Abstract
Background
Infant medulloblastoma represents an enormous challenge in neuro-oncology, due to their simultaneous high-risk of recurrence and high risk of severe neurodevelopmental sequelae with craniospinal irradiation. Currently infant medulloblastoma are treated with intensified protocols, either comprising intraventricular methotrexate or autologous transplant, both of which carry significant morbidity and are not feasible in the majority of the world. We sought to evaluate the molecular predictors of outcome in a cohort of infants homogeneously treated with induction chemotherapy, focal radiation and maintenance chemotherapy.
Methods
In a retrospective analysis, 29 young children treated with a craniospinal irradiation sparing strategy from Hospital Garrahan in Buenos Aires were profiled using Illumina HumanMethylationEPIC arrays, and correlated with survival.
Results
Twenty-nine children (range, 0.3–4.6 y) were identified, comprising 17 sonic hedgehog (SHH), 10 Group 3/4, and 2 non-medulloblastomas. Progression-free survival (PFS) across the entire cohort was 0.704 (95% CI: 0.551–0.899). Analysis by t-distributed stochastic neighbor embedding revealed 3 predominant groups, SHHβ, SHHγ, and Group 3. Survival by subtype was highly prognostic with SHHγ having an excellent 5-year PFS of 100% (95% CI: 0.633–1) and SHHβ having a PFS of 0.56 (95% CI: 0.42–1). Group 3 had a PFS of 0.50 (95% CI: 0.25–1). Assessment of neurocognitive outcome was performed in 11 patients; the majority of survivors fell within the low average to mild intellectual disability, with a median IQ of 73.5.
Conclusions
We report a globally feasible and effective strategy avoiding craniospinal radiation in the treatment of infant medulloblastoma, including a robust molecular correlation along with neurocognitive outcomes.
Keywords: brain tumor, infant, medulloblastoma, SHH, radiation
Key points.
1. Treatment of infant SHH medulloblastoma without intensification is feasible and effective.
2. Survival of SHHγ/SHH-II is 100% using multimodal therapy.
3. Whole posterior fossa irradiation results in significant neurocognitive sequelae.
Importance of the Study.
Our study provides a framework for a globally feasible and cost-effective approach to the treatment of infant medulloblastoma. With the recent publications of SJYC07 and ACNS1221 suggesting de-escalation of therapy results in an unacceptable number of relapses across all SHH infants, the standard of care for even low-risk SHH has remained intensification of therapy with either intraventricular methotrexate or autologous transplant, both of which are not feasible or practical in the majority of the world. Our strategy could be considered using conformal radiotherapy across most low to middle income countries.
Infant medulloblastoma constitutes a heterogeneous group, currently defined as being under age 3–5 (age cutoff varies by cooperative group), and one of the most daunting therapeutic challenges for pediatric neuro-oncologists.1 This is a unique population at highest risk for neurodevelopmental sequelae as a result of craniospinal irradiation (CSI) therapy.2 The main challenge in the management of infants with medulloblastoma has been to balance the fine line between improved overall survival (OS) and impaired functional outcomes. Indeed the inability of survivors to live independently, owing to severe intellectual impairment resulting from the neurotoxicity of radiation exposure, limits the adoption of CSI for high-risk disease subgroups such as in older children.3,4 Although a variety of factors play a role in the development of such sequelae, it has been demonstrated that age at diagnosis, perioperative complications, and CSI are the major causes of this impairment.5,6 Many North American and European cooperative group clinical trials attempted to reduce, delay, or avoid CSI either with intrathecal chemotherapy (HIT-SKK92) or myeloablative chemotherapy with high-dose chemotherapy and stem cell rescue (Head Start studies, CCG-99703) with variable degrees of success.7–16 However, the risks are not insignificant of CNS infection, leukoencephalopathy with intraventricular therapy, and the morbidity and toxic mortality of a myeloablative regimen.17
Half to two-thirds of infant medulloblastoma are SHH-driven tumors, one-third are Group 3 or, much less commonly, Group 4 tumors, with the WNT subgrouping almost nonexistent.18–20 The majority of medulloblastomas histologically defined as desmoplastic are SHH-driven tumors. Infant SHH tumors are mainly distributed across SHHβ and SHHγ, with disparate outcomes and copy-number profiles. SHHβ tumors are frequently metastatic and have a worse OS compared with SHHγ. Moreover, SHHγ are enriched for the medulloblastoma with extensive nodularity (MBEN) histology, which is known to portend more indolent clinical behavior.18,19,21,22 A similar trend was observed in the prospective trial of infant medulloblastoma ACNS1221, where 6 of 7 MBEN tumors belonged to the SHHγ/SHH-II group, and MBEN histology as a whole had a 100% survival. Although almost all SHH tumors with MBEN histology are assigned to SHHγ, only a minority of SHHγ tumors have MBEN histology, demonstrating that histology alone is an inadequate surrogate to identify SHHγ tumors.23 Despite this enormous molecular heterogeneity, there is not a robust molecular risk stratification system with adapted treatment correlation in infant medulloblastoma.22
The recent observation that a substantial proportion of both low- and high-risk infants treated with non-intensified regimens relapse has resulted in either autologous transplant or intraventricular methotrexate (MTX) being considered the standard of care for infant medulloblastoma.24 Autologous transplant-based strategies and intraventricular MTX carry challenges with feasibility across the majority of low- to middle-income settings due to limited resources, including the high cost of transplant, the high cost of thiotepa, and difficulties with neurosurgical expertise, including insertion of intraventricular devices. As such, new globally feasible strategies for these groups are urgently needed. Here, we evaluate the molecular predictors of outcome in a cohort of infants homogeneously treated with induction chemotherapy, focal radiation, and maintenance chemotherapy at Hospital Garrahan in Buenos Aires, Argentina.
