Abstract
Background
Advanced radiotherapeutic treatment techniques limit the cognitive morbidity associated with whole-brain radiotherapy (WBRT) for brain metastasis through avoidance of hippocampal structures. However, achieving durable intracranial control remains challenging.
Methods
We conducted a single-institution single-arm phase II trial of hippocampal-sparing whole brain irradiation with simultaneous integrated boost (HSIB-WBRT) to metastatic deposits in adult patients with brain metastasis. Radiation therapy consisted of intensity-modulated radiation therapy delivering 20 Gy in 10 fractions over 2–2.5 weeks to the whole brain with a simultaneous integrated boost of 40 Gy in 10 fractions to metastatic lesions. Hippocampal regions were limited to 16 Gy. Cognitive performance and cancer outcomes were evaluated.
Results
A total of 50 patients, median age 60 years (interquartile range, 54–65), were enrolled. Median progression-free survival was 2.9 months (95% CI: 1.5–4.0) and overall survival was 9 months. As expected, poor survival and end-of-life considerations resulted in a high exclusion rate from cognitive testing. Nevertheless, mean decline in Hopkins Verbal Learning Test–Revised delayed recall (HVLT-R DR) at 3 months after HSIB-WBRT was only 10.6% (95% CI: −36.5‒15.3%). Cumulative incidence of local and intracranial failure with death as a competing risk was 8.8% (95% CI: 2.7‒19.6%) and 21.3% (95% CI: 10.7‒34.2%) at 1 year, respectively. Three grade 3 toxicities consisting of nausea, vomiting, and necrosis or headache were observed in 3 patients. Scores on the Multidimensional Fatigue Inventory 20 remained stable for evaluable patients at 3 months.
Conclusions
HVLT-R DR after HSIB-WBRT was significantly improved compared with historical outcomes in patients treated with traditional WBRT, while achieving intracranial control similar to patients treated with WBRT plus stereotactic radiosurgery (SRS). This technique can be considered in select patients with multiple brain metastases who cannot otherwise receive SRS.
Keywords: brain metastases, cognition, hippocampus, radiation, whole brain
Key Points.
In a single-arm phase II trial, mean declines in verbal delayed recall at 3 months after HSIB-WBRT was 10.6%, significantly less than prior studies showing a 60% decrease with standard WBRT.
Local recurrence after HSIB-WBRT was 8.8% and intracranial recurrence was 21.3% at 1 year with few treatment-related toxicities.
Importance of the Study.
HSIB-WBRT allows preservation of cognition while providing excellent intracranial control in patients with multiple brain metastases. Randomized trials are required to confirm superiority to current standard treatments. If successful, HSIB-WBRT would enable effective treatment of brain metastases with a better safety profile than WBRT with technology already available at most modern centers.
Whole-brain radiotherapy (WBRT) has been used for decades for the treatment of brain metastases. Unfortunately, WBRT can be associated with significant decline in neurocognitive function (NCF) across numerous measurable domains, as well as different time points.1–4 NCF decline in this context has been linked to radiation exposure of neural stem cells within the hippocampal dentate gyrus, and several clinical trials support that dose reduction to hippocampal structures reduces NCF decline.5–7 To avoid this, focused treatment approaches such as stereotactic radiosurgery (SRS) have been used in selected patients, particularly those with good performance status and limited disease.8,9 However, many patients are excluded from eligibility for SRS because of a high number of lesions, increased risk of intracranial relapses compared with WBRT, lack of SRS availability, and other medical reasons.3,10,11 Multiple randomized trials have demonstrated local control benefits in patients with multiple metastases who receive an SRS boost.12–14 New approaches that combine advantageous features of WBRT and SRS are now feasible due to advancements in conformal radiation techniques such as intensity-modulated radiation therapy (IMRT). Specifically, it is now possible to deliver fractionated cranial radiation that simultaneously boosts dose to tumors, avoids hippocampal structures, and treats at-risk brain.15–17 This integrated approach may maximize therapeutic efficacy and minimize morbidity in this select patient population.
