Abstract
For malaria parasites balancing sexual commitment, the frequency with which asexual bloodstream forms differentiate into non-replicative male and female gametocytes is critical because asexual replication is required to maintain a persistent infection of the human host while the non-replicative gametocytes are essential for infection of the mosquito vector and transmission. Here we describe recent advances in understanding of the regulatory mechanisms controlling this key developmental decision. These include new insights into the mechanistic roles of the transcriptional master switch AP2-G and the epigenetic modulator GDV1, as well as the identification of defined metabolic signals that modulate their activity. Many of these metabolites are linked to parasite phospholipid biogenesis and we propose a model linking this pathway to the epigenetic regulation underlying sexual commitment in P. falciparum.
Keywords: malaria, parasites, protozoa, differentiation, metabolism, gene regulation, heterochromatin
Graphical Abstract:
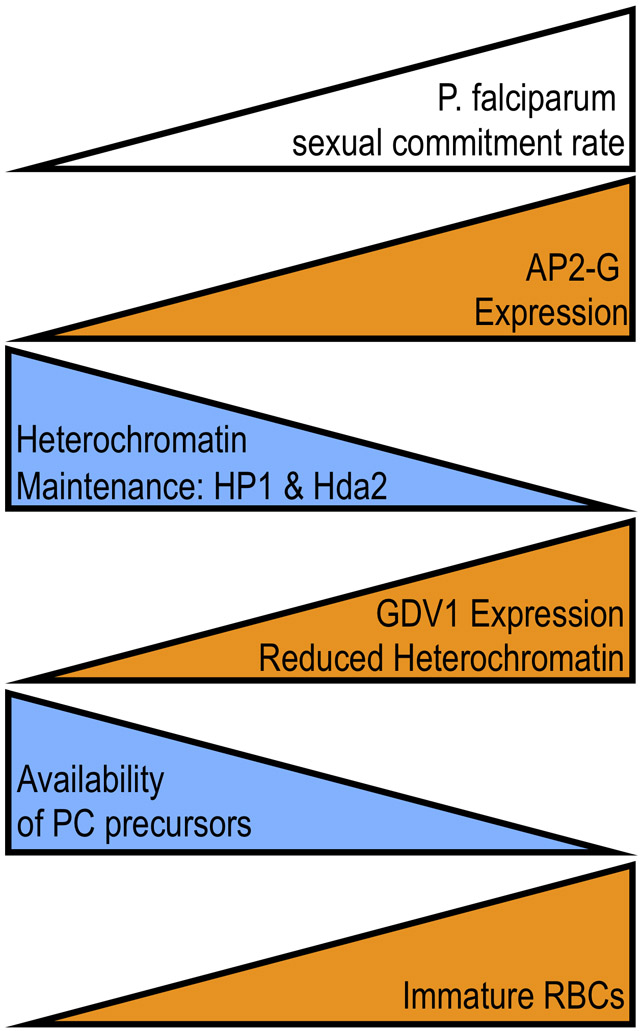
Factors promoting (orange) and suppressing (blue) the frequency of sexual commitment rate in Plasmodium falciparum.
Introduction
The lifecycle of human malaria parasites is complex and requires differentiation into multiple distinct stages within both us and the mosquito vector and failure to advance to the next lifecycle stage is fatal for the parasite. The lone exception to this rule occurs once parasites begin replicating with the blood stream. Many apicomplexan parasites, including other closely-related haemosporida such as Haemoproteus and Leucocytozoidae spp., are limited in the number of asexual replication cycles prior to initiating sexual differentiation [1]. The asexual stages of malaria parasites, however, can continue to replicate indefinitely within the bloodstream (barring treatment, immune clearance, or the death of their host). Each replication cycle only a small fraction of the parasite population commits to differentiate into non-replicating male and female gametocytes that can infect the mosquito vector.
Parasite replication within red blood cells occurs by schizogony, whereby continuous rounds of nuclear replication produce a single multi-nucleated cell that gives rise to 16-30 new merozoite progeny through a single round of cell division near the end of the lytic cycle. Experiments in P. falciparum showed that merozoites from the same schizont are generally committed to the same developmental fate, meaning that upon re-invasion they will either all initiate a new round of asexual replication or will differentiate into either all male or all female gametocytes [2-4]. This implied that the developmental decisions that determine asexual replication vs. gametocytogenesis, as well as male vs. female, are made during the preceding round of replication but will only manifest upon re-invasion when sexually committed parasites undergo gametocyte development.
