Abstract
Humans and animals use mental representations of the spatial structure of the world to navigate. The classical view is that these representations take the form of Euclidean cognitive maps, but alternative theories suggest they are cognitive graphs consisting of locations connected by paths. Here we review evidence suggesting that both map-like and graph-like representations exist in the mind/brain, relying on partially overlapping neural systems. Maps and graphs can operate simultaneously or separately, and they may be applied to both spatial and nonspatial knowledge. By providing structural frameworks for complex information, cognitive maps and cognitive graphs may provide fundamental organizing schemata that allow us to navigate in physical, social, and conceptual spaces.
Keywords: spatial navigation, memory, semantic knowledge, hippocampus, visual scenes, grid cells, retrosplenial complex
Spatial navigation and spatial knowledge
To navigate efficiently from place to place, we rely on internal representations of the spatial structure of the world [1]. These are sometimes referred to as “cognitive maps” because they play a functional role that is similar to physical maps. The study of cognitive maps has been a central concern of psychology and neuroscience, stretching back to the dawn of the cognitive revolution [2] (Box 1). The importance of these representational structures is emphasized by the fact that they are believed to be used not just for spatial navigation, but also for reasoning, inference, and memory in a wide variety of knowledge domains [3-6].
Box 1 -. Historical conceptions of cognitive maps.
The concept of a cognitive map dates back to Tolman [2], who presented it as an alternative to behaviorist approaches to psychology. Tolman suggested that animals form representations of environments that are more than an interlinked series of associations and whose learning does not depend on immediate reward. The idea that humans and animals have internal spatial representations is now widely accepted, but there has been less consensus about the nature of “cognitive maps” - their degree of accuracy, the inputs required to form them, and how they integrate separately experienced environments.
In 1978, O’Keefe and Nadel proposed that a cognitive map is Euclidean, and this has become the dominant model for neuroscientists [7]. A precise definition comes from Langille and Gallistel [178]: “A map is a set of vectors in a 2- or 3-dimensional vector space, on which navigation-relevant vector functions are defined.” Under this definition, the key feature of a map is that it establishes coordinates for each point in space, thus allowing a navigator to associate non-location information (e.g. terrain characteristics, visual snapshots, reward values) with any location, and to set a course between different locations [1]. O’Keefe and Nadel contrasted the flexibility of the Euclidean “locale” system with the inflexibility of the action-based “taxon” system, which they postulated mediated a habit-like following of routes.
However, in parallel to the Euclidean map hypothesis, other suggestions for very different types of cognitive maps were put forth, some of which have graph-like aspects. Kuipers suggested that in addition to Euclidean cognitive maps, people store representations that are based on topological knowledge - connectivity between locations through routes and their hierarchical organization into regions [42,43]. Subsequent authors have developed other models of graph-like spatial representations [19,26,36,38,41,45,47,179-182], with additional elements such as a skeleton of major routes on which the graph is constructed [44], labels at nodes and edges indicating directions and distances [8,9,30], and node-specific reference frames [37,39]. It remains debated whether spatial knowledge is Euclidean, graph-based, or a combination of both. In this paper, we use the term cognitive map to refer to Euclidean cognitive maps, while referring to graph-like structures as cognitive graphs.
But, what is the nature of the cognitive map? Under the classical conception, a cognitive map encodes environmental elements (places, landmarks, goals) in a Euclidean spatial coordinate system [7]. However, an alternative conception is that spatial knowledge takes a graph-like form, with locations (nodes) connected by paths (links/edges), but with no information about their orientation relative to a global reference frame [8]. These two ideas are often considered to be in opposition to each other [8,9]. Here we review evidence that suggests that both map-like and graph-like knowledge structures are encoded by the human brain (Figure 1). We explore the neural basis of both kinds of representations, and consider how they might be applied to both spatial and nonspatial information.
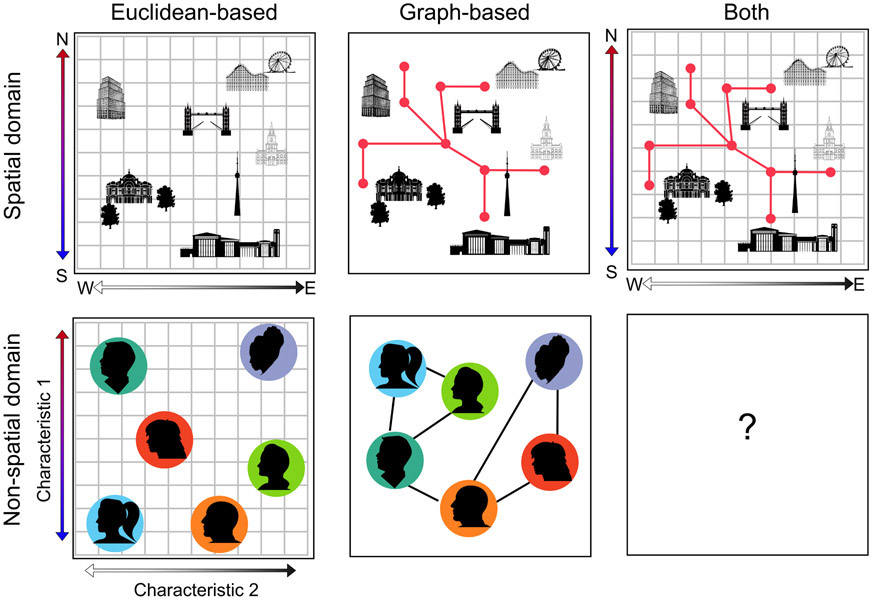
Figure 1: Map-based vs. graph-based representations.
In the spatial domain (top row), knowledge can be purely map-based with locations coded in terms of Euclidean coordinates (e.g. latitude and longitude), or purely graph-based with locations as nodes and paths between locations as links. It is also possible for map- and graph-based representations to exist simultaneously, allowing us to switch flexibly between the two. In non-spatial domains (bottom row), knowledge is map-based when information is coded in terms of continuous dimensions and graph-based when it is coded in terms of distinct links between items. For example, the individuals in a social group might be represented in terms of their personality characteristics (map-based) or in terms of the social connections within the group (graph-based). Currently, it is unclear whether a flexible combination of graph- and map-like representations exists in non-spatial domains.
What form does spatial knowledge take?
Cognitive maps are typically conceptualized as encoding environmental elements in terms of their positions in Euclidean space (see Glossary) [1,7,10]. A representation of this kind enables a navigator to orient toward unseen locations, identify novel shortcuts, and detour around obstacles--tasks that require the flexible use of spatial knowledge. This formulation received major support from the discovery of grid cells, which may provide a neural substrate for representing locations on a 2D Euclidean coordinate system [11,12].
However, a challenge to this idea comes from evidence that people seem to form mental representations that violate Euclidean rules. There are many examples in the literature. People tend to distort route angles in memory toward 90 degrees, in a way that can create impossible configurations [13-16]. Distance and direction estimates between locations are warped by the number of turns in the routes connecting them [15,17-21], and can differ between forward and backward route directions [19,21-25]. Moreover, people often maintain only a very schematic and distorted representation of their environment [14,16,26,27], and may be unable to point in the direction of unseen landmarks even after years of experience [28]. Nevertheless, people are often able to confidently and accurately navigate and take novel shortcuts even when they do not possess a full metric mapping of the environment [26,29]. People can even navigate in virtual environments that have an impossible, non-Euclidean structure, while remaining seemingly unaware of those inconsistencies [30-34] (see Box 2, Figure 2).
Box 2: Environments - Real, Virtual and Impossible.
When the study of cognitive maps began, investigators used real physical environments. In the lab, they constructed wooden mazes or filled tubs with milky water. In natural environments, they tracked the countryside over which bees flew or asked students questions about the layout of their campuses. The lab environments provided experimental control, whereas the natural environments had the advantage of ecological validity--but both kinds of environment were three-dimensional layouts that provided a full range of sensory input and in which the participant could move. Along with these strengths came weaknesses. The constructed environments were rarely large in scale, and the natural environments did not allow for standardization across studies.
Advances in technology now permit the use of virtual reality (VR) environments in the study of navigation. VR technology comes in a variety of flavors, ranging from desktop computer displays to fully immersive head-mounted displays with bodily motion tracking, and it has been used to study navigation in both humans and non-human animals. Although VR and real navigation differ in some important respects (especially the lack of kinesthetic and proprioceptive feedback in desktop situations [183]), VR navigation experiments have been found to involve many of the same neural mechanisms implicated in real-world navigation [184].
One advantage of virtual environments is that they allow participants to experience layouts that are impossible in the real world. For instance, participants can pass through wormholes taking them immediately to spots they would normally take time to get to, or they can go around “five-sided squares” (Figure 2, [30-34]). Experimenters have often reported that participants do not even notice these impossibilities, and have used this fact to argue against Euclidean cognitive maps [8,30,31]. However, it is also possible that participants expect environments like those they encounter in real life; faced with difficulty in making sense of their experience, they do their best, attributing uncertainty to their possible lapses of attention or insufficient spatial ability. Indeed, there are sometimes indications that participants are aware of oddities, such as in the swirling notations on sketch maps in a study by Muryy and Glennerster [34]. As that paper notes, future work should directly examine whether people are able to distinguish possible from impossible environments when directly instructed to do so. However, irrespective of the question of whether subjects are aware of the impossible nature of virtual environments, these VR studies suggest the possibility that people can form non-Euclidean spatial representations and use them for navigation.
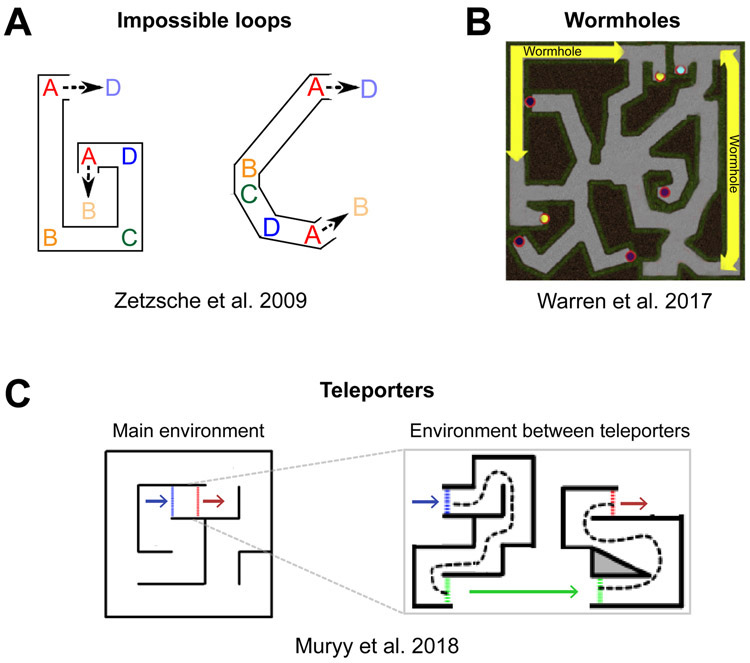
Figure 2: Impossible virtual environments provide evidence for non-Euclidean spatial representations.
