Abstract
Some form of regeneration occurs in all lifeforms and extends from single-cell organisms to humans. The degree to which regenerative ability is distributed across different taxa, however, is harder to ascertain given the potential for phylogenetic constraint or inertia, and adaptive processes to shape this pattern. Here, we examine the phylogenetic history of regeneration in two groups where the trait has been well-studied: arthropods and reptiles. Because autotomy is often present alongside regeneration in these groups, we performed ancestral state reconstructions for both traits to more precisely assess the timing of their origins and the degree to which these traits coevolve. Using an ancestral trait reconstruction, we find that autotomy and regeneration were present at the base of the arthropod and reptile trees. We also find that when autotomy is lost it does not re-evolve easily. Lastly, we find that the distribution of regeneration is intimately connected to autotomy with the association being stronger in reptiles than in arthropods. While these patterns suggest that decoupling autotomy and regeneration at a broad phylogenetic scale may be difficult, the available data provides useful insight into their entanglement. Ultimately, our reconstructions provide important groundwork to explore how selection may have played a role during the loss of regeneration in specific lineages.
Keywords: arthropod, squamate reptiles, regeneration, autotomy, ancestral state reconstruction, phylogenetic constraint/inertia
Graphical Abstract

Introduction
Long before On the Origin of Species (Darwin, 1859) introduced the world to the theory of natural selection, naturalists were busy exploring regenerative phenomena in animals and plants (Réaumur, 1712; Lenhoff and Lenhoff, 1986; Dinsmore, 1996; Hijmans and Elith, 2016). As Darwin’s ideas took root among different scientists, evolutionary thinking came to influence regenerative biologists who began offering explanations for the persistence or loss of regenerative ability across the tree of life (Cravens and Allen, 1980). In parallel with the influence of evolutionary thinking, a shifting trend in embryological research catalyzed a more rigorous experimental approach to the study of embryonic development and regeneration. At its height, the dichotomy between naturalists and experimentalists embodied a key argument concerning the distribution of regenerative abilities among animals. The argument centered on whether regeneration was an adaptive trait or instead, an irreducible and fundamental property of life. During this period, August Weismann and Thomas Hunt Morgan embodied opposing viewpoints in this debate: “…whereas for Weismann, animal regeneration was a contingent, naturally selected adaptation, Morgan insisted that animal regeneration was neither contingent nor a selected adaptation but rather a general property of the organism as a whole: a universal organic quality characterizing any organism occurring in different degrees” (page 516, Esposito 2013).
Evidence in favor of tissue regeneration as a fundamental organismal property comes from the fact that on its surface, regeneration has close links to embryogenesis (reviewed in Sánchez Alvarado, 2000) and asexual reproduction (reviewed in Bely and Nyberg, 2010); these links suggest regeneration has deep evolutionary origins dating back to the advent of multicellularity or even earlier. Given that pattern restoration extends to the scale of single-cell organisms and their internal structures (Needham, 1953a; Tartar, 2011), regeneration may simply be the expression or augmentation of a more basic process of pattern formation and elaboration. This idea rests with regeneration predating the evolution of the embryo where basic cellular-level regenerative processes are amplified in postembryonic animals as tissue (multi-cell) regeneration. Regardless of whether regenerative processes originated before or after the evolution of multicellular organisms, it likely emerged early in the evolution of complex life on earth. Thus, the distribution of regenerative ability in different lineages is likely shaped by phylogenetic constraint or inertia where the physical and genetic mechanisms underlying early regenerative processes constrained the evolution of later regenerative mechanisms. Arguments for regeneration as an adaptive trait rely on fitness advantages gained by regenerating a whole organism or a part thereof. For example, if a piece of a planaria or Hydra is severed, regrowth via regeneration can be so substantial that it can create new progeny where the parent organism regenerates and the severed piece regenerates into a daughter organism. Agonistic interactions with predators or with conspecifics can cause tissue damage which regeneration can serve to mitigate, thus minimizing fitness declines (Morgan, 1901a; Maginnis, 2006; Slack, 2010). In the context of appendages, the loss of limbs or tails is so common, and minimizing the damage so important, that in some species these tissues are preferentially lost through autotomy. During autotomy, the structure is removed at a weak point or breakage plane allowing a more limited wound which facilitates regeneration of the structure. In the absence of direct testing, there is a potential role for both selection and phylogenetic inertia in the evolution of regeneration.
How can we determine the relative strength of selection and constraint in the evolution of regeneration? One tool is the comparative method from evolutionary biology where we look for patterns that emerge from analyzing the presence and extent of tissue regeneration among different taxa across the tree of life. When considering multicellular animals, regenerative ability varies substantially (Needham, 1953a; Bely and Nyberg, 2010). For instance, some cnidarians show near limitless regenerative ability with the power to regenerate entire individuals from dissociated cells (Goss, 1969). This stands in contrast to animals that can only regenerate specific structures, such as the tail but not limbs in lizards (Vitt et al., 1977), and further still to animals like mites that appear to lack any appreciable regenerative capacity whatsoever (Maruzzo et al., 2005). This variation suggests that either the adaptive value of regeneration varies across taxa, resulting in its selective maintenance in some taxa and selective elimination in others, or the developmental and pattern formation processes that produce regeneration in some taxa evolve complexities that lead to its elimination in other groups. Thus, distinguishing the relative roles of adaptation and constraint remains a crucial task in understanding the evolution of regeneration (Sánchez Alvarado, 2000; Bely and Nyberg, 2010; Tiozzo and Copley, 2015).
Here we attempt to address this issue by looking at the phylogenetic history of regeneration and its co-occurrence with autotomy. Studying autotomy is crucial for understanding if (or how) the fitness costs and benefits of regeneration vary across different species because it is necessary to separate the potential costs and benefits of regeneration from the costs of autotomy that may precede regeneration in many species. Numerous arthropods are capable of autotomizing their limbs following damage or predation attempts (Fleming et al., 2007). Since the growth of many arthropods is tied to molting, many of them develop a regenerated limb in a papilla sac that later unfolds after molting (e.g., fiddler crab, Hopkins 1993; cockroach, Truby 1983). Moreover, autotomy can be beneficial in arthropods since molting can lead to appendages becoming stuck in the exuviate (i.e., shed exoskeleton) and autotomy ensures that the exoskeleton is shed even in these cases. Reptiles are another group of organisms heavily studied for their regenerative capacities and a group in which autotomy is widespread (Bellairs and Bryant, 1985). Notably, lizards are well-known to autotomize their tail when attacked by predators and a number of scincid and gekkonid lizards can shed parts of their integument in response to physical contact (Bauer et al., 1989; Zani, 1996; Scherz et al., 2017). Although lizards can regenerate their autotomized tail, the replacement is not a perfect facsimile since the missing vertebrae and spinal cord are replaced by a cartilaginous rod and ependymal tube respectively (Simpson, 1968; Bateman and Fleming, 2009; Gilbert et al., 2013; Lozito and Tuan, 2016). While not perfect, autotomy followed by regeneration would seem to offer an advantage as tail autotomy alone causes diminished locomotor performances (McElroy and Bergmann, 2013). However, other reptiles, such as chameleons, are neither capable of tail autotomy nor tail regeneration, which highlights the phylogenetic variation in both of these traits (Bellairs and Bryant, 1985; Anderson and Higham, 2014).
Here we use this variation in regenerative ability and autotomy to reconstruct the ancestral states of these two traits across arthropods and reptiles. Knowing these ancestral states is crucial for determining the evolutionary timing for the origin of autotomy relative to regeneration, and this timing in turn provides evidence for the value of regeneration in rebuilding structures lost by autotomy. For example, if autotomy evolved first, this could have set the stage for the adaptive evolution of regeneration. On the other hand, if regeneration evolved first, this would suggest the function of regenerative abilities must be broader than simply appendage replacement. In fact, our results show that autotomy and regeneration were present at the base of the arthropod and reptile trees. While this result suggests that untangling the relative role of adaptation and constraint in the evolution of tissue regeneration will require a more sophisticated approach than the one used here, our reconstructions provide important groundwork to explore how selection may play a role in the loss of regeneration in particular lineages.
Methods
Definitions
Regeneration
The term regeneration refers to the functional replacement of tissue that is lost or damaged. Importantly, we consider reparative regeneration in this paper as it occurs in response to injury or autotomy, either self-induced or caused by an external agent. More precisely, we refer to regeneration as the restoration of a pattern that occurs when the organism’s body system is disrupted. This definition separates regeneration from scar formation that occurs in some animals (e.g., replacement of lost tissue with dissimilar, non-functional tissue). This definition also applies across multiple levels of biological organization and unites regeneration in single-cell organisms with the higher-level combination of processes that occurs during replacement of an appendage. As a singular event, regeneration encompasses individual processes at different levels of biological organization. For example, barrier restoration, homeostasis, and morphogenesis broadly describe regeneration from beginning to end. Thus, for the purposes of identifying regeneration as a trait, this necessitates considering these processes together.