Successful parasite transmission therefore requires balancing the investments in asexual reproduction and gametocyte formation under variable conditions, including transmission intensity [5], competition in multiply-infected hosts [6], fitness under drug treatment [7], and anatomical niche [8]. The ability to vary the frequency of sexual commitment is fundamental to this evolutionary bet-hedging strategy, allowing the parasite to persist substantially longer within the intermediate host and generating future chances for transmission.
This is especially important since completion of its life-cycle requires interaction of both host species, something the parasite has limited control over and the frequency of which can vary greatly depending on the transmission environment. The genome of malaria asexual blood stages is haploid and lacks sex chromosomes haploid and lacks sex chromosomes [9]. Malaria parasites are self-fertile as single clones are able to produce mating-compatible male and female gametocytes, thus allowing the parasite to be transmitted even in low transmission conditions when the probability of co-infection is low.
In laboratory culture, the frequency of P. falciparum sexual commitment can vary greatly depending on both the genetic background and culture conditions. Numerous factors have been described that alter the balance of asexual to gametocyte progeny as well as the male to female ratio (recently reviewed in [10]). In this review, we will discuss recent advances into the molecular nature of the signals controlling the frequency of sexual commitment in P. falciparum and the parasite’s mechanisms for sensing and integrating these environmental signals.
Molecular Regulation of Sexual Commitment
Identification of the transcription factor AP2-G as required for sexual commitment offered insights into the molecular regulation of the asexual versus sexual decision [11••,12••]. Robust expression of the ap2-g locus is necessary and sufficient for sexual commitment [11-12,13•,14-15] but transcription of the locus is silenced by heterochromatin in asexually replicating parasites [16]. This silencing requires trimethylation on lysine 9 of histone H3 (H3K9me3), a canonical mark of heterochromatin, and other factors necessary for general heterochromatin maintenance, including the histone deacetylase Hda2 and recognition of H3K9me3 by heterochromatin protein 1 (HP1) [17,18]. Failure to maintain effective silencing of the ap2-g promoter leads to high level expression as the ap2-g upstream region contains multiple AP2-G binding sites, creating a transcriptional positive feedback loop once AP2-G starts being expressed [19•,20•].
The outcome of AP2-G expression depends on how soon it is expressed following merozoite invasion. If ap2-g expression is activated during early ring stages, which occurs only at very low frequency in wildtype parasites or at high frequency in engineered lines, sexual commitment occurs and gametocyte development begins without another replication cycle (same cycle conversion) [13-15]. However, same cycle conversion is a rare event in vitro and in the wide majority of cells AP2-G expression is activated after this ring-stage checkpoint, instead giving rise to sexually committed merozoites that will initiate gametocyte development following re-invasion (next cycle conversion). The frequency of ap2-g activation appears to be set around 36 hours post-invasion [21•], approximately the time of peak nuclear replication, when demands on heterochromatin-maintenance are greatest. When activated at this time, AP2-G primarily drives its own transcription along with a limited number of other regulators of transcription and chromatin structure [19,20]. By preloading AP2-G on its own promoter, ap2-g is poised in the committed merozoite for high-level expression immediately following re-invasion, ensuring efficient activation of the gametocyte development transcriptional program in the early ring-stage [19,20].
In P. falciparum, the activation of the AP2-G expression and subsequent sexual commitment depends on gametocyte-development protein 1 (GDV1) [22•,23-24]. Like AP2-G, GDV1 is only expressed within a subset of asexual blood stages where it localizes to regions of heterochromatin, reduces overall levels of HP1 at these sites, and increases sexual commitment [23]. Regulatable overexpression of GDV1 resulted in high levels of sexual commitment, and like ap2-g, loss of function mutations in gdv1 are frequently seen in culture adapted strains. It remains unclear whether GDV1 plays a role in actively disassembling heterochromatin or impairs its maintenance during genome replication. Unlike ap2-g, stochastic expression of gdv1 is not directly regulated via heterochromatin but via complementary non-coding RNA, though the detailed mechanism of this regulation remains to be determined. This gdv1-antisense RNA is transcribed from a large downstream region that encodes multiple non-coding RNAs. Intriguingly, a substantial fraction of this downstream region also bears the H3K9me3 silencing mark, raising the possibility of a second regulatory circuit involving heterochromatin.
