference with hERG trafficking. |
(Chorin et al., 2020; Gautret et al., 2020; Juurlink, 2020; Yang et al., 2017; Zhang et al., 2017) |
| Lopinavir/ritonavir | Reduced length of hospital stay for severe patients. | Potential QT prolongation. | hERG channel blockade. No evidence of interference with hERG trafficking. |
(Anson et al., 2005; Cao et al., 2020a) |
4.1. CQ and HCQ
CQ and HCQ are quinoline antimalarial drugs, among which CQ is the most widely used antimalarial drug in history (Haeusler et al., 2018). In the treatment of COVID-19, CQ and HCQ have attracted a great deal of interest. An open-label, non-randomized study involving the application of HCQ (combined with azithromycin in some patients) showed that HCQ treatment is significantly associated with reduced/disappeared viral load in COVID-19 patients (Gautret et al., 2020). A small, prospective, observational study also suggested that CQ/CQ ± azithromycin may be effective in controlling SARS-CoV-2 infection, but the use of these drugs alone or in combination may prolong the QT interval and therefore increase the incidence of TdP (Saleh et al., 2020). An in vitro study also found that CQ and HCQ were effective in controlling SARS-CoV-2 infection (Wang et al., 2020), and HCQ was more effective than CQ in inhibiting SARS-CoV-2 (Yao et al., 2020).
There are now more than 100 clinical trials aimed at testing CQ or HCQ in the treatment of COVID-19, and CQ and HCQ have even been used as standard treatments for COVID-19 patients in hospitals in some countries, such as China (Ledford, 2020). Unfortunately, CQ publicity is derailing the search for coronavirus treatments, even leading to difficulty in treating patients with autoimmune diseases, which can result in potentially life-threatening manifestations, such as lupus nephritis (Jakhar and Kaur, 2020; Ledford, 2020; Yazdany and Kim, 2020). Despite this situation, the data supporting the treatment of COVID-19 with CQ or HCQ are limited (Ledford, 2020; Moore, 2020; Yazdany and Kim, 2020). And as mentioned earlier, a large RCT showed that COVID-19 patients receiving HCQ treatment did not benefit, or even be harmful (Horby et al., 2020). The NIH noted that there are insufficient data to recommend or oppose the use of CQ and HCQ for COVID-19 populations, and in COVID-19 patients receiving HCQ or azithromycin, 11%–25% of them have excessively prolonged QT intervals (i.e., greater than 500 ms) (Chorin et al., 2020; Panel, 2020). Therefore, better RCTs of CQ or HCQ are needed to effectively treat COVID-19 (Ferner and Aronson, 2020).
It is well known that antimalarial drugs are cardiotoxic (White, 2007), and QT prolongation is the most common adverse reaction to antimalarial drugs (Llanos-Cuentas et al., 2014). Although small doses of CQ and HCQ are generally safe, both can block the hERG channel (Giudicessi et al., 2020; Naksuk et al., 2020). Long-term use of CQ and HCQ has been reported to induce QT prolongation and malignant arrhythmia (Chen et al., 2006; Stas et al., 2008), and CQ-induced TdP in a COVID-19 patient was also reported (Szekely et al., 2020). Antimalarial drugs likely block the hERG channel in heterologous expression models and animal models (Sánchez-Chapula et al., 2001; Traebert et al., 2004). In feline ventricular cardiomyocytes, CQ blocked several inward and outward membrane currents, and the order of potency is inwardly rectifying potassium current (IK1)> IKr > INa > ICaL (Sánchez-Chapula et al., 2001). In contrast, CQ significantly slowed the rate of hERG channel deactivation, reflecting the inability of drug-bound channels to close (Sánchez-Chapula et al., 2002). Furthermore, as reported by hERG-lite (Wible et al., 2005), a novel systematic high-throughput screen for diLQTS risk, CQ increases hERG protein transport (Borsini et al., 2012). These different results indicate that a more sophisticated intracellular view to illuminate hERG channel inhibition induced by CQ is required. In addition, both CQ and HCQ are metabolized by CYP3A4, and the risk of QT prolongation might increase if CQ and/or HCQ are combined with CYP3A4 inhibitors such as ritonavir/lopinavir or azithromycin (Naksuk et al., 2020, Wu et al., 2020).
4.2. Azithromycin
Azithromycin, as a macrolide antibiotic, is thought to enhance the therapeutic effect of HCQ in COVID-19 patients (Gautret et al., 2020). Similarly, a combination of azithromycin and CQ/HCQ was shown to be helpful for COVID-19 patients, and there were no reports of death from fatal arrhythmia (Saleh et al., 2020). However, given the cardiotoxicity of antimalarial drugs themselves, whether azithromycin when combined with them increases adverse reactions and whether we should use these drugs alone or in combination to treat COVID-19 is worth considering. As previously mentioned in a cohort study, the effect of azithromycin combined with CQ/HCQ on prolonging the QT interval is more obvious (Mercuro et al., 2020), and approximately one-quarter of the QT interval is prolonged excessively (Chorin et al., 2020). Perhaps azithromycin itself does not usually cause a clinically significant prolongation of the QT interval (Thomsen et al., 2006), but its use in combination with CQ or HCQ may theoretically increase the risk of TdP (Juurlink, 2020). These conflicting and poor-quality studies suggest that clinicians should carefully weigh the risks and benefits of azithromycin, CQ and HCQ, and it may be advisable to avoid these drugs.
Among macrolides, azithromycin is considered to be the least likely to cause arrhythmia because it is the least cardiotoxic, with an estimated 47 added cardiovascular deaths per million courses according to a report (Ray et al., 2012). Indeed, studies have confirmed that the rank order of arrhythmogenicity is estimated to be erythromycin > clarithromycin > roxithromycin > azithromycin (Milberg et al., 2002; Ohtani et al., 2000). Specifically, azithromycin suppresses the IKr current only at 50 times the clinically related concentration (2075 mg/L), and the inhibition rate is approximately 30% (Zhang et al., 2017). Unsurprisingly, compared with dofetilide, which has been proven to be an hERG channel blocker, azithromycin has no electrophysiological effects; thus, azithromycin is safe (Avedissian et al., 2019; Thomsen et al., 2006). However, based on the evidence of macrolides (such as erythromycin) targeting hERG channels (Volberg et al., 2002), it is necessary to further investigate the interaction of azithromycin with the hERG channel, even though azithromycin mainly increases cardiac Na+ current and only slightly blocks IKr (Yang et al., 2017). Moreover, azithromycin, as a weaker CYP3A4 inhibitor than homologous antibiotics, may increase the risk of QT prolongation when used in combination with antimalarial drugs in treating COVID-19 patients (Wu et al., 2020). This seems to explain why azithromycin in combination with CQ or HCQ has a more significant QT prolongation effect.