Methods
Patients and Treatment
The study and sample collection were performed in accordance with the approval of the Hospital Garrahan and Hospital for Sick Children Research ethics boards, with a waiver of consent granted for retrospective studies. A retrospective review was conducted at Hospital Garrahan to identify and diagnose medulloblastoma in infants to be treated with CSI sparing strategies. All patients received initial maximum safe surgical tumor resection. The treatment strategy was based on the standard of care treatment administered between 2002 and 2020 at Hospital Garrahan, based on a modified POG-9934 strategy. The assessment of presence or absence of residual tumor was based on brain CT with enhancement (15 patients) or MRI with gadolinium (14 patients) within 48 hours after surgery. All patients had histologically confirmed primary medulloblastoma and were staged with imaging of the craniospinal axis and cytological examination of the lumbar cerebrospinal fluid (CSF) after 14 days following the surgery at time of diagnosis. All patients received the first postoperative treatment course according to schedule of treatment within 4 weeks after surgery. Postsurgical adjuvant therapy was based on a modified POG9934 regimen. Specifically, induction therapy consisted of 5 cycles of chemotherapy given at 21-day intervals. The induction therapy consisted of (i) cisplatin 3.5 mg/kg day 0, (ii) vincristine (VCR) 0.05 mg/kg on days 0, 7, and 14 (days 7 and 14 were included only in cycles 1, 2, and 3), (iii) cyclophosphamide (CPM) 30 mg/kg on days 1 and 2, (iv) etoposide 4 mg/kg (VP-16) on days 1 and 2, (v) MTX 266 mg/kg on day 3 only for metastatic medulloblastoma at diagnosis or residual tumor more than 1.5 cm2. Conformal focal radiation to the posterior fossa (3D/intensity-modulated radiotherapy) with concurrent daily temozolomide was initiated after induction in patients aged at least 18 months, whereas those younger than 18 months received the maintenance regimen to delay focal radiotherapy until the age of 18 months. A total dose of focal post fossa radiation was 54 Gy in 1.8 Gy fractions over 6 weeks. Temozolomide at 2.5 mg/kg/day was administered daily during radiation and for a maximum of 42 days. Maintenance chemotherapy was indicated after focal radiation and consisted of 4 cycles given at 56-day intervals: (i) carboplatin 18 mg/kg on day 0, (ii) VCR 0.05 mg/kg on days 0 and 28, (iii) CPM 50 mg/kg on day 28, (iv) etoposide 1.6 mg/kg orally daily from days 1–14 and days 29–43. A comparison of regimens to POG-9934 and SJYC07 is provided in Supplementary Table 1.
MR imaging of the entire neuroaxis was performed at completion of induction, after 3–4 weeks of completion of radiation, and every 3 months during maintenance chemotherapy and during regular intervals post completion of therapy.
Functional Outcomes
Intellectual functioning after treatment was assessed by several different commonly applied intelligence test versions due to the longitudinal nature of this study, and school assessments were performed through telephone encounter. Test versions used included the Wechsler Intelligence Scale for Children (WISC-IV and WISC-V), the Wechsler Preschool and Primary Scale of Intelligence (WPPSI-III and WPPSI-IV), and the Stanford-Binet Intelligence Scale. We analyzed the following components of these intelligence scales: Full Scale Intelligence Quotient (FSIQ) is a reliable measure of overall cognitive functioning, the Verbal Comprehension Index (VCI) measures verbal reasoning, the Perceptual Reasoning Index (PRI) evaluates the interpretation/organization of visual/nonverbal information, the Working Memory Index (WMI) measures attentional and concentration abilities, and the Processing Speed Index (PSI) evaluates the speed of graph motor and mental processing. School assessment was performed by telephone encounter.