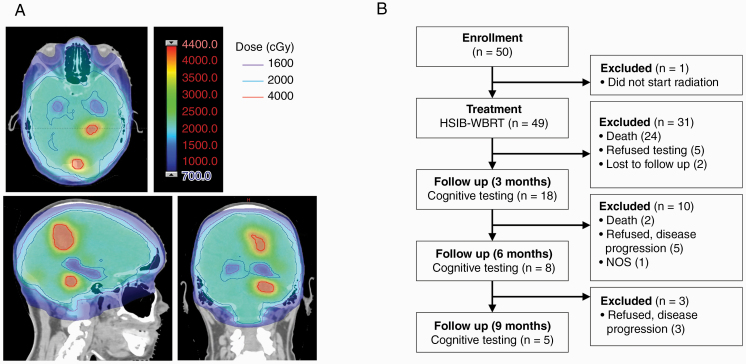
To evaluate the feasibility and efficacy of hippocampal-sparing whole brain irradiation with simultaneous integrated boost (HSIB-WBRT) (Fig. 1A), we conducted a single-institution single-arm phase II trial in patients with brain metastasis. We monitored patients for changes in cognitive function, including verbal delayed recall at 3 months as assessed by the Hopkins Verbal Learning Test–Revised (HVLT-R), intracranial cancer indices such as local control, adverse events according to the Common Terminology Criteria for Adverse Events (CTCAE), and health-related quality of life.
Fig. 1.
(A) Example HSIB-WBRT plan showing sparing of hippocampus (1600 centigray [cGy]), dose to normal brain (2000 cGy), and boost to metastasis (4000 cGy). (B) CONSORT diagram showing patient exclusion from neurocognitive testing.
Methods
Patients
Eligible for enrollment were adult patients (age ≥18 y) with a pathologic diagnosis of a non-hematopoietic malignancy other than small cell lung cancer or germ cell malignancy, ≤8 untreated brain metastases visible on contrast-enhanced brain MRI outside a 5 mm margin around the bilateral hippocampi, Karnofsky performance status ≥70, Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis class I or II, and treating physician assessed life expectancy of at least 3 months. Ineligible were those with evidence of leptomeningeal metastases, a contraindication to MRI, serum creatinine >1.4 mg/dL ≤28 days prior to study entry, or ≥3 uncontrolled or untreated extracranial sites of gross disease. All patients provided written informed consent and the study was approved by institutional review boards (#STU 042011-050).
Study Design and Treatment
A noncontrast treatment-planning CT scan of the entire head region with a 1.25 mm slice thickness was required within 2 weeks of initiating treatment. Patients were immobilized in the supine position using a thermoplastic mask. For lesion targeting and hippocampal contouring, brain MRI with T1 contrast and T2 images with 1 mm slice thickness were acquired and fused to the planning CT images. IMRT (static or volumetric modulated arc therapy) was used to deliver 20 Gy in 10 fractions over 2–2.5 weeks to the whole brain with a simultaneous integrated boost of 40 Gy in 10 fractions to identified metastatic lesions. Each brain metastasis was given a unique, physician defined, gross tumor volume (GTV). This was followed by a uniform 2 mm expansion to create the planning target volume. Hippocampal avoidance regions were manually generated by 3D expansion of the hippocampal contours by 5 mm.18 Per protocol, maximum dose to the hippocampus itself should not exceed 16 Gy. However, maximum dose ≤17 Gy was deemed variation acceptable. All final treatment plans and contours were reviewed after initiation of HSIB-WBRT and, if unacceptable on final quality assurance analysis, rendered unevaluable on final data analysis.
Study Assessments
Cognitive endpoints were measured using validated cognitive assessments at baseline and at 3, 6, 9, and 12 months follow-up. The primary endpoint of the study was delayed verbal recall, as determined by the relative change in HVLT-R delayed recall (DR) score19 from the start of treatment to 3 months after the start of treatment.3 The HVLT-R incorporates 6 different forms, each including 12 nouns (targets) with 4 words drawn from 3 semantic categories. Raw scores were then derived for total recall, DR, percent retained, and a recognition discrimination index. The Trail Making Test (TMT),20 Controlled Word Association Test (COWAT),21 Medical Outcomes Scale–Cognitive Functioning Subscale (MOS),22 and Mini-Mental State Examination (MMSE)23 were also used to assess changes in NCF from baseline in non-memory domains. Cancer outcomes were also included as secondary endpoints. These included physician-assessed radiographic recurrence, overall survival, and adverse events based on CTCAE.
Fatigue was assessed with the Multidimensional Fatigue Inventory (MFI) 20.24 The MFI-20 is a multidimensional, self-reporting instrument designed to measure fatigue. It covers the following dimensions: general fatigue, physical fatigue, mental fatigue, reduced motivation, and reduced activity. A subscore from 4 to 20 is reported for each dimension, with 20 corresponding to maximal fatigue.
