Abstract
Aim: To determine the prevalence of pulmonary hypertension (PH) and its associated factors among end-stage renal disease (ESRD) patients who underwent maintenance dialysis.
Methods: A total of 491 patients received echocardiography examinations and underwent pulmonary artery systolic pressure (PASP) assessments. A subgroup of 283 patients were subjected to plasma creatinine (Cr) and blood urea nitrogen concentration (BUN) tests, routine blood examinations and electrolyte analysis. First, we compared the differences in echocardiographic, Cr and BUN, blood routine and electrolyte parameters between PH and non-PH groups. The correlations between PASP and the parameters mentioned above were also analyzed. Furthermore, univariate and adjusted logistic regression analyses were performed to identify the independent associated factors.
Results: The incidence of PH among ESRD patients who were treated with maintenance dialysis was 34.6%. Most of the echocardiographic parameters, including end-diastolic internal diameters of the left atrium, left ventricle, right atrium, and pulmonary artery, as well as interventricular septum mobility, left ventricular posterior wall mobility, fractional shortening, stroke volume and left ventricle ejection fraction (LVEF), were associated with PH. Furthermore, Mg2+ (p = 0.037) and Cl− (p = 0.043) were significantly associated with PASP. However, after adjustments were made in the regression analysis, only internal diameters of the left atrium, right atrium, and LVEF were independently associated with PH.
Conclusion: PH is prevalent, with a relatively high incidence among ESRD patients who undergo maintenance dialysis. The sizes of the left and right atria as well as LVEF were independently associated with PH, but further cohort and basic mechanistic studies are needed to confirm this finding.
Keywords: prevalence, pulmonary hypertension, maintenance dialysis, end-stage renal disease (ESRD), a retrospective study
Introduction
Pulmonary hypertension (PH) is reported to be one of the most frequent complications among various diseases including end-stage renal disease (ESRD) and is characterized by progressively increased pulmonary arterial pressure (PAP) (1–4). PH is the leading cause of right heart failure among cardiovascular diseases (1, 5, 6). Additionally, PH is recognized to be a novel threat for ESRD patients (7). It is reported that the incidence of PH is between 9 and 39% among patients with stage 5 chronic kidney disease, this value ranges from 8.8 to 68.8% among ESRD patients who undergo hemodialysis (2). Whilst, the incidence of PH is up to 42% among peritoneal dialysis-treated patients (8–11).
However, the associated risk factors and precise mechanisms underlying PH in ESRD patients have not been specifically addressed. Some researchers have suggested that the volume overload, severe anemia, hemodynamic modifications, hyperparathyroidism induced by renal failure may be involved in the development of PH (4, 10, 12, 13). Left heart dysfunction in ESRD patients has also been considered to be the underlying cause of PH (14). Furthermore, the respiratory system related diseases such as chronic obstructive pulmonary disease have also been indicated to the important causes for PH.
Although a few previous studies have reported some risk factors for PH in ERSD which belong to the V classification PH (PH with multiple-reason/unclear mechanisms/ caused by renal dysfunction) (15). This kind of PH may be caused by toxicity of the metabolic wastes which cannot been excreted in renal failure patients (16, 17). Furthermore, the blood components and electrolytes may be also the causes of PH (18). There are still many factors have not been reported in renal failure patients with PH.
Thus, we conducted this retrospective study with a large size of population from March 2011 to June 2019 in our dialysis center to determine the prevalence of PH and its associated factors.
Methods
Study Design and Population
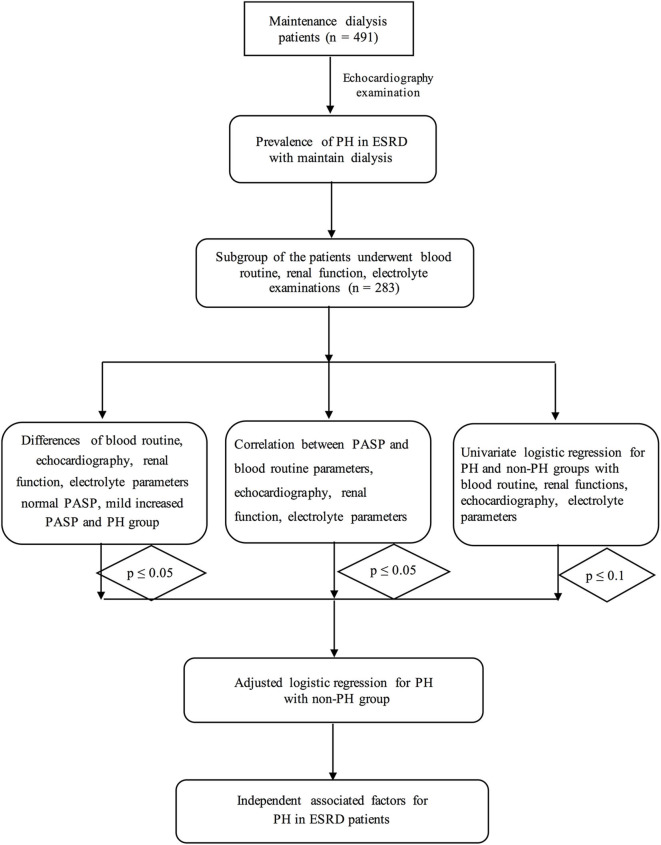
This was a retrospective study of patients with ESRD who underwent maintenance dialysis from March 2011 to June 2019 in the Nephrology of Chongqing and Kidney Center of PLA, Xinqiao Hospital, Army Medical University (Third Military Medical University). A total of 491 ERSD patients who underwent hemodialysis were selected for this study and underwent echocardiography examination according to the inclusion and exclusion criteria. Exclusion criteria included: previous pulmonary embolism, collagen vascular disease, obstructive sleep apnea, chronic obstructive pulmonary disease (COPD) as well as the moderate/severe mitral or aortic valve diseases. Furthermore, patients with liver failure or heart failure have also been excluded. A subgroup of 283 patients received Cr and BUN tests, routine blood examinations and electrolyte analysis. The procedure is shown in Figure 1.
Figure 1.
The flow chart of this study.
Each patient was completely informed of the purpose and procedure of the study and provided written informed consent. The current study was designed in compliance with the Declaration of Helsinki with respect to human research and was approved by the ethics committee of The Second Affiliated Clinic Hospital (Xinqiao Hospital), Army Medical University (Third Military Medical University).