Genome-wide DNA Methylation Profiling
Formalin-fixed paraffin embedded–derived samples were analyzed on the Illumina Infinium HumanMethylationEPIC at the Princess Margaret Cancer Centre–OICR Translational Genomics Laboratory (Toronto, Ontario) according to manufacturer’s instructions and as previously described; all analysis was conducted in the R Statistical Environment (v3.6.0).25 Molecular subgroup was determined using the Heidelberg brain tumor classifier (https://www.molecularneuropathology.org/mnp) as previously described. For prediction of SHH subtypes, we used a bootstrapping k-nearest neighbor classifier (using 1000 bootstraps). The training dataset was composed of Illumina Infinium HumanMethylation450 BeadChip array data downloaded for 223 MBSHH samples previously subgroup and subtype classified.24,25 For the classification we restricted the datasets to the 500 most important probes based on ExtraTreesClassifier importance scores predicted from the training cohort. Sample similarity was visualized utilizing t-distributed stochastic neighbor embedding (t-SNE) based on a weighted Pearson correlation distance matrix. DNA copy number variants were inferred from Illumina Infinium HumanMethylationEPIC arrays using the Conumee R package with default parameters as previously described.26 Assignment of SHH subtypes was performed as previously described, with SHHβ corresponding to SHH-I, and SHHγ corresponding to SHH-II.24
Statistical Analysis
Progression-free survival (PFS) and OS were analyzed by the Kaplan–Meier method and P-values reported using the log-rank test. Associations between covariates and risk groups were tested by Fisher’s exact test. All statistical analyses were performed in the R statistical environment (v3.6.0), using the R packages survival (v2.41–3), survminer (v0.4.6), and ggplot2 (v3.3.0).
Results
Demographics of the Entire Cohort
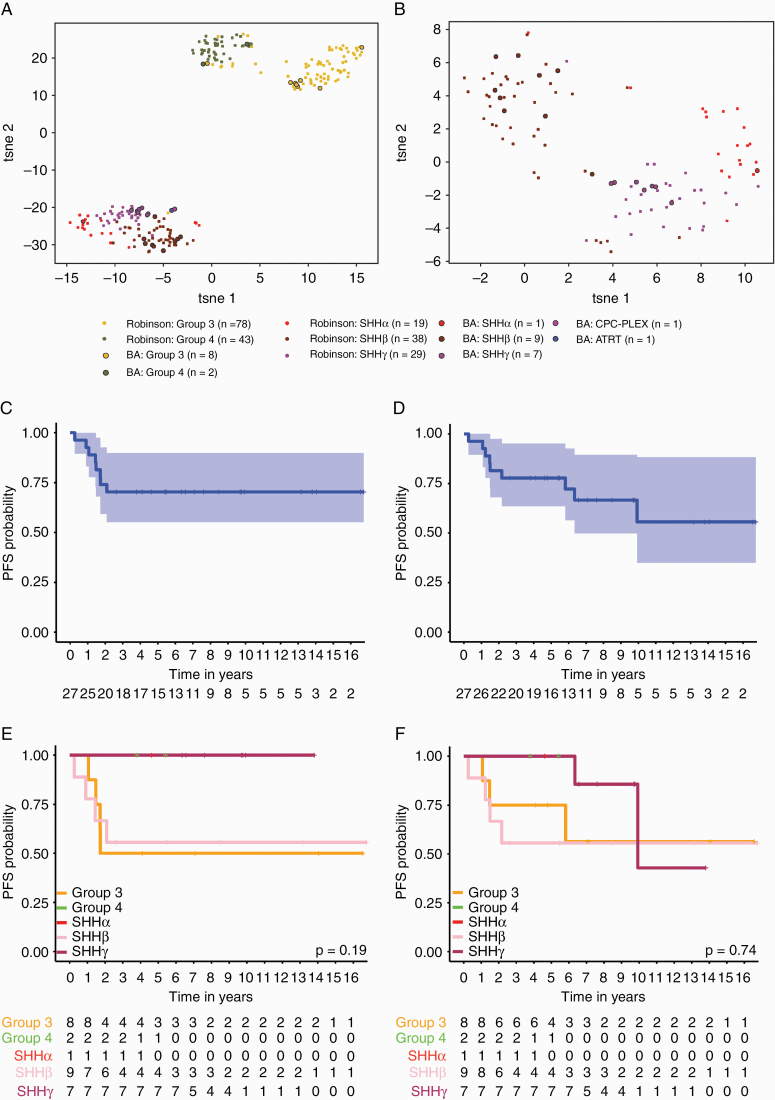
Twenty-nine infants treated between October 2002–November 2016 were identified and analyzed using genome wide methylation profiling. Applying the Molecular Neuropathology 2.0 classifier (https://www.molecularneuropathology.org/mnp), samples were subgrouped into 17 SHH, 10 Group 3/4 (eight Group 3, two Group 4) and 2 non-medulloblastomas (1 atypical teratoid rhabdoid tumor–SHH, 1 choroid plexus carcinoma). These 2 non-medulloblastomas were excluded from all subsequent analyses. Analysis by t-SNE revealed 3 predominant groups, SHHβ, SHHγ, and Group 3, with 1 SHHα, 9 SHHβ, and 7 SHHγ (Fig. 1A, B). Applying the recently identified Groups 3 and 4 subtypes, we identified 1 Group I, 1 Group II, 6 Group IV and 2 Group VII. Demographic and disease characteristics of the 27 infant medulloblastoma patients are summarized in Table 1 (Supplementary Fig. 1 and Supplementary Table 2). Median age at diagnosis was 2.2 years (range, 0.4–4.6 y). Four patients were metastatic at diagnosis, 3 in Group 3 and 1 in SHHβ group. Fourteen patients had hydrocephalus at diagnosis who required shunting or a third ventriculostomy procedure (Supplementary Table 3). Five patients had residual tumor >1.5 cm2. Two of them were in SHHγ, 2 in SHHβ, and 1 in Group 3. Median age at focal radiation was 3.5 years (range, 1.5–5). Three patients younger than 18 months received the maintenance regimen after induction to delay focal radiotherapy until the age of 18 months.
