Abstract
Background
“Head Start” III, was a prospective clinical trial using intensive induction followed by myeloablative chemotherapy and autologous hematopoietic cell rescue (AuHCR) to either avoid or reduce the dose/volume of irradiation in young children with medulloblastoma.
Methods
Following surgery, patients received 5 cycles of induction followed by myeloablative chemotherapy using carboplatin, thiotepa, and etoposide with AuHCR. Irradiation was reserved for children >6 years old at diagnosis or with residual tumor post-induction.
Results
Between 2003 and 2009, 92 children <10 years old with medulloblastoma were enrolled. Five-year event-free survival (EFS) and overall survival (OS) rates (±SE) were 46 ± 5% and 62 ± 5% for all patients, 61 ± 8% and 77 ± 7% for localized medulloblastoma, and 35 ± 7% and 52 ± 7% for disseminated patients. Nodular/desmoplastic (ND) medulloblastoma patients had 5-year EFS and OS (±SE) rates of 89 ± 6% and 89 ± 6% compared with 26 ± 6% and 53 ± 7% for classic and 38 ± 13% and 46 ± 14% for large-cell/anaplastic (LCA) medulloblastoma, respectively. In multivariate Cox regression analysis, histology was the only significant independent predictor of EFS after adjusting for stage, extent of resection, regimen, age, and sex (P <0.0001). Five-year irradiation-free EFS was 78 ± 8% for ND and 21 ± 5% for classic/LCA medulloblastoma patients. Myelosuppression was the most common toxicity, with 2 toxic deaths. Twenty-four survivors completed neurocognitive evaluation at a mean of 4.9 years post-diagnosis. IQ and memory scores were within average range overall, whereas processing speed and adaptive functioning were low-average.
Conclusion
We report excellent survival and preservation of mean IQ and memory for young children with ND medulloblastoma using high-dose chemotherapy, with most patients surviving without irradiation.
Keywords: desmoplastic, infants, medulloblastoma, myeloablative chemotherapy
Key Points.
1. Young children with medulloblastoma have poor prognosis when treated with conventional chemotherapy.
2. This trial shows that when treated with intensive induction chemotherapy followed by high-dose myeloablative chemotherapy, a significant proportion of children with ND medulloblastoma can be cured without cranial irradiation with mean preservation of IQ and memory.
Importance of the Study.
This is a prospective clinical trial using high-dose chemotherapy with an intent of avoiding craniospinal irradiation (CSI), and hence long-term neurocognitive sequelae, in young children with medulloblastoma. Patients with ND medulloblastoma enrolled on this trial enjoyed 89% EFS, with most patients being able to avoid CSI. Previously, best survival figures for this disease entity had been reported by Rutkowski et al, with 5-year progression-free survival (PFS) of 85% using conventional chemotherapy, including intravenous and intraventricular methotrexate.1 However, neurocognitive outcome of these children was significantly lower than their age-matched controls. Due to this concern, Children’s Oncology Group and St Jude Children’s Research Hospital recently completed 2 studies treating ND medulloblastoma with conventional chemotherapy only and without intrathecal methotrexate.2,3 Both studies resulted in significantly inferior PFS: 52% and 60%, respectively. Hence, it is imperative that data from our clinical trial be published to provide patients with another potential therapeutic option.
Medulloblastoma is the most common malignant brain tumor occurring in children 0–14 years of age.4 The treatment of infants and young children with medulloblastoma is particularly challenging due to severe long-term side effects associated with craniospinal irradiation (CSI), a standard component of treatment for children >3 years old in North America, with this disease.5–11
We previously reported encouraging survival, quality of life (QoL) and neurocognitive outcome data for medulloblastoma patients treated on “Head Start” (HS) I and II studies, where we investigated whether the use of intensive chemotherapy, without brain irradiation, could improve survival and preserve QoL and intellectual function in young children with malignant brain tumors.12–16 Here, we present survival and neurocognitive outcome data for medulloblastoma patients enrolled on HSIII clinical trial.
Patients and Methods
Children with newly diagnosed, localized (M0) medulloblastoma <4 years old and children with either disseminated (M1+) medulloblastoma or with postoperative residual tumor <10 years old were enrolled between 2003 and 2009 at 39 participating institutions. Adequate organ function was required prior to study entry. Chemotherapy was scheduled to begin within 42 days of initial surgical resection. Patients were required to have disease evaluation with MRI of the brain and spine and lumbar puncture for cerebrospinal fluid (CSF) cytology within 21 days of starting chemotherapy.
Staging Criteria, Surgical Resection, and Pathologic Diagnosis
MRIs of brain and spine with/without gadolinium and lumbar CSF cytology were required at diagnosis. Stage was assigned according to the modified Chang tumor M-staging system.17 Extent of resection was defined as follows: gross total resection (GTR), if no residual tumor was present on postoperative MRI (R0); subtotal resection (STR) if >50% tumor resection but visible residual tumor on MRI; partial resection, 10–50% tumor resection, and biopsy if <10% tumor resection. For the purpose of this analysis, patients with any residual tumor (<GTR) were evaluated as one group (R1). Central review of pathology was not required for study entry. Fifty-two of 92 cases were reviewed retrospectively by the study pathologist (F.G.), mainly from institutions lacking a pediatric neuropathologist. The remaining 40 cases were from institutions where a pediatric neuropathologist had interpreted the slides and were not reviewed centrally. For cases that were centrally reviewed, specimen was marked as ND medulloblastoma if there was evidence of diffuse nodularity, and included cases of medulloblastoma with extreme nodularity.