Statistical Analysis
The primary endpoint was DR, as measured by the relative change in HVLT-R DR score from the start of treatment to 3 months after the start of treatment. A prior randomized study established that the decline in HVLT-R DR score was 64% at 4 months for those receiving WBRT for brain metastases.3 We estimated that a 50% relative improvement in HVLT-R DR at 3 months would result from HSIB-WBRT. Therefore, the null (H0) and alternative hypotheses (Ha) were H0: ΔHVLT-R DR = 0.60 and Ha: ΔHVLT-R DR ≤0.30. ΔHVLT-R DR is the mean of relative decline between baseline and 3 months after treatment in this patient population. For patient individual i, the relative decline was calculated as follows:
HVLTRi0 and HVLTRi3 denote individual patient recall scores at baseline and 3 months after treatment, respectively. Using these estimates, we calculated that a sample size of 50 patients would be required to reject the null hypothesis with 80% statistical power, assuming a 60% death rate prior to 3 months and a 10% unevaluable rate. Target enrollment was calculated assuming a standard deviation of 51%, with α of 0.05 (one-sided sample t-test).
Cognitive decline after HSIB-WBRT at 3 months was computed along with the corresponding 95% confidence interval. Cumulative incidence with death as a competing risk was used to model local and intracranial control in the brain. The Kaplan–Meier estimator was used to determine the median time to death for this patient population. A log-rank test was conducted to examine if there was significant difference (α < 0.05) in overall survival between patients with low and high number of brain metastases.
Results
Study Population Characteristics
Between December 2011 and August 2016, fifty patients were enrolled on study, although 1 patient did not initiate treatment (Fig. 1B). Characteristics of the 49 evaluable patients are shown in Table 1. Eighty percent of patients had brain metastases from lung primary and 10% from breast primary. Eastern Cooperative Oncology Group (ECOG) performance status was <2 in 84% of cases. Nineteen patients had 1–3 lesions, 20 had 4–6 lesions, and 10 had 7–8 lesions in the brain. The average lesion size was 0.8 cc (interquartile range [IQR], 0.4–1.8). Educational attainment varied, with 22 patients having attended college, 21 high school, and 7 with less than a high school education. Baseline cognitive scores were slightly below what would be expected in the normal population based on published series (Table 1). Nevertheless, this is consistent with previous measures of cognitive performance in patients with brain tumors.25
Table 1.
Demographics and baseline patient characteristics
| Baseline Characteristics | |||
|---|---|---|---|
| Median age, y (IQR) | 60 (54–65) | ||
| n | |||
| Sex | Male | 24 | |
| Female | 25 | ||
| Ethnicity | |||
| White | 31 | ||
| African American | 11 | ||
| Other | 7 | ||
| Education | |||
| No high school | 7 | ||
| High school | 21 | ||
| College | 22 | ||
| Number of brain metastases | |||
| 1 to 3 | 18 | ||
| 4 to 6 | 24 | ||
| 7 to 8 | 8 | ||
| ECOG | |||
| 0 | 11 | ||
| 1 | 30 | ||
| 2 | 8 | ||
| Primary site | |||
| Breast | 5 | ||
| Lung | 39 | ||
| Other | 5 | ||
| Lesion volume, cc (IQR) | 0.6 (0.3–1.9) | ||
| Cognitive test scores (SD) | Mean T score (SD) | z-Score (SD) | |
| HVLT–Immediate Recall | 41.3 (12.7) | −0.87 (1.27) | |
| HVLT-DR | 43.7 (13.7) | −0.67 (1.37) | |
| HVLT-R | 45.8 (13.2) | −0.42 (1.32) | |
| COWAT | 41.4 (11.5) | −0.86 (1.15) | |
| TMT-A | 37.4 (13.4) | −1.26 (1.34) | |
| TMT-B | 43.0 (9.8) | −0.70 (0.98) | |
| MOS | 28.8 (7.5) | ||
| MMSE | 51.1 (16.9) | 0.11 (1.69) | |
| MFI-20 | 55.3 (22.8) |
Hippocampal Segmentation and Dosimetry
Prior studies involving hippocampal sparing revealed that ~25% of cases required contouring revision upon central review.7 However, as contouring atlases have become widely available,26 delineation of the hippocampus is expected to be more reliable, and recent studies support that compliance with hippocampal contouring guidelines has risen.18,27 We assessed contouring variation among practitioners for the current protocol. Fifty hippocampal contours from 13 providers were individually interrogated using the Hausdorff distance, which is the greatest of all the distances from a point on the reference contour to the closest point on the investigator contour.28 Of the 49 hippocampal contours, 45 had no variation and 5 had acceptable variation, suggesting that anatomical segmentation of the hippocampus is generally achievable by most practitioners given current reference materials and resources.27 For our study population, the mean dose, minimum dose, and maximum dose achieved for the hippocampus were 13.2 Gy (± 1.3), 11.5 Gy (± 1.5), and 15.8 Gy (± 1.5) Gy, respectively. Median minimum and maximum doses to brain were 13.5 Gy (IQR, 12.7–15.0) and 42.6 Gy (IQR, 41.8–43.6), respectively. Median minimum and maximum doses to metastases were 39.0 Gy (IQR, 38.2–39.6) and 42.5 Gy (IQR, 42.0–43.3). In 2 patients, the maximum dose to a single hippocampus was not met.