Impact of External Signals on Sexual Differentiation
That the rate of sexual commitment is responsive to culture conditions was first observed shortly after the development of the P. falciparum culture system in 1976 [25]. Since then a wide variety of factors have been described as impacting the commitment rate. Here we will focus on the role of metabolite signaling, but hemoglobinopathies [26,27], drug treatment [28,29], hormones levels [30-32], and cholera toxin [33] have also been described to influence commitment rates.
Effects of complex alteration to the culture environment
It was noted early on that the rate of P. falciparum sexual commitment in culture increases with the concentration of infected erythrocytes [25,34] or the use of conditioned or spent media [35-37]. This density dependence clearly indicated that the commitment rate was responsive to external stimuli but it remained unclear whether this was mediated by an increase in parasite-produced factors analogous to bacterial quorum sensing, faster depletion of nutrient from the culture medium, or both. Extracellular vesicles derived from conditioned media have also been implicated in increasing the commitment rate [38,39]. However, extracellular vesicles only increased commitment at high concentrations and conditioned media retained its commitment-promoting effect even when depleted of extracellular vesicles [21]. Additionally, cultures grown under serum starvation (minimal fatty acid media containing only oleate and palmitate) had substantially increased rates of sexual commitment, implying that depletion of a serum component is involved [21,40].
Effects of Individual Metabolites
In addition to the complex changes in media composition associated with conditioned media or serum starvation, a number of studies have identified more targeted metabolic changes that can alter the commitment rate of P. falciparum in vitro. Treatment of cultures with DTT or thapsigargin, compounds known to induce the unfolded protein response and ER stress, have been reported to increase the commitment rate [41]. Pre-loading of erythrocytes with homocysteine, another possible trigger of ER stress [42], also increased commitment [43], as did media supplementation with excess serine [40]. Finally, Brancucci and colleagues identified lysophosphatidylcholine (LysoPC) phospholipids as the active serum component regulating sexual commitment [21]. Their landmark study showed that LysoPC is taken up by infected erythrocytes, depleted from the media, and that the addition of LysoPC to conditioned media or minimal fatty acid media was sufficient to suppress increased commitment under these conditions. Additionally, they showed that the choline head group of LysoPC was the critical component for suppression of commitment as supplementation with choline alone mirrored the effects of LysoPC.
Recently, the patatin-like phospholipase PNPLA1 was also implicated as promoting sexual commitment [44]. Inducible knockdown of PNPLA1 during the commitment cycle blocked the increase in sexual differentiation in response to serum starvation, yielding similar commitment rate to cultures supplemented with LysoPC or choline. Conversely, overexpression of PNPLA1 resulted in increased commitment when driven by a promoter specific for asexual blood stages but not when driven by a gametocyte-specific promoter. Since loss of this phospholipase mimics supplementation with LysoPC while overexpression mimic LysoPC depletion this suggests that PNPLA1 counters the suppressive effects of LysoPC, possibly by degrading it.
A connection between phosphatidylcholine synthesis and sexual commitment
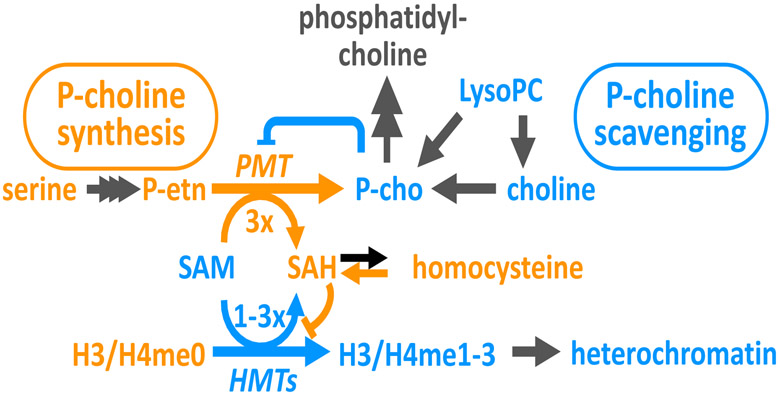
Intriguingly, several of the other metabolites that alter commitment can also be linked to phosphatidylcholine synthesis. As the parasite grows and replicates, the phospholipid content of infected erythrocytes increases 6-fold during each lytic cycle, with membrane phosphatidylcholine comprising more than half of this increase [40,45]. Plasmodium falciparum can either scavenge the required phospho-choline head group from exogenous sources or synthesize it de novo. When available, LysoPC and other choline-containing lipids are converted into phospho-choline and comprise a major source of parasite phosphatidylcholine [21,45]. However, in the absence of sufficient precursors, phospho-choline is synthesized by successive tri-methylation of phospho-ethanolamine (reviewed in [46]). Catalyzed by phospho-ethanolamine methyltransferase (PMT), this reaction consumes three equivalents of the S-adenosylmethionine (SAM) methyl donor and produces three equivalents of S-adenosylhomocysteine (SAH) for each phospho-choline generated. Both serine and homocysteine are directly linked to this reaction. Serine is the source for the phospho-ethanolamine precursor while homocysteine is generated by cleavage of SAH.