Autotomy
Autotomy has been broadly defined as the loss of tissue induced directly by an individual itself (as a reflex severance – autotomy – or by pulling on the appendage – autotilly) or indirectly by the action of outside agent (autospasy) (Wood and Wood 1932). In this paper we refer to autotomy broadly, including all of the aforementioned definitions. Autotomy can occur voluntarily or as the result of extensive damage to a limb or appendage. In either case, an autotomy plane prevents hemolymph or blood loss and facilitates regeneration of the missing appendage (Bellairs and Bryant, 1985; Maginnis, 2006; Fleming et al., 2007). Following autotomy, tissue either regenerates or heals with a scar. Regeneration in arthropods and reptiles generally proceeds via hemostasis, inflammation, re-epithelialization, blastema formation, and morphogenesis (Seifert et al., 2012).
Ancestral State Reconstruction
Ancestral state or trait reconstruction in a phylogenetic context was proposed as early as 1938 (Dobzhansky and Sturtevant, 1938) and is an approach that improves on similar methodologies appropriated from cladistics. What distinguishes ancestral state reconstruction is how it considers shared evolutionary history between taxa. Character mapping traits on a cladogram using parsimony to manually reconstruct ancestral states ignores shared evolutionary history and favors a mild view of a traits’ history because it ignores the effect of branch lengths in shaping the topology of the tree. Formal ancestral state reconstruction uses a more standardized approach in that it applies a hypothetical model of evolution to a phylogeny and in doing so takes into account evolutionary time.
Character Coding
In our data gathering, we code each experimental or observational report of bonafide regeneration, or its absence, with a value of 1 or 0, respectively; autotomy was similarly recorded. In the absence of experimental or observational information explicitly specifying trait presence of absence, we attributed a value of NA. If genera or species within a higher taxon level scored differently (e.g., some regenerate, others do not) then a branch tip on the tree would receive a 1/0 as was the case for Pterygota (see Figure 1). Although some authors use regeneration in referring to embryos, for the purposes of our reconstructions we only considered regeneration for post-embryonic stages of development. Most embryos can regenerate parts and tissue regeneration in embryos can be influenced by the degree of autonomous or conditional development present in a given taxa. Because life stage, tissue type, and organismal traits can influence regenerative ability (Seifert et al., 2012b), we considered data for presence or absence of autotomy and regeneration when it was reported for (1) any post-embryonic life stage and (2) for any appendage or body part. An exception to these criteria applied to the Pterygota where we only considered regeneration if reported for adults due to the role of regenerative ability in early development for these insects, which undergo partial or complete metamorphosis. While regenerative ability can vary across life-stage, it should be noted that the majority of data we found scored the presence or absence of these traits in adults. Even when life stages clearly separate regenerative capacities in some clades (as in holometabolous insects), we decided to embrace this fact and explore the differences between those groups and other closely related groups (see below). Since many species within a clade lack direct information on whether they autotomize or regenerate, we assigned presence or absence values for autotomy and regeneration to a tip of a clade even when only one or two species in the clade had been investigated. A potential bias in our dataset is that studies are more likely to report presence rather than absence of regeneration and autotomy. This particular limitation cannot be avoided until more research is conducted as advocated for below.
Figure 1:
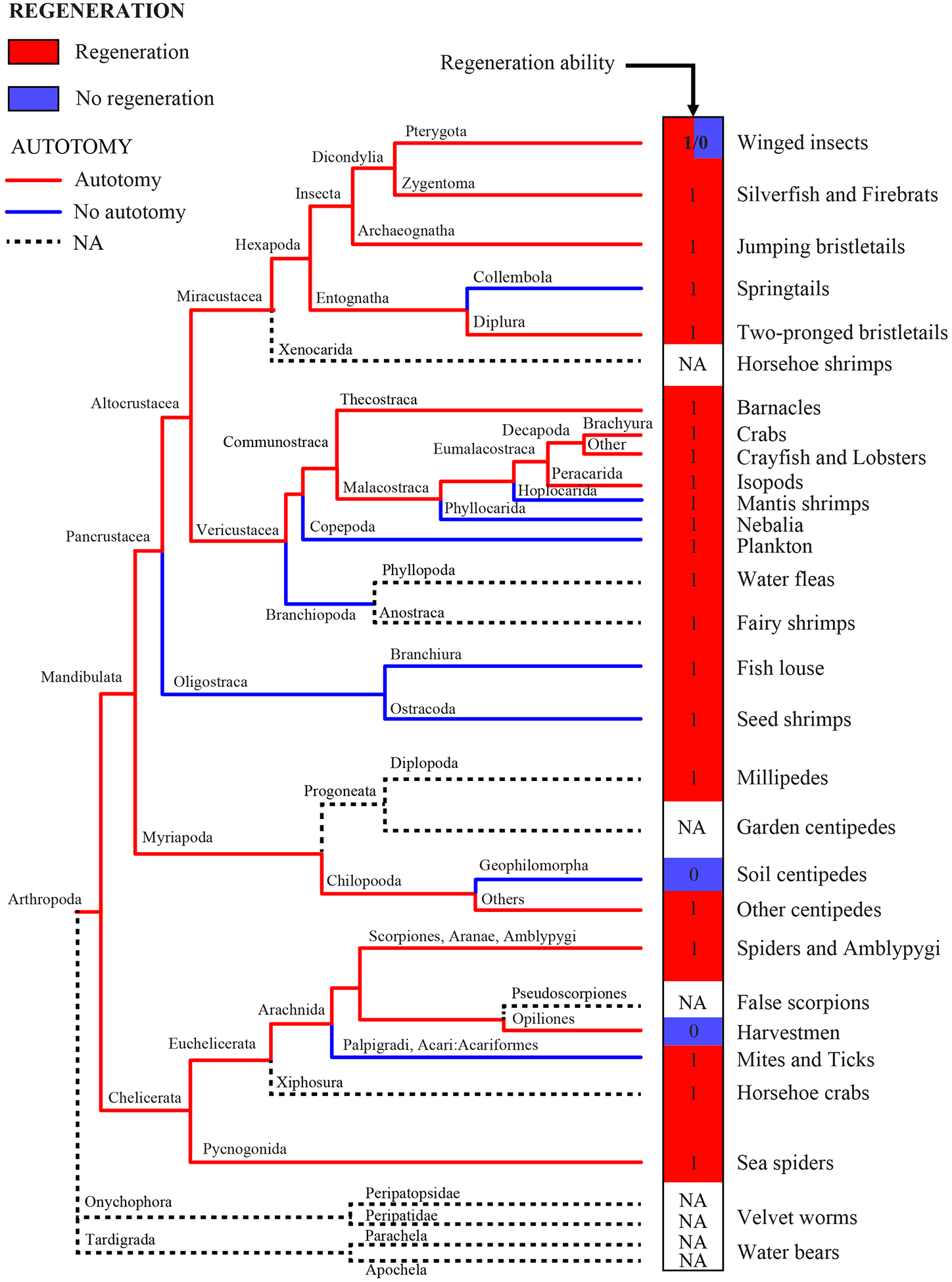
Evidence of regeneration and autotomy manually mapped onto an arthropod phylogeny (Regier et al., 2010). Common names of organisms at each branch tip are on the right-hand side for convenience (see Table 1 for data). Red and blue boxes at the tips of the tree indicate presence or absence, respectively, for regeneration. Red and blue branches on the phylogeny indicate the presence or absence, respectively, of autotomy. Dashed branches indicate data are not available (NA).
In surveying the existing literature for robust information about autotomy and regeneration in arthropods, we relied on comprehensive reviews for arthropods (Maruzzo et al., 2005), invertebrates (Fleming et al., 2007), and extensive compendiums of these traits (Morgan, 1901b; McVean, 1982). Similarly, in order to determine the ancestral state for autotomy and regeneration in squamate reptiles, we surveyed the existing literature and relied heavily on a monograph that compiled information on these traits (Bellairs and Bryant, 1985) which we supplemented with species-level studies when possible (see below).
Phylogenies
In order to map both autotomy and regeneration in arthropods, we selected one of the most recent arthropod phylogenies (Regier et al., 2010). Although this phylogeny provides the most complete coverage of arthropod families and is congruent with recent discussions of arthropod relationships, it is not a chronogram whose branch lengths are proportional to time, which are preferred when reconstructing ancestral states using maximum likelihood (Litsios and Salamin, 2012). Thus, for the ancestral state reconstruction, we used the most recent arthropod phylogeny with calibrated divergence times (Rehm et al., 2011). Ultimately, this phylogeny was trimmed to include only species for which we had regeneration and autotomy data from the literature search before running the ancestral state reconstruction. However, this new tree did not include any species where regeneration was absent rendering it impossible for us to run a formal ancestral state reconstruction for regeneration. Nevertheless, this analysis might be unnecessary in view of the lack of variation in regeneration capacities across the arthropod tree.
For squamate reptiles, we used a recent phylogeny based on morphological and genetic data as our backbone for character mapping and for the ancestral state reconstruction of autotomy and regeneration (Pyron, 2017). Due to a lack of data on regeneration and autotomy for the Serpentes, we omitted this infra-order from our analysis. Regeneration is probably absent in snakes, but there are sporadic reports of caudal autotomy (or tail breakage) in a few colubrids (Arnold, 1984). Whether a tail fragility in these species represents true autotomy or a unique trait remains unresolved (Etheridge, 1967) and underscored the decision to omit them from our analyses. When focusing on Iguanidae, we used a less recent, but more specific phylogeny to manually map autotomy and regeneration in this group (Wiens and Hollingsworth, 2000).