Treatment
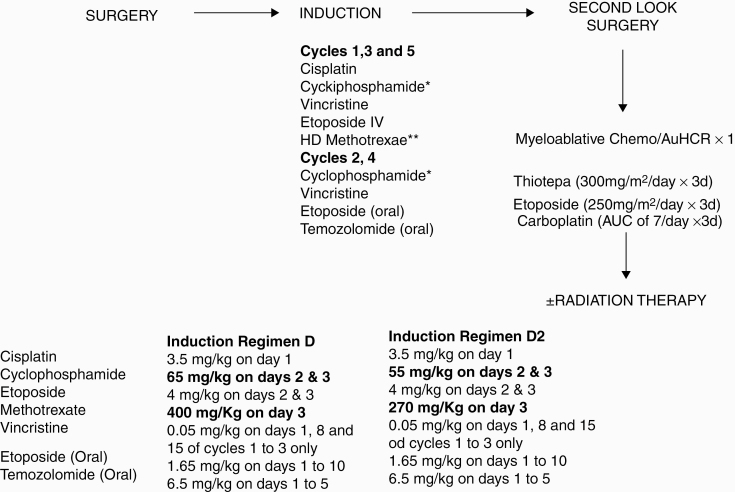
All medulloblastoma patients enrolled on HSIII Regimen D were to receive 5 cycles of induction chemotherapy. Second-look surgery was strongly advocated for residual tumor after completion of induction. If there was no evidence of disease (NED) after induction or second-look surgery, 1 cycle of myeloablative chemotherapy was administered with autologous hematopoietic cell rescue (AuHCR) and no irradiation. In January 2007, HSIII (Regimen D) was suspended pending review by the HSIII Data Safety Monitoring Committee (DSMC) of toxic deaths in children <18 months old treated on this arm (one in medulloblastoma cohort). The study was reopened in October 2007 after DSMC approval with changes in chemotherapy doses as well as more rigorous supportive care requirements. Regimen D2 was initiated with chemotherapy dose reductions for high-dose methotrexate (HD-MTX) and cyclophosphamide, since it has been shown convincingly in the literature that critical cytotoxic levels of methotrexate in the CSF (10–6 M) can be achieved with intravenous doses >5 g/m2 and the dose reduction for cyclophosphamide was modest.18,19 HD-MTX was administered over 4 hours with leucovorin rescue (intravenous or oral) initiated 24 hours after beginning of the methotrexate infusion and administered every 6 hours until the methotrexate level was <0.1 μmol. Drugs, schedule, and dosing for all the drugs are outlined in Figure 1.
Fig. 1.
Dose and schedule for chemotherapy drugs
Only children between 6 and 10 years of age or children younger than 6 years old with residual tumor post-induction were to receive irradiation following consolidation. The recommended dose and volumes of irradiation to be administered based on age, extent of disease at diagnosis, and end-of-induction response status, post-consolidation, are listed in Supplementary Table 1.
Response Evaluation
Criteria for response by CSF cytology were defined as follows: complete response (CR), complete clearance of all malignant cells; and no response, incomplete clearance of malignant cells. Radiographic response was determined (using classic MacDonald criteria) by reviewing MRI scans performed prior to initiation of treatment and sequential scans thereafter: end of induction, following consolidation, and following irradiation if applicable.20 CR was defined as disappearance of all radiographic disease. Partial response (PR) was defined as >50% decrease in the sum of the product of the greatest diameter and perpendicular diameter of all tumor lesions. Progressive disease (PD) was defined as a >25% increase in the sum of the product of diameters of a lesion or appearance of tumor in a previously uninvolved area. Stable disease (SD) was defined as neither sufficient decrease nor increase in the size of a lesion to meet response or progression criteria. Only presence or absence of leptomeningeal spread was to be noted as it is difficult to measure quantitatively. A local radiologist’s assessment was used for response evaluation. Central radiology review was not required.
Supportive Care
Platelet count and hemoglobin were required to be maintained >20 000/mm3 and >8 g/dL, respectively, while on treatment and >40 000/mm3 and >10 g/dL during febrile neutropenic episodes and in the presence of moderate to severe mucositis, unless higher parameters were clinically indicated. All patients were to be maintained on Pneumocystis carinii pneumonia prophylaxis during induction chemotherapy and then restarted following day +42 post-AuHCR. After approval of amendment #3, palivizumab administration was recommended for all children <2 years of age during the respiratory syncytial virus season and it was also recommended to screen these children for serum quantitative immunoglobulin (IgG, IgA, IgM) levels prior to each cycle of chemotherapy and, if low, receive intravenous immune globulin as per institutional guidelines.
Toxicity
Toxicities were graded in accordance with the Common Terminology Criteria for Adverse Events v3.0.21 The site, measure, and grade for all grade 3, 4, and 5 toxicities were reported.
Informed Consent
The patient’s parents/legal guardian were required to sign an informed consent approved by the treating centers’ institutional review board or equivalent committee to become a participant in this trial in accordance with institutional policies in accord with the US Department of Health and Human Services.
Statistical Analysis
Primary aim of the study was to determine 2-year event-free survival (EFS) and overall survival (OS) from time of study enrollment for children with localized (M0) medulloblastoma <4 years old at study enrollment and disseminated (M1+) medulloblastoma <10 years old at study enrollment, treated with induction chemotherapy Regimen D and Regimen D2 (post–amendment #3) followed by consolidation with myeloablative chemotherapy and AuHCR. The primary endpoint for analysis was EFS, which was defined as the duration in time between date on study and date of progression, relapse, or death from any cause, with patients who did not have any events censored at the last follow-up date. The secondary endpoint included OS, defined as duration in time between date on study and date of death, with patients alive censored at the last follow-up date. For irradiation-free event-free survival (rEFS), tumor recurrence, progression, receiving irradiation (either as part of the protocol treatment plan or as a salvage therapy), and death were defined as failures. We acknowledge that this definition of rEFS could be conservative, since for this study irradiation could be given as part of the protocol treatment before a patient experienced progression or tumor recurrence, and including all irradiation as failure events could underestimate the rEFS rates. All time durations are as of last contact with the patient or as of June 2015. Survival probabilities were estimated by the Kaplan–Meier method. Analyses of EFS, OS, and rEFS were based on log-rank tests, product-limit estimator, and Cox regression analyses.22 All analyses were performed in Stata software.23
Neurocognitive Evaluation
Twenty-four survivors from 12 institutions completed neurocognitive assessments, examining IQ, working memory, and processing speed, as well as verbal and nonverbal memory. Only neurocognitive assessments prior to relapse were included in the analysis. As 11 of 24 patients completed only one evaluation, results from assessments conducted farthest from diagnosis were analyzed, using IBM SPSS Statistics v25.0.24 One-sample t-tests were used to compare the entire sample with the expected population mean based on the test publishers’ nonmedical normative data. Independent 2-sample t-tests were also used to compare groups: chemotherapy regimen; radiation exposure (RT), unless assumptions were violated. For nonnormal distributions, Mann–Whitney U-test with mean ranks was used given nonsimilar distribution between groups. Neurocognitive outcome variables and group comparisons were corrected for multiple comparisons using probability cutoffs and false discovery rates. An alpha level of 0.05 was used to indicate statistical significance for analyses using means (x̄), standard deviations (s), and minimum to maximum values for continuous variables.