Cognitive Decline
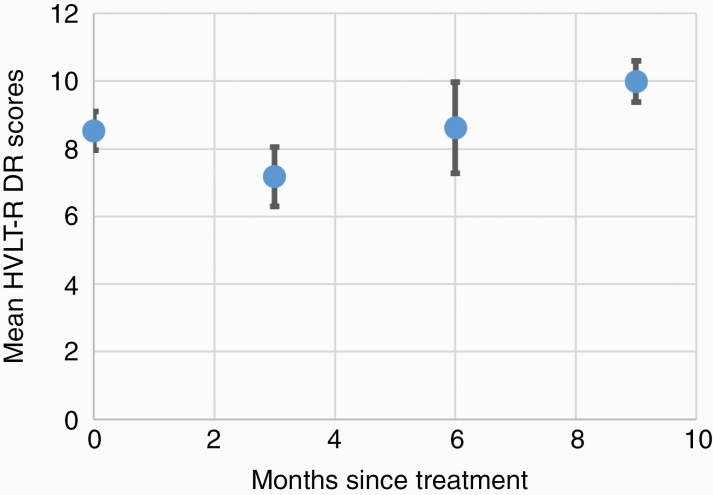
Two patients were unable to complete baseline cognitive testing upon enrollment because of illiteracy. Of the 23 evaluable patients at 3 months follow-up, 18 (78%) completed cognitive testing. Of the patients who did not complete testing, the most common reasons were patient refusal or disease progression leading to a change in goals of care and cancellation of follow-up. Compliance with cognitive testing further declined, for similar reasons, to 52% at 6 months follow-up, and further to 36% at 9 months (Fig. 1B). Mean decline in HVLT-R DR at 3 months after HSIB-WBRT was 10.6% (± 50.43), better than the ~60% decline noted in our prespecified historical control (P < 0.0001).3 At the individual patient level, 3 patients experienced declines in DR greater than standard deviations from the mean (Supplementary Figure 1). Additional measures of cognitive performance 3 months after HSIB-WBRT did not show statistical differences in mean change from baseline, although error estimates were high (Table 2). Interestingly, of the patients who survived beyond 6 months, mean relative HVLT-R DR scores recovered to baseline and trended above baseline scores (Fig. 2).
Table 2.
Change in cognitive performance at 3 months*
| Variable | N | Mean Change (%)* | SD |
|---|---|---|---|
| HVLT-DR | 17 | −10.6 | 50.4 |
| HVLT–Immediate Recall | 17 | 39.6 | 129.4 |
| HVLT-R | 17 | −0.4 | 19.9 |
| COWAT | 17 | 3.7 | 40.0 |
| TMT-A | 16 | 17.7 | 86.6 |
| TMT-B | 14 | 12.0 | 34.9 |
| MOS | 17 | 3.2 | 18.2 |
| MMSE | 17 | −0.1 | 7.0 |
| MFI-20 | 16 | 6.6 | 32.2 |
* Mean calculation is the average of ‘ 100*(3 month − baseline) / baseline
Fig. 2.
Mean raw HVLT-R DR scores of patients surviving to 9 months.
