Abstract
Evidence has implicated oxidative stress (OS) and inflammation as drivers of neurodegenerative pathologies. We previously reported on the beneficial effects of (−)-epicatechin (Epi) treatment, on aging-induced OS and its capacity to restore modulators of mitochondrial biogenesis in the prefrontal cortex of 26-month-old male mice. In the present study using the same mouse model of aging, we examined the capacity of Epi to mitigate hippocampus OS, inflammation, hyperphosphorylation of tau protein, soluble β-amyloid protein levels, cell survival, memory, anxiety-like behavior levels as well as systemic inflammation. Mice underwent 4 weeks of Epi treatment (1 mg/kg/day) and samples of hippocampus were obtained. Assessments of the OS markers protein carbonyls and malondialdehyde levels demonstrated significant increases (~3 fold) with aging that were partially suppressed by Epi. Protein levels of the glial fibrillary acidic protein, inflammatory factor 1 (Iba1), proinflammatory cytokines, interleukins (IL-1β, IL-3, 5, 6 and 15), ciclooxygenase 2, tumor necrosis factor α, nuclear factor activated B cells and interferon γ increase with aging and were also significantly decreased with Epi treatment. However, anti-inflammatory cytokines, IL-1ra, Il-10 and 11 were decreased in aging and were restored with Epi. Epi also reversed aging effects on the hyperphosporylation of tau, increased soluble β-amyloid levels (~2 fold), cell death, (as per caspase 3 and 9 activity), and reductions in nerve growth factor and triggering receptor myeloid cells 2 levels. Measures of anxiety like behavior and memory demonstrated improvements with Epi treatment. Indicators of systemic inflammation were elevated with aging and Epi was capable to decrease blood inflammatory markers. Altogether, results evidence a significant capacity of Epi to mitigate hippocampus OS and inflammation leading to improved brain function.
Keywords: Aging, oxidative stress, memory, anxiety
Introduction
Aging is associated with cognitive decline which has been linked to a progressive loss of specific neuronal cell populations and to the presence of protein aggregates in the brain1. In the setting of aging, common features include high levels of local (i.e. brain) and systemic oxidative stress (OS) as well as inflammation which can promote neurodegeneration and/or neuronal cell death1–3. In the aged brain, extracellular amyloid plaques (senile plaques) and intracellular neurofibrillary tangles (NFTs) develop secondary to the β-A aggregates and hyperphosphorylation (hp) of the microtubule-binding protein tau respectively4. Critical to the preservation of neuronal health is the presence and activity of nerve growth factor (NGF) and triggering receptor expressed on myeloid cells2 (TREM2) whose levels also fall with aging5,6. Conversely, with aging and when there is trauma or disease, glial fibrillary acidic protein (GFAP) expression increases, therefore it can serve as a biomarker of neurotoxicity7,8. To date there are not effective therapies aimed at the preservation of neuronal health and thus, the identification of safe and effective agents that exert these actions is of high priority.
Preclinical, clinical and epidemiological evidence indicates that the consumption of cacao products or specific flavanols found in cacao, can ameliorate age-related cognitive decline. A study using aged rats demonstrated positive effects of a flavonoid-rich cocoa extract on performance in discrimination and spatial tasks9. In normal aged human subjects, the consumption of flavanol rich cocoa for 8 weeks was associated to improvements in cognitive function, linked to enhanced hippocampal function and/or brain blood flow10–16. Epidemiological studies also report on the association between the sustained consumption of cocoa products (most prominently dark chocolate) and improved cognitive function17.
The most abundant flavanol present in cocoa is (−)-epicatechin (Epi), which stimulates nitric oxide production in endothelial cells and thus, increases blood flow. Epi can cross the blood-brain barrier and potentially act directly on neurons and supporting systems18. In mice, Epi favorably impacts anxiety19, learning and memory20,21 and such effects are associated with increased angiogenesis and neuronal spine density as well as with the upregulation of mRNA levels of proteins associated with learning and downregulation of markers of neurodegeneration in the hippocampus. We reported on the beneficial effects of Epi on OS levels and indicators of mitochondrial biogenesis, structure and function in the prefrontal cortex of aged mice (26-month-old)22. Thus, Epi recapitulates the beneficial actions of cocoa in blood vessels and brain. Nevertheless, no studies have examined the effects of Epi treatment on indicators of OS, neuroinflammation and neurodegeneration in the senile hippocampus and on systemic indicators of inflammation.
With these goals in mind, a study was implemented in young and aged mice assessing the effects of Epi treatment on blood cytokines levels, hippocampus OS markers, neuroinflammation modulators, hp-tau, soluble β-amyloid levels (and related signaling pathways) as well as markers of neuronal health and cell survival. Behavioral studies evaluated Epi effects on cognitive function and anxiety like behavior measures.
Materials and Methods
Animal studies
C57BL6 male mice were purchased from Jackson laboratories and divided into four groups. Animals were housed under controlled conditions, with 12 h light/dark cycles. Group 1, young control (YC) mice (6-month old), n = 7; group 2, aged control (AC) mice (26-month old), n = 7; group 3, Y mice treated with Epi (YE), n = 7; group 4, aged (A) mice treated with Epi (AE), n = 7. Groups 1 and 2 were provided vehicle (water) by oral gavage. As previously described23 groups 3 and 4 were treated with Epi (1 mg/kg/day) by oral gavage during 4 weeks. Open field and object recognition tasks were performed in all four groups of mice. Results compared the effects of vehicle vs. Epi treatment measured at 4 weeks of treatment. At the end of treatment, blood samples and brain were collected, and the hippocampal region was dissected and stored at −80°C until used. All animal handling procedures were approved by the UCSD’s Institutional Animal Care and Use Committee (approval number S10223) and according to NIH guidelines on the use of animals.
Protein Carbonylation
Protein carbonylation was used as a surrogate indicator of tissue OS levels25. Tissue samples were homogenized with a polytron tissue homogenizer (Brinkmann Instruments, Westbury, New York) in 500μl of cold buffer (50 mM 4-morpholineethanesulfonic acid, pH 6.7, containing 1 mM EDTA) and centrifuged (10,000 g) for 15 min at 4 °C. Supernatants were collected and incubated at room temperature for 15 min with streptomycin sulfate at a final concentration of 1%. Samples were centrifuged (6,000 g) for 10 min at 4 °C. Total protein carbonylation was measured in supernatants using a colorimetric protein carbonyl assay kit in accordance with the manufacturer’s instructions (Cayman Chemicals intra-assay coefficient of variation of 4.7%) at 360 nm using a SpectroQuant spectrophotometer (BioTek Instruments Inc. Winooski, VT, USA). All samples were tested in duplicate at room temperature.
Measurement of Malondialdehyde (MDA) for Lipid Peroxidation
Lipid peroxidation was measured by MDA quantitation. Tissue samples (10 mg) were washed in cold PBS, homogenized with a polytron in 303μl of lysis solution (Abcam, San Diego, CA, USA) while on ice and then centrifuged at 13,000 g for 10 min. Supernatants were collected and used to measure malondyaldehyde bound to thiobarbituric acid (TBA) by a colorimetric assay kit (TBARS) according to the manufacturer’s instructions (Cayman Chemicals) at 360 nm using a μQuant spectrophotometer (BioTek Instruments Inc. Winooski, VT, USA). All samples were tested in duplicate and measured at 540 nm wavelength at room temperature.
Western blotting
Tissue samples were homogenized with a polytron in 250 μl of lysis buffer (1% Triton X-100, 200 mM Tris, 140 mM NaCl, 2 mM EDTA, and 0.1% sodium dodecyl sulfate) with protease and phosphatase inhibitor cocktails (P2714; Sigma-Aldrich), supplemented with 0.15 mM PMSF, 5 mM Na3VO4 and 3 mM NaF, as previously described22,23,25,26. Homogenates were sonicated in a tank sonicator (Fisherbrand USA) for 15 min at 4 °C and centrifuged (10,000 g) for 10 min at 4 °C22. The total protein content was measured in the supernatant using the Bradford method. A total of 40 μg of protein were loaded onto a 4–15% Mini-PROTEAN® TGX™ Precast Protein Gel (Bio-Rad, Hercules, CA, USA)26, electrotransferred to a PVDF membrane using a Trans-Blot® Semi-Dry system (16 V, 60 min) for low molecular weight proteins, or using a Mini Trans-Blot® Cell system (70 V, 2 h) (Bio-Rad, Hercules, CA, USA) for high molecular weight proteins. The membranes were incubated for 1 h in blocking solution (5% nonfat dry milk in Tris-buffered saline plus 0.1% Tween 20), followed by overnight incubation at 4 °C26 or 3 h of incubation at room temperature22 with primary antibodies. Membranes were washed (3 X for 5 min) in Tris-buffered saline plus 0.1% Tween 20 and incubated for 1 h at room temperature with specific horseradish peroxidase (HRP)-conjugated secondary antibodies diluted 1:10,000 in blocking solution. The immunoblots were developed using an enhanced chemiluminescence (ECL) detection kit (Amersham-GE, USA). The band densitometric analyses were achieved using the ImageJ software.
Antibodies
Anti-TNFα, COX2, NFκβ, INFγ, IL-1β, IL-5 and IL-6 antibodies were purchased from Cell Signaling Technologies (San Diego, CA) and used as pro-inflammation markers. Anti-IL-10 and IL-11 antibodies were from Santa Cruz BioTech, (Santa Cruz, CA) and used as anti-inflammatory markers. To measure memory surrogates anti-AKT, p-AKT (Ser473), GSK3β, p-GSK3β (Ser9), Amyloid-β (Aβ−42) (Cell Signaling), TREM2, NGF, TAU and p-TAU (Ser396) were obtained from Abcam (San Diego, CA) while GFAP was from Santa Cruz Biotech. and Iba1 antibody was purchased from Abcam (San Diego, CA). Anti-GAPDH, used for signal normalization, as well as anti-rabbit and anti-mouse HRP-conjugated secondary antibodies were from Cell Signaling. (San Diego, CA).