A Full Scale Intelligence Quotient (FSIQ-4) was derived using primary subtests from the age-appropriate Wechsler scale: Vocabulary, Similarities, Block Design, and Matrix Reasoning.25–27 The Adaptive Behavior Assessment System‒Second Edition (ABAS-2)28 provided parent report of overall daily living skills. Working memory was obtained with the Digit Span subtest and Processing speed (PSI) from the composite of the Coding and Symbol Search subtests of the age-appropriate Wechsler scale.25,26 Memory was reported from the Dot Locations and Stories subtests of the Children’s Memory Scale (CMS)29 and the California Verbal Learning Test‒Children’s Version (CVLT-C).30
Results
Patient Characteristics
Between May 2003 and December 2009, 92 children with newly diagnosed medulloblastoma were enrolled: 45 patients were enrolled on Regimen D and 47 on Regimen D2. Thirty-nine patients (43%) had M0 and 51 (57%) had M1+ disease (M1: 8; M2: 5; M3: 38). M-stage could not be assessed for 2 patients as they did not have a lumbar puncture due to medical reasons. Fifty-two patients had classical, 27 had ND, and 13 had large-cell/anaplastic (LCA) medulloblastoma subtype. Additional patient characteristics are described in Supplementary Table 2 and clinical characteristics by each histological subtype are outlined in Supplementary Table 3.
Response to Induction and Consolidation Chemotherapy
Of the 92 patients, 26 had a CR to induction chemotherapy, 20 had a continued complete response (CCR), 12 had a PR, 4 had a minor response (MR), 9 had SD, 18 developed PD, 2 patients were non-evaluable (NE; parents withdrew consent for 1 patient and 1 patient died due to CNS hemorrhage from an accidental fall), and there was 1 toxic death (TD). Sixty-eight patients proceeded to receive myeloablative consolidation chemotherapy and AuHCR, of which 43 had CCR, 7 CR, 8 PR, 3 MR, 2 SD, 4 PD, and 1 TD. Twenty-four patients were non-evaluable: in addition to 18 PD, 1 TD, and 2 NE during induction, 3 patients were NE during consolidation—1 received irradiation, 1 patient withdrew consent, and 1 transferred to a non-“Head Start” institution post-induction.
Outcomes
Although the primary aim of the protocol was to determine 2-year EFS and OS for M0 medulloblastoma patients <4 years of age and M1+ patients <10 years of age at the time of enrollment, 5-year survival estimates are also provided as we have longer follow-up on all patients enrolled on the trial (Table 1).
Table 1.
EFS and OS rates at 2, 3, and 5 years, by baseline variables
| Cohorts | n | EFS Rates (±SE) | OS Rates (±SE) | ||
|---|---|---|---|---|---|
| 2 year | 5 year | 2 year | 5 year | ||
| All patients | 92 | 54 ± 5% | 46 ± 5% | 71 ± 5% | 62 ± 5% |
| Regimen | |||||
| D | 45 | 56 ± 7% | 46 ± 7% | 69 ± 7% | 57 ± 7% |
| D2 | 47 | 53 ± 7% | 46 ± 8% | 72 ± 7% | 67 ± 7% |
| M-stage* | |||||
| M0 | 39 | 64 ± 8% | 61 ± 8% | 79 ± 6% | 77 ± 7% |
| M1+ | 51 | 47 ± 7% | 35 ± 7% | 65 ± 7% | 52 ± 7% |
| Extent of resection pre-induction | |||||
| R0 | 50 | 60 ± 7% | 52 ± 7% | 74 ± 6% | 65 ± 7% |
| R1 | 42 | 48 ± 8% | 40 ± 8% | 67 ± 7% | 58 ± 8% |
| M-stage and extent of resection | |||||
| M0/R0 | 24 | 71 ± 9% | 66 ± 10% | 83 ± 8% | 83 ± 8% |
| M0/R1+ | 15 | 53 ± 13% | 53 ± 13% | 73 ± 11% | 65 ± 13% |
| M1+ | 51 | 47 ± 7% | 35 ± 7% | 65 ± 7% | 52 ± 7% |
| Histology | |||||
| Classic | 52 | 37 ± 7% | 26 ± 6% | 62 ± 7% | 53 ± 7% |
| Nodular/desmoplastic | 27 | 89 ± 6% | 89 ± 6% | 93 ± 5% | 89 ± 6% |
| LCA | 13 | 54 ± 14% | 38 ± 13% | 62 ± 13% | 46 ± 14% |
| M0 patients: Histology | |||||
| Classic | 18 | 44 ± 12% | 38 ± 12% | 72 ± 11% | 66 ± 11% |
| Nodular/desmoplastic | 15 | 93 ± 6% | 93 ± 6% | 93 ± 6% | 93 ± 6% |
| LCA | 6 | 50 ± 20% | 50 ± 20% | 67 ± 19% | 67 ± 19% |
| M1 patients: Histology | |||||
| Classic | 33 | 33 ± 8% | 21 ± 7% | 58 ± 9% | 48 ± 9% |
| Nodular/desmoplastic | 11 | 82 ± 12% | 82 ± 12% | 91 ± 9% | 81 ± 12% |
| LCA | 7 | 57 ± 19% | 29 ± 17% | 57 ± 19% | 29 ± 17% |
| Sex | |||||
| Female | 40 | 55 ± 8% | 45 ± 8% | 75 ± 7% | 63 ± 8% |
| Male | 52 | 54 ± 7% | 48 ± 7% | 67 ± 7% | 61 ± 7% |
| Age, y | |||||
| <6 | 83 | 54 ± 5% | 50 ± 6% | 69 ± 5% | 65 ± 5% |
| <1.5 | 23 | 65 ± 10% | 65 ± 10% | 65 ± 10% | 65 ± 10% |
| 1.5 to <3 | 33 | 58 ± 9% | 58 ± 9% | 76 ± 7% | 72 ± 8% |
| 3 to <6 | 27 | 41 ± 9% | 28 ± 9% | 63 ± 9% | 56 ± 10% |
| ≥6 | 9 | 56 ± 17% | 11 ± 10% | 89 ± 10% | 36 ± 19% |
* Two patients were excluded from this analysis as a lumbar puncture for CSF cytology was not performed for medical reasons.