Layouts for each experiment are depicted from a survey (bird’s-eye) view, although participants only experienced the environments from an eye-level perspective. A) Impossible looping corridors used in [31]. Letters indicate distinct corners. Each environment formed a closed, continuous loop from the participants’ perspective (that is, walking from A to B to C to D and continuing leads back to A). In the environment on the left, distances were non-Euclidean (e.g. the distance between points A and B was larger than between points C and D); in the environment on the right, angles were non-Euclidean. B) Maze with wormholes used in [30]. Yellow lines indicate the points connected by the wormholes - when participants moved into a wormhole they immediately emerged on its other side. However, the views at the entrance and exit of each wormhole were seamlessly matched, so that there was no cue to participants that they had passed into a different part of the environment. C) Teleporters used in [33]. On the left is the layout of the main environment, with two teleporters marked by blue and red lines. When participants entered a teleporter, they were transported to the environment on the right. After following the route indicated by the dashed line, they emerged back into the main environment from the other teleporter. Therefore, a long and complex route replaced the short Euclidean distance between the blue and red lines. In all three studies (A-C), participants successfully learned to navigate the environments, despite their impossible structure. Furthermore, participants were not informed that the environments were irregular and many did not report noticing anything amiss (but see Box 2 for discussion). These findings suggest that people can form non-Euclidean representations of space, possibly taking the form of cognitive graphs.
Results such as these suggest that people might use a non-Euclidean kind of spatial knowledge to represent their environment, created by the combination of familiar routes into a network of path segments. Memory of how to follow a specific route is often thought to be procedural - an ordered sequence of stimuli and responses (“when seeing X, turn right”), not necessarily incorporating any explicit spatial knowledge [35]. However, learning how routes connect at intersections might allow a navigator to encode a flexible representation of the route network, thus enabling the recombination of path segments in novel ways during navigation [8,19,26,36-39]. Such a flexible representation of path segments is referred to as a cognitive graph.
In a graph, only a limited number of spatial locations are represented as nodes. These may be locations of navigational importance (e.g. turns in the road, intersections between routes, prominent landmarks, or locations of prolonged stay), or places with special personal meaning to the navigator [10,36,39]. Spatial relationships between nodes are represented as links that include only the information needed to travel between the nodes - specific action sequences to take, relevant metric information (e.g. lengths of path segments), or route characteristics such as slope and general direction [36,39]. Cognitive graphs can be thought of as a set of state transitions, where specific action sequences lead from one state to another - similar to the mechanisms underlying performance of non-spatial tasks [36,40].
An important aspect of a cognitive graph--distinguishing it in our conception from a cognitive map--is that locations are not referenced to a global coordinate system. Opinions differ, however, as to whether cognitive graphs incorporate local metric information. Some investigators argue for topological graphs that do not use any spatial coordinate system [26,39,41-45]. Supporting this view is the fact that people usually know the correct topological structure among landmarks (order, containment, and connectivity), even when they have very distorted Euclidean environmental representations [14]. Others have argued for labeled graphs that encode the angles and lengths of the paths extending from each node [8,9,30,37,46]. This kind of graph might enable finding novel shortcuts by the sequential summing of local spatial information, explaining how people can make complex navigational maneuvers without possessing a global Euclidean cognitive map [8,30,47].
The evidence we review below suggests that cognitive graphs and cognitive maps do not have to be mutually exclusive. Instead, maps and graphs can be learned in parallel in the same environment (Box 3) [48], and may complement each other.
Box 3 -. How are cognitive maps and graphs acquired and lost?
To understand the interplay between cognitive graphs and maps, it is important to consider their order of acquisition. There are two possibilities: graph-first or map-first.
In the first scenario, environments are initially encoded in terms of individual locations and connecting routes. With prolonged experience, more paths in the environment are traversed and more connections are established. This increase of information allows the construction of an integrated representation with a global reference frame that encompasses the entire space, in a manner that is consistent with the classical view of spatial microgenesis [10] (although see [185]). Evidence for this scenario comes from reports that grid and HD cell firing fields are initially anchored by the walls of individual subspaces [186], but with experience extend across boundaries to encompass a larger space [187,188]. In non-spatial domains, this scenario might play out as initial learning of one-to-one associations between stimuli, followed by integration as global organizing principles are discovered.
In the second scenario, environments are initially encoded using a Euclidean map, which might be created through path integration. Graph representations are formed later as part of a consolidation process that involves the identification of key locations, and the storing of paths between these locations in memory, so that they can be directly retrieved rather than being computed anew each time they are required. Consistent with this scenario are findings indicating that activity during navigation shifts from the hippocampus to cortical regions, especially RSC, as an environment becomes familiar [189-191]. The graph-like nature of these cortical representations can be inferred from examination of patients with hippocampal damage. When these patients are asked to draw sketch maps of neighborhoods they have lived in for decades, the maps tend to be schematic, only including the main roads and the few most prominent landmarks [192]. In one particularly illuminating case, a former London taxi driver was able to navigate along primary roads in virtual London, but tended to get lost when the route involved more side streets, suggesting that only a skeleton of primary locations and routes remained [193]. Although there is no direct connection to non-spatial domains, these findings are reminiscent of observations in semantic dementia patients, who retain a skeleton of core concepts with a shrinking network of specific exemplars as the disease progresses [194].
Neural systems supporting cognitive maps and cognitive graphs
In this section, we review the neural systems that support cognitive maps and cognitive graphs. Figure 3A summarizes our conclusions in graphical format.
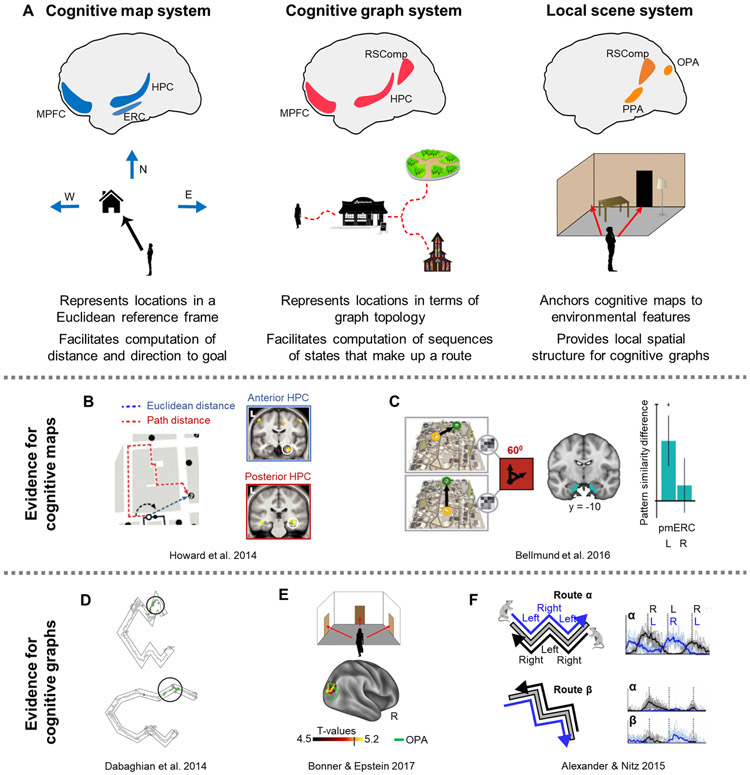
Figure 3: Neural systems supporting map-based and graph-based representations.
A) Putative brain systems supporting each type of representation, and their suggested functions. B-F) Evidence for cognitive map and cognitive graph representations. B) Results from an fMRI study showing Euclidean distance representations in the anterior hippocampus and path distance representations in the posterior hippocampus [54]. Participants learned the layout of London’s Soho district and navigated toward different goal locations. Activity in the anterior hippocampus scaled with Euclidean distance to the current navigational goal, while activity in the posterior hippocampus scaled with path distance to the goal. C) Results from an fMRI study showing representation of Euclidean direction to goal [86]. Subjects learned the layout of a virtual city, and then on each trial they were asked to imagine themselves standing in front one building while facing in the direction of another building. Activity patterns in ERC exhibited a six-fold symmetry with respect to the imagined vector, demonstrating the existence of a direction code related to entorhinal grid cells. D) Example of a topological representation in the rat hippocampus [94]. Rats navigated a U-shaped route, which could be folded such that the angles between route segments and their positions within the surrounding room were changed, without changing the overall route length. Hippocampal place cells fired at the same route segments regardless of the manipulation, demonstrating that the firing is sensitive to route topology and not to absolute Euclidean location in space. E) Results of an fMRI study showing sensitivity to the navigational affordances of scenes [115]. Participants viewed rooms with exits (doors) at different locations, as well as naturalistic scenes. Navigational affordances (i.e. pathways for movement) in both types of scenes were represented by similarity in multi-voxel patterns in the OPA. F) Examples of route information coding in the rat retrosplenial cortex [126]. Rats navigated two W-shaped routes located in different parts of a room, in both route directions. Some retrosplenial neurons coded route action sequences (e.g. right-left-right turn) while distinguishing the direction of route movement, while other retrosplenial neurons additionally distinguished between the two routes. PPA - parahippocampal place area, OPA - occipital place area, RSComp - retrosplenial complex, HPC - hippocampus, ERC - entorhinal cortex, MPFC - medial prefrontal and orbitofrontal cortex, R - right, L - left.
Evidence for Euclidean spatial representations
The discovery of place cells in the rodent hippocampus provided the first evidence for a spatial code in the brain [49]. O’Keefe and Nadel [7] hypothesized that these neurons, whose firing depends on the position of the animal, support a Euclidean map. Consistent with this idea, neuroimaging studies have found that the hippocampus exhibits a key feature of a map: encoding of distances between locations [3,50-54]. Because Euclidean and path-based distances tend to be correlated in most realistic environments, it has not always been clear whether these distance effects are evidence for map-like or graph-like representations. However, one study that attempted to dissociate these factors found that activity in the anterior hippocampus and entorhinal cortex was related to Euclidean distance, whereas activity in posterior hippocampus was related to path distance [54] (Figure 3B).