4.3. Lopinavir/ritonavir
The HIV protease inhibitor class of antiretroviral drugs has obvious benefits for HIV. As an important CYP3A4 inhibitor, ritonavir can enhance the effect of other protease inhibitors, such as lopinavir and atazanavir (Soliman et al., 2011; Zhou et al., 2005). The combined preparation of lopinavir/ritonavir has aroused widespread interest after a clinical trial for its use in COVID-19 was conducted (Cao et al., 2020a). The randomized trial found that for severe COVID-19 patients, lopinavir/ritonavir (400 mg and 100 mg, respectively) treatment does not significantly promote clinical improvement, reduce mortality or reduce the detectability of throat RNA of SARS-CoV-2, but it is beneficial for some secondary outcomes (e.g., a shorter time to stay in the intensive care unit (ICU)) (Cao et al., 2020a; Stower, 2020). Subsequently, although researchers advocated that lopinavir/ritonavir can be used as an alternative treatment for COVID-19 before the completion of the World Health Organization SOLIDARITY trial (Cao et al., 2020b), the side effects, including QT prolongation, have raised concerns about the higher dose or longer treatment of this programme. It is uncertain whether lopinavir/ritonavir and other antiretroviral drugs can ameliorate clinical outcomes or prevent infection in patients with high-risk COVID-19 (Ford et al., 2020). More recently, it has been reported that a triple combination of interferon beta-1b, lopinavir/ritonavir, and ribavirin is safer than and superior to lopinavir/ritonavir alone in alleviating symptoms in patients with mild to moderate COVID-19 (Hung et al., 2020). However, the same question regarding how to balance the risks and benefits remains.
The evidence for HIV protease inhibitor-induced arrhythmia is sufficient (Anson et al., 2005; Cao et al., 2020a; Gallagher et al., 2008; Han et al., 2015; Kikuchi et al., 2002; Soliman et al., 2011; Vicente et al., 2019). In vitro, lopinavir, nelfinavir, ritonavir, and saquinavir caused dose-dependent hERG channel blockade (Anson et al., 2005). A RCT showed that treatment with ritonavir-enhanced protease inhibitors and non-enhanced regimens had similar effects on QT duration (Soliman et al., 2011), suggesting that combined treatment regimens may be more beneficial with fewer side effects, and for QT prolongation, the role of ritonavir as an enhancer might not be as important. In particular, 100 mg ritonavir did not cause QT prolongation in healthy subjects (Sarapa et al., 2008); therefore, as a CYP3A4 inhibitor, it may generate side effects only when it increases the blood concentration of related drugs.
Except for atazanavir, there is no relevant evidence to support whether other protease inhibitors affect hERG protein maturation and expression on the plasma membrane (Han et al., 2015). It has been confirmed that HIV protease inhibitors induce ER stress (ERS) in intestinal epithelial cells (Wu et al., 2010), and importantly, ERS can downregulate cardiac ion channel expression (Liu et al., 2018). Referring to the effect of rosuvastatin on the hERG channel (Feng et al., 2019), ERS may play an important role in diLQTS. Whether the hERG channel is affected by COVID-19-related drugs through ERS requires further verification.
5. Future directions
Various factors can affect the hERG channel to induce QT prolongation, and QT prolongation may also be the result of multiple ion channel actions. In the course of COVID-19, in addition to affecting the lungs, causing interstitial pneumonia and severe acute respiratory distress syndrome (ARDS), SARS-CoV-2 also damages multiple organs due to a massive increase in inflammatory factors, especially in the cardiovascular system, leading to a variety of cardiac problems such as arrhythmia and myocarditis (Guzik et al., 2020; Inciardi et al., 2020; Zheng et al., 2020). In such cases, the cardiotoxicity of relevant therapeutic drugs may be obscured by the disease itself; therefore, it is necessary to conduct comprehensive clinical management to consider whether to use these drugs, and if necessary, to monitor ECGs. Most drug interactions with the hERG channel induce a prolonged QT interval, which is considered a major risk factor in pharmaceutical drug development, and in addition to the hERG channel, drugs actions on many ion channels causing severe arrhythmias have become a limiting factor for their clinical use (Denning et al., 2016). Therefore, it may be necessary to determine the cardiotoxicity of a drug before it enters clinical use in the treatment of COVID-19, which requires a sensitive and effective experimental platform to carry out rigorous in vitro experiments as well as tremendous effort for preclinical management.
In 2013, the U.S. FDA proposed an international initiative termed the Comprehensive In Vitro Proarrhythmia Assay (CiPA), which recommends that a multi-electrode array (MEA) be used as a measurement tool, combined with human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs), to conduct a preclinical assessment of a drug to evaluate the risk of TdP. The CiPA initiative requires a comprehensive ion current effect, not just IKr, to evaluate preclinically drug safety, and hiPSC-CMs will become a new chapter in safety assessment guidelines (Denning et al., 2016). Compared with heterologous systems and animal models in research on cLQTS or diLQTS, hiPSC-CMs, as an autologous source of reprogrammed cells, show a major advantage. Although still immature (Veerman et al., 2015), hiPSC-CMs almost completely reproduce the phenotype of cardiomyocytes in vitro because they contain multiple ion channels that participate in the action potentials of cardiomyocytes (Ma et al., 2011). The results of hiPSC-CMs for proarrhythmia prediction under CiPA would serve as predictive indicators for the ion channel and in silico modelling prediction of proarrhythmic risk (Blinova et al., 2017). More importantly, individual-derived somatic cells with gene mutations can also be reprogrammed to differentiate into disease-specific cardiomyocytes, such as LQTS-hiPSC-CMs (Egashira et al., 2012; Itzhaki et al., 2011; Portero et al., 2017; Sala et al., 2019; Wuriyanghai et al., 2018) and Brugada syndrome (BrS)-hiPSC-CMs (Belbachir et al., 2019; Kosmidis et al., 2016), from LQTS and BrS patients, respectively, which has considerable significance for the study of gene-drug interactions. An MEA is a high-throughput screening tool for cellular electrical activity, whose measured field potential duration (FPD) reflects the QT interval and, to some extent, the activities of various ion channels (Nozaki et al., 2014).
