Abstract
Sickle cell disease (SCD) is a monogenic hemoglobinopathy associated with significant morbidity and mortality. Cardiopulmonary, vascular and sudden death underlies the majority of young adult mortality in SCD. To better understand the clinical importance of multi-level vascular dysfunction, in 2009 we assessed cardiac function including tricuspid regurgitant jet velocity (TRV), tissue velocity in systole(S’) and diastole (E’), inflammatory, rheologic and hemolytic biomarkers as predictors of mortality in patients with SCD. With up to 9 years of follow up, we determined survival in 95 children, adolescents and adults with SCD. Thirty-eight patients (40%) were less than 21 years old at initial evaluation. Survival and Cox proportional-hazards analysis were performed. There was 19% mortality in our cohort, with median age at death of 35 years. In the pediatric subset, there was 11% mortality during the follow up period. The causes of death included cardiovascular and pulmonary complications in addition to other end-organ failure. On Cox proportional-hazards analysis, our model predicts that a 0.1 m/s increase in TRV increases risk of mortality 3%, 1 cm/s increase in S’ results in a 91% increase and 1 cm/s decrease in E’ results in a 43% increase in mortality. In conclusion, Elevated TRV and altered markers of cardiac systolic and diastolic function predict mortality in a cohort of adolescents and young adult patients with SCD. These predictors should be considered when counseling cardiovascular risk and therapeutic optimization at transition to adult providers.
Keywords: Tricuspid regurgitant velocity, diastolic function, Sickle cell disease, mortality, pediatrics
Introduction
Sickle cell disease (SCD) is a genetic disease caused by a single point mutation in the beta globin gene and affects 1 out of every 365 African-Americans1,2. Median survival for all genotypes has improved but it has also exposed chronic cardiovascular disease that worsens with age and leads to multiple end-organ dysfunction3–6. Access to comprehensive care in early childhood has improved survival of SCD children with widespread use of newborn screening programs, use of penicillin prophylaxis and vaccinations for encapsulated organisms7,8. Despite improved survival, mortality rates increase yearly by 1%, especially in young adults after the age of 18 years4. The most common SCD related causes of death are acute chest syndrome, renal disease, stroke, multi-organ failure syndrome, pulmonary hypertension, and sudden unexplained death9,10. Large single and multicenter studies have shown that increased tricuspid regurgitant jet velocity (TRV) is an independent predictor of mortality in adults,10–16 but this has not been shown frequently in children and adolescents16–18. Increases in TRV represent contributions from abnormal pulmonary vascular resistance and left ventricular (LV) diastolic dysfunction19,20. The presence of elevated right ventricular pressure and LV diastolic dysfunction are both additive and independent risk factors of mortality in older adults with SCD21. The utility of cardiac screening, especially in adolescent and young adult patients, is controversial due to the lack of a single mechanistic and therapeutic target. The role of echocardiography and TRV as a predictive marker of cardiovascular mortality in the pediatric, adolescent and young adult SCD population has recently been reported in a large cohort22. The American Thoracic Society recently cited long term studies of TRV and risk for cardiovascular morbidity and mortality in the adolescent and young adult population as a critical unmet area of research23.
TRV is correlated with lactate dehydrogenase (LDH) and plasma free hemoglobin, two biomarkers of intravascular hemolysis that are associated with endothelial dysfunction and death in patients with SCD12,24. While LDH is an inert plasma biomarker of hemolysis, plasma free hemoglobin and arginase lead to direct intravascular free radical toxicity, nitric oxide depletion, endothelial dysfunction and vascular remodeling25,26. Increased circulating plasma free hemoglobin levels are associated with impaired flow mediated dilation (FMD) and tissue oxygenation in SCD patients. While FMD is associated with pulmonary hypertension and VOC in SCD27, as well as with idiopathic primary pulmonary hypertension in children28, it has never been directly linked to mortality.
Interestingly, FMD impairment is independent of microvascular dysfunction, suggesting separate mechanistic etiologies27. Abnormal blood rheology, vascular inflammation, and recurring ischemia reperfusion are thought to represent the primary cause of microvascular damage29. To better understand the interplay among these variables and the multiple levels of vascular dysfunction found in SCD, we measured both macro-vascular and micro-vascular function, echocardiographic parameters of systolic and diastolic function, erythrocyte deformability, whole blood viscosity, markers of inflammation, and hemolytic profile in a cohort of pediatric and adult SCD patients between 2009 and 2012. We then followed them longitudinally to determine predictors of mortality as they transitioned from pediatric to adult care. This study addresses a critical question concerning modifiable cardiovascular risk factors for long term cardiovascular morbidity and mortality in pediatric, adolescent, young adult and adult patients with sickle cell disease.
Methods
Patient Population
We recruited non-transfused and chronically transfused SCD patients from the Children’s Hospital of Los Angeles (CHLA), the University of Southern California and the larger southern California sickle cell anemia community between the years of 2009 and 2012. This was designed as a generalized screening study; there were no cardiovascular disease inclusion or exclusion criteria. The study was approved by the CHLA Institutional Review Board and all patients provided informed consent. Non-transfused SCD patients underwent a single study visit to collect blood biomarkers, comprehensive vascular testing and echocardiography. Chronically transfused patients had study visits immediately prior to schedule transfusion (Tx) and repeat study visit within 4 days of completing their Tx. Chronically transfused patients included both simple and exchange Tx. The measurements were made during steady state when the patient was free of any pain crisis or hospitalization for at least a month or prior Tx. We followed them until September 2018 and assessed our primary outcome, survival.
The following data points were collected during a single study visit at CHLA between 2009 and 2012:
Clinical and Laboratory data
complete vital signs including oxygen(O2) saturation from pulse oximetry, SCD genotype, treatment status, complete blood count, hemoglobin (Hgb) electrophoresis, lactate dehydrogenase, and plasma free Hgb, high sensitivity CRP (hs-CRP).