Other Adverse Events and Quality of Life
A total of three grade 3 treatment toxicities consisting of nausea, vomiting, and necrosis or headache were observed. All grade 3 toxicities were felt to be possibly related to treatment by an external review board composed of physician experts. All toxicities related to treatment are listed in Supplementary Table 1. Mean relative fatigue, as assessed by MFI-20 scores, remained stable for patients evaluable at 3 months. However, at 6 and 9 months, MFI-20 scores increased to 37% and 83%, respectively, from baseline, suggesting progressive fatigue over time (Supplementary Figure 2).
Cancer-Related Outcomes
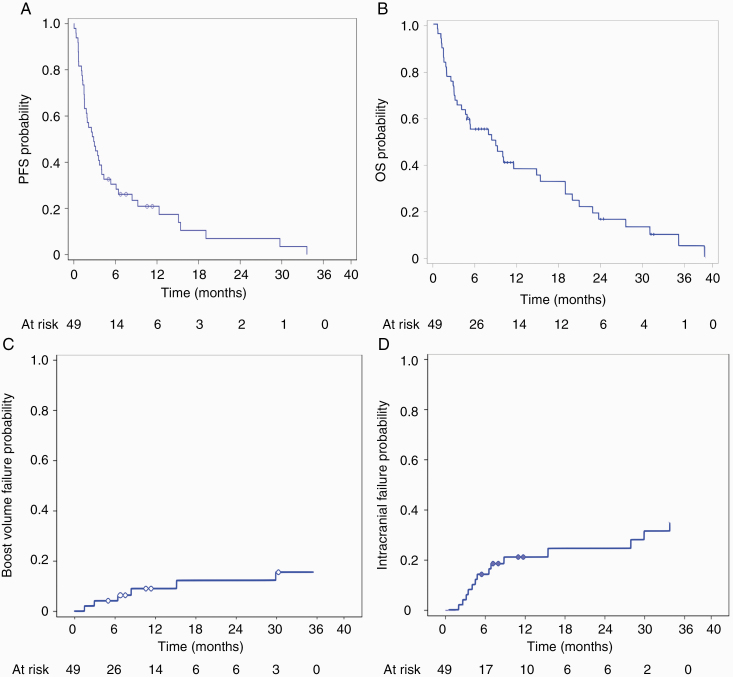
With a mean follow-up of 10.5 months (range, 1–39 mo), median progression-free survival was 2.9 months and median overall survival for the cohort was 9 months (Fig. 3A, B), comparable to historical controls.3,7,14,29 No patient died from treatment-related toxicity or disease progression in the brain. However, one patient had pathologically confirmed radiation necrosis after undergoing resection of a large cerebellar lesion after concerns of radiographic progression. When stratified for 1–3 versus >4 metastases, there was no significant difference in overall survival (P = 0.922). Cumulative incidence of local failure, defined as tumor recurrence within 5 mm, was 8.8% (95% CI: 2.7‒19.6%) at 1 year (Fig. 3C). Cumulative incidence of intracranial failure was 21.3% (95% CI: 10.7‒34.2%) at 1 year (Fig. 3D). One patient was noted to have a recurrence in the hippocampus 5 months after HSIB-WBRT, which was successfully salvaged with radiosurgery.
Fig. 3.
(A) Kaplan–Meier curve for progression-free survival. (B) Kaplan–Meier curve for overall survival. (C) Cumulative incidence of local recurrence (failure at boost site). (D) Cumulative incidence of intracranial recurrence.
Discussion
RTOG 0933 investigated the impact of sparing hippocampal brain, aiming to reduce toxicity, particularly cognitive decline. Here we build on that experience by showing the feasibility and efficacy of a hippocampal sparing approach, HSIB-WBRT, which incorporates a simultaneous integrated boost to visible brain metastases. We also reduced the dose to at-risk brain to 20 Gy in 10 fractions, as opposed to the standard 30 Gy dose. This dose was chosen based on the hypothesis that MRI is sufficiently sensitive to detect brain metastases requiring higher doses to control, and that 20 Gy may be sufficient to treat MRI-undetectable microscopic disease. This resulted in a 10.6% mean decline in verbal memory performance as assessed by HVLT-R DR at 3 months without sacrificing intracranial control. Our rate was similar to the 7% mean decline in HVLT-R DR at 4 months seen in RTOG 0933, although it should be noted that our endpoint was measured at 3 months instead of 4.7 This is also comparable to previous outcomes in patients treated with SRS alone. For example, a single-institution phase III trial of patients with 1–3 brain metastases showed that HVLT-R DR declined 64% at 4 months in those who underwent WBRT + SRS compared with 20% for those who received SRS alone.3 Brown and colleagues observed similar differences in rates of cognitive decline with WBRT + SRS versus SRS alone in patients with 1–3 brain metastases.14
Over two-thirds of patients treated on RTOG 0933 developed intracranial progression.7 In contrast, intracranial and local control after our integrated boost of 40 Gy to gross disease in HSIB-WBRT was 79% and 91%, respectively, at 1 year. These results are similar to the SRS boost arm of RTOG 9508, which randomized patients to WBRT ± SRS boost. In patients treated with WBRT alone, the risk of local recurrence was 43% greater than those treated with WBRT + SRS.13 We observed an intracranial failure rate outside boosted gross disease volumes, of 12.5% at 1 year despite delivery of 20 and 16 Gy in 10 fractions to normal brain and hippocampus, respectively. This is substantially lower than rates seen with SRS alone and agrees with RTOG 9508 and N0574.13,30 Our results also agree with RTOG 0933 with respect to the low rate of hippocampal failures. In our study, the single patient who recurred in the hippocampus received salvage radiosurgery and survived an additional 2 years.