The spatial metric underlying these distance codes is believed to be implemented not in the hippocampus itself, but upstream in entorhinal cortex (ERC). This region contains grid cells, whose multi-peaked firing fields provide a regular grid for representing space [11]. Evidence for grid-cell-like representations has also been reported in human fMRI [55], single neuron activity [56], and intracranial theta oscillations [57], suggesting a cross-species Euclidean representational mechanism. The integrity of these grid-cell-like patterns relates to navigational ability and is reduced in individuals at genetic risk of developing Alzheimer’s disease and older adults [55,58,59]. Interestingly, grid-cell-like patterns in humans have also been observed outside of ERC, notably in the medial prefrontal cortex [55,56,60], suggesting that frontal lobe mechanisms also support the encoding of continuous dimensions. Within ERC, grid cells are organized into discrete modules along the dorsal-ventral axis, with more ventral modules exhibiting larger grid fields [61]; a similar organization by scale is observed in hippocampal place cells in rodents [62] and hippocampal fMRI signals in humans [63-65]. The use of multiple scales may allow the hippocampal-entorhinal system to support maps of different sizes, and also to code locations with greater robustness by combining information across scales [66].
Recording and modelling studies suggest that the grid system is intrinsically wired to support a representation of a continuous 2-D manifold, with its own, self-organizing, internal dynamics [67]. Activity in this manifold might be updated based on inputs from head direction cells [68] and speed cells [69], thus providing a mechanism for path integration [12]. However, it is worth noting that grid patterns often exhibit distortions from regularity, which are tied in a predictable manner to the geometry of the environment as defined by its boundaries [70-72]. Behavioral studies in humans have shown that spatial memories exhibit biases that are consistent with the geometry-based distortions observed in rodent grid cells [73-75]. According to one theory, grid cells may provide accurate measures of distance to the most-recently encountered boundary, but only an approximate reference system for the environment as a whole [76]. Interactions with environmental boundaries or with distal cues can reduce navigational errors by correcting the drift in spatial codes that is inevitable when grid and place fields are updated solely from internal self-motion cues [77-80]. These findings suggest that path integration may rely primarily on a Euclidean grid signal, but goal-directed navigation ultimately relies on representations that are a compromise between this Euclidean signal and information obtained from local spatial features such as boundaries and landmarks.
Besides encoding distances, another key aspect of a Euclidean map is that it allows the calculation of the direction to the navigational goal. Theoretical work suggests that grid cells can perform such computations [81-83]. Neuroimaging studies have found evidence for coding of direction to a navigational goal in the ERC and presubiculum. In two studies multivoxel patterns in these regions distinguished uniquely between directions in a manner reminiscent of rodent head direction cells [84,85], while in another study these patterns exhibited a 6-fold symmetry that is characteristic of grid cells [86]. Notably, the latter study used a complex virtual city where the straight-line vectors between various locations did not correspond to the navigational paths (Figure 3C). When taken together with the evidence for distance coding, these findings point to a Euclidean representational system in the brain, centered on ERC and hippocampus.
Evidence for graph-based spatial representations
In contrast to the flexible navigation afforded by a cognitive map, route-based navigation is often conceptualized as a rigid, procedure-bound strategy. Consistent with this view, numerous studies report a distinction between map-based navigation supported by the hippocampus and route following supported by the caudate nucleus [87-90]. However, graph-based navigation can also be conceptualized as not merely following an overlearned trajectory, but as a navigational system that enables flexible recombination of path segments in planning. This kind of navigation is unlikely to involve the caudate, but rather may recruit brain networks that partially overlap with the cognitive map system [91].
Proposals that the hippocampal formation represents graph-like spatial codes, instead of (or in addition to) cognitive maps, have existed in parallel with cognitive map theory for some time [38,92]. Although they did not use the term, Eichenbaum and Cohen [93] characterized the hippocampus as encoding relational elements (e.g. A is east of B) that could form the elements of a spatial graph. Recent evidence from rodent neurophysiology suggests that hippocampal place fields in constrained mazes are determined by position relative to the topological structure of the maze, rather than distance along the path or position in Euclidean space [94] (Figure 3D). More broadly, hippocampal place cells have been shown to divide space and time into segments [95,96], encode the hierarchical structure of experience [97], and represent schematic relationships between environmental elements [98], all of which can be conceptualized in terms of graph coding.
During navigational planning, hippocampal place cells are believed to support a path-based mechanism that respects environmental topology [92,99-101], and the firing rates of place cells are modulated by past and future paths [102-104]. Evidence for such a path-based planning mechanism has been observed in both rodents [105] and humans [54,106]. Hippocampal replay (i.e. sequential reactivation of place cells when the rodent is stationary) also appears to be sensitive to maze topology, with reversal of replay direction observed at intersections [107]. Related results have been reported in human neuroimaging: as participants entered a new street in a virtual environment, their posterior hippocampal signal reflected the number of network connections possible for future travel, while anterior hippocampal signal reflected the global network topology of the environment [108]. These data are consistent with the idea that the hippocampus supports a graph-like representation of locations in the environment and the links connecting them.
The role of scene regions
As discussed previously, behavioral studies suggest that people might not just encode topological graphs of which nodes are connected to each other; they might also encode labeled graphs that contain local spatial information about the distance and direction of the links or the angles that the links form at each node. What are the neural mechanisms that would give graphs this kind of local spatial structure?
To our knowledge, there have been no studies directly investigating this question. That said, a relevant observation is the fact that there is an intimate relationship between these local spatial features of a graph node and the spatial features of the local visual scene. This fact is well known to roboticists, who have exploited it when building artificial navigation systems [109]. Thus, we hypothesize that global topological representations, supported by the hippocampus, are given local spatial structure by the network of cortical regions known to represent the local scene. These scene-selective regions include the parahippocampal place area (PPA), occipital place area (OPA), and retrosplenial complex (RSComp) [110]. In previous reviews, we have discussed a possible role for these regions in anchoring cognitive maps by providing geometric and contextual inputs to the hippocampal-entorhinal system [3,111]. Here we argue that they may also play a central role in representing elements of cognitive graphs.
Both OPA and PPA respond strongly during the viewing of spatial scenes such as landscapes, cityscapes, and rooms [110]. Several lines of recent work suggest that OPA may be particularly important for processing information about the spatial layout of the local scene [111,112]. For example, OPA has been shown to represent scene geometry as defined by environmental boundaries [113,114] and the navigational affordances of the scene as defined by the locations of egress points and pathways through the scene [115] (Figure 3E). The latter result is particularly relevant because it is the egress points that define the local environment’s structure within a labelled graph (e.g. a room with two exits on opposite walls has a different graph structure than a room with two exits on adjoining walls or a room with 3 exits). PPA, on the other hand, appears to process the visual features, geometric features, and objects that define a scene as a specific place or context [116,117]. In map-based navigation, these representations of the geometric structure (OPA) and identifying features (PPA) of the local scene provide the perceptual inputs that allow a navigator to determine their orientation and location within the reference frame of the cognitive map. In graph-based navigation, these same representations would allow a navigator to understand the angles between the links converging on a node (using the OPA) and the discriminating features that give a node its unique identity (using the PPA).
These local representations might be integrated into the larger graph by RSComp, a region that encompasses retrosplenial cortex proper (BA 29/30), and adjoining territory in the parieto-occipital sulcus. Like OPA and PPA, RSComp responds strongly when people view scenes, and recent work suggests that its posterior portion may contain a retinotopic map, similar to those found in OPA and PPA [118]. However, RSComp is also implicated in global spatial processes that may be crucial for understanding the relationship between the local scene and the wider world [119].
In real-world or realistic virtual environments, RSComp represents spatial quantities such as location and heading [120-122] defined relative to stable environmental features such as environmental boundaries and landmarks [123-125]. In linear mazes, neurons in rodent retrosplenial cortex represent path elements that could potentially be used to construct a graph, such as turns, position along the path, and path identity [126-128] (see also [129] for related results in monkeys). These cells also represent relationships between path elements [130] and the allocentric location of the path within the broader environment [126] (Figure 3F). These integrative functions of RSComp contrast with the egocentric and route-centered coding observed in lateral parietal cortex neurons, which fire in response to specific route actions (e.g. turning left or right) and route positions, but do not incorporate information about the allocentric location of the route within the larger spatial context [131]. In an fMRI study, we observed that RSComp was sensitive to the order that buildings were encountered along a newly learned route through an urban neighborhood, responding more when buildings were presented in the correct order than when they were presented in the reverse order [132]. Notably, this effect was only found for buildings at intersections, suggesting that RSComp might be encoding routes in a graph-like manner with the intersections as nodes.
Taken together, this evidence suggests that graph-based navigation is supported in part by a network of cortical regions typically thought of as scene-selective, with OPA and PPA primarily involved in representing the nodes, and RSComp primarily involved in representing the links. The functions of these regions in graph-based navigation are related to their functions in map-based navigation, but they are not always directly analogous. The role of RSComp is particularly instructive. In map-based navigation, RSComp is believed to mediate between scene-based codes in the cortex and map-based codes in the hippocampal-entorhinal system [133]. This translation function allows a navigator to anchor their cognitive map to the local scene and understand directional relationships to unseen locations. Indeed, an inability to understand the relationship between the local scene and distal locations is an oft-reported consequence of the “heading disorientation” observed after RSComp damage [134]. Graph-based navigation, on the other hand, might utilize vectors that are stored directly in RSComp rather than being recalculated through hippocampal-ERC-RSComp interactions each time they are required (Box 3). In this way, a brain region that is used for active computation during map-based navigation might also come to support graph knowledge.
Factors that may affect the encoding and use of maps and graphs
Although cognitive maps and cognitive graphs can develop in parallel, different factors may influence the genesis and use of each. Consideration of these factors may help to clarify previous findings that appeared to support one kind of representation over the other.
Environmental constraints
To keep track of their location in a Euclidean coordinate system while moving about the world, navigators must maintain a sense of direction within a consistent reference frame. This task is easier in environments that contain clear global directional cues, such as prominent distal landmarks or slopes, and clear organizing axes, such as prominent straight routes (Figure 4). Thus, map-like codes are more likely to be used in open arenas or in cities with a grid-like street structure, especially when prominent landmarks such as large buildings or mountains are visible. In contrast, graph-based navigation is aided by distinct local landmarks that help identify nodes or trigger route action sequences [24,135], and may be more efficient than map-based navigation in environments where movement is constrained to a small number of routes [19,21,26,31,32,36]. Prototypical environments that would facilitate graph-like navigation include complex buildings, cities with limited visibility and curving streets, natural cave systems, and dense forests; in such environments, people may learn to navigate by following and combining path segments, without being aware of their global orientation or metric location [28,29].