Analyses
To examine the evolutionary history of autotomy and regeneration in arthropods and reptiles, ancestral state reconstruction analyses were done using R version 3.0.1 (R Core Team, 2013) and the ape (Paradis et al., 2004), caper (Orme et al., 2012), geiger (Harmon et al., 2008), phytools (Revell, 2010), phangorn (Schliep, 2011), and xlsx (Dragulescu, 2014) packages. Each ancestral reconstruction figure depicts a density map of 100 stochastic trait histories generated from our dataset using an MCMC approach (stochastic character mapping; Huelsenbeck et al. 2003). The reconstructed ancestral state at an internal node of the tree for autotomy and regeneration is the probability of that traits occurring among the 100 stochastic trait histories.
Results
Ancestral state for autotomy and regeneration in arthropods
Mapping regeneration and autotomy for all arthropods revealed that regeneration, while nearly ubiquitous across arthropod orders, appears to have been independently lost in three orders; in the Opiliones (harvestmen), Geophilomorpha (soil centipedes) and then again within the Endopterygota comprising all the holometabolous insects (Figure 1 and Table 1). However, based on the phylogenetic resolution of our branches, there are a few additional fine scale losses (i.e., genus and species) that are not present on the tree and represent isolated losses of regenerative ability (e.g., mites). In contrast with regeneration, autotomy was lost in ten major lineages containing approximately 23,000 species (Figure 1 and Table 1; Resh and Cardé 2009).
Table 1.
Data for autotomy and regeneration in arthropods associated with Figure 1. Each tree branch is presented with the corresponding order and class. Autotomy and regeneration are characterized as present (1), absent (0), or unknown (NA). Unless indicated by other superscripts, the reference for the character state is 1Maruzzo et al., 2005; 2Wood and Wood, 1932; 3Korschelt, 1907; 4McVean, 1982 and 5Fleming et al., 2007.
| Tree branch | Class | Subclass or Order or Infraorder or Family | Genus | Regen1 | Autotomy1 |
|---|---|---|---|---|---|
| Winged insects | Insecta | Pterygota, Paleoptera | NA | 1 | 1 |
| Pterygota, Neoptera | NA | 0/1 | 0/1 | ||
| Silverfish & Firebrats | Insecta | Zygentoma | NA | 1 | 15 |
| Jumping bristletails | Insecta | Archaeognatha | Machilis, Thermobia & Lepisma | 1 | 15 |
| Springtails | Entognatha | Entomobryomorpha | Orchesella & Tomocerus | 1 | 0 |
| Two-pronged bristletails | Entognatha | Diplura | Campodea | 1 | 15 |
| Horseshoe shrimps | Cephalocarida | Brachypoda | NA | NA | NA |
| Barnacles | Maxillopoda | Thecostraca | Lepas | 1 | NA |
| Crabs | Malacostraca | Brachyura | Uca | 13 | 12 |
| Crayfish & Lobster | Malacostraca | Pleocyemata | Cambarus & Homarus | 13 | 12 |
| Isopods | Malacostraca | Peracarida | Asellus & Porcellio | 15 | 14 |
| Mantis shrimps | Malacostraca | Stomatopoda | Squilla | 1 | 0 |
| Nebalia | Malacostraca | Leptostraca | Nebalia | 1 | 0 |
| Plankton | Hexanauplia | Cyclopoida | NA | 1 | 0 |
| Water fleas | Branchiopoda | Cladocera | Triops | 1 | NA |
| Fairy shrimps | Branchiopoda | Anostraca | Branchipus | 1 | NA |
| Fish lices | Maxillopoda | Arguloida | Argulus | 1 | 0 |
| Seed shrimps | Ostracoda | Halocyprida | NA | 12 | 04 |
| Millipedes | Diplopoda | Spirostreptida | Julus & Spirostreptus | 15 | NA |
| Garden centipedes | Symphyla | Scolopendrellidae | NA | NA | NA |
| Soil centipedes | Chilopoda | Geophilomorpha | NA | 03/4 | 04 |
| Other centipedes | Chilopoda | Scutigeromorpha | Scutigera | 1 | 14 |
| Spiders & Amblypygi | Arachnida | Amblypygi | Centaurus | 1 | 1 |
| False scorpions | Arachnida | Pseudoscorpiones | NA | NA | NA |
| Harvestmen | Arachnida | Opiliones | Leiobunum | 0 | 1 |
| Mites & Ticks | Arachnida | Opilioacarida | Opilioacarus | 1 | 0 |
| Horseshoe crabs | Merostomata | Xiphosura | Limulus | 1 | NA |
| Sea spiders | Pycnogonida | Pantopoda | Phoxichilus & Nymphon | 1 | 1 |
| Velvet worms* | Udeonychophora | Peripatopsidae | NA | NA | NA |
| Velvet worms* | Udeonychophora | Peripatidae | NA | NA | NA |
| Water bears* | Eutardigrada | Parachaela | NA | NA | NA |
| Eutardigrada | Apochela | NA | NA | NA |
Velvet worms and water bears are outgroups in this phylogeny.
We next used ancestral state reconstruction to resolve the phylogenetic history of autotomy. In the case of regeneration, there was no variation in regenerative ability (all remaining taxa were capable of regeneration) using the Rehm et al. (2011) tree; thus, the basal condition was presence of regeneration. Even in the Regier et al. (2010) tree, regeneration is the most parsimonious ancestral state for arthropods because only three derived groups showed a lack of regenerative ability (Figure 1 and Table 1). We determined that autotomy was the ancestral state in arthropods based on the available data (scaled likelihood of autotomy at the root: presence > 0.99, absence = 5.9×10−4; Figure 2).
Figure 2:

Ancestral state reconstruction of autotomy (see text for methodological details) using a recent arthropod phylogenetic tree (Rehm et al. 2011 based on Meusemann et al. 2010). Red and black circles at the tips of the tree indicate autotomy or no autotomy, respectively (see Table 1 for data). The ancestral reconstruction recovers probabilities for the presence or absence of autotomy along the tree branches showing an ancestral state of autotomy in the present phylogeny. Along those tree branches, absence of autotomy is represented in blue and presence in red. Major arthropods’ phyla or orders have been layered on top of the phylogeny for convenience.
Next, we sought to investigate two arthropod groups where autotomy and regeneration appeared to be more evolutionarily labile. First, we examined the insects (class Insecta) in more detail (Figure 3 and 4). Despite limited data given the number of species within this class, the available data for adult Odonata (Figure 3; Table 2) strongly suggests that most, if not all, hemimetabolous insects (incomplete metamorphosis) are capable of autotomy and regeneration. In contrast, examining holometabolous insects (complete metamorphosis), we found no instances of regeneration in adults (note that Frankliniella and Dictyoptera are hemimetabolous and outgroups in this tree; Figure 4; Table 3). As for autotomy, although the data are incomplete, they support that all holometabolous insects conserve their capacity to autotomize limbs (Figure 4; Table 3). That some holometabolous insects exhibit regenerative ability as larvae suggests that the radical developmental shift to complete metamorphosis created a negative trade-off with regenerative ability.
Figure 3:

Modified phylogeny of adults Odonata (hemimetabolous insects; from Figure 2 in Dumont et al., 2010) onto which both regeneration and autotomy have been mapped (see text for methodological details and Table 2 for data). Taxonomic groups are on the right-hand side for convenience. Red and blue boxes at the tips of the tree indicate presence or absence, respectively, for regeneration. Red and blue branches on the phylogeny indicate the presence or absence, respectively, of autotomy. Dashed branches indicate data are not available (NA).
Figure 4:

Modified phylogeny of adults holometabolus insects (from Figure 2 in McKenna and Farrell, 2010) onto which both regeneration and autotomy has been mapped (see text for methodological details and Table 3 for data). Taxonomic groups are on the right-hand side for convenience and genera are included where known. Red and blue boxes at the tips of the tree indicate presence or absence, respectively, for regeneration. Red and blue branches on the phylogeny indicate the presence or absence, respectively, of autotomy. Dashed branches indicate data are not available (NA).
Table 2.