Pro- and anti-inflammatory cytokines in plasma
Blood was draw from the inferior vena cava using a syringe containing 50 mM EDTA pH 8 and centrifuged at 1700 g for 10 min at 4° C. Plasma was recovered and used for Luminex xMAP technology with the Milliplex brand of magnetic-bead fluorescent immunoassay for TNF-α, IFN-γ, IL-1ra IL-15, IL-3 and IL-1β (MilliporeSigma EMD). Plasma samples for the assay were processed according to the manufacturer instructions and evaluated in the Bioplex plate reader (Bio-Rad, Hercules, CA, USA). Concentration of cytokines was calculated using the standard curve and validated using the ProcartaPlex internal controls of the assay.
Amyloid-β (Aβ 42) measurements by ELISA
APP null mouse hippocampus samples were kindly provided by Dr. Hemal Patel. Hippocampus was homogenized in cold buffer of 5 M guanidine-HCl/50 mM Tris, pH 8.0, protease inhibitors and incubated with shaking for 3 h at room temperature and then processed for Aβ 42 assessments according to the manufacturer instructions (Invitrogen ThermoFisher). Homogenized samples were centrifuged at 15000 g for 20 min at 4° C. Supernatants were collected and diluted in cold PBS with protease inhibitors and used for Aβ 42 measurements in a microplate reader (SpectroQuant) at 450 nm wavelength using a SpectroQuant spectrophotometer (BioTek Instruments Inc. Winooski, VT, USA). Aβ 42 concentrations were calculated using the standard curve from the assay.
Caspase 3 and 9 activity measurements
The activity of caspases as surrogates of apoptosis was measured using the caspase-3 and caspase-9 kits both from Abcam. Hippocampal samples were homogenized and processed as per manufacturer instructions. Caspases 3 and 9 recognize DEVD and LEHD sequences, respectively, and cleave the labeled substrate p-NA developing color reaction which was measured in a microplate reader al 405 nm wavelength using a SpectroQuant spectrophotometer (BioTek Instruments Inc. Winooski, VT, USA).
Locomotor activity and Open Field Task (OFT)
To avoid stressful conditions that could affect the performance or consolidation of each task, animals were transported from the vivarium to the experimental room during 3 consecutive days and left in the testing room for 1 h prior starting the test and were left at the same location for an additional 2 h at the end of the test27. General locomotor activity and anxiety-like behavioral responses to a novel environment were measured in an OF apparatus, consisting of an acrylic 50 cm (length) X 50 cm (width) surrounded by a 50 cm high wall. Two areas were virtually differentiated in the OF: the periphery (outer zone 10 cm from the wall) and the central area (the rest of the OF) (Fig. 7A). Animals were gently placed in the center of the area at the beginning of the test, allowed to move free and uninterrupted in the quadrants of the OF to explore the environment for 10 min period. Animal movements and paths were recorded by a Sony Cyber Shot video camera (Sony, USA). The video recordings were used to measure total distance traveled and the time spent in each area28.
Figure 7.
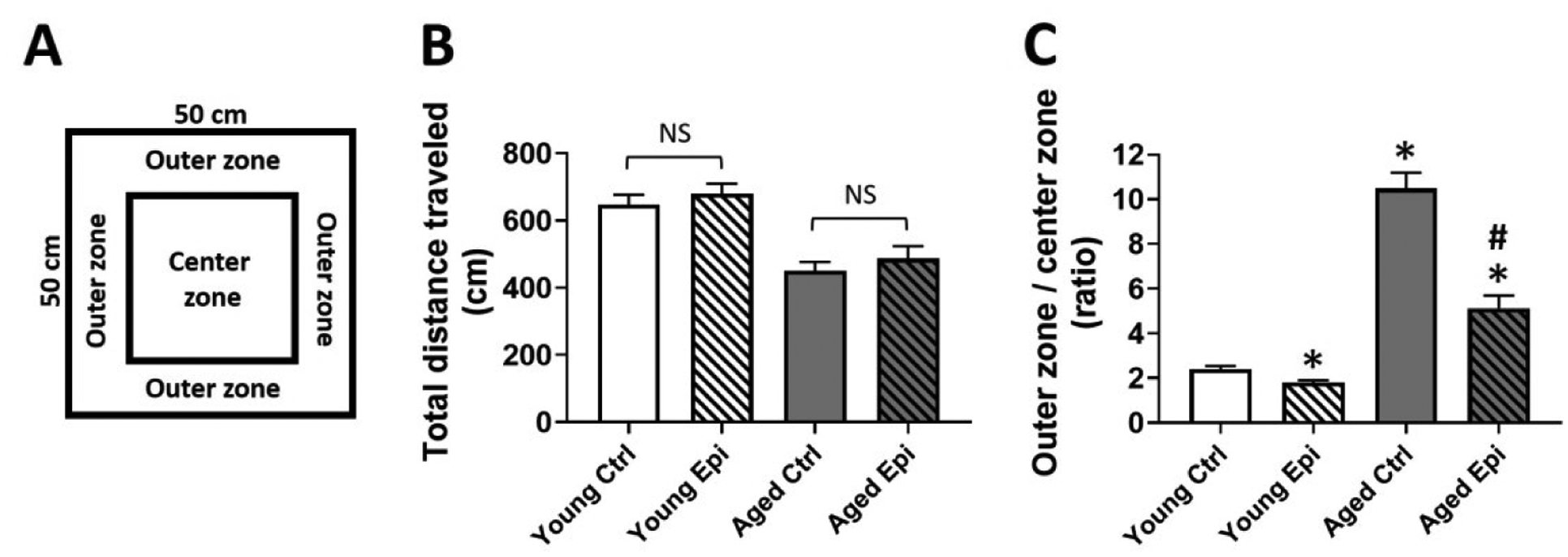
Open field testing of young and aged animals treated with vehicle (control) or epicatechin for 4 weeks. (A) Schematic representation of open field test. (B) Total distance traveled (in cm) in the open field. (C) outer zone vs centrer zone ratio of young and aged control (YC and AC) and epicatechin treated mice (YE and AE). Results are reported as outer vs center time in seconds (s) ratio. (n=7/group, *p<0.05 vs. YC), #p < 0.05 vs. AC).
Object Recognition Task (ORT)
To avoid behavior alterations due to exposure to a new environment the animals were transported to the testing room as in OFT. Animals were habituated individually to the open empty arena to free exploration for 5 min in two consecutive days. On the following day animals were familiarized during 5 min to two identical aligned objects (A + A) placed in the open field at 10cm from each wall. Then, mice were sent back to their home cages for 1 h and reintroduced to the arena after one object was exchanged (A + B) and allow them to explore for 5 min (short-term memory). After 24 h object B was exchanged for object C and allow them for an exploratory period of 5 min (long-term memory) (Fig.8A). Exploration was considered as pointing the nose toward an object at a distance of less than 1 cm and/or touching it with the nose. Turning around or sitting close to the object was not considered as exploratory behavior29. The trials were video recorded using a Sony Cyber Shot camera (Sony, USA). for subsequent scoring by two different individuals blind to the experimental conditions. Object recognition was defined as time spent between novel (TN) and the time sum of both objects novel and familiar (TF) [(TN)/(TN+TF)].
Figure 8.
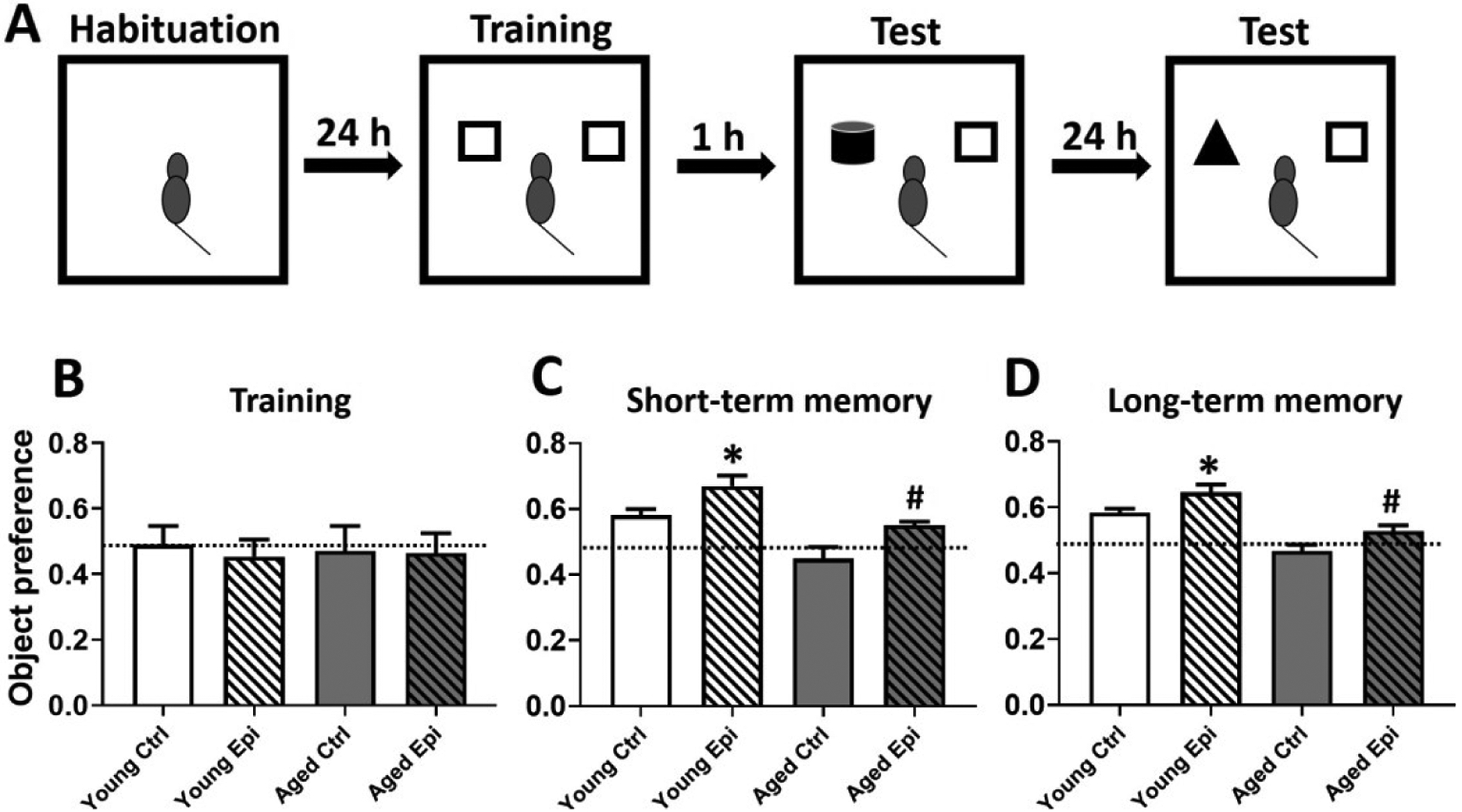
Object recognition test of aged animals treated with vehicle or epicatechin for 4 weeks. Schematic representation of the object recognition test (A). Graphic representation of the object preference score during the training (B), during the short-term memory (1h) test (C) and during the long-term memory (24h) test (D). Values compare chance score of the object preference (0.5) vs. those recorded at day 28. (B) Discrimination index obtained from 28th recorded values divided by baseline values. Results are reported as latency time in seconds (s). (n=7/group, *p<0.05 vs. before, #p < 0.05 vs. AC).