Rheologic parameters: Elongation Index for deformability (LORCA, Mechatronics Instruments, The Netherlands) was measured over a shear stress range of 0.3 to 50 pascals(Pa) and oxygenated and deoxygenated whole blood viscosity (Rheolog, Health Vector Co., Pennsauken, NJ) were measured over a shear rate range of 5 to 1000 sec−1 using a tube viscometer as described previously30. The hematocrit: viscosity ratio at all shear rates and under deoxygenated and oxygenated conditions was calculated using a spun hematocrit measurement and the viscosity as described previously31.
Cardiac and Vascular measurements
Echocardiography: Peak TRV, systolic and diastolic function parameters, and cardiac index were measured by standard transthoracic echocardiography using a Philips IE-33 ultrasound machine (Philips Healthcare, Andover, MA). Measured variables included shortening fractionS, ejection fractionS, myocardial performance index by tissue DopplerS&D, mitral valve early diastolic transmitral flow velocity (MV E)D, lateral mitral annulus early diastolic tissue velocity (lat E’)D, lateral mitral annular systolic tissue velocity (lat S’)S, MV E to lat E’ ratio (E/E’)D, average E/E’, left atrial (LA) volume indexed to body surface areaD, and ratio of mitral valve early to late filling (MV E/A)D. (The superscript S=systolic parameter and D=diastolic parameter)
- Shear-mediated endothelial nitic oxide release as a measure of conduit vessel endothelial dysfunction was measured using flow mediated dilation (FMD) as described previously27. Percent change in brachial artery diameter after 3 minute of cuff occlusion was calculated as:
- Resting and peak microcirculatory blood flow was measured using Laser Doppler flowmetry in the finger at the nailbed as described previously30 and reported in perfusion units (PU). Post-occlusive hyperemia was calculated by percent change in blood flow after 3 minutes of cuff occlusion:
Near infrared spectroscopy (NIR): Regional oxygen saturation (rSO2) was collected in the dorsum of both hands to reflect the balance of arterial and venous hemoglobin saturation in the peripheral circulation using INVOS™ 5100C Cerebral/Somatic Oximeter.
Cause of death was obtained in 2018 from public records available at Los Angeles (LA) County. Death certificate information was obtained from the medical examiner when available.
Statistical analysis
Independent variables: Patient age, sex, race, type of SCD, treatment status, hemoglobin, white-cell count (WBC), plasma free Hgb, LDH, oxygen saturation, TRV, ΔFMD%, rSO2, lat E’, lat S’, E/E’ ratio, MV E/A ratio, LA volume index, resting PU and ΔPU% were included in the survival analysis.
Statistical analyses, including evaluation of means/medians, standard deviation/interquartile range (IQR), the t-test (or Wilcoxon rank sum) and the χ2 test, were performed for survivors versus non survivors. P-value <0.05 was considered statistically significant. The Kaplan–Meier curve and log-rank test was used to estimate survival probabilities. Entry into the cohort was defined by the date of vascular assessment. The cutoff values for Kaplan-Meier were TRV >250 cm/s, plasma free Hgb >10 and FMD<6% based on published normal values27. We used Cox proportional-hazards regression model to predict the risk factors associated with mortality in patients with SCD. Right censoring was performed for the Cox proportional-hazards model. Variables were selected by stepwise backward elimination at 5% level of significance to obtain the final model. Schoenfeld residuals were used to check the Cox proportional-hazards assumption. Data analysis was performed using Stata/IC version 14.0 statistical software (Release 14.College Station, TX: StataCorp LP.)
Results
Clinical characteristics of deceased and living patients
A total of 101 patients were studied for vascular and hematological assessment between 2009–2012. We were able to follow up a total of 95 patients with SCD until September 2018 and 6 patients were lost to follow up. Those six patients completed the initial cardiovascular assessment but we don’t have any further follow up data so we have excluded them from survival analysis. Of these 95 patients with follow up, 18 patients (19%) died. Median follow up time was 7.7 years (6.97–8.22 IQR) and a total of 698 person-years. Median age at follow up was 31 years (26–43 IQR). Patient demographics and clinical characteristics comparing the group of patients who died to the patients who lived are listed in table 1. Patients in the living group were significantly younger at initial assessment but there was no difference in the age of death or at the time of last follow up. The median age at death or last follow up was not different between males and females. There was no difference in race, gender, type of SCD or chronic transfusion/hydroxyurea between two groups. Patients who died showed greater hemolysis, were more anemic, had higher WBC, higher LDH and higher plasma free Hgb (p<0.05) at the time of assessment. Among vascular markers, 88% of deceased patients had abnormal FMD and 81% had elevated plasma hemoglobin. Oxygen saturation trended lower in the deceased group. Among cardiac markers, TRV was higher in deceased group of patients (p=0.01). After categorization of TRV, 63% of deceased patients had elevated TRV>250 cm/sec. Deceased patients also had significantly lower lat E’ and MV E/A ratio and their LA volume index trended higher. Rheologic variables were not associated with risk of mortality, thus the data is not shown here.