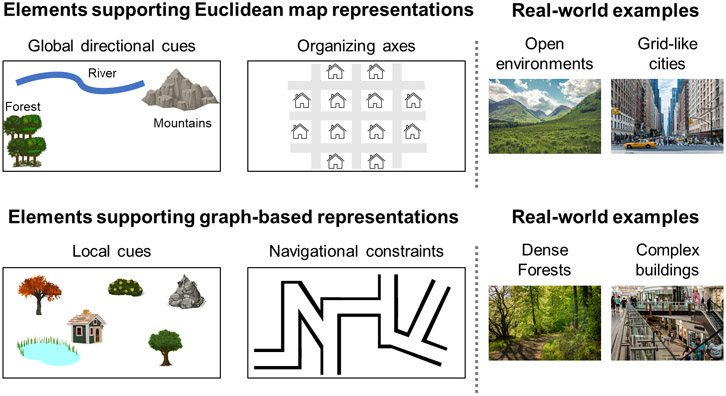
Figure 4: Environmental features supporting map-based and graph-based representations.
Euclidean representations are supported when the environment contains global directional cues (e.g. distal landmarks, slopes) or prominent defining axes. These representations may be favored in open environments, small co-visible spaces like single rooms, and in cities with a grid-like structure. In contrast, graph-based representations are supported by the presence of local landmarks and may be optimal when navigation is constrained to specific routes. Therefore, graph-based representations might be favored in dense forests, complex buildings, and cities without clear organizing axes.
Spatial scale
Navigable spaces vary in scale, from small rooms to large cities. These different scales may require different navigational strategies [136,137] that rely on partially separable neural systems [64,65]. Smaller environments (such as single rooms) might be easily coded as a Euclidean reference frame, especially if they are entirely co-visible. In contrast, larger spaces containing several subcompartments or subregions have an inherent hierarchical structure that lends itself to representation as a graph, and boundaries between these subcompartments may limit visibility and movements, making it difficult to create a global Euclidean map [18,138,139]. Indeed, several theories of spatial cognition emphasize the coding of a Euclidean reference frame for small environments, and a graph of connecting routes for larger environments [8,37,39,42,43]. The scale of space, by itself, need not be the only determinant of the form of representation; a large open park with clear axes and boundaries might be represented using a Euclidean coordinate frame, while a small building with several corridors might be represented as a cognitive graph. However, all other things being equal, smaller spaces may tend to be represented as Euclidean maps, while larger spaces may tend to be represented as cognitive graphs.
Individual and cultural variability
People appear to vary in their ability (and tendency) to form Euclidean and route-based representations. Most individuals can learn to follow specific routes, but many perform poorly when they are asked to point in the direction of unseen landmarks (i.e., create a Euclidean representation), or integrate intersecting routes (i.e., create a cognitive graph). These capacities depend on factors such as mental rotation ability and associative memory [140,141]. In addition, people have consistent individual tendencies to follow familiar routes vs. attempting to go straight in the general direction of their goal [29,89,142]. Social and cultural factors may also have an effect: for example, Americans rely more on global (cardinal) directions and less on local landmarks than Europeans when giving route descriptions [143,144], and men use cardinal directions and global directional cues (such as slopes) more than women when navigating and giving directions (and this ratio also varies by country) [145-150].
Despite the above-mentioned factors, in many environments people can maintain both map and graph representations, and use them interchangeably. The differential use of each may depend on the type of problem encountered [29,142]. For example, in a city built as a grid but with one-way streets, a route-based representation may be used when driving, but Euclidean navigation may be more useful when walking. Different types of problems will require different types of representations - and this parallels the different types of knowledge organization used in other kinds of problems, outside the spatial domain.
Beyond space: Maps and graphs in abstract domains
To what extent does the map-graph distinction extend to domains that are not inherently spatial, such as social or semantic knowledge? An increasing number of researchers have argued that spatial cognitive maps can be generalized to form maps of nonspatial information (for reviews, see [3-6]). In contrast, parallels between spatial and nonspatial graphs have been less discussed.
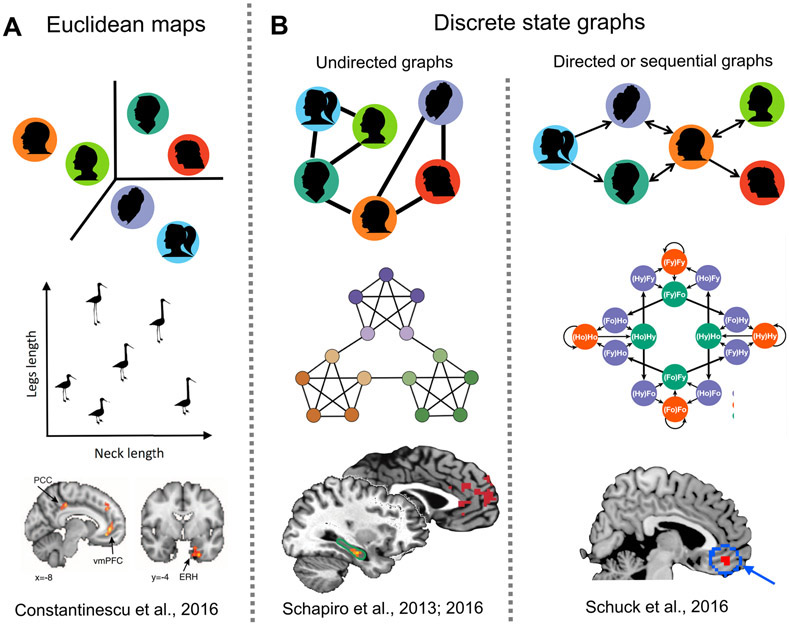
In our everyday lives, we are likely to use a wide variety of map-like and graph-like knowledge structures [4,151]. For example, we may represent the people we know in terms of continuous variables such as various abilities that are naturally encoded as a map-like attribute space (Figure 5A), or we may represent them in terms of discrete relationships between individuals that are naturally encoded in graph-like formats (e.g. social networks or family trees; Figure 5B). Similarly, we might represent living creatures in terms of continuous variables such as size and ferociousness, or in terms of a semantic network of discrete associations [152]. In experimental settings, the details of the paradigm may determine whether participants form map-like or graph-like representations: some tasks require participants to organize information along continuous dimensions, while others involve ordered transitions between discrete states. To date, most experiments have used only one task at a time, leading to an intriguing discrepancy between the spatial and nonspatial literatures: whereas spatial results can often be explained equally well by Euclidean or graph-based representations, nonspatial results usually come from paradigms that constrain the participant to use one representation or the other. Yet despite this difference, the observed neural substrates from spatial and nonspatial experiments are similar.
Figure 5: Maps and graphs in abstract spaces.
A) Some kinds of nonspatial knowledge might be organized into Euclidean spaces based on continuous feature dimensions - for example, the people working together on a project may be represented as points in a space based on their abilities or characteristics. Entorhinal cortex and medial prefrontal cortex have been shown to represent such non-spatial maps of continuous dimensions--for example, the length of bird legs and necks in a shape space [160]; figure from [5]. B) Other kinds of nonspatial knowledge might be organized into graphs representing connections or transitions between states. The middle column shows an example of an undirected graph indicating which people in the group know each other socially. The hippocampus and medial prefrontal cortex have been implicated in representing such undirected graphs [169,171]. The right column shows an example of a directed graph, indicating a set workflow, where task are passed from particular team members to other team members. A representative study investigating this type of knowledge structure has implicated the orbitofrontal cortex as the locus of ‘task space’ graphs, containing the transitions between different nodes in the graph [147]. Note that the relationships contained in the graphs in panel B can be independent of the placement of the individuals in Euclidean space in panel A.
Several studies have examined the coding of continuous representational dimensions in nonspatial domains. These studies have revealed a remarkable correspondence between the neural systems involved in the coding of spatial and nonspatial maps. Hippocampal cells in rodents have been shown to code several linear dimensions, including time [153-155] and auditory pitch [156]. In fMRI studies, hippocampal activity has been shown to track Euclidean vectors along experimentally-defined dimensions, including the angle of a power vs. affiliation vector derived from social interactions [157], distance within a 2-dimensional social attribute space defined by popularity and competence [158], and distance in a 2-dimensional stimulus space defined by visual size and opacity [159]. Entorhinal activity has also been shown to track Euclidean distance [158], and grid-like signals (in the form of hexadirectional modulation) have been found in ERC for several abstract spaces, including a continuous shape space defined by bird beak and leg length [160] (Figure 5A), an olfactory space defined by the blending of two odors [161], and a semantic space in which symbolic labels were used to parse two continuous stimulus dimensions into categorical regions [162]. Grid cells in monkey ERC have been shown to encode the visual space on a display screen [163] and a similar effect has been observed in human ERC [164,165]. As previously observed for physical spaces, neuroimaging studies have observed grid-like signals in medial prefrontal cortex [160-162]. Taken together, these results provide strong evidence for a common neural system for representing Euclidean spaces across a wide variety of physical and abstract domains.
Fewer studies have directly investigated graph coding in nonspatial domains, but evidence for graph-based coding comes from studies that present participants with sequences of items corresponding to transition matrices with an underlying graph structure (Figure 5B). Behaviorally, the learning of such graphs can be demonstrated by evaluating participants’ response times in terms of distances within the graph [166-168], or by testing their ability to parse items into “communities” of closely linked stimuli [169,170]. These studies have found evidence for graph coding and hierarchical organization using both explicit [168,170] and implicit [166,167,169] measures. Neuroimaging studies suggest that the hippocampus, prefrontal cortex, and RSComp support the ability to extract graph structure [166,168,169], but the pattern of results differs depending on specific task requirements. In a virtual subway network where participants made navigational decisions, but in the absence of first-person navigation experience, the hippocampus and ventromedial prefrontal cortex jointly tracked proximity to goal as the number of stops along the route, while dorsomedial prefrontal cortex tracked distance-to-goal in terms of the number of subway lines [168]. Interestingly, though it was not emphasized by the authors, this study also found effects of graph distance in RSComp. Similarly, in a network structure that participants learned by observing sequences of items corresponding to possible transitions between graph nodes, medial prefrontal cortex and hippocampal patterns were more similar for items closer together in the underlying structure [169,171]. However, another study found that activation patterns in a different frontal region adjacent and connected to the mPFC --orbitofrontal cortex--distinguished between nodes of a directed graph that represented the hidden states of a behavioral task [172]. Interestingly, this ability to represent the contingencies between abstract states seems to depend crucially on intact communication between the hippocampus and the orbitofrontal cortex [173]. This evidence is broadly consistent with the idea that the orbitofrontal cortex represents tasks as an abstracted graph of the goal-directed transitions between actions or states [40,174].
