Abstract
Magnetic nanoparticles are currently the focus of investigation for a wide range of biomedical applications that fall into the categories of imaging, sensing, and therapeutics. A deep understanding of nanoparticle magnetization dynamics is fundamental to optimization and further development of these applications. Here, a summary of theoretical models of nanoparticle dynamics is presented, and computational nonequilibrium models are outlined, which currently represent the most sophisticated methods for modeling nanoparticle dynamics. Nanoparticle magnetization response is explored in depth; the effect of applied field amplitude, as well as nanoparticle size, on the resulting rotation mechanism and timescale is investigated. Two applications in biomedicine, magnetic particle imaging and magnetic fluid hyperthermia, are highlighted.
1. Introduction
In recent years, the use of magnetic nanoparticles (MNPs) for a range of biomedical applications, specifically in the areas of medical imaging, diagnostics, and treatment, has been the focus of significant research. Their small size allows MNPs to infiltrate biological systems, and their magnetic properties allow them to be controlled and detected by externally applied magnetic fields and field gradients, allowing for a wide range of potential clinical uses [1, 2] which include targeted drug delivery [3, 4, 5, 6], stem cell tracking [7, 8, 9, 10, 11, 12], molecular detection through magnetic separation [13, 14, 15, 16, 17, 18, 19, 20], contrast enhancement for magnetic resonance imaging [21, 22, 23, 24, 25, 26], hyperthermia therapy in cancer treatment [27, 28, 29, 30, 31, 32], and magnetic particle imaging [33, 34, 1], among others [1, 2, 35]. For further development of these applications, specifically for those involving the application of alternating magnetic fields, a complete understanding of the dynamics of magnetic nanoparticles under nonequilibrium conditions is required.
1.1. Superparamagnetic nanoparticles
Magnetic nanoparticles, comprised of ferromagnetic, ferrimagnetic, or in some cases antiferromagnetic materials, have a permanent magnetic moment in the absence of an applied magnetic field. When the size of a magnetic nanoparticle is reduced to below the single domain limit (e.g. around 30 nm for iron oxide), it can become superparamagnetic at room temperature, in which case the MNP will have a single net magnetic moment that can spontaneously flip within a defined time period. The MNP will behave as a paramagnet under an applied field, but with a magnetic moment magnitude much larger (by as many as four orders of magnitude) than those of typical paramagnets. The lack of remanent magnetization enables the MNPs to avoid agglomeration and maintain colloidal stability, which is critical for use in biomedical applications, while their large magnetic moment allows them to be manipulated by external magnetic fields and remotely detected even at low concentrations. The motion of the magnetic moment of a superparamagnetic particle under an applied field will be a complex stochastic process, due to coupling of the magnetic moment to the microscopic degrees of freedom of its environment (e.g. conducting electrons, phonons, nuclear spins) resulting in thermal fluctuations. The dynamics can also be affected by macroscopic parameters such as the non-magnetic coatings, often used for biocompatibility or for specific functionalization. Iron oxide, particularly the magnetite phase (Fe3O4), is a commonly used material for MNPs for biomedical applications, due to its biocompatibility.
Developments to the chemical synthesis procedure have now enabled superparamagnetic iron oxide nanoparticles (SPIONs) to be synthesized with a high degree of control over their phase, size, size distribution, and shape [36, 37, 38, 39]. SPIONs are typically coated with a surfactant, such as a polymer [40, 41, 42, 43, 44, 45, 46, 47], for stabilization in water. Additional functional groups or targeting molecules can then be added to the surface of the nanoparticle, if required. With proper surface functionalization, SPIONs can bind to proteins, genes, or viruses; interact with single cell-receptors; target cancerous tissue; or penetrate cells [13, 48, 49, 23, 50, 19, 2]. The therapeutic and diagnostic power of SPIONs becomes evident when these functionalities are considered in combination with their ability to be magnetically controlled and detected.
1.2. Applications of MNPs in biomedicine
The biomedical applications of MNPs generally fall into the categories of imaging, sensing, and therapeutics. MNPs are currently used as contrast agents for magnetic resonance imaging [23, 25, 26, 51], but are also the key component of magnetic particle imaging (MPI), a new medical imaging system in the preclinical stage [33, 34, 1]. In MPI, a combination of static gradient and alternating current magnetic fields allows the concentration and distribution of magnetic nanoparticles to be detected, enabling reconstruction of a three-dimensional image [33, 52, 34, 1, 53]. MPI relies on the nonlinearity of the magnetization curve for a superparamagnetic particle and the fact that its magnetization is eventually saturated for any applied magnetic field greater than the saturation field. If the MNPs are subject to an oscillating magnetic field, the magnetization response, M(t), will vary with time. The time-varying magnetization will induce a voltage in the receive coil, which will be proportional to the rate of change of the magnetization, M′(t). The spatial localization of the signal is achieved by superimposing a static magnetic gradient field on top of the alternating field, such that the MNP magnetization is saturated outside of the field-free region [33].
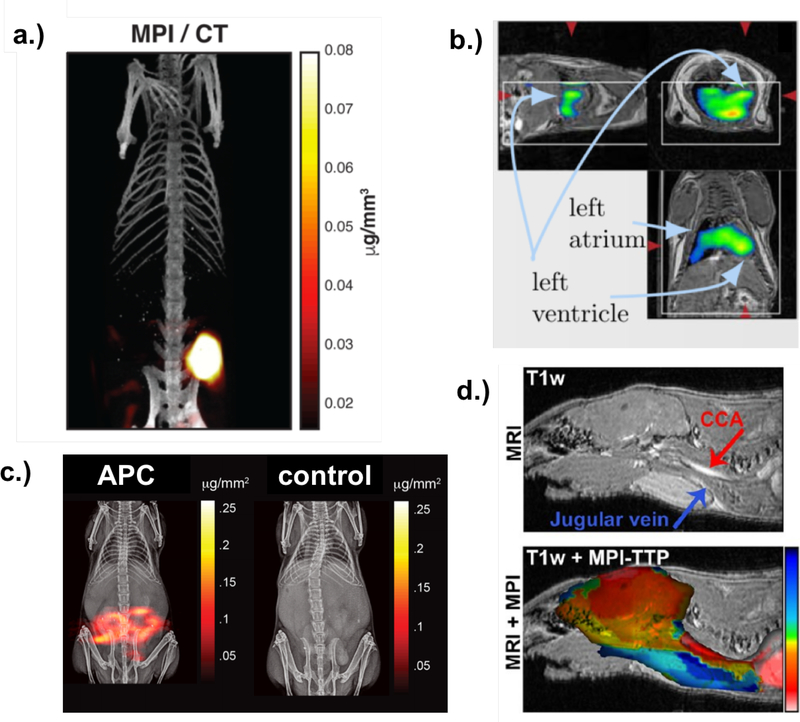
SPIONs are currently used as contrast agents to enhance signal in MRI [54], and so their safety and viability have already been well-tested. However, in MRI, SPIONs generally provide T2 (dark) contrast, whereas they provide positive (bright) contrast in MPI, which is favored by radiologists and clinicians (although it has been shown that bright T1 contrast can be achieved with very small SPIONs 4–7 nm in diameter [51]). Since MPI is a tracer-based imaging system, it produces a quantitative image signal with no background signal from surrounding tissue, since biological tissue is weakly diamagnetic and generates no MPI signal [1]. MPI has high mass sensitivity, and therefore large signal generating efficiency, many orders of magnitude greater per volume than protons detected with MRI, for example, resulting in a high signal-to-noise ratio (SNR) [52] which enables fast image acquisition times. Real-time imaging with MPI over short timescales (on the order of tens of milliseconds) has already been demonstrated [52, 55]. The high SNR also enables MPI to detect very low concentrations (on the order of nanograms [56]) of MNPs, reducing the quantity needed for accurate imaging [57]. The MPI signal is linear with tracer concentration, providing a quantitative image. Furthermore, MPI may be both safer and more cost efficient than other leading medical imaging methods, since it requires only a magnetic field gradient, rather than an expensive highly uniform superconducting magnet, as required for MRI. MPI SPION tracers can be stored for months and are cheaper to produce than radioactive tracers used in positron emission tomography (PET), for example, and are much safer than iodine and gadolinium contrast agents, which are are used in other imaging modalities. Iron oxide magnetic nanoparticle formulations have been approved by the FDA for doses as large as 500 mg/day [58] and shown to have no adverse effects on the body, in contrast to iodine, used for computed tomography (CT), and gadolinium, used in MRI [59], particularly for the small quantities required for MPI. Again due to its high SNR, MPI only requires a very small amount of these tracers, compared to the doses approved. Specific clinical applications for MPI include in vivo cancer imaging [60] (Fig. 1a), real-time cardiovascular imaging [56] (Fig. 1b), gut bleed detection [61] (Fig. 1c), acute stroke detection [62] (Fig. 1d), as well as stem cell tracking [63].
Figure 1:
a. MPI image of a mouse tumor acquired 6 hours after tracer injection with CT underlay. Reproduced with permission from [60]. b. MPI images of nanoparticles passing through a mouse heart with MRI underlay. Reproduced with permission from [56]. c. MPI image of a mouse with GI bleed (left) vs. control (right) with MRI underlay. Reproduced with permission from [61]. d. MPI stroke imaging of a mouse with MRI underlay. Reproduced with permission from [62].
MNP dynamics are highly sensitive to their surrounding environment, which enables the use of MNPs as sensors. For example, changes in viscosity can be detected using the technique of magnetization spectroscopy of nanoparticle Brownian motion (MSB), developed by John Weaver and Adam Rauwerdink [64]. Other biologically relevant parameters, such as temperature, can be detected and even quantified using such a technique [65, 66]. With proper surface functionalization, sensing of specific biomolecules is also possible, due to changes in dynamics that result from differences in the binding state of MNPs, enabling a range of diagnostic applications [67, 68, 69]. These sensing techniques can in principle be combined with imaging, via MPI, to create multi-channel or multicolor images, in which color represents a parameter such as viscosity or temperature, in theory enabling simultaneous real-time imaging and diagnostics.
Another biomedical application of MNPs is magnetic fluid hyperthermia (MFH), in which high-frequency excitation fields are used to induce nonlinear MNP rotations in cancer cells, generating cytotoxic heat [29, 30, 70, 71, 32, 72]. MNPs can be localized in tumors, either with the use of targeting ligands or direct injection. Under application of an alternating magnetic field, magnetic hysteresis losses will result in localized heating of the tumor, triggering the onset of apoptosis under correct conditions. Localized hyperthermia can also be used in conjunction with conventional cancer treatments, such as chemotherapy and radiation, to improve their efficiency [73]. In theory, MFH could also be combined with MPI and temperature sensing to create a combined imaging and therapeutic platform, in which the MNPs and resulting heat generation could be quantitatively tracked. A related use is targeted delivery of chemotherapy drugs, in which antitumor drugs are attached to the nanoparticle surface and then released via thermal activation from application of an external magnetic field. In a similar way, MPI combined with sensing of temperature or binding-state could enable a monitored targeted drug-delivery system.
While the biological applications of MNPs extend beyond these, the above applications have been highlighted because they require a detailed understanding of MNP dynamics in vivo, including their dependence on environmental and particle properties under nonequilibrium conditions. Outlining the current theoretical models of MNP dynamics up to the most recent developments in computational modeling in the context of imaging and therapy will be the focus of this work.
2. Equilibrium and linear models of nanoparticle relaxation
Superparamagnetic nanoparticles are single-domain, and so have a single net magnetic moment. Under an applied magnetic field, the MNP magnetic moment will rotate to align with the field. We define the equilibrium relaxation time constant τ, which refers to the time required for the particle moment to return to equilibrium after an external perturbation. When applying a field, we first consider the equilibrium case, where the timescale of the change in field is large enough that the particle can reach equilibrium at every step. Under an oscillating magnetic field, for example, this condition requires 1/f ≫ τ, where f is the frequency of the applied field. While this condition typically does not apply for biomedical applications of MNPs, it is still instructive to examine the equilibrium case before moving on to nonequilibrium scenarios.
2.1. The Langevin function
Superparamagnetic nanoparticles can be modeled as dipoles with magnetic moment magnitude μ = MsVc, where Ms is the saturation magnetization of the material (∼ 420 kA/m for magnetite), and Vc is the volume of the magnetic core. We distinguish this from the hydrodynamic volume, Vh, which includes the non-magnetic ligands that are applied to the surface of the core particle. In an ensemble of isotropic non-interacting magnetic nanoparticles, the equilibrium energy of an individual particle will be:
| (1) |
Here, μ is , with a unit vector pointing in the direction of the magnetic moment, μ0 is the permeability of free space, and H is the applied magnetic field. Without loss of generality, for the remainder of this section we define the field to be applied in the z-direction. With the partition function of an ensemble of such particles, the average particle energy and magnetization in the z-direction can be extracted. The average particle magnetization is proportional to the Langevin function:
| (2) |
where
| (3) |
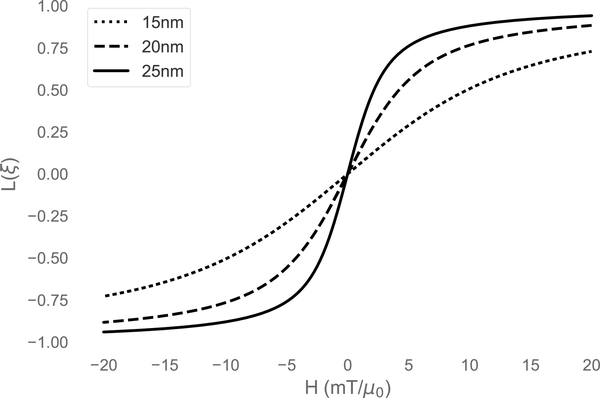
Here, ξ is the unitless field, μ0μH/kBT, where T is the temperature and kB is Boltzmann’s constant. This well-known equation describes the magnetization of an ensemble of isotropic particles at equilibrium, i.e. when the particle dipole moments are allowed to fully relax at every step of the applied field. The Langevin function for three different particle core sizes is shown in Fig. 2. It should be emphasized that the Langevin function does not incorporate particle anisotropy, and so is only applicable as an approximation for small or nearly isotropic particles in a nonequilibrium case.
Figure 2:
The Langevin function for three different particle core diameters, with Ms = 420 kA/m at 300 K. Note that the derivative, dM/dH at H = 0, increases with increasing particle size.
2.2. Anisotropic partition function
In reality, superparamagnetic nanoparticles will have some degree of anisotropy, resulting from combined magnetocrystalline, shape, and surface anisotropies. Typically, uniaxial anisotropy is assumed, indicating that there is a single easy axis along which the magnetic moment prefers to align. In that case, we can define σ = KVc/kBT, where K is the effective anisotropy constant, incorporating all magnetocrystalline and shape anisotropy. We can then express the energy of a single particle as
| (4) |
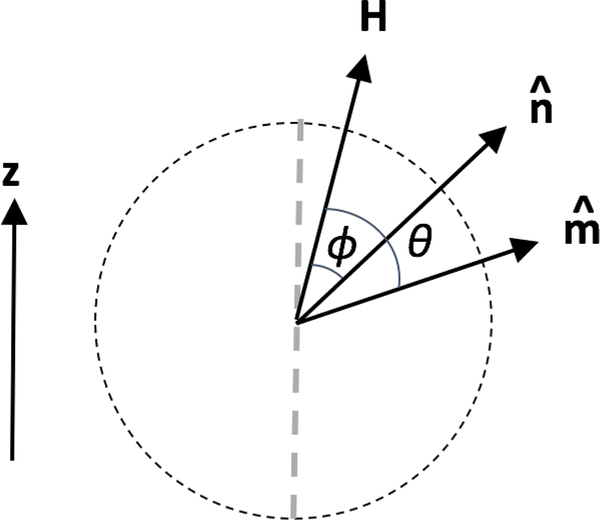
The first term represents the internal anisotropy energy, and the second term represents the energy from the Zeeman interaction with the external applied field. We define θ to be the angle between the moment and ξ (which points in the direction of the applied field), and ϕ to be the angle between the easy axis and ξ (see Fig. 4). If the assumption is made that the easy axis is aligned with the magnetic field ξ in the z-direction and lies in the same plane as the magnetization, following Stoner and Wolhfarth [74], the system becomes two-dimensional. The energy, in unitless form, is then:
| (5) |
Figure 4:
Angles defined in the general case. The direction of the applied field is indicated by H, the direction of the easy axis is indicated by , and the direction of the magnetic moment is indicated by . In many approximations, the applied field is assumed to lie along the z-direction (not shown here).