Table 1:
Clinical characteristics of study population
| Deceased [n=18] | Living [n=77] | p-value | |
|---|---|---|---|
| Patient demographics | |||
| Age at initial assessment, years* | 30 [22–42] | 23 [17–34] | 0.03 |
| Age at death or last follow-up, years* | 35 [28–50] | 30 [25–42] | 0.09 |
| Sex** | |||
| Male | 7 [39] | 31 [40] | 0.9 |
| Female | 11[61] | 46 [60] | |
| Race** | |||
| African-American | 18 [100] | 66 [86] | 0.12 |
| Hispanic | 0 [0] | 11 [14] | |
| SCD genotype** | |||
| SS | 17[94] | 61 [79] | |
| SC | 1[6] | 12 [16] | 0.3 |
| S-Beta thalassemia | 0[0] | 4 [5] | |
| Treatment status at study** | |||
| Chronically transfused | 8 [44] | 18 [23] | 0.08 |
| Non-transfused | 10[56] | 59[77] | |
| Blood and Hemolytic markers | |||
| Hemoglobin (g/dL)* | 8.3 [7.1–9.8] | 9.3 [8.6–10.1] | 0.02 |
| White-cell count (K/uL)* | 10.9 [9.6–16.1] | 8.5 [6.8–12.2] | 0.03 |
| Reticulocyte count (%)* | 9.5[6.8–18.8] | 7.9[4.5–12.8] | 0.07 |
| Hemoglobin S (%)* | 66.7[31–79.9] | 69.5[49.9–84.2] | 0.4 |
| Plasma free hemoglobin (mg/dL)* | 25 [18–35] | 14 [11–23] | 0.02 |
| Plasma free hemoglobin category | |||
| >10 (abnormal) | 13[81] | 57[76] | 0.7 |
| <=10 (normal) | 3[19] | 18[24] | |
| Lactate dehydrogenase (U/L) * | 1248 [814–1982] | 959 [765–1313] | 0.04 |
| hs-CRP (mg/L) * | 6.3[1.9–14.6] | 3.4[1.6–7.6] | 0.2 |
| Vascular markers | |||
| Oxygen saturation – pulse oximetry (%)* | 94 [91–97] | 96 [94–98] | 0.05 |
| Δ Flow mediated dilation (%)* | 4.7 [3.1–5.3] | 5.4 [3.8–6.9] | 0.2 |
| Δ Flow mediated dilation category | |||
| <6% (abnormal) | 14[88] | 41[64] | 0.07 |
| >=6% (normal) | 2 [12] | 23[36] | |
| rSO2 by NIRs (%)Δ | 39 [29–41] | 40 [33–48] | 0.3 |
| Resting microcirculatory blood flow (PU) * | 41 [38–69] | 51 [24–82] | 0.9 |
| Post-Occlusive Reactive Hyperemia (%ΔPU) Δ | 204.6 [150.5–270.9] | 163.6 [104.9–257.3] | 0.4 |
| Cardiac markers | |||
| TRV (cm/sec)Δ | 268 [225–301] | 234 [208–257] | 0.01 |
| TRV category | |||
| >250 cm/sec (abnormal) | 10[62.5] | 22[31] | 0.02 |
| <=250 cm/sec (normal) | 6[37.5] | 49[69] | |
| Lat E’ cm/sec* | 12.95[11.2–15.8] | 15.8[12.9–19.8] | 0.01 |
| Lat S’ cm/sec* | 10.5[8.95–12.6] | 9.6[8.3–11.5] | 0.09 |
| Average E/E’* | 8.6[6.9–9.9] | 7.7[6.3–8.7] | 0.17 |
| MV E/A ratio | 1.65 [1.40–2.14] | 2.04 [1.73–2.48] | 0.02 |
| LA volume index* (ml/m2) | 38.2[28.1–49.9] | 31.8[24.5–38.6] | 0.06 |
Results are represented as median [IQR]; Wilcoxon rank sum test
Results are represented as n (%); X2 test
Results are represented as median [IQR]; T-test
Survival and Cox proportional-hazards analysis
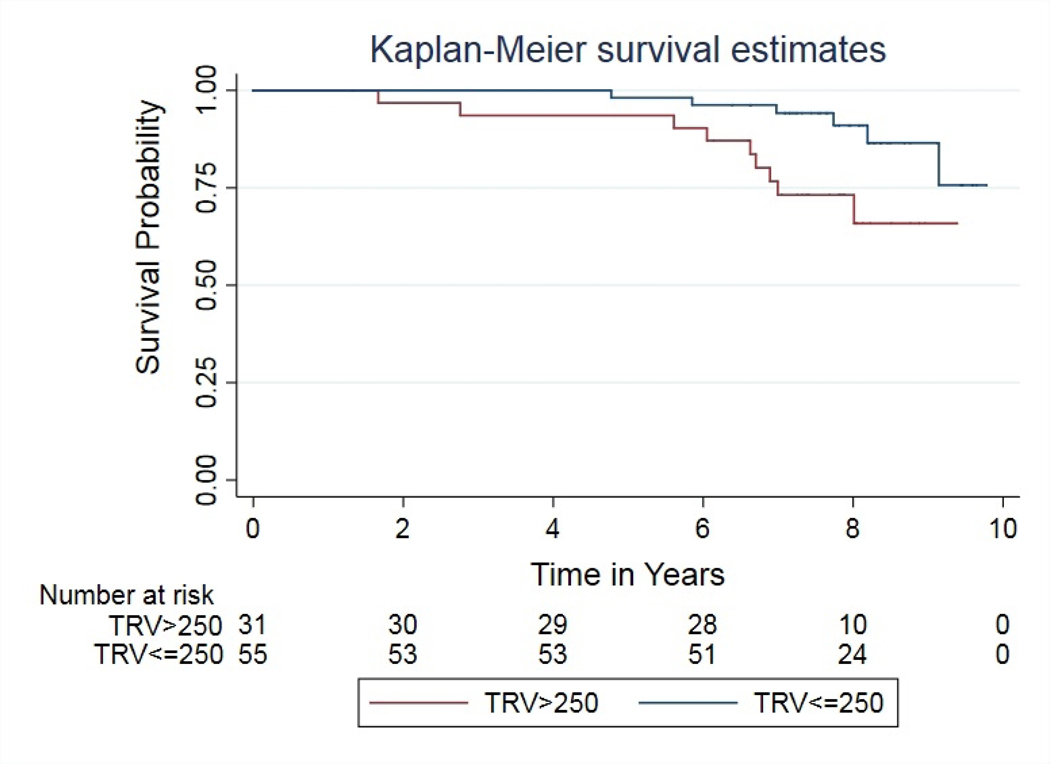
One patient was excluded from survival analysis because the exact date of death was unknown. Median survival age was not different by sex or SCD genotype. SCD patients with TRV >250 cm/sec were at greater risk of mortality (p=0.018) (Figure 1). Patients on chronic transfusion therapy had a lower TRV at the initial visit (Supplemental Figure 1) but had a similar risk of death compared with the non-transfused cohort at long term follow up. The detail about the SCD treatment effect is available in on-line supplemental results-Treatment Effects.