Fig. 1.
Molecular and clinical characterization of the Buenos Aires (BA) infant medulloblastoma cohort. (A) tSNE visualization of genome-wide methylation profiling from 29 infants treated in BA in comparison with Robinson et al cohort from St Jude’s. (B) Plot by tSNE of SHH samples only. Samples are colored according to their respective consensus cluster affiliation. Kaplan‒Meier survival curve of (C) PFS and (D) OS for 27 BA-infant medulloblastoma. Shaded areas around curve represent 95% CIs. Kaplan‒Meier estimates of (E) PFS and (F) OS for 27 BA-infant medulloblastoma stratified by molecular subgroup and subtype. P-values are determined using the log-rank method.
Table 1.
Subgroup-specific demographics across the entire cohort
| SHH | Group 3 | Group 4 | |
|---|---|---|---|
| n = 17 | n = 8 | n = 2 | |
| Age at Diagnosis | |||
| Median, y (interquartile range) | 2.2 (1.01–2.64) | 2.3 (1.07–2.63) | 3.1 (2.35–3.88) |
| <3 y | 16 | 7 | 0 |
| 3–5 y | 1 | 1 | 2 |
| Sex | |||
| Female | 10 | 2 | 2 |
| Male | 7 | 6 | 0 |
| Histological Group | |||
| Nodular/desmoplastic | 15 | 1 | 1 |
| Classic | 1 | 6 | 1 |
| Large cell/anaplastic | 1 | 1 | 0 |
| Metastatic Status | |||
| M0 | 16 | 5 | 2 |
| M+ | |||
| M1 | 0 | 1 | 0 |
| M 2–3 | 1 | 2 | 0 |
| Residual Disease | |||
| <1.5 cm 2 | 13 | 7 | 2 |
| ≥1.5 cm 2 | 4 | 1 | 0 |
| Pattern of Relapse | |||
| Metastatic | 4 | 4 | 0 |
| Local | 0 | 0 | 0 |
| Secondary Tumor | |||
| 2 | 0 | 0 |
Subgroup- and Subtype-Specific Outcomes
Five-year PFS across the cohort was 0.704 (95% CI: 0.551–0.899) and 5-year OS was 0.778 (95% CI: 0.636–0.952) (Fig. 1C, D). Two patients progressed during induction chemotherapy, both with disseminated disease (M3) at diagnosis, 1 in Group 3 and 1 in the SHHβ group. Only one patient with residual tumor had tumor progression, this patient was a metastatic (M3), group SHHβ at diagnosis. Survival by subtype was highly prognostic, with SHHγ having an excellent 5-year PFS of 100% (95% CI: 0.633–1.000), and SHHβ having an inferior PFS of 0.56 (95% CI: 0.420–1). Group 3 had an intermediate PFS of 0.50 (95% CI: 0.25–1) (Fig. 1E, F). No local relapses were observed in this cohort specifically; all SHHβ and Group 3 relapsed in the metastatic compartment. Two late deaths due to secondary tumors were identified in SHHγ, 1 osteosarcoma and 1 undifferentiated sarcoma, 5.9 years and 9.8 years, respectively, after completed therapy. Both secondary tumors were located within the radiation treatment volume and no family history or physical stigma of hereditary predisposition syndrome was found. Germline analyses of SUFU, PTCH1, and TP53 were not performed clinically, and could not be performed retrospectively due to a lack of germline DNA. Consistent with previous descriptions, we observed 3 patients with chromosome 2 gain in the SHHβ subtype (1 progressed and 2 are alive with complete remission).