With the combination of hiPSC-CMs and MEAs, many drugs can be screened sensitively and efficiently (Nozaki et al., 2014), with great potential and advantages in reducing the cost of drug development compared with the use of immortalized cell lines or animal models. Furthermore, disease-specific hiPSC-CMs can be used to detect effective treatments in vitro that can be effectively translated into clinical trials (Mehta et al., 2018; Schwartz et al., 2019). In the future, related work should focus more on systemizing and standardizing hiPSC-CMs/MEA applications for comprehensive preclinical drug safety screening and generating systematic, large-scale, and available drug safety data to further guide clinical practice. For drugs that must be used but are cardiotoxic, inducing QT prolongation, it is still necessary to further clarify the molecular mechanism behind possible rescue strategies. LUF7346, as an hERG channel allosteric modulator, was recently shown to reverse congenital and drug-induced hERG channel blockade in hiPSC-CMs and heterologous expression models through binding to sites in the channel that are different from traditional drug-binding sites to induce a conformational change in the channel (Sala et al., 2016). However, an excessively shortened QT interval caused by related rescue strategies is also a problem worthy of attention. In summary, the goal of precision medicine may be truly achieved through clinical translation studies only after a comprehensive assessment of risks and benefits as well as a deeper understanding of underlying mechanisms.
CRediT authorship contribution statement
Zheng Zequn: Conceptualization, Data curation, Writing - original draft, Software, Resources. Wu Yujia: Conceptualization, Data curation, Writing - original draft, Software, Resources. Qian Dingding: Investigation, Supervision. Lian Jiangfang: Conceptualization, Writing - review & editing.
Declaration of competing interest
None.
References
- Albert R.K., Schuller J.L. Macrolide antibiotics and the risk of cardiac arrhythmias. Am. J. Respir. Crit. Care Med. 2014;189:1173–1180. doi: 10.1164/rccm.201402-0385CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anson B.D., Weaver J.G.R., Ackerman M.J., Akinsete O., Henry K., January C.T., Badley A.D. Blockade of HERG channels by HIV protease inhibitors. Lancet. 2005;365:682–686. doi: 10.1016/S0140-6736(05)17950-1. [DOI] [PubMed] [Google Scholar]
- Avedissian S.N., Rhodes N.J., Ng T.M.H., Rao A.P., Beringer P.M. The potential for QT interval prolongation with chronic azithromycin therapy in adult cystic fibrosis patients. Pharmacotherapy. 2019;39:718–723. doi: 10.1002/phar.2270. [DOI] [PubMed] [Google Scholar]
- Barros F., de la Peña P., Domínguez P., Sierra L.M., Pardo L.A. The EAG voltage-dependent K+ channel subfamily: similarities and differences in structural organization and gating. Front. Pharmacol. 2020;11:411. doi: 10.3389/fphar.2020.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros F., Domínguez P., de la Peña P. Cytoplasmic domains and voltage-dependent potassium channel gating. Front. Pharmacol. 2012;3:49. doi: 10.3389/fphar.2012.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belbachir N., Portero V., Al Sayed Z.R., Gourraud J.B., Dilasser F., Jesel L., Guo H., Wu H., Gaborit N., Guilluy C., Girardeau A., Bonnaud S., Simonet F., Karakachoff M., Pattier S., Scott C., Burel S., Marionneau C., Chariau C., Gaignerie A., David L., Genin E., Deleuze J.F., Dina C., Sauzeau V., Loirand G., Baro I., Schott J.J., Probst V., Wu J.C., Redon R., Charpentier F., Le Scouarnec S. RRAD mutation causes electrical and cytoskeletal defects in cardiomyocytes derived from a familial case of Brugada syndrome. Eur. Heart J. 2019;40:3081–3094. doi: 10.1093/eurheartj/ehz308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinova K., Stohlman J., Vicente J., Chan D., Johannesen L., Hortigon-Vinagre M.P., Zamora V., Smith G., Crumb W.J., Pang L., Lyn-Cook B., Ross J., Brock M., Chvatal S., Millard D., Galeotti L., Stockbridge N., Strauss D.G. Comprehensive translational assessment of human-induced pluripotent stem cell derived cardiomyocytes for evaluating drug-induced arrhythmias. Toxicol. Sci. : Off. J. Soc. Toxicol. 2017;155:234–247. doi: 10.1093/toxsci/kfw200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsini F., Crumb W., Pace S., Ubben D., Wible B., Yan G.X., Funck-Brentano C. In vitro cardiovascular effects of dihydroartemisin-piperaquine combination compared with other antimalarials. Antimicrob. Agents Chemother. 2012;56:3261–3270. doi: 10.1128/aac.05688-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A., Helliwell M.V., Zhang Y., Hancox J.C., Dempsey C.E. An update on the structure of hERG. Front. Pharmacol. 2019;10:1572. doi: 10.3389/fphar.2019.01572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li H., Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li H., Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang J., Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Zhang D., Wang C. A trial of lopinavir-ritonavir in covid-19. Reply. N. Engl. J. Med. 2020;382 doi: 10.1056/NEJMc2008043. [DOI] [PubMed] [Google Scholar]
- Chen C.Y., Wang F.L., Lin C.C. Chronic hydroxychloroquine use associated with QT prolongation and refractory ventricular arrhythmia. Clin. Toxicol. 2006;44:173–175. doi: 10.1080/15563650500514558. Philadelphia, Pa. [DOI] [PubMed] [Google Scholar]
- Chorin E., Dai M., Shulman E., Wadhwani L., Bar-Cohen R., Barbhaiya C., Aizer A., Holmes D., Bernstein S., Spinelli M., Park D.S., Chinitz L.A., Jankelson L. The QT interval in patients with COVID-19 treated with hydroxychloroquine and azithromycin. Nat. Med. 2020;26:808–809. doi: 10.1038/s41591-020-0888-2. [DOI] [PubMed] [Google Scholar]