Figure 1:
Kaplan Meier survival curve shows Higher TRV (>250 cm/s) (p=0.018) is associated with the risk of mortality in patients with Sickle Cell Disease. 7-Years survival for the category TRV>250 is 73% (CI 53%–86%) and for the category TRV ≤ 250 is 94% (CI 83%–98%); TRV= Tricuspid Regurgitant Jet Velocity.
Supplemental Table 1 summarizes the univariate Cox proportional-hazards ratios of various parameters predicting the risk of mortality. Age and oxygen saturation were the only significant predictors from history and physical examination. Significant blood biomarkers were Hgb and LDH levels. Among the cardiac variables, higher TRV, higher lat S’, reduced lat E’ and MV E/A ratio predicted mortality.
On multivariate analysis, we found out that multiple predictors were correlated with each other (supplemental table 2); therefore we analyzed several different multivariate models and presented the best model here (More details on multivariate analysis can be found in on-line supplemental results-Modeling). Out of all models from stepwise backward elimination, we found that higher TRV, decreased lateral mitral annulus early diastolic tissue velocity (lat E)’ and increased lateral mitral annular systolic tissue velocity (lat S’) were significant predictors of mortality (p<0.0001, Table 2).
Table 2:
Multivariate Cox proportional-hazards regression analysis of variables predicting mortality
| Predictors | Model | |
|---|---|---|
| LR chi2(3)=34.58 | ||
| P(chi2) <0.0001 | ||
| Hazard Ratio (95% CI) | p-value | |
| TRV cm/sec | 1.03(1.01–1.04) | 0.003 |
| Lat S’ cm/sec | 1.91(1.46–2.61) | <0.001 |
| Lat E’ cm/sec | 0.70(0.58–0.83) | <0.001 |
Cause of death
There was 19% mortality in our cohort. The youngest patient who died in our cohort was 25 years of age and had sudden death. Eleven patients (61%) died before the age of 40. Cause of death as obtained from their LA county death records are listed in supplemental Table 4. SCD related cardiovascular and pulmonary complications were the most common causes of death, followed by iron overload, kidney failure and liver failure. The cause of death for four of the patients was unknown (22%).
On sub-analysis of the pediatric cohort, there were 38 patients (40%) under the age of 21 at the time of assessment. A total of 10 pediatric patients in this cohort (26%) had a TRV of >250cm/s. Four of the pediatric patients (11%) died during follow up but only one of the four patients who died had a TRV>250cm/s at presentation. All of the pediatric patients who died were on chronic transfusion therapy at the time of study assessment, with 2 of those patients continuing transfusion therapy into adulthood and two stopping therapy. Of the two patients continuing on chronic transfusions, one died from gram negative sepsis at 27 years of age, with co-morbid cardiac iron overload despite prescribed chelation therapy. This was the patient with TRV >250cm/s at the time of study assessment. The second patient continuing transfusion therapy developed metastatic cancer and died from it. One patient discontinued chronic transfusion therapy despite having a subsequent TRV >300 cm/s and died from sudden death. The last patient had normal TRV on multiple assessments while under our care but stopped chronic transfusion therapy after transition to an adult care provider and died from pulmonary hypertension at 28 years of age. We did not have a record of that patient’s TRV after leaving our care.
Discussion
Sickle cell disease is a model of diffuse endothelial disease characterized by multi-organ dysfunction and increased risk of death in the adult population. The primary aim of our study was to assess cardiovascular function, inflammatory, rheologic and hemolytic biomarkers as risk factors for mortality in a pediatric and adult cohort with up to 9 years of follow up. The present cohort had a median age of 24 years at the time of enrollment and a median follow up time of 7.7 years which is younger and longer duration than most published studies10,13. More recently, a study of 510 pediatric patients with median 7.3 years follow up demonstrated that TRV, ferritin, FEV1/FVC and neutrophil count were significant predictors of mortality22. In our study, the overall mortality rate of 19% and the median age at death of 35 years suggests that there is clinically significant mortality in early adulthood that may not have been captured in the previously reported older cohorts11,12,32 but similar to the recently published study in pediatric patients. We found that higher TRV, higher systolic myocardial tissue velocity (S’), and lower diastolic myocardial tissue velocity during passive filling (E’) were independent predictors of death in pediatric, adolescent and young adults with SCD. Similar to other studies, all of the patients in the deceased group were homozygous SS genotype10,11.