Additionally, genetic evidence also ties LysoPC and PMT to sexual commitment in P. falciparum. Targeted deletion of pmt results in impaired growth as well as both reduced sexual commitment and gametocyte maturation[47]. All of these phenotypes were reversed upon genetic complementation. We have observed similar effects in conditional pmt knockdown but found that long-term growth of pmt deficient parasites selects for compensatory mutations (unpublished results). This may explain why similar effects were not seen when pmt was knocked out in another strain [48]. Additionally, the activity of PMT is closely tied to the availability of phospho-choline precursors, which suppress pmt transcript levels and target the enzyme for degradation [21,49]. Interestingly, the pmt gene was lost from the genomes of rodent malaria parasites like Plasmodium berghei, which are insensitive to the suppressive effects of LysoPC [21]. This loss of de novo phosphatidylcholine synthesis in the rodent parasite lineages coincided with a large expansion of fam-a genes, which have the ability to transport phosphatidylcholine directly [50], likely compensating for the loss of de novo synthesis.
A possible link to histone methylation
A key outstanding question is how these metabolites implicated in phosphatidylcholine synthesis link to the maintenance of heterochromatin that controls the frequency of ap2-g activation. One clear connection is that de novo methylation of histones necessary for heterochromatin maintenance and methylation of phospho-ethanolamine for phospho-choline by PMT both consume the SAM as the methyldonor and produce SAH, a potent feedback inhibitor of many methyltransferases (Fig. 1). Under conditions of limiting phospho-choline precursors, the parasite would rely heavily on PMT for synthesis of membrane phosphatidylcholine. With each reaction consuming or generating three times the amount of SAM or SAH for every molecule of phospho-choline produced, PMT activity would likely impair the activity of other methyltransferases by reducing the available SAM pool and increasing intracellular SAH levels. Due to their ability to down-regulate PMT, abundant phospho-choline precursors would have the converse effect of increasing SAM availability and decreasing levels of SAH, thereby promoting the activity of other methyltransferases including those involved in heterochromatin maintenance. Under this model, the pro-commitment effects of serine can be explained by increasing phospho-ethanolamine levels and flux through PMT. Similarly, the pro-commitment effects of homocysteine can be explained by increasing SAH levels as the energetics of the reaction catalyzed by SAH hydrolase actually favor SAH generation when the reaction products are abundant. Moreover, the observations that homocysteine also increases commitment in rodent parasites [43] while LysoPC fails to suppress it [21], are explained by the fact that rodent-malaria parasites lack PMT and thus the link between phospho-choline precursors and SAM/SAH, while homocysteine would still impair methylation activity by increasing SAH.
Figure 1:
Metabolic model connecting the availability of phosphatidylcholine precursors to heterochromatin maintenance via the shared methylation donor and inhibitors SAM and SAH used in both histone methylation and de novo phospho-choline synthesis. Factors known or expected to increase the frequency of sexual commitment are in orange and those known or expected to repress sexual commitment are shown in blue.
Implications in vivo
The physiological implications of the parasite’s modulation of gametocyte formation in response to phospho-choline precursors remains unclear. It has been suggested that increasing the production of transmission stages in response to nutrient depletion, high-parasitemia, or other stresses allows the parasite to “escape” a host likely be a dead-end for the parasite as the result of imminent death or drug treatment. This may make evolutionary sense for malaria parasites with rapid gametocyte developments but significantly less so for P. falciparum, which has a prolonged period of gametocyte maturation, meaning that the increased opportunity for transmission will not be manifested for nearly two weeks.
A more convincing hypothesis put forward is that monitoring changes in phosphatidylcholine precursors allows the parasite to increase sexual commitment within an anatomical site more suited for gametocyte development, such as the bone marrow (expertly reviewed recently in [8]). Indeed, immature P. falciparum gametocytes are highly enriched at sites of hematopoiesis [51,52], the presence of immature erythrocytes increases the commitment rate [27,53,54], and tissue levels of LysoPC were lower in the bone-marrow of mice [21].