Autotomy and regeneration data for adult Odonata associated with Figure 3. Each taxon is presented with the associated literature reference used for characterization of autotomy and regeneration as present (1), absent (0), or unknown (NA). 1Child and Young, 1903; 2Robinson et al., 2006; 3Maruzzo et al., 2005; 4Fleming et al., 2007; 5Stoks, 2003; 6Tennessen, 2009; 7Madhavan and Schneiderman, 2007; 8Moore and Tabashnik, 1989; 9French, 1976 and 10Maginnis, 2006. Outgroups used for Figure 3 are: Pyralidae, Curculionidae, Trichogrammatidae, Ectobiidae, Acrididae, Sminthuridae and Baetidae.
| Genus or Species name | Suborder | Family | Regeneration | Autotomy |
|---|---|---|---|---|
| Argia moesta | Zygoptera | Coenagrionidae | 11/10 | 12 |
| NA | Zygoptera | Calopterygidae | NA | 13 |
| Burmargiolestes sp. | Zygoptera | Megapodagrionidae | NA | 13 |
| Noguchiphaea sp. | Zygoptera | Calopterygidae | NA | 13 |
| Pseudolestes sp. | Zygoptera | Pseudolestidae | NA | 13 |
| NA | Zygoptera | Hetaerinidae | NA | 13 |
| NA | Zygoptera | Rimanellidae | NA | 13 |
| NA | Zygoptera | Heliocharitidae | NA | 13 |
| NA | Zygoptera | Protoneuridae | NA | 13 |
| NA | Zygoptera | Platycnemididae | NA | 13 |
| NA | Zygoptera | Philogeniidae | NA | 13 |
| Stenocnemis sp. | Zygoptera | Platycnemididae | NA | 13 |
| Ceriagrion sp. | Zygoptera | Coenagrionidae | 11/10 | 14 |
| NA | Zygoptera | Pseudostigmatidae | NA | 13 |
| Nephallenia sp. | Zygoptera | Coenagrionidae | 11/10 | 14 |
| Devadatta sp. | Zygoptera | Amphipterygidae | NA | 13 |
| Rhinagrion sp. | Zygoptera | Megapodagrionidae | NA | 13 |
| Coeliccia sp. | Zygoptera | Platycnemididae | 110 | 13 |
| Philoganga sp. | Zygoptera | Amphipterygidae | NA | 13 |
| NA | Zygoptera | Euphaeidae | NA | 13 |
| NA | Zygoptera | Chlorocyphidae | NA | 13 |
| NA | Zygoptera | Megapodagrionidae | NA | 13 |
| NA | Zygoptera | Megapodagrionidae | NA | 13 |
| NA | Zygoptera | Plastystictidae | NA | 13 |
| Lestes viridis | Zygoptera | Lestidae | 15 | 14 |
| NA | Epiprocta | Libellulidae | NA | 16 |
| NA | Epiprocta | Macromiidae | NA | 16 |
| Macromidia sp. | Epiprocta | Corduliidae | NA | 16 |
| NA | Epiprocta | Corduliidae | NA | 16 |
| NA | Epiprocta | Gomphidae | NA | 16 |
| Anax imperator / A. cyanea | Epiprocta | Aeshnidae | 13 | 14 |
| NA | Epiprocta | Petaluridae | NA | 16 |
| NA | Epiprocta | Chlorogomphidae | NA | 16 |
| NA | Epiprocta | Neopetaliidae | NA | 16 |
| NA | Epiprocta | Cordulegastridae | NA | 16 |
| NA | Epiprocta | Epiophlebiidae | NA | 16 |
| Galleria mellonella | Heteroneura | Pyralidae | 17 | 18 |
| Sitophilus zeamais | Polyphaga | Curculionidae | NA | NA |
| Trichogramma minutum | Apocrita | Trichogrammatidae | NA | NA |
| Blattella germanica | Cockroaches | Ectobiidae | 19 | 19 |
| Oxya chinensis | Caelifera | Acrididae | 13 | 13 |
| Sminthurus viridis | Collembola (order) | Sminthuridae | 13 | 03 |
| Callibaetis ferrugineus | Pisciforma | Baetidae | 13 | 13 |
Table 3.
Autotomy and regeneration data for adult holometabolous insects associated with Figure 4. Each taxon is presented with the associated literature reference used for characterization of autotomy and regeneration as present (1), absent (0), or unknown (NA). 1Wigglesworth, 1944; 2Maginnis, 2006; 3Fleming et al., 2007; 4Haynie and Bryant, 1976; 5Marchand, 1917; 6Shcherbakov et al., 1995; 7 Yang et al., 2016 and 8Hermann, 1971. 9Frankliniella and Dictyoptera are hemimetabolous insects and outgroups in this phylogeny.
| Genus or Species name | Order or Suborder | Family | Regeneration | Autotomy |
|---|---|---|---|---|
| Pterostichus | Adephaga | Carabidae | NA | NA |
| Bembidion | Adephaga | Carabidae | NA | NA |
| Laccophilus | Adephaga | Dytiscidae | NA | NA |
| Hydroscapha | Myxophaga | Hydroscaphidae | NA | NA |
| Tenomerga | Archostemata | Cupedidae | NA | NA |
| Strangalia | Polyphaga | Cerambycidae | 01 | NA |
| Tribolium | Polyphaga | Tenebrionidae | 02 | 13 |
| Chauliognathus | Polyphaga | Cantharidae | 01 | NA |
| Halictophagus sp. | Strepsiptera | Halictophagidae | NA | NA |
| Mengenilla | Strepsiptera | Mengenillidae | NA | NA |
| Kempynus | Hemerobiiformia | Osmylidae | NA | NA |
| Austroneurorthus | Neuroptera | Nevrorthidae | NA | NA |
| Platystoechotes | Neuroptera | Polystoechotidae | NA | NA |
| NA | Megaloptera | NA | NA | NA |
| Mongoloraphidia | Raphidioptera | Raphidiidae | NA | NA |
| Microchorista | Mecoptera | Nannochoristidae | NA | NA |
| Nannochorista | Mecoptera | Nannochoristidae | NA | NA |
| Panorpa | Mecoptera | Panorpidae | NA | NA |
| Bittacidae | Raptipeda | Bittacidae | NA | NA |
| Boreus | Mecoptera | Boreidae | NA | NA |
| Neotyphloceras | Hystrichopsyllomorpha | Hystrichopsyllidae | NA | NA |
| Ctenocephalides | Pulicomorpha | Pulicidae | NA | 15 |
| Drosophila | Brachycera | Drosophilidae | 04 | 13 |
| Musca | Brachycera | Muscidae | NA | NA |
| Tipulidae | Nematocera | Tipulidae | NA | 13 |
| Anopheles | Nematocera | Culicidae | NA | 16 |
| Noctuidae | Glossata | Noctuidae | 07 | 13 |
| Bombyx | Glossata | Bombycidae | 02 | 13 |
| Hydropsyche | Annulipalpia | Hydropsychidae | NA | NA |
| Pteromalidae | Apocrita | Pteromalidae | NA | 18 |
| Tenthredinidae | Symphyta | Tenthredinidae | NA | 18 |
| Apis | Apocrita | Apidae | NA | 18 |
| Frankliniella9 | Terebrantia | Thripidae | NA | NA |
| NA | Dictyoptera9 | NA | 13 | 12 |
Ancestral state for autotomy and regeneration in reptiles
We next investigated the evolutionary history of these two traits in reptiles and assigned each tip of the phylogeny with a value for regeneration and autotomy based on family and infra-order information (Figure 5; Table 4). Character mapping of both traits revealed that they were widespread across reptiles (Figure 5; Table 4). Similar to arthropods, we determined autotomy and regeneration as the ancestral state for squamate reptiles based on our data (autotomy: scaled likelihood of autotomy at the root: presence = 0.9747, absence = 0.0253, standard error = 7.74×10−4; regeneration: Scaled likelihood of regeneration at the root: presence = 0.9942, absence = 0.0058, standard error = 4.940×10−4; Figure 6 and Figure 7). To investigate the association of regeneration and autotomy in more detail, we examined the Iguanidae family where both traits showed independent losses (Figure 8; Table 5). Our analysis revealed that loss of one trait did not necessarily predict the other. For example, Iguana delicatissima is capable of regeneration but not autotomy, whereas a sister species Iguana iguana is capable of autotomy but not regeneration (Figure 8). Together, our results show that regeneration and autotomy are the basal condition in squamate reptiles and that tail regeneration, albeit imperfect, has persisted in most major reptilian lineages.
Figure 5:

Modified phylogeny of squamate reptiles (Pyron, 2017) onto which regeneration and autotomy have been mapped (see text for methodological details and Table 4 for data). Full names of the taxa are on the right-hand side with family color coded for convenience. Red and blue boxes at the tips of the tree indicate presence or absence, respectively, for regeneration. Red and blue branches on the phylogeny indicate the presence or absence, respectively, of autotomy. Dashed branches indicate data are not available (NA).
Table 4.
Autotomy and regeneration data for squamate reptiles associated with Figures 5, 6, and 7. Each taxon is presented with the associated literature reference used for characterization of autotomy and regeneration as present (1), absent (0), or unknown (NA). 1The reptile database (http://www.reptile-database.org/). Unless indicated by other superscripts, the reference for the character state is from 2Bellairs and Bryant 1985; 3Arnold 1994; 4Anderson and Higham 2014; 5Cope 1967; 6Zug et al. 2006; 7Wiens and Etheridge 2006; 8Zani 1996; 9Laspiur et al. 2007 and 10Poe 2006.