The SRS literature suggests that achievement of intracranial local control, as was demonstrated in this trial, will have clinical benefits. Specifically, if brain metastases can be managed, they are no longer primary drivers of prognosis for certain cancers.31 This is exemplified by recent studies on renal cell carcinoma where there was no difference in survival in patients treated with SRS for brain metastases with >5 brain metastases versus <5 metastases.32,33 Additional support comes from a multi-institutional prospective observational study of SRS for brain metastases by Yamamoto and colleagues, which showed that survival in patients with 5–10 brain metastases was non-inferior to that of patients with 2–4 brain metastasis.34 Indeed, our study demonstrated a median survival of 9 months, slightly better than similar studies, such as those reported by Chang (6 mo, WB + SRS) and Gondi (6.8 mo).3,7,14,29 This is remarkable given the high representation of lung cancer in our cohort. Additionally, 7.3% of the patients in the Gondi series died because of progression in the brain, whereas we observed none.
Continued advancements in the ability to control systemic disease,35 such as new immunomodulatory and targeted systemic therapies,36–39 will likely further elevate the importance of achieving long-term intracranial control while preserving quality of life. HSIB-WBRT was well tolerated and generally without evidence of significant direct acute or late toxicity. Of note, we observed a near complete recovery to baseline in HLVT-R DR scores beyond 3 months following treatment. A similar recovery pattern was observed in the RTOG 0933 as well as RTOG 0614 studies. However, this observation was noted only in patients who lived beyond 6 months.7 In contrast, WBRT without hippocampal avoidance strategies showed minimal neurocognitive recovery.40 Therefore, HSIB-WBRT may provide additional opportunities for continued neurological recovery as brain metastasis patients survive longer.
HSIB-WBRT is complementary to other efforts to innovate in the WBRT space. Although currently only available in abstract form, NRG CC001, a phase III investigation of Ha-WBRT plus memantine versus WBRT plus memantine, confirms the benefits seen in earlier Ha-WBRT trials.41 However, local control in our trial appears to be better, suggesting a possible benefit from dose escalation at the site of metastasis. It is conceivable that dose reduction to at-risk brain and integrated tumor boost may provide additional benefits to the NRG CC001 concept. HSIB-WBRT may also offer an efficient alternative to the use of whole brain with a radiosurgical boost, as advocated by some. Indeed, SRS + WBRT often requires additional procedures, expertise, and cost and is associated with worse patient-reported quality of life.14 Additionally, SRS appears to provide no discernible survival advantage for multiple metastases.3,10,11,13,14 These points are reflected in the American Society for Radiation Oncology’s Choosing Wisely campaign recommendations against combining SRS with whole brain radiation.42 HSIB-WBRT is a promising alternative that circumvents many of these issues, while offering many of the benefits of intensified local therapy.