Data and Statistical Analysis
Results are expressed as mean ± standard error (SEM). Statistical analysis was pursued using two-way analysis of variance followed by a Tukey post-hoc tests and unpaired t-test when applicable using GraphpadPrism version 8.0 (GraphPad Prism, San Diego, CA, USA). Statistical significance was defined when p < 0.05.
Results
Protein Carbonylation and MDA Levels
Total protein carbonylation was significantly increased (p<0.05) by ~2.5 fold in AC (8.45 ±1.3 nmol/mg of protein) vs. YC (3.25 ±1.1 nmol/mg of protein). Epi treatment reduced protein carbonylation levels in AE animals by ~25% (6.4 ±0.9 nmol/mg of protein) vs. AC (p<0.05) (Fig. 1A). Lipid peroxidation as measured by the presence of MDA increased in AC by ~2 fold (3.4 ±0.6 nmol/mg of protein) vs. YC mice (1.7 ±0.3 nmol/mg of protein) (p<0.05). Epi treatment also significantly reduced MDA levels by ~30% in AE (2.35 ±0.4 nmol/mg of protein) vs. AC (Fig. 1B).
Figure 1.
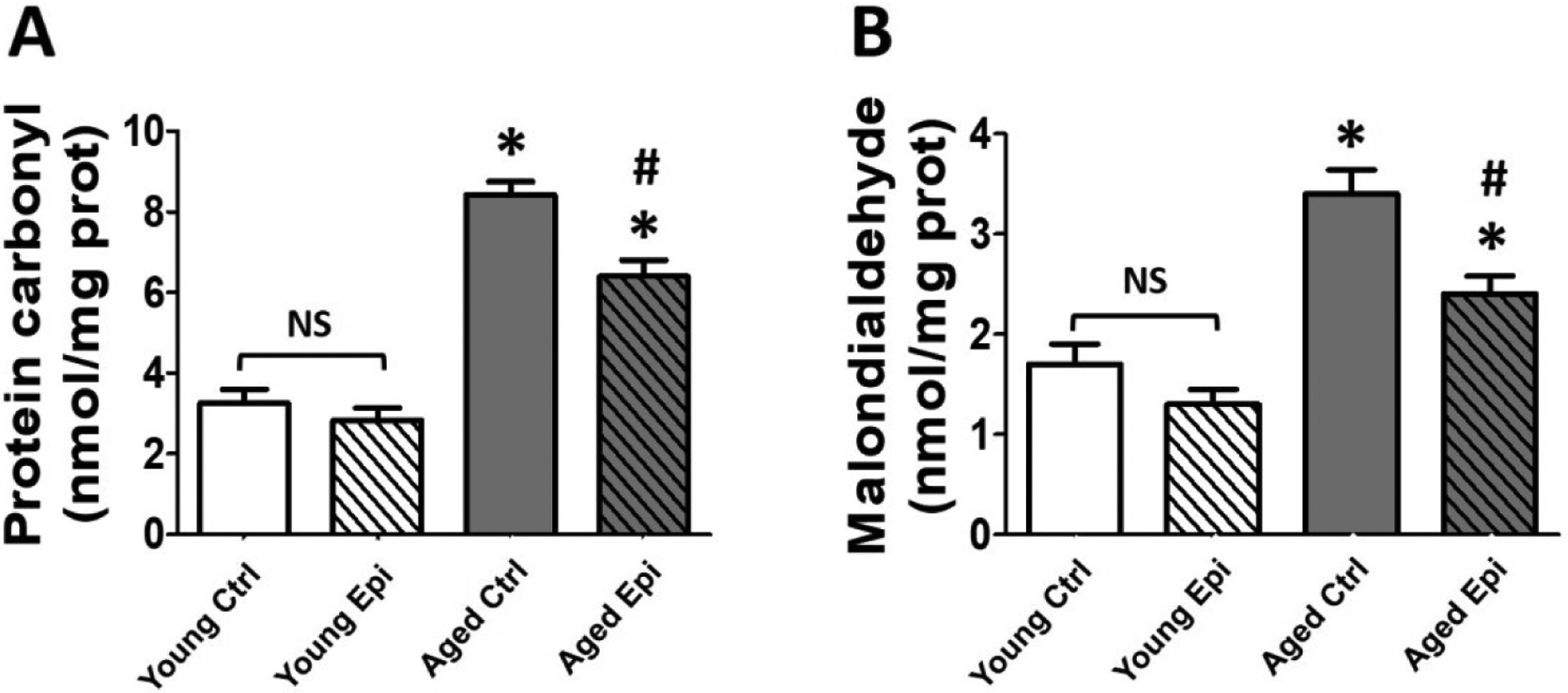
Oxidative stress indicators level in the hippocampus of young and aged control (YC and AC) and epicatechin treated mice (YE and AE). (A) Changes observed in total protein carbonylation levels. (B) Changes observed in malondialdehyde-bound thiobarbituric acid (MDA) levels. Values were normalized by sample protein content (n = 7/ group, *p < 0.05 vs. YC, #p < 0.05 vs. AC).
Pro- and anti-inflammatory endpoints
To investigate the impact of aging and Epi treatment on inflammation, biomarkers were assessed by Western blot in hippocampus (Fig. 2) and using the Milliplex immunoassay system (plasma) (Fig. 3). In hippocampus of AC animals, all pro-inflammatory markers (COX2, TNFα, NFκβ, IFN-γ, IL-1β, IL-5, and IL-6) increased significantly (vs. YC) ranging ~18% (IL-5) to 30% (COX2), while anti-inflammatory IL-10 and IL-11 were decreased (~20 and 30% respectively). In YE animals, treatment was able to significantly decrease IFN-γ protein levels by ~20% vs. YC. In AE animals, treatment significantly reduced the protein levels for all examined molecules (COX2, NFκβ, TNFα, IFN-γ, IL-5, IL-6 and IL-1β) by ~15–20%, while increased IL-10 and IL-11 by ~30% vs. AC group. Similar results were obtained from the Milliplex system assays in plasma of AC animals in all pro-inflammatory markers tested. TNFα, IFN-γ, IL-15, IL-3 and IL-1β, were increased significantly, while anti-inflammatory IL-1ra was decreased (vs. YC) ranging from ~150% for TNFα and IL-15; and up to ~200% for IL-1β, IL-3 and IFN-γ. In AE animals, treatment was able to significantly decrease all the cytokines almost at YC levels with the exception of IFN-γ, which only shown an important decrease trend and significantly increase the IL-1ra.
Figure 2.