To find the equilibrium state of the particle, we take the derivative of the energy with respect to the moment direction θ, which simplifies to
| (6) |
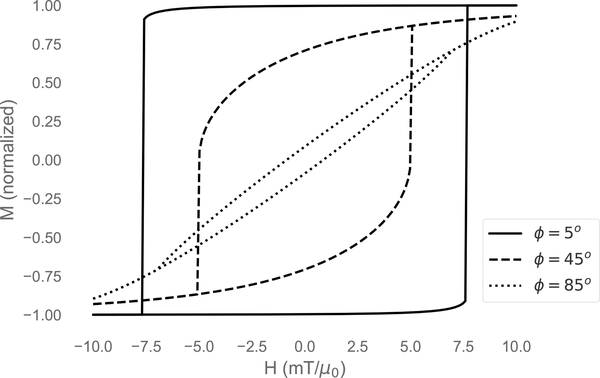
To find a minimum we must also ensure . The Stoner-Wohlfarth hysteresis loop emerges from solving for the magnetization (cosθ) and plotting with respect to the field (see Fig. 3). The width of the hysteresis loop depends on the angle ϕ between the easy axis and the applied field. This model has an advantage over the simple Langevin function in that it accounts for particle anisotropy, but still assumes equilibrium conditions, and therefore does not account for particle relaxation. Furthermore, in this form, the Stoner-Wohlfarth model does not incorporate thermal energy, and is only fully valid at T = 0. Extensions to the Stoner-Wohlfarth model which incorporate temperature dependency have been made [75, 76], but are not discussed here as they still do not incorporate particle relaxation.
Figure 3:
Hysteresis curves calculated from the Stoner-Wohlfarth model at different angles of the easy axis with respect to the applied field.
2.3. Nanoparticle rotation mechanisms and equilibrium timescales
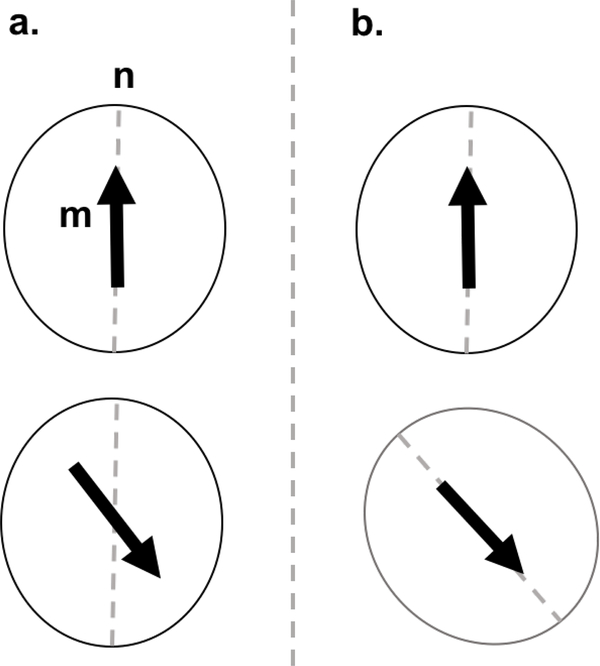
There are two mechanisms by which a superparamagnetic nanoparticle can reverse its magnetic moment. Brownian rotation refers to the physical rotation of the particle to align its magnetic moment with the applied magnetic field, and Néel rotation refers to the reversal of the magnetic moment within the particle itself, assuming that the physical particle remains stationary (see Fig. 5). The particle will rotate via either the Brownian or Néel process or some combination of both, although the faster mechanism will typically dominate. In MPI, nanoparticle dipole moments rotate under the influence of an external oscillating field, generating a measurable signal by induction in a pickup coil resulting from the changing magnetic field. The particle dynamics are typically discussed in terms of timescales of nanoparticle rotations, particularly the equilibrium relaxation time [77, 78, 79].
Figure 5:
Néel (a.) vs. Brownian (b.) rotation mechanisms. With Néel rotation, the easy axis n stays stationary, while only the magnetic moment m rotates. Under Brownian rotation, both the axis and the moment rotate.
Due to the magnetocrystalline anisotropy and shape asymmetry of MNPs, their magnetic moment will preferentially align with a particular crystallographic direction (the easy axis), a result of coupling between the atomic spin and orbital angular moments. The energy required to rotate the magnetization from the easy axis is denoted the anisotropy energy. The anisotropy energy, when uniaxial, has two minima, which correspond to alignment with the easy axis , separated by an energy barrier KVc. When this energy barrier is smaller than the thermal energy kBT, the particle magnetic moment is free to rotate between energy minima. The Néel process occurs when the particle orientation (the easy axis) remains fixed while the magnetic moment flips, and is characterized by the equilibrium Néel relaxation time [80, 32, 81],
| (7) |
where the attempt time τ0 is equal to Ms/(2αγK). α is a dimensionless damping constant, assumed to be between 0.1 and 1, and γ is the gyromagnetic ratio.
When measuring MNP magnetization, the measurement time τM must be defined. The particle is in a superparamagnetic state when τM ≫ τN. In this state, the magnetization will flip multiple times over the course of the measurement resulting in zero net remanent magnetization. Alternatively, if τM ≪ τN, then the magnetization will not reverse during the measurement; the particle will be in a blocked state. The blocking temperature is defined as the temperature at which τM = τN, when the transition between the blocked and the superparamagnetic state occurs [80]. If the particle is in the blocked state, the particle physically rotates along with the magnetic moment, and is then described by the Brownian rotation mechanism. We can similarly quantify the characteristic equilibrium Brownian relaxation time [32, 30]:
| (8) |
where η is the viscosity of the surrounding medium and again Vh is the hydrodynamic volume including any ligands attached to the surface. The mechanism by which the particle reverses its magnetic moment will be determined by the particle anisotropy and size (smaller particles exhibit only Néel rotation, while larger particles exhibit primarily Brownian rotation). The effective magnetic equilibrium relaxation time is often expressed as [32, 30]:
| (9) |
from which it can be seen that the mechanism with the lowest relaxation time for a given particle size and anisotropy will dominate. In reality, the two rotation mechanisms are coupled in a non-trivial way, which is not fully described by this effective relaxation time [82, 83]. Furthermore, when a sufficiently large external driving field is applied (ξ > 5), these equilibrium time constants can no longer completely describe the subsequent dynamics [79, 83]. Under a strong applied field, the magnetic moments of the particles will align faster than in the equilibrium case, and so the true timescales of rotation will be shorter than the equilibrium relaxation times.
2.4. Linear response theory
One of the most common models of dynamic MNP magnetization is the Debye/Fannin model [84], which describes the linear response of a dipole to an oscillating field. In linear response theory (LRT), it is assumed that the applied field amplitude is low enough (or that the frequency is high enough) so that magnetic saturation, when all particle moments are fully aligned with the field, is not reached. We define χ to be the magnetic susceptibility, which will be constant with increasing applied field H (so M = χH, where M is the ensemble magnetization in the direction of the field). According to the Langevin model, the equilibrium (or DC) susceptibility, χ0, of a system of non-interacting MNPs will be [85]:
| (10) |
where is the Langevin function (Eq. 3). In the Debye model [84, 86], to account for the lag in magnetization with respect to the applied field, the magnetic susceptibility is broken down into real (in-phase) and imaginary (out-of-phase) components:
| (11) |
τ represents the effective magnetic relaxation time, from Eq. 9, and ω is the angular frequency of the applied field. The out-of-phase component will have a maximum at ωτ = 1, at which point the phase shift between the particle’s magnetic moment and the applied field is maximum, resulting in energy loss. The Debye model assumes symmetry in the AC susceptibility spectrum (when plotted vs. log frequency), which is often not seen experimentally. The Havriliak-Negami model [87] provides a phenomenological model for the susceptibility:
| (12) |
Here, χinf is the real susceptibility at high frequencies and χamp is the amplitude of the remainder of the frequency-dependent regime. The parameter α describes the width of the spectrum, and β incorporates the asymmetry. The model reduces to the Debye model in the limits of α = 0 and β = 1. This model, in addition to incorporating particle size distributions, can be used for more accurate fitting of AC susceptibility spectra to obtain the effective relaxation time.
Finally, the magnetization in the direction of the field is then described by:
| (13) |
Here we have included a size distribution g(d) for the particles, where d is the particle diameter, typically considered to be a log-normal distribution [88]. This model for the magnetization is theoretically valid as long as linear response theory holds, i.e. for high frequencies or low fields (typically when ξ is less than 10). However, for the frequencies and magnetic field strengths typically employed in MPI and MFH (frequencies on the order of tens or hundreds of kHz, and amplitudes on the order of tens of mT/μ0), nonlinear behavior will arise. Consequently, while the Debye model is instructive, future sections will focus almost exclusively on nonequilibrium and nonlinear models of nanoparticle dynamics.
3. Nonequilibrium nanoparticle rotation timescales
The magnetic fields required for magnetic particle imaging and magnetic fluid hyperthermia are AC fields with frequencies on the order of tens or hundreds of kilohertz. The short timescales involved give rise to nonequilibrium conditions and resulting nonlinear nanoparticle dynamics. For these clinical applications of MNPs, it is essential to quantify properties of the nanoparticle dynamics as a function of the applied field and characteristics of the nanoparticles and surrounding medium. MNP dynamics are often described in terms of zero-field relaxation timescales; however, the effective rotation timescales have been shown to be highly dependent on field strength (for both AC and DC fields) [89, 79]. Moreover, the physical regime where both Néel rotation and Brownian rotation may occur has yet to be described in any quantitative manner for the relevant field strengths and frequencies required for the above mentioned applications.
Brownian and Néel mechanisms are each characterized by respective timescales. The equilibrium relaxation time constant predicts the time required for thermal fluctuations to reorient the net magnetization to zero after an applied magnetic field is removed. In reality, the Brownian and Néel mechanisms are coupled, and the respective rotation timescales are dependent on parameters like temperature, core and hydrodynamic size, and viscosity. The equilibrium relaxation timescales, which are often used to describe MNP dynamics, ignore applied fields and so have limited applicability for many biomedical applications of MNPs which employ applied magnetic fields. The rotational time constant will change with field and frequency, and for high fields the external magnetic field may dominate over thermal terms.
3.1. Field dependence of rotation timescales
The effective rotation timescale is often expressed as by Eq. 9, which assumes that the rotation mechanism with the lowest rotation timescale will dominate. It should again be emphasized here that τN and τB refer to zero-field relaxation timescales; once a magnetic field is applied, these equilibrium expressions will no longer hold. We first consider the case of MNPs subjected to a static magnetic field. Brown developed an expression for a DC field-dependent Néel relaxation time [90]:
| (14) |
with dimensionless parameter h = H/Hk, with H as the applied magnetic field, the unitless anisotropic field σ = KVc/kBT, and Hk = 2K/μ0Ms. This expression holds as long as the magnetic field is not too large (h < 0.4), and only when the easy axes of the particles are parallel to the field. When σ ≥ 2, Eq. 14 can be approximated as:
| (15) |
To properly generalize this expression, averaging over all directions of the easy axis would be necessary, assuming that easy axes in a realistic sample would be randomly oriented. Coffey et al. [91] showed that the Néel rotation timescale under a DC field is highly dependent on the angle between the easy axis and the field. However, to date, no generalized expression has been derived. Furthermore, the above expressions for the Néel rotation timescale are functions of σ, which is expressed in terms of K, the effective uniaxial magnetocrystalline anisotropy constant. For spherical magnetite nanoparticles, the magnetocrystalline anisotropy is cubic rather than uniaxial, and uniaxial anisotropy may not necessarily be an accurate approximation, particularly when considering the relative orientations of the easy axis to the field [92].
Martsenyuk et al. [93] developed the following expressions for the Brownian relaxation time in a static magnetic field, when relaxation is considered parallel and perpendicular to a small probing static field:
| (16) |
| (17) |
The above expressions are derived by treating the probing field as a small perturbation to the equilibrium field. From Eq. 17, we can find low-field (ξ < 5) and high-field (ξ > 5) approximations [93]:
| (18) |
| (19) |
The above expressions hold when ξ ≪ 𝜎 and σ ≥ 10, i.e. when Brownian rotation is clearly dominant and the field strength is still relatively small (compared to the anisotropy energy of the particle). It should be noted that DC magnetization timescales will have limited applicability to AC magnetic fields, except in the adiabatic limit of slowly varying magnetic fields (i.e. when the timescale of rotation is much smaller than the timescale of changes in the magnetic field).
Yoshida and Enpuku [94] derived the following expression for the Brownian rotation timescale under a large DC magnetic field, from fitting to the Fokker-Planck equation:
| (20) |
They further determined the following set of equations to describe the dynamics of thermally blocked MNPs under relatively large AC magnetic fields (ξ0 ≥ 20):
| (21) |
and
| (22) |
where
| (23) |
and
| (24) |
Using these expressions, the dynamics of purely Brownian particles under large AC fields can be described. For high DC fields, Eqs. 19 and 20 give similar results [83].
Dieckhoff et al. [89] developed an expression in the form of Eq. 20 for the effective Néel rotation timescale under an AC field, based on fitting to experimental data:
| (25) |
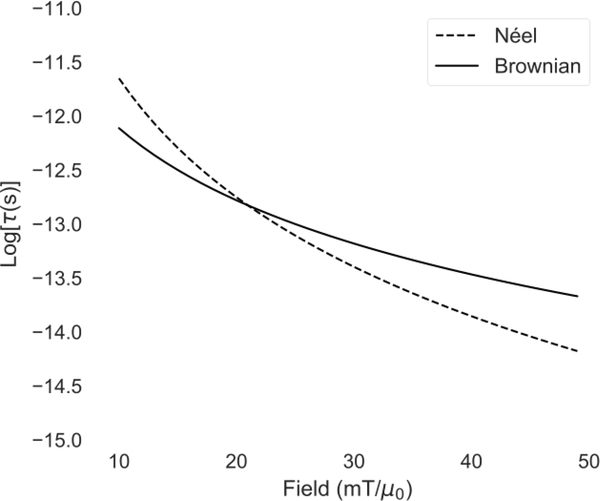
Fig. 6 is a plot of the rotation timescale calculated from Eqs. 20 and 25 as a function of applied field, with parameters dc = 25 nm, dh = 40 nm, α = 1, K = 5 kJ/m3, Ms = 420 kA/m, T = 300 K, and η = 1 cp. As can be seen from the figure, the Néel rotation time has a much stronger dependence on field strength. Making the assumption that the rotation mechanism with the shorter associated timescale will typically dominate, it can be inferred that for nanoparticles with these properties, for low fields, the Brownian mechanism will dominate, and for high fields, the Néel mechanism will dominate.
Figure 6:
Brownian and Néel rotation timescales (τB,H and τN,H from Eqs. 20 and 25) as a function of applied field amplitude, for nanoparticles with 25 nm core diameter and 40 nm hydrodynamic diameter.
The Néel mechanism may be much faster under high applied fields than the Brownian mechanism, even if the zero-field timescales are much higher. For example, with the parameters listed above, the zero-field relaxation timescales τN = 354 μs, whereas τB = 21.6 μs, a full order of magnitude lower. To understand this, we can look at the internal energy density, when the field is along the z-axis:
| (26) |
Again, θ is the angle between and H, and ϕ is the angle between and H (see Fig. 4). The energy is a function of the orientations of the magnetic dipole moment, the crystalline lattice, and the external field. Without an external field, the energy density is symmetric with two minima at the locations of the easy axes. The moment will flip when the thermal energy exceeds the energy barrier, KVc, and in this case the zero-field relaxation time τN will define the magnetization dynamics. When an external field is applied, the energy density will no longer be symmetric. One minimum will be lower, and the energy barrier will be reduced in that direction. For 0 < H < 2K/μ0Ms, there will be two unequal minima, and for H ≥ 2K/μ0Ms there will be only one minimum, resulting in a lowered average time to transition. So, for example, there will be only one minimum in the energy density once the magnetic field strength reaches 25 mT/μ0 for Ms = 420 kA/m and for K = 5 kJ/m3, indicating that for fields above 25 mT/μ0, the dynamics will be purely driven by the field, since spontaneous flipping of the magnetic moment is not possible.
In applications such as MPI, both static and alternating magnetic fields are employed. As shown above, expressions for field-dependent rotation timescales have been derived for purely Brownian and purely Néel-dominated MNPs, with certain restrictions (particularly, for Néel rotation, that the field is applied parallel to the easy axis). However, as Diessler et al. [79] demonstrated, the Brownian and Néel rotation mechanisms are coupled in a complex way that depends on applied field strength. With increasing field strength, both rotation timescales decrease, but the Néel rotation timescale decreases much more rapidly, as seen in Fig. 6.
Consequently, the zero-field relaxation timescales may indicate that particles are purely Brownian, but for stronger magnetic fields, Néel rotation may dominate the dynamics. This will be especially relevant for high frequency fields, where the period of the AC field is smaller than either the zero-field Néel or Brownian timescales. In that case, the Brownian contribution may be very small, while the amplitude of the field may be strong enough to sufficiently decrease the effective Néel rotation time so that a magnetic response is observed.