The Echocardiography Examination
The echocardiographic examinations were performed by our skilled echocardiographist, Dr. Rongsheng Rao, according to the American Society of Echocardiography recommendations (19) in the morning after dialysis after been puncture to obtain fast venous blood samples. The patients were positioned in the supine position after a 10-min rest period while undergoing the echocardiography examination. Two-dimensional echocardiographically guided M-mode images were recorded from standardized views by using an ultrasonography system (CX50, Philips, USA) with a S5 probe. The end-diastolic internal diameters of the left atrium (LA), left ventricle (LVDD), right atrium (RA), right ventricle (RV), and pulmonary artery (PA) were measured. Furthermore, the interventricular septum (IVS) and its mobility, left ventricular posterior wall (LVPW) and its mobility, fractional shortening (FS), stroke volume (SV), and ejection fraction (EF) were also measured and recorded. In addition, tricuspid regurgitation (TR)-related parameters including TR area (TRA), TR velocity (TRV was used to calculate the PAP), and TR pressure were also examined.
Biomarker Variable Determination
The patients' venous blood samples were collected in the early morning after fasting (>12 h). First, routine blood examinations were performed. The RBC count (RBC), mean corpuscular volume (MCV), hematocrit (HCT), hemoglobin concentration (Hb), red blood cell distribution width (RDW), white blood cell count (WBC), platelet (PLT) count, mean corpuscular hemoglobin concentration (MCHC), and mean corpuscular hemoglobin (MCH) were tested via an automated hematology corpuscle analyzer (AU400; Olympus Optical, Co., Tokyo, Japan). In addition, the lymphocyte ratio (LYM), monocyte ratio (MONO), neutrophil ratio (NEU), eosinophil ratio (EO), and basophil ratio (BAS) were calculated.
Next, plasma creatinine (Cr, enzyme assay) was also measured using Roche Diagnostics GmbH productions (Abbott, i2000, America). The sodium concentration (Na+) was determined by using indirect ion selective electrode assay (EX-Z, JOKOH, Japan). Tri-azo methods were used to measure the serum calcium concentration (Ca2+), while the phosphomolybdate ultraviolet method was employed to determine phosphate concentration (P) (Roche Diagnostics GmbH, America). In addition, the blood urea nitrogen concentration (BUN) and serum potassium concentration (K+) were also measured (FERENE methods, Beckman AU5821).
Each biochemical variable was measured from blood specimens in the Clinical Laboratory Department of the Second Affiliated Clinical College (Xinqiao Hospital).
Variable Definitions
First, pulmonary artery systolic pressure (PASP) was calculated by the modified Bernoulli equation using the tricuspid systolic jet according to the recommendation by the American Society of Echocardiography: PASP = 4 × (TRV)2+ estimated right atrial pressure.
The estimated right atrial pressure was 5, 10, and 15 mmHg when the right atrium was normal, mildly enlarged, and significantly enlarged, respectively.
PH was defined as a PASP greater than 35 mmHg, while a PASP less than 35 mmHg is defined as normal non-PH patients according to the American Society of Echocardiography and other studies (19–22).
The severe increased PASP group is confirmedly defined PASP is more than 50 mmHg while PASP is between 36 and 50 mmHg is considered as mild increased PASP, other patients have been included into the normal PASP group according to the American Society of Echocardiography and other researches.
Statistical Analysis
A continuous variable was described as the mean ± standard deviation (SD) if it was normally distributed. These variables were compared by using independent student's t-tests between non-PH and PH groups. The non-normally distributed variables are expressed as medians (25–75%), and these variables were compared with Non-parametric tests between PH and non-PH groups.
Univariate logistic regression analyses were primarily used to screen the associated factors for PH. A variable with a p-value < 0.05 in the univariate logistic regression was included in the multivariate (adjusted) logistic regression analyses to identify independent associated factors for PH. All of the statistical analyses were performed with the statistical software SPSS 22.0 (California, USA) for MAC.
Results
The Basic Information and Prevalence of PH in the Different Populations
A total of 491 patients were included in our study. The mean age was 52.95 ± 14.21 years, and the mean BMI was 21.89 ± 4.89 kg/m2. The incidence of PH among ESRD patients who were treated with maintenance dialysis was 34.6% (170/491). Most of the patients had a normal PASP (65.4%, 321/491). These results are shown in Figure 2.
Figure 2.
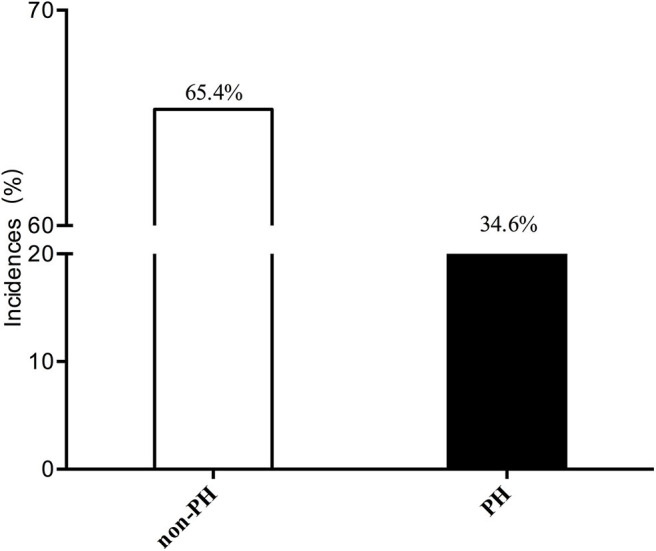
Incidence of PH in ESRD patients.
The Differences in Cr and BUN, Echocardiographic, Blood Routine, and Electrolyte Parameters Between PH and non-PH Groups
In our present study, there were no differences in demographic data (age and BMI, p > 0.05) between the non-PH and PH groups.