- Cubeddu L.X. Drug-induced inhibition and trafficking disruption of ion channels: pathogenesis of QT abnormalities and drug-induced fatal arrhythmias. Curr. Cardiol. Rev. 2016;12:141–154. doi: 10.2174/1573403x12666160301120217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning C., Borgdorff V., Crutchley J., Firth K.S., George V., Kalra S., Kondrashov A., Hoang M.D., Mosqueira D., Patel A., Prodanov L., Rajamohan D., Skarnes W.C., Smith J.G., Young L.E. Cardiomyocytes from human pluripotent stem cells: from laboratory curiosity to industrial biomedical platform. Biochim. Biophys. Acta. 2016;1863:1728–1748. doi: 10.1016/j.bbamcr.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis A., Wang L., Wan X., Ficker E. hERG channel trafficking: novel targets in drug-induced long QT syndrome. Biochem. Soc. Trans. 2007;35:1060–1063. doi: 10.1042/BST0351060. [DOI] [PubMed] [Google Scholar]
- Dennis A.T., Nassal D., Deschenes I., Thomas D., Ficker E. Antidepressant-induced ubiquitination and degradation of the cardiac potassium channel hERG. J. Biol. Chem. 2011;286:34413–34425. doi: 10.1074/jbc.M111.254367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresser G.K., Spence J.D., Bailey D.G. Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin. Pharmacokinet. 2000;38:41–57. doi: 10.2165/00003088-200038010-00003. [DOI] [PubMed] [Google Scholar]
- Egashira T., Yuasa S., Suzuki T., Aizawa Y., Yamakawa H., Matsuhashi T., Ohno Y., Tohyama S., Okata S., Seki T., Kuroda Y., Yae K., Hashimoto H., Tanaka T., Hattori F., Sato T., Miyoshi S., Takatsuki S., Murata M., Kurokawa J., Furukawa T., Makita N., Aiba T., Shimizu W., Horie M., Kamiya K., Kodama I., Ogawa S., Fukuda K. Disease characterization using LQTS-specific induced pluripotent stem cells. Cardiovasc. Res. 2012;95:419–429. doi: 10.1093/cvr/cvs206. [DOI] [PubMed] [Google Scholar]
- Eichelbaum M., Burk O. CYP3A genetics in drug metabolism. Nat. Med. 2001;7:285–287. doi: 10.1038/85417Dresser. [DOI] [PubMed] [Google Scholar]
- Feng P., Zhao L., Guo F., Zhang B., Fang L., Zhan G., Xu X., Fang Q., Liang Z., Li B. The enhancement of cardiotoxicity that results from inhibiton of CYP 3A4 activity and hERG channel by berberine in combination with statins. Chem. Biol. Interact. 2018;293:115–123. doi: 10.1016/j.cbi.2018.07.022. [DOI] [PubMed] [Google Scholar]
- Feng P.F., Zhang B., Zhao L., Fang Q., Liu Y., Wang J.N., Xu X.Q., Xue H., Li Y., Yan C.C., Zhao X., Li B.X. Intracellular mechanism of rosuvastatin-induced decrease in mature hERG protein expression on membrane. Mol. Pharm. 2019;16:1477–1488. doi: 10.1021/acs.molpharmaceut.8b01102. [DOI] [PubMed] [Google Scholar]
- Ferner R.E., Aronson J.K. Chloroquine and hydroxychloroquine in covid-19. BMJ. 2020;369:m1432. doi: 10.1136/bmj.m1432. [DOI] [PubMed] [Google Scholar]
- Ficker E., Kuryshev Y.A., Dennis A.T., Obejero-Paz C., Wang L., Hawryluk P., Wible B.A., Brown A.M. Mechanisms of arsenic-induced prolongation of cardiac repolarization. Mol. Pharmacol. 2004;66:33–44. doi: 10.1124/mol.66.1.33. [DOI] [PubMed] [Google Scholar]
- Ford N., Vitoria M., Rangaraj A., Norris S.L., Calmy A., Doherty M. Systematic review of the efficacy and safety of antiretroviral drugs against SARS, MERS or COVID-19: initial assessment. J. Int. AIDS Soc. 2020;23 doi: 10.1002/jia2.25489. e25489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher D.P., Kieran J., Sheehan G., Lambert J., Mahon N., Mallon P.W. Ritonavir-boosted atazanavir, methadone, and ventricular tachycardia: 2 case reports. Clin. Infect. Dis. 2008;47:e36–38. doi: 10.1086/589869. [DOI] [PubMed] [Google Scholar]
- Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honoré S., Colson P., Chabrière E., La Scola B., Rolain J.-M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Giudicessi J.R., Noseworthy P.A., Friedman P.A., Ackerman M.J. Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19) Mayo Clin. Proc. 2020;95:1213–1221. doi: 10.1016/j.mayocp.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzik T.J., Mohiddin S.A., Dimarco A., Patel V., Savvatis K., Marelli-Berg F.M., Madhur M.S., Tomaszewski M., Maffia P., D'Acquisto F., Nicklin S.A., Marian A.J., Nosalski R., Murray E.C., Guzik B., Berry C., Touyz R.M., Kreutz R., Wang D.W., Bhella D., Sagliocco O., Crea F., Thomson E.C., McInnes I.B. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. 2020. Cardiovascular research. [DOI] [PMC free article] [PubMed]
- Haeusler I.L., Chan X.H.S., Guérin P.J., White N.J. The arrhythmogenic cardiotoxicity of the quinoline and structurally related antimalarial drugs: a systematic review. BMC Med. 2018;16:200. doi: 10.1186/s12916-018-1188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S.-n., Sun X.-y., Zhang Z., Zhang L.-r. The protease inhibitor atazanavir blocks hERG K+ channels expressed in HEK293 cells and obstructs hERG protein transport to cell membrane. Acta Pharmacol. Sin. 2015;36:454–462. doi: 10.1038/aps.2014.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Zhang Y., Chen Q., Duan Y., Zheng T., Hu X., Zhang Z., Zhang L. Fluconazole inhibits hERG K(+) channel by direct block and disruption of protein trafficking. Eur. J. Pharmacol. 2011;650:138–144. doi: 10.1016/j.ejphar.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Hancox J.C., Mitcheson J.S. Combined hERG channel inhibition and disruption of trafficking in drug-induced long QT syndrome by fluoxetine: a case-study in cardiac safety pharmacology. Br. J. Pharmacol. 