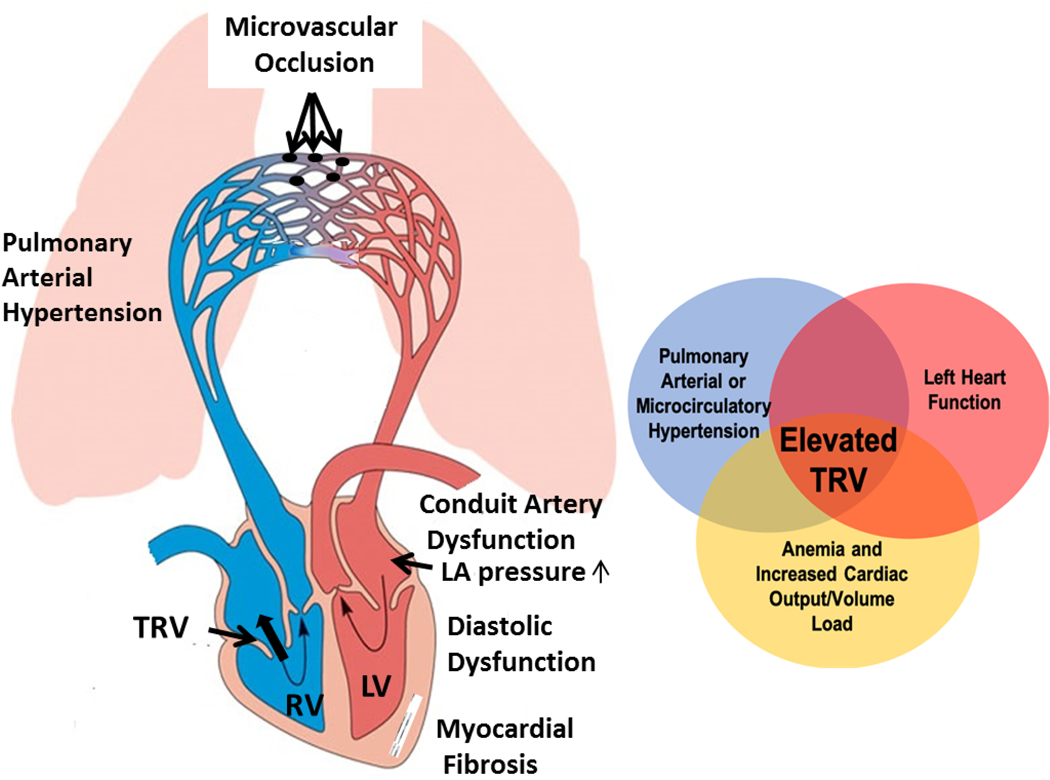
TRV continues to be a strong, independent predictor of mortality, even in our adolescent and young adult population, with an optimal TRV threshold for mortality of 258 cm/s, which mirrors prior studies33. Based on current WHO clinical guidelines for pulmonary hypertension (TRV >280 cm/s), 15% of our population met criteria for pulmonary hypertension and the association with mortality was still significant. Based on PUSH study cutoff TRV≥270 cm/sec22, 50% of patient who died had abnormal TRV. Since the original publication by Gladwin and colleagues, controversy has surrounded classification of pulmonary hypertension and the cutoff of TRV >250 cm/s as a predictor of mortality in SCD10,12,34 for two reasons. Firstly, a definitive all-cause pulmonary hypertension diagnosis is made by cardiac catheterization with a mean pulmonary artery pressure of >25 mmHg35,36. TRV is an estimate of right ventricle and pulmonary artery pressure and 250 cm/sec is lower than the conservative cutoff 280 cm/sec used by most pulmonary hypertension experts37 and many subjects with TRV >250 cm/s have normal pulmonary artery pressures upon cardiac catheterization34,38. Second, approximately half of SCD patients with increased pulmonary artery pressure are due to a combination of subclinical changes related to increased left atrial pressure and/or decreased left heart diastolic function, pulmonary microcirculatory disease and volume overload39(Figure 2). Echocardiographic parameters in the left heart: lat E’, lat S’, E/E’ ratio, MV E/A ratio, and LA volume, are used to predict left atrial pressure, which contributes to peak TRV. Therefore, TRV in SCD should not be considered solely as diagnostic criteria of pulmonary arterial hypertension but as a marker of diffuse vascular and cardiac function changes, prompting further workup to define the etiology.
Figure 2:
Multifactorial etiology of elevated TRV. Elevated TRV represents the superposition of increased cardiac output on micro/macrovascular disease in the lungs and poor left ventricular compliance. (Figure adapted from The Open University); TRV= Tricuspid Regurgitant Jet Velocity; LV=Left ventricle; RV=Right ventricle; LA=Left atrium
Endothelial dysfunction, specifically shear-mediated nitric oxide related dysfunction, is prevalent in patients with sickle cell disease and has a strong non-linear association with plasma free hemoglobin27. While the final multivariate model did not include markers of hemolysis or abnormal FMD, they are both correlated with TRV, thus representing one mechanism of disease that underlies the risk of mortality but is less sensitive to prediction of mortality. Plasma free hemoglobin is associated with mortality when TRV is not included in the model; adding TRV removes plasma free hemoglobin (supplemental table 3). Lastly, TRV is a sensitive predictor of mortality because it reports on diffuse, multilevel cardiovascular dysfunction, which includes endotheliopathy, microcirculatory disease and diastolic dysfunction (Figure 2).
LV diastolic dysfunction and pulmonary hypertension can develop independently but the mortality risk is additive if both are present in adults with SCD21. Diastolic function parameters that predict mortality do not fit neatly into a traditional definition of diastolic dysfunction based on accepted criteria in 200721 and the 2016 American Society of Echocardiography (ASE) released revised diastolic function guidelines40. Stratifying patients by the presence or absence of diastolic dysfunction as defined by the 2007 or the 2016 criteria did not predict mortality. There are several reasons why diastolic function assessment is challenging in SCD patients. First, chronic anemia causes volume expansion, increased cardiac output and ventricular dilation, independent of any other pathology. Second, total peripheral vascular resistance is actually decreased in SCD, therefore, preload is increased, and afterload is decreased, both of which impact Doppler-based diastolic metrics. Lastly, SCD patients appear to suffer from a unique myocardial phenotype that is characterized by markedly increased extracellular volume fraction (ECV) measured by cardiac MRI, consistent with diffuse myocardial fibrosis41. SCD myocardial fibrosis was first described in mouse models, with MRI findings validated by tissue histology42. Subsequent MRI studies in children and young adults demonstrated that increased ECV begins in the first two decades of life and is accompanied by decreased E’, increased BNP, and decreased exercise performance43.
We were somewhat surprised that higher systolic tissue velocity (S’) was a predictor of mortality in our cohort because it has not previously been reported in SCD patients. Tissue Doppler reflects annular velocity during ejection and could be impacted by several hemodynamic changes in patients with SCD. Increased stroke volume, increased cardiac volume/mass, increased fibrosis and decreased afterload all result in increased myocardial active force generation. Tissue S’ is also sensitive to inotropic state and SCD patients are known to have disproportionately elevated sympathetic to vagal balance. In fact, patients undergoing head up tilt table testing had 3 distinct autonomic response phenotypes. The severe phenotype was characterized by diminished parasympathetic activity, decreased heart rate variability, and exaggerated peripheral vasoconstriction to maintain blood pressure all signifying increased sympathetic drive in SCD44.