3.2. Size dependence of rotation timescales
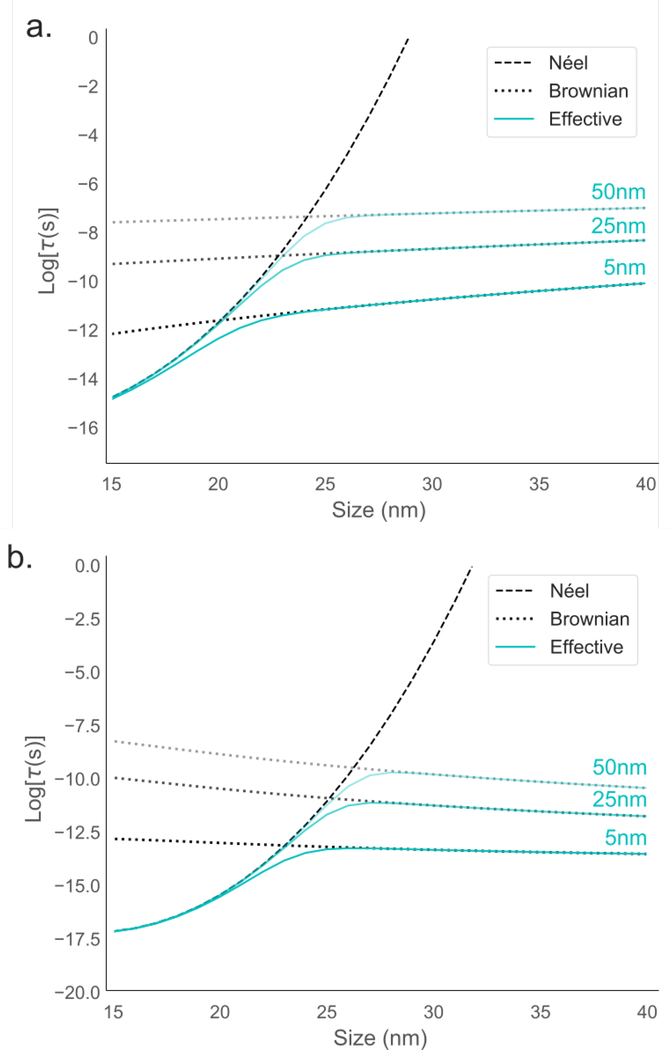
When both rotation mechanisms are considered, the rotation timescale is often described by the reduced relaxation time, τeff (Eq. 9). The reduced time implies that the mechanism with the shortest equilibrium relaxation time will dominate. The magnetic core size and hydrodynamic size of the particle will affect which mechanism will dominate, in addition to the field amplitude, as shown in the previous section. The reduced relaxation time τeff is plotted as a function of particle core size in Fig. 7a.
Figure 7:
a. τN, τB, and τeff from Eqs. 7, 8 and 9 as a function of core size. b. τN,H and τB,H from Eqs. 25 and 20 as a function of core size. In both cases, results for three different coating sizes are shown and indicated in cyan. The following parameters were used in both: H0 = 20 mT/μ0, K = 5 kJ/m3, Ms = 420 kA/m, T = 300 K, α = 1, and η = 1 cp. It should be noted that as this figure shows calculations for DC fields, it cannot necessarily be generalized to AC fields, such as those used in MPI or MFH.
Since the timescale of the Brownian rotation mechanism is highly dependent on the non-magnetic coating (τB scales as Vh), three different coatings have been plotted. The hydrodynamic diameter will be the sum of the core diameter and the non-magnetic coating on each side, so for a core diameter of 20 nm and coating of 5 nm, the hydrodynamic diameter will be 30 nm. For MPI applications, where iron oxide nanoparticles must be dispersed in water, there will always be an additional non-magnetic coating, for hydrophilicity as well as functionalization for in vivo medical purposes, such as long-term blood circulation or cancer targeting. The increase in size due to the added coating is often ignored, and Brownian rotation timescales are often compared to Néel rotation timescales with Vh = Vc [79], which is inaccurate for realistic MPI purposes. While the Néel mechanism is unaffected by added non-magnetic coatings, the Brownian mechanism is dependent on hydrodynamic size.
Using Eqs. 20 and 25, we can plot the field-dependent rotation timescales as a function of size as well. From these, we can also calculate an effective reduced rotation timescale, using the same formula as Eq. 9, but with τB,H and τN,H substituted for τB and τN. This is shown in Fig. 7b, also for three different coating sizes.
In the equilibrium (zero-field) case, the relaxation time increases with size. In the field-dependent case, the Néel rotation timescale increases with size, but the Brownian rotation timescale decreases with core size, in contrast to the zero-field case. In both cases, the coating size shifts the intersection point between Brownian and Néel rotation. Larger coatings indicate that the Néel mechanism will dominate through larger sizes. With Eqs. 20 and 25, we can extract the core size at which the two timescales intersect, which indicates the size at which the Brownian mechanism starts to dominate, as a function of field amplitude and coating size. For low field amplitudes (< 7 mT/μ0) and small coatings (< 5 nm), Brownian rotation starts to dominate when the core size is around 22 nm. As both the field amplitude increases and the coating size increases, Néel rotation remains dominant for increasing core diameters. For high field amplitudes (> 50 mT/μ0) and large coatings (> 50 nm), Néel rotation dominates up to particles that are larger than 27 nm. It should be emphasized again that these calculations are performed assuming DC fields, rather than AC fields.
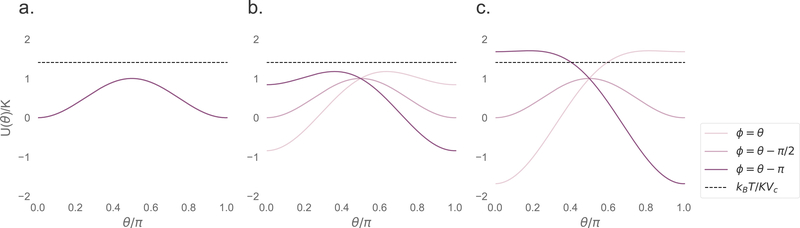
To understand this behavior, we study the energy density, described by Eq. 26. The energy density is shown in Fig. 8 for a range of different AC field strengths at different points in the field cycle, when the axis is aligned with the field (so when ϕ = 0 in Eq. 26). An AC field H = H0 cos(ωt) is assumed. For small applied fields, the energy density has two minima at all times, and so the particle has enough thermal energy to spontaneously flip. Increasing the particle size will lower the effective thermal energy density kBT/KVc, which will increase the rotation timescale. As the field is increased, asymmetry is introduced, lowering the effective energy barrier and shortening the rotation timescale. For high fields, larger particles will no longer have enough thermal energy to spontaneously flip, and the rotation becomes purely driven by the field. The timescale of rotations will become highly dependent on applied field frequency, rather than the core volume.
Figure 8:
Energy density (Eq. 26) for a 10 nm particle as a function of θ, the angle between the particle magnetic moment and the applied field, for different points in the AC field cycle, ωt. The thermal energy density kBT/KVc is also shown. a. No external applied field b. 10 mT/μ0 applied field. c. 20 mT/μ0 applied field.
4. Nonlinear models of nanoparticle dynamics
The equilibrium and linear models described in the previous section are seriously limited in their ability to describe the dynamics of nanoparticles under the fields employed in MPI and MFH. MPI, for example, relies on nanoparticles reaching magnetic saturation outside of the field-free point, which results in nonlinear behavior outside of the limits of the Debye model. The frequencies used (on the order of tens of kHz for MPI and hundreds of kHz for MFH) are high enough that equilibrium conditions cannot be assumed. Furthermore, the AC field amplitudes used for MPI (on the order of tens of mT/μ0) result in driven nanoparticle rotation rather than simple relaxation. In this section we proceed to the exploration of nonequilibrium, nonlinear models of nanoparticle dynamics.
4.1. Fokker-Planck equations
The first models we study are the Fokker-Planck equations, which describe the time evolution of the distribution of dipole-moment orientations W(x, t), where x ≡ cosϑ, so that ϑ and the azimuthal angle φ represent the direction of magnetic moment in spherical coordinates. The Fokker-Planck equation was first developed by Brown in 1963 [90]. In this interpretation, a nanoparticle’s magnetization is represented as an arrow pointing to a spot on the unit sphere. The function W(ϑ, φ, t) describes the probability distribution of moments on the surface of the sphere, given the restriction that the total number of particles must be conserved. When the surface density of the magnetization changes, a surface current J(ϑ, φ, t) will arise. The continuity equation will then be enforced:
| (27) |
In the absence of thermal fluctuations, J will only depend on the velocity of points. When thermal fluctuations are incorporated, the distribution function acquires a diffusion term, which is manifested as a term proportional to a diffusion constant D. This results in:
| (28) |
The diffusion constant D is determined by the conditions at equilibrium, where there is no applied field and ∂W/∂t = 0. If we first assume only Brownian rotation, with relaxation time constant τB, the velocity of the magnetic moment can be written as a differential equation:
| (29) |
Rewriting the numerator using vector identities and inserting the result into Eq. 28 results in:
| (30) |
This equation does not have an analytical solution for general directions of the applied field, but if we assume that the applied magnetic field is aligned with the polar axis , the dependence on the azimuthal angle φ will drop out, and Eq. 30 simplifies to:
| (31) |
Considering equilibrium conditions (∂W/∂t → 0), we find D = 1/2τB. We can then substitute x = cosϑ, and so
| (32) |
| (33) |
where ξ(t) ≡ μ0μH(t)/kBT. The average magnetization is found by integrating the probability distribution W(x, t) over the unit sphere.
To solve this equation, W(x, t) can be expanded in terms of Legendre polynomials:
| (34) |
The coefficients an(t) can then be solved recursively, with a0(t) = 1/2:
| (35) |
Considering Néel rotation only, the corresponding Fokker-Planck equation can be derived in a similar way including an anisotropy term [95]:
| (36) |
where αK = 2KVc/kBT. This form again assumes azimuthal symmetry, with the easy axis aligned with the applied field in the z-direction. Solutions to this equation are again Legendre polynomials with coefficients an(t):
| (37) |
| (38) |
Using the decoupled Fokker-Planck equations (Eq. 33 and Eq. 36), we can extract the rotation timescale separately for pure Brownian or pure Néel rotation. By truncating Eq. 35 or Eq. 37 after N terms, we can write the following matrix equation:
| (39) |
where A is an N × N matrix, and b and y are column vectors with N elements. The equilibrium timescales are represented by τ0, so for Brownian rotation τ0 = τB, and for Néel rotation τ0 = τN. For pure Brownian rotation (Eq. 33), A will be a tridiagonal matrix with components [79]:
| (40) |
| (41) |
| (42) |
The remaining components are zero. As before, ξ = μ0μH/kBT. The first component of b will be b1 = ξ/2, with remaining components equal to zero.
For Néel rotation, the components of b are b1 = ξ/2 and b2 = αK/2, and remaining components zero. In this case, A is a pentadiagonal matrix with matrix elements defined by [79]:
| (43) |
| (44) |
| (45) |
| (46) |
| (47) |
Again, the remaining components are zero. For both of these cases, the solution to Eq. 39 will be dominated by the eigenvalue of A with the largest real part, λmax. We can then find the effective rotation timescale in terms of λmax and the equilibrium relaxation time [79]. For Brownian rotation, then:
| (48) |
and for Néel rotation,
| (49) |
These solutions can be generated quickly, and provide some analytical information, which makes the Fokker-Planck formulation a useful tool for studying nonlinear nanoparticle dynamics. However, there are two major limitations to this method which must be emphasized. First, these equations are restricted to exclusively either Néel or Brownian rotation, rather than allowing for a combination of both. For nanoparticles with core diameter ∼25 nm, such as those typically used for MPI, there is some indication that both mechanisms play an important role [92] and are coupled in a non-trivial way. Second, the Fokker-Planck equation for Néel rotation assumes that the easy axes of the particles are aligned in the direction of the field. However, this approximation does not generally apply, and the relative angle between the easy axis and the field will significantly affect the nanoparticle dynamics.
Recently, Jürgen Weizenecker derived a Fokker-Planck equation for coupled Néel and Brownian rotation, allowing for full generalization of the direction of the easy axis, moment, and applied field [96]. The results will be presented here, but for the full derivation and additional details, refer to Ref. [96].
The average direction of the particle easy axis is here represented in spherical coordinates as , and the average direction of the particle moment is (note that here θ and ϕ have been redefined from previous sections to now represent the direction of the easy axis in spherical coordinates). Similarly, the direction of the magnetic field is in spherical coordinates, and in Cartesian coordinates.
This system will have only four degrees of freedom, represented by the four angles, since and are unit vectors and therefore have fixed unit radial length. The probability density can then be expressed as W(ϑ, φ, θ, ϕ). The fully generalized, coupled Fokker-Planck equation is presented here without derivation:
| (50) |
Here, α is the dimensionless damping constant which has appeared before, ui are the direction cosines for the particle moment, and pi are the direction cosines for the particle axis, defined below:
| (51) |
| (52) |
| (53) |
| (54) |
| (55) |
| (56) |
Since each component of and depends on all four angles, the above equation is fully coupled. To find solutions to this equation, we can perform a series expansion into spherical harmonics:
| (57) |
Here, the expansion coefficients are represented by , and and are the Legendre polynomials associated with the spherical harmonics and . The variables l and m are associated with the angular momentum of the particle axis, while r and q describe the angular momentum of the particle moment. It will also be necessary to calculate the derivatives of the spherical harmonics, which will have the effect of raising and lowering the indices, represented by Δ (e.g., l → l + Δl):
| (58) |
Since acting with an operator, such as taking the derivative, can only change the indices by one, our system will be simplified significantly; the operators in the Fokker-Planck equation act a maximum of two times. The full solution to the Fokker-Planck equation can then be written as a system of differential equations:
| (59) |
where represent matrix elements. In the fully general case, there will be 81 coefficients per row, with a total of 131 terms. Since we are only interested in systems where the magnetic field is applied along a single direction, here the z-direction, we can simplify this significantly by imposing azimuthal symmetry, which will send all m, q = 0. The system of differential equations then becomes:
| (60) |
Now, the matrix elements will contain only 11 coefficients with 28 total terms per row. The matrix elements are explicitly listed in Ref. [96]. To generate solutions, the matrix must be truncated, which is done by setting a maximum value for L and R. For simplicity, we will choose the maximum value for both L and R to be represented by Lmax. The matrix represented by will have dimension N × N, where . In a case of Lmax = 25, for example, we will have 625 coupled differential equations to solve.
Once the elements of are determined, we can extract the relevant physical properties, in particular, the average value of the particle magnetization in the z-direction:
| (61) |
The time evolution of will therefore represent the average particle magnetization over time. Again, further details of the derivation and solution can be found in Ref. [96].While the fully general coupled Fokker-Planck equation itself is quite complicated, once azimuthal symmetry is imposed and the required matrix is generated, solutions can be generated fairly easily and quickly, although the time required is highly dependent on Lmax, as the number of coupled differential equations scales as .
4.2. Effective field model
For relatively low applied fields, another useful model, that we will mention briefly but not discuss in detail, comes from an approximation to the Fokker-Planck equation, and is called the effective field model. In this model, the frequency of oscillation is assumed to be low enough so the distribution remains in the Maxwell-Boltzmann (equilibrium) form, so that , where ξe is an effective field. This is able to produce nonlinear dynamics for the MNP magnetization [97]:
| (62) |
where τ is again the effective rotation timescale. Here, the effective field can be calculated at every time step using the inverse Langevin function, and will change in time but not necessarily be equal to the applied cosinusoidal field. This model is similarly limited in that it assumes that MNPs experience pure magnetic relaxation, rather than driven magnetic reversal. Consequently, this approximation, as well as the Debye model, diverges when the field applied is large (greater than 4K/3Ms).