Regarding the echocardiographic parameters, we first found differences in the structure and function of the left heart. We discovered that LA (42.06 ± 5.50 vs. 36.38 ± 4.56 mm) and LVDD (52.83 ± 6.71 vs. 47.73 ± 5.02 mm) were significantly higher in the PH group than in the normal PASP group (all p-values < 0.001, Table 1). The LVPW was significantly thinner in the PH group than that in the non-PH group between non-PH and PH groups (p =0.014), its mobility was more dispersive in the PH group than in the non-PH group (p < 0.001). However, the IVS was not different between the abovementioned two groups, and its mobility showed significantly more dispersion in the PH populations (all p-values < 0.001). The left heart function and LVEF were lower in the PH group (55.78 ± 12.06%) group than in the non-PH group (63.39 ± 7.26% p < 0.001). In accordance with LVEF, FS was lower in the PH group [22(28.0–35.0)], while it was significantly higher in the non-PH group [35.00 (32.25–37.00), p < 0.001]. However, the SV of the PH population was significantly higher than that of the non-PH group (p < 0.001). Regarding the right heart, RA (35.13 ± 3.42 vs. 41.05 ± 5.46 mm) and RV (34.01 ± 3.09 vs. 38.42 ± 4.83 mm) were significantly smaller in the non-PH patients than that in the PH ones (both p < 0.001, Table 1). Furthermore, the TR-related parameters, including TRA, TRV and ΔP, also revealed statistically significant differences between PH and non-PH groups (Table 1).
Table 1.
Differences of the baseline parameters between PH and non-PH patients.
| PH group (n = 111) | Non-PH group (n = 172) | p-value | |
|---|---|---|---|
| DEMOGRAPHIC DATA | |||
| Age (years) | 52.42 ± 15.07 | 52.77 ± 13.79 | 0.844 |
| BMI (kg/m2) | 22.10 ± 3.35 | 22.66 ± 3.80 | 0.202 |
| Gender (female, %) | 38(34.2%) | 69(40.1%) | 0.380 |
| MEDICAL HISTORY | |||
| Hypertension (n, %) | 74(66.7%) | 110(64.0%) | 0.799 |
| ACEI/ARB | 27(24.3%) | 35(20.3%) | 0.463 |
| CCB | 48(43.2%) | 67(38.9%) | 0.536 |
| Diabetes (n, %) | 19(17.1%) | 25(14.5%) | 0.615 |
| ECHOCARDIOGRAPHIC PARAMETERS | |||
| LA (mm) | 42.06 ± 5.50 | 36.38 ± 4.56 | <0.001** |
| LVDD (mm) | 52.83 ± 6.71 | 47.73 ± 5.02 | <0.001** |
| RA (mm) | 41.05 ± 5.46 | 35.13 ± 3.42 | <0.001** |
| RV (mm) | 38.42 ± 4.83 | 34.01 ± 3.09 | <0.001** |
| PA (mm) | 24.90 ± 2.54 | 23.27 ± 2.32 | <0.001** |
| IVS (mm) | 12.48 ± 1.54 | 12.49 ± 1.67 | 0.951 |
| IVS mobility | 6.0(6.0–6.0) | 6.0(6.0–6.0) | <0.001** |
| LVPW (mm) | 12.0(10.0–12.4) | 11.0(10.0–12.0) | 0.014* |
| LVPW mobility | 10.0(9.6–10.0) | 10.0(10.0–10.0) | <0.001** |
| FS (%) | 22(28.0–35.0) | 35.00 (32.25–37.00) | <0.001** |
| LVEF (%) | 55.78 ± 12.06 | 63.39 ± 7.26 | <0.001** |
| SV(mL) | 80.40 ± 21.66 | 74.40 ± 17.91 | 0.012* |
| TRA(cm2) | 4.00(2.00–6.60) | 1.00(1.00–1.88) | <0.001** |
| TRV (cm/s) | 324.0(298.0–362.0) | 245.00(228.75–255.00) | <0.001** |
| ΔP(mmHg) | 42.0(35.0–53.0) | 24.00(21.00–25.25) | <0.001** |
| CR AND BUN | |||
| Cr (μmol/L) | 814.40 (626.90–1019.30) | 821.80 (613.38–1016.85) | 0.799 |
| BUN (μmoI/L) | 21.90 ± 8.11 | 21.66 ± 7.66 | 0.805 |
| ELECTROLYTE PARAMETERS | |||
| Mg2+ (mmol/L) | 1.000 ± 0.142 | 0.970 ± 0.167 | 0.123 |
| K+ (mmol/L) | 4.80 ± 0.83 | 4.79 ± 0.76 | 0.893 |
| Ca2+ (mmol/L) | 2.15 (1.98–2.31) | 2.15 (2.04–2.28) | 0.343 |
| Na+(mmol/L) | 137.8 (136.6–139.7) | 137.9(136.2–140.0) | 0.743 |
| Cl− (mmol/L) | 103.60 (100.70–106.20) | 102.15 (99.10–104.75) | 0.016* |
| TCO2 (mmol/L) | 20.21 ± 3.69 | 20.95 ± 4.30 | 0.136 |
| P (mmol/L) | 1.87 ± 0.64 | 1.94 ± 0.64 | 0.398 |
| Dialysis time (days) | 1314.0 (522.00–2635.0) | 1216.0 (529.50–2300.50) | 0.225 |
BAS, basophil ratio; BUN, blood urea nitrogen concentration; Ca2+, serum calcium concentration; Cr, plasma creatinine; ESRD, end-stage renal disease; EO, eosinophil ratio; FS, fractional shortening; Hb, hemoglobin concentration; HCT, hematocrit; IVS, interventricular septum; K+, serum potassium concentration; LA, left atrium end-diastolic internal diameter; LVDD, left ventricle end-diastolic internal diameter; LVEF, ejection fraction of left ventricle; LVPW, left ventricular posterior wall; LYM, lymphocyte ratio; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; MONO, monocyte ratio; Na+, sodium concentration; NEU, neutrophil ratio; P, phosphate concentration; PA, pulmonary artery end-diastolic internal diameter; PAP, pulmonary arterial pressure; PASP, pulmonary artery systolic pressure; PH, pulmonary hypertension; PLT, platelet; RA, right atrium end-diastolic internal diameter; RBC, red blood cell count; RDW, red blood cell distribution width; RV, right ventricle end-diastolic internal diameter; SV, stroke volume; TR, tricuspid regurgitation; TRA, TR area; TRV, TR velocity; WBC, white blood cell count; ACEI,ARB, CCB.
p < 0.05,
p < 0.01.