Studies using multivoxel pattern analyses have not typically found evidence for graph-like coding in ERC (e.g. [171]). However, in one study where participants viewed objects in temporal sequences governed by a specific graph structure, ERC exhibited fMRI adaptation related to link distance and other graph measures [166]. This finding suggested that objects associated with nearby nodes in the graph recruited more overlapping neural populations relative to objects associated with nodes further apart. This result is consistent with theoretical views that the hippocampus and ERC work together as a system that represents both the details and the structural regularities of the environment [175-177]. Under this perspective, the hippocampus establishes a set of states that can be linked to perceptual inputs while ERC represents general geometric rules that govern transitions from one state to another—rules that could potentially take either a map-like or graph-like form. This intriguing idea suggests the possibility that maps and graphs may be variants of an underlying representational continuum rather than categorically different knowledge structures (Box 4).
Box 4: Are maps and graphs fundamentally different?
Are maps and graphs truly different, or are they just different manifestations of the same underlying neuronal code? Some recent proposals suggest that all representations in the hippocampal-entorhinal system are fundamentally graph-like. One influential idea draws on reinforcement learning to argue that the hippocampus does not primarily code for one’s current state (i.e. location), but rather the distribution of possible states that could be visited in the future, termed the successor representation (SR) [177]. Knowledge of the available state space is acquired via exploration [195], and includes the likelihood of visiting each state and the probability of transitioning between specific states [196]. SRs therefore correspond to graph structure learning, where each state is a node, and the transition probability between any pair of adjacent states determines the weight of the connecting link. These transition probabilities can be affected by both spatial factors (such as boundaries) and non-spatial factors (such as the current goal). This approach has been used to explain hippocampal responses ranging from rodent electrophysiology to human conceptual learning [177]. In this scheme, grid fields in the entorhinal cortex are argued to represent principal components of hippocampal state representations [177,197], which can support planning across multiple spatial scales.
How might the brain build a Euclidean representation out of this inherently graph-like structure? A relevant observation is that the SR is agnostic about how states and transitions between them are defined. Existing models assume that spatial information is inherent in this definition; for example, by defining states in terms of their Euclidean locations [177] or transitions in terms of compass directions [175]. One possibility is that this information is provided by cell types whose firing is inherently spatial, such as head direction cells, or boundary vector cells (BVCs) [198,199]. These neural responses to one’s immediate environment (and additional local scene input from scene-selective areas) can thus be used to compute SR states. An open question is whether non-spatial inputs (perhaps from image spaces in the posterior parietal cortex [200]) might be used in a similar way to define states and transitions for abstract spaces.
Concluding remarks and future perspectives
Cognitive maps and cognitive graphs provide two powerful methods for organizing spatial and nonspatial knowledge. Whereas cognitive maps have been a topic of extensive discussion in the literature, cognitive graphs have garnered comparatively less attention--there is much still to be learned about how they operate and how they are instantiated by neural systems (see Outstanding Questions). Meanwhile, the issue of whether true Euclidean maps exist [8,26,36,47] and how they might support nonspatial knowledge remains a topic of intense debate.
OUTSTANDING QUESTIONS.
Are maps and graphs fundamentally distinct forms of representation, or are they two facets of the same underlying code (Box 4)? For example, can Euclidean maps be conceptualized as very dense graphs—or, alternatively, can cognitive graphs be conceptualized as maps that have higher dimensional topologies?
How do individual differences in map vs. graph use manifest across the population? Are people who are better at Euclidean navigation also better at graph-based navigation, or do people tend to favor one representation or the other? Do preferences for map-like or graph-like codes when navigating in space transfer to non-spatial tasks?
Many studies have investigated the neural systems that support cognitive maps. In contrast, the neural basis of cognitive graphs is less well established. To what extent are the hypotheses presented here about the neural basis of cognitive graphs borne out by studies that specifically investigate this question?
Physical space has three orthogonal dimensions that are continuous and equipotential. In contrast, semantic “spaces” typically have many more dimensions, which may not be orthogonal and are often more categorical than continuous. Do these differences have implications for understanding how cognitive maps and graphs are used to represent nonphysical spaces?
How extensive is the overlap between the brain systems that encode spatial vs. nonspatial knowledge? Do non-spatial representations rely on additional systems beyond the ones used in spatial navigation, for example linguistic or compositional codes? To what extent does non-spatial thinking employ other “spatial” cell types beyond place cells and grid cells--for example, head direction cells, or boundary cells?
One crucial unresolved issue is whether cognitive maps and cognitive graphs can truly be distinguished. As we have highlighted, most studies in spatial cognition use paradigms that can lead participants to develop both graph and map representations of the same environments. Participants may then use these representations interchangeably and according to their individual preferences, complicating the interpretation of the results. An important avenue for future research would be to develop experimental paradigms that are specifically designed to tease apart graph and map representations, both in space and in abstract domains. For example, different spatial environments could be constructed that are balanced for most features, but have different geometries that encourage formation of either graph or map representations. Alternatively, behavioral and fMRI similarity measures could be used to identify competing representations of cognitive graphs and Euclidean maps in the same environment, for example by contrasting coding of path distances and Euclidean distances between locations (e.g. [54]). The same procedures could be used in non-spatial domains, for example by investigating representations of items connected as a graph (e.g. social network connectivity between people) vs. coding of the same stimuli on a Euclidean space (e.g. people’s location on a coordinate system defined by continuous personality traits).
Irrespective of whether maps and graphs turn out to be fundamentally different, or two sides of the same coin, the distinction between these knowledge structures provides a useful framework for thinking about how complex information is represented in the mind-brain. Future investigations of these structures will be important not only for understanding how neural systems mediate spatial navigation, a core cognitive ability essential to survival and human flourishing, but also other fundamental elements of thought, including reasoning, memory, and prospection.
HIGHLIGHTS.
Spatial navigation has been suggested to rely either on Euclidean cognitive maps, or on graph-like representations of routes between locations.
Rather than being competing hypotheses, cognitive maps and cognitive graphs may coexist in the same individuals, with availability and use depending on environmental characteristics and navigational demands.
Cognitive maps and cognitive graphs are instantiated by partially distinct, partially overlapping neural systems in the hippocampal formation, frontal lobes, and scene-selective cortical regions.
Both representational systems can likely support abstract thought, with Euclidean maps suited for representing content varying along continuous dimensions, and cognitive graphs suited for representing state transitions and discrete associations between items.
Acknowledgments:
This project was supported by NIH (R01 EY022350 to RE), NSF (EHR 1660996 to NN) and a Zuckerman STEM Leadership fellowship to MP. We thank Randy Gallistel and Lynn Nadel for helpful discussions.
GLOSSARY
- Cognitive graph
a representation of space in terms of nodes (locations) connected by links (path segments). A cognitive graph can be topological (representing only whether locations are connected to each other or not), or labeled (representing local metric information such as distances and directions of each link, or the angles that links form at a node).
- Entorhinal cortex (ERC)
A brain region found in both rodents and humans that serves as a major input/output structure for the hippocampus. ERC contains both grid cells and head direction cells.
- Euclidean space
a continuous space defined by reference axes (usually two or three). Locations in a Euclidean space can be specified by coordinates, and relationships can be expressed in terms of distances and angles.
- Grid cells
neurons that represent space in a distributed manner, by firing in a regular array of locations that tile the environment in a hexagonal lattice.
- Head direction cells
neurons that fire as a function of the direction that the animal is facing, independent of its location, similar to the behavior of a compass.
- Hexadirectional modulation
a phenomenon where brain activity is modulated by the subject’s current direction of movement, with a six-fold symmetry - that is, firing is maximal for headings with angular spacing of 60 degrees. Believed to be a marker for grid cells, whose fields exhibit a similar six-fold organization.
- Local visual scene (or vista space)
a space that can be perceived from a single point without the need to navigate. Sometimes contrasted with environmental spaces, which cannot be perceived from a single point of view.
- Path integration
a strategy in which a navigator keeps track of the distances and directions they have travelled in order to compute a straight-line vector to the starting point. Path integration is believed to be crucial for learning Euclidean coordinates for locations in the environment, but it can become inefficient in large environments due to accumulated errors.
- Place cells
neurons that represent space in a localized manner, by firing when the animal is in a specific location.
- Route
a distinct path connecting two locations, which may have multiple path segments. Routes can be specified either by the lengths and directions of the path segments, and/or by the navigational actions (e.g. turns) to be performed along the path.