4.3. Stochastic Langevin equations
To investigate the dynamics of particles which experience both Néel and Brownian rotation, we implement stochastic simulations of Langevin equations. Computational simulations are used to model the magnetic response of nanoparticles exposed to an external magnetic field. The nanoparticles are assumed to be spherical and single-domain, and so can be characterized by a single magnetic moment, as the sizes of particles that we typically consider for biosensing applications (5–30 nm in diameter) are well within the superparamagnetic limit [98]. The size of the nanoparticles as well as the anisotropy constants will vary according to a log-normal distribution, as is typically assumed [99]:
| (63) |
where x0 is the median value and σ is the distribution shape parameter. Since some degree of particle agglomeration is common for nanoparticles in liquid suspensions, and particularly in biological environments [100], inclusion of these distributions is necessary to approximate potential agglomeration effects, as well as natural variation among particles from the fabrication process, as observed by TEM images [99, 101]. As nanoparticles are typically coated with a non-magnetic coating as functionalization for specific biomedical purposes, we differentiate between the magnetic core diameter and the hydrodynamic diameter. The particles are able to rotate via both Brownian and Néel rotation. The internal magnetization direction will rotate according to the Landau-Lifshitz-Gilbert equation. This differential equation will be coupled with the differential equation describing the general torque exerted on the easy axis of the particle [102, 82]. The coupled equations for a single particle are as follows:
| (64) |
| (65) |
where γ is the electron gyromagnetic ratio in bulk iron [103], α is the damping coefficient, η is the viscosity of the surrounding medium, and Vh is the hydrodynamic volume. The effective magnetic field, H, and the effective torque, Θ, are determined from the total particle energy:
| (66) |
where μ represents the magnitude of the particle’s magnetic moment, equal to MsVc, where Ms is the saturation magnetization and Vc is the core volume. K is the effective anisotropy constant, which includes magnetocrystalline and shape anisotropy. Subscript i denotes particle number. Here, the positions ri are initialized randomly based on the desired nanoparticle concentration, and the positions do not vary in time. The first term in Eq. 66 represents the Zeeman interaction with the applied field, Happ, the second term is the energy contribution from the effective particle anisotropy, and the third term represents the magnetic dipole-dipole interaction with all other particles i in the simulation. The vector r pointing to the center of each particle has magnitude r and direction . For applications we are considering, the applied field is sinusoidal, with amplitude H0 and angular frequency ω. We apply the field in the z-direction, and so:
| (67) |
The effective magnetic field and torque can then be found by taking derivatives of the energy, and adding thermal fluctuations:
| (68) |
| (69) |
Hth represents the fluctuation field, which accounts for microscopic effects (interactions with nuclear spins, phonons, etc.) that cause fluctuations of the orientation of the magnetic moment. It is generally assumed [104, 105, 82] that this fluctuation field is a Gaussian stochastic process whose components have the following statistical properties:
| (70) |
| (71) |
where kB is Boltzmann’s constant and T is the temperature. Similarly, Θth represents a thermally-generated torque with statistical properties:
| (72) |
| (73) |
The resulting equations can then be numerically integrated using stochastic calculus; typically, the Stratanovich-Heun scheme is used [92, 104]. Example simulation results using this method (here abbreviated LLG for Landau-Lifshitz-Gilbert), along with comparison to measured magnetic particle spectroscopy (MPS) data, is shown in Fig. 9. Often, significant computational power is required in order to generate solutions for a large number of averages using a sufficiently small time step.
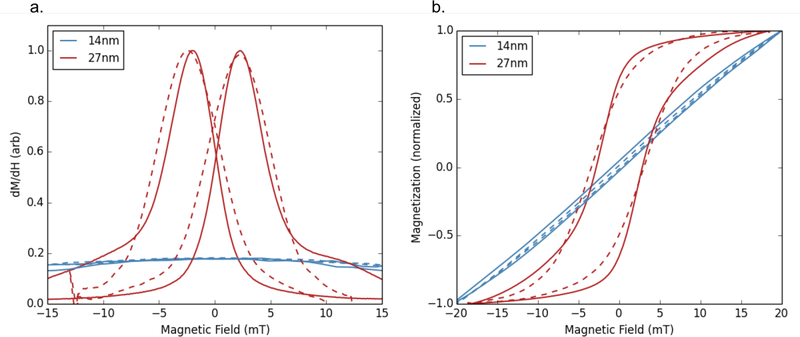
Figure 9:
a. Experimental MPS data (solid lines) and simulated data (dashed lines) for two core sizes of nanoparticles. b. Integrated MPS data (solid lines) and simulated LLG data (dashed lines) for two core sizes. Measurements were taken under an applied field of amplitude 20 mT/μ0 and frequency 26 kHz. The magnetization has been normalized for better comparisons between simulated and measured data. The 14 nm particle shows essentially no MPS signal.
These stochastic simulations, as well as the coupled Fokker-Planck equation, currently represent the most sophisticated methods of modeling nanoparticle dynamics. Fig. 10 shows simulations of the magnetic moment of monodisperse uniaxial particles with identical characteristics, for two different core sizes, plotted against time and applied field. It can be seen that the simulations match very closely, especially for the smaller particles. The remainder of this work will focus on applications of magnetic nanoparticles, specifically MPI and MFH, which both heavily depend on a detailed understanding of nanoparticle rotation dynamics. In both cases, the nonlinear simulation methods described above have been used to improve performance and efficiency.
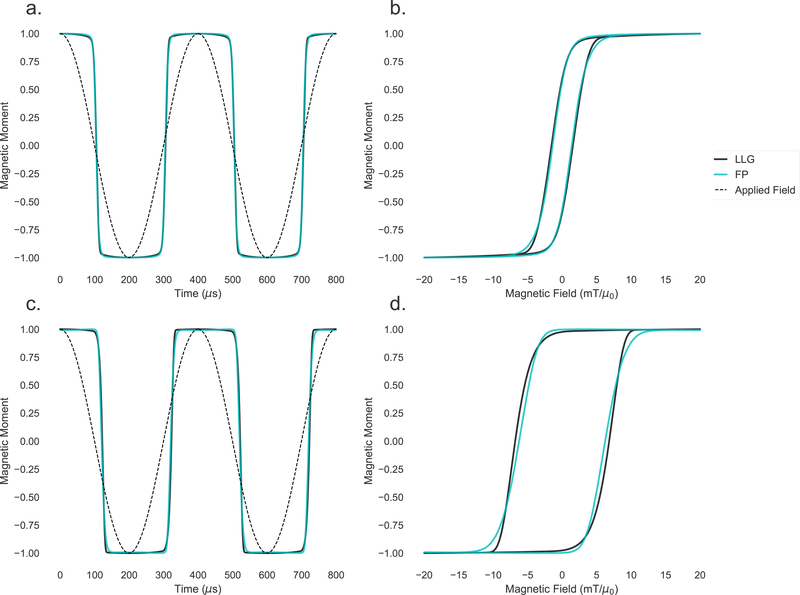
Figure 10:
Top: simulations of the magnetic moment over time (a.) and plotted against magnetic field (b.) for a 22 nm particle. Bottom: simulations of the magnetic moment over time (c.) and plotted against magnetic field (d.) for a 29 nm particle. Simulations from the stochastic Langevin equations (labeled LLG and shown in black) agree well with simulations of the coupled Fokker-Planck equation (labeled FP and shown in cyan). In the left figures, the applied cosinusoidal field is also shown for reference.
5. Magnetic particle imaging
Although substantial improvements in MPI image quality have been made in recent years, an ongoing challenge in MPI research is improving tracer detection limits and image resolution. The rotation mechanism and magnetization dynamics of MNP tracers plays a significant role in the sensitivity and resolution of the resulting MPI image, and so modeling MNP rotation is critical for optimization in MPI.
5.1. Signal generation in MPI
Although there are different specific setups of current MPI systems, the main principle behind all is the same. A strong static magnetic gradient field, generally called the selection field, is superimposed onto an oscillating drive field. The selection field gradient generates a small well-defined region at its origin where the magnetic field is zero, referred to as the field-free point (FFP). Outside of the FFP, the static field increases in magnitude very rapidly, and therefore the magnetization of the particles is saturated in any region outside of the central point. The FFP can then be scanned across the sample. The resulting induced signal will be nonzero only when the FFP is at the location of the nanoparticle tracers. A tomographic image is formed as the FFP is scanned across the sample, causing any tracers in that voxel to reverse their magnetization and induce a signal in the detection coil. The shape of the differential susceptibility M′(H(t)) determines the point-spread function (PSF) of the MPI imaging system. Understanding the factors that influence the shape of the PSF is key to optimizing the signal and the entire MPI system.
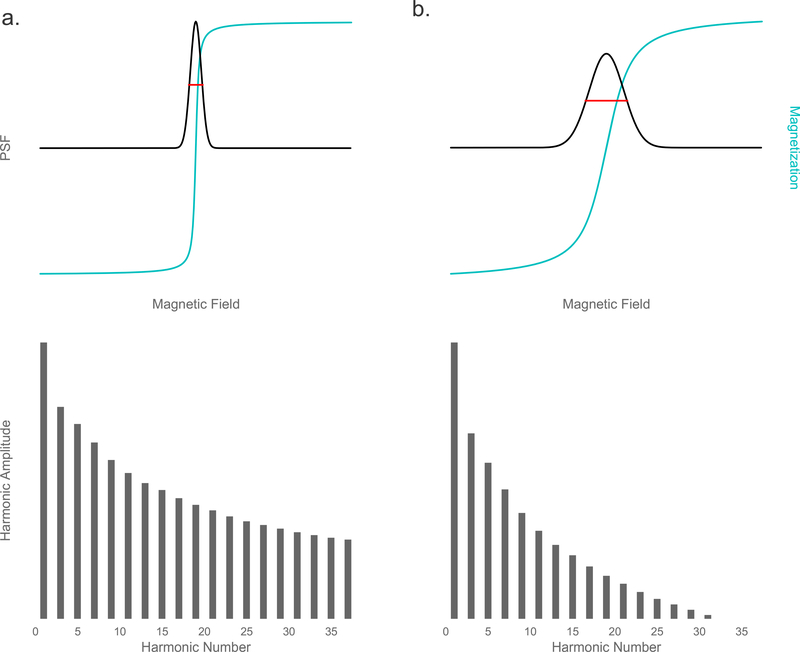
There are two primary MPI system setups currently in development. The first is f-space (frequency-space) MPI, developed by Philips Hamburg, which first measures a harmonic spectrum system function for each sample and reconstructs the image in frequency space [33]. The second is f-space MPI, developed by Goodwill and Conolly at Berkeley, which measures M′(H(t)) directly as the point-spread function (PSF) [106, 34, 53]. The resulting image is then a convolution of the tracer distribution and the PSF. In both methods, the image resolution is defined by the width of the PSF, often described by the full-width at half-maximum (FWHM), and the signal magnitude is defined by the height of the PSF. Resolution is also indicated by the harmonic spectrum (the Fourier transform of M′(H(t))); the wider the PSF, the faster the harmonic amplitudes will drop off (see Fig. 11). For this reason, ratios of the harmonic amplitudes (for example, the ratio of the 5th to the 3rd harmonic, A5/A3) can be used as a concentration-independent measurement of signal quality.
Figure 11:
Nearly ideal (a.) and more realistic (b.) measurements of the point-spread function and resulting harmonics. Top: Particle magnetization (blue) and resulting PSF (black), with FWHM indicated in red. Bottom: Harmonic spectrum.
Magnetic particle spectroscopy (MPS) is functionally a zero-dimensional MPI system that applies an AC field uniformly across the sample and records the inductive signal in a receive coil. With MPS, the PSF of a given sample can be directly extracted. The discussion in this section will be limited to zero-and one-dimensional systems, but the principles apply generally to two- and three-dimensional systems as well. When the particle magnetization is approximated as the Langevin function (Eq. 3), the derivative of the magnetization is given by:
| (74) |
with n as the particle concentration. As expected, the signal scales linearly with concentration. The derivative of the Langevin function is
| (75) |
for ξ > 0, and equal to 1/3 when ξ = 0. M′(H(t)) therefore has a maximum at ξ = 0, and decreases rapidly for increasing ξ, until it approaches zero in the saturation region. The FWHM here is
| (76) |
The FWHM of M′(H(t)), which can be used to predict the image resolution that can be obtained by the sample, is then given by:
| (77) |
The signal-to-noise ratio (SNR) is indicated by the height of the PSF, which is proportional to the concentration and saturation magnetization, as well as the slope of the magnetization. In this idealized case, the peak of the PSF (where the slope of the magnetization is highest) will be given when the field strength is zero:
| (78) |
The width and height of the PSF (predicting resolution and SNR) are highly affected by properties of the sample itself, the sample environment, and the applied field. To optimize both, highly mondisperse particle sizes are required, as well as strong magnetic gradients.
For the remainder of the discussion, we will examine the one-dimensional case in the x-space formulation, following [34], although the principles apply equally to the Fourier space construction. In a 1D MPI system with linear gradient −G and time-dependent field H0(t), the magnetic field at a position x will be:
| (79) |
The location of the field-free point, xs(t), can be found by solving this equation for H(x, t) = 0:
| (80) |
Or equivalently, the magnetic field at position x can be written in terms of the location of the FFP:
| (81) |
If we assume a continuous distribution of nanoparticles ρ(x) in the adiabatic limit (assuming that the particles’ magnetization reaches equilibrium at every time step of the applied field), the sample magnetization follows the Langevin function:
| (82) |
The magnetization flux can be obtained by integrating over x, which can be written as a convolution:
| (83) |
The changing magnetic flux will be recorded by an inductive detector that will have some sensitivity, σs. The received signal will then be:
| (84) |
Defining β ≡ kBT/σsμ0μ2G and the PSF , the signal can be written as a simple spatial convolution (again emphasizing that this is the signal received in the adiabatic limit):
| (85) |
The velocity of the FFP, , is simply the scanning rate of the FFP of the system.
5.2. Relaxation effects in image reconstruction
For realistic MPI systems, the adiabatic approximation will not hold. The MNP moments will not align instantaneously with the field; instead, there will be some time required for alignment. This relaxation can be modeled using a Debye (exponential) relaxation kernel r(t) [107]:
| (86) |
Plugging in Eq. 85, this becomes
| (87) |
This expression for the total signal now contains two convolutions; the inner convolution here is spatial, and the outer convolution is temporal.
The MPI image can be generated by mapping the signal equation to the spatial location of the FFP. The resulting image will be a convolution of the adiabatic image with the relaxation kernel [108, 107]:
| (88) |
If we assume that the FFP is scanned linearly across the sample with a constant scanning rate vs, then the location of the FFP can be expressed as xs(t) = vst, and so t = xs(t)/vs. When this is inserted into Eq. 88, the total MPI image equation, including relaxation, becomes [107]:
| (89) |
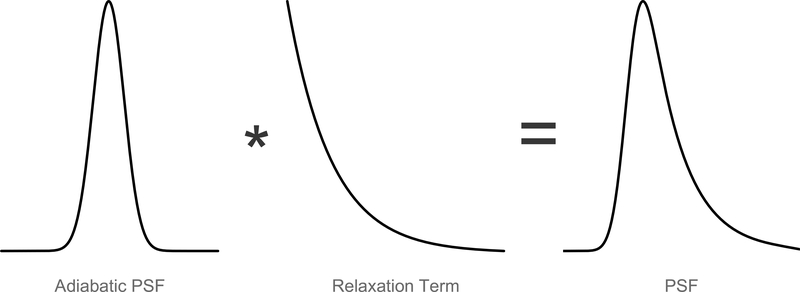
The convolution is now purely spatial, with unit area r(xs(t)/vs)/vs, which allows us to examine the effects of relaxation on the image. As seen in Fig. 12, the particle relaxation increases the width of the PSF, which decreases the image resolution, causing additional blur. The Debye exponential relaxation kernel is:
| (90) |
where u(t) is the Heaviside step function (equal to 0 for t < 0, and 1 for t > 0), which serves the function of “turning on” the relaxation at t = 0. As before, τ is the effective relaxation time, which will be a function of particle characteristics as well as properties of the drive field. Again substituting t = xs(t)/vs, the relaxation kernel becomes:
| (91) |
Figure 12:
Non-adiabatic PSF shown as a convolution of the adiabatic PSF and a relaxation term. The effect is a widening of the PSF, resulting in lower image resolution.
Inserting this into Eq. 89 gives the MPI image (where ):
| (92) |
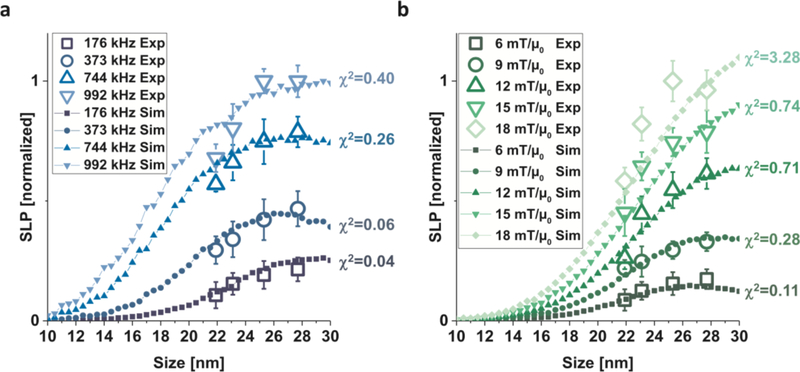
This important result gives an indication of the trade-offs encountered when attempting to optimize the MPI signal. While the adiabatic signal scales with MNP core size, indicating that increasing particle size should uniformly improve SNR and resolution, the relaxation time τ will monotonically increase with particle size, as indicated by Eqs. 7, 8 and 9. Consequently, increasing particle size will also contribute to increased relaxation effects, causing additional blur in the image. This suggests that there will be an optimal particle core size which maximizes signal and resolution; experimentally, the optimal size has been found to be around 28 nm for magnetite particles [57].