2006;149:457–459. doi: 10.1038/sj.bjp.0706890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell M.V., Zhang Y., El Harchi A., Du C., Hancox J.C., Dempsey C.E. Structural implications of hERG K channel block by a high-affinity minimally structured blocker. J. Biol. Chem. 2018;293:7040–7057. doi: 10.1074/jbc.RA117.000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P., Mafham M., Linsell L., Bell J.L., Staplin N., Emberson J.R., Wiselka M., Ustianowski A., Elmahi E., Prudon B., Whitehouse T., Felton T., Williams J., Faccenda J., Underwood J., Baillie J.K., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Lim W.S., Montgomery A., Rowan K., Tarning J., Watson J.A., White N.J., Juszczak E., Haynes R., Landray M.J. Effect of hydroxychloroquine in hospitalized patients with covid-19. N. Engl. J. Med. 2020;383:2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung I.F., Lung K.C., Tso E.Y., Liu R., Chung T.W., Chu M.Y., Ng Y.Y., Lo J., Chan J., Tam A.R., Shum H.P., Chan V., Wu A.K., Sin K.M., Leung W.S., Law W.L., Lung D.C., Sin S., Yeung P., Yip C.C., Zhang R.R., Fung A.Y., Yan E.Y., Leung K.H., Ip J.D., Chu A.W., Chan W.M., Ng A.C., Lee R., Fung K., Yeung A., Wu T.C., Chan J.W., Yan W.W., Chan W.M., Chan J.F., Lie A.K., Tsang O.T., Cheng V.C., Que T.L., Lau C.S., Chan K.H., To K.K., Yuen K.Y. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/s0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inciardi R.M., Lupi L., Zaccone G., Italia L., Raffo M., Tomasoni D., Cani D.S., Cerini M., Farina D., Gavazzi E., Maroldi R., Adamo M., Ammirati E., Sinagra G., Lombardi C.M., Metra M. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhaki I., Maizels L., Huber I., Zwi-Dantsis L., Caspi O., Winterstern A., Feldman O., Gepstein A., Arbel G., Hammerman H., Boulos M., Gepstein L. Modelling the long QT syndrome with induced pluripotent stem cells. Nature. 2011;471:225–229. doi: 10.1038/nature09747. [DOI] [PubMed] [Google Scholar]
- Jakhar D., Kaur I. Potential of chloroquine and hydroxychloroquine to treat COVID-19 causes fears of shortages among people with systemic lupus erythematosus. Nat. Med. 2020;26:632. doi: 10.1038/s41591-020-0853-0. [DOI] [PubMed] [Google Scholar]
- Jan H., Faisal S., Khan A., Khan S., Usman H., Liaqat R., Shah S.A. COVID-19: review of epidemiology and potential treatments against 2019 novel coronavirus. Discoveries. 2020;8:e108. doi: 10.15190/d.2020.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jie L.-J., Wu W.-Y., Li G., Xiao G.-S., Zhang S., Li G.-R., Wang Y. Clemizole hydrochloride blocks cardiac potassium currents stably expressed in HEK 293 cells. Br. J. Pharmacol. 2017;174:254–266. doi: 10.1111/bph.13679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juurlink D.N. Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS-CoV-2 infection. CMAJ (Can. Med. Assoc. J.) 2020;192:E450–E453. doi: 10.1503/cmaj.200528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannankeril P., Roden D.M., Darbar D. Drug-induced long QT syndrome. Pharmacol. Rev. 2010;62:760–781. doi: 10.1124/pr.110.003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapplinger J.D., Tester D.J., Salisbury B.A., Carr J.L., Harris-Kerr C., Pollevick G.D., Wilde A.A., Ackerman M.J. Spectrum and prevalence of mutations from the first 2,500 consecutive unrelated patients referred for the FAMILION long QT syndrome genetic test. Heart Rhythm. 2009;6:1297–1303. doi: 10.1016/j.hrthm.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y., Genka I., Ishizaki A., Sunagawa K., Yasuoka A., Oka S. Serious bradyarrhythmia that was possibly induced by lopinavir-ritonavir in 2 patients with acquired immunodeficiency syndrome. Clin. Infect. Dis. 2002;35:488–490. doi: 10.1086/341975. [DOI] [PubMed] [Google Scholar]
- Kosmidis G., Veerman C.C., Casini S., Verkerk A.O., van de Pas S., Bellin M., Wilde A.A., Mummery C.L., Bezzina C.R. Readthrough-promoting drugs gentamicin and PTC124 fail to rescue Nav 1.5 function of human-induced pluripotent stem cell-derived cardiomyocytes carrying nonsense mutations in the sodium channel gene SCN5A. Circulation. Arrhythmia Electrophysiol. 2016:E4227. doi: 10.1161/circep.116.004227. [DOI] [PubMed] [Google Scholar]
- Kupferschmidt K., Cohen J. Race to find COVID-19 treatments accelerates. Science. 2020;367:1412–1413. doi: 10.1126/science.367.6485.1412. [DOI] [PubMed] [Google Scholar]
- Kuryshev Y.A., Ficker E., Wang L., Hawryluk P., Dennis A.T., Wible B.A., Brown A.M., Kang J., Chen X.-L., Sawamura K., Reynolds W., Rampe D. Pentamidine-induced long QT syndrome and block of hERG trafficking. J. Pharmacol. Exp. Therapeut. 2005;312:316–323. doi: 10.1124/jpet.104.073692. [DOI] [PubMed] [Google Scholar]
- Ledford H. Chloroquine hype is derailing the search for coronavirus treatments. Nature. 2020;580:573. doi: 10.1038/d41586-020-01165-3. [DOI] [PubMed] [Google Scholar]
- Liu M., Shi G., Zhou A., Rupert C.E., Coulombe K.L.K., Dudley S.C. Activation of the unfolded protein response downregulates cardiac ion channels in human induced pluripotent stem cell-derived cardiomyocytes. J. Mol. Cell. Cardiol. 2018;117:62–71. doi: 10.1016/j.yjmcc.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Xu X., Zhang Y., Li M., Guo J., Yan C., Wang F., Li Y., Ding Y., Li B., Fan P. Thioridazine induces cardiotoxicity via reactive oxygen species-mediated hERG channel deficiency and L-type calcium channel activation. Oxid Med Cell Longev. 2020;2020 doi: 10.1155/2020/3690123. 3690123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llanos-Cuentas A., Lacerda M.V., Rueangweerayut R., Krudsood S., Gupta S.K., Kochar S.K., Arthur P., Chuenchom N., Möhrle J.J., Duparc S., Ugwuegbulam C., Kleim J.-P., Carter N., Green J.A., Kellam L. Tafenoquine plus chloroquine for the treatment and relapse prevention of Plasmodium vivax malaria (DETECTIVE): a multicentre, double-blind, randomised, phase 2b dose-selection study. Lancet. 2014;383:1049–1058. doi: 10.1016/S0140-6736(13)62568-4. [DOI] [PubMed] [Google Scholar]