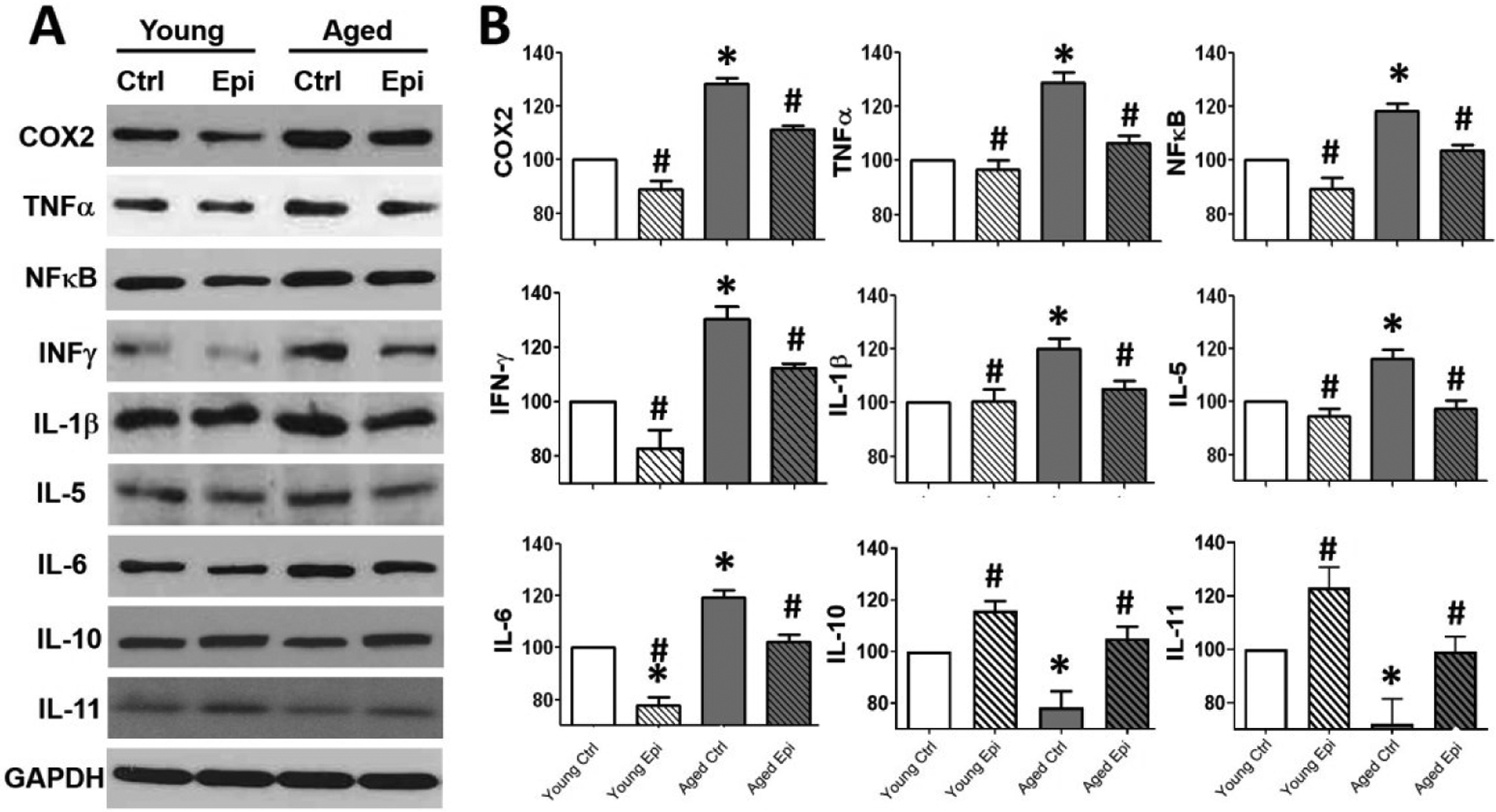
Inflammatory modulators and Interleukin(s) relative protein levels in the hippocampus of young and aged control (YC and AC) and epicatechin treated mice (YE and AE). (A) Representative Western blot images observed in COX2, TNF-α, NFκB, INF-γ, IL-1β, IL-5, IL-6, IL-10 and IL-11 protein levels. (B) Relative changes noted with YC values set as 100%. Protein levels were normalized using GAPDH values (n = 7/ group, *p < 0.005 vs. YC, #p < 0.05 vs. AC).
Figure 3.
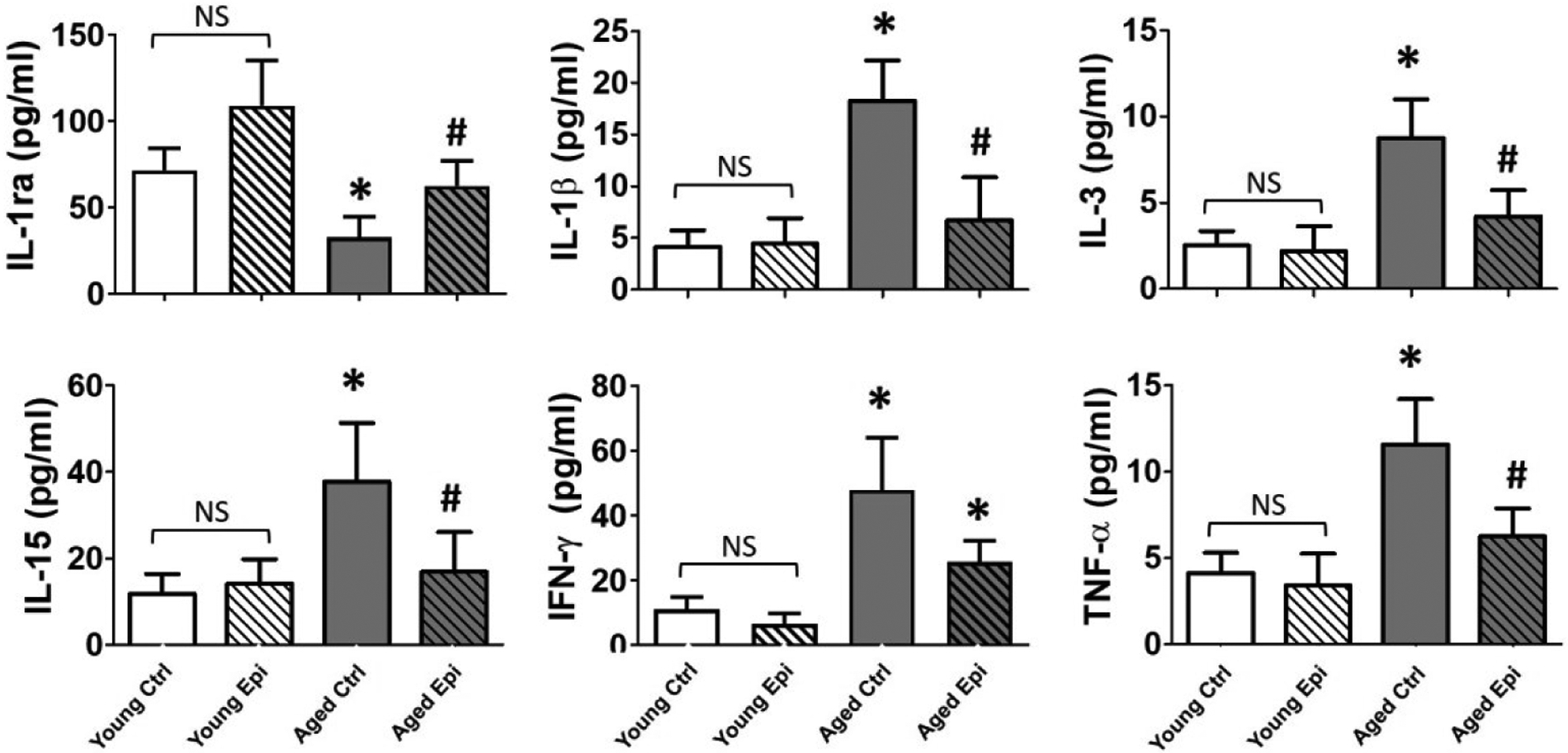
Pro and anti-inflammatory cytokines levels in plasma. Quantification of IL-1ra, IL-1β, IL-3, IL-15, IFN-γ and TNF-α plasma levels in young and aged control (YC and AC) and epicatechin treated mice (YE and AE) values (n = 7/ group, *p < 0.005 vs. YC, #p < 0.05 vs. AC).
Tau/β-amyloid pathway related endpoints
Tau hyperphosphorylation, (including its pathway modulators glycogen synthase kinase 3-β [GSK3β] and AKT) (Fig. 4A–C) and soluble β-amyloid (Aβ 42) (Fig. 5A–B) protein levels were measured. In AC animals, p-AKT and p-GSK3β protein levels decreased ~38% and ~47% (vs. YC) respectively (Fig. 4A and B), while hp-tau increased significantly ~90% (Fig. 4C). In young mice, Epi treatment (YE) significantly increased p-AKT and inhibitory p-Ser9-GSK3β levels to ~110% and ~95% respectively. In AE animal treatment significantly stimulated p-AKT (~40%) and p-GSK3β (~50%), while hp-tau was decreased (~60%) vs. AC. On the other hand, in AC animals, Aβ 42 increased significantly ~150% vs YC. While in AE animal treatment significantly decreased Aβ 42 by ~70% (Fig 5A–B). As a control for the Aβ 42 presence, APP knock out mice were used, and as expected Aβ 42 protein levels were undetectable (Fig 5A–B).
Figure 4.