Although these results are promising, limitations exist. First, this was a phase II trial, comparing historical controls, and is therefore subject to possible bias. Second, our sample size was limited to the minimum enrollment required to address our primary hypothesis, leading to few patients with long-term follow-up. Third, we had poor compliance with neurocognitive testing in follow-up. However this was anticipated because of disease prognosis, and in fact, many subjects lost motivation in the setting of end-of-life care where goals of care had changed. Had these patients agreed to participate, it is possible that these subjects would have shown declines in neurocognitive metrics. Nevertheless, these data may not have been meaningful, given that patients in hospice care often receive strong medications with cognitive effects, such as narcotics, in end-of-life settings. Indeed, we noted a high degree of variability in the change of neurocognitive performance that we speculate may be driven by this clinical aspect. Finally, neuroprotective drugs, which have shown positive effects in the setting both of standard and of hippocampal sparing WBRT, were not used.40,43 Future studies of HSIB-WBRT with these drugs appear warranted. In summary, our data support that HSIB-WBRT offers a safe and accessible treatment option that provides excellent intracranial tumor control while preserving neurocognition in patients with brain metastases.
Funding
This work was supported by the University of Texas Southwestern Medical Center.
Conflict of interest statement. K.D.W. has received consulting fees from Sanofi Oncology, serves on the scientific advisory board of Vibliome Therapeutics and had sponsored research agreements with Astellas Pharmaceuticals and Revolution Medicine.
Authorship statement. All authors contributed equally to data interpretation and manuscript composition.
Supplementary Material
References
- 1. Roman DD, Sperduto PW. Neuropsychological effects of cranial radiation: current knowledge and future directions. Int J Radiat Oncol Biol Phys. 1995;31(4):983–998. [DOI] [PubMed] [Google Scholar]
- 2. DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39(6):789–796. [DOI] [PubMed] [Google Scholar]
- 3. Chang EL, Wefel JS, Hess KR, et al. . Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–1044. [DOI] [PubMed] [Google Scholar]
- 4. Soffietti R, Kocher M, Abacioglu UM, et al. . A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol. 2013;31(1):65–72. [DOI] [PubMed] [Google Scholar]
- 5. Gondi V, Tomé WA, Mehta MP. Why avoid the hippocampus? A comprehensive review. Radiother Oncol. 2010;97(3):370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gondi V, Hermann BP, Mehta MP, Tomé WA. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys. 2012;83(4):e487–e493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gondi V, Pugh SL, Tome WA, et al. . Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kalkanis SN, Kondziolka D, Gaspar LE, et al. . The role of surgical resection in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsao MN, Rades D, Wirth A, et al. . Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2(3):210–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aoyama H, Shirato H, Tago M, et al. . Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483–2491. [DOI] [PubMed] [Google Scholar]
- 11. Kocher M, Soffietti R, Abacioglu U, et al. . Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29(2):134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45(2):427–434. [DOI] [PubMed] [Google Scholar]
- 13. Andrews DW, Scott CB, Sperduto PW, et al. . Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665–1672. [DOI] [PubMed] [Google Scholar]
- 14. Brown PD, Jaeckle K, Ballman KV, et al. . Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(4):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gutiérrez AN, Westerly DC, Tomé WA, et al. . Whole brain radiotherapy with hippocampal avoidance and simultaneously integrated brain metastases boost: a planning study. Int J Radiat Oncol Biol Phys. 2007;69(2):589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hsu F, Carolan H, Nichol A, et al. . Whole brain radiotherapy with hippocampal avoidance and simultaneous integrated boost for 1-3 brain metastases: a feasibility study using volumetric modulated arc therapy. Int J Radiat Oncol Biol Phys. 2010;76(5):1480–1485. [DOI] [PubMed] [Google Scholar]
- 17. Jiang A, Sun W, Zhao F, et al. . Dosimetric evaluation of four whole brain radiation therapy approaches with hippocampus and inner ear avoidance and simultaneous integrated boost for limited brain metastases. Radiat Oncol. 2019;14(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gondi V, Tolakanahalli R, Mehta MP, et al. . Hippocampal-sparing whole-brain radiotherapy: a “how-to” technique using helical tomotherapy and linear accelerator-based intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(4):1244–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shapiro AM, Benedict RH, Schretlen D, Brandt J. Construct and concurrent validity of the Hopkins Verbal Learning Test-revised. Clin Neuropsychol. 1999;13(3):348–358. [DOI] [PubMed] [Google Scholar]
- 20. Bowie CR, Harvey PD. Administration and interpretation of the Trail Making Test. Nat Protoc. 2006;1(5):2277–2281. [DOI] [PubMed] [Google Scholar]
- 21. Ross TP, Calhoun E, Cox T, Wenner C, Kono W, Pleasant M. The reliability and validity of qualitative scores for the controlled oral word association test. Arch Clin Neuropsychol. 2007;22(4):475–488. [DOI] [PubMed] [Google Scholar]
- 22. Stewart AL, Hays RD, Ware JE Jr. The MOS short-form general health survey. Reliability and validity in a patient population. Med Care. 1988;26(7):724–735. [DOI] [PubMed] [Google Scholar]
- 23. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 24. Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39(3):315–325. [DOI] [PubMed] [Google Scholar]
- 25. Gerstenecker A, Nabors LB, Meneses K, et al. . Cognition in patients with newly diagnosed brain metastasis: profiles and implications. J Neurooncol. 2014;120(1):179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gondi V, Tome WA, Rowley HA, Mehta MP. Hippocampal Contouring: A Contouring Atlas for RTOG 0933.https://www.rtog.org/CoreLab/ContouringAtlases/HippocampalSparing.aspx.