- Scene-selective regions
brain regions that respond more strongly in fMRI when people view spatial scenes (e.g. landscapes, cityscapes, rooms) than when they view other visual stimuli. Three scene regions have been identified in the posterior portion of the brain, near the apex of the visual system: the parahippocampal place area (PPA), retrosplenial complex (RSComp) and occipital place area (OPA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gallistel CR (1990) The organization of learning, The MIT Press. [Google Scholar]
- 2.Tolman EC (1948) Cognitive maps in rats and men. Psychol. Rev 55, 189–208 [DOI] [PubMed] [Google Scholar]
- 3.Epstein RA et al. (2017) The cognitive map in humans: spatial navigation and beyond. Nat. Neurosci 20, 1504–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellmund JLS et al. (2018) Navigating cognition: Spatial codes for human thinking. Science 362, eaat6766. [DOI] [PubMed] [Google Scholar]
- 5.Behrens TEJ et al. (2018) What Is a Cognitive Map? Organizing Knowledge for Flexible Behavior. Neuron 100, 490–509 [DOI] [PubMed] [Google Scholar]
- 6.Schafer M and Schiller D (2018) Navigating Social Space. Neuron 100, 476–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Keefe J and Nadel L (1978) The hippocampus as a cognitive map, Clarendon Press ; Oxford University Press. [Google Scholar]
- 8.Warren WH (2019) Non-Euclidean navigation. J. Exp. Biol 222, [DOI] [PubMed] [Google Scholar]
- 9.Chrastil ER and Warren WH (2014) From cognitive maps to cognitive graphs. PloS One 9, e112544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siegel AW and White SH (1975) The Development of Spatial Representations of Large-Scale Environments In Advances in Child Development and Behavior 10 (Reese HW, ed), pp. 9–55, JAI [DOI] [PubMed] [Google Scholar]
- 11.Hafting T et al. (2005) Microstructure of a spatial map in the entorhinal cortex. Nature 436, 801–806 [DOI] [PubMed] [Google Scholar]
- 12.McNaughton BL et al. (2006) Path integration and the neural basis of the “cognitive map.” Nat. Rev. Neurosci 7, 663–678 [DOI] [PubMed] [Google Scholar]
- 13.Moar I and Bower GH (1983) Inconsistency in spatial knowledge. Mem. Cognit 11, 107–113 [DOI] [PubMed] [Google Scholar]
- 14.Lynch K (1960) The Image of the City, MIT Press. [Google Scholar]
- 15.Byrne RW (1979) Memory for urban geography. Q. J. Exp. Psychol 31, 147–154 [Google Scholar]
- 16.Casakin H et al. (2000) Schematic Maps as Wayfinding Aids In Spatial Cognition II: Integrating Abstract Theories, Empirical Studies, Formal Methods, and Practical Applications (Freksa C et al. , eds), pp. 54–71, Springer [Google Scholar]
- 17.Brunec IK et al. (2017) Contracted time and expanded space: The impact of circumnavigation on judgements of space and time. Cognition 166, 425–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newcombe N et al. (1999) What do misestimations and asymmetries in spatial judgement indicate about spatial representation? J. Exp. Psychol. Learn. Mem. Cogn 25, 986–996 [Google Scholar]
- 19.Meilinger T et al. (2018), Spatial Survey Estimation Is Incremental and Relies on Directed Memory Structures , in Spatial Cognition XI, Cham, pp. 27–42 [Google Scholar]
- 20.Allen GL (1981) A developmental perspective on the effects of “subdividing” macrospatial experience. J. Exp. Psychol. [Hum. Learn.] 7, 120–132 [Google Scholar]
- 21.Meilinger T et al. (2018), Humans Construct Survey Estimates on the Fly from a Compartmentalised Representation of the Navigated Environment , in Spatial Cognition XI, Cham, pp. 15–26 [Google Scholar]
- 22.Sadalla EK et al. (1980) Reference points in spatial cognition. J. Exp. Psychol. [Hum. Learn.] 6, 516. [PubMed] [Google Scholar]
- 23.Holyoak KJ and Mah WA (1982) Cognitive reference points in judgments of symbolic magnitude. Cognit. Psychol 14, 328–352 [Google Scholar]
- 24.Ruddle RA et al. (2011) The effect of landmark and body-based sensory information on route knowledge. Mem. Cognit 39, 686–699 [DOI] [PubMed] [Google Scholar]
- 25.Allen GL et al. (1979) Developmental Issues in Cognitive Mapping: The Selection and Utilization of Environmental Landmarks. Child Dev. 50, 1062–1070 [PubMed] [Google Scholar]
- 26.Passini R (1984) Spatial representations, a wayfinding perspective. J. Environ. Psychol 4, 153–164 [Google Scholar]
- 27.Meilinger T et al. (2006), How much information do you need? Schematic maps in wayfinding and self localisation. , presented at the International Conference on Spatial Cognition, pp. 381–400 [Google Scholar]
- 28.Moeser SD (1988) Cognitive Mapping in a Complex Building. Environ. Behav 20, 21–49 [Google Scholar]
- 29.Hölscher C et al. (2006) Up the down staircase: Wayfinding strategies in multi-level buildings. J. Environ. Psychol 26, 284–299 [Google Scholar]
- 30.Warren WH et al. (2017) Wormholes in virtual space: From cognitive maps to cognitive graphs. Cognition 166, 152–163 [DOI] [PubMed] [Google Scholar]
- 31.Zetzsche C et al. (2009) Representation of space: image-like or sensorimotor? Spat. Vis 22, 409–424 [DOI] [PubMed] [Google Scholar]
- 32.Ericson JD and Warren WH (2020) Probing the invariant structure of spatial knowledge: Support for the cognitive graph hypothesis. Cognition 200, 104276. [DOI] [PubMed] [Google Scholar]
- 33.Muryy A and Glennerster A (2018), Pointing Errors in Non-metric Virtual Environments , in Spatial Cognition XI, Cham, pp. 43–57 [Google Scholar]
- 34.Muryy AA and Glennerster A (2020) Route selection in non-Euclidean virtual environments. bioRxiv [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thorndyke PW and Hayes-Roth B (1982) Differences in spatial knowledge acquired from maps and navigation. Cognit. Psychol 14, 560–589 [DOI] [PubMed] [Google Scholar]
- 36.Lieblich I and Arbib MA (1982) Multiple representations of space underlying behavior. Behav. Brain Sci 5, 627–640 [Google Scholar]
- 37.Meilinger T (2008), The Network of Reference Frames Theory: A Synthesis of Graphs and Cognitive Maps , in Spatial Cognition VI. Learning, Reasoning, and Talking about Space, Berlin, Heidelberg, pp. 344–360 [Google Scholar]
- 38.Poucet B (1993) Spatial cognitive maps in animals: New hypotheses on their structure and neural mechanisms. Psychol. Rev 100, 163–182 [DOI] [PubMed] [Google Scholar]
- 39.Werner S et al. (2000) Modelling Navigational Knowledge by Route Graphs In Spatial Cognition II: Integrating Abstract Theories, Empirical Studies, Formal Methods, and Practical Applications (Freksa C et al. , eds), pp. 295–316, Springer [Google Scholar]
- 40.Wilson RC et al. (2014) Orbitofrontal Cortex as a Cognitive Map of Task Space. Neuron 81, 267–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chown E et al. (1995) Prototypes, location, and associative networks (PLAN): Towards a unified theory of cognitive mapping. Cogn. Sci 19, 1–51 [Google Scholar]
- 42.Kuipers B (1982) The “Map in the Head” Metaphor. Environ. Behav 14, 202–220 [Google Scholar]
- 43.Kuipers B (1978) Modeling spatial knowledge. Cogn. Sci 2, 129–153 [Google Scholar]
- 44.Kuipers B et al. (2003) The Skeleton In The Cognitive Map: A Computational and Empirical Exploration. Environ. Behav 35, 81–106 [Google Scholar]
- 45.Trullier O et al. (1997) BIOLOGICALLY BASED ARTIFICIAL NAVIGATION SYSTEMS: REVIEW AND PROSPECTS. Prog. Neurobiol 51, 483–544 [DOI] [PubMed] [Google Scholar]
- 46.Chrastil ER and Warren WH (2015) Active and passive spatial learning in human navigation: Acquisition of graph knowledge. J. Exp. Psychol. Learn. Mem. Cogn 41, 1162. [DOI] [PubMed] [Google Scholar]
- 47.Glennerster A (2016) A moving observer in a three-dimensional world. Philos. Trans. R. Soc. B Biol. Sci 371, 20150265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacobs LF and Schenk F (2003) Unpacking the cognitive map: The parallel map theory of hippocampal function. Psychol. Rev 110, 285–315 [DOI] [PubMed] [Google Scholar]
- 49.O’Keefe J and Dostrovsky J (1971) The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 34, 171–175 [DOI] [PubMed] [Google Scholar]
- 50.Morgan LK et al. (2011) Distances between Real-World Locations Are Represented in the Human Hippocampus. J. Neurosci 31, 1238–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deuker L et al. (2016) An event map of memory space in the hippocampus. eLife 5, e16534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vass LK et al. (2016) Oscillations Go the Distance: Low-Frequency Human Hippocampal Oscillations Code Spatial Distance in the Absence of Sensory Cues during Teleportation. Neuron 89, 1180–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chrastil ER et al. (2015) There and Back Again: Hippocampus and Retrosplenial Cortex Track Homing Distance during Human Path Integration. J. Neurosci 35, 15442–15452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Howard LR et al. (2014) The Hippocampus and Entorhinal Cortex Encode the Path and Euclidean Distances to Goals during Navigation. Curr. Biol 24, 1331–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doeller CF et al. (2010) Evidence for grid cells in a human memory network. Nature 463, 657–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jacobs J et al. (2013) Direct recordings of grid-like neuronal activity in human spatial navigation. Nat. Neurosci 16, 1188–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maidenbaum S et al. (2018) Grid-like hexadirectional modulation of human entorhinal theta oscillations. Proc. Natl. Acad. Sci 115, 10798–10803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kunz L et al. (2015) Reduced grid-cell-like representations in adults at genetic risk for Alzheimer’s disease. Science 350, 430–433 [DOI] [PubMed] [Google Scholar]
- 59.Stangl M et al. (2018) Compromised Grid-Cell-like Representations in Old Age as a Key Mechanism to Explain Age-Related Navigational Deficits. Curr. Biol 28, 1108–1115.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Horner AJ et al. (2016) Grid-like Processing of Imagined Navigation. Curr. Biol 26, 842–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stensola H et al. (2012) The entorhinal grid map is discretized. Nature 492, 72–78 [DOI] [PubMed] [Google Scholar]
- 62.Kjelstrup KB et al. (2008) Finite Scale of Spatial Representation in the Hippocampus. Science 321, 140–143 [DOI] [PubMed] [Google Scholar]
- 63.Evensmoen HR et al. (2013) The Anterior Hippocampus Supports a Coarse, Global Environmental Representation and the Posterior Hippocampus Supports Fine-grained, Local Environmental Representations. J. Cogn. Neurosci 25, 1908–1925 [DOI] [PubMed] [Google Scholar]
- 64.Brunec IK et al. (2018) Multiple Scales of Representation along the Hippocampal Anteroposterior Axis in Humans. Curr. Biol 28, 2129–2135.e6 [DOI] [PubMed] [Google Scholar]
- 65.Peer M et al. (2019) Processing of different spatial scales in the human brain. eLife 8, e47492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fiete IR et al. (2008) What Grid Cells Convey about Rat Location. J. Neurosci 28, 6858–6871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoon K et al. (2013) Specific evidence of low-dimensional continuous attractor dynamics in grid cells. Nat. Neurosci 16, 1077–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Winter SS et al. (2015) Disruption of the head direction cell network impairs the parahippocampal grid cell signal. Science 347, 870–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kropff E et al. (2015) Speed cells in the medial entorhinal cortex. Nature 523, 419–424 [DOI] [PubMed] [Google Scholar]