As observed in supplemental Table 4, pulmonary hypertension and diastolic dysfunction are not considered the primary cause of death in most patients although they could certainly have complicated the management of otherwise survivable lesions, such as sepsis or contribute to sudden cardiac death, estimated to be as high as 41% of the deaths in SCD45. Histologic studies in sickle cell mice demonstrate ongoing myocyte injury and repair, mitochondrial damage, abnormal ion channel activity, prolonged QT interval and cardiac repolarization abnormalities42. Hence, the same process that is triggering progressive extracellular fibrosis appears to be creating a substrate for lethal ventricular arrhythmias and diastolic dysfunction. Several human autopsy studies have shown fibrotic change in the myocardium46, including specific deposition and fibrotic changes noted in the conduction tissue of the heart.
In May 2019, the American Thoracic Society released a document laying out critically needed areas of research: “increased tricuspid regurgitant jet velocity (TRV) observed in 10% to 20% of children and adolescents with SCD have unclear prognostic significance, whereas in adults these echocardiographic findings have been repeatedly associated with early mortality23.” Echocardiographic screening remains controversial in pediatric and adolescent patients mainly due to lack of single mechanistic or therapeutic target47. Longitudinal assessment is necessary to understand individual cardiovascular disease trajectory. 10% of the pediatric patients with TRV > 250 cm/s and 10.7% of patients with TRV < 250 cm/s at our assessment died within the follow up period. One pediatric patient who died had TRV <=250 cm/s at the time of study assessment but found later to have a TRV >300 cm/s. The chronic progressive nature of cardiovascular disease should be acknowledged when using TRV as a screening tool in pediatric patients. Therefore, longitudinal assessment is necessary to understand the individual disease trajectory and for multidisciplinary counseling of cardiovascular risk.
TRV and markers of systolic and diastolic heart function are strong predictors in our cohort that spans a large age range, including pediatric patients and young adults at the time of transition. All four pediatric patients who died during follow up were on chronic transfusion therapy at the time of study assessment. These patients are at remarkably high risk of severe vascular disease if not on transfusion therapy, and they are at risk for iron overload if they remain on transfusion therapy. This is problematic at the time of transition and when determining continuation of transfusion therapy vs transition to hydroxyurea treatment48, initiation of exchange transfusion therapy that may stabilize cell free hemoglobin49 and improve TRV50 or consideration of curative therapies. Patients can be cured by marrow transplant and gene therapies are on the horizon. New supportive therapies such as L-glutamine, oxygen dissociation modulation, and P-selectin inhibition are now approved and are being utilized. Metrics that identify which adolescents and young adults are destined for premature death are still needed. The data presented here tell us that markers of cardiac dysfunction related to subsequent mortality may be present in young patients and knowledge of this may significantly influence the intensity therapy recommended by the clinician. Our data supports incorporation of routine, longitudinal cardiovascular screening in adolescence and young adults and incorporation of these metrics into multidisciplinary counseling of cardiovascular risk and therapeutic optimization.
Limitations
The data was limited to one visit at our center with some enrollment from community groups throughout southern California, therefore, our sample may skew toward a more severe disease phenotype and the number of patients assessed for mortality was relatively small. We do not know the onset of cardiovascular disease in those patients with TRV > 250cm/s; however, given the chronic nature of the diffuse vasculopathy, inclusion of age as a variable should partially account for this. Patients were transitioned to different facilities/care-providers so we could not obtain detailed information on all subjects’ current health status, comorbidities or treatment modalities such as continuation of chronic transfusion therapy or hydroxyurea at time of last follow up. A majority of death certificates have limited information concerning the cause of death and only listed SCD as the cause of death, which is not always accurate. The markers analyzed in this cohort represent a single point in time and ideally longitudinal measurements should be made in these patients.
Conclusion
Elevated tricuspid regurgitant jet velocity and myocardial tissue Doppler parameters of both systolic and diastolic function are strong markers of cardiovascular disease severity and predict mortality in pediatric, adolescent, young adult and adult patients with sickle cell disease. Critically needed predictors of cardiovascular mortality in pediatric patients with sickle cell disease can be used to better counsel patients about long term cardiovascular risk and may guide difficult discussions about future curative therapies.
Supplementary Material
Acknowledgments
Funding Source
This research was supported by National Institute of Health funded grants K12 HD52954-6 A1 (JAD), U01 HL 117718-01 (TDC/JCW), U54 HL090511-01 (JAD), U56 HL117718 (TDC/JCW), K23 HL 119627-01A1 (JAD), R03 HL 138321-01 (JAD), 1RC HL099412-01 (JCW). The study was presented at 60th Annual American Society of Hematology Conference 2018, San Diego, California.
