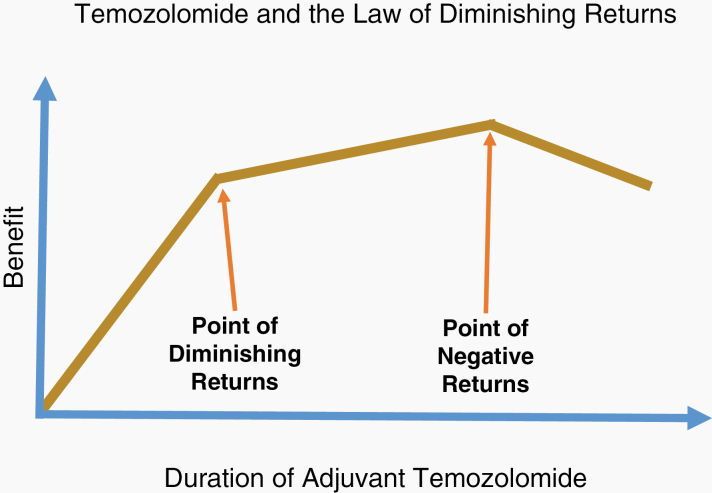
The law of diminishing returns is a highly regarded principle of economics and business which states that not every unit of input will lead to a proportional increase in output. Furthermore, it asserts that recurrent investments in one input, while holding all other inputs constant, yield progressively smaller output results. This principle is frequently taught using an agricultural example. While one application of fertilizer may dramatically increase crop yields, with successive applications the increase in yield falls and eventually productivity diminishes while fertilizer-related toxicities and costs rise. Thus, it is critically important to identify the “point of diminishing returns” if the ultimate goal is to continually improve outcomes while focusing on a single interventional strategy. This principle also applies to chemotherapy and survival outcomes.
The pivotal study that led to the approval of temozolomide (TMZ) in patients with newly diagnosed glioblastoma randomized patients to 6 weeks of radiation or 6 weeks of radiation with concurrent TMZ followed by 6 months of adjuvant TMZ.1 The results demonstrated a significant increase in median survival (12 vs 14.5 mo) and survival at 2 years (10% vs 26%). However, the survival benefit from adding TMZ is largely restricted to patients with O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation.2 The design of this important study was simple and appropriate. Survival was compared in patients receiving no TMZ and those receiving extensive TMZ (concurrent and adjuvant). With regulatory approval, the full complement of TMZ was fixed as the standard of care for all patients with newly diagnosed glioblastoma. Fifteen years have now passed since the original publication of these results. Multiple attempts have been made to improve this regimen. Higher dose adjuvant TMZ was studied in a large, randomized, prospective study.3 This resulted in significant increases in toxicity, but no improvement in survival. In addition, several retrospective analyses have concluded that prolonging the duration of adjuvant TMZ also increases toxicities without a survival benefit.4,5 It is of particular importance that even in MGMT methylated patients, who might be expected to benefit most from TMZ, no survival benefit was observed in these studies exploring higher doses or longer durations of adjuvant TMZ.
The GEINO 14-01 study group is to be commended for conducting the first prospective randomized trial designed specifically to address the risks and benefits of prolonged adjuvant TMZ.6 Patients who had no evidence of tumor progression after completing standard therapy (6 weeks of concurrent radiation and TMZ and 6 months of adjuvant TMZ) were randomized to stop TMZ or to receive an additional 6 months of adjuvant TMZ. In retrospect, there are several aspects of this study design which were not ideal. The primary endpoint of the study was progression-free survival (PFS) rather than overall survival. Unfortunately, PFS is difficult to accurately quantify, especially in the post-radiation setting and in a population of patients rich in MGMT methylation who are prone to pseudoprogression. The investigators were asking a non-inferiority question but chose to approach this using a “phase II” design. The overall sample size was too small given the many important variables that need to be addressed, such as age, performance status, MGMT methylation status, isocitrate dehydrogenase (IDH) mutational status, and extent of resection. In addition, IDH mutational status was incompletely assessed. There are reasonable explanations for these shortcomings. Accruing patients with a terminal illness to this type of study is difficult as clinicians and patients prefer promising novel therapies intended to improve survival rather than non-inferiority and dose de-escalation studies. Knowing this, the investigators chose a phase II design rather than a non-inferiority design, which would have required much higher accrual numbers. They also chose a PFS endpoint which shortens the time to reach the primary endpoint. The lack of complete IDH evaluations is also understandable given that the study was designed and initiated before the importance of IDH mutations was fully understood.
In spite of these shortcomings, this prospective, randomized, multicenter study confirms what has been reported in retrospective studies posing the same question. They found no improvement in PFS or overall survival associated with longer durations of adjuvant TMZ, even in MGMT methylated patients. They also found that prolonged TMZ was associated with higher toxicity rates. Other factors to be considered with prolonged TMZ administration include: (i) higher medical costs for TMZ, anti-emetics, pneumocystis jerovecii pneumonia prophylaxis, blood counts, and complications of therapy; (ii) prolonged TMZ-induced lymphopenia which may affect outcomes with immunotherapy or infections; and (iii) extended disruption of quality time for some patients who instinctively limit travel and important social interactions while receiving chemotherapy.
Given the outcomes of studies documenting that higher doses and longer durations of adjuvant TMZ result in more harm than good, it is reasonable to wonder if even the standard 6 months of adjuvant TMZ is beyond the “point of diminishing returns” described by economists (Fig. 1). There are no studies that convincingly document the value of the adjuvant TMZ. In the study by the European Organisation for Research and Treatment of Cancer that led to FDA approval of TMZ, patients received a median of 3 rather than 6 cycles of adjuvant TMZ and yet the results were positive.1 Is it possible that adjuvant TMZ adds nothing to the 6 weeks of daily TMZ with radiation? This is important to consider as all of us would quickly move to explore novel adjuvant regimens in this patient population if we knew that adjuvant TMZ added little to survival. Although dose de-escalation and non-inferiority studies are often discouraged in diseases where outcomes are poor, it is imperative that we understand which components of this 15-year-old treatment regimen are truly critical. As astutely noted by economists: More is not always better—sometimes it is just more.
Fig. 1.
TMZ and the Law of Diminishing Returns. Data from GEINO 14-01 and several retrospective studies strongly suggests that 12 months of adjuvant TMZ adds toxicity without additional survival benefit. As a result, this should be considered beyond the “point of negative returns.” Additional information is needed to determine how much adjuvant TMZ is required to reach the “point of diminishing returns.”
Acknowledgment
This text is the sole product of the author. No third party had input or gave support to its writing. There are no potential conflicts of interest.
References
- 1. Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 2. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 3. Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blumenthal DT, Gorlia T, Gilbert MR, et al. Is more better? The impact of extended adjuvant temozolomide in newly diagnosed glioblastoma: a secondary analysis of EORTC and NRG Oncology/RTOG. Neuro Oncol. 2017;19(8):1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gramatzki D, Kickingereder P, Hentschel B, et al. Limited role for extended maintenance temozolomide for newly diagnosed glioblastoma. Neurology. 2017;88(15):1422–1430. [DOI] [PubMed] [Google Scholar]
- 6. Balana C, Vaz MA, Sepúlveda JM, et al. A phase II randomized, multicenter, open-label trial of continuing adjuvant temozolomide beyond six cycles in patients with glioblastoma (GEINO 14-01). Neuro Oncol. 2020. doi: 10.1093/neuonc/noaa107. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]