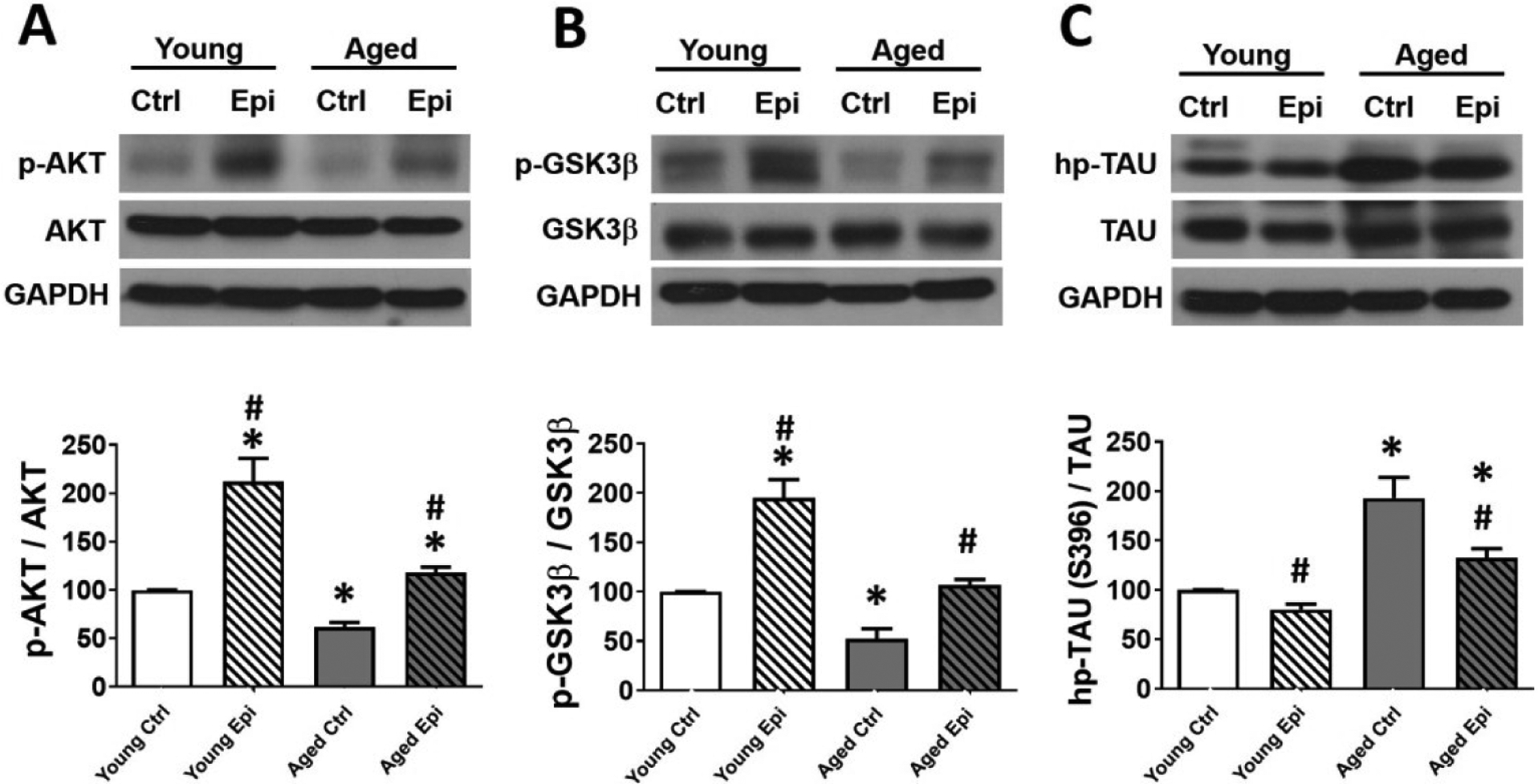
AKT, GSK3β and TAU total and phosphorylated relative protein levels in the hippocampus of young and aged control (YC and AC) and epicatechin treated mice (YE and AE). (A) Representative Western blot images observed in p-AKT (A), p-GSK3β (B) and hp-Tau (C). Protein levels are reported relative to total protein with YC values set as 100%. Protein levels were normalized using GAPDH values (n = 7/ group, *p < 0.05 vs. YC, #p < 0.05 vs. AC).
Figure 5.
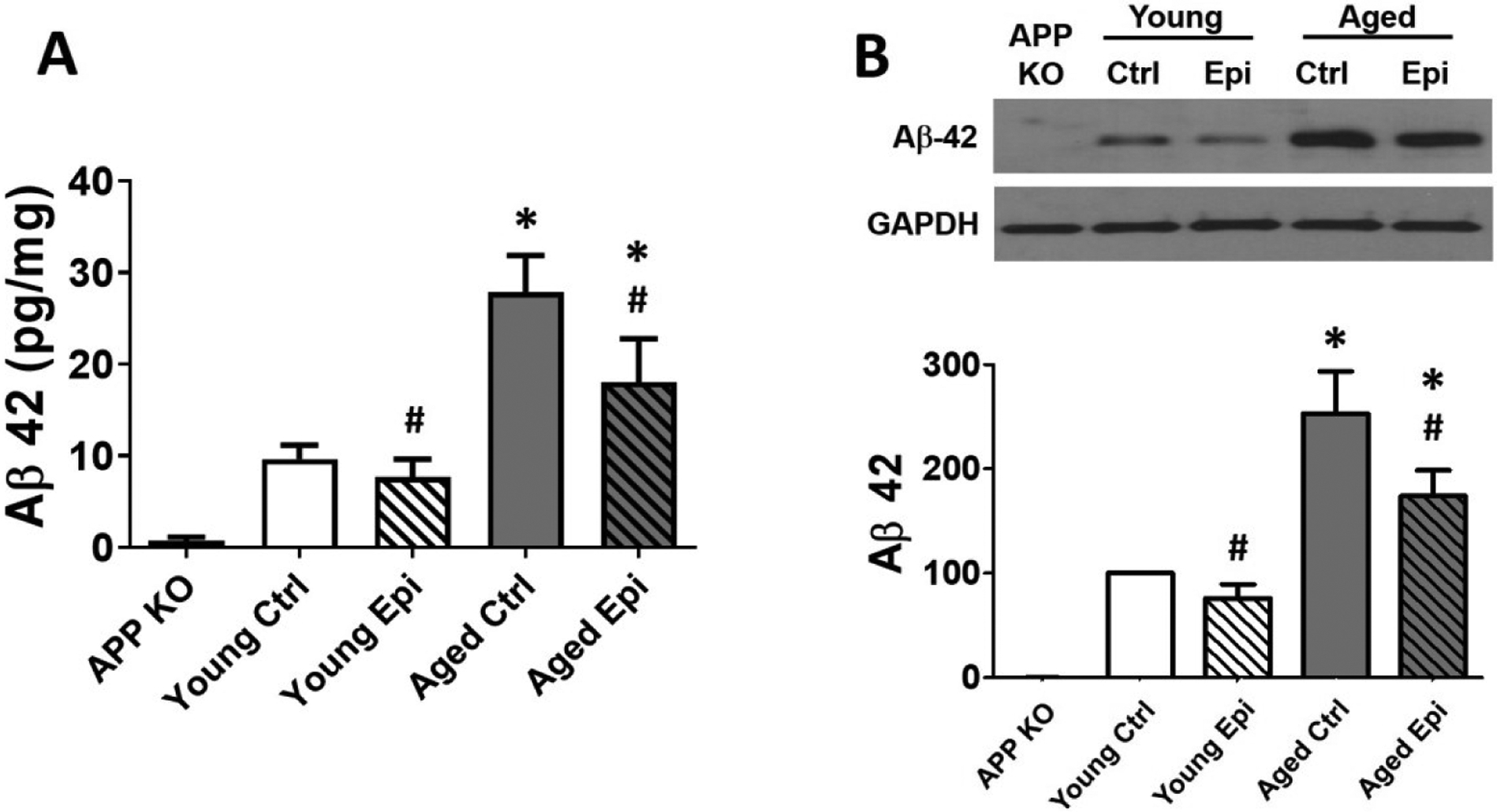
Aβ 42 protein levels of young and aged control (YC and AC) and epicatechin treated mice (YE and AE). (A) Aβ 42 protein levels in plasma samples by ELISA reported as pg of Aβ 42/mg of total protein. (B) Representative Western blot images and relative changes noted with YC values set as 100%. Protein levels were normalized using GAPDH values (n = 7/ group, *p < 0.005 vs. YC, #p < 0.05 vs. AC).
Neuronal viability/health
As an indicator of neuronal health, nerve growth factor (NGF) and TREM2 were measured (Fig. 6A). AC animals showed a ~50% and ~60% decrease in NGF and TREM2 levels respectively (vs. YC). However, Epi treatment significantly recovered NGF, and TREM2 levels in AE (~35%) vs. controls. Contrary glial associated activators GFAP and Iba1 protein levels increased ~30% and ~45% respectively in AC vs YC. (Fig. 6B); while Epi treatment significantly decreased GFAP and Iba1 by ~30% and ~40% respectively. Caspases (3, 9) activity as a surrogate of apoptosis was measured (Fig. 6C). In AC animals, caspase activity was increased (~180%) vs YC. AE animals showed a decreased activity in caspase 3 and 9, (~40% and ~45% respectively); however, such decrement does not reach levels as YC.
Figure 6.