5.3. MNP optimization for performance in vivo
MPI image quality will be dependent on many factors, including hardware parameters (e.g. gradient field strength and coil sensitivity) and tracer properties. Development of highly monodisperse single-phase spherical magnetite MNPs, as well as hardware improvements, has so far resulted in significant improvement in MPI image quality [109, 110, 38]. Controlling drive field conditions (e.g. frequency and amplitude) can minimize particle relaxation behavior, resulting in substantial improvements to MPI signal and resolution [107]. Remaining areas for improvement include optimizing MNP size and monodispersity, as well as optimizing for their specific performance in vivo, which involves an understanding of the physical in vivo environment.
According to Langevin theory, the image resolution should be inversely proportional to the MNP core volume (Eq. 77), and signal strength should be proportional to the square of the volume (Eq. 78) [34, 106]. In this model, then, increasing the nanoparticle core size should result in a dramatic effect on image quality, and so it is often assumed that increasing particle core size will have a unilaterally positive effect on image quality, as long as the particles remain within the superparamagnetic limit. However, as outlined above, relaxation effects increase with core size [109, 111]. Practical limits on performance improvement will therefore arise from increasing core size indefinitely. Previous results have indicated that large core sizes result in image blurring [109, 108, 112, 113, 114] and reduced signal-to-noise ratio (SNR) [57, 115, 108]. Increased interparticle magnetostatic interaction strength and increased polydispersity are additional practical difficulties that arise when increasing MNP core size.
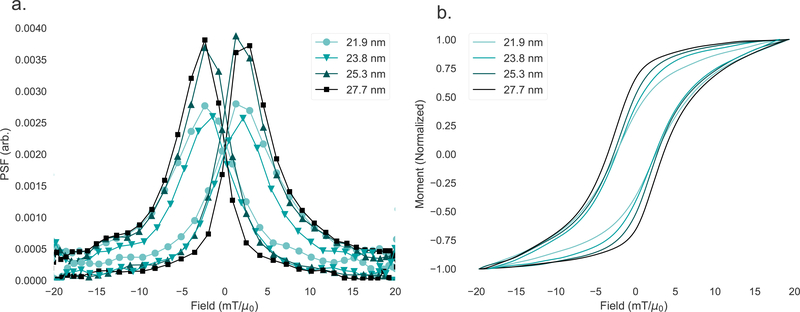
There has been significant recent work into optimizing particles, particularly their size, for MPI, aided by a progressing understanding of nanoparticle rotation dynamics [57, 115]. Examples of measured point-spread functions for different sized MNP tracers are shown in Fig. 13. The two smaller samples (21.9 nm and 23.8 nm) indicate significantly worse signal intensity (indicated by the peak of the PSF) and resolution (indicated by the FWHM of the PSF) than the larger two samples (25.3 nm and 27.7 nm). The differences in magnetization response among the four samples is indicated by the hysteresis loops (M(H)) generated by integrating the time-domain MPS data. Following field reversal, the larger particles’ magnetic moments will take longer to follow the field, resulting in an increased phase lag with size which has the effect of increasing the width of the hysteresis loop. While the PSF for the two smaller samples and two larger samples each look similar, the M(H) curve clearly shows that there is a continuous progression with size, indicated by the increasing steepness of the curve and width of the loop.
Figure 13:
a. The point-spread function for four nanoparticle samples as measured by MPS. The smaller samples show decreased peak height and broader FWHM compared to the larger samples. b. The integrated M(H) magnetization curves.
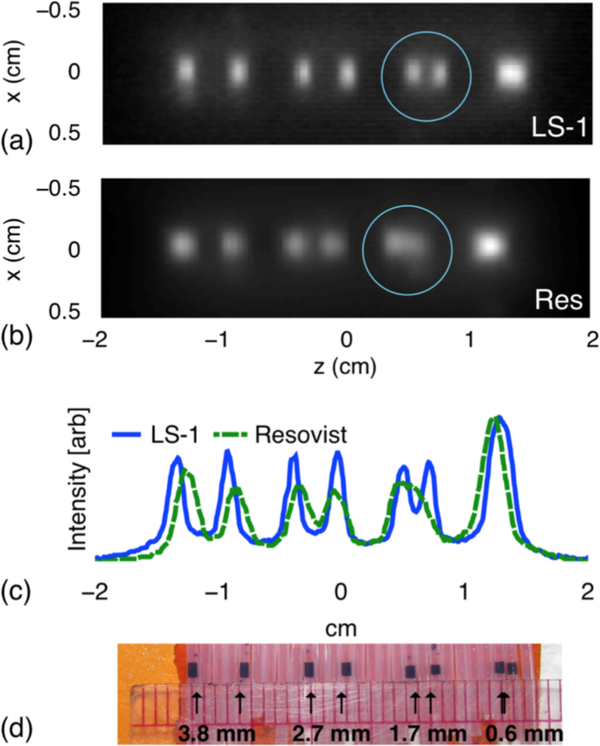
The optimal core size has been found, from experiment and simulation, to be between 27 and 28 nm [110, 116]; larger core sizes result in significant relaxation effects and potential for agglomeration due to interparticle interactions. We can see, for example, that MPI resolution is significantly improved with optimized particles (LS-1) compared to the commercially-available Resovist tracers (Fig. 14). Sensitivity also improves with size-optimized particles; detection of nanogram concentrations of iron oxide has been successfully achieved [56].
Figure 14:
Resolution phantoms. MPI images of phantoms containing LS-1 (a) and Resovist (b); blue circles were overlaid for emphasis. c. Cross sections. d. Photograph of the LS-1 phantom. Reproduced with permission from [116].
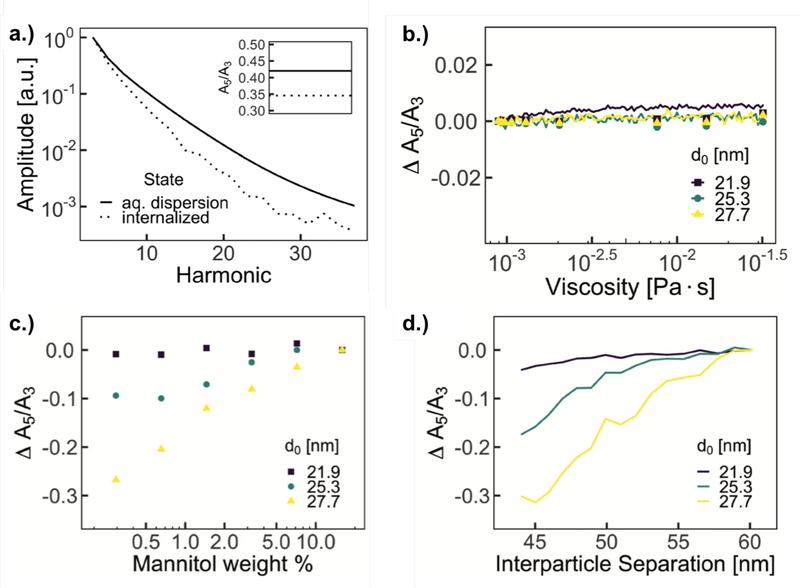
When optimizing for performance in vivo, it is important to understand environmental factors, like viscosity and pH, which may affect MNP dynamics. Brownian rotation, in particular, is influenced by the viscosity of the surrounding medium, as is evident from the equilibrium Brownian relaxation time (Eq. 8). The intracellular magnetic performance of MNPs has been studied [117], and a significant drop in MPI signal quality was found after internalization in cells (Fig. 15a). No evidence of physical degradation of the particles was found.
Figure 15:
a. Comparison of pre- and post-internalization MPS harmonic distribution in cells (with inset A5/A3) b. Magnetic particle spectroscopy performance, A5/A3, as a function of solution viscosity of three different batches of magnetite nanoparticles. Simulation results, shown as lines, of equivalent nanoparticle characteristics show consistent performance over expected range of viscosities. c. Magnetic particle spectroscopy performance, A5/A3, as a function of mannitol weight % of three different batches of MNPs with increasing average core diameters. d. Simulations of A5/A3 of equivalent nanoparticle characteristics for different average interparticle separations. Reproduced with permission from [117].
In Ref. [117], nonlinear simulations of stochastic Langevin equations were performed, with input values of nanoparticle size, size distribution, hydrodynamic size, and saturation magnetization matching measurements of each nanoparticle sample. A range of viscosity values and interparticle separations were input directly into the simulations. For each set of simulations, all other values (e.g. temperature and physical particle characteristics) were assumed to be constant. A minimal dependence on signal quality with viscosity was found, based on experimental measurements of glycerol solutions and simulations of different viscosity values (Fig. 15b). Interparticle interaction strength, however, did have a significant impact on the signal, as studied by mannitol matrix measurements and simulations (Fig. 15c and d). TEM images indicate that MNPs are localized in vesicles within cells, which has the effect of physically bringing particles closer together, compared to when free in solution, which increases average dipole interaction strength, decreasing overall magnetic performance [117]. Minimizing interparticle interactions, then, for example through surface modification, is key to improving performance when internalized in cells, such as required for cell tracking applications in MPI.
5.4. Multicolor imaging and sensing
In conventional MPI, the signal from the nanoparticle tracer response creates a single contrast image, in which the strength of the tracer signal at each spatial location is translated to image intensity of the corresponding pixel during the image reconstruction process. The result of this is a grayscale (or equivalent) image. There have been several recent studies investigating the development multicolor (also referred to as “multi-contrast” or “multi-channel”) MPI, which involves separating the signals generated from different tracers or different environments [118]. If the signal from different particle types or environments can be separately reconstructed on individual channels, a multicolor image can be generated in which each channel corresponds to one color, enabling a new range of therapeutic and diagnostic applications that could function in combination with MPI.
When considering potential theranostic applications of multicolor MPI, a major enabling factor is the wide potential for functionalization of the nanoparticle surface. Iron oxide nanoparticles are particularly versatile. When synthesized via thermolysis of organometallic precursors, superparamagnetic iron oxide nanoparticles (SPIONs) need to be coated with a capping ligand, e.g. oleic acid, which renders them hydrophobic. In order to be dispersed in water for use in biomedical applications, they must undergo a phase transfer process, typically either by ligand exchange [119, 120, 121] or with the addition of a polymer coating, for example, poly(maleic anhydride alt-1-octadecene) (PMAO), which interacts hydrophobically with the capping ligand [122]. Once SPIONs are coated with a polymer, additional molecules can be attached to the surface.
When SPIONs are delivered into the bloodstream, extracellular serum proteins adsorb onto the nanoparticle surface, forming a protein corona [123, 124], which may decrease nanoparticle stability and facilitate nanoparticle removal from the bloodstream and body [125]. To reduce protein adsorption and improve biocompatibility and stability, SPIONs can be coated with poly(ethylene glycol) (PEG) containing co-polymer [38] in addition to PMAO, which enables long term circulation of particles in the blood [126, 61], necessary for applications like cardiovascular imaging. Alternatively, SPIONs can be coated with silica [42, 43], on top of which ligands can be attached, such as ligands for cancer targeting [48, 49, 127, 128, 129]. Cancer targeting ligands include antibodies, oligosaccharides, oligopeptides, and folic acid [130, 131].
In addition to functionalization for long-term blood circulation and cancer targeting, SPIONs can be coated on medical devices; for example, on diagnostic catheters and guide wires used for cardiovascular interventions [132, 133, 134]. A possible application of multicolor MPI, then, would involve distinguishing between particles coated on a catheter and guide wire (used for inserting stents in the heart or diagnosing heart disease) and particles flowing in the blood, on which preliminary studies have been done [134]. This technique could, in theory, replace coronary catheter angiography. Coronary catheter angiograms, which require threading a catheter through the femoral artery, are performed at a rate of approximately 1 million per year in the United States [135]. The catheters are used to inject iodine into the heart, which is then used as a contrast agent in order to allow diagnosis with x-ray imaging or CT. However, iodine can have serious health consequences, particularly for elderly patients or patients with chronic kidney disease. For these patients, the injection of iodine can result in renal failure, and the insertion of the catheter through the femoral artery itself carries a finite risk [135]. Multicolor MPI could therefore provide a significantly safer alternative to coronary angiography.
To create a multi-channel image with MPI, the signal from MNPs of different sizes or in varying chemical, thermal, or viscous environments must be distinguished, in contrast to single-channel MPI which produces a monochromatic image. This is possible due to the sensitivity of the rotation behavior of MNP tracers. Properties that affect the MNP rotation behavior, and therefore resulting MPI signal, as we have seen, include viscosity, interparticle interaction strength, temperature, material, mobility state, core size, and hydrodynamic size. Sensing of any of these properties could in principle be integrated into MPI, and there has already been investigation into many of these [136, 137, 64, 138, 139, 118, 140, 141, 142], including viscosity mapping in [143], and temperature mapping in [144] and [66]. These studies show that the MPI signal is highly sensitive to particle properties and environment, which can enable discrimination based on particle type [118, 140, 142] and in some cases quantification of environmental properties.
Because of this, multicolor MPI could be used to combine medical imaging with disease diagnostics and treatment. For example, quantifying the viscosity of the MNP environment can provide information about nanoparticle binding to or internalization in cells, which would be useful for applications like targeted cancer treatment. Blood viscosity could also be detected as an indication of disease [145], such as a brain hemorrhage, during which blood coagulation occurs [146]. Temperature can be another significant marker of disease; malignant cells have a higher temperature than normal cells, by possibly up to 1 degree Celsius, and general inflammation results in temperature changes [147, 148, 149, 150]. Detecting local temperature changes during an imaging scan could therefore provide information for disease diagnostics, and detection sensitivity of < 1°C should be possible with MPI [66]. Magnetic fluid hyperthermia (MFH), in which magnetic nanoparticles are used to locally heat cancer cells [29, 30, 70, 151, 152, 32, 72], discussed in the following section, could also be combined with active monitoring of temperature with MPI, allowing for a thermally-controlled cancer treatment system. Additionally, cancer detection via protease sensing with MPI could be achieved by detecting changes in interparticle interaction strength using linker peptides that are cleaved in the presence of proteases expressed by malignant cells [69].
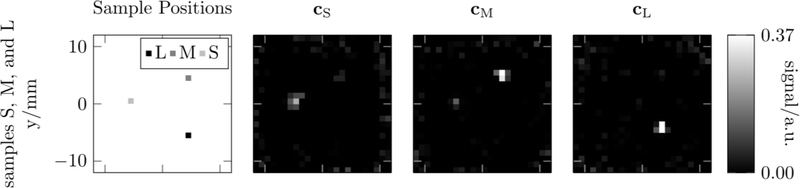
To highlight one example, multicolor MPI based on core size discrimination was realized in [142]. As seen in Fig. 13, there is a clear dependence of MPI signal on core size as a result of differing rotation behavior, which enables signal separation. This behavior can be supported and predicted by nonlinear simulations [153], as seen, for example, in Fig. 10, providing a basis for signal separation with MPI based on core size discrimination. Results of a multi-channel reconstruction are shown in Fig. 16. In this example, each particle type could be separately functionalized, e.g. to circulate in the blood or coated on a catheter or guide wire, enabling simultaneous imaging of each element as a replacement for coronary angiography.
Figure 16:
Multi-channel reconstruction with Mavg = 10000 of the multi-sample datasets with all three samples in the field of view. The location of the samples is indicated in the first column. Reconstructions on each channel cS, cM, and cL are shown in the second to fourth columns. Reproduced with permission from [142].
6. Magnetic fluid hyperthermia
In magnetic fluid hyperthermia (MFH), magnetic nanoparticles are dispersed throughout cancerous tissue, either through direct injection [154] or biological targeting [155, 156]. Under subsequent application of an appropriate AC magnetic field, the particles’ magnetic moments will rotate, dissipating energy and transferring heat to the tumor, resulting in cell damage or death. MFH, consequently, provides the benefit of specific localized cancer treatment.
Cytotoxic heat is generated from power losses that result from the lagging of particle magnetizations behind the applied field. As with MPI, iron oxide nanoparticles are typically used because of their magnetic properties and biocompatibility [1, 152]. In order to be effective as a means of therapy, hyperthermia requires heating of tumor cells to between 42° and 45° C from a baseline of 37° C, so tailoring of nanoparticle properties and applied fields for optimal heating is critical [152]. The efficiency of a given nanoparticle sample to generate heat in MFH will be dependent on the MNP concentration, saturation magnetization, and core size [31, 157]. The viability of MFH has been questioned, since most clinical trials have required a high dose of iron oxide nanoparticles [158, 159], and the long-term toxicity of high MNP doses has not yet been thoroughly studied [160]. It is therefore imperative to the success of MFH that particle heating at low iron concentrations be maximized. Understanding the nonlinear magnetization behavior of nanoparticles under an AC magnetic field, which result in power losses, is crucial to this goal.