Footnotes
Disclosure
The authors do not have any financial disclosure.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1002/ajh.26003
References:
- 1.CDC. Data & Statistics on Sickle Cell Disease | CDC. Centers for Disease Control and Prevention. Published August 31, 2016. Accessed February 18, 2019 https://www.cdc.gov/ncbddd/sicklecell/data.html
- 2.Sickle Cell Disease | National Heart, Lung, and Blood Institute (NHLBI). Accessed July 10, 2019 https://www.nhlbi.nih.gov/health-topics/sickle-cell-disease
- 3.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality In Sickle Cell Disease – Life Expectancy and Risk Factors for Early Death. N Engl J Med. 1994;330(23):1639–1644. doi: 10.1056/NEJM199406093302303 [DOI] [PubMed] [Google Scholar]
- 4.Lanzkron S, Carroll CP, Haywood C. Mortality rates and age at death from sickle cell disease: U.S., 1979–2005. Public Health Rep Wash DC 1974. 2013;128(2):110–116. doi: 10.1177/003335491312800206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elmariah H, Garrett ME, De Castro LM, et al. Factors associated with survival in a contemporary adult sickle cell disease cohort. Am J Hematol. 2014;89(5):530–535. doi: 10.1002/ajh.23683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeBaun MR, Ghafuri DL, Rodeghier M, et al. Decreased median survival of adults with sickle cell disease after adjusting for left truncation bias: a pooled analysis. Blood. 2019;133(6):615–617. doi: 10.1182/blood-2018-10-880575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood. 2010;115(17):3447–3452. doi: 10.1182/blood-2009-07-233700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanni E, Grosse SD, Yang Q, Olney RS. Trends in pediatric sickle cell disease-related mortality in the United States, 1983–2002. J Pediatr. 2009;154(4):541–545. doi: 10.1016/j.jpeds.2008.09.052 [DOI] [PubMed] [Google Scholar]
- 9.Darbari DS, Hildeshem M, Minniti C, Kato GJ, Taylor JG. Health Care Utilization for Painful Events Is Associated with Early Mortality in a Contemporary Population of Adults with Sickle Cell Anemia. Blood. 2011;118(21):2115. [Google Scholar]
- 10.Maitra P, Caughey M, Robinson L, et al. Risk factors for mortality in adult patients with sickle cell disease: a meta-analysis of studies in North America and Europe. Haematologica. 2017;102(4):626–636. doi: 10.3324/haematol.2016.153791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzhugh C, Lauder N, Jonassaint J, et al. Cardiopulmonary Complications Leading to Premature Deaths in Adult Patients with Sickle Cell Disease. Am J Hematol. 2010;85(1). doi: 10.1002/ajh.21569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gladwin MT, Sachdev V, Jison ML, et al. Pulmonary Hypertension as a Risk Factor for Death in Patients with Sickle Cell Disease. N Engl J Med. 2004;350(9):886–895. doi: 10.1056/NEJMoa035477 [DOI] [PubMed] [Google Scholar]
- 13.Mehari A, Gladwin MT, Tian X, Machado RF, Kato GJ. Mortality in Adults With Sickle Cell Disease and Pulmonary Hypertension. JAMA. 2012;307(12):1254–1256. doi: 10.1001/jama.2012.358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parent F, Bachir D, Inamo J, et al. A Hemodynamic Study of Pulmonary Hypertension in Sickle Cell Disease. N Engl J Med. 2011;365(1):44–53. doi: 10.1056/NEJMoa1005565 [DOI] [PubMed] [Google Scholar]
- 15.Vichinsky EP. Pulmonary Hypertension in Sickle Cell Disease. N Engl J Med. 2004;350(9):857–859. doi: 10.1056/NEJMp038250 [DOI] [PubMed] [Google Scholar]
- 16.Hagar RW, Michlitsch JG, Gardner J, Vichinsky EP, Morris CR. Clinical differences between children and adults with pulmonary hypertension and sickle cell disease. Br J Haematol. 2008;140(1):104–112. doi: 10.1111/j.1365-2141.2007.06822.x [DOI] [PubMed] [Google Scholar]
- 17.Lee MT, Small T, Khan MA, Rosenzweig EB, Barst RJ, Brittenham GM. Doppler-defined pulmonary hypertension and the risk of death in children with sickle cell disease followed for a mean of three years. Br J Haematol. 2009;146(4):437–441. doi: 10.1111/j.1365-2141.2009.07779.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hebson C, New T, Record E, et al. Elevated tricuspid regurgitant velocity as a marker for pulmonary hypertension in children with sickle cell disease: less prevalent and predictive than previously thought? J Pediatr Hematol Oncol. 2015;37(2):134–139. doi: 10.1097/MPH.0000000000000184 [DOI] [PubMed] [Google Scholar]
- 19.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2016;29(4):277–314. doi: 10.1016/j.echo.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 20.Skinner GJ. Echocardiographic Assessment of Pulmonary Arterial Hypertension for Pediatricians and Neonatologists. Front Pediatr. 2017;5:168. doi: 10.3389/fped.2017.00168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sachdev V, Machado RF, Shizukuda Y, et al. Diastolic Dysfunction Is an Independent Risk Factor for Death in Patients With Sickle Cell Disease. J Am Coll Cardiol. 2007;49(4):472–479. doi: 10.1016/j.jacc.2006.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nouraie M, Darbari DS, Rana S, et al. Tricuspid regurgitation velocity and other biomarkers of mortality in children, adolescents and young adults with sickle cell disease in the United States: The PUSH study. Am J Hematol. Published online April 3, 2020. doi: 10.1002/ajh.25799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruhl AP, Sadreameli SC, Allen JL, et al. Identifying Clinical and Research Priorities in Sickle Cell Lung Disease. An Official American Thoracic Society Workshop Report. Ann Am Thorac Soc. 2019;16(9):e17–e32. doi: 10.1513/AnnalsATS.201906-433ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato GJ, McGowan V, Machado RF, et al. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006;107(6):2279–2285. doi: 10.1182/blood-2005-06-2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kato GJ, Steinberg MH, Gladwin MT. Intravascular hemolysis and the pathophysiology of sickle cell disease. J Clin Invest. 127(3):750–760. doi: 10.1172/JCI89741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris CR, Kato GJ, Poljakovic M, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA. 2005;294(1):81–90. doi: 10.1001/jama.294.1.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Detterich JA, Kato RM, Rabai M, Meiselman HJ, Coates TD, Wood JC. Chronic transfusion therapy improves but does not normalize systemic and pulmonary vasculopathy in sickle cell disease. Blood. Published online January 1, 2015:blood-2014–12-614370. doi: 10.1182/blood-2014-12-614370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman D, Szmuszkovicz J, Rabai M, Detterich JA, Menteer J, Wood JC. Systemic endothelial dysfunction in children with idiopathic pulmonary arterial hypertension correlates with disease severity. J Heart Lung Transplant Off Publ Int Soc Heart Transplant. 2012;31(6):642–647. doi: 10.1016/j.healun.2012.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hebbel RP, Boogaerts MAB, Eaton JW, Steinberg MH. Erythrocyte Adherence to Endothelium in Sickle-Cell Anemia. N Engl J Med. 1980;302(18):992–995. doi: 10.1056/NEJM198005013021803 [DOI] [PubMed] [Google Scholar]