- 27. Martinage G, Hong AM, Fay M, et al. . Quality assurance analysis of hippocampal avoidance in a melanoma whole brain radiotherapy randomized trial shows good compliance. Radiat Oncol. 2018; 13(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aslian H, Sadeghi M, Mahdavi SR, et al. . Magnetic resonance imaging-based target volume delineation in radiation therapy treatment planning for brain tumors using localized region-based active contour. Int J Radiat Oncol Biol Phys. 2013;87(1):195–201. [DOI] [PubMed] [Google Scholar]
- 29. Mehta MP, Rodrigus P, Terhaard CH, et al. . Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol. 2003;21(13):2529–2536. [DOI] [PubMed] [Google Scholar]
- 30. Buckner JC, Shaw EG, Pugh SL, et al. . Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016;374(14):1344–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mary K. Dean AAA, Perry J, Nagy E. Distribution of dedicated stereotactic radiosurgery systems in the United States. Appl Radiat Oncol. 2019;8(1):26–30. [Google Scholar]
- 32. Wardak Z, Christie A, Bowman A, et al. . Stereotactic radiosurgery for multiple brain metastases from renal-cell carcinoma. Clin Genitourin Cancer. 2019;17(2):e273–e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bowman IA, Bent A, Le T, et al. . Improved survival outcomes for kidney cancer patients with brain metastases. Clin Genitourin Cancer. 2019;17(2):e263–e272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yamamoto M, Serizawa T, Shuto T, et al. . Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15(4):387–395. [DOI] [PubMed] [Google Scholar]
- 35. Miller KD, Weathers T, Haney LG, et al. . Occult central nervous system involvement in patients with metastatic breast cancer: prevalence, predictive factors and impact on overall survival. Ann Oncol. 2003;14(7):1072–1077. [DOI] [PubMed] [Google Scholar]
- 36. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bhullar KS, Lagarón NO, McGowan EM, et al. . Kinase-targeted cancer therapies: progress, challenges and future directions. Mol Cancer. 2018;17(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wardak Z, Choy H. Improving treatment options for brain metastases from ALK-positive non-small-cell lung cancer. J Clin Oncol. 2016;34(34):4064–4065. [DOI] [PubMed] [Google Scholar]
- 39. Gabani P, Fischer-Valuck BW, Johanns TM, et al. . Stereotactic radiosurgery and immunotherapy in melanoma brain metastases: patterns of care and treatment outcomes. Radiother Oncol. 2018;128(2):266–273. [DOI] [PubMed] [Google Scholar]
- 40. Brown PD, Pugh S, Laack NN, et al. ; Radiation Therapy Oncology Group (RTOG) Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15(10):1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brown PD, Gondi V, Pugh S, et al. . Hippocampal avoidance during whole-brain radiotherapy plus memantine for patients with brain metastases: phase III trial NRG Oncology CC001. J Clin Oncol. 2020;38(10):1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choosing Wisely. Don’t Routinely Add Adjuvant Whole Brain Radiation Therapy to Stereotactic Radiosurgery for Limited Brain Metastases. The ABIM Foundation/ASTRO. https://www.choosingwisely.org/clinician-lists/american-society-radiation-oncology-adjuvant-whole-brain-radiation-therapy/. Updated June 9, 2017. Accessed July 21, 2019.
- 43. Gondi V, Pugh S, D Brown P, et al. . NCOG-01. Preservation of neurocognitive function (NCF) with hippocampal avoidance during whole-brain radiotherapy (WBRT) for brain metastases: preliminary results of phase III trial NRG oncology CC001. Neuro Oncol. 2018;20(suppl_6):vi172–vi172. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.