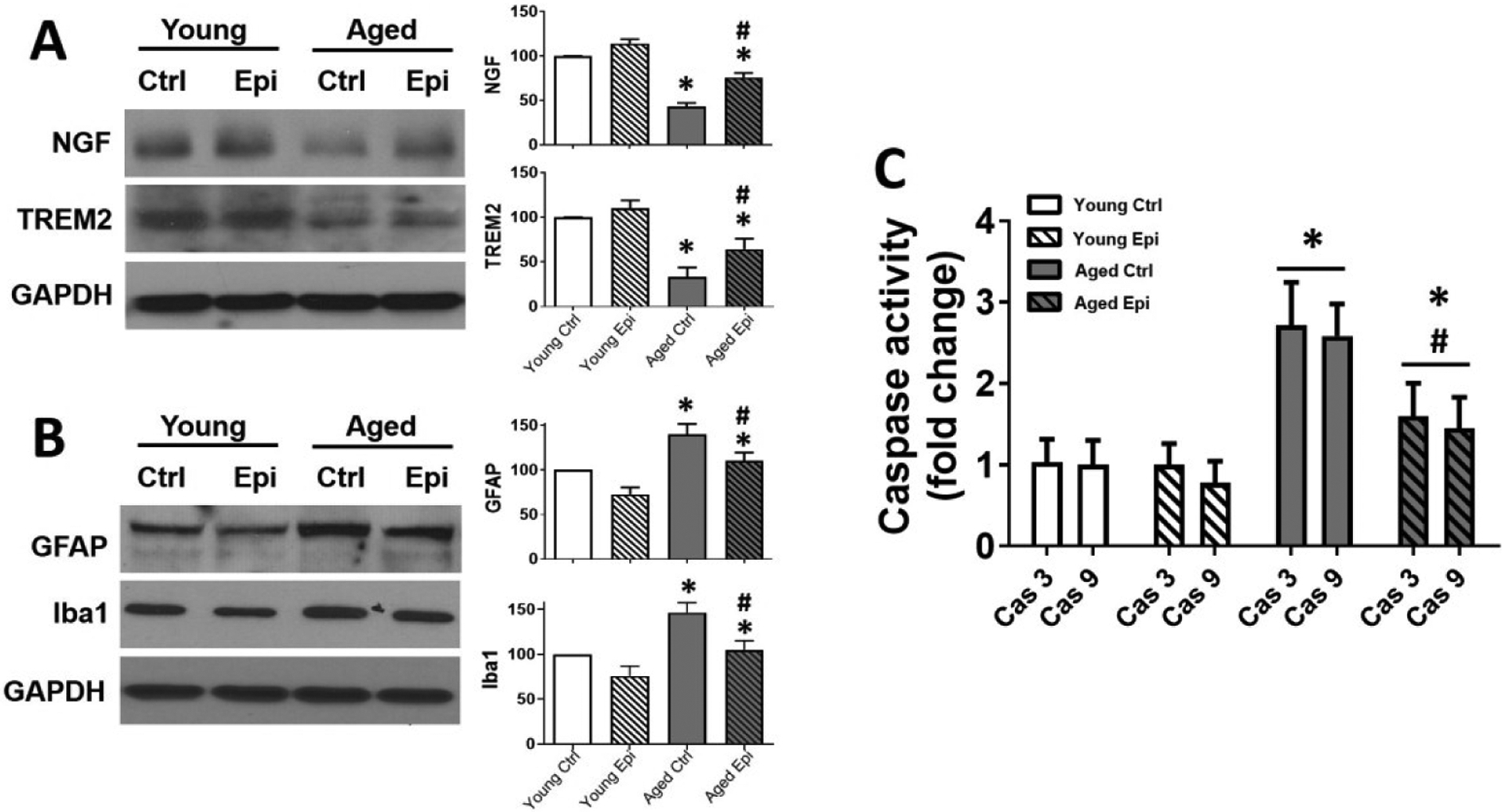
Protein levels of NGF, TREM2, GFAP and Iba1 and Caspase activity in hippocampus of young and aged control (YC and AC), and epicatechin treated mice (YE and AE). Representative Western blot images observed, for NGF, TREM2 (A) and GFAP and Iba1 (B). Relative changes noted with YC values set as 100%. Protein levels were normalized using GAPDH values. Caspase (Cas) 3 and 9 activity is reported as fold change (C) values (n = 7/ group, *p < 0.05 vs. YC, #p < 0.05 vs. AC).
OFT and ORT tests
The first important parameter in OFT (Fig. 7A) measurements is the total ambulatory distance in order to remove any confusing activity behavioral in test. Total distance traveled results demonstrated no differences in locomotor activity between control and Epi treated animals (Fig. 7B). OFT, used as an indicator of anxiety-like behavior, demonstrated a significant reduction in the outer zone vs. the center zone time with Epi treatment either in young or aged animals (Fig 7C).
ORT was performed to evaluate short and long-term memory in mice as shown in Fig. 8A. Epi treatment significantly improved the object preference score in young and aged animals vs. controls either in short (Fig. 8C) or long-term memory (Fig. 8D).
Discussion
Unique results provide evidence for the beneficial effects of Epi in alleviating OS, inflammation (including systemic indicators), markers of neurodegeneration and cell survival in the hippocampus leading to improved cognitive performance and anxiety measures in aged mice (Fig. 9). The described Epi effects may thus, partly account for the beneficial impact that cocoa consumption has on measures of brain health in mammals including humans.
Figure 9.
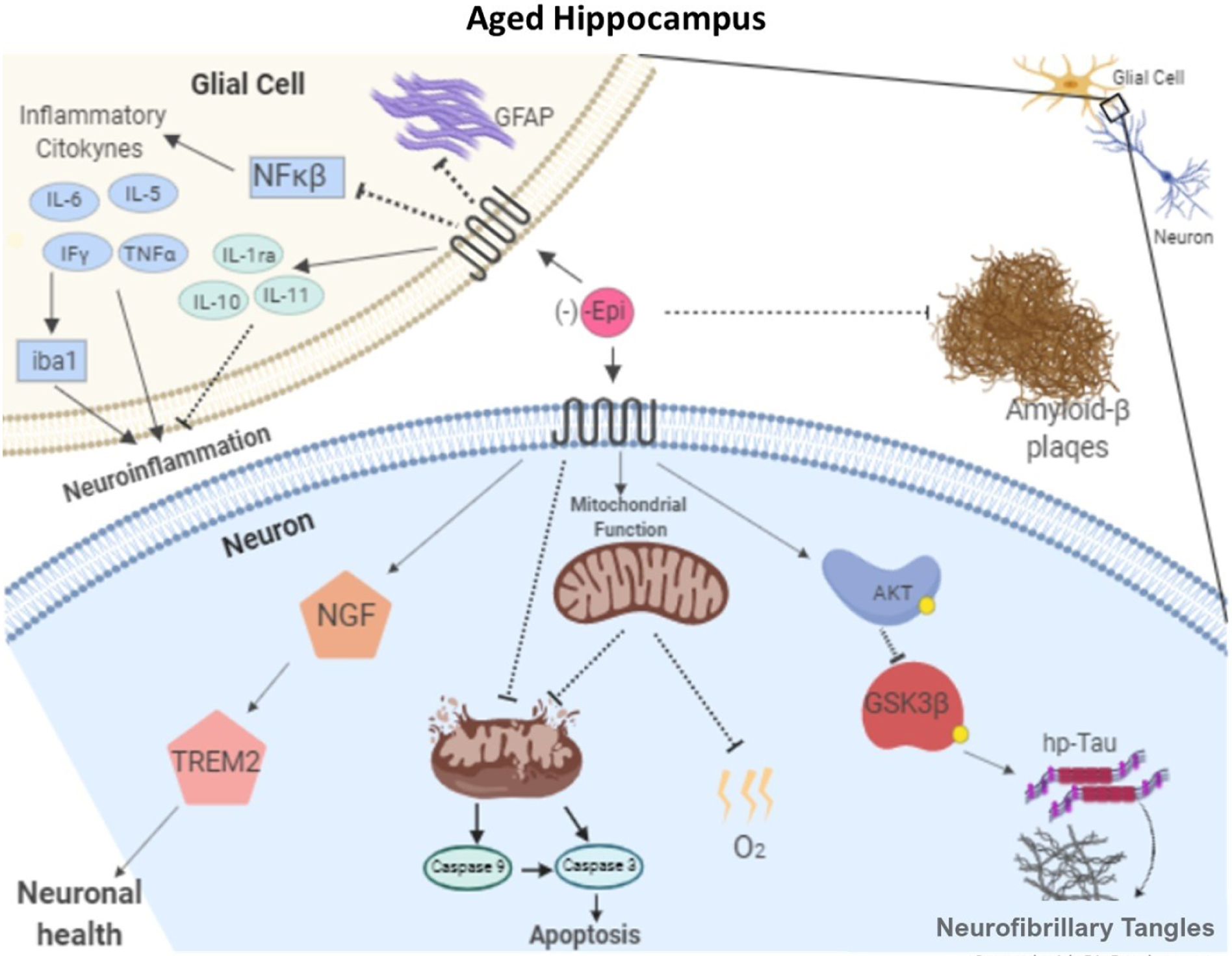
Schematic representation of proposed mechanisms for which epicatechin reduces neuroinflammation and Tau-hp, improving the noted endpoints such as memory and learning.
It is well accepted that longstanding OS can promote the development of neurodegeneration30,31. The aged brain may be particularly susceptible to oxidative damage due to high levels of ROS production32 and age-associated decline in ROS buffering capacity33. In this study, we provide evidence as to the beneficial effects that Epi has on protein and lipid oxidation endpoints both in young and senile mice. It is widely believed that antioxidants may exert beneficial effects on OS and that such actions may be secondary to the direct neutralization of ROS. In a previous publication, we demonstrated that in aged mice, Epi (at the same doses used in this study) can stimulate in a highly effective manner, frontal cortex protein and activity levels of the ROS catalysis system which is mainly comprised of Nrf2, sirtuin3, glutathione peroxidase, thioredoxin, catalase and superoxide dismutase 2 (SOD2). Furthermore, the levels of buffering systems such a reduced glutathione are also enhanced22. Thus, tissues that are exposed to high levels of ROS production when treated with Epi, can more effectively neutralize their reactivity. These results are similar to those reported using green tea epicatechin (EGCG) at 2 mg/kg/day which lowered lipid peroxidation and protein carbonylation levels in whole brain tissue from aged rats where proposed mechanisms were ascribed to the upregulation of SOD and catalase levels34. Similarly, studies using quercetin (albeit at higher doses, 50 mg/kg/day) in rats attenuate hippocampus and striatum swimming induced OS35.
The enhanced local and systemic production and release of pro-inflammatory molecules such as COX2, interferon-γ, TNF-α, IL-1β, IL-5 and IL-6 has been shown to increase as a function of aging and likely contribute to the development of neurodegeneration21. Key control elements such as NF-κB and AP-1 appear to play central roles in stimulating the production of such molecules. Published in vitro studies have reported on the anti-inflammatory potential of cocoa flavanols via the suppression of molecules such as IL-4 and TGF-β36. In fact, pure flavonoids (e.g., quercetin, genistein, hesperetin, epigallocatechin-3-gallate) or enriched-extracts, can reduce the expression of pro-inflammatory cytokines (IL-6, TNF-α, IL-1β and COX-2), down-regulate inflammatory markers and prevent neural damage. Studies using young (3 month old) female mice, have reported on the beneficial effects that Epi (2 weeks at a concentration equivalent to 100 mg/kg/day) has on genes associated with learning, neuroinflammation, neurodegeneration and cell death as per the microarray analysis of hippocampus samples21. The beneficial effects of Epi on neuroinflammation and neurodegeneration endpoints are similar to those noted on pro-inflammatory COX2, NFκβ, TNFα, INF-γ, IL-5, IL-6 and IL-1β and anti-inflammatory IL-10, IL-11 and IL-1ra in our study. However, there is a 100-fold difference in the dose used as we only treated animals with 1 mg/kg/day. Other flavonoids such as ECGC have demonstrated to possess anti-inflammatory potential. In a study, using a mouse model of amyotrophic lateral sclerosis treatment with ECGC at 10 mg/kg/day leads to the suppression of NF-kB protein levels in the spinal cord37. Quercetin also suppresses proinflammatory gene expression and reduces apoptosis in cocultures of microglial and neuronal cells38.