- 70.Krupic J et al. (2018) Local transformations of the hippocampal cognitive map. Science 359, 1143–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krupic J et al. (2015) Grid cell symmetry is shaped by environmental geometry. Nature 518, 232–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stensola T et al. (2015) Shearing-induced asymmetry in entorhinal grid cells. Nature 518, 207–212 [DOI] [PubMed] [Google Scholar]
- 73.Keinath AT et al. (2020) Environmental deformations dynamically shift human spatial memory. Hippocampus at <https://onlinelibrary.wiley.com/doi/full/10.1002/hipo.23265?af=R> [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen X et al. (2015) Bias in Human Path Integration Is Predicted by Properties of Grid Cells. Curr. Biol 25, 1771–1776 [DOI] [PubMed] [Google Scholar]
- 75.Bellmund JLS et al. (2020) Deforming the metric of cognitive maps distorts memory. Nat. Hum. Behav 4, 177–188 [DOI] [PubMed] [Google Scholar]
- 76.Keinath AT et al. (2018) Environmental deformations dynamically shift the grid cell spatial metric. eLife 7, e38169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Savelli F et al. (2017) Framing of grid cells within and beyond navigation boundaries. eLife 6, e21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Giocomo LM (2016) Environmental boundaries as a mechanism for correcting and anchoring spatial maps. J. Physiol 594, 6501–6511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hardcastle K et al. (2015) Environmental Boundaries as an Error Correction Mechanism for Grid Cells. Neuron 86, 827–839 [DOI] [PubMed] [Google Scholar]
- 80.Jayakumar RP et al. (2019) Recalibration of path integration in hippocampal place cells. Nature 566, 533–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bush D et al. (2015) Using Grid Cells for Navigation. Neuron 87, 507–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Erdem UM and Hasselmo M (2012) A goal-directed spatial navigation model using forward trajectory planning based on grid cells: Forward linear look-ahead trajectory model. Eur. J. Neurosci 35, 916–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kubie JL and Fenton AA (2012) Linear Look-Ahead in Conjunctive Cells: An Entorhinal Mechanism for Vector-Based Navigation. Front. Neural Circuits 6, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chadwick MJ et al. (2015) A Goal Direction Signal in the Human Entorhinal/Subicular Region. Curr. Biol 25, 87–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shine JP et al. (2019) Evidence for allocentric boundary and goal direction information in the human entorhinal cortex and subiculum. Nat. Commun 10, 4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bellmund JL et al. (2016) Grid-cell representations in mental simulation. eLife 5, e17089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hartley T et al. (2003) The well-worn route and the path less traveled: distinct neural bases of route following and wayfinding in humans. Neuron 37, 877–888 [DOI] [PubMed] [Google Scholar]
- 88.Iaria G et al. (2003) Cognitive Strategies Dependent on the Hippocampus and Caudate Nucleus in Human Navigation: Variability and Change with Practice. J. Neurosci 23, 5945–5952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marchette SA et al. (2011) Cognitive mappers to creatures of habit: differential engagement of place and response learning mechanisms predicts human navigational behavior. J. Neurosci 31, 15264–15268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van der Meer MA et al. (2010) Triple dissociation of information processing in dorsal striatum, ventral striatum, and hippocampus on a learned spatial decision task. Neuron 67, 25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iglói K et al. (2010) Lateralized human hippocampal activity predicts navigation based on sequence or place memory. Proc. Natl Acad. Sci. U. S. A 107, 14466–14471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Muller RU et al. (1996) The hippocampus as a cognitive graph. J. Gen. Physiol 107, 663–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eichenbaum H and Cohen NJ (2004) From Conditioning to Conscious Recollection: Memory Systems of the Brain, Oxford University Press, USA. [Google Scholar]
- 94.Dabaghian Y et al. (2014) Reconceiving the hippocampal map as a topological template. eLife 3, e03476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang C-H et al. (2020) Hippocampal Place Cells Encode Local Surface-Texture Boundaries. Curr. Biol 30, 1397–1409.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bulkin DA et al. (2020) Hippocampal state transitions at the boundaries between trial epochs. Hippocampus 30, 582–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sun C et al. (2020) Hippocampal neurons represent events as transferable units of experience. Nat. Neurosci 23, 651–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baraduc P et al. (2019) Schema cells in the macaque hippocampus. Science 363, 635–639 [DOI] [PubMed] [Google Scholar]
- 99.Redish AD and Touretzky DS (1998) The Role of the Hippocampus in Solving the Morris Water Maze. Neural Comput. 10, 73–111 [DOI] [PubMed] [Google Scholar]
- 100.Edvardsen V et al. (2020) Navigating with grid and place cells in cluttered environments. Hippocampus 30, 220–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Blum KI and Abbott LF (1996) A Model of Spatial Map Formation in the Hippocampus of the Rat. Neural Comput. 8, 85–93 [DOI] [PubMed] [Google Scholar]
- 102.Wood ER et al. (2000) Hippocampal Neurons Encode Information about Different Types of Memory Episodes Occurring in the Same Location. Neuron 27, 623–633 [DOI] [PubMed] [Google Scholar]
- 103.Frank LM et al. (2000) Trajectory Encoding in the Hippocampus and Entorhinal Cortex. Neuron 27, 169–178 [DOI] [PubMed] [Google Scholar]
- 104.Grieves RM et al. (2016) Place cells on a maze encode routes rather than destinations. eLife 5, e15986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pfeiffer BE and Foster DJ (2013) Hippocampal place-cell sequences depict future paths to remembered goals. Nature 497, 74–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brown TI et al. (2016) Prospective representation of navigational goals in the human hippocampus. Science 352, 1323–1326 [DOI] [PubMed] [Google Scholar]
- 107.Wu X and Foster DJ (2014) Hippocampal Replay Captures the Unique Topological Structure of a Novel Environment. J. Neurosci 34, 6459–6469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Javadi A-H et al. (2017) Hippocampal and prefrontal processing of network topology to simulate the future. Nat. Commun 8, 14652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Beeson P et al. (2010) Factoring the Mapping Problem: Mobile Robot Map-building in the Hybrid Spatial Semantic Hierarchy. Int. J. Robot. Res 29, 428–459 [Google Scholar]
- 110.Epstein RA and Baker CI (2019) Scene Perception in the Human Brain. Annu. Rev. Vis. Sci 5, 373–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Julian JB et al. (2018) The Neurocognitive Basis of Spatial Reorientation. Curr. Biol 28, R1059–R1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dilks DD et al. (2011) Mirror-Image Sensitivity and Invariance in Object and Scene Processing Pathways. J. Neurosci 31, 11305–11312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Julian JB et al. (2016) The Occipital Place Area Is Causally Involved in Representing Environmental Boundaries during Navigation. Curr. Biol 26, 1104–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Henriksson L et al. (2019) Rapid Invariant Encoding of Scene Layout in Human OPA. Neuron 103, 161–171.e3 [DOI] [PubMed] [Google Scholar]
- 115.Bonner MF and Epstein RA (2017) Coding of navigational affordances in the human visual system. Proc. Natl. Acad. Sci 114, 4793–4798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Marchette SA et al. (2015) Outside Looking In: Landmark Generalization in the Human Navigational System. J. Neurosci 35, 14896–14908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Janzen G and van Turennout M (2004) Selective neural representation of objects relevant for navigation. Nat. Neurosci 7, 673–677 [DOI] [PubMed] [Google Scholar]
- 118.Silson EH et al. (2016) Scene-Selectivity and Retinotopy in Medial Parietal Cortex. Front. Hum. Neurosci 10, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Epstein RA et al. (2007) Where Am I Now? Distinct Roles for Parahippocampal and Retrosplenial Cortices in Place Recognition. J. Neurosci 27, 6141–6149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vass LK and Epstein RA (2013) Abstract Representations of Location and Facing Direction in the Human Brain. J. Neurosci 33, 6133–6142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Baumann O and Mattingley JB (2010) Medial Parietal Cortex Encodes Perceived Heading Direction in Humans. J. Neurosci 30, 12897–12901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shine JP et al. (2016) The Human Retrosplenial Cortex and Thalamus Code Head Direction in a Global Reference Frame. J. Neurosci 36, 6371–6381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Marchette SA et al. (2014) Anchoring the neural compass: coding of local spatial reference frames in human medial parietal lobe. Nat. Neurosci 17, 1598–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Auger SD et al. (2012) Retrosplenial Cortex Codes for Permanent Landmarks. PLoS ONE 7, e43620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jacob P-Y et al. (2017) An independent, landmark-dominated head-direction signal in dysgranular retrosplenial cortex. Nat. Neurosci 20, 173–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Alexander AS and Nitz DA (2015) Retrosplenial cortex maps the conjunction of internal and external spaces. Nat. Neurosci 18, 1143–1151 [DOI] [PubMed] [Google Scholar]
- 127.Mao D et al. (2017) Sparse orthogonal population representation of spatial context in the retrosplenial cortex. Nat. Commun 8, 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vedder LC et al. (2016) Retrosplenial Cortical Neurons Encode Navigational Cues, Trajectories and Reward Locations During Goal Directed Navigation. Cereb. Cortex DOI: 10.1093/cercor/bhw192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sato N et al. (2006) Navigation-associated medial parietal neurons in monkeys. Proc. Natl. Acad. Sci 103, 17001–17006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Alexander AS and Nitz DA (2017) Spatially Periodic Activation Patterns of Retrosplenial Cortex Encode Route Sub-spaces and Distance Traveled. Curr. Biol 27, 1551–1560.e4 [DOI] [PubMed] [Google Scholar]
- 131.Nitz D (2009) Parietal cortex, navigation, and the construction of arbitrary reference frames for spatial information. Neurobiol. Learn. Mem 91, 179–185 [DOI] [PubMed] [Google Scholar]
- 132.Schinazi VR and Epstein RA (2010) Neural correlates of real-world route learning. NeuroImage 53, 725–735 [DOI] [PubMed] [Google Scholar]
- 133.Byrne P et al. (2007) Remembering the past and imagining the future: A neural model of spatial memory and imagery. Psychol. Rev 114, 340–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Aguirre GK and D’Esposito M (1999) Topographical disorientation: a synthesis and taxonomy. Brain 122, 1613–1628 [DOI] [PubMed] [Google Scholar]