Conclusions
This model predicts that other conditions that alter intracellular SAM and SAH levels or directly impact the activity of relevant downstream methyltransferases should therefore also lead to changes in the commitment frequency. A major step forward would be the identification of the relevant enzymes regulating methylation states of histones and other targets. Additionally, high levels of choline were also found to reduce levels of GDV1, which would also be expected to reduce commitment [23]. Given that the gdv1 locus contains a large downstream region encoding regulatory non-coding RNAs that is partially heterochromatinized, it remains to be seen whether this reduction is also dependent on methyltransferase activity or via alternative mechanisms. Interestingly, the genomes of rodent malaria parasites also lack homologs of gdv1 in addition to pmt, which, together with the responsiveness of GDV1 protein levels in P. falciparum, raises the question whether GDV1 acts as the sensor of phospho-choline levels in human malaria parasites.
Highlights.
Sexual commitment requires expression of the silenced transcriptional master-switch AP2-G.
Factors promoting (HP1, Hda2) and reducing heterochromatin (GDV1) control the activation frequency.
Metabolites linked to phosphatidylcholine synthesis regulate the frequency of commitment.
Heterochromatin maintenance and phosphatidylcholine synthesis are linked by methylation metabolites.
Acknowledgements
We apologize to all the research that we could not include for space limitations. We would like to thank Jessica King, Kirk Deitsch, and members of the Kafsack Lab for careful reading and thoughtful feedback. This work was supported by funding to BK from 1R01AI138499 (NIAID), 1R01AI141965 (NIAID), and the Alice Bohmfalk Charitable Trust.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Garnham P: Malaria Parasites and Other Haemosporidia. J. B. Lippincott Company; 1966. [Google Scholar]
- 2.Inselburg J: Gametocyte Formation by the Progeny of Single Plasmodium falciparum Schizonts. J Parasitol 1983, 69:584. [PubMed] [Google Scholar]
- 3.Bruce MC, Alano P, Duthie S, Carter R: Commitment of the malaria parasite Plasmodium falciparum to sexual and asexual development. Parasitology 1990, 100 Pt 2:191–200. [DOI] [PubMed] [Google Scholar]
- 4.Silvestrini F, Alano P, Williams JL: Commitment to the production of male and female gametocytes in the human malaria parasite Plasmodium falciparum. Parasitology 2000, 121 Pt 5:465–471. [DOI] [PubMed] [Google Scholar]
- 5.Stepniewska K, Price RN, Sutherland CJ, Drakeley CJ, Seidlein von L, Nosten F, White NJ: Plasmodium falciparum gametocyte dynamics in areas of different malaria endemicity. Malar J 2008, 7:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollitt LC, Mideo N, Drew DR, Schneider P, Colegrave N, Reece SE: Competition and the evolution of reproductive restraint in malaria parasites. The American Naturalist 2011, 177:358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes KI, Little F, Mabuza A, Mngomezulu N, Govere J, Durrheim D, Roper C, Watkins B, White NJ: Increased Gametocytemia after Treatment: An Early Parasitological Indicator of Emerging Sulfadoxine-Pyrimethamine Resistance in Falciparum Malaria. J INFECT DIS 2008, 197:1605–1613. [DOI] [PubMed] [Google Scholar]
- 8.Venugopal K, Hentzschel F, Valkiūnas G, Marti M: Plasmodium asexual growth and sexual development in the haematopoietic niche of the host. Nat Rev Micro 2020, 18:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paul REL, Coulson TN, Raibaud A, Brey PT: Sex Determination in Malaria Parasites. Science 2000, 287:128–131. [DOI] [PubMed] [Google Scholar]
- 10.Henry NB, Serme SS, Siciliano G, Sombie S, Diarra A, Sagnon N, Traore AS, Sirima SB, Soulama I, Alano P: Biology of Plasmodium falciparum gametocyte sex ratio and implications in malaria parasite transmission. Malar J 2019, 18:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kafsack BFC, Rovira-Graells N, Clark TG, Bancells C, Crowley VM, Campino SG, Williams AE, Drought LG, Kwiatkowski DP, Baker DA, et al. : A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature 2014, 507:248–252.•• Identified the transcription factor PfAP2-G as the essential master switch from asexual replication to gametocyte formation in the human malaria parasite Plasmodium falciparum.