LCA, large cell/anaplastic.
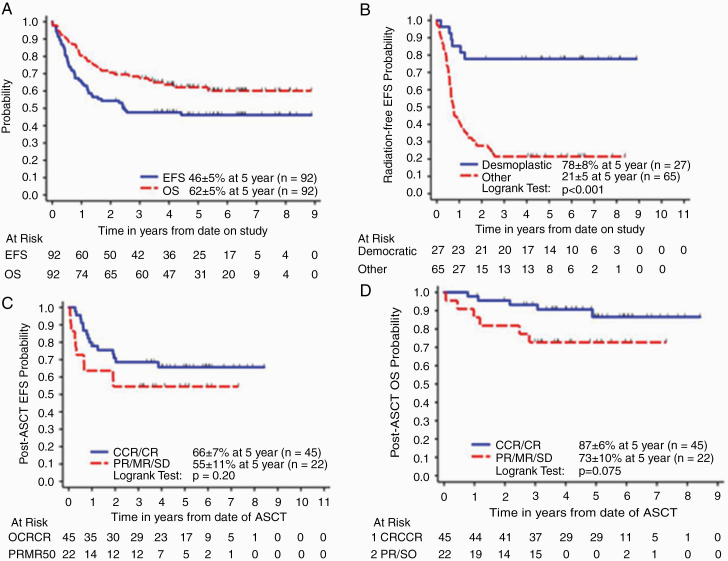
The 5-year EFS and OS (±SE) rates for all medulloblastoma patients enrolled on the study were 46 ± 5% and 62 ± 5%, respectively (Figure 2A). Thirteen patients received irradiation according to protocol guidelines, of whom 4 progressed. One patient received irradiation in protocol violation and 4 did not receive irradiation in protocol violation. The 5-year rEFS rates were 38 ± 5% and 41 ± 5% for all patients enrolled on study and patients who were <6 years of age at diagnosis, respectively. There were 43 relapses. There is no information on patterns of relapse as no further data were collected after patients developed progressive disease.
Fig. 2.
(A) All medulloblastoma, EFS and OS, (B) irradiation-free EFS (rEFS) by histology , and (C, D) post-consolidation EFS and OS of patients by end-induction response status.
Prognostic Factors Histology
Nodular/desmoplastic medulloblastoma. —The 5-year EFS and OS rates (±SE) for 27 patients with ND medulloblastoma were 89 ± 6% and 89 ± 6%, respectively. For 15 patients with M0 ND medulloblastoma, the 5-year EFS and OS rates were 93 ± 6% and 93 ± 6%, respectively (Table 1). Only 1 patient died from CNS hemorrhage related to an accidental fall during induction. The remaining 14 patients are alive without having received any irradiation. For 11 patients with M1+ ND medulloblastoma, the 5-year EFS and OS rates were 82 ± 12% and 81 ± 12%, respectively (Table 1). One patient died from progressive disease post-transplant and 1 patient died of toxicity. Only 3 of the remaining 9 surviving patients with M1+ disease received 2340 cGy, 2160 cGy, and 2700 cGy CSI, plus boost to a total dose of 5400 cGy, respectively. The 5-year rEFS rate for ND medulloblastoma patients was 78 ± 8% (Figure 2B). In multivariate Cox regression analysis (Supplementary Table 4), ND medulloblastoma histology was the only significant independent predictor of survival after adjusting for metastatic status, extent of resection at diagnosis, regimen, age, and sex (P < 0.001 for both EFS and OS). After controlling other variables, patients with ND medulloblastoma had significantly better EFS than patients with classic tumors (hazard ratio [HR] = 0.093, 95% CI = 0.026, 0.33). Patients with ND medulloblastoma also did significantly better in OS than patients with classic tumors (HR = 0.098, 95% CI = 0.024, 0.41).
Classic and Large Cell/Anaplastic Medulloblastoma
The 5-year EFS and OS rates (±SE) for patients with classic medulloblastoma (n = 52) were 26 ± 6% and 53 ± 7% and for patients with LCA histology (n = 13) were 38 ± 13% and 46 ± 14%, respectively. Five-year EFS and OS rates for M0 as well as M1+ classic and LCA medulloblastoma are listed in Table 1. In multivariate Cox regression analysis, no significant difference in EFS and OS was found between classic and LCA tumors. Ten patients (2 M0, 2 M1, 6 M3) received irradiation according to protocol guidelines (9 received 2340 cGY CSI and 5580 cGY tumor-bed boost and 1 received 3600 cGY CSI plus 5580 cGY tumor bed boost), of which 5 progressed. Two patients with M1 disease received CSI (2340 cGY), of which 1 is alive progression free. Both patients with M0 disease progressed. Twenty-two M0 patients did not received irradiation, of which 11 are alive progression free. Twelve of 32 patients with M1+ disease who did not receive irradiation as per protocol are alive; 4 without progression. The 5-year rEFS for classic/LCA medulloblastoma patients was 21 ± 5% (Figure 2B).
Stage and Extent of Resection
Patients with M0 medulloblastoma fared significantly better than M1+ patients; 5-year EFS and OS (±SE) of 61 ± 8% and 77 ± 7% for M0 patients compared with 35 ± 7% and 52 ± 7%, for M1+ patients (EFS, P = 0.022; OS, P = 0.017). There was no significant difference in survival between R0 and R1 patients (EFS, P = 0.22; OS, P = 0.66, Table 2). When we combined the stage and extent of resection, 5-year EFS and OS (±SE) rates for M0R0 (n = 24) and M0R1 (n = 15) patients were 66 ± 10% and 83 ± 8%, 53 ± 13% and 65 ± 13%, respectively (Table 1).
Table 2.