- 135.Evans GW et al. (1984) The effects of pathway configuration, landmarks and stress on environmental cognition. J. Environ. Psychol 4, 323–335 [Google Scholar]
- 136.Wolbers T and Wiener JM (2014) Challenges for identifying the neural mechanisms that support spatial navigation: the impact of spatial scale. Front. Hum. Neurosci 8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Montello DR (1993), Scale and multiple psychologies of space. , presented at the European conference on spatial information theory, pp. 312–321 [Google Scholar]
- 138.Brunec IK et al. (2018) Boundaries Shape Cognitive Representations of Spaces and Events. Trends Cogn. Sci 22, 637–650 [DOI] [PubMed] [Google Scholar]
- 139.Wiener JM et al. (2004) Use and interaction of navigation strategies in regionalized environments. J. Environ. Psychol 24, 475–493 [Google Scholar]
- 140.Weisberg SM et al. (2014) Variations in cognitive maps: Understanding individual differences in navigation. J. Exp. Psychol. Learn. Mem. Cogn 40, 669–682 [DOI] [PubMed] [Google Scholar]
- 141.Weisberg SM and Newcombe NS (2018) Cognitive Maps: Some People Make Them, Some People Struggle. Curr. Dir. Psychol. Sci 27, 220–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Anggraini D et al. (2018) Neural signatures of reinforcement learning correlate with strategy adoption during spatial navigation. Sci. Rep 8, 10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Haun DBM et al. (2011) Plasticity of human spatial cognition: Spatial language and cognition covary across cultures. Cognition 119, 70–80 [DOI] [PubMed] [Google Scholar]
- 144.Hund AM et al. (2012) The impact of culture and recipient perspective on direction giving in the service of wayfinding. J. Environ. Psychol 32, 327–336 [Google Scholar]
- 145.Weisberg SM and Newcombe NS (2014) A slippery directional slope: Individual differences in using slope as a directional cue. Mem. Cognit 42, 648–661 [DOI] [PubMed] [Google Scholar]
- 146.Ward SL et al. (1986) Turn Left at the Church, Or Three Miles North: A Study of Direction Giving and Sex Differences. Environ. Behav 18, 192–213 [Google Scholar]
- 147.Coutrot A et al. (2018) Global determinants of navigation ability. Curr. Biol 28, 2861–2866 [DOI] [PubMed] [Google Scholar]
- 148.Nardi D et al. (2015) Sex differences and errors in the use of terrain slope for navigation. Cogn. Process 16, 323–326 [DOI] [PubMed] [Google Scholar]
- 149.Nazareth A et al. (2019) A meta-analysis of sex differences in human navigation skills. Psychon. Bull. Rev 26, 1503–1528 [DOI] [PubMed] [Google Scholar]
- 150.Lawton CA and Kallai J (2002) Gender Differences in Wayfinding Strategies and Anxiety About Wayfinding: A Cross-Cultural Comparison. Sex Roles 47, 389–401 [Google Scholar]
- 151.Kemp C and Tenenbaum JB (2008) The discovery of structural form. Proc. Natl. Acad. Sci 105, 10687–10692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Collins AM and Quillian MR (1969) Retrieval time from semantic memory. J. Verbal Learn. Verbal Behav 8, 240–247 [Google Scholar]
- 153.Pastalkova E et al. (2008) Internally Generated Cell Assembly Sequences in the Rat Hippocampus. Science 321, 1322–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.MacDonald CJ et al. (2011) Hippocampal “Time Cells” Bridge the Gap in Memory for Discontiguous Events. Neuron 71, 737–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kraus BJ et al. (2015) During Running in Place, Grid Cells Integrate Elapsed Time and Distance Run. Neuron 88, 578–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Aronov D et al. (2017) Mapping of a non-spatial dimension by the hippocampal–entorhinal circuit. Nature 543, 719–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tavares RM et al. (2015) A Map for Social Navigation in the Human Brain. Neuron 87, 231–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Park SA et al. (2020) Map Making: Constructing, Combining, and Inferring on Abstract Cognitive Maps. Neuron 0, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Theves S et al. (2019) The Hippocampus Encodes Distances in Multidimensional Feature Space. Curr. Biol 29, 1226–1231.e3 [DOI] [PubMed] [Google Scholar]
- 160.Constantinescu AO et al. (2016) Organizing conceptual knowledge in humans with a gridlike code. Science 352, 1464–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bao X et al. (2019) Grid-like Neural Representations Support Olfactory Navigation of a Two-Dimensional Odor Space. Neuron 102, 1066–1075.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Viganò S and Piazza M (2020) Distance and Direction Codes Underlie Navigation of a Novel Semantic Space in the Human Brain. J. Neurosci 40, 2727–2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Killian NJ et al. (2012) A map of visual space in the primate entorhinal cortex. Nature 491, 761–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Julian JB et al. (2018) Human entorhinal cortex represents visual space using a boundary-anchored grid. Nat. Neurosci 21, 191–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nau M et al. (2018) Hexadirectional coding of visual space in human entorhinal cortex. Nat. Neurosci 21, 188–190 [DOI] [PubMed] [Google Scholar]
- 166.Garvert MM et al. (2017) A map of abstract relational knowledge in the human hippocampal–entorhinal cortex. eLife 6, e17086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Karuza EA et al. (2017) Process reveals structure: How a network is traversed mediates expectations about its architecture. Sci. Rep 7, 12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Balaguer J et al. (2016) Neural Mechanisms of Hierarchical Planning in a Virtual Subway Network. Neuron 90, 893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Schapiro AC et al. (2013) Neural representations of events arise from temporal community structure. Nat. Neurosci 16, 486–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Solway A et al. (2014) Optimal Behavioral Hierarchy. PLoS Comput. Biol 10, e1003779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Schapiro AC et al. (2016) Statistical learning of temporal community structure in the hippocampus: STATISTICAL LEARNING OF TEMPORAL COMMUNITY STRUCTURE. Hippocampus 26, 3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Schuck NW et al. (2016) Human Orbitofrontal Cortex Represents a Cognitive Map of State Space. Neuron 91, 1402–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wikenheiser AM et al. (2017) Suppression of Ventral Hippocampal Output Impairs Integrated Orbitofrontal Encoding of Task Structure. Neuron 95, 1197–1207.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wikenheiser AM and Schoenbaum G (2016) Over the river, through the woods: cognitive maps in the hippocampus and orbitofrontal cortex. Nat. Rev. Neurosci 17, 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Whittington JC et al. (2019) The Tolman-Eichenbaum Machine: Unifying space and relational memory through generalisation in the hippocampal formation. BioRxiv [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bush D et al. (2014) What do grid cells contribute to place cell firing? Trends Neurosci. 37, 136–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Stachenfeld KL et al. (2017) The hippocampus as a predictive map. Nat. Neurosci 20, 1643–1653 [DOI] [PubMed] [Google Scholar]
- 178.Langille JJ and Gallistel CR (2020) Locating the engram: Should we look for plastic synapses or information-storing molecules? Neurobiol. Learn. Mem 169, 107164. [DOI] [PubMed] [Google Scholar]
- 179.Montello DR (1998) A new framework for understanding the acquisition of spatial knowledge in large-scale environments. Spat. Temporal Reason. Geogr. Inf. Syst [Google Scholar]
- 180.Arbib M and Lieblich I (1977) Motivational learning of spatial behavior. Syst. Neurosci [Google Scholar]
- 181.Tversky B (1993), Cognitive maps, cognitive collages, and spatial mental models. , presented at the European conference on spatial information theory, pp. 14–24 [Google Scholar]
- 182.Golledge RG (1999) Human wayfinding and cognitive maps. Wayfinding Behav. Cogn. Mapp. Spat. Process [Google Scholar]
- 183.Taube JS et al. (2013) Is navigation in virtual reality with FMRI really navigation? J. Cogn. Neurosci 25, 1008–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Diersch N and Wolbers T (2019) The potential of virtual reality for spatial navigation research across the adult lifespan. J. Exp. Biol 222, [DOI] [PubMed] [Google Scholar]
- 185.Ishikawa T and Montello DR (2006) Spatial knowledge acquisition from direct experience in the environment: Individual differences in the development of metric knowledge and the integration of separately learned places. Cognit. Psychol 52, 93–129 [DOI] [PubMed] [Google Scholar]
- 186.Derdikman D et al. (2009) Fragmentation of grid cell maps in a multicompartment environment. Nat. Neurosci 12, 1325–1332 [DOI] [PubMed] [Google Scholar]
- 187.Carpenter F et al. (2015) Grid Cells Form a Global Representation of Connected Environments. Curr. Biol 25, 1176–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wernle T et al. (2018) Integration of grid maps in merged environments. Nat. Neurosci 21, 92–101 [DOI] [PubMed] [Google Scholar]
- 189.Patai EZ et al. (2019) Hippocampal and Retrosplenial Goal Distance Coding After Long-term Consolidation of a Real-World Environment. Cereb. Cortex [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mao D et al. (2018) Hippocampus-dependent emergence of spatial sequence coding in retrosplenial cortex. Proc. Natl. Acad. Sci 115, 8015–8018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wolbers T and Buchel C (2005) Dissociable Retrosplenial and Hippocampal Contributions to Successful Formation of Survey Representations. J. Neurosci 25, 3333–3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rosenbaum RS et al. (2000) Remote spatial memory in an amnesic person with extensive bilateral hippocampal lesions. Nat. Neurosci 3, 1044–1048 [DOI] [PubMed] [Google Scholar]
- 193.Maguire EA et al. (2006) Navigation around London by a taxi driver with bilateral hippocampal lesions. Brain 129, 2894–2907 [DOI] [PubMed] [Google Scholar]
- 194.Hodges JR et al. (1995) Charting the progression in semantic dementia: Implications for the organisation of semantic memory. Memory 3, 463–495 [DOI] [PubMed] [Google Scholar]
- 195.Dayan P (1993) Improving generalization for temporal difference learning: The successor representation. Neural Comput. 5, 613–624 [Google Scholar]
- 196.Momennejad I (2020) Learning Structures: Predictive Representations, Replay, and Generalization. Curr. Opin. Behav. Sci 32, 155–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dordek Y et al. (2016) Extracting grid cell characteristics from place cell inputs using non-negative principal component analysis. Elife 5, e10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Barry C et al. (2006) The Boundary Vector Cell Model of Place Cell Firing and Spatial Memory. Rev. Neurosci 17, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.de Cothi W and Barry C (2020) Neurobiological successor features for spatial navigation. Hippocampus DOI: 10.1002/hipo.23246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bottini R and Doeller CF (2020) Knowledge across reference frames: cognitive maps and image spaces. Trends Cogn. Sci [DOI] [PubMed] [Google Scholar]