- 12.Sinha A, Hughes KR, Modrzynska KK, Otto TD, Pfander C, Dickens NJ, Religa AA, Bushell E, Graham AL, Cameron R, et al. : A cascade of DNA-binding proteins for sexual commitment and development in Plasmodium. Nature 2014, 507:253–257.•• Identified the transcription factor PbAP2-G as the essential master switch from asexual replication to gametocyte formation in the rodent malaria parasite Plasmodium berghei.
- 13.Bancells C, Llorà-Batlle O, Poran A, Nötzel C, Rovira-Graells N, Elemento O, Kafsack BFC, Cortés A: Revisiting the initial steps of sexual development in the malaria parasite Plasmodium falciparum. Nat. Microbiol 2019, 4:144–154.• Demonstrated that PfAP2-G expression in early ring stages is sufficient to initiate gametocyte development without the formation of a sexually committed schizont.
- 14.Kent RS, Modrzynska KK, Cameron R, Philip N, Billker O, Waters AP: Inducible developmental reprogramming redefines commitment to sexual development in the malaria parasite Plasmodium berghei. Nat. Microbiol 2018, 3:1206–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Llorà-Batlle O, Michel-Todó L, Witmer K, Toda H, Fernandez-Becerra C, Baum J, Cortés A: Conditional expression of PfAP2-G for controlled massive sexual conversion in Plasmodium falciparum. Science advances 2020, 6:eaaz5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez-Rubio JJ, Mancio-Silva L, Scherf A: Genome-wide analysis of heterochromatin associates clonally variant gene regulation with perinuclear repressive centers in malaria parasites. Cell Host Microbe 2009, 5:179–190. [DOI] [PubMed] [Google Scholar]
- 17.Coleman BI, Skillman KM, Jiang RHY, Childs LM, Altenhofen LM, Ganter M, Leung Y, Goldowitz I, Kafsack BFC, Marti M, et al. : A Plasmodium falciparum Histone Deacetylase Regulates Antigenic Variation and Gametocyte Conversion. Cell Host Microbe 2014, 16:177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brancucci NMB, Bertschi NL, Zhu L, Niederwieser I, Chin WH, Wampfler R, Freymond C, Rottmann M, Felger I, Bozdech Z, et al. : Heterochromatin protein 1 secures survival and transmission of malaria parasites. Cell Host Microbe 2014, 16:165–176. [DOI] [PubMed] [Google Scholar]
- 19.Poran A, Notzel C, Aly O, Mencia-Trinchant N, Harris CT, Guzman ML, Hassane DC, Elemento O, Kafsack BFC: Single-cell RNA sequencing reveals a signature of sexual commitment in malaria parasites. Nature 2017, 551:95–99.• Demonstrated that sexual commitment is locked via a transcriptional positive feedback upon AP2-G expression and characterized the PfAP2-g-driven transcriptional program in committed schizonts.
- 20.Josling GA, Russell TJ, Venezia J, Orchard L, van Biljon R, Painter HJ, Llinás M: Dissecting the role of PfAP2-G in malaria gametocytogenesis. Nature Communications 2020, 11:1–13.• Identified PfAP2-G binding sites in committed schizonts, gametorings, and early gametocytes. Confirmed that positive feedback is directly mediated by PfAP2-g binding its own promoter.
- 21.Brancucci NMB, Gerdt JP, Wang C, De Niz M, Philip N, Adapa SR, Zhang M, Hitz E, Niederwieser I, Boltryk SD, et al. : Lysophosphatidylcholine Regulates Sexual Stage Differentiation in the Human Malaria Parasite Plasmodium falciparum. Cell 2017, 171:1532–1544.el5.•• Identified LysoPC as the missing component mediating increased sexual commitment during serum starvation. Showed the LysoPC and choline supplementation is sufficient for suppressing commitment.
- 22.Eksi S, Morahan BJ, Haile Y, Furuya T, Jiang H, Ali O, Xu H, Kiattibutr K, Suri A, Czesny B, et al. : Plasmodium falciparum gametocyte development 1 (Pfgdv1) and gametocytogenesis early gene identification and commitment to sexual development. PLoS Pathog 2012, 8:e1002964.• Identified GDV1 as essential for gametocyte formation.
- 23.Filarsky M, Fraschka SA, Niederwieser I, Brancucci NMB, Carrington E, Carrió E, Moes S, Jenoe P, Bartfai R, Voss TS: GDV1 induces sexual commitment of malaria parasites by antagonizing HP1-dependent gene silencing. Science 2018, 359:1259–1263.•• Demonstrated that GDV1 interacts HP1 in heterochromatin and reduces HP1 levels, that GDV1 expression is responsive to choline supplementation, and the GDV1 is regulated by an anti-sense non-coding RNA.