Sample characteristics and outcomes for subjects with neurocognitive evaluation
| Categorical Variable | Frequency | Percent | |
|---|---|---|---|
| Gender | Male | 13 | 54.2 |
| Female | 11 | 45.8 | |
| Ethnicity | Non-Hispanic Caucasian | 17 | 70.8 |
| Hispanic | 5 | 20.8 | |
| Other | 2 | 8.3 | |
| Regimen | D | 11 | 45.8 |
| D2 | 13 | 54.2 | |
| RT | Yes+ | 4 | 16.7 |
| No | 20 | 83.3 |
| Continuous Variable | Minimum | Maximum | Mean | SD | P-value | |
|---|---|---|---|---|---|---|
| Age at diagnosis, y | 0.17 | 5.92 | 2.35 | 1.48 | n/a | |
| Time to NC assessment, y | 0.92 | 10.75 | 4.93 | 2.29 | n/a | |
| FSIQ-4 (n=15) (SS) | 77 | 127 | 95.40 | 12.97 | 0.191 | |
| Regimen | Regimen D (n=8) | 77 | 107 | 94.63 | 11.50 | 0.771 |
| Regimen D2 (n=7) | 85 | 127 | 96.29 | 15.38 | ||
| RT exposure | No (n=12) | 77 | 127 | 94.33 | 13.36 | n/a |
| Yes (n= 3) | 85 | 107 | 99.67 | 12.70 | ||
| PSI (n=16) (SS) | 70 | 110 | 89.19 | 9.61 | 0.0004* | |
| Regimen | Regimen D (n=7) | 80 | 110 | 89.29 | 10.48 | 0.973 |
| Regimen D2 (n=9) | 70 | 100 | 89.11 | 9.52 | ||
| RT exposure | No (n=13) | 70 | 110 | 89.46 | 10.65 | n/a |
| Yes (n=3) | 85 | 91 | 88.00 | 3.00 | ||
| ABAS GAC (n=14) (SS) | 67 | 111 | 85.93 | 13.27 | 0.002* | |
| Regimen | Regimen D (n=5) | 67 | 111 | 89.60 | 16.70 | 0.463 |
| Regimen D2 (n=9) | 68 | 98 | 83.89 | 11.56 | ||
| RT exposure | No (n=12) | 67 | 111 | 86.08 | 14.36 | n/a |
| Yes (n=2) | 82 | 88 | 85.00 | 4.24 | ||
| Digit Span (n=13) (ss) | 5 | 15 | 8.92 | 2.72 | 0.179 | |
| Regimen | Regimen D (n=5) | 5 | 12 | 7.80 | 2.59 | 0.256 |
| Regimen D2 (n=8) | 7 | 15 | 9.63 | 2.72 | ||
| RT exposure | No (n=9) | 5 | 15 | 9.00 | 3.00 | 0.886 |
| Yes (n=4) | 7 | 12 | 8.75 | 2.36 | ||
| CMS Stories (n=14) (ss) | 5 | 16 | 10.21 | 3.26 | 0.810 | |
| Regimen | Regimen D (n=7) | 5 | 16 | 9.14 | 3.67 | 0.233 |
| Regimen D2 (n=7) | 6 | 14 | 11.29 | 2.63 | ||
| RT exposure | No (n=11) | 5 | 16 | 10.27 | 3.26 | n/a |
| Yes (n=3) | 6 | 14 | 10.00 | 4.00 | ||
| CMS Dot Locations (n=15) (ss) | 5 | 15 | 10.40 | 3.09 | 0.624 | |
| Regimen | Regimen D (n=7) | 5 | 15 | 9.43 | 3.31 | 0.200 |
| Regimen D2 (n=8) | 5 | 14 | 11.25 | 2.82 | ||
| RT exposure | No (n=11) | 5 | 15 | 10.09 | 3.39 | 0.541 |
| Yes (n=4) | 9 | 14 | 11.25 | 2.22 | ||
| CVLT (n=14) (z-score) | −2.5 | 1.0 | 0.36 | 1.18 | 0.279 | |
| Regimen | Regimen D (n=7) | −2.5 | 1.0 | 1.00 | 1.26 | 0.036 |
| Regimen D2 (n=7) | −1.0 | 1.0 | 0.29 | 0.70 | ||
| RT exposure | No (n=11) | −2.5 | 1.0 | 0.32 | 1.29 | n/a |
| Yes (n=3) | −1.0 | 0.5 | 0.50 | 0.87 |
NC: Neurocognitive Assessment; SS: Standard Score (mean = 100; SD = 15); ss: scaled score (mean = 10; SD = 3); z-score (mean = 0.0; SD = 1.0); FSIQ-4: Full-Scale IQ-Four Subscale; PSI: Processing Speed Index; ABAS: GAC: Adaptive Behavior Assessment System: General Ability Composite; CMS: Children’s Memory Scale; CVLT: California Verbal Learning Test—Children’s Version; n/a = not applicable, RT group too small for group comparison; +These 4 patients are among 13 patients who received irradiation as per protocol guidelines. *Significant at .05 level using corrected probability cutoffs and false discovery rates.
Response to Induction Chemotherapy
Analyses were restricted to patients who finished the induction therapy and had a CR, PR, or SD response at the end of induction. Patients who died of toxicity or progressed during induction were excluded from the analyses. The 5-year EFS post-consolidation and OS post-consolidation rates for patients with CR post-induction were 66 ± 7% and 87 ± 6% compared with 55 ± 11% and 73 ± 10% for patients with residual disease (EFS, P = 0.2; OS, P = 0.075, Figure 2C and 2D).
Regimen, Age, and Sex
There was a significant difference in EFS between different age groups, with older patients doing worse than younger patients (P = 0.008, Table 1) but there was no significant difference in OS between various age groups (P = 0.24, Table 1). There was no significant difference in EFS and OS between regimen D and D2 (EFS, P = 0.99; OS, P = 0.29) and sex (EFS, P = 0.94; OS, P = 0.73).
AuHCR and Engraftment
The median neutrophil engraftment time was 11 days and median platelet engraftment time was 21 days following AuHCR. The date of neutrophil engraftment was defined as first day of absolute neutrophil count ≥500/mm3 for 3 consecutive days and date of platelet engraftment was defined as >20 000/mm3 without transfusion for at least 7 days.
Toxicity
Grade 3 toxicities both during induction and consolidation were expected and related to myelosuppression, infection, mucositis, nausea/vomiting, hearing loss, and electrolyte abnormalities (Supplementary Tables 5 and 6). Grade 4/5 toxicities during induction were mainly related to myelosuppression, electrolyte abnormalities, and infection, whereas during consolidation, myelosuppression was the most common grade 4/5 toxicity and one patient had reversible encephalopathy, one had veno-occlusive disease (VOD), one gastrointestinal (GI) hemorrhage, one pulmonary hemorrhage, and one patient had multi-organ system failure (Supplementary Tables 7 and 8). There were no significant differences in hematologic toxicities between Regimen D and D2. As expected with reduced doses of methotrexate and cyclophosphamide on Regimen D2, rate of mucositis (1.1% vs 12%), transaminitis (2% vs 9%), infection (11% vs 22%), and acute respiratory distress syndrome (1.3% vs 11%) were lower as compared with Regimen D.