We further investigated the differences in Cr and BUN among the three groups. However, we did not find any differences in Cr or BUN between the PH and non-PH populations (all p-values > 0.05). Similarly, regarding electrolytes, the levels of Mg2+, K+, Ca2+, Na+, TCO2, and P were not different between the two groups. However, we found that the PH patients were characterized with a higher Cl− concentration [103.60 (100.70–106.20) vs. 102.15 (99.10–104.75), p = 0.016, Table 1].
Finally, we examined the differences in routine blood examination parameters. The RBC and Hb-related parameters, RBC, MCV, HCT, MCH, MCHC, RDW-CV, and RDW-SD showed no differences PH and non-PH groups (all p-values > 0.05). In addition, the WBC and PLT count were not significantly different between the two groups mentioned above. The ratios of the other cell types, including the EO, BASO, MONO, NEU, and LYMP, in the PH group did not differ from non-PH group (all p-values > 0.05, Supplementary Table 1).
Furthermore, we have also compared the abovementioned parameters among normal PASP, mild increased PASP and severe increased PASP groups. The results have been listed in Supplementary Table 2.
Correlations Between PASP and Demographic, Echocardiographic, Blood Routine, and Electrolyte Parameters as Well as Cr and BUN
We further analyzed the associations between PASP and demographic, echocardiographic, blood routine, and electrolyte parameters as well as Cr and BUN. First, regarding the demographic data, neither age (r = −0.034, p = 0.637) nor BMI (r = −0.052, p = 0.385) were correlated with PASP. Similarly, the dialysis time showed no association with PASP (r = 0.098, p = 0.101).
Importantly, we found that most of the echocardiographic parameters were associated with PASP. The structures of both the left and right heart, including LA (r = 0.549, p < 0.001), LVDD (r = 0.515, p < 0.001), FS (r = −0.323, p < 0.001), RA (r = 0.600, p < 0.001), and RV (r = 0.588, p < 0.001), showed a statistically significant relationship with PASP. Furthermore, the functions of the left heart, including LVEF (r = −0.421, p < 0.001) and SV (r = 0.158, p < 0.027), as well as the mobility of IVS (r = −0.333, p < 0.001), were also significantly correlated with PASP. TR-related parameters, TRA (r = 0.640, p < 0.001), TRV (r = 0.996, p < 0.001), and ΔP (r = 0.997, p < 0.001), were also related to PASP.
In our study, neither Cr nor BUN were correlated with PASP (all p-values were greater than 0.05). However, regarding the serum sodium ion concentrations, we found that Mg2+ (r = 0.149, p = 0.037) and Cl− (r = 0.144, p = 0.043) were significantly associated with PASP. None of the following ion concentrations showed statistical associations with PASP: K+, Ca2+, Na+, TCO2, or P (all of the p-values were greater than 0.05, Table 2).
Table 2.
Relationship between PASP and other parameters.
| Relationship with PASP | ||
|---|---|---|
| Parameters | r | p-value |
| Demographic data | ||
| Age (years) | −0.034 | 0.637 |
| BMI (kg/m2) | −0.052 | 0.385 |
| ECHOCARDIOGRAPHIC PARAMETERS | ||
| LA (mm) | 0.549 | <0.001 |
| LVDD (mm) | 0.515 | <0.001 |
| RA (mm) | 0.600 | <0.001 |
| RV (mm) | 0.588 | <0.001 |
| PA (mm) | 0.383 | <0.001 |
| IVS (mm) | 0.026 | 0.714 |
| IVS mobility | −0.333 | <0.001 |
| LVPW (mm) | 0.116 | 0.104 |
| FS (%) | −0.323 | <0.001 |
| LVEF (%) | −0.421 | <0.001 |
| SV(mL) | 0.158 | 0.027 |
| TRA(cm2) | 0.640 | <0.001 |
| TRV (cm/s) | 0.996 | <0.001 |
| ΔP(mmHg) | 0.997 | <0.001 |
| CR AND BUN | ||
| Cr (μmol/L) | −0.007 | 0.921 |
| BUN (μmoI/L) | −0.012 | 0.864 |
| ELECTROLYTE PARAMETERS | ||
| Mg2+ (mmol/L) | 0.149 | 0.037 |
| K+ (mmol/L) | 0.040 | 0.576 |
| Ca2+ (mmol/L) | 0.007 | 0.927 |
| Na+(mmol/L) | −0.015 | 0.830 |
| Cl− (mmol/L) | 0.144 | 0.043 |
| TCO2 (mmol/L) | −0.110 | 0.125 |
| P (mmol/L) | −0.008 | 0.927 |
| Dialysis time (days) | 0.098 | 0.101 |
This table shows the Spearman's correlations between PASP and the related factors.
Finally, neither the RBC-related parameters nor WBC-related parameters showed any correlations with PASP. Additionally, neither the PLT count nor the ratios of other cell types were correlated with PASP (all p-values > 0.05, Supplementary Table 3).
Logistic Regression for PH Using Demographic, Echocardiographic, Blood Routine, and Electrolyte Parameters as Well as Cr and BUN
First, we performed univariate logistic regression for PH using each variable. Neither age nor BMI showed any association with PH.
In the univariate logistic regression analysis, we found that LA, LVDD, RA, RV, PA, FS, IVS mobility, and LVEF were screened to be potential associated factors for PH (all of the p-values were less than 0.001, Table 3). Additionally, SV (OR: 1.016, 95%CI: 1.003–1.027, p = 0.013), LVPW (OR: 1.100, 95%CI: 1.028–1.399, p = 0.021), and its mobility (OR: 0.693, 95%CI: 0.567–0.848, p < 0.001) were also potential associated factors for PH.
Table 3.