The mechanism of heat generation in MFH was first studied by Rosensweig [30] in the context of linear response theory. In this model, the power dissipation due to hysteresis losses resulting from the lagging of the magnetization behind the applied field is given by:
| (93) |
where, as before, H0 is the externally applied field amplitude, f is the applied field frequency, g(R) is the lognormal distribution of particle sizes, and χ′′ is the out-of-phase susceptibility. As χ′′ is strongly dependent on the field frequency and effective MNP rotation timescale, as indicated in Eq. 11, it is evident that particle relaxation effects play a crucial role in determining the power dissipation. In this model, which inherently assumes small anisotropy and core size, the power loss is maximized at:
| (94) |
This would be the theoretically optimal value for the rotation timescale in order to maximize dissipation due to magnetic viscosity, and corresponds to a core size of 14 nm for magnetite, assuming equilibrium relaxation times [30]. If the rotation timescale is shorter than this optimal value, the shorter phase lag will result in a reduction of the area of the hysteresis loop. If τ is longer than 1/2πf, the particle magnetization will rotate too slowly, and will not be able to follow the applied field. While this model is instructive and attractive due to its simplicity, linear response theory is not applicable for all particle core sizes and applied fields, as discussed previously.
Alternatively, heat generation can be predicted directly from the area of simulated (e.g. using the nonlinear methods described in section 4) or measured hysteresis loops. The heat generated from a given nanoparticle during a field-driven rotation process will be equal to the magnetic energy required for magnetic alignment over one cycle of the applied field [161], which is represented by the hysteresis loop M(H). The heat generated will therefore be proportional to the area of the hysteresis loop [76]:
| (95) |
From this, we can identify the specific loss power (SLP), also called the specific absorption rate (SAR), which indicates the rate of energy loss per unit mass:
| (96) |
where ρ is the mass density of the nanoparticles. The SLP can also be expressed in terms of the specific heat capacity c of the surrounding medium (e.g. water):
| (97) |
where dT/dt is the measured temperature change over time. This second expression, then, is used to experimentally measure the SLP, while Eq. 96 can be used to predict the SLP from nonlinear simulations, for example.
In MFH, high frequency fields are applied (on the order of hundreds of kHz), and therefore Néel rotation will be the dominant mechanism for nanoparticle rotation. The energy generated will then be related to the anisotropic energy barrier that the particles must overcome, KVc, and so the heating efficiency will therefore be highly dependent on particle size. As outlined in section 3, however, the effective rotation timescale will also be strongly dependent on the applied field. Biological safety constraints limit the applied field amplitudes and frequencies that can be used for MFH; the “Brezovich-Criterion” requires that H0·f0 ≤ 615 kHz·mT/μ0 in order to limit peripheral nerve stimulation due to induced eddy currents in the body [162].
There have been many studies attempting to optimize nanoparticles for maximal heating rate, particularly investigations into the effect of size [70, 71, 151, 152, 72], structure [161, 163, 164], and aggregates [165, 166]. Stochastic Langevin simulations were used in Ref. [72] to study size and field dependence of heating rate. Results are shown in Fig. 17, with corresponding experimental data. There is a general increase in SLP with increasing field frequency and amplitude, as well as an increase in SLP with core size even up to core sizes as large as 28 nm, in stark contrast to the predictions from linear response theory. From these results, it is clear that nonlinear models, which incorporate field and frequency dependence of nanoparticle rotation dynamics, are necessary in order to accurately model heat generation in MFH.
Figure 17:
Comparison of normalized size-dependent SLP values from experiment (open symbols) and dynamic Monte Carlo simulations (closed symbols) for various frequencies at fixed H0 = 6 mT/μ0 (a) and field amplitudes at fixed f = 176 kHz (b). Simulations were performed with an effective anisotropy K = 4 kJ/m3 and damping parameter α = 0.7. The individual fitting parameters (χ2) are shown next to each simulated curve. Reproduced with permission from [72].
7. Conclusion
Here we have presented an overview of the theoretical models describing the dynamics of magnetic nanoparticles, including their applications to magnetic particle imaging and magnetic fluid hyperthermia. The dependence of various factors on nanoparticle magnetization dynamics, such as nanoparticle size and applied field, has been explored. Applied field strength affects the Néel and Brownian rotation mechanisms differently, such that even for Brownian particles (under equilibrium conditions), Néel rotation may dominate for strong field amplitudes. Equilibrium and linear models are useful tools which can be used to gain a basic understanding of the dependence of the rotation timescale on certain parameters, but they are clearly incomplete. Fokker-Planck equations or simulations of stochastic Langevin equations are typically required in order to fully simulate the nonlinear behavior that arises in both MPI and MFH.
Acknowledgments
This work was supported in part by NIH/NIBIB grants EB008192, EB013689, EB013520. CS was supported by a National Science Foundation Graduate Research Fellowship under Grant DGE-1256082. We would like to acknowledge Dr. Amit Khandhar, Dr. Mathew Ferguson, Dr. Hamed Arami, Dr. Eric Teeman, Dr. Ryan Hufschmid, Dr. Ulrich Engelmann, and Alyssa Troksa for contributions to the work described here. We would also like to acknowledge our collaborators, particularly Dr. Tobias Knopp, Dr. Martin Möodel, Dr. Matthias Gräser, Dr. Thorsten M. Buzug, Dr. Steven Conolly, and Dr. Patrick W. Goodwill, as well as Dr. Jürgen Weizenecker and Dr. Daniel Reeves.
References
- [1].Krishnan Kannan M. Biomedical nanomagnetics: a spin through possibilities in imaging, diagnostics, and therapy. Magnetics, IEEE Transactions on, 46(7):2523–2558, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Colombo Miriam, Carregal-Romero Susana, Casula Maria F, Gutiérrez Lucía, Morales María P, Böhm Ingrid B, Heverhagen Johannes T, Prosperi Davide, and Parak Wolfgang J. Biological applications of magnetic nanoparticles. Chemical Society Reviews, 41(11):4306–4334, 2012. [DOI] [PubMed] [Google Scholar]
- [3].Widder Kenneth J, Senyei Andrew E, and Ranney David F. Magnetically responsive microspheres and other carriers for the biophysical targeting of antitumor agents. In Advances in Pharmacology, volume 16, pages 213–271. Elsevier, 1979. [DOI] [PubMed] [Google Scholar]
- [4].Dobson Jon. Magnetic nanoparticles for drug delivery. Drug development research, 67(1):55–60, 2006. [Google Scholar]
- [5].McBain Stuart C, Yiu Humphrey HP, and Dobson Jon. Magnetic nanoparticles for gene and drug delivery. International journal of nanomedicine, 3(2):169, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Prijic Sara and Sersa Gregor. Magnetic nanoparticles as targeted delivery systems in oncology. Radiology and oncology, 45(1):1–16, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bulte Jeff WM, Douglas Trevor, Witwer Brian, Zhang Su-Chun, Strable Erica, Lewis Bobbi K, Zywicke Holly, Miller Brad, Van Gelderen Peter, Moskowitz Bruce M, et al. Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nature biotechnology, 19(12):1141, 2001. [DOI] [PubMed] [Google Scholar]
- [8].Kraitchman Dara L, Heldman Alan W, Atalar Ergin, Amado Luciano C, Martin Bradley J, Pittenger Mark F, Hare Joshua M, and Bulte Jeff WM. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation, 107(18):2290–2293, 2003. [DOI] [PubMed] [Google Scholar]
- [9].Amado Luciano C, Saliaris Anastasios P, Schuleri Karl H, St John Marcus, Xie Jin-Sheng, Cattaneo Stephen, Durand Daniel J, Fitton Torin, Kuang Jin Qiang, Stewart Garrick, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proceedings of the National Academy of Sciences, 102(32):11474–11479, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Arai Takayasu, Kofidis Theo, Bulte Jeff WM, De Bruin Jorg, Venook Ross D, Berry Gerald J, Mcconnell Michael V, Quertermous Thomas, Robbins Robert C, and Yang Phillip C. Dual in vivo magnetic resonance evaluation of magnetically labeled mouse embryonic stem cells and cardiac function at 1.5 t. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 55(1):203–209, 2006. [DOI] [PubMed] [Google Scholar]
- [11].Au Ka-Wing, Liao Song-Yan, Lee Yee-Ki, Lai Wing-Hon, Ng Kwong-Man, Chan Yau-Chi, Yip Mei-Chu, Ho Chung-Yee, Wu Ed X, Li Ronald A, et al. Effects of iron oxide nanoparticles on cardiac differentiation of embryonic stem cells. Biochemical and biophysical research communications, 379(4):898–903, 2009. [DOI] [PubMed] [Google Scholar]
- [12].Chithrani Devika B. Nanoparticles for improved therapeutics and imaging in cancer therapy. Recent patents on nanotechnology, 4(3):171–180, 2010. [DOI] [PubMed] [Google Scholar]
- [13].Ugelstad J, Kilaas L, Stenstad P, Ellingsen T, Bjørgum J, Aune O, Nils Nilsen T, Schmid R, and Berge A. Magnetic separation techniques applied to cellular and molecular biology. In Preparation and Application of Monosized Composite Polymer Particles. Herausgegeben von JT Kemshead. Wordsmith’s Conference Publications, Bristol, UK, pages 235–254, 1991. [Google Scholar]
- [14].Weber Christoph and Falkenhagen Dieter. Specific blood purification by means of antibody-conjugated magnetic microspheres. In Scientific and Clinical Applications of Magnetic Carriers, pages 371–378. Springer, 1997. [Google Scholar]
- [15].Nam Jwa-Min, Thaxton C Shad, and Mirkin Chad A. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. science, 301(5641):1884–1886, 2003. [DOI] [PubMed] [Google Scholar]
- [16].Lee Hakho, Sun Eric, Ham Donhee, and Weissleder Ralph. Chip–nmr biosensor for detection and molecular analysis of cells. Nature medicine, 14(8):869, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Polito Laura, Monti Diego, Caneva Enrico, Delnevo Eleonora, Russo Giovanni, and Prosperi Davide. One-step bioengineering of magnetic nanoparticles via a surface diazo transfer/azide–alkyne click reaction sequence. Chemical communications, (5):621–623, 2008. [DOI] [PubMed] [Google Scholar]
- [18].Colombo Miriam, Ronchi Silvia, Monti Diego, Corsi Fabio, Trabucchi Emilio, and Prosperi Davide. Femtomolar detection of autoantibodies by magnetic relaxation nanosensors. Analytical biochemistry, 392(1):96–102, 2009. [DOI] [PubMed] [Google Scholar]
- [19].Kim Jaeyun, Piao Yuanzhe, Lee Nohyun, Park Yong Il, Lee In-Hwan, Lee Jung-Ho, Paik Seung R, and Hyeon Taeghwan. Magnetic nanocomposite spheres decorated with nio nanoparticles for a magnetically recyclable protein separation system. Advanced Materials, 22(1):57–60, 2010. [DOI] [PubMed] [Google Scholar]
- [20].Cai Shaoyu, Liang Guohai, Zhang Peng, Chen Hui, Zhang Song, Liu Baohong, and Kong Jilie. Rational strategy of magnetic relaxation switches for glycoprotein sensing. Analyst, 136(1):201–204, 2011. [DOI] [PubMed] [Google Scholar]
- [21].Harisinghani Mukesh G, Barentsz Jelle, Hahn Peter F, Deserno Willem M, Tabatabaei Shahin, Hulsbergen van de Kaa Christine, de la Rosette Jean, and Weissleder Ralph. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. New England Journal of Medicine, 348(25):2491–2499, 2003. [DOI] [PubMed] [Google Scholar]
- [22].Kircher Moritz F, Mahmood Umar, King Raymond S, Weissleder Ralph, and Josephson Lee. A multimodal nanoparticle for preoperative magnetic resonance imaging and intraoperative optical brain tumor delineation. Cancer research, 63(23):8122–8125, 2003. [PubMed] [Google Scholar]
- [23].Billotey C, Wilhelm C, Devaud M, Bacri JC, Bittoun J, and Gazeau F. Cell internalization of anionic maghemite nanoparticles: quantitative effect on magnetic resonance imaging. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 49(4):646–654, 2003. [DOI] [PubMed] [Google Scholar]
- [24].Morawski Anne M, Winter Patrick M, Crowder Kathryn C, Caruthers Shelton D, Fuhrhop Ralph W, Scott Michael J, David Robertson J, Abendschein Dana R, Lanza Gregory M, and Wickline Samuel A. Targeted nanoparticles for quantitative imaging of sparse molecular epitopes with mri. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 51(3):480–486, 2004. [DOI] [PubMed] [Google Scholar]
- [25].Bellin Marie-France. Mr contrast agents, the old and the new. European journal of radiology, 60(3):314–323, 2006. [DOI] [PubMed] [Google Scholar]
- [26].Sun Conroy, Lee Jerry SH, and Zhang Miqin. Magnetic nanoparticles in mr imaging and drug delivery. Advanced drug delivery reviews, 60(11):1252–1265, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gilchrist RK, Medal Richard, Shorey William D, Hanselman Russell C, Parrott John C, and Taylor C Bruce. Selective inductive heating of lymph nodes. Annals of surgery, 146(4):596, 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hiergeist R, Andrä W, Buske N, Hergt R, Hilger I, Richter U, and Kaiser W. Application of magnetite ferrofluids for hyperthermia. Journal of Magnetism and Magnetic Materials, 201(1):420–422, 1999. [Google Scholar]
- [29].Jordan Andreas, Scholz Regina, Wust Peter, Fähling Horst, and Felix Roland. Magnetic fluid hyperthermia (mfh): Cancer treatment with ac magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. Journal of Magnetism and Magnetic materials, 201(1–3):413–419, 1999. [Google Scholar]
- [30].Rosensweig Ronald E. Heating magnetic fluid with alternating magnetic field. Journal of magnetism and magnetic materials, 252:370–374, 2002. [Google Scholar]
- [31].Hergt Rudolf, Dutz Silvio, Müller Robert, and Zeisberger Matthias. Magnetic particle hyperthermia: nanoparticle magnetism and materials development for cancer therapy. Journal of Physics: Condensed Matter, 18(38):S2919, 2006. [Google Scholar]
- [32].Deatsch Alison Eand Evans Benjamin A. Heating efficiency in magnetic nanoparticle hyperthermia. Journal of Magnetism and Magnetic Materials, 354:163–172, 2014. [Google Scholar]
- [33].Gleich Bernhard and Weizenecker Jürgen. Tomographic imaging using the nonlinear response of magnetic particles. Nature, 435(7046):1214, 2005. [DOI] [PubMed] [Google Scholar]
- [34].Goodwill Patrick W and Conolly Steven M. The x-space formulation of the magnetic particle imaging process: 1-d signal, resolution, bandwidth, snr, sar, and magnetostimulation. IEEE transactions on medical imaging, 29(11):1851–1859, 2010. [DOI] [PubMed] [Google Scholar]
- [35].Nie Shuming, Xing Yun, Kim Gloria J, and Simons Jonathan W. Nanotechnology applications in cancer. Annu. Rev. Biomed. Eng, 9:257–288, 2007. [DOI] [PubMed] [Google Scholar]
- [36].Ferguson Richard Mathew, Khandhar Amit P, Arami Hamed, Hua Loc, Hovorka Ondrej, and Krishnan Kannan M. Tailoring the magnetic and pharmacokinetic properties of iron oxide magnetic particle imaging tracers. Biomedizinische Technik/Biomedical Engineering, 58(6):493–507, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hufschmid Ryan, Arami Hamed, Ferguson R Matthew, Gonzales Marcela, Teeman Eric, Brush Lucien N, Browning Nigel D, and Krishnan Kannan M. Synthesis of phase-pure and monodisperse iron oxide nanoparticles by thermal decomposition. Nanoscale, 7(25):11142–11154, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kemp Scott J, Ferguson R Matthew, Khandhar Amit P, and Krishnan Kannan M. Monodisperse magnetite nanoparticles with nearly ideal saturation magnetization. RSC Advances, 6(81):77452–77464, 2016. [Google Scholar]