There were 2 toxic deaths: one during induction and one during consolidation. One patient expired during induction from severe mucositis and GI hemorrhage leading to cardiorespiratory failure. Another patient expired on day +21 following AuHCR from complications of VOD and multisystem organ failure.
Neurocognitive Outcomes
At a mean of 4.93 years post-diagnosis, patients’ overall IQ, working memory, and verbal and nonverbal memory were within the average range for the group (Table 2). Processing speed and overall adaptive functioning were both within the low-average range (Table 2). Of note, no statistically significant differences in test performance were noted between cumulative methotrexate received as part of Regimen D and D2 on any measure, or between those who received irradiation versus those who did not, on the Digit Span subtest and CMS Dot Locations; however, these analyses were limited by small numbers of patients in the external beam radiotherapy group. There was no significant difference in age as well as M-stage between patients who underwent neurocognitive testing (n = 24) versus who did not (data not shown).
Discussion
As the long-term deleterious consequences of CSI were recognized in the 1970s and 1980s, many North American and European cooperative group clinical trials attempted to tackle the issue of reducing, delaying, or avoiding CSI by using either conventional chemotherapy alone (CCG9921, SFOP, SJYC07, ACNS1221, UKCCSG CNS9204),2,3,31–34 or with intrathecal chemotherapy (HIT-SKK92),1 or with/without focal irradiation (COG P9934),34 or myeloablative chemotherapy with AuHCR (“Head Start” studies, CCG-99703).12,13,35 Survival on most clinical trials utilizing conventional chemotherapy doses without irradiation has been pretty dismal with the exception of ND medulloblastoma in some studies.
Although the HSIII clinical trial was not powered to make comparisons to historical controls, survival data for patients with medulloblastoma are comparable to other contemporary irradiation-avoiding “baby” protocols, including the HIT SKK92 study,1 which had the best reported outcomes for children with medulloblastoma using conventional chemotherapy, including intermediate-dose intravenous and intrathecal methotrexate. Similar to HIT SKK92 study, patients with ND medulloblastoma did exceptionally well on HSIII with a 5-year EFS and OS of 89% and 93%, respectively. Patients with R0M0 ND medulloblastoma on HSIII enjoyed a 100% 5-year OS (data not shown). This is especially important, because 2 recently completed multi-institutional studies, specifically designed for ND medulloblastoma patients, utilizing conventional dose chemotherapy and no intraventricular methotrexate, failed to show a significant improvement in survival for these patients.2,3 A recently closed Children’s Oncology Group (COG) study, ACNS1221, used the HIT SKK92 approach but without the use of intraventricular methotrexate with study being closed prematurely due to a poor 2-year progression-free survival (PFS) rate of 52 ± 10%% (expected 90%).2 Similarly, another study, from St Jude Children’s Research Hospital (SJYC07), used reduced-intensity chemotherapy and oral maintenance chemotherapy to treat infants with non-metastatic ND medulloblastoma and reported 5-year EFS rates of 52.5%.3 Encouraging survival of ND medulloblastoma patients was also reported on the CCG99703 study using 3 cycles of induction chemotherapy followed by 3 cycles of high-dose consolidation chemotherapy using thiotepa and carboplatin; however, the number of ND medulloblastoma was even smaller on this trial (n = 14, 1 M1+) and almost 50% of the patients either received irradiation post-AuHCR or did not have radiation therapy data available.35
Avoiding CSI is one of the primary objectives for which high-dose chemotherapy is utilized for young children with medulloblastoma. While 21% of classical/LCA medulloblastoma patients were surviving event free without irradiation at 5 years, 5-year rEFS rate for ND medulloblastoma patients was 78% with none of the M0 ND medulloblastoma patients and 8 of 11 patients with M1+ ND medulloblastoma not having received irradiation. These findings suggest that high-dose chemotherapy by itself might not be enough to provide a cure for young patients with classical/LCA medulloblastoma and these patients might require additional therapy such as reduced-dose/volume irradiation or intracavitatory/intra-Ommaya chemotherapy, or metronomic biologic therapy post-consolidation. Older patients >6 years old at diagnosis with disseminated disease also did not fare well on this study despite having received reduced-dose CSI post-AuHCR, suggesting that these patients might be better served by treatment strategies using full-dose CSI upfront.
Though patients with CR appeared to have better post-consolidation EFS and OS than patients with <CR (PR/SD), the association was not statistically significant for post-consolidation EFS (P = 0.20), and was marginally significant for post-consolidation OS (P = 0.075). Given the small sample size and the small number of failure events in this group of the patients, multivariable analyses on the associations between various factors and post-consolidation EFS or OS were not very informative.
Toxicity associated with intensive induction chemotherapy and myeloablative chemotherapy can be considerable when compared with conventional chemotherapy, and use of such an approach for modest benefit in survival can be questioned. We had 2 toxic deaths on study, which were similar to other high-dose chemotherapy approaches35 but deaths have not been observed with conventional dose chemotherapy approaches.2,3,31–34
There are several limitations of this study. Despite neuropsychological evaluation being a secondary objective of the study, only 12 institutions participated in collecting neuropsychological data, with institutions reporting the lack of a neuropsychologist or funding as barriers to participation. Though limited by small sample size and lack of longitudinal data, the finding of average overall IQ and working memory, as well as verbal and nonverbal memory, suggests that the HSIII treatment approach of reducing or eliminating cranial irradiation may preserve aspects of neurocognitive functioning in some young children treated for medulloblastoma. However, the findings of low-average processing speed and overall adaptive functioning suggest these patients display areas of difficulty that would benefit from supportive interventions. It is also important to note the wide range in test performance, from well above to well below the average range, across patients in this small sample, indicating variability in individual outcomes.