According to the “amyloid hypothesis” of AD progression39, the overproduction or failure of clearance of β-amyloid in the brain leads to its deposition and the progression of pathological events, including the formation of neurofibrillary tangles. Both processes are linked to the hp-tau. Signaling pathways associated with these events include GSK3β which appears to activate tau hyper-phosphorylation leading to the formation of neurofibrillary tangles in vivo. Opposite to this, is the inactivation of GSK3β through the PI3-kinase/AKT signaling pathway which is believed to prevent tau hyperphosphorylation and apoptosis40. Interestingly, previous studies have linked AKT-induced inhibition of GSK3β as a mechanism for Epi induced anxiolytic effects in young healthy mice following a 14-week treatment with Epi19. Our results are in accordance with such reports as Epi treatment significantly improved hippocampus p-AKT/AKT ratios and decreased p-GSK3β/GSK3β ratio in aged mice, demonstrating Epi effects of this pathway along with the decreased levels of Aβ 42 and hp-tau. A study by Phan et al., 2019, proved that flavonoids completely inhibited fibrillation of Aβ monomers and induced the formation of spherical, unstructured aggregates with less toxic fibrillary species in an in vitro model. Our results are consistent with them, in the extent of the reduced levels of Aβ 42 shown in treated animals; nevertheless could be interesting to evaluate the formation of these so called “unstructured aggregates”41.
NGF which promotes neuron growth is known to decrease in aged and AD brains, particularly in memory and cognitive-related areas such as the forebrain and hippocampus42 and is recognized as an important therapeutic target. Flavonoids such as quercetin and Epi have proven to induce neurite outgrowth in PC12 cells43. Moreover, studies of the diabetic retina of rats treated with quercetin evidence a significant increase in neurotrophic factors and reduced cytochrome c and caspase-3 activity44. Our results are in accordance with the aforementioned data, as NGF protein levels increased in Epi treated mice. TREM2 expression is important in limiting neuronal toxicity during the early stages of amyloid deposition. The absence of TREM2 expression on microglia impairs their capacity to phagocytose cell membrane debris and increases their production of pro-inflammatory cytokines6 Epi was capable to increase the levels of TREM2 in YE and SE in a significant manner indicating that flavonoids could have a direct role in the expression of TREM2 during neuroinflammation processes. More work needs to be done in that regard.
GFAP protein and Iba-1 are specific markers for activated astrocytes and microglia, respectively. Quercetin shows a strong anti-inflammatory action by suppressing activated astrocytosis and microgliosis45. Since our results showed decreased levels of GFAP and Iba-1 as seen in45 we can state that Epi treated groups have a suppressed activation of glial cells, therefore reducing antiinflamtory endpoints promoted by age.
Flavonoids have been observed to block oxidative-induced neuronal damage by preventing the activation of caspase-3, providing evidence in support of their potent anti-apoptotic action. In particular, Epi and 3-O-methyl-epicatechin also protect neurons against oxidative damage via a mechanism involving the suppression of JNK, and downstream partners, cjun and pro-caspase-316. Our results also show a decrease in Caspase 3 and Caspase 9 when animals were treated with Epi, promoting as is, neuronal cell survival even during aging. The loss of memory and increase in anxiety are hallmarks of the aging process and onset of neurodegenerative diseases and follow the pathophysiological changes described above including OS, neuroinflammation/degeneration, formation of β-amyloid plaques, hp-tau and formation of tangles. Evidence in humans has linked cocoa consumption to improved cognitive performance as well as increased cortical blood flow11. Published studies indicate that Epi, enhances mouse retention of spatial memory in water maze tests and the effects can be reinforced by the combination with exercise. Other flavonoids appear to exert similar effects. Two-month-old mice treated for 2 months with ECGC at 20 mg/kg/day increased proliferation labeled cells in the dentate gyrus leading to improved spatial recognition in mice46. In our model, Epi treatment significantly improved anxiety-like behavior as measured in OFT in aged animals, demonstrating a significant reduction in the outer zone vs. center zone time ratio. Also, in the ORT, Epi improved the object preference/recognition score, as a measure of short and long-term memory. Thus, Epi appears to have a role in preserving short and long-term memory while decreasing anxiety-like behavior levels as seen with aging.
Study results support a neuroprotective potential for the use of low dose Epi in the setting of aging induced brain function decline. Such effects can be attributed to the potential of the flavanol to effectively mitigate OS, inflammation and promoters of brain degeneration while encouraging those associated with neuron growth leading to functional improvements. Noteworthy, is that effects can also be noted in young animals thus, potentially promoting the preservation of brain health. The clinical relevance of such observations needs to be explored in properly designed clinical trials.
Acknowledgments
We kindly appreciate the technical support of Dr. T. Neri and Dr G. Manjarrez
Funding
This work was supported by NIH (DK98717, AG47326) and Department of Defense (PR150090) to Dr. Villarreal and Consejo Nacional de Ciencia y Tecnologia (CONACyT) 253769 to Dr. Ceballos and Instituto Politecnico Nacional (SIP-IPN 20180897) and CONACyT 283938 to Dr. Ramirez-Sanchez
Abbreviations
- TNFα
tumor necrosis factor α
- COX2
cyclooxygenase 2
- NF-κβ
nuclear factor kappa-light-chain-enhancer of activated B cells
- IL
interleukin
- AKT
protein kinase B
- p-AKT
phosphorylated protein kinase B
- AD
Alzheimer’s Disease
- PHF
paired helical filaments
- OXPHOS
oxidative phosphorylation
- ATP
adenosine triphosphate
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GSK3β
Glycogen synthase kinase 3 beta
- p-GSK3β
phosphorylated glycogen synthase kinase 3 beta
- ROS
reactive oxygen species
- CNS
central nervous system
- NFT
neurofibrillar tangles
- OS
oxidative stress
- Epi
(−)-epicatechin
- MDA
Malondialdehyde
- TBA
thiobarbituric acid
- PVDF
polyvinylidene difluoride
- HRP
horseradish peroxidase
- NGF
Nerve Growth Factor
- EDTA
ethylenediaminetetraacetic acid
- TBA
thiobarbituric acid
- TREM2
triggerin receptor expressed on myeloid cells2
- Cas
caspase
- IFN-γ
interferon gamma
- β-A
beta amyloid
- GFAP
glial fibrillary acidic protein
- WB
Western blot
- PMSF
phenylmethane sulfonyl fluoride
- ECL
enhanced chemiluminescence
- OFT
Open Field Task
- ORT
Object Recognition Task
- AU
arbitrary units
- Aβ
amyloid beta
- MAP
microtubule-associated protein
- IL-1ra
interleukin 1 receptor antagonist
- Iba1
ionized calcium-binding protein or allograft inflammatory factor 1
Footnotes
Conflicts of Interest
Dr. Villarreal (co-founder) and Dr. Ceballos are sotckholders of Epirium Inc
References
- 1.Barnham KJ, Masters CL and Bush AI, Neurodegenerative diseases and oxidatives stress, Nat. Rev. Drug Discov, 2004, 3, 205–214. [DOI] [PubMed] [Google Scholar]
- 2.Glass CK, Saijo K, Winner B, Marchetto MC and Gage H, Mechanisms Underlying Inflammation in Neurodegeneration, Nih, 2010, 140, 918–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harada CN, Natelson Love MC and Triebel K, Normal Cognitive Aging. Public Access, Clin. Geriatr. Med, 2013, 29, 737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckert A, Keil U, Marques CA, Bonert A, Frey C, Schüssel K and Müller WE, Mitochondrial dysfunction, apoptotic cell death, and Alzheimer’s disease, Biochem. Pharmacol, 2003, 66, 1627–1634. [DOI] [PubMed] [Google Scholar]
- 5.Terry AV, Kutiyanawalla A and Pillai Anilkumar, Age-dependent alterations in nerve growth factor (NGF)-related proteins, sortilin, and learning and memory in rats, Bone, 2008, 23, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raha AA, Henderson JW, Stott SRW, Vuono R, Foscarin S, Friedland RP, Zaman SH and Raha-Chowdhury R, Neuroprotective effect of TREM-2 in aging and Alzheimer’s disease model, J. Alzheimer’s Dis, 2016, 55, 199–217. [DOI] [PubMed] [Google Scholar]
- 7.O’Callaghan JP and Sriram K, Glial fibrillary acidic protein and related glial proteins as biomarkers of neurotoxicity, Expert Opin. Drug Saf, 2005, 4, 433–442. [DOI] [PubMed] [Google Scholar]
- 8.Kamphuis W, Mamber C, Moeton M, Kooijman L and Sluijs JA, GFAP Isoforms in Adult Mouse Brain with a Focus on Neurogenic Astrocytes and Reactive Astrogliosis in Mouse Models of Alzheimer Disease, PLoS One, 2012, 7, 42823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bisson JF, Nejdi A, Rozan P, Hidalgo S, Lalonde R and Messaoudi M, Effects of long-term administration of a cocoa polyphenolic extract (Acticoa powder) on cognitive performances in aged rats, Br. J. Nutr, 2008, 100, 94–101. [DOI] [PubMed] [Google Scholar]