- Ma J., Guo L., Fiene S.J., Anson B.D., Thomson J.A., Kamp T.J., Kolaja K.L., Swanson B.J., January C.T. High purity human-induced pluripotent stem cell-derived cardiomyocytes: electrophysiological properties of action potentials and ionic currents. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H2006–H2017. doi: 10.1152/ajpheart.00694.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta A., Ramachandra C.J.A., Singh P., Chitre A., Lua C.H., Mura M., Crotti L., Wong P., Schwartz P.J., Gnecchi M., Shim W. Identification of a targeted and testable antiarrhythmic therapy for long-QT syndrome type 2 using a patient-specific cellular model. Eur. Heart J. 2018;39:1446–1455. doi: 10.1093/eurheartj/ehx394. [DOI] [PubMed] [Google Scholar]
- Mercuro N.J., Yen C.F., Shim D.J., Maher T.R., McCoy C.M., Zimetbaum P.J., Gold H.S. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1036–1041. doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milberg P., Eckardt L., Bruns H.J., Biertz J., Ramtin S., Reinsch N., Fleischer D., Kirchhof P., Fabritz L., Breithardt G., Haverkamp W. Divergent proarrhythmic potential of macrolide antibiotics despite similar QT prolongation: fast phase 3 repolarization prevents early afterdepolarizations and torsade de pointes. J. Pharmacol. Exp. Therapeut. 2002;303:218–225. doi: 10.1124/jpet.102.037911. [DOI] [PubMed] [Google Scholar]
- Mitcheson J.S., Chen J., Lin M., Culberson C., Sanguinetti M.C. A structural basis for drug-induced long QT syndrome. Proc. Natl. Acad. Sci. U.S.A. 2000;97:12329–12333. doi: 10.1073/pnas.210244497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mladěnka P., Applová L., Patočka J., Costa V.M., Remiao F., Pourová J., Mladěnka A., Karlíčková J., Jahodář L., Vopršalová M., Varner K.J., Štěrba M. Comprehensive review of cardiovascular toxicity of drugs and related agents. Med. Res. Rev. 2018;38:1332–1403. doi: 10.1002/med.21476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore N. Chloroquine for COVID-19 infection. Drug Saf. 2020;43:393–394. doi: 10.1007/s40264-020-00933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naksuk N., Lazar S., Peeraphatdit T.B. Cardiac safety of off-label COVID-19 drug therapy: a review and proposed monitoring protocol. Eur. Heart J. Acute Cardiovascular Care. 2020;9:215–221. doi: 10.1177/2048872620922784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogawa H., Kawai T. hERG trafficking inhibition in drug-induced lethal cardiac arrhythmia. Eur. J. Pharmacol. 2014;741:336–339. doi: 10.1016/j.ejphar.2014.06.044. [DOI] [PubMed] [Google Scholar]
- Nozaki Y., Honda Y., Tsujimoto S., Watanabe H., Kunimatsu T., Funabashi H. Availability of human induced pluripotent stem cell-derived cardiomyocytes in assessment of drug potential for QT prolongation. Toxicol. Appl. Pharmacol. 2014;278:72–77. doi: 10.1016/j.taap.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Ohtani H., Taninaka C., Hanada E., Kotaki H., Sato H., Sawada Y., Iga T. Comparative pharmacodynamic analysis of Q-T interval prolongation induced by the macrolides clarithromycin, roxithromycin, and azithromycin in rats. Antimicrob. Agents Chemother. 2000;44:2630–2637. doi: 10.1128/aac.44.10.2630-2637.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panel C.-T.G. Coronavirus diseases 2019 (COVID-19) treatment guidelines. 2020. https://www.covid19treatmentguidelines.nih.gov/ accessed June 20, 2020)
- Portero V., Casini S., Hoekstra M., Verkerk A.O., Mengarelli I., Belardinelli L., Rajamani S., Wilde A.A.M., Bezzina C.R., Veldkamp M.W., Remme C.A. Anti-arrhythmic potential of the late sodium current inhibitor GS-458967 in murine Scn5a-1798insD+/- and human SCN5A-1795insD+/- iPSC-derived cardiomyocytes. Cardiovasc. Res. 2017;113:829–838. doi: 10.1093/cvr/cvx077. [DOI] [PubMed] [Google Scholar]
- Rajamani S., Eckhardt L.L., Valdivia C.R., Klemens C.A., Gillman B.M., Anderson C.L., Holzem K.M., Delisle B.P., Anson B.D., Makielski J.C., January C.T. Drug-induced long QT syndrome: hERG K+ channel block and disruption of protein trafficking by fluoxetine and norfluoxetine. Br. J. Pharmacol. 2006;149:481–489. doi: 10.1038/sj.bjp.0706892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray W.A., Murray K.T., Hall K., Arbogast P.G., Stein C.M. Azithromycin and the risk of cardiovascular death. N. Engl. J. Med. 2012;366:1881–1890. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala L., Gnecchi M., Schwartz P.J. Long QT syndrome modelling with cardiomyocytes derived from human-induced pluripotent stem cells. Arrhythmia Electrophysiol. Rev. 2019;8:105–110. doi: 10.15420/aer.2019.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala L., Yu Z., Ward-van Oostwaard D., van Veldhoven J.P., Moretti A., Laugwitz K.L., Mummery C.L., Ap I.J., Bellin M. A new hERG allosteric modulator rescues genetic and drug-induced long-QT syndrome phenotypes in cardiomyocytes from isogenic pairs of patient induced pluripotent stem cells. EMBO Mol. Med. 2016;8:1065–1081. doi: 10.15252/emmm.201606260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh M., Gabriels J., Chang D., Kim B.S., Mansoor A., Mahmood E., Makker P., Ismail H., Goldner B., Willner J., Beldner S., Mitra R., John R., Chinitz J., Skipitaris N., Mountantonakis S., Epstein L.M. The effect of chloroquine, hydroxychloroquine and azithromycin on the corrected QT interval in patients with SARS-CoV-2 infection. Circulation. Arrhythmia Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.120.008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sănchez-Chapula J.A., Ferrer T., Navarro-Polanco R.A., Sanguinetti M.C. Voltage-dependent profile of human ether-a-go-go-related gene channel block is influenced by a single residue in the S6 transmembrane domain. Mol. Pharmacol. 2003;63:1051–1058. doi: 10.1124/mol.63.5.1051. [DOI] [PubMed] [Google Scholar]