Univariate logistic analysis for PH with non-PH patients.
| β | p-value | OR | 95CI% | ||
|---|---|---|---|---|---|
| Parameters | Lower borderline | Upper borderline | |||
| DEMOGRAPHIC DATA | |||||
| Age (years) | −0.002 | 0.843 | 0.998 | 0.982 | 1.015 |
| BMI (kg/m2) | −0.044 | 0.202 | 0.957 | 0.895 | 1.024 |
| ECHOCARDIOGRAPHIC PARAMETERS | |||||
| LA (mm) | 0.239 | <0.001 | 1.270 | 1.191 | 1.355 |
| LVDD (mm) | 0.148 | <0.001 | 1.159 | 1.106 | 1.302 |
| RA (mm) | 0.309 | <0.001 | 1.362 | 1.258 | 1.475 |
| RV (mm) | 0.301 | <0.001 | 1.351 | 1.241 | 1.471 |
| PA (mm) | 0.277 | <0.001 | 1.319 | 1.181 | 1.473 |
| IVS (mm) | −0.005 | 0.951 | 0.958 | 0.858 | 1.154 |
| IVS mobility | −0.563 | <0.001 | 0.570 | 0.422 | 0.769 |
| LVPW (mm) | 0.182 | 0.021 | 1.100 | 1.028 | 1.399 |
| LVPW mobility | −0.366 | <0.001 | 0.693 | 0.567 | 0.848 |
| FS (%) | −0.125 | <0.001 | 0.883 | 0.843 | 0.924 |
| LVEF (%) | −0.086 | <0.001 | 0.917 | 0.889 | 0.946 |
| SV(mL) | 0.016 | 0.013 | 1.016 | 1.003 | 1.027 |
| TRA(cm2) | 1.227 | <0.001 | 3.410 | 2.511 | 4.633 |
| Dialysis time (days) | <0.001 | 0.192 | 1.000 | 1.000 | 1.000 |
| CR AND BUN | |||||
| Cr (μmol/L) | 0.000 | 0.717 | 1.000 | 0.999 | 1.001 |
| BUN (μmoI/L) | 0.004 | 0.804 | 1.004 | 0.974 | 1.035 |
| ELECTROLYTE PARAMETERS | |||||
| Mg2+ (mmol/L) | 1.9192 | 0.125 | 3.295 | 0.718 | 15.115 |
| K+ (mmol/L) | −0.021 | 0.893 | 1.021 | 0.754 | 1.384 |
| Ca2+ (mmol/L) | −0.363 | 0.477 | 0.695 | 0.255 | 1.893 |
| Na+(mmol/L) | −0.033 | 0.423 | 0.968 | 0.893 | 1.048 |
| Cl− (mmol/L) | 0.047 | 0.097 | 1.048 | 0.992 | 1.107 |
| TCO2 (mmol/L) | −0.045 | 0.136 | 0.956 | 0.900 | 1.014 |
| P (mmol/L) | −0.163 | 0.397 | 0.849 | 0.583 | 1.239 |
| BLOOD ROUTINE EXAMINATION PARAMETERS | |||||
| RBC (1012 /L) | 0.086 | 0.610 | 1.090 | 0.783 | 1.517 |
| Hb (g/dL) | 0.005 | 0.411 | 1.005 | 0.993 | 1.018 |
| Hct (L/L) | 0.018 | 0.362 | 1.018 | 0.979 | 1.059 |
| MCV(fl) | −0.010 | 0.512 | 1.010 | 0.980 | 1.041 |
| MCH (pg) | −0.019 | 0.681 | 1.019 | 0.932 | 1.114 |
| MCHC(g/dL) | −0.004 | 0.663 | 0.996 | 0.978 | 1.014 |
| RDW-CV (%) | 0.043 | 0.559 | 1.044 | 0.904 | 1.206 |
| RDW-SD (%) | 0.018 | 0.289 | 1.019 | 0.985 | 1.054 |
| PLT (109/L) | −0.003 | 0.159 | 0.997 | 0.994 | 1.001 |
| WBC (109/L) | −0.047 | 0.363 | 0.954 | 0.862 | 1.056 |
| EO (%) | −0.013 | 0.674 | 0.988 | 0.932 | 1.047 |
| LYMP (%) | −0.008 | 0.644 | 0.992 | 0.960 | 1.026 |
| BASO (%) | 0.096 | 0.776 | 1.101 | 0.568 | 2.134 |
| MONO (%) | 0.029 | 0.527 | 1.030 | 0.940 | 1.154 |
| NEU (%) | 0.005 | 0.724 | 1.005 | 0.980 | 1.030 |
Furthermore, TRA (OR: 3.410, 95%CI: 2.511–4.633, p < 0.001) was also associated with PH.
Among the various ions, only Cl− (OR: 1.048, 95%CI: 0.992–1.107, p = 0.097) showed associations with PH.
Finally, from the routine blood examination, none of them was included in the adjusted regression.
In the adjusted regression, we found that only LA (OR: 1.132, 95%CI: 1.047–1.224, p = 0.002), RA (OR: 1.236, 95%CI: 1.126–1.357, p < 0.001), and LVEF (OR: 0.945, 95%CI: 0.894–0.999) were independently associated with PH (Table 4).
Table 4.
Adjusted logistic regressions for PH.
| β | p-value | OR | 95CI% | ||
|---|---|---|---|---|---|
| Parameters | Lower borderline | Upper borderline | |||
| LA | 0.124 | 0.002 | 1.134 | 1.048 | 1.225 |
| RA | 0.212 | <0.001 | 1.236 | 1.126 | 1.357 |
| LVEF | −0.056 | 0.046 | 0.945 | 0.894 | 0.999 |
Discussion
In the present study, we found that PH is prevalent, with a relatively high incidence of 34.6% among 491 ESRD patients who undergo maintenance dialysis. We also investigated the associations between PASP/PH and Cr, BUN, serum ions, and routine blood parameters. Finally, we showed that only cardiac structure parameters, namely, LA, RA, and LVEF were independently associated with PH.
PH Is Prevalent Among ESRD Patients Who Undergo Dialysis Treatment
PH is one of most frequent complications of numerous diseases, such as left heart failure, systemic lupus erythematosus (SLE) and renal dysfunction (1, 3, 16, 23). PH has been classified into two categories (primary PH or secondary PH) based on the presence of causes or risk factors (1). In ESRD patients, PH may be caused by the side effects of renal dysfunction (2). Furthermore, in many disease contexts, PH is considered one of the leading causes of death due to the associated complication of right heart failure (16, 24, 25). Thus, data on the prevalence of PH or PASP among dialysis patients are valuable toward informing guidelines for the management of complications in ESRD patients who undergo hemodialysis. In our present study, we found that PH occurs in maintenance dialysis ESRD patients. Previous studies have reported that the incidence of PH is high among ESRD patients, being as high as 39% among patients with stage 5 CKD and the incidence of PH is almost 68.8% among hemodialysis patients (9, 10, 16, 23, 26). PH is the basic physiopathologic process of many fatal diseases such as severe right heart failure and pulmonary edema (15).