- 10.Field DT, Williams CM and Butler LT, Consumption of cocoa flavanols results in an acute improvement in visual and cognitive functions, Physiol. Behav, 2011, 103, 255–260. [DOI] [PubMed] [Google Scholar]
- 11.Fisher NDL, Sorond FA and Hollenberg NK, Cocoa flavanols and brain perfusion, J. Cardiovasc. Pharmacol, 2006, 47, 210–214. [DOI] [PubMed] [Google Scholar]
- 12.Francis ST, Head K, Morris PG and Macdonald IA, The effect of flavanol-rich cocoa on the fMRI response to a cognitive task in healthy young people, J. Cardiovasc. Pharmacol, 2006, 47, 215–220. [DOI] [PubMed] [Google Scholar]
- 13.Engler MMB, Chen CY, Malloy MJ, Browne A, Chiu EY, Kwak H-K, Milbury P, Paul SM, Blumberg J and Mietus-Snyder ML, Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults., J. Am. Coll. Nutr, 2004, 23, 197–204. [DOI] [PubMed] [Google Scholar]
- 14.Heiss C, Dejam A, Kleinbongard P, Schewe T, Sies H and Kelm M, Vascular Effects of Cocoa Rich in Flavan-3-ols [6], J. Am. Med. Assoc, 2003, 290, 1030–1031. [DOI] [PubMed] [Google Scholar]
- 15.Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, Sies H, Kwik-uribe C, Schmitz HH and Kelm M, (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. [DOI] [PMC free article] [PubMed]
- 16.Vauzour D, Vafeiadou K, Rodriguez-Mateos A, Rendeiro C and Spencer JPE, The neuroprotective potential of flavonoids: A multiplicity of effects, Genes Nutr, 2008, 3, 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crichton GE, Elias MF and Alkerwi A, Chocolate intake is associated with better cognitive function: The Maine-Syracuse Longitudinal Study, Appetite, 2016, 100, 126–132. [DOI] [PubMed] [Google Scholar]
- 18.Faria A, Pestana D, Teixeira D, Couraud PO, Romero I, Weksler B, De Freitas V, Mateus N and Calhau C, Insights into the putative catechin and epicatechin transport across blood-brain barrier, Food Funct., 2011, 2, 39–44. [DOI] [PubMed] [Google Scholar]
- 19.Stringer TP, Guerrieri D, Vivar C and Van Praag H, Plant-derived flavanol (−)epicatechin mitigates anxiety in association with elevated hippocampal monoamine and BDNF levels, but does not influence pattern separation in mice, Transl. Psychiatry, 2015, 5, e493–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rendeiro C, Vauzour D, Rattray M, Waffo-Téguo P, Mérillon JM, Butler LT, Williams CM and Spencer JPE, Dietary Levels of Pure Flavonoids Improve Spatial Memory Performance and Increase Hippocampal Brain-Derived Neurotrophic Factor, PLoS One, 2013, 8, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Praag H, Lucero MJ, Yeo GW, Stecker K, Heivand N, Zhao C, Yip E, Afanador M, Schroeter H, Hammerstone J and Gage FH, Plant-derived flavanol (−)epicatechin enhances angiogenesis and retention of spatial memory in mice, J. Neurosci, 2007, 27, 5869–5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreno-Ulloa A, Nogueira L, Rodriguez A, Barboza J, Hogan MC, Ceballos G, Villarreal F and Ramirez-Sanchez I, Recovery of Indicators of Mitochondrial Biogenesis, Oxidative Stress, and Aging With (−)-Epicatechin in Senile Mice, Journals Gerontol. - Ser. A Biol. Sci. Med. Sci, 2015, 70, 1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nogueira L, Ramirez-Sanchez I, Perkins GA, Murphy A, Taub PR, Ceballos G, Villarreal FJ, Hogan MC and Malek MH, (−)-Epicatechin enhances fatigue resistance and oxidative capacity in mouse muscle, J. Physiol, 2011, 589, 4615–4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nogueira L, Ramirez-Sanchez I, a Perkins G, Murphy A, Taub PR, Ceballos G, Villarreal FJ, Hogan MC and Malek MH, (−)-Epicatechin enhances fatigue resistance and oxidative capacity in mouse muscle., J. Physiol, 2011, 589, 4615–4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stadtman E, Protein oxidation and aging, Science (80-. )., 1992, 257, 1220–1224. [DOI] [PubMed] [Google Scholar]
- 26.Ramírez-Sánchez I, Rodríguez A, Moreno-Ulloa A, Ceballos G and Villarreal F, (−)-Epicatechin-induced recovery of mitochondria from simulated diabetes: Potential role of endothelial nitric oxide synthase, Diabetes Vasc. Dis. Res, 2016, 13, 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roy V and Chapillon P, Further evidences that risk assessment and object exploration behaviours are useful to evaluate emotional reactivity in rodents, Behav. Brain Res, 2004, 154, 439–448. [DOI] [PubMed] [Google Scholar]
- 28.Seibenhener ML and Wooten MC, Use of the open field maze to measure locomotor and anxiety-like behavior in mice, J. Vis. Exp, 2015, 52434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balderas I, Rodriguez-Ortiz CJ, Salgado-Tonda P, Chavez-Hurtado J, McGaugh JL and Bermudez-Rattoni F, The consolidation of object and context recognition memory involve different regions of the temporal lobe, 2015, 618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ichiro Ikebe S, Tanaka M, Ohno K, Sato W, Hattori K, Kondo T, Mizuno Y and Ozawa T, Increase of deleted mitochondrial DNA in the striatum in Parkinson’s disease and senescence, Biochem. Biophys. Res. Commun, 1990, 170, 1044–1048. [DOI] [PubMed] [Google Scholar]
- 31.Pappolla MA, Omar RA, Kim KS and Robakis NK, Immunohistochemical evidence of oxidative [corrected] stress in Alzheimer’s disease., Am. J. Pathol, 1992, 140, 621–8. [PMC free article] [PubMed] [Google Scholar]
- 32.Gilgun-Sherki Y, Melamed E and Offen D, Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier, Neuropharmacology, 2001, 40, 959–975. [DOI] [PubMed] [Google Scholar]
- 33.Cho CG, Kim HJ, Chung SW, Jung KJ, Shim KH, Yu BP, Yodoi J and Chung HY, Modulation of glutathione and thioredoxin systems by calorie restriction during the aging process, Exp. Gerontol, 2003, 38, 539–548. [DOI] [PubMed] [Google Scholar]
- 34.Srividhya R, Jyothilakshmi V, Arulmathi K, Senthilkumaran V and Kalaiselvi P, Attenuation of senescence-induced oxidative exacerbations in aged rat brain by (−)-epigallocatechin-3-gallate, Int. J. Dev. Neurosci, 2008, 26, 217–223. [DOI] [PubMed] [Google Scholar]
- 35.Ishisaka A, Ichikawa S, Sakakibara H, Piskula MK, Nakamura T, Kato Y, Ito M, Miyamoto KI, Tsuji A, Kawai Y and Terao J, Accumulation of orally administered quercetin in brain tissue and its antioxidative effects in rats, Free Radic. Biol. Med, 2011, 51, 1329–1336. [DOI] [PubMed] [Google Scholar]
- 36.Selmi C, Mao TK, Keen CL, Schmitz HH and Gershwin ME, The Anti-inflammatory Properties of Cocoa Flavanols, Inflammation, 2006, 47, 163–171. [DOI] [PubMed] [Google Scholar]
- 37.Xu Z, Chen S, Li X, Luo G, Li L and Le W, Neuroprotective effects of (−)-epigallocatechin-3-gallate in a transgenic mouse model of amyotrophic lateral sclerosis, Neurochem. Res, 2006, 31, 1263–1269. [DOI] [PubMed] [Google Scholar]
- 38.Bureau G, Longpre F and Martinoli M-G, Resveratrol and Quercetin, Two Natural Polyphenols, Reduce Apoptotic Neuronal Cell Death Induced by Neuroinflammation Genevieve, J. Neurosci. Res, 2007, 2639, 2631–2639. [DOI] [PubMed] [Google Scholar]
- 39.Hardy J and Selkoe DJ, The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics, Science (80-. )., 2002, 297, 353–356. [DOI] [PubMed] [Google Scholar]
- 40.Hooper C, Killick R and Lovestone S, The GSK3 hypothesis of Alzheimer’s disease, J. Neurochem, 2008, 104, 1433–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phan HTT, Samarat K, Takamur Y, Azo-Oussou AF, Nakazono Y and Vestergaard MC, Polyphenols modulate alzheimer’s amyloid beta aggregation in a structure-dependent manner, Nutrients, , DOI: 10.3390/nu11040756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Middlemore-Risher ML, Adam BL, Lambert NA and Terry AV, Effects of chlorpyrifos and chlorpyrifos-oxon on the dynamics and movement of mitochondria in rat cortical neurons, J. Pharmacol. Exp. Ther, 2011, 339, 341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakajima KI, Niisato N and Marunaka Y, Quercetin stimulates NGF-induced neurite outgrowth in PC12 cells via activation of Na/K + /2Cl - cotransporter, Cell. Physiol. Biochem, 2011, 28, 147–156. [DOI] [PubMed] [Google Scholar]
- 44.Ola MS, Ahmed MM, Shams S and Al-Rejaie SS, Neuroprotective effects of quercetin in diabetic rat retina, Saudi J. Biol. Sci, 2017, 24, 1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khan A, Ali T, Rehman SU, Khan MS, Alam SI, Ikram M, Muhammad T, Saeed K, Badshah H and Kim MO, Neuroprotective effect of quercetin against the detrimental effects of LPS in the adult mouse brain, Front. Pharmacol, 2018, 9, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang JF, Schramm DD, Holt RR, Ensunsa JL, Fraga CG, Schmitz HH and Keen CL, A Dose-Response Effect from Chocolate Consumption on Plasma Epicatechin and Oxidative Damage, J. Nutr, 2000, 130, 2115S–2119S. [DOI] [PubMed] [Google Scholar]