| Species name | Suborder1 | Family1 | Regeneration2 | Autotomy2 |
|---|---|---|---|---|
| Acontias_percivali | Scincomorpha | Scincidae | 1 | 1 |
| Aeluroscalabotes_felinus | Gekkota | Eublepharidae | 1 | 1 |
| Agama_agama | Iguania | Agamidae | 1 | 1 |
| Amphiglossus_splendidus | Scincidae | Amphiglossus | 1 | 1 |
| Amphisbaena_fuliginosa | Lacertoidea | Amphisbaenidae | 1 | 1 |
| Anniella_pulchra | Anguimorpha | Anniellidae | 1 | 1 |
| Anolis_carolinensis | Iguania | Dactyloidae | 1 | 1 |
| Aspidoscelis_tigris | Sauria | Teiidae | 1 | 1 |
| Basiliscus_basiliscus | Iguania | Corytophanidae | 1 | 1 |
| Bipes_biporus | Lacertoidea | Bipedidae | 1 | 1 |
| Bipes_canaliculatus | Lacertoidea | Bipedidae | 1 | 1 |
| Brachylophus_fasciatus | Iguania | Iguanidae | 1 | 03 |
| Brachymeles_gracilis | Scincidae | Scincidae | 1 | 1 |
| Brookesia_brygooi | Iguania | Chamaeleonidae | 0 | 04 |
| Callopistes_maculatus | Sauria | Teiidae | 1 | 1 |
| Calotes_emma | Iguania | Agamidae | 16 | 1 |
| Celestus_enneagrammus | Anguimorpha | Diploglossidae | 1 | 1 |
| Chalarodon_madagascariensis | Iguania | Opluridae | 1 | 1 |
| Chamaeleo_laevigatus | Iguania | Chamaeleonidae | 0 | 04 |
| Coleonyx_variegatus | Gekkota | Eublepharidae | 1 | 1 |
| Corytophanes_cristatus | Iguania | Corytophanidae | 0 | 08 |
| Cricosaura_typica | Scincoidea | Xantusiidae | 1 | 1 |
| Crotaphytus_collaris | Iguania | Crotaphytidae | 0 | 03 |
| Delma_borea | Gekkota | Pygopodidae | 1 | 1 |
| Diplometopon_zarudnyi | Amphisbaenia | Trogonophidae | 1 | 1 |
| Dipsosaurus_dorsalis | Iguania | Iguanidae | 1 | 1 |
| Elgaria_multicarinata | Anguimorpha | Anguidae | 1 | 1 |
| Enyalioides_laticeps | Iguania | Hoplocercidae | 1 | 17 |
| Eublepharis_macularius | Gekkota | Eublepharidae | 1 | 1 |
| Eugongylus_rufescens | Scincoidea | Scincidae | 1 | 1 |
| Feylinia_polylepis | Scincoidea | Scincidae | 1 | 1 |
| Gambelia_wislizenii | Iguania | Crotaphytidae | 1 | 1 |
| Gekko_gecko | Gekkota | Gekkonidae | 1 | 1 |
| Geocalamus_acutus | Lacertoidea | Amphisbaenidae | 1 | 1 |
| Gonatodes_albogularis | Gekkota | Sphaerodactylidae | 1 | 1 |
| Heloderma_horridum | Anguimorpha | Helodermatidae | 1 | 03 |
| Heloderma_suspectum | Anguimorpha | Helodermatidae | 1 | 03 |
| Lacerta_viridis | Lacertoidea | Lacertidae | 1 | 1 |
| Lanthanotus_borneensis | Anguimorpha | Lanthanotidae | 1 | 1 |
| Leiocephalus_barahonensis | Iguania | Leiocephalidae | 1 | 1 |
| Leiolepis_belliana | Iguania | Agamidae | 0 | 07 |
| Leiosaurus_catamarcensis | Iguania | Leiosauridae | 0 | 09 |
| Lepidophyma_flavimaculatum | Scincoidea | Xantusiidae | 1 | 1 |
| Lialis_burtonis | Gekkota | Pygopodidae | 1 | 1 |
| Liolaemus_bellii | Iguania | Liolaemidae | 1 | 1 |
| Morunasaurus_annularis | Iguania | Hoplocercidae | 1 | 1 |
| Oplurus_cyclurus | Iguania | Opluridae | 1 | 1 |
| Petrosaurus_mearnsi | Iguania | Phrynosomatidae | 1 | 1 |
| Phelsuma_lineata | Gekkota | Gekkonidae | 1 | 1 |
| Phrynosoma_platyrhinos | Iguania | Phrynosomatidae | 0 | 0 |
| Phymaturus_palluma | Iguania | Liolaemidae | 1 | 1 |
| Physignathus_cocincinus | Iguania | Agamidae | 1 | 07 |
| Platysaurus_pungweensis | Scincoidea | Cordylidae | 1 | 1 |
| Plestiodon_fasciatus | Scincoidea | Scincidae | 1 | 1 |
| Plica_plica | Iguania | Tropiduridae | 1 | 1 |
| Pogona_vitticeps | Iguania | Agamidae | 1 | 1 |
| Polychrus_marmoratus | Iguania | Polychrotidae | 0 | 07 |
| Pristidactylus_torquatus | Iguania | Leiosauridae | 1 | 1 |
| Pseudopus_apodus | Anguimorpha | Anguidae | 1 | 1 |
| Rhacodactylus_auriculatus | Gekkota | Diplodactylidae | 1 | 1 |
| Rhineura_floridana | Amphisbaenia | Rhineuridae | 1 | 05 |
| Saltuarius_cornutus | Gekkota | Carphodactylidae | 1 | 1 |
| Sauromalus_ater | Iguania | Iguanidae | 1 | 1 |
| Sceloporus_variabilis | Iguania | Phrynosomatidae | 1 | 1 |
| Scincus_scincus | Scincoidea | Scincidae | 1 | 1 |
| Shinisaurus_crocodilurus | Anguimorpha | Shinisauridae | 1 | 1 |
| Smaug_mossambicus | Scincoidea | Cordylidae | 1 | 1 |
| Sphenodon_punctatus | Rhynchocephalia | Sphenodontidae | 1 | 1 |
| Sphenomorphus_solomonis | Scincoidea | Scincidae | 1 | 1 |
| Stenocercus_guentheri | Iguania | Tropiduridae | 1 | 1 |
| Strophurus_ciliaris | Gekkota | Diplodactylidae | 1 | 1 |
| Takydromus_sexlineatus | Lacertoidea | Lacertidae | 1 | 1 |
| Teius_teyou | Gymnophthalmoidea | Teiidae | 1 | 1 |
| Teratoscincus_przewalskii | Gekkota | Sphaerodactylidae | 1 | 1 |
| Tiliqua_scincoides | Scincoidea | Scincidae | 1 | 1 |
| Trachylepis_quinquetaeniata | Scincoidea | Scincidae | 1 | 1 |
| Trogonophis_wiegmanni | Lacertoidea | Trogonophidae | 1 | 1 |
| Tupinambis_teguixin | Gymnophthalmoidea | Teiidae | 1 | 1 |
| Uma_scoparia | Iguania | Phrynosomatidae | 1 | 1 |
| Uranoscodon_superciliosus | Iguania | Tropiduridae | 1 | 1 |
| Uromastyx_aegyptia | Iguania | Agamidae | 1 | 1 |
| Urostrophus_vautieri | Iguania | Leiosauridae | 0 | 010 |
| Uta_stansburiana | Iguania | Phrynosomatidae | 1 | 1 |
| Varanus_acanthurus | Anguimorpha | Varanidae | 1 | 03 |
| Varanus_exanthematicus | Anguimorpha | Varanidae | 1 | 03 |
| Varanus_salvator | Anguimorpha | Varanidae | 1 | 03 |
| Xantusia_vigilis | Scincoidea | Xantusiidae | 1 | 1 |
| Xenosaurus_grandis | Anguimorpha | Xenosauridae | 1 | 03 |
| Xenosaurus_platyceps | Anguimorpha | Xenosauridae | 1 | 03 |
Figure 6:

Ancestral state reconstruction of autotomy (see text for methodological details and Table 4 for data) using a recent reptile phylogenetic tree (Pyron, 2017). Colors are the same as Figure 2 in terms of presence and absence of the trait. The ancestral reconstruction shows an ancestral state of autotomy for the whole phylogeny. Major squamate reptile families have been layered on top of the phylogeny for convenience; (*) denotes Lacertoidae and (**) denotes Rhynchocephalia.
Figure 7:

Ancestral state reconstruction of regeneration (see text for methodological details and Table 4 for data) using the reptile phylogenetic tree from Figure 6 (Pyron, 2017). Colors are the same as Figure 2 in terms of presence and absence of the trait. The ancestral reconstruction shows an ancestral state of regeneration for the whole phylogeny. Major squamate reptiles families have been layered on top of the phylogeny for convenience; (*) denotes Lacertoidae and (**) denotes Rhynchocephalia.
Figure 8:

Modified phylogeny of Iguanidae (from “combined data” in Figure 1 in Wiens and Hollingsworth, 2000) onto which both regeneration and autotomy has been mapped (see text for methodological details and Table 5 for data). Full names of the taxa are on the right-hand side for convenience. Red and blue boxes at the tips of the tree indicate presence or absence, respectively, for regeneration. Red and blue branches on the phylogeny indicate the presence or absence, respectively, of autotomy. Dashed branches indicate data are not available (NA).
Table 5.