In particular, some of the patients in this study showed a significantly higher PASP, reaching 90 mmHg, which requires further examination and treatment (to the outpatient department of cardiovascular diseases). The incidence of PH is similar to those of previous studies of hemodialysis patients. However, other meta-analysis-based studies have reported a higher incidence of PH in maintenance dialysis (hemodialysis and peritoneal dialysis). The incidence of PH in maintenance dialysis patients may be higher than that in the general population, and this difference may be due to Cr and BUN (26).
The patients who have been diagnosed with PH by the non-invasive method will be followed up in cardiovascular diseases department, they may need right heart catheterization examination and treatment.
Associations Between PASP and Electrolytes Parameters, Cr and BUN
As discussed above, in ESRD patients, PH may be caused by imbalances in BUN, Cr and other toxins (including macromolecule toxins) that are induced by renal dysfunction (7, 25, 27). Furthermore, excessive serum ions also play adverse roles in PH (25). Thus, we examined the associations between PASP/PH and Cr and BUN as well as serum ions.
Previous studies have reported a relationship between PH and renal dysfunction (4, 13). Renal dysfunction is thought to contribute to the development of PH (2). However, we found that there were no differences in BUN or Cr among the various PASP groups. In addition, these parameters were not closely correlated with PASP.
It has been demonstrated that ions have various biological functions in the processes of cell proliferation, migration, and differentiation (28). In particular, K+ and Ca2+ have been reported to participate in pulmonary vasculature remodeling (29, 30). Thus, these ions may potentially play critical roles in PH. Our present study has also focused on the associations between ions and PASP/PH. However, in the first analysis, no significant differences were found among the three groups.
Emerging investigations of Mg2+ have focused on its effects on CREB proteins in neurons (31). In addition, its roles in cell proliferation and protein synthesis have been uncovered. However, the roles of Mg2+ in cardiovascular diseases have not been characterized completely. We tried to find an association between Mg2+ and PH, but instead, we found associations that need to be confirmed by further basic mechanistic studies.
PH Has No Close Relationship With Routine Blood Parameters
Anemia is considered to be the most important complication in ESRD patients who undergo dialysis treatment (32). This is mainly caused by insufficient EPO production. It is also thought to lead to various diseases that may be caused by reductions in the oxygen supply, with consequent local and systemic hypoxia (32, 33). Thus, we also explored the associations between Hb- or RBC-related parameters and PASP or PH. However, we did not find any differences among the PASP groups. Additionally, these parameters were not closely related to PASP. Moreover, relationship between Hb- or RBC-related parameters and PH were not identified by further logistic regression analyses including the univariate and adjusted regressions. This may explain why the effects of Hb and RBC on PASP require a relatively long period of time. The lower Hb or RBC may induce hypoxia resulting in the proliferation of pulmonary artery vascular smooth muscle cells (34, 35).
We also examined the WBC- and PLT-count-related parameters as well as the ratios of other cells. However, no associations were identified.
PH Is Independently Associated With RA, LA, and LVEF
Regarding the structure and function of the heart, PH patients had relatively high LA, RA, LVDD, and PA and relatively low left heart function. This finding is consistent with previous views on the structure and function of the heart in other cardiovascular diseases associated with PH (19, 36, 37). In the further analysis, only LA and RA were independently associated with PH. A large number of studies have shown that PH often accompanies changes in heart structure (19, 36, 37). The changes of right atrium may lead the secondary tricuspid regurgitation. The tricuspid regurgitation further increased the right atrium as a negative feedback. Both of the abovementioned conditions may induce the onset of PH. Thus, PH may be secondary to the enlargement of the right heart as well as its functions, leading to an enlarged tricuspid orifice or its relative insufficiency. Furthermore, the interactions between the left heart and right heart may also cause TR and PH. Many studies have indicated that changes in the structure and function of the left heart may lead to PH (38). Our study also found an association between LA and PH that warrants further cohort studies to confirm the role of the left heart in PH in the special subgroup of ESRD patients who undergo maintenance dialysis. The dialysis-influenced hemodynamics (left and right heart) may also be a reason for the development of PH. Thus, to inhabit the increase of left and right heart such as the use of Angiotensin-Converting Enzyme Inhibitors or Angiotensin Receptor Blockers may be a novel strategy to prevent PH. Finally, we found that the LVEF was closely associated with PH (may be showed a protective role). Thus, the future cohort study may be performed to identify the protective or predictive role of LVEF for PH.
Limitations
Our present study was a retrospective study aimed at determining the prevalence of PH and its associated factors. There are several limitations in the study that may be improved in our future studies. First, it was a retrospective study that could identify only the associated factors for PH. The causality between clinical factors and PH/PASP should be determined by further cohort studies. Second, we included some of the frequently used parameters; however, there may be other more valuable factors that should receive attention. The state of nutrition, chronic inflammation and history have also been demonstrated to be associated with the onset of PH. Finally, pre-dialysis-associated parameters were not included in this study but will be included in our future studies.
Conclusions
PH is prevalent, with a relatively high incidence among ESRD patients who undergo maintenance dialysis. The sizes of the left and right atria as well as LVEFF are independently associated with PH, but further cohort and basic mechanistic studies are needed to confirm this finding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of The Second Affiliated Clinic Hospital (Xinqiao Hospital), Army Medical University (Third Military Medical University). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
S-ZB participated in the design of the study. YZ and S-ZB also drafted the manuscript and performed the statistical analysis. X-HD reviewed and revised this manuscript critically for important intellectual content. RR performed the echocardiography examination. RR performed the echocardiography examination and the analysis of PASP-related measurements. YZ and ST carried out the collection of demographic data and routine blood examination data. The other laboratory measurements were performed by YW and FP. The dialysis-related data were obtained by YZ, YW, and LN. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank all the individuals who participated in this study for their support. We also would like to thank Jianfei Chen for his help in the analysis of PASP-related measurement. Finally, I (S-ZB) will thank my wife Meng Zhou for her help and care during the preparation of the manuscript.