- 24.Usui M, Prajapati SK, Ayanful-Torgby R, Acquah FK, Cudjoe E, Kakaney C, Amponsah JA, Obboh EK, Reddy DK, Barbeau MC, et al. : Plasmodium falciparum sexual differentiation in malaria patients is associated with host factors and GDV1-dependent genes. Nature Communications 2019, 10:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carter R, Miller LH: Evidence for environmental modulation of gametocytogenesis in Plasmodium falciparum in continuous culture. Bull. World Health Organ. 1979, 57 Suppl 1:37–52. [PMC free article] [PubMed] [Google Scholar]
- 26.Robert V, Tchuinkam T, Mulder B, Bodo JM, Verhave JP, Carnevale P, Nagel RL: Effect of the sickle cell trait status of gametocyte carriers of Plasmodium falciparum on infectivity to anophelines. Am J Trop Med Hyg 1996, 54:111–113. [DOI] [PubMed] [Google Scholar]
- 27.Trager W, Gill GS, Lawrence C, Nagel RL: Plasmodium falciparum: enhanced gametocyte formation in vitro in reticulocyte-rich blood. Experimental Parasitology 1999, 91:115–118. [DOI] [PubMed] [Google Scholar]
- 28.Buckling A, Ranford-Cartwright LC, Miles A, Read AF: Chloroquine increases Plasmodium falciparum gametocytogenesis in vitro. Parasitology 1999, 118 ( Pt 4):339–346. [DOI] [PubMed] [Google Scholar]
- 29.Peatey CL, Skinner-Adams TS, Dixon MWA, McCarthy JS, Gardiner DL, Trenholme KR: Effect of antimalarial drugs on Plasmodium falciparum gametocytes. J INFECT DIS 2009, 200:1518–1521. [DOI] [PubMed] [Google Scholar]
- 30.Maswoswe SM, Peters W, Warhurst DC: Corticosteroid stimulation of the growth of Plasmodium falciparum gametocytes in vitro. Ann Trop Med Parasitol 1985, 79:607–616. [DOI] [PubMed] [Google Scholar]
- 31.Lingnau A, Margos G, Maier WA, Seitz HM: The effects of hormones on the gametocytogenesis of Plasmodium falciparum in vitro. Applied parasitology 1993, 34:153–160. [PubMed] [Google Scholar]
- 32.Paul RE, Doerig C, Brey PT: Erythropoiesis and molecular mechanisms for sexual determination in malaria parasites. IUBMB Life 2000, 49:245–248. [DOI] [PubMed] [Google Scholar]
- 33.Peatey CL, Dixon MW, Gardiner DL, Trenholme KR: Temporal evaluation of commitment to sexual development in Plasmodium falciparum. Malar J 2013, 12:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carter R, Ranford-Cartwright L, Pietro Alano: The Culture and Preparation of Gametocytes of Plasmodium falciparum for Immunochemical, Molecular, and Mosquito Infectivity Studies. 1993, 21:67–88. [DOI] [PubMed] [Google Scholar]
- 35.Williams JL: Stimulation of Plasmodium falciparum gametocytogenesis by conditioned medium from parasite cultures. Am J Trop Med Hyg 1999, 60:7–13. [DOI] [PubMed] [Google Scholar]
- 36.Fivelman QL, McRobert L, Sharp S, Taylor CJ, Saeed M, Swales CA, Sutherland C, Baker DA: Improved synchronous production of Plasmodium falciparum gametocytes in vitro. Mol Biochem Parasitol 2007, 154:119–123. [DOI] [PubMed] [Google Scholar]
- 37.Brancucci NMB, Goldowitz I, Buchholz K, Werling K, Marti M: An assay to probe Plasmodium falciparum growth, transmission stage formation and early gametocyte development. Nature Protocols 2015, 10:1131–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mantel P-Y, Hoang AN, Goldowitz I, Potashnikova D, Hamza B, Vorobjev I, Ghiran I, Toner M, Irimia D, Ivanov AR, et al. : Malaria-Infected Erythrocyte-Derived Microvesicles Mediate Cellular Communication within the Parasite Population and with the Host Immune System. Cell Host Microbe 2013, 13:521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Regev-Rudzki N, Wilson DW, Carvalho TG, Sisquella X, Coleman BM, Rug M, Bursac D, Angrisano F, Gee M, Hill AF, et al. : Cell-Cell Communication between Malaria-Infected Red Blood Cells via Exosome-like Vesicles. Cell 2013, 153:1120–1133. [DOI] [PubMed] [Google Scholar]