- [39].Hufschmid Ryan, Landers Joachim, Shasha Carolyn, Salamon Soma, Wende Heiko, and Krishnan Kannan M. Nanoscale physical and chemical structure of iron oxide nanoparticles for magnetic particle imaging. physica status solidi (a), 216(2):1800544, 2019. [Google Scholar]
- [40].Yaacob Iskandar I, Nunes Anthony C, and Bose Arijit. Magnetic nanoparticles produced in spontaneous cationic-anionic vesicles: room temperature synthesis and characterization. Journal of colloid and interface science, 171(1):73–84, 1995. [Google Scholar]
- [41].Portet David, Denizot Benoît, Rump Elmar, Lejeune Jean-Jacques, and Jallet Pierre. Nonpolymeric coatings of iron oxide colloids for biological use as magnetic resonance imaging contrast agents. Journal of colloid and interface science, 238(1):37–42, 2001. [DOI] [PubMed] [Google Scholar]
- [42].Caruso Frank. Nanoengineering of particle surfaces. Advanced materials, 13(1):11–22, 2001. [Google Scholar]
- [43].Tartaj Pedro, del Puerto Morales Maria, Veintemillas-Verdaguer Sabino, González-Carreño Teresita, and Serna Carlos J. The preparation of magnetic nanoparticles for applications in biomedicine. Journal of physics D: Applied physics, 36(13):R182, 2003. [Google Scholar]
- [44].Yoon Tae-Jong, Yu Kyeong Nam, Kim Eunha, Kim Jun Sung, Geol Kim Byung, Yun Sang-Hyun, Sohn Byeong-Hyeok, Cho Myung-Haing, Lee Jin-Kyu, and Park Seung Bum. Specific targeting, cell sorting, and bioimaging with smart magnetic silica core–shell nanomaterials. Small, 2(2):209–215, 2006. [DOI] [PubMed] [Google Scholar]
- [45].Xie Jin, Xu Chenjie, Kohler Nathan, Hou Yanglong, and Sun Shouheng. Controlled pegylation of monodisperse fe3o4 nanoparticles for reduced non-specific uptake by macrophage cells. Advanced Materials, 19(20):3163–3166, 2007. [Google Scholar]
- [46].Hatakeyama Mamoru, Kishi Hiroshi, Kita Yoshinori, Imai Kensuke, Nishio Kosuke, Karasawa Satoki, Masaike Yuka, Sakamoto Satoshi, Sandhu Adarsh, Tanimoto Akihiro, et al. A two-step ligand exchange reaction generates highly water-dispersed magnetic nanoparticles for biomedical applications. Journal of Materials Chemistry, 21(16):5959–5966, 2011. [Google Scholar]
- [47].Tassa Carlos, Shaw Stanley Y, and Weissleder Ralph. Dextran-coated iron oxide nanoparticles: a versatile platform for targeted molecular imaging, molecular diagnostics, and therapy. Accounts of chemical research, 44(10):842–852, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Moghimi S Moein, Hunter A Christy, and Murray J Clifford. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacological reviews, 53(2):283–318, 2001. [PubMed] [Google Scholar]
- [49].Allen Theresa M. Ligand-targeted therapeutics in anticancer therapy. Nature Reviews Cancer, 2(10):750, 2002. [DOI] [PubMed] [Google Scholar]
- [50].Villanueva Angeles, Cañete Magdalena, Roca Alejandro G, Calero Macarena, Veintemillas-Verdaguer Sabino, Serna Carlos J, del Puerto Morales María, and Miranda Rodolfo. The influence of surface functionalization on the enhanced internalization of magnetic nanoparticles in cancer cells. Nanotechnology, 20(11):115103, 2009. [DOI] [PubMed] [Google Scholar]
- [51].Khandhar Amit P, Wilson Gregory J, Kaul Michael G, Salamon Johannes, Jung Caroline, and Krishnan Kannan M. Evaluating size-dependent relaxivity of pegylated-uspios to develop gadolinium-free t1 contrast agents for vascular imaging. Journal of Biomedical Materials Research Part A, 106(9):2440–2447, 2018. [DOI] [PubMed] [Google Scholar]
- [52].Weizenecker J, Gleich B, Rahmer J, Dahnke H, and Borgert J. Three-dimensional real-time in vivo magnetic particle imaging. Physics in Medicine & Biology, 54(5):L1, 2009. [DOI] [PubMed] [Google Scholar]
- [53].Goodwill Patrick W and Conolly Steven M. Multidimensional x-space magnetic particle imaging. IEEE transactions on medical imaging, 30(9):1581–1590, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wang Yi-Xiang J, Hussain Shahid M, and Krestin Gabriel P. Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in mr imaging. European radiology, 11(11):2319–2331, 2001. [DOI] [PubMed] [Google Scholar]
- [55].P Vogel, Rückert MA, Klauer P, Kullmann WH, Jakob PM, and Behr VC. First in vivo traveling wave magnetic particle imaging of a beating mouse heart. Physics in Medicine & Biology, 61(18):6620, 2016. [DOI] [PubMed] [Google Scholar]
- [56].Graeser Matthias, Knopp Tobias, Szwargulski Patryk, Friedrich Thomas, von Gladiss Anselm, Kaul Michael, Krishnan Kannan M, Ittrich Harald, Adam Gerhard, and Buzug Thorsten M. Towards picogram detection of superparamagnetic iron-oxide particles using a gradiometric receive coil. Scientific reports, 7(1):6872, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ferguson R Matthew, Minard Kevin R, and Krishnan Kannan M. Optimization of nanoparticle core size for magnetic particle imaging. Journal of magnetism and magnetic materials, 321(10):1548–1551, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Bobo Daniel, Robinson Kye J, Islam Jiaul, Thurecht Kristofer J, and Corrie Simon R. Nanoparticle-based medicines: a review of fda-approved materials and clinical trials to date. Pharmaceutical research, 33(10):2373–2387, 2016. [DOI] [PubMed] [Google Scholar]
- [59].Singh Ajay, Patel Tejas, Hertel Joachim, Bernardo Marializa, Kausz Annamaria, and Brenner Louis. Safety of ferumoxytol in patients with anemia and ckd. American Journal of Kidney Diseases, 52(5):907–915, 2008. [DOI] [PubMed] [Google Scholar]
- [60].Yu Elaine Y, Bishop Mindy, Zheng Bo, Ferguson R Matthew, Khandhar Amit P, Kemp Scott J, Krishnan Kannan M, Goodwill Patrick W, and Conolly Steven M. Magnetic particle imaging: a novel in vivo imaging platform for cancer detection. Nano letters, 17(3):1648–1654, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Yu Elaine Y, Chandrasekharan Prashant, Berzon Ran, Tay Zhi Wei, Zhou Xinyi Y, Khandhar Amit P, Ferguson R Matthew, Kemp Scott J, Zheng Bo, Goodwill Patrick W, et al. Magnetic particle imaging for highly sensitive, quantitative, and safe in vivo gut bleed detection in a murine model. ACS nano, 11(12):12067–12076, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ludewig Peter, Gdaniec Nadine, Sedlacik Jan, Nils D Forkert Patryk Szwargulski, Graeser Matthias, Adam Gerhard, Kaul Michael G, Krishnan Kannan M, Ferguson R Matthew, et al. Magnetic particle imaging for real-time perfusion imaging in acute stroke. ACS nano, 11(10):10480–10488, 2017. [DOI] [PubMed] [Google Scholar]
- [63].Bulte Jeff WM, Walczak Piotr, Gleich Bernhard, Weizenecker Jürgen, Markov Denis E, Aerts Hans CJ, Boeve Hans, Borgert Jörn, and Kuhn Michael. Mpi cell tracking: what can we learn from mri? In Medical Imaging 2011: Biomedical Applications in Molecular, Structural, and Functional Imaging, volume 7965, page 79650Z. International Society for Optics and Photonics, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Rauwerdink Adam M and Weaver John B. Viscous effects on nanoparticle magnetization harmonics. Journal of Magnetism and Magnetic Materials, 322(6):609–613, 2010. [Google Scholar]
- [65].Weaver John B, Rauwerdink Adam M, and Hansen Eric W. Magnetic nanoparticle temperature estimation. Medical physics, 36(5):1822–1829, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zhong Jing, Schilling Meinhard, and Ludwig Frank. Magnetic nanoparticle temperature imaging with a scanning magnetic particle spectrometer. Measurement Science and Technology, 29(11):115903, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zhang Xiaojuan, Reeves Daniel B, Perreard Irina M, Kett Warren C, Griswold Karl E, Gimi Barjor, and Weaver John B. Molecular sensing with magnetic nanoparticles using magnetic spectroscopy of nanoparticle brownian motion. Biosensors and Bioelectronics, 50:441–446, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Dieckhoff Jan, Lak Aidin, Schilling Meinhard, and Ludwig Frank. Protein detection with magnetic nanoparticles in a rotating magnetic field. Journal of Applied Physics, 115(2):024701, 2014. [Google Scholar]
- [69].Gandhi Sonu, Arami Hamed, and Krishnan Kannan M. Detection of cancer-specific proteases using magnetic relaxation of peptide-conjugated nanoparticles in biological environment. Nano letters, 16(6):3668–3674, 2016. [DOI] [PubMed] [Google Scholar]
- [70].Gonzales-Weimuller Marcela, Zeisberger Matthias, and Krishnan Kannan M. Size-dependant heating rates of iron oxide nanoparticles for magnetic fluid hyperthermia. Journal of magnetism and magnetic materials, 321(13):1947–1950, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Khandhar Amit P, Ferguson R Matthew, and Krishnan Kannan M. Monodispersed magnetite nanoparticles optimized for magnetic fluid hyperthermia: Implications in biological systems. Journal of applied physics, 109(7):07B310, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Engelmann Ulrich M, Shasha Carolyn, Teeman Eric, Slabu Ioana, and Krishnan Kannan M. Predicting size-dependent heating efficiency of magnetic nanoparticles from experiment and stochastic néel-brown langevin simulation. Journal of Magnetism and Magnetic Materials, 471:450–456, 2019. [Google Scholar]
- [73].Vorotnikova Ekaterina, Ivkov Robert, Foreman Allan, Tries Mark, and Braunhut Susan J. The magnitude and time-dependence of the apoptotic response of normal and malignant cells subjected to ionizing radiation versus hyperthermia. International journal of radiation biology, 82(8):549–559, 2006. [DOI] [PubMed] [Google Scholar]
- [74].Stoner Edmund Cand Wohlfarth EP. A mechanism of magnetic hysteresis in heterogeneous alloys. Philosophical Transactions of the Royal Society of London A: Mathematical, Physical and Engineering Sciences, 240(826):599–642, 1948. [Google Scholar]
- [75].Pfeiffer H. Determination of anisotropy field distribution in particle assemblies taking into account thermal fluctuations. physica status solidi (a), 118(1):295–306, 1990. [Google Scholar]
- [76].Carrey Julian, Mehdaoui Boubker, and Respaud Marc. Simple models for dynamic hysteresis loop calculations of magnetic single-domain nanoparticles: Application to magnetic hyperthermia optimization. Journal of Applied Physics, 109(8):083921, 2011. [Google Scholar]
- [77].Martens Michael A, Deissler Robert J, Wu Yong, Bauer Lisa, Yao Zhen, Brown Robert, and Griswold Mark. Modeling the brownian relaxation of nanoparticle ferrofluids: Comparison with experiment. Medical physics, 40(2), 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Reeves Daniel B and Weaver John B. Simulations of magnetic nanoparticle brownian motion. Journal of applied physics, 112(12):124311, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Deissler Robert J, Wu Yong, and Martens Michael A. Dependence of brownian and néel relaxation times on magnetic field strength. Medical physics, 41(1), 2014. [DOI] [PubMed] [Google Scholar]
- [80].Coffey William Tand Kalmykov Yuri P. Thermal fluctuations of magnetic nanoparticles: Fifty years after brown. Journal of Applied Physics, 112(12):121301, 2012. [Google Scholar]
- [81].Dennis Cindi L and Ivkov Robert. Physics of heat generation using magnetic nanoparticles for hyperthermia. International Journal of Hyperthermia, 29(8):715–729, 2013. [DOI] [PubMed] [Google Scholar]
- [82].Reeves Daniel Band Weaver John B. Combined néel and brown rotational langevin dynamics in magnetic particle imaging, sensing, and therapy. Applied physics letters, 107(22):223106, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Reeves Daniel B and Weaver John B. Comparisons of characteristic timescales and approximate models for brownian magnetic nanoparticle rotations. Journal of applied physics, 117(23):233905, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Fannin PC, Scaife BKP, and Charles W. New technique for measuring the complex susceptibility of ferrofluids. Journal of Physics E: Scientific Instruments, 19(3):238, 1986. [Google Scholar]
- [85].Morrish Allan H. The physical principles of magnetism. The Physical Principles of Magnetism, by Morrish Allan H., pp. 696. ISBN 0–7803-6029-X. Wiley-VCH, January 2001., page 696, 2001. [Google Scholar]
- [86].Debye Peter. Polar molecules New York, The Chemical Catalog Co., Inc., 1929. [Google Scholar]
- [87].Havriliak Sand Negami S . A complex plane representation of dielectric and mechanical relaxation processes in some polymers. Polymer, 8:161–210, 1967. [Google Scholar]
- [88].Chantrell R, Popplewell J, and Charles S. Measurements of particle size distribution parameters in ferrofluids. IEEE Transactions on Magnetics, 14(5):975–977, 1978. [Google Scholar]
- [89].Dieckhoff Jan, Eberbeck Dietmar, Schilling Meinhard, and Ludwig Frank. Magnetic-field dependence of brownian and néel relaxation times. Journal of Applied Physics, 119(4):043903, 2016. [Google Scholar]
- [90].Brown William Fuller Jr. Thermal fluctuations of a single-domain particle. Physical Review, 130(5):1677, 1963. [Google Scholar]
- [91].Coffey WT, Crothers DSF, Dormann JL, Geoghegan LJ, Kalmykov Yu P, Waldron JT, and Wickstead AW. Effect of an oblique magnetic field on the superparamagnetic relaxation time. Physical Review B, 52(22):15951, 1995. [DOI] [PubMed] [Google Scholar]
- [92].Shah Saqlain A, Reeves Daniel B, Ferguson R Matthew, Weaver John B, and Krishnan Kannan M. Mixed brownian alignment and néel rotations in superparamagnetic iron oxide nanoparticle suspensions driven by an ac field. Physical Review B, 92(9):094438, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Martsenyuk MA, Raikher Yu L, and Shliomis MI. On the kinetics of magnetization of suspension of ferromagnetic particles. Journal of Experimental and Theoretical Physics, 38(2):413–416, 1974. [Google Scholar]
- [94].Yoshida Takashi and Enpuku Keiji. Simulation and quantitative clarification of ac susceptibility of magnetic fluid in nonlinear brownian relaxation region. Japanese Journal of Applied Physics, 48(12R):127002, 2009. [Google Scholar]
- [95].Coffey William Tand Kalmykov Yuri P. The Langevin equation: with applications to stochastic problems in physics, chemistry and electrical engineering. World Scientific, 2004. [Google Scholar]
- [96].Weizenecker Jürgen. The fokker–planck equation for coupled brown–néel-rotation. Physics in Medicine & Biology, 63(3):035004, 2018. [DOI] [PubMed] [Google Scholar]
- [97].Raikher Yuriy L and Shliomis Mark I. The effective field method in the orientational kinetics of magnetic fluids. Adv. Chem. Phys, 87:595–751, 1994. [Google Scholar]
- [98].Krishnan Kannan M, Pakhomov Alexandre B, Bao Yuping, Blomqvist Peter, Chun Yoonsoo, Gonzales Marcela, Griffin Kelli, Ji Xiaosong, and Roberts Bradley K. Nanomagnetism and spin electronics: materials, microstructure and novel properties. Journal of materials science, 41(3):793–815, 2006. [Google Scholar]
- [99].Ferguson R Matthew, Khandhar Amit P, Jonasson Christian, Blomgren Jakob, Johansson Christer, and Krishnan Kannan M. Size-dependent relaxation properties of monodisperse magnetite nanoparticles measured over seven decades of frequency by ac susceptometry. IEEE transactions on magnetics, 49(7):3441–3444, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Branquinho Luis C, Carrião Marcus S, Costa Anderson S, Zufelato Nicholas, Sousa Marcelo H, Miotto Ronei, Ivkov Robert, and Bakuzis Andris F. Effect of magnetic dipolar interactions on nanoparticle heating efficiency: Implications for cancer hyperthermia. Scientific reports, 3:2887, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Jamet Matthieu, Wernsdorfer Wolfgang, Thirion Christophe, Dupuis Veronique, Patrice Mélinon Alain Pérez, and Mailly Dominique. Magnetic anisotropy in single clusters. Physical Review B, 69(2):024401, 2004. [DOI] [PubMed] [Google Scholar]
- [102].Coffey WT. Ferromagnetic particles. 1993. [Google Scholar]