Autotomy and regeneration data for Iguanidae associated with Figure 8. Each tree tip is presented with the associated literature reference used for characterization of autotomy and regeneration as present (1), absent (0), or unknown (NA). 1The reptile database (http://www.reptile-database.org/); 2Bellairs and Bryant 1985; 3Arnold 1994; 4Smith 2016; 5Burger and Gochfeld 2006; 6de Queiroz 1987; 7Robyn 2013; 8Ariano-Sanchez and Gil-Escobedo 2016; 9Koleska and Jablonski 2018; 10Wu et al. 2014 11Koleska et al. 2017 and 12Carter and Hayes 2004.
| Species name | Suborder1 | Family1 | Regeneration | Autotomy |
|---|---|---|---|---|
| Dipsosaurus_dorsalis | Iguania | Iguanidae | 12 | 12 |
| Brachylophus_fasciatus | Iguania | Iguanidae | 12 | 03 |
| Amblyrhynchus_cristatus | Iguania | Iguanidae | 12 | 03/6 |
| Conolophus_pallidus | Iguania | Iguanidae | 02 | 03/6 |
| Conolophus_subcristatus | Iguania | Iguanidae | 02 | 06 |
| Ctenosaura_hemilopha | Iguania | Iguanidae | 14 | 02 |
| Ctenosaura_palearis | Iguania | Iguanidae | 17 | 16 |
| Ctenosaura_quinquecarinata | Iguania | Iguanidae | 18 | 16 |
| Ctenosaura_similis | Iguania | Iguanidae | 15 | 15 |
| Iguana delicatissima | Iguania | Iguanidae | 19 | 03/6 |
| Iguana iguana | Iguania | Iguanidae | 010 | 12/3 |
| Sauromalus ater | Iguania | Iguanidae | 111 | 16 |
| Sauromalus varius | Iguania | Iguanidae | NA | 16 |
| Cyclura cychlura | Iguania | Iguanidae | 112 | 16 |
| Cyclura nubila | Iguania | Iguanidae | 112 | 16 |
| Cyclura ricordii | Iguania | Iguanidae | 112 | 16 |
Discussion
With few exceptions, biologists continue to debate the adaptive nature of regeneration in the absence of formal evolutionary tests or experiments to directly assess the fitness consequences when regeneration is lost (Sánchez Alvarado 2000; Brockes and Kumar 2008; Bely and Nyberg 2010; Lai and Aboobaker 2018; Maden 2018). Directly measuring fitness can be difficult in regenerative species where lifespans extend for many years (with reproduction occurring over a large portion of the lifespan) and reproductive output can be large. Analyzing specific traits using ancestral state reconstruction provides a rigorous test to assess the evolutionary history for a particular trait. Strong inference is possible when species relationships are supported by robust phylogenies and sampling for a particular character is represented widely across the tree branches. In an effort to begin addressing whether the presence or absence of regenerative ability might be under selection, we endeavored to perform an ancestral state reconstruction of appendage regeneration in two groups where it has been relatively well-studied (arthropods and reptiles). We also reconstructed the evolutionary history of autotomy in order to ascertain whether regenerative ability might be constrained or influenced by this trait since the two are often associated for any given species in these taxa (Tiozzo and Copley, 2015).
First, our data demonstrated that regeneration and autotomy were present in the basal arthropod ancestor and that these traits have persisted during the evolution of most arthropod lineages. However, the data also showed that forces, known and unknown, can lead to loss of regenerative ability across entire clades. For example, a major shift in life-history strategy from incomplete to complete metamorphosis was accompanied by a complete loss of regenerative ability among adult holometabolous insects. In the case of arthropods, we could not find an instance where regeneration was lost, only to later remerge in a more recent lineage. Compared to regeneration, our data also showed that autotomy has been lost more frequently and that once lost within a lineage this change tends to persist in all descendent lineages. Although autotomy and regeneration are present in many arthropod lineages, the data suggest that the association of these traits has relaxed during the evolution of more recent taxa.
In contrast, regeneration and autotomy appear tightly linked in reptiles and our ancestral state reconstructions show that autotomy and regeneration were present in the common ancestor of all reptiles. Thus, when autotomy is lost in a particular reptilian lineage, regenerative ability is often also lost and vice versa. We did find exceptions to this general trend within the Iguanidae and Anguimorpha. The Anguimorpha were the only group in which autotomy was repeatedly lost but where regeneration remained. Similar to our finding in arthropods, when autotomy was lost at deep nodes it did not appear to re-evolve. It is important to note that conflicting molecular and morphological information exists concerning the phylogenetic relationships among reptiles and thus it is possible that topological inaccuracies within the tree may ultimately explain observed discrepancies (Wiens and Hollingsworth, 2000). Nevertheless, that these patterns were present in both trees adds strong support to our general conclusions. We did find intriguing counterexamples to the tight linkage between these traits, and investigating these counterexamples may help disentangle the evolution of autotomy and regeneration.
Broadly examining when autotomy and regeneration were lost in certain lineages, we find that autotomy is generally lost in more basal lineages, whereas loss of regeneration appears to be a more recent phenomenon. Furthermore, when autotomy is lost it appears very difficult to regain in descendant lineages (with two exceptions in each tree). This suggests that loss of autotomy is relatively permanent and does not leave organisms with dormant processes that are easily re-activated under the right conditions. This may reflect how autotomy is dependent on the presence of complex morphological and physiological components (e.g., precisely located fracture plane, contractile mechanism of muscle, nervous induction, etc.) that may be constrained by similar evolutionary pressures. The case of fragile tails in some snakes offers support for this view. Although some authors have referred to caudal autotomy among a few colubrids, other authors reject the presence of autotomy and suggest that their unique mechanism of intervertebral breakage has most likely been independently derived (Etheridge, 1967; Arnold, 1984).
Interestingly, while autotomy could be uncoupled from regeneration, loss of regenerative ability appears strongly linked to autotomy in both groups. In general, in every reptilian clade where regeneration was lost, so too was autotomy. Although the converse pattern, loss of autotomy leading to loss of regeneration, was common, Iguanas are an exception where autotomy has been lost several times but regeneration persists (Arnold, 1994; Bateman and Fleming, 2009; Gilbert et al., 2013). Aside from these losses, autotomy is widespread among iguana species, and these losses provide fertile ground for more empirical studies focused on why species have lost the ability to autotomize but are still able to regenerate. Similarly, the persistence or loss of regeneration in Pterygota (winged insects), while not necessarily connected to autotomy, was highly correlated with another trait: incomplete or complete metamorphosis (Maruzzo et al., 2005; Seifert et al., 2012b). In this case, the association between metamorphosis and regeneration suggests that the selective pressures associated with the radical life history change to complete metamorphosis imposed alterations that were antagonistic to regeneration in adult holometabolous insects.
The discrepancy between the timing observed for autotomy and regeneration losses could be explained in several different ways. First, it is possible that in those taxa that regenerate ancestrally, regeneration provides significant fitness benefits and thus those taxa that lose it are more likely to go extinct. In this case, only species who have lost regeneration relatively recently will be present in a particular phylogenetic tree. This is difficult to test unless a phylogeny contains closely related species with and without regeneration where fitness consequences can be directly measured (Maginnis 2006). That autotomy is lost more frequently in the trees may reflect a lower cost associated with its loss and thus, taxa that lose it persist for longer and are represented in the trees. It is also possible that the more frequent loss of autotomy reflects different rates of gain and loss between autotomy and regeneration. In this scenario regeneration is more easily gained and lost, whereas autotomy may be lost more slowly but is rarely re-gained. As a complex trait with many interacting processes, regeneration can be easily lost and gained in some taxa when mutations inactivate a signaling pathway required for regeneration. Examples include loss (and gain) of head regeneration in annelids and some flatworms (Bely and Sikes 2010). However, it is also possible that mutations which restore regeneration may be likely if the inactivated process can be replaced by the augmented function of a different but related process. Moreover, such mutations might be likely to sweep through populations if regeneration is under strong positive selection. Thus, for those species that lose and regain regeneration, the gain might occur before the species that lost it diversifies, which would make inferring the gain difficult using contemporary data. The loss of regenerative ability has also been associated with the evolution of increased cellular diversification, specific life-histories, differences in growth mode, and alterations in adaptive immunity (rev. in Needham 1953; Sánchez Alvarado 2000; Harty et al. 2003; Mescher and Neff 2005). In contrast, autotomy may be a trait more susceptible to complete loss without easy re-enabling options besides evolving the trait altogether anew. Coupled with weaker selection for mutations that do cause gain of autotomy, this could result in slower rates for gaining autotomy and thus losses would persist in the phylogeny when they do occur.
When considering inferences derived from data presented in the current paper, several cautionary notes are appropriate. One problem concerning comparative regeneration studies has always been a paucity of data from non-model species. Data concerning the absence or presence of autotomy and regeneration are lacking for many species, which underscores the need for more empirical studies collecting data for both traits. Data indicating true absences of autotomy or regeneration are particularly important. Indeed, inconspicuous appendages might be capable of autotomy or regeneration or both despite reports to the contrary for a more prominent appendage. Thus, only a systemic approach testing several appendages and organs can truly reject the presence of autotomy or regeneration or both in one species compared to another. For these reasons, we caution against strong inference based on the data presented here and instead present our conclusions as testable hypotheses in need of more systematic data.
To conclude, we found convincing support for the hypothesis that regeneration and autotomy were present in the common ancestors of arthropods and squamate reptiles and neither likely re-evolved in lineages where they were lost. In addition, our work supports the hypothesis that regeneration is lost relatively quickly possibly due to trade-offs with other costly capacities (Giangrande and Licciano, 2014). Recalling the views of Weismann and Morgan who believed, respectively, that regeneration is adaptive or that it is an ancestral and fundamental property, we suggest that our work finds evidence for both of these views. Finally, we emphasize the need for extensive empirical studies that collect data on regenerative ability and autotomy such that stronger inferences can be drawn from phylogenetic reconstructions. Similarly, we encourage researchers to expand exploration of the ancestral states of autotomy and regeneration in other clades such as birds, amphibians, and mammals. For instance, we know that complex tissue regeneration has evolved independently within mammals at least twice (rabbits and spiny mice) (Vorontsova and Liosner, 1960; Joseph and Dyson, 1966; Seifert et al., 2012a). With these data in hand, we will be better equipped to answer a myriad of questions including: have cases of appendage regeneration across vertebrate lineages evolved independently; does autotomy drive selection for regeneration; does the loss of regeneration always lead to the loss of autotomy; and why is regeneration seemingly more essential to maintaining autotomy than autotomy is to maintaining regeneration? Only with a broader understanding of the origins of regeneration and autotomy will we be able to enhance our understanding of those traits and one day harness the power of regeneration for the benefits of human beings.