Glossary
Abbreviations
- BAS
basophil ratio
- BUN
blood urea nitrogen concentration
- Ca2+
serum calcium concentration
- Cr
plasma creatinine
- ESRD
end-stage renal disease
- EO
eosinophil ratio
- FS
fractional shortening
- Hb
hemoglobin concentration
- HCT
hematocrit
- IVS
interventricular septum
- K+
serum potassium concentration
- LA
left atrium end-diastolic internal diameter
- LVDD
left ventricle end-diastolic internal diameter
- LVEF
ejection fraction of left ventricle
- LVPW
left ventricular posterior wall
- LYM
lymphocyte ratio
- MCH
mean corpuscular hemoglobin
- MCHC
mean corpuscular hemoglobin concentration
- MCV
mean corpuscular volume
- MONO
monocyte ratio
- Na+
sodium concentration
- NEU
neutrophil ratio
- P
phosphate concentration
- PA
pulmonary artery end-diastolic internal diameter
- PAP
pulmonary arterial pressure
- PASP
pulmonary artery systolic pressure
- PH
pulmonary hypertension
- PLT
platelet
- RA
right atrium end-diastolic internal diameter
- RBC
red blood cell count
- RDW
red blood cell distribution width
- RV
right ventricle end-diastolic internal diameter
- SV
stroke volume
- TR
tricuspid regurgitation
- TRA
TR area
- TRV
TR velocity
- WBC
white blood cell count.
Footnotes
Funding. This study was supported by the National Natural Science Foundation of China (grants No. 81400736 and 81901916) and Social and Clinical Program from Xinqiao Hospital, Army Medical University (2016YLC16).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2020.570874/full#supplementary-material
References
- 1.Barbera JA, Roman A, Gomez-Sanchez MA, Blanco I, Otero R, Lopez-Reyes R, et al. Guidelines on the diagnosis and treatment of pulmonary hypertension: summary of recommendations. Arch Bronconeumol. (2018) 54:205–15. 10.1016/j.arbr.2017.11.017 [DOI] [PubMed] [Google Scholar]
- 2.Bolignano D, Pisano A, D'Arrigo G. Pulmonary hypertension: a neglected risk condition in renal patients? Rev Cardiovasc Med. (2018) 19:117–21. 10.31083/j.rcm.2018.04.3188 [DOI] [PubMed] [Google Scholar]
- 3.Genctoy G, Arikan S, Gedik O. Secondary hyperparathyroidism is associated with pulmonary hypertension in older patients with chronic kidney disease and proteinuria. Int Urol Nephrol. (2015) 47:353–8. 10.1007/s11255-014-0889-5 [DOI] [PubMed] [Google Scholar]
- 4.Han BG, Kim J, Jung IY, Son JW. Relationship between volume status and possibility of pulmonary hypertension in dialysis naive CKD5 patients. PLoS ONE. (2019) 14:e0221970. 10.1371/journal.pone.0221970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maron BA, Hess E, Maddox TM, Opotowsky AR, Tedford RJ, Lahm T, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the veterans affairs clinical assessment, reporting, and tracking program. Circulation. (2016) 133:1240–8. 10.1161/CIRCULATIONAHA.115.020207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenkranz S, Ghofrani HA, Grunig E, Klose H, Olschewski H, Hoeper MM. Cologne consensus conference on pulmonary hypertension - Update 2018. Int J Cardiol. (2018) 272S:1–3. 10.1016/j.ijcard.2018.09.064 [DOI] [PubMed] [Google Scholar]
- 7.O'Leary JM, Assad TR, Xu M, Birdwell KA, Farber-Eger E, Wells QS, et al. Pulmonary hypertension in patients with chronic kidney disease: invasive hemodynamic etiology and outcomes. Pulm Circ. (2017) 7:674–83. 10.1177/2045893217716108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nasri H. Unexplained pulmonary hypertension in peritoneal dialysis and hemodialysis patients. Rev Port Pneumol. (2013) 19:238–9. 10.1016/j.rppneu.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 9.Shang W, Li Y, Ren Y, Li W, Wei H, Dong J. Prevalence of pulmonary hypertension in patients with chronic kidney disease without dialysis: a meta-analysis. Int Urol Nephrol. (2018) 50:1497–504. 10.1007/s11255-018-1853-6 [DOI] [PubMed] [Google Scholar]
- 10.Sise ME, Courtwright AM, Channick RN. Pulmonary hypertension in patients with chronic and end-stage kidney disease. Kidney Int. (2013) 84:682–92. 10.1038/ki.2013.186 [DOI] [PubMed] [Google Scholar]
- 11.Suresh H, Arun BS, Moger V, Vijayalaxmi PB, Murali Mohan KTK. A prospective study of pulmonary hypertension in patients with chronic kidney disease: a new and pernicious complication. Indian J Nephrol. (2018) 28:127–34. 10.4103/ijn.IJN_36_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah SJ, Thenappan T, Rich S, Tian L, Archer SL, Gomberg-Maitland M. Association of serum creatinine with abnormal hemodynamics and mortality in pulmonary arterial hypertension. Circulation. (2008) 117:2475–83. 10.1161/CIRCULATIONAHA.107.719500 [DOI] [PubMed] [Google Scholar]
- 13.Shoukat Rehman IU, Sumera Idrees MK, Tanweer. Pulmonary hypertension and leading factors in patients undergoing dialysis. J Coll Phys Surg Pak. (2014) 24:836–9. [PubMed] [Google Scholar]
- 14.Rosenkranz S, Lang IM, Blindt R, Bonderman D, Bruch L, Diller GP, et al. Pulmonary hypertension associated with left heart disease: updated Recommendations of the Cologne Consensus Conference 2018. Int J Cardiol. (2018) 272S:53–62. 10.1016/j.ijcard.2018.08.080 [DOI] [PubMed] [Google Scholar]
- 15.Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Rev Esp Cardiol. (2016) 69:177. 10.1016/j.rec.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 16.Miri M, Hejazi S, Maghsoudlou F, Ahmadi M. Prevalence of pulmonary hypertension in end-stage renal disease patients undergoing hemodialysis and peritoneal dialysis at a referral center in Mashhad, Iran, From 2015 to 2016. Iran J Kidney Dis. (2018) 12:364–8. [PubMed] [Google Scholar]