- [103].Song Ning-Ning, Yang Hai-Tao, Liu Hao-Liang, Ren Xiao, Ding Hao-Feng, Zhang Xiang-Qun, and Cheng Zhao-Hua. Exceeding natural resonance frequency limit of monodisperse fe3o4 nanoparticles via superparamagnetic relaxation. Scientific reports, 3, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].García-Palacios José Luis and Lázaro Francisco J. Langevin-dynamics study of the dynamical properties of small magnetic particles. Physical Review B, 58(22):14937, 1998. [Google Scholar]
- [105].Berkov DV and Gorn NL. Susceptibility of the disordered system of fine magnetic particles: a langevin-dynamics study. Journal of Physics: Condensed Matter, 13(41):9369, 2001. [Google Scholar]
- [106].Rahmer Jürgen, Weizenecker Jürgen, Gleich Bernhard, and Borgert Jörn. Signal encoding in magnetic particle imaging: properties of the system function. BMC medical imaging, 9(1):4, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Croft Laura R, Goodwill Patrick W, Konkle Justin J, Arami Hamed, Price Daniel A, Li Ada X, Saritas Emine U, and Conolly Steven M. Low drive field amplitude for improved image resolution in magnetic particle imaging. Medical physics, 43(1):424–435, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Croft Laura R, Goodwill Patrick W, and Conolly Steven M. Relaxation in x-space magnetic particle imaging. IEEE transactions on medical imaging, 31(12):2335–2342, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Goodwill PW, Tamrazian A, Croft LR, Lu CD, Johnson EM, Pidaparthi R, Ferguson RM, Khandhar AP, Krishnan KM, and Conolly SM. Ferrohydrodynamic relaxometry for magnetic particle imaging. Applied Physics Letters, 98(26):262502, 2011. [Google Scholar]
- [110].Ferguson R Matthew, Khandhar Amit P, and Krishnan Kannan M. Tracer design for magnetic particle imaging. Journal of applied physics, 111(7):07B318, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Arami Hamed, Ferguson RM, Khandhar Amit P, and Krishnan Kannan M. Size-dependent ferro-hydrodynamic relaxometry of magnetic particle imaging tracers in different environments. Medical physics, 40(7), 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Goodwill Patrick William, Saritas Emine Ulku, Croft Laura Rose, Kim Tyson N, Krishnan Kannan M, Schaffer David V, and Conolly Steven M. X-space mpi: magnetic nanoparticles for safe medical imaging. Advanced Materials, 24(28):3870–3877, 2012. [DOI] [PubMed] [Google Scholar]
- [113].Saritas Emine U, Goodwill Patrick W, Croft Laura R, Konkle Justin J, Lu Kuan, Zheng Bo, and Conolly Steven M. Magnetic particle imaging (mpi) for nmr and mri researchers. Journal of Magnetic Resonance, 229:116–126, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Bente Klaas, Weber Matthias, Graeser Matthias, Sattel Timo F, Erbe Marlitt, and Buzug Thorsten M. Electronic field free line rotation and relaxation deconvolution in magnetic particle imaging. IEEE transactions on medical imaging, 34(2):644–651, 2015. [DOI] [PubMed] [Google Scholar]
- [115].Ferguson R Matthew, Minard Kevin R, Khandhar Amit P, and Krishnan Kannan M. Optimizing magnetite nanoparticles for mass sensitivity in magnetic particle imaging. Medical physics, 38(3):1619–1626, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Ferguson R Matthew, Khandhar Amit P, Kemp Scott J, Arami Hamed, Saritas Emine U, Croft Laura R, Konkle Justin, Goodwill Patrick W, Halkola Aleksi, Rahmer Jürgen, et al. Magnetic particle imaging with tailored iron oxide nanoparticle tracers. IEEE transactions on medical imaging, 34(5):1077–1084, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Teeman Eric, Shasha Carolyn, Evans James E, and Krishnan Kannan M. Intracellular dynamics of superparamagnetic iron oxide nanoparticles for magnetic particle imaging. Nanoscale, 2019. [DOI] [PubMed] [Google Scholar]
- [118].Rahmer J, Halkola A, Gleich B, Schmale I, and Borgert J. First experimental evidence of the feasibility of multi-color magnetic particle imaging. Physics in medicine and biology, 60(5):1775, 2015. [DOI] [PubMed] [Google Scholar]
- [119].Hostetler Michael J, Green Stephen J, Stokes Jennifer J, and Murray Royce W. Monolayers in three dimensions: synthesis and electrochemistry of ω-functionalized alkanethiolate-stabilized gold cluster compounds. Journal of the American Chemical Society, 118(17):4212–4213, 1996. [Google Scholar]
- [120].Ingram Roychelle S, Hostetler Michael J, and Murray Royce W. Poly-hetero-ω-functionalized alkanethiolate-stabilized gold cluster compounds. Journal of the American Chemical Society, 119(39):9175–9178, 1997. [Google Scholar]
- [121].Lattuada Marco and Alan Hatton T. Functionalization of monodisperse magnetic nanoparticles. Langmuir, 23(4):2158–2168, 2007. [DOI] [PubMed] [Google Scholar]
- [122].Pellegrino Teresa, Manna Liberato, Kudera Stefan, Liedl Tim, Koktysh Dmitry, Rogach Andrey L, Keller Simon, Rädler Joachim, Natile Giovanni, and Parak Wolfgang J. Hydrophobic nanocrystals coated with an amphiphilic polymer shell: a general route to water soluble nanocrystals. Nano letters, 4(4):703–707, 2004. [Google Scholar]
- [123].Lundqvist Martin, Stigler Johannes, Elia Giuliano, Lynch Iseult, Cedervall Tommy, and Dawson Kenneth A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proceedings of the National Academy of Sciences, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Walkey Carl D and Chan Warren CW. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chemical Society Reviews, 41(7):2780–2799, 2012. [DOI] [PubMed] [Google Scholar]
- [125].Hill Alexandra and Payne Christine K. Impact of serum proteins on mri contrast agents: cellular binding and t 2 relaxation. RSC advances, 4(60):31735–31744, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Ferrari Mauro. Cancer nanotechnology: opportunities and challenges. Nature reviews cancer, 5(3):161, 2005. [DOI] [PubMed] [Google Scholar]
- [127].Hood John D, Bednarski Mark, Frausto Ricardo, Guccione Samira, Reisfeld Ralph A, Xiang Rong, and Cheresh David A. Tumor regression by targeted gene delivery to the neovasculature. Science, 296(5577):2404–2407, 2002. [DOI] [PubMed] [Google Scholar]
- [128].Tomitaka Asahi, Arami Hamed, Gandhi Sonu, and Krishnan Kannan M. Lactoferrin conjugated iron oxide nanoparticles for targeting brain glioma cells in magnetic particle imaging. Nanoscale, 7(40):16890–16898, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Arami Hamed, Teeman Eric, Troksa Alyssa, Bradshaw Haydin, Saatchi Katayoun, Tomitaka Asahi, Gambhir Sanjiv Sam, Häfeli Urs O, Liggitt Denny, and Krishnan Kannan M. Tomographic magnetic particle imaging of cancer targeted nanoparticles. Nanoscale, 9(47):18723–18730, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Goren Dorit, Horowitz Aviva T., Tzemach Dina, Tarshish Mark, Zalipsky Samuel, and Gabizon Alberto. Nuclear delivery of doxorubicin via folate-targeted liposomes with bypass of multidrug-resistance efflux pump. Clinical Cancer Research, 6(5):1949–1957, 2000. [PubMed] [Google Scholar]
- [131].Sudimack Jennifer and Lee Robert J. Targeted drug delivery via the folate receptor. Advanced Drug Delivery Reviews, 41(2):147–162, 2000. [DOI] [PubMed] [Google Scholar]
- [132].Troksa Alyssa. Superparamagnetic iron oxide nanoparticle coating enhanced visualization of vascular catheters. Master’s thesis, University of Washington, 2016. [Google Scholar]
- [133].Haegele Julian, Biederer Sven, Wojtczyk Hanne, Gräser Matthias, Knopp Tobias, Buzug Thorsten M, Barkhausen Jörg, and Vogt Florian M. Toward cardiovascular interventions guided by magnetic particle imaging: First instrument characterization. Magnetic resonance in medicine, 69(6):1761–1767, 2013. [DOI] [PubMed] [Google Scholar]
- [134].Haegele Julian, Vaalma Sarah, Panagiotopoulos Nikolaos, Barkhausen Jörg, Vogt Florian M, Borgert Jörn, and Rahmer Jürgen. Multi-color magnetic particle imaging for cardiovascular interventions. Physics in medicine and biology, 61(16):N415, 2016. [DOI] [PubMed] [Google Scholar]
- [135].Barrett Brendan J, Katzberg Richard W, Thomsen Henrik S, Chen Nan, Sahani Dushyant, Soulez Gilles, Heiken Jay P, Lepanto Luigi, Ni Zhao-hui, and Nelson Rendon. Contrast-induced nephropathy in patients with chronic kidney disease undergoing computed tomography: a double-blind comparison of iodixanol and iopamidol. Investigative radiology, 41(11):815–821, 2006. [DOI] [PubMed] [Google Scholar]
- [136].Weaver John B, Rauwerdink Adam M, Sullivan Charles R, and Baker Ian. Frequency distribution of the nanoparticle magnetization in the presence of a static as well as a harmonic magnetic field. Medical physics, 35(5):1988–1994, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Rauwerdink Adam M, Hansen Eric W, and Weaver John B. Nanoparticle temperature estimation in combined ac and dc magnetic fields. Physics in medicine and biology, 54(19):L51, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Rauwerdink Adam M, Giustini Andrew J, and Weaver John B. Simultaneous quantification of multiple magnetic nanoparticles. Nanotechnology, 21(45):455101, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Wawrzik T, Kuhlmann C, Ludwig F, and Schilling M. Estimating particle mobility in mpi In Magnetic Particle Imaging (IWMPI), 2013 International Workshop on, pages 1–1. IEEE, 2013. [Google Scholar]
- [140].Hensley Daniel, Goodwill Patrick, Croft Laura, and Conolly Steven. Preliminary experimental x-space color mpi. In Magnetic Particle Imaging (IWMPI), 2015 5th International Workshop on, pages 1–1. IEEE, 2015. [Google Scholar]
- [141].Utkur Mustafa, Muslu Yavuz, and Saritas Emine Ulku. Relaxation-based viscosity mapping for magnetic particle imaging. Physics in Medicine & Biology, 62(9):3422, 2017. [DOI] [PubMed] [Google Scholar]
- [142].Shasha Carolyn, Teeman Eric, Krishnan Kannan M, Szwargulski Patryk, Knopp Tobias, and Möddel Martin. Discriminating nanoparticle core size using multi-contrast mpi. Physics in Medicine & Biology, 64(7):074001, 2019. [DOI] [PubMed] [Google Scholar]
- [143].Möddel Martin, Meins Christian, Dieckhoff Jan, and Knopp Tobias. Viscosity quantification using multi-contrast magnetic particle imaging. New journal of physics, 20(8):083001, 2018. [Google Scholar]
- [144].Stehning Christian, Gleich Bernhard, and Rahmer Jürgen. Simultaneous magnetic particle imaging (mpi) and temperature mapping using multi-color mpi. International Journal on Magnetic Particle Imaging, 2(2), 2016. [Google Scholar]
- [145].Lee DH, Jung JM, Kim SY, Kim KT, and Cho YI. Comparison tests for plasma viscosity measurements. International Communications in Heat and Mass Transfer, 39(10):1474–1477, 2012. [Google Scholar]
- [146].Lee KR, Betz AL, Kim S, Keep RF, and Hoff JT. The role of the coagulation cascade in brain edema formation after intracerebral hemorrhage. Acta neurochirurgica, 138(4):396–401, 1996. [DOI] [PubMed] [Google Scholar]
- [147].DeBerardinis Ralph J, Lum Julian J, Hatzivassiliou Georgia, and Thompson Craig B. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell metabolism, 7(1):11–20, 2008. [DOI] [PubMed] [Google Scholar]
- [148].Mace Thomas A, Zhong Lingwen, Kilpatrick Casey, Zynda Evan, Lee Chen-Ting, Capitano Maegan, Minderman Hans, and Repasky Elizabeth A. Differentiation of cd8+ t cells into effector cells is enhanced by physiological range hyperthermia. Journal of leukocyte biology, 90(5):951–962, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Rajendra Acharya U, Yin-Kwee Ng Eddie, Tan Jen-Hong, and Vinitha Sree S. Thermography based breast cancer detection using texture features and support vector machine. Journal of medical systems, 36(3):1503–1510, 2012. [DOI] [PubMed] [Google Scholar]
- [150].Vreugdenburg Thomas D, Willis Cameron D, Mundy Linda, and Hiller Janet E. A systematic review of elastography, electrical impedance scanning, and digital infrared thermography for breast cancer screening and diagnosis. Breast cancer research and treatment, 137(3):665–676, 2013. [DOI] [PubMed] [Google Scholar]
- [151].Khandhar Amit P, Ferguson R Matthew, Simon Julian A, and Krishnan Kannan M. Enhancing cancer therapeutics using size-optimized magnetic fluid hyperthermia. Journal of applied physics, 111(7):07B306, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Khandhar Amit P, Ferguson R Matthew, Simon Julian A, and Krishnan Kannan M. Tailored magnetic nanoparticles for optimizing magnetic fluid hyperthermia. Journal of Biomedical Materials Research Part A, 100(3):728–737, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Shasha Carolyn, Teeman Eric, and Krishnan Kannan M. Harmonic simulation study of simultaneous nanoparticle size and viscosity differentiation. IEEE Magnetics Letters, 8:1–5, 2017. [Google Scholar]
- [154].Giustini Andrew J, Petryk Alicia A, Cassim Shiraz M, Tate Jennifer A, Baker Ian, and Hoopes P Jack. Magnetic nanoparticle hyperthermia in cancer treatment. Nano Life, 1(01n02):17–32, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Veiseh Omid, Gunn Jonathan W, and Zhang Miqin. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Advanced drug delivery reviews, 62(3):284–304, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Giustini Andrew J, Perreard Irina, Rauwerdink Adam M, Hoopes P Jack, and Weaver John B. Noninvasive assessment of magnetic nanoparticle–cancer cell interactions. Integrative Biology, 4(10):1283–1288, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Blanco-Andujar C, Teran FJ, and Ortega D. Current outlook and perspectives on nanoparticle-mediated magnetic hyperthermia. In Iron Oxide Nanoparticles for Biomedical Applications, pages 197–245. Elsevier, 2018. [Google Scholar]
- [158].Roussakow Sergey. The history of hyperthermia rise and decline. In Conference Papers in Science, volume 2013. Hindawi, 2013. [Google Scholar]
- [159].Southern Paul and Pankhurst Quentin A. Commentary on the clinical and preclinical dosage limits of interstitially administered magnetic fluids for therapeutic hyperthermia based on current practice and efficacy models. International Journal of Hyperthermia, 34(6):671–686, 2018. [DOI] [PubMed] [Google Scholar]
- [160].Schiller Brigitte, Bhat Premila, and Sharma Amit. Safety and effectiveness of ferumoxytol in hemodialysis patients at 3 dialysis chains in the united states over a 12-month period. Clinical therapeutics, 36(1):70–83, 2014. [DOI] [PubMed] [Google Scholar]
- [161].Mamiya Hiroaki and Jeyadevan Balachandran. Hyperthermic effects of dissipative structures of magnetic nanoparticles in large alternating magnetic fields. Scientific reports, 1:157, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Atkinson William J, Brezovich Ivan A, and Chakraborty Dev P. Usable frequencies in hyperthermia with thermal seeds. IEEE Transactions on Biomedical Engineering, (1):70–75, 1984. [DOI] [PubMed] [Google Scholar]
- [163].Lee Jae-Hyun, Jang Jung-tak, Choi Jin-sil, Moon Seung Ho, Noh Seung-hyun, Kim Ji-wook, Kim JinGyu, Kim Il-Sun, Park Kook In, and Cheon Jinwoo. Exchange-coupled magnetic nanoparticles for efficient heat induction. Nature nanotechnology, 6(7):418, 2011. [DOI] [PubMed] [Google Scholar]
- [164].Abenojar Eric C, Wickramasinghe Sameera, Bas-Concepcion Jesbaniris, and Samia Anna Cristina S. Structural effects on the magnetic hyperthermia properties of iron oxide nanoparticles. Progress in Natural Science: Materials International, 26(5):440–448, 2016. [Google Scholar]
- [165].Landi Gabriel T. Role of dipolar interaction in magnetic hyperthermia. Physical Review B, 89(1):014403, 2014. [Google Scholar]
- [166].Saville Steven L, Qi Bin, Baker Jonathon, Stone Roland, Camley Robert E, Livesey Karen L, Ye Longfei, Crawford Thomas M, and Mefford O Thompson. The formation of linear aggregates in magnetic hyperthermia: Implications on specific absorption rate and magnetic anisotropy. Journal of colloid and interface science, 424:141–151, 2014. [DOI] [PubMed] [Google Scholar]