Acknowledgements
We thank all members of the Seifert lab for insightful discussions. AWS is supported by grants from the National Science Foundation (NSF) and the Office for International Science and Engineering (OISE) (IOS #1353713) and from the National Institute of Musculoskeletal, Arthritis and Skin Diseases (NIAMS) (R01AR070313). JVC is supported by NSF CAREER Award #1846260. The content in this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Competing Financial Interests
The authors declare no competing financial interests.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Bibliography
- Anderson CV, Higham TE. 2014. Chameleon Anatomy. In: Tolley K, Herrel A, editors. The Biology of Chameleons. Berkeley and Los Angeles, California: University of California Press. p 7–56. [Google Scholar]
- Ariano-Sanchez D, Gil-Escobedo J. 2016. Tail Trifurcation. Herpetol Rev 47:463. [Google Scholar]
- Arnold EN. 1984. Evolutionary aspects of tail shedding in lizards and their relatives. J Nat Hist 18:127–169. [Google Scholar]
- Arnold EN. 1994. Investigating the evolutionary effects of one feature on another: does muscle spread suppress caudal autotomy in lizards? J Zool 232:505–523. [Google Scholar]
- Bateman PW, Fleming PA. 2009. To cut a long tail short: A review of lizard caudal autotomy studies carried out over the last 20 years. J Zool 277:1–14. [Google Scholar]
- Bauer AM, Russell AP, Shadwick RE. 1989. Mechanical properties and morphological correlates of fragile skin in gekkonid lizards. J Exp Biol 145:79–102. [Google Scholar]
- Bellairs A, Bryant SV. 1985. Autotomy and regeneration in reptiles. In: Gans C, Billett F, editors. Biology of the Reptilia, Vol. 15: Development B. New York, NY: John Wiley and Sons. p 301–410. [Google Scholar]
- Bely AE, Nyberg KG. 2010. Evolution of animal regeneration: re-emergence of a field. Trends Ecol Evol 25:161–170. [DOI] [PubMed] [Google Scholar]
- Brockes JP, Kumar A. 2008. Comparative Aspects of Animal Regeneration. Annu Rev Cell Dev Biol 24:525–549. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. 1993. The Importance of the Human Face in Risk Perception by Black Iguanas, Ctenosaura similis. J Herpetol 27:426. [Google Scholar]
- Carter RL, Hayes WK. 2004. Conservation of an endangered Bahamian rock iguana, II: Morphological variation and conservation priorities. In: Iguanas: Biology and Conservation. . p 258–273. [Google Scholar]
- Child CM, Young AN. 1903. Regeneration of the appendages in nymphs of the agrionidae. Arch für Entwicklungsmechanik der Org 15:543–602. [Google Scholar]
- Cope R 1967. Wide soouted worm-lizard. Cat Am Amphib Reptil:1–2. [Google Scholar]
- Cravens H, Allen GE. 1980. Thomas Hunt Morgan: The Man and His Science. Princeton, NJ: Princeton University Press. [Google Scholar]
- Darlington PJ. 1929. Notes on the Habits of Amphizoa. Psyche (New York) 36:383–385. [Google Scholar]
- Darwin C 1859. On the Origin of Species by Means of Natural Selection. London: John Murray. [Google Scholar]
- De Queiroz K 1987. Phylogenetic systematics of iguanine lizards : a comparative osteological study. Berkeley: University of California Press. [Google Scholar]
- Dinsmore CE. 1996. Urodele limb and tail regeneration in early biological thought: An essay on scientific controversy and social change. Int J Dev Biol 40:621–627. [PubMed] [Google Scholar]
- Dobzhansky T, Sturtevant AH. 1938. Inversions in the chromosomes of Drosophila pseudoobscura. Genetics 23:28–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragulescu AA. 2014. xlsx: Read, write, format Excel 2007 and Excel 97/2000/XP/2003 files. R package version 0.5.7. https://CRANR-project.org/package=xlsx.
- Dumont HJ, Vierstraete A, Vanfleteren JR. 2010. A molecular phylogeny of the Odonata (Insecta). Syst Entomol 35:6–18. [Google Scholar]
- Esposito M 2013. Weismann Versus Morgan Revisited: Clashing Interpretations on Animal Regeneration. J Hist Biol 46:511–541. [DOI] [PubMed] [Google Scholar]
- Etheridge R 1967. Lizard Caudal Vertebrae. Copeia 1967:699–721. [Google Scholar]
- Fleming PA, Muller D, Bateman PW. 2007. Leave it all behind: A taxonomic perspective of autotomy in invertebrates. Biol Rev 82:481–510. [DOI] [PubMed] [Google Scholar]
- French V 1976. Leg regeneration in the cockroach, Blattella germanica - I. Regeneration from a congruent tibial graft/host junction. Wilhelm Roux’s Arch Dev Biol 179:57–76. [DOI] [PubMed] [Google Scholar]
- Galis F, Wagner GP, Jockusch EL. 2003. Why is limb regeneration possible in amphibians but not in reptiles, birds, and mammals? Evol Dev 5:208–220. [DOI] [PubMed] [Google Scholar]
- Giangrande A, Licciano M. 2014. Regeneration and clonality in Metazoa. The price to pay for evolving complexity. Invertebr Reprod Dev 58:1–8. [Google Scholar]
- Gilbert EAB, Payne SL, Vickaryous MK. 2013. The anatomy and histology of caudal autotomy and regeneration in lizards. Physiol Biochem Zool 86:631–644. [DOI] [PubMed] [Google Scholar]
- Goss RJ. 1969. Principles of Regeneration. Academic Press. [Google Scholar]
- Harmon LJ, Weir JT, Brock CD, Glor RE, Challenger W. 2008. GEIGER: Investigating evolutionary radiations. Bioinformatics 24:129–131. [DOI] [PubMed] [Google Scholar]
- Harty M, Neff AW, King MW, Mescher AL. 2003. Regeneration or scarring: An immunologic perspective. Dev Dyn 226:268–279. [DOI] [PubMed] [Google Scholar]
- Haynie JL, Bryant PJ. 1976. Intercalary regeneration in imaginal wing disk of drosophila melanogaster. Nature 259:659–662. [DOI] [PubMed] [Google Scholar]
- Hermann HR. 1971. Sting autotomy, a defensive mechanism in certain social Hymenoptera. Insectes Soc 18:111–120. [Google Scholar]
- Hijmans RJ, Elith J. 2016. Species distribution modeling with R. http://www.idg.pl/mirrors/CRAN/web/packages/dismo/vignettes/sdm.pdf:87.