- Sánchez-Chapula J.A., Navarro-Polanco R.A., Culberson C., Chen J., Sanguinetti M.C. Molecular determinants of voltage-dependent human ether-a-go-go related gene (HERG) K+ channel block. J. Biol. Chem. 2002;277:23587–23595. doi: 10.1074/jbc.M200448200. [DOI] [PubMed] [Google Scholar]
- Sánchez-Chapula J.A., Salinas-Stefanon E., Torres-Jácome J., Benavides-Haro D.E., Navarro-Polanco R.A. Blockade of currents by the antimalarial drug chloroquine in feline ventricular myocytes. J. Pharmacol. Exp. Therapeut. 2001;297:437–445. [PubMed] [Google Scholar]
- Sapp J.L., Alqarawi W., MacIntyre C.J., Tadros R., Steinberg C., Roberts J.D., Laksman Z., Healey J.S., Krahn A.D. Guidance on minimizing risk of drug-induced ventricular arrhythmia during treatment of COVID-19: a statement from the Canadian heart rhythm society. Can. J. Cardiol. 2020;36:948–951. doi: 10.1016/j.cjca.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarapa N., Nickens D.J., Raber S.R., Reynolds R.R., Amantea M.A. Ritonavir 100 mg does not cause QTc prolongation in healthy subjects: a possible role as CYP3A inhibitor in thorough QTc studies. Circulation. Arrhythmia Electrophysiol. 2008;83:153–159. doi: 10.1038/sj.clpt.6100263. [DOI] [PubMed] [Google Scholar]
- Saxena P., Zangerl-Plessl E.M., Linder T., Windisch A., Hohaus A., Timin E., Hering S., Stary-Weinzinger A. New potential binding determinant for hERG channel inhibitors. Sci. Rep. 2016;6 doi: 10.1038/srep24182. 24182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schewe M., Sun H., Mert Ü., Mackenzie A., Pike A.C.W., Schulz F., Constantin C., Vowinkel K.S., Conrad L.J., Kiper A.K., Gonzalez W., Musinszki M., Tegtmeier M., Pryde D.C., Belabed H., Nazare M., de Groot B.L., Decher N., Fakler B., Carpenter E.P., Tucker S.J., Baukrowitz T. A pharmacological master key mechanism that unlocks the selectivity filter gate in K channels. Science. 2019;363:875–880. doi: 10.1126/science.aav0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz P.J., Ackerman M.J. The long QT syndrome: a transatlantic clinical approach to diagnosis and therapy. Eur. Heart J. 2013;34:3109–3116. doi: 10.1093/eurheartj/eht089. [DOI] [PubMed] [Google Scholar]
- Schwartz P.J., Gnecchi M., Dagradi F., Castelletti S., Parati G., Spazzolini C., Sala L., Crotti L. From patient-specific induced pluripotent stem cells to clinical translation in long QT syndrome Type 2. Eur. Heart J. 2019;40:1832–1836. doi: 10.1093/eurheartj/ehz023. [DOI] [PubMed] [Google Scholar]
- Schwartz P.J., Woosley R.L. Predicting the unpredictable: drug-induced QT prolongation and Torsades de Pointes. J. Am. Coll. Cardiol. 2016;67:1639–1650. doi: 10.1016/j.jacc.2015.12.063. [DOI] [PubMed] [Google Scholar]
- Shi Y.P., Thouta S., Claydon T.W. Modulation of hERG K+ channel deactivation by voltage sensor relaxation. Front. Pharmacol. 2020;11:139. doi: 10.3389/fphar.2020.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.L., Anderson C.L., Burgess D.E., Elayi C.S., January C.T., Delisle B.P. Molecular pathogenesis of long QT syndrome type 2. J. Arrhythmia. 2016;32:373–380. doi: 10.1016/j.joa.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman E.Z., Lundgren J.D., Roediger M.P., Duprez D.A., Temesgen Z., Bickel M., Shlay J.C., Somboonwit C., Reiss P., Stein J.H., Neaton J.D. Boosted protease inhibitors and the electrocardiographic measures of QT and PR durations. AIDS (Lond.) 2011;25:367–377. doi: 10.1097/QAD.0b013e328341dcc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stas P., Faes D., Noyens P. Conduction disorder and QT prolongation secondary to long-term treatment with chloroquine. Int. J. Cardiol. 2008;127:e80–82. doi: 10.1016/j.ijcard.2007.04.055. [DOI] [PubMed] [Google Scholar]
- Stower H. Lopinavir-ritonavir in severe COVID-19. Nat. Med. 2020;26:465. doi: 10.1038/s41591-020-0849-9. [DOI] [PubMed] [Google Scholar]
- Szekely Y., Lichter Y., Shrkihe B.A., Bruck H., Oster H.S., Viskin S. Chloroquine-induced torsade de pointes in a COVID-19 patient. Heart Rhythm. 2020;17:1452–1455. doi: 10.1016/j.hrthm.2020.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemasa H., Nagatomo T., Abe H., Kawakami K., Igarashi T., Tsurugi T., Kabashima N., Tamura M., Okazaki M., Delisle B.P., January C.T., Otsuji Y. Coexistence of hERG current block and disruption of protein trafficking in ketoconazole-induced long QT syndrome. Br. J. Pharmacol. 2008;153:439–447. doi: 10.1038/sj.bjp.0707537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M.B., Beekman J.D., Attevelt N.J., Takahara A., Sugiyama A., Chiba K., Vos M.A. No proarrhythmic properties of the antibiotics Moxifloxacin or Azithromycin in anaesthetized dogs with chronic-AV block. Br. J. Pharmacol. 2006;149:1039–1048. doi: 10.1038/sj.bjp.0706900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traebert M., Dumotier B., Meister L., Hoffmann P., Dominguez-Estevez M., Suter W. Inhibition of hERG K+ currents by antimalarial drugs in stably transfected HEK293 cells. Eur. J. Pharmacol. 2004;484:41–48. doi: 10.1016/j.ejphar.2003.11.003. [DOI] [PubMed] [Google Scholar]
- van Noord C., Eijgelsheim M., Stricker B.H.C. Drug- and non-drug-associated QT interval prolongation. Br. J. Clin. Pharmacol. 2010;70:16–23. doi: 10.1111/j.1365-2125.2010.03660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg J.I., Perozo E., Allen T.W. Towards a structural view of drug binding to hERG K+ channels. Trends Pharmacol. Sci. 2017;38:899–907. doi: 10.1016/j.tips.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg J.I., Perry M.D., Perrin M.J., Mann S.A., Ke Y., Hill A.P. hERG K+ channels: structure, function, and clinical significance. Physiol. Rev. 2012;92:1393–1478. doi: 10.1152/physrev.00036.2011. [DOI] [PubMed] [Google Scholar]