Secondly, transcriptional profiling of medulloblastoma tumors has identified 4 molecular subgroups: wingless (WNT), sonic hedgehog (SHH), Group 3, and Group 4, with prognostic significance in favor of WNT and SHH (particularly infant SHH).36–38 Additionally, Cavalli et al reported the existence of 4 SHH medulloblastoma subtypes; SHHα, SHHβ, SHHγ, and SHHδ. SHHβ and SHHγ comprised the majority of infant SHH tumors, with SHHβ having more patients with metastatic disease and carrying a worse prognosis.39 Robinson et al confirmed the presence of these 2 SHH medulloblastoma subgroups (iSHH-I and iSHH-II) for patients enrolled on SJYC07 with iSHH-II patients having a significantly better PFS than iSHH-I; 5-year PFS of 27.8% versus 75.4%.3 A major limitation of our study is that we do not have molecular subgroup data for this group of patients. This is mainly due to the fact that tumor tissue was only collected for retrospective central pathology review but not for research on this clinical trial and was not available for testing. Although it is easier to speculate that most patients with ND medulloblastoma belong to the SHH medulloblastoma subgroup, including iSHH-I and II, as the overlap of ND medulloblastoma and SHH subgroup was found to be 100% for patients enrolled on the HIT200040 as well as on SJYC07,3 it is harder to comment on the molecular subgroup of classical/LCA patients enrolled on HSIII.
Additionally, though a majority of cases were reviewed by the study neuropathologist or an experienced neuropathologist at external sites, we would also like to acknowledge the lack of central review of pathology on our trial being a limitation, due to the lack of precise definition of ND medulloblastoma and discordance between multiple neuropathologists. However, excellent survival of such patients on this trial suggests that maybe the pathologists were more aware of this entity and were careful in describing these tumors. A combination of histopathology and molecular/genomic features will help classify these tumors better in the future. Similarly, lack of central radiology review is a limitation as well. Although response criteria were listed in detail in the protocol, interreader variability in interpretation of images is well described in the literature.41,42
In conclusion, histology was the only significant independent predictor of EFS after adjusting for metastatic status, initial extent of resection of the primary tumor, regimen, age, and sex (P < 0.001), with ND medulloblastoma patients having significantly better EFS than patients with classic tumors. No significant difference was found between classic and LCA tumors. A significant proportion of ND medulloblastoma patients are surviving without any irradiation with preservation of mean IQ and memory. Lack of correlation with molecular subgroups due to absence of prospective tissue collection and sparse longitudinal neuropsychological outcome date are significant limitations.
Funding
This clinical trial was supported in part by Alex’s Lemonade Stand, Pediatric Cancer Research, Soccer for Hope, Michael Hoefflin, McAllister, Martel, Making Headway, and From Maddi’s Closet Foundations, Children’s Hospital Los Angeles/Saban Research Institute, and University of Southern California Norris Cancer Center award number P30CA014089 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest statement. None of the authors are aware of any conflict of interest.
Authorship statement. Conceptualization, data curation, methodology, investigation, project administration, supervision, writing-original draft, review, editing, and funding acquisition: Girish Dhall, Jonathan Finlay. Data curation, statistical analysis, methodology, writing-review, and editing: Lingyun Ji, Sharon H. O’Neil, Ashley M. Whitaker, Kelley Haley, Richard Sposto. Investigation, resources, methodology, validation, writing-review, and editing: Marvin Nelson, Floyd Gilles, Sharon Gardner, Jeffrey Allen, Albert Cornelius, Kamnesh Pradhan, James Garvin, Randal Olshefski, Juliette Hukin, Melanie Comito, Stewart Goldman, Mark Atlas, Andrew Walter, Stephen Sands.
Supplementary Material
References
- 1. Rutkowski S, Bode U, Deinlein F, et al. . Treatment of early childhood medulloblastoma by postoperative chemotherapy alone N Engl J Med. 2005;352(10):978–986. [DOI] [PubMed] [Google Scholar]
- 2. Lafay-Cousin L, Bouffet E, Strother D, et al. . Phase II study of nonmetastatic desmoplastic medulloblastoma in children younger than 4 years of age: a report of the Children’s Oncology Group (ACNS1221). J Clin Oncol. 2020;38(3):223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Robinson GW, Rudneva VA, Buchhalter I, et al. . Risk-adapted therapy for young children with medulloblastoma (SJYC07): therapeutic and molecular outcomes from a multicentre, phase 2 trial Lancet Oncol. 2018;19(6):768–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ostrom QT, Cioffi G, Gittleman H, et al. . CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 2019;21(S5):1–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chapman CA, Waber DP, Bernstein JH, et al. . Neurobehavioral and neurologic outcome in long-term survivors of posterior fossa brain tumors: role of age and perioperative factors. J Child Neurol. 1995;10(3):209–212. [DOI] [PubMed] [Google Scholar]
- 6. Radcliffe J, Bunin GR, Sutton LN, Goldwein JW, Phillips PC. Cognitive deficits in long-term survivors of childhood medulloblastoma and other noncortical tumors: age-dependent effects of whole brain radiation. Int J Dev Neurosci. 1994;12(4):327–334. [DOI] [PubMed] [Google Scholar]
- 7. Ris MD, Noll RB. Long-term neurobehavioral outcome in pediatric brain tumor patients: review and methodological critique. J Clin Exp Neuropsychol. 1994;16:21–42. [DOI] [PubMed] [Google Scholar]
- 8. Constine LS, Woolf PD, Cann D, et al. . Hypothalamic-pituitary dysfunction after radiation for brain tumors. N Engl J Med. 1993;328(2):87–94. [DOI] [PubMed] [Google Scholar]
- 9. Kiltie AE, Lashford LS, Gattamaneni HR. Survival and late effects in medulloblastoma patients treated with craniospinal irradiation under three years old. Med Pediatr Oncol. 1997;28(5):348–354. [DOI] [PubMed] [Google Scholar]
- 10. Walter AW, Mulhern RK, Gajjar A, et al. . Survival and neurodevelopmental outcome of young children with medulloblastoma at St Jude Children’s Research Hospital. J Clin Oncol. 1999;17(12):3720–3728. [DOI] [PubMed] [Google Scholar]