- Hopkins PM. 1993. Regeneration of walking legs in the fiddler crab Uca pugilator. Integr Comp Biol 33:348–356. [Google Scholar]
- Huelsenbeck JP, Nielsen R, Bollback JP. 2003. Stochastic mapping of morphological characters. Syst Biol 52:131–158. [DOI] [PubMed] [Google Scholar]
- Joseph J, Dyson M. 1966. Tissue replacement in the rabbit’s ear. Br J Surg 53:372–380. [DOI] [PubMed] [Google Scholar]
- Koleska D, Jablonski D. 2018. A four-tailed iguana delicatissima (Squamata: Iguanidae) on petite terre, guadeloupe (Lesser antilles, caribbean region). Phyllomedusa 17:157–159. [Google Scholar]
- Koleska D, Svobodová V, Husák T, Kulma M, Jablonski D. 2017. Tail bifurcation recorded in sauromalus ater. Herpetol Notes 10:363–364. [Google Scholar]
- Korschelt E 1907. Regeneration und transplantation. Korschelt Von Dr. E.. Jena: Gustav Fischer. [Google Scholar]
- Lai AG, Aboobaker AA. 2018. EvoRegen in animals: Time to uncover deep conservation or convergence of adult stem cell evolution and regenerative processes. Dev Biol 433:118–131. [DOI] [PubMed] [Google Scholar]
- Laspiur A, Acosta JC, Abdala CS. 2007. A new species of Leiosaurus (Iguania: Leiosauridae) from central-western Argentina. Zootaxa 1470:47–57. [Google Scholar]
- Lenhoff SG, Lenhoff HM. 1986. Hydra and the Birth of Experimental Biology - 1744. Pacific Grove, CA: The Boxwood Press. [Google Scholar]
- Litsios G, Salamin N. 2012. Effects of phylogenetic signal on ancestral state reconstruction. Syst Biol 61:533–538. [DOI] [PubMed] [Google Scholar]
- Lozito TP, Tuan RS. 2016. Lizard tail skeletal regeneration combines aspects of fracture healing and blastema-based regeneration. Dev 143:2946–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden M 2018. The evolution of regeneration – Where does that leave mammals? Int J Dev Biol 62:369–372. [DOI] [PubMed] [Google Scholar]
- Madhavan K, Schneiderman HA. 1969. Hormonal control of imaginal disc regeneration in Galleria mellonella (Lepidoptera). Biol Bull 137:321–331. [Google Scholar]
- Maginnis TL. 2006. The costs of autotomy and regeneration in animals: A review and framework for future research. Behav Ecol 17:857–872. [Google Scholar]
- Maruzzo D, Bonato L, Brena C, Fusco G, Minelli A. 2005. Appendage loss and regeneration in arthropods. In: Crustacea and Arthropod Relationships. . p 215–245. [Google Scholar]
- McElroy EJ, Bergmann PJ. 2013. Tail autotomy, tail size, and locomotor performance in lizards. Physiol Biochem Zool 86:669–679. [DOI] [PubMed] [Google Scholar]
- McKenna DD, Farrell BD. 2010. 9-Genes Reinforce the Phylogeny of Holometabola and Yield Alternate Views on the Phylogenetic Placement of Strepsiptera. PLoS One 5:e11887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVean A 1982. Autotomy. In: Sandeman DC, Atwood HL, editors. The Biology of Crustacea. Vol. 4. New York, NY: Academic Press. p 107–132. [Google Scholar]
- Mescher AL, Neff AW. 2005. Regenerative capacity and the developing immune system. Adv Biochem Eng Biotechnol 93:39–66. [DOI] [PubMed] [Google Scholar]
- Meusemann K, Von Reumont BM, Simon S, Roeding F, Strauss S, Kück P, Ebersberger I, Walzl M, Pass G, Breuers S, Achter V, Von Haeseler A, Burmester T, Hadrys H, Wägele JW, Misof B. 2010. A phylogenomic approach to resolve the arthropod tree of life. Mol Biol Evol 27:2451–2464. [DOI] [PubMed] [Google Scholar]
- Moore A, Tabashnik BE. 1989. Leg Autotomy of Adult Diamondback Moth (Lepidoptera: Plutellidae) in Response to Tarsal Contact with Insecticide Residues. J Econ Entomol 82:381–384. [Google Scholar]
- Morgan TH. 1901a. Regeneration and liability to injury. Science (80- ) 14:235–248. [DOI] [PubMed] [Google Scholar]
- Morgan TH. 1901b. Regeneration. London, UK: The Macmillan Company. [Google Scholar]
- Needham AE. 1953a. Regeneration and Wound-healing. Methuen. [Google Scholar]
- Needham AE. 1953b. The incidence and adaptive value of autotomy and of regeneration in Crustacea. Proc Zool Soc London 123:111–122. [Google Scholar]
- Orme CDL, Freckleton RP, Thomas GH, Petzoldt T, Fritz SA. 2012. caper: Comparative analyses of phylogenetics and evolution in R (http://R-Forge.R-project.org/projects/caper/).
- Paradis E, Claude J, Strimmer K. 2004. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 20:289–290. [DOI] [PubMed] [Google Scholar]
- Poe S 2004. Phylogeny of Anoles. Herpetol Monogr 18:37. [Google Scholar]
- Pyron RA. 2017. Novel approaches for phylogenetic inference from morphological data and total-evidence dating in squamate reptiles (Lizards, Snakes, and Amphisbaenians). Syst Biol 66:38–56. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2013. R: A Language and Environment for Statistical Computing. 2:https://www.R-project.org. [Google Scholar]
- Réaumur R-AF de. 1712. Sur les diverses reproductions qui se font dans les Ecrevisse, les Omars, les Crabes, etc. et entr’autres sur celles de leurs Jambes et de leurs Ecailles. Mem Acad R Sci:223–241. [Google Scholar]
- Regier JC, Shultz JW, Zwick A, Hussey A, Ball B, Wetzer R, Martin JW, Cunningham CW. 2010. Arthropod relationships revealed by phylogenomic analysis of nuclear protein-coding sequences. Nature 463:1079–1083. [DOI] [PubMed] [Google Scholar]
- Rehm P, Borner J, Meusemann K, von Reumont BM, Simon S, Hadrys H, Misof B, Burmester T. 2011. Dating the arthropod tree based on large-scale transcriptome data. Mol Phylogenet Evol 61:880–887. [DOI] [PubMed] [Google Scholar]
- Resh VH. 2009. Encyclopedia of Insects. In: Resh VH, Cardé RT, editors. Encyclopedia of Insects. 2nd ed. Oxford, UK: Academic Press. p 721–729. [Google Scholar]
- Revell LJ. 2010. Phylogenetic signal and linear regression on species data. Methods Ecol Evol 1:319–329. [Google Scholar]
- Robinson JV, Hayworth DA, Harvey MB. 1991. The Effect of Caudal Lamellae Loss on Swimming Speed of the Damselfly Argia moesta (Hagen) (Odonata: Coenagrionidae). Am Midl Nat 125:240. [Google Scholar]
- Robyn. 2013. Regrown Leg? Reptil Rep. [Google Scholar]
- Sánchez Alvarado A 2000. Regeneration in the metazoans: Why does it happen? BioEssays 22:578–590. [DOI] [PubMed] [Google Scholar]
- Scherz MD, Daza JD, Köhler J, Vences M, Glaw F. 2017. Off the scale: a new species of fish-scale gecko (Squamata: Gekkonidae: \textit{Geckolepis}) with exceptionally large scales. PeerJ 5:e2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliep KP. 2011. phangorn: Phylogenetic analysis in R. Bioinformatics 27:592–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert AW, Kiama SG, Seifert MG, Goheen JR, Palmer TM, Maden M. 2012a. Skin Shedding and Tissue Regeneration in African Spiny Mice (Acomys). Nature 489:561–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert AW, Monaghan JR, Smith MD, Pasch B, Stier AC, Michonneau F, Maden M. 2012b. The influence of fundamental traits on mechanisms controlling appendage regeneration. Biol Rev 87:330–345. [DOI] [PubMed] [Google Scholar]
- Shcherbakov DE, Lukashevich ED, Blagoderov VA. 1995. Triassic Diptera and initial radiation of the order. Int J Dipterological Res 6:75–115. [Google Scholar]
- Simpson SB. 1968. Morphology of the regenerated spinal cord in the lizard, Anolis carolinensis. J Comp Neurol 134:193–209. [DOI] [PubMed] [Google Scholar]
- Slack JMW. 2010. Evolution of Regeneration. Hear Dev Regen 123:827–837. [Google Scholar]
- Smith HM. 1972. The sonoran subspecies of the lizard Ctenosaura hemilopha. Gt Basin Nat 32:104–111. [Google Scholar]
- Stoks R 1998. Indirect Monitoring of Agonistic Encounters in Larvae of Lestes viridis (Odonata: Lestidae) Using Exuviae Lamellae Status. Aquat Insects 20:173–180. [Google Scholar]
- Tartar V 2011. The biology of Stentor. Pergammon Press. [Google Scholar]
- Tiozzo S, Copley RR. 2015. Reconsidering regeneration in metazoans: An evo-devo approach. Front Ecol Evol 3:1–12. [Google Scholar]
- Truby PR. 1983. Blastema formation and cell division during cockroach limb regeneration. J Embryol Exp Morphol Vol. 75:151–164. [PubMed] [Google Scholar]
- Vitt LJ, Congdon JD, Dickson NA. 1977. Adaptive Strategies and Energetics of Tail Autonomy in Lizards. Ecology 58:326–337. [Google Scholar]
- Vorontsova MA, Liosner LD. 1960. Asexual Propagation and Regeneration. London: Pergamon Press. [Google Scholar]
- Wiens JJ, Etheridge RE. 2003. Phylogenetic Relationships of Hoplocercid Lizards: Coding and Combining Meristic, Morphometric, and Polymorphic Data Using Step Matrices. Herpetologica 59:375–398. [Google Scholar]
- Wiens JJ, Hollingsworth BD. 2000. War of the iguanas: Conflicting molecular and morphological phylogenies and long-branch attraction in iguanid lizards. Syst Biol 49:143–159. [DOI] [PubMed] [Google Scholar]
- Wigglesworth VB. 1944. The Principles of Insect Physiology. Second. New York: Chapman and Hall. [Google Scholar]
- Wood FD, Wood HE. 1932. Autotomy in decapod Crustacea. J Exp Zool 62:1–55. [Google Scholar]
- Wu P, Alibardi L, Chuong C-M. 2014. Regeneration of reptilian scales after wounding: neogenesis, regional difference, and molecular modules. Regeneration 1:15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Li Z, Li H, Li Y, Yang Y, Zhang Q, Liu X. 2016. Comparison of leg regeneration potency between holometabolous helicoverpa armigera (Lepidoptera: Noctuidae) and hemimetabolous locusta migratoria manilensis (Orthoptera: Acrididae). Environ Entomol 45:1552–1560. [DOI] [PubMed] [Google Scholar]
- Zani PA. 1996. Patterns of cuadal-autotomy evolution in lizards. J Zool 240:201–220. [Google Scholar]
- Zug G, Brown H, Schulte J, Vindum J. 2006. Systematics of the garden lizards, Calotes versicolor group (Reptilia, Squamata, Agamidae), in Myanmar: central dry zone populations. Proc Calif Acad Sci 57:35–68. [Google Scholar]