- 17.Zhang L, Zhao S, Ma J, Gong J, Qiu G, Ren Y, et al. Prevalence and risk factors for pulmonary arterial hypertension in end-stage renal disease patients undergoing continuous ambulatory peritoneal dialysis. Ren Fail. (2016) 38:815–21. 10.3109/0886022X.2015.1103637 [DOI] [PubMed] [Google Scholar]
- 18.Zeng Y, Yang DD, Feng S, Shen HY, Wang Z, Jiang S, et al. Risk factors for pulmonary hypertension in patients receiving maintenance peritoneal dialysis. Braz J Med Biol Res. (2016) 49:e4733. 10.1590/1414-431X20154733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mansour IN, Lang RM, Aburuwaida WM, Bhave NM, Ward RP. Evaluation of the clinical application of the ACCF/ASE appropriateness criteria for stress echocardiography. J Am Soc Echocardiogr. (2010) 23:1199–204. 10.1016/j.echo.2010.07.008 [DOI] [PubMed] [Google Scholar]
- 20.Collins N, Bastian B, Quiqueree L, Jones C, Morgan R, Reeves G. Abnormal pulmonary vascular responses in patients registered with a systemic autoimmunity database: pulmonary Hypertension Assessment and Screening Evaluation using stress echocardiography (PHASE-I). Eur J Echocardiogr. (2006) 7:439–46. 10.1016/j.euje.2005.12.002 [DOI] [PubMed] [Google Scholar]
- 21.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. (2010) 23:685–713; quiz 786–8. 10.1016/j.echo.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 22.Centurion OA, Garcia LB. Pulmonary artery hypertension and related complications associated to left atrial myxoma. Open Cardiovasc Med J. (2017) 11:156–8. 10.2174/1874192401711010156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta KS, Shirkande AK, Bhurke SP, Pajai AE, Swami RS, Jadhav SN. Pulmonary hypertension in various stages of chronic kidney disease in Indian patients. Indian J Nephrol. (2019) 29:95–101. 10.4103/ijn.IJN_407_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huston JH, Maron BA, French J, Huang S, Thayer T, Farber-Eger EH, et al. Association of mild echocardiographic pulmonary hypertension with mortality and right ventricular function. JAMA Cardiol. (2019) 4:1112–21. 10.1001/jamacardio.2019.3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reque J, Garcia-Prieto A, Linares T, Vega A, Abad S, Panizo N, et al. Pulmonary hypertension is associated with mortality and cardiovascular events in chronic kidney disease patients. Am J Nephrol. (2017) 45:107–14. 10.1159/000453047 [DOI] [PubMed] [Google Scholar]
- 26.Yang QM, Bao XR. Pulmonary hypertension in patients with stage 1-3 chronic kidney disease. Genet Mol Res. (2014) 13:5695–703. 10.4238/2014.July.25.25 [DOI] [PubMed] [Google Scholar]
- 27.Pabst S, Hammerstingl C, Hundt F, Gerhardt T, Grohe C, Nickenig G, et al. Pulmonary hypertension in patients with chronic kidney disease on dialysis and without dialysis: results of the PEPPER-study. PLoS ONE. (2012) 7:e35310. 10.1371/journal.pone.0035310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lambert M, Capuano V, Olschewski A, Sabourin J, Nagaraj C, Girerd B, et al. Ion channels in pulmonary hypertension: a therapeutic interest? Int J Mol Sci. (2018) 19:3162. 10.3390/ijms19103162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lai N, Lu W, Wang J. Ca(2+) and ion channels in hypoxia-mediated pulmonary hypertension. Int J Clin Exp Pathol. (2015) 8:1081–92. [PMC free article] [PubMed] [Google Scholar]
- 30.Peth S, Karle C, Dehnert C, Bärtsch P, Mairbäurl H. K+ channel activation with minoxidil stimulates nasal-epithelial ion transport and blunts exaggerated hypoxic pulmonary hypertension. High Alt Med Biol. (2006) 7:54–63. 10.1089/ham.2006.7.54 [DOI] [PubMed] [Google Scholar]
- 31.Wu G, Yu J, Wang L, Ren S, Zhang Y. PKC/CREB pathway mediates the expressions of GABAA receptor subunits in cultured hippocampal neurons after low-Mg(2+) solution treatment. Epilepsy Res. (2018) 140:155–61. 10.1016/j.eplepsyres.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 32.Santoro A, Canova C. Anemia and erythropoietin treatment in chronic kidney diseases. Minerva Urol Nefrol. (2005) 57:23–31. [PubMed] [Google Scholar]
- 33.Ruifrok WP, Lipsic E, de Boer RA, van Gilst WH, van Veldhuisen DJ. Anemia and erythropoietin in heart failure. Heart Fail Monit. (2008) 6:28–33. [PubMed] [Google Scholar]
- 34.Zhao Y, Wang B, Zhang J, He D, Zhang Q, Pan C, et al. ALDH2 (Aldehyde dehydrogenase 2) protects against hypoxia-induced pulmonary hypertension. Arterioscler Thromb Vasc Biol. (2019) 39:2303–19. 10.1161/ATVBAHA.119.312946 [DOI] [PubMed] [Google Scholar]
- 35.Hu CJ, Poth JM, Zhang H, Flockton A, Laux A, Kumar S, et al. Suppression of HIF2 signalling attenuates the initiation of hypoxia-induced pulmonary hypertension. Eur Respir J. (2019) 54:1900378. 10.1183/13993003.00378-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Condon DF, Nickel NP, Anderson R, Mirza S, de Jesus Perez VA. The 6th World Symposium on Pulmonary Hypertension: what's old is new. F1000Res. (2019) 8:F1000 Faculty Rev-888. 10.12688/f1000research.18811.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramasubbu K, Deswal A, Herdejurgen C, Aguilar D, Frost AE. A prospective echocardiographic evaluation of pulmonary hypertension in chronic hemodialysis patients in the United States: prevalence and clinical significance. Int J Gen Med. (2010) 3:279–86. 10.2147/IJGM.S12946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Selvaraj S, Shah SJ, Ommerborn MJ, Clark CR, Hall ME, Mentz RJ, et al. Pulmonary hypertension is associated with a higher risk of heart failure hospitalization and mortality in patients with chronic kidney disease: the Jackson Heart Study. Circ Heart Fail. (2017) 10:e003940. 10.1161/CIRCHEARTFAILURE.116.003940 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.