- 30.Detterich JA, Kato R, Bush A, et al. Sickle cell microvascular paradox-oxygen supply-demand mismatch. Am J Hematol. 2019;94(6):678–688. doi: 10.1002/ajh.25476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Detterich J, Alexy T, Rabai M, et al. Low-shear red blood cell oxygen transport effectiveness is adversely affected by transfusion and further worsened by deoxygenation in sickle cell disease patients on chronic transfusion therapy. Transfusion (Paris). 2013;53(2):297–305. doi: 10.1111/j.1537-2995.2012.03822.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schimmel M, van Beers EJ, van Tuijn CFJ, et al. N-terminal pro-B-type natriuretic peptide, tricuspid jet flow velocity, and death in adults with sickle cell disease. Am J Hematol. 2015;90(4):E75–E76. doi: 10.1002/ajh.23944 [DOI] [PubMed] [Google Scholar]
- 33.Gladwin MT, Barst RJ, Gibbs JSR, et al. Risk factors for death in 632 patients with sickle cell disease in the United States and United Kingdom. PloS One. 2014;9(7):e99489. doi: 10.1371/journal.pone.0099489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ataga KI, Moore CG, Jones S, et al. Pulmonary hypertension in patients with sickle cell disease: a longitudinal study. Br J Haematol. 2006;134(1):109–115. doi: 10.1111/j.1365-2141.2006.06110.x [DOI] [PubMed] [Google Scholar]
- 35.Abman SH, Hansmann G, Archer SL, et al. Pediatric Pulmonary Hypertension: Guidelines From the American Heart Association and American Thoracic Society. Circulation. 2015;132(21):2037–2099. doi: 10.1161/CIR.0000000000000329 [DOI] [PubMed] [Google Scholar]
- 36.Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC), European Respiratory Society (ERS), International Society of Heart and Lung Transplantation (ISHLT), et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34(6):1219–1263. doi: 10.1183/09031936.00139009 [DOI] [PubMed] [Google Scholar]
- 37.Augustine DX, Coates-Bradshaw LD, Willis J, et al. Echocardiographic assessment of pulmonary hypertension: a guideline protocol from the British Society of Echocardiography. Echo Res Pract. 2018;5(3):G11–G24. doi: 10.1530/ERP-17-0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fonseca GHH, Souza R, Salemi VMC, Jardim CVP, Gualandro SFM. Pulmonary hypertension diagnosed by right heart catheterisation in sickle cell disease. Eur Respir J. 2012;39(1):112–118. doi: 10.1183/09031936.00134410 [DOI] [PubMed] [Google Scholar]
- 39.Gladwin MT, Sachdev V. Cardiovascular Abnormalities in Sickle Cell Disease. J Am Coll Cardiol. 2012;59(13). doi: 10.1016/j.jacc.2011.10.900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiogra... - PubMed - NCBI. Accessed October 23, 2019 https://www.ncbi.nlm.nih.gov/pubmed/27422899
- 41.Association between diffuse myocardial fibrosis and diastolic dysfunction in sickle cell anemia | Blood | American Society of Hematology. Accessed October 23, 2019 https://ashpublications.org/blood/article-lookup/doi/10.1182/blood-2017-02–767624 [DOI] [PMC free article] [PubMed]
- 42.Bakeer N, James J, Roy S, et al. Sickle cell anemia mice develop a unique cardiomyopathy with restrictive physiology. Proc Natl Acad Sci U S A. 2016;113(35):E5182–5191. doi: 10.1073/pnas.1600311113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niss O, Fleck R, Makue F, et al. Association between diffuse myocardial fibrosis and diastolic dysfunction in sickle cell anemia. Blood. 2017;130(2):205–213. doi: 10.1182/blood-2017-02-767624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chalacheva P, Khaleel M, Sunwoo J, et al. Biophysical markers of the peripheral vasoconstriction response to pain in sickle cell disease. PLOS ONE. 2017;12(5):e0178353. doi: 10.1371/journal.pone.0178353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manci EA, Culberson DE, Yang Y-M, et al. Causes of death in sickle cell disease: an autopsy study. Br J Haematol. 2003;123(2):359–365. doi: 10.1046/j.1365-2141.2003.04594.x [DOI] [PubMed] [Google Scholar]
- 46.Desai Ankit A., Patel Amit R., Homaa Ahmad, et al. Mechanistic Insights and Characterization of Sickle Cell Disease–Associated Cardiomyopathy. Circ Cardiovasc Imaging. 2014;7(3):430–437. doi: 10.1161/CIRCIMAGING.113.001420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liem RI, Lanzkron S, D Coates T, et al. American Society of Hematology 2019 guidelines for sickle cell disease: cardiopulmonary and kidney disease. Blood Adv. 2019;3(23):3867–3897. doi: 10.1182/bloodadvances.2019000916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pashankar FD, Carbonella J, Bazzy-Asaad A, Friedman A. Longitudinal follow up of elevated pulmonary artery pressures in children with sickle cell disease. Br J Haematol. 2009;144(5):736–741. doi: 10.1111/j.1365-2141.2008.07501.x [DOI] [PubMed] [Google Scholar]
- 49.Detterich JA, Liu H, Suriany S, et al. Erythrocyte and plasma oxidative stress appears to be compensated in patients with sickle cell disease during a period of relative health, despite the presence of known oxidative agents. Free Radic Biol Med. 2019;141:408–415. doi: 10.1016/j.freeradbiomed.2019.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsitsikas DA, Seligman H, Sirigireddy B, Odeh L, Nzouakou R, Amos RJ. Regular automated red cell exchange transfusion in the management of pulmonary hypertension in sickle cell disease. Br J Haematol. 2014;167(5):707–710. doi: 10.1111/bjh.13031 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.