- 11. Ris MD, Packer R, Goldwein J, Jones-Wallace D, Boyett JM. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a Children’s Cancer Group study. J Clin Oncol. 2001;19(15):3470–3476. [DOI] [PubMed] [Google Scholar]
- 12. Dhall G, Grodman H, Ji L, et al. . Outcome of children less than three years old at diagnosis with non-metastatic medulloblastoma treated with chemotherapy on the “Head Start” I and II protocols. Pediatr Blood Cancer. 2008;50(6):1169–1175. [DOI] [PubMed] [Google Scholar]
- 13. Chi SN, Gardner SL, Levy AS, et al. . Feasibility and response to induction chemotherapy intensified with high-dose methotrexate for young children with newly diagnosed high-risk disseminated medulloblastoma. J Clin Oncol. 2004;22(24):4881–4887. [DOI] [PubMed] [Google Scholar]
- 14. Sands SA, Pasichow KP, Weiss R, et al. . Quality of life and behavioral follow-up study of Head Start I pediatric brain tumor survivors. J Neurooncol. 2011;101(2):287–295. [DOI] [PubMed] [Google Scholar]
- 15. Saha A, Salley CG, Saigal P, et al. . Late effects in survivors of childhood CNS tumors treated on Head Start I and II protocols. Pediatr Blood Cancer. 2014;61(9):1644–1652; quiz 1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sands SA, Oberg JA, Gardner SL, Whiteley JA, Glade-Bender JL, Finlay JL. Neuropsychological functioning of children treated with intensive chemotherapy followed by myeloablative consolidation chemotherapy and autologous hematopoietic cell rescue for newly diagnosed CNS tumors: an analysis of the Head Start II survivors. Pediatr Blood Cancer. 2010;54(3):429–436. [DOI] [PubMed] [Google Scholar]
- 17. Chang CH, Housepian EM, Herbert C Jr. An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology. 1969;93(6):1351–1359. [DOI] [PubMed] [Google Scholar]
- 18. Milano G, Thyss A, Serre Debeauvais F, et al. . CSF drug levels for children with acute lymphoblastic leukemia treated by 5 g/m2 methotrexate. A study from the EORTC Children’s Leukemia Cooperative Group. Eur J Cancer. 1990;26(4):492–495. [DOI] [PubMed] [Google Scholar]
- 19. Csordas K, Hegyi M, Eipel OT, Muller J, Erdelyi DJ, Kovacs GT. Comparison of pharmacokinetics and toxicity after high-dose methotrexate treatments in children with acute lymphoblastic leukemia. Anticancer Drugs. 2013;24(2):189–197. [DOI] [PubMed] [Google Scholar]
- 20. Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 21. Trotti A, Colevas AD, Setser A, et al. . CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176–181. [DOI] [PubMed] [Google Scholar]
- 22. Cox DR, Oakes D.. Analysis of Survival Data. London; New York: Chapman and Hall; 1984. [Google Scholar]
- 23. StataCorp. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP; 2009. [Google Scholar]
- 24. IBM SPSS Statistics for Macintosh, Version 25.0. Armonk, NY: IBM Corp; 2017. [Google Scholar]
- 25. Crawford JR, Anderson V, Rankin PM, MacDonald J. An index-based short-form of the WISC-IV with accompanying analysis of the reliability and abnormality of differences. Br J Clin Psychol. 2010;49(Pt 2):235–258. [DOI] [PubMed] [Google Scholar]
- 26. Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. 4th ed San Antonio, TX: Pearson; 2012. [Google Scholar]
- 27. Wechsler D, Kaplan E, Fein D, et al. . Wechsler Intelligence Scale for Children. 4th ed San Antonio, TX: Pearson; 2003. [Google Scholar]
- 28. Oakland T, Harrison P.. Adaptive Behavior Assessment System. 2nd ed Torrance, CA: WPS; 2003. [Google Scholar]
- 29. Cohen M. Children’s Memory Scale. San Antonio, TX: Pearson; 1994. [Google Scholar]
- 30. Delis DC, Kramer JH, Kaplan E, Ober BA.. California Verbal Learning Test‒Children’s Version. San Antonio, TX: Pearson; 1994. [Google Scholar]
- 31. Geyer JR, Sposto R, Jennings M, et al. . Children’s Cancer Group. Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: a report from the Children’s Cancer Group. J Clin Oncol. 2005;23(30):7621–7631. [DOI] [PubMed] [Google Scholar]
- 32. Grill J, Sainte-Rose C, Jouvet A, et al. . French Society of Paediatric Oncology. Treatment of medulloblastoma with postoperative chemotherapy alone: an SFOP prospective trial in young children. Lancet Oncol. 2005;6(8):573–580. [DOI] [PubMed] [Google Scholar]
- 33. Grundy RG, Wilne SH, Robinson KJ, et al. ; Children’s Cancer and Leukaemia Group (formerly UKCCSG) Brain Tumour Committee Primary postoperative chemotherapy without radiotherapy for treatment of brain tumours other than ependymoma in children under 3 years: results of the first UKCCSG/SIOP CNS 9204 trial. Eur J Cancer. 2010;46(1):120–133. [DOI] [PubMed] [Google Scholar]
- 34. Ashley DM, Merchant TE, Strother D, et al. . Induction chemotherapy and conformal radiation therapy for very young children with nonmetastatic medulloblastoma: Children’s Oncology Group Study P9934. J Clin Oncol. 2012;19:3470–3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cohen BH, Geyer JR, Miller DC, et al. ; Children’s Oncology Group Pilot study of intensive chemotherapy with peripheral hematopoietic cell support for children less than 3 years of age with malignant brain tumors, the CCG-99703 phase I/II study. A report from the Children’s Oncology Group. Pediatr Neurol. 2015;53(1):31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thompson MC, Fuller C, Hogg TL, et al. . Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol. 2006;24(12):1924–1931. [DOI] [PubMed] [Google Scholar]
- 37. Northcott PA, Korshunov A, Witt H, et al. . Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29(11):1408–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kool M, Korshunov A, Remke M, et al. . Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012;123(4):473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cavalli FMG, Remke M, Rampasek L, et al. . Intertumoral heterogeneity within medulloblastoma subgroups. Cancer Cell. 2017;31(6): 737–754.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pietsch T, Schmidt R, Remke M, et al. . Prognostic significance of clinical, histopathological, and molecular characteristics of medulloblastomas in the prospective HIT2000 multicenter clinical trial cohort. Acta Neuropathol. 2014;128(1):137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tang PA, Pond GR, Chen EX. Influence of an independent review committee on assessment of response rate and progression-free survival in phase III clinical trials. Ann Oncol. 2010;21(1):19–26. [DOI] [PubMed] [Google Scholar]
- 42. Yoon SH, Kim KW, Goo JM, et al. . Observer variability in RECIST-based tumor burden measurements: a meta-analysis. Eur J Cancer. 2016;53:5–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.