- Veerman C.C., Kosmidis G., Mummery C.L., Casini S., Verkerk A.O., Bellin M. Immaturity of human stem-cell-derived cardiomyocytes in culture: fatal flaw or soluble problem? Stem Cell. Dev. 2015;24:1035–1052. doi: 10.1089/scd.2014.0533. [DOI] [PubMed] [Google Scholar]
- Vicente J., Zusterzeel R., Johannesen L., Ochoa-Jimenez R., Mason J.W., Sanabria C., Kemp S., Sager P.T., Patel V., Matta M.K., Liu J., Florian J., Garnett C., Stockbridge N., Strauss D.G. Assessment of multi-ion channel block in a phase I randomized study design: results of the CiPA phase I ECG biomarker validation study. Circulation. Arrhythmia Electrophysiol. 2019;105:943–953. doi: 10.1002/cpt.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volberg W.A., Koci B.J., Su W., Lin J., Zhou J. Blockade of human cardiac potassium channel human ether-a-go-go-related gene (HERG) by macrolide antibiotics. J. Pharmacol. Exp. Therapeut. 2002;302:320–327. doi: 10.1124/jpet.302.1.320. [DOI] [PubMed] [Google Scholar]
- Wang D.T., Hill A.P., Mann S.A., Tan P.S., Vandenberg J.I. Mapping the sequence of conformational changes underlying selectivity filter gating in the KV11.1 potassium channel. Nat. Struct. Mol. Biol. 2011;18:35–41. doi: 10.1038/nsmb.1966. [DOI] [PubMed] [Google Scholar]
- Wang L., Wible B.A., Wan X., Ficker E. Cardiac glycosides as novel inhibitors of human ether-a-go-go-related gene channel trafficking. J. Pharmacol. Exp. Therapeut. 2007;320:525–534. doi: 10.1124/jpet.106.113043. [DOI] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., MacKinnon R. Cryo-EM structure of the open human ether-à-go-go-related K+ channel hERG. Cell. 2017;169:422–430. doi: 10.1016/j.cell.2017.03.048. e410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmke J.W., Ganetzky B. A family of potassium channel genes related to eag in Drosophila and mammals. Proc. Natl. Acad. Sci. U.S.A. 1994;91:3438–3442. doi: 10.1073/pnas.91.8.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whicher J.R., MacKinnon R. Structure of the voltage-gated K⁺ channel Eag 1 reveals an alternative voltage sensing mechanism. Science. 2016;353:664–669. doi: 10.1126/science.aaf8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N.J. Cardiotoxicity of antimalarial drugs. Lancet Infect. Dis. 2007;7:549–558. doi: 10.1016/S1473-3099(07)70187-1. [DOI] [PubMed] [Google Scholar]
- Wible B.A., Hawryluk P., Ficker E., Kuryshev Y.A., Kirsch G., Brown A.M. HERG-Lite: a novel comprehensive high-throughput screen for drug-induced hERG risk. J. Pharmacol. Toxicol. Methods. 2005;52:136–145. doi: 10.1016/j.vascn.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Witchel H.J., Hancox J.C.J.C., Pharmacology E., Physiology Familial and acquired long QT syndrome and the cardiac rapid delayed. Rectifier Potassium Curr. 2001;27:753–766. doi: 10.1046/j.1440-1681.2000.03337.x. [DOI] [PubMed] [Google Scholar]
- Wu C.-I., Postema P.G., Arbelo E., Behr E.R., Bezzina C.R., Napolitano C., Robyns T., Probst V., Schulze-Bahr E., Remme C.A., Wilde A.A.M. SARS-CoV-2, COVID-19 and inherited arrhythmia syndromes. Heart Rhythm. 2020;17:1456–1462. doi: 10.1016/j.hrthm.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Sun L., Zha W., Studer E., Gurley E., Chen L., Wang X., Hylemon P.B., Pandak W.M., Jr., Sanyal A.J., Zhang L., Wang G., Chen J., Wang J.Y., Zhou H. HIV protease inhibitors induce endoplasmic reticulum stress and disrupt barrier integrity in intestinal epithelial cells. Gastroenterology. 2010;138:197–209. doi: 10.1053/j.gastro.2009.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuriyanghai Y., Makiyama T., Sasaki K., Kamakura T., Yamamoto Y., Hayano M., Harita T., Nishiuchi S., Chen J., Kohjitani H., Hirose S., Yokoi F., Gao J., Chonabayashi K., Watanabe K., Ohno S., Yoshida Y., Kimura T., Horie M. Complex aberrant splicing in the induced pluripotent stem cell-derived cardiomyocytes from a patient with long QT syndrome carrying KCNQ1-A344Aspl mutation. Heart Rhythm. 2018;15:1566–1574. doi: 10.1016/j.hrthm.2018.05.028. [DOI] [PubMed] [Google Scholar]
- Yang Z., Prinsen J.K., Bersell K.R., Shen W., Yermalitskaya L., Sidorova T., Luis P.B., Hall L., Zhang W., Du L., Milne G., Tucker P., George A.L., Campbell C.M., Pickett R.A., Shaffer C.M., Chopra N., Yang T., Knollmann B.C., Roden D.M., Murray K.T. Azithromycin causes a novel proarrhythmic syndrome. Circulation. Arrhythmia Electrophysiol. 2017;10 doi: 10.1161/CIRCEP.115.003560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Liu X., Zhao L., Dong E., Song C., Zhan S., Lu R., Li H., Tan W., Liu D. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdany J., Kim A.H.J. Use of hydroxychloroquine and chloroquine during the COVID-19 pandemic: what every clinician should know. Ann. Intern. Med. 2020;172:754–755. doi: 10.7326/m20-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Xie M., Li S., Gao Y., Xue S., Huang H., Chen K., Liu F., Chen L. Electrophysiologic studies on the risks and potential mechanism underlying the proarrhythmic nature of azithromycin. Cardiovasc. Toxicol. 2017;17:434–440. doi: 10.1007/s12012-017-9401-7. [DOI] [PubMed] [Google Scholar]
- Zheng Y.-Y., Ma Y.-T., Zhang J.-Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi D., Feng P.-F., Sun J.-L., Guo F., Zhang R., Zhao X., Li B.-X. The enhancement of cardiac toxicity by concomitant administration of Berberine and macrolides. Eur. J. Pharmaceut. Sci. 2015;76:149–155. doi: 10.1016/j.ejps.2015.05.009. [DOI] [PubMed] [Google Scholar]
- Zhou S., Yung Chan S., Cher Goh B., Chan E., Duan W., Huang M., McLeod H.L. Mechanism-based inhibition of cytochrome P450 3A4 by therapeutic drugs. Clin. Pharmacokinet. 2005;44:279–304. doi: 10.2165/00003088-200544030-00005. [DOI] [PubMed] [Google Scholar]