- 40.Gulati S, Ekland EH, Ruggles KV, Chan RB, Jayabalasingham B, Zhou B, Mantel P-Y, Lee MCS, Spottiswoode N, Coburn-Flynn O, et al. : Profiling the Essential Nature of Lipid Metabolism in Asexual Blood and Gametocyte Stages of Plasmodium falciparum. Cell Host Microbe 2015, 18:371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaubey S, Grover M, Tatu U: Endoplasmic Reticulum Stress Triggers Gametocytogenesis in the Malaria Parasite. Journal of Biological Chemistry 2014, 289:16662–16674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tehlivets O, Malanovic N, Visram M, Pavkov-Keller T, Keller W: S-adenosyl-L-homocysteine hydrolase and methylation disorders: yeast as a model system. Biochim Biophys Acta 2013, 1832:204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beri D, Balan B, Chaubey S, Subramaniam S, Surendra B, Tatu U: A disrupted transsulphuration pathway results in accumulation of redox metabolites and induction of gametocytogenesis in malaria. Sci. Rep 2017, 7:40213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flammersfeld A, Panyot A, Botté YY, Aurass P, Przyborski JM, Flieger A, Botte C, Pradel G: A patatin-like phospholipase functions during gametocyte induction in the malaria parasite Plasmodium falciparum. Cell Microbiol 2020, 22. [DOI] [PubMed] [Google Scholar]
- 45.Wein S, Ghezal S, Bure C, Maynadier M, Perigaud C, Vial HJ, Lefebvre-Tournier I, Wengelnik K, Cerdan R: Contribution of the precursors and interplay of the pathways in the phospholipid metabolism of the malaria parasite. J. Lipid Res 2018, 59:1461–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kilian N, Choi J-Y, Voelker DR, Ben Mamoun C: Role of phospholipid synthesis in the development and differentiation of malaria parasites in the blood. Journal of Biological Chemistry 2018, 293:17308–17316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bobenchik AM, Witola WH, Augagneur Y, Nic Lochlainn L, Garg A, Pachikara N, Choi J-Y, Zhao YO, Usmani-Brown S, Lee A, et al. : Plasmodium falciparum phosphoethanolamine methyltransferase is essential for malaria transmission. Proceedings of the National Academy of Sciences 2013, 110:18262–18267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brancucci NMB, De Niz M, Straub TJ, Ravel D, Sollelis L, Birren BW, Voss TS, Neafsey DE, Marti M: Probing Plasmodium falciparum sexual commitment at the single-cell level. Wellcome Open Research 2018, 3:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Witola WH, Ben Mamoun C: Choline induces transcriptional repression and proteasomal degradation of the malarial phosphoethanolamine methyltransferase. Eukaryotic Cell 2007, 6:1618–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fougere A, Jackson AP, Bechtsi DP, Braks JAM, Annoura T, Fonager J, Spaccapelo R, Ramesar J, Chevalley-Maurel S, Klop O, et al. : Variant Exported Blood-Stage Proteins Encoded by Plasmodium Multigene Families Are Expressed in Liver Stages Where They Are Exported into the Parasitophorous Vacuole. PLoS Pathog 2016, 12:e1005917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farfour E, Charlotte F, Settegrana C, Miyara M, Buffet P: The extravascular compartment of the bone marrow: a niche for Plasmodium falciparum gametocyte maturation? Malar J 2012, 11:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joice R, Nilsson SK, Montgomery J, Dankwa S, Egan E, Morahan B, Seydel KB, Bertuccini L, Pietro Alano, Williamson KC, et al. : Plasmodium falciparum transmission stages accumulate in the human bone marrow. Science Translational Medicine 2014, 6:244re5–244re5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trager W, Gill GS: Enhanced gametocyte formation in young erythrocytes by Plasmodium falciparum in vitro. J. Protozool 1992, 39:429–432. [DOI] [PubMed] [Google Scholar]
- 54.Peatey CL, Watson JA, Trenholme KR, Brown CL, Nielson L, Guenther M, Timmins N, Watson GS, Gardiner DL: Enhanced gametocyte formation in erythrocyte progenitor cells: a site-specific adaptation by Plasmodium falciparum. J INFECT DIS 2013, 208:1170–1174. [DOI] [PubMed] [Google Scholar]