Assessment of Intellectual Functioning
Assessment of neurocognitive outcome was performed in 11 patients with a median time of 6.8 years after diagnosis (range, 3.4–13), 5 of them at various timepoints post completion of therapy. The majority of survivors fell within the low average to mild intellectual disability range in the Wechsler Intelligence Scale, with a median IQ at last assessment of 73.5 (range, 47–93) (Fig. 2, Supplementary Tables 3 and 4). A school assessment was performed in 19 patients where 13 of 19 patients required special education needs. Specifically, 8 were enrolled in regular schools on an adapted curriculum with or without additional learning support and 5 were enrolled in dedicated special education schools. Audiometric assessments were available in 10 patients, of whom 6 demonstrated significant toxicity with Common Terminology Criteria for Adverse Events v4 grade 2 ototoxicity at the end of treatment (Supplementary Table 3). No trend toward differences in functional outcome were observed between those patients with or without hydrocephalus requiring shunting.
Fig. 2.
Declines in neurocognitive status over time in patients with infant medulloblastoma treated with posterior fossa radiotherapy. Estimated declines in (A) the Full-Scale Intelligence Quotient (FSIQ) over time (years) since diagnosis. (B) Processing Speed Index (PSI), (C) Perceptual Reasoning/Organization Index (PRI), (D) Working Memory index (WMI), and (E) Verbal Comprehension Index (VCI) in linear term model. Lines represent patients who were seen for longitudinal intellectual assessments; each dot represents a patient who was seen once. The dark gray line at 80 represents the delineation between borderline and low average.
Discussion
Herein we report an effective and feasible strategy that avoids CSI in the treatment of infant SHH medulloblastoma. This strategy is one of the first to avoid autologous transplant or intraventricular MTX and achieve high survival rates, suggesting this may be a feasible strategy to be implemented in the developing world across all subtypes and subgroups of infant medulloblastoma. This study is one of the first studies of infant medulloblastoma to incorporate robust molecular correlates along with neurocognitive outcomes in a subset of patients.
Our study reports far superior outcomes to 2 recently reported trial cohorts omitting intraventricular MTX or high-dose chemotherapy with autologous stem cell support. Indeed, both studies, ACNS1221 and SJYC07, performed post-hoc molecular profiling, and revealed inferior outcomes across all SHH medulloblastoma, but most importantly inferior outcome within both SHHβ and SHHγ, suggesting that these strategies are woefully inadequate for either subtype. Specifically in the SHHγ group, both ACNS1221 and SJYC07 report survivals below 70%, suggesting that even for this seemingly good performing subtype, intensification of therapy will be required.24,27 The propensity for local relapses particularly in SHHγ in both ACNS1221 and SJYC07 suggests that additional measures for local control are necessary and may account for the superior outcomes we are observing. Indeed, we have previously shown that SHH medulloblastoma recur locally in 60% of cases, suggesting that unlike Groups 3 and 4, local control is necessary in the majority of infants.28 Our study is in keeping with the results of both ACNS1221 and SJYC07, where SHHβ tumors relapse with metastasis, and our previous observations that Groups 3 and 4 relapse almost exclusively with metastatic dissemination.24,27,28 This suggests that microscopic leptomeningeal metastases not visible by neuroimaging or CSF examination are resistant to therapy where additional local therapies targeting the tumor bed are unlikely to improve survival.
The disappointing PFS observed in both ACNS1221 and SJYC07 had led to the incorporation of either autologous transplant such as in the Head Start studies and CCG-99703 or intraventricular MTX as being the gold standard therapy for SHH medulloblastoma. Previously, a retrospective cohort of 53 patients treated as per CCG-99703 revealed excellent radiation-free survival for the SHH subgroup, with PFS rising to 86.2% at 5 years.13 Similar results have been for both SHH and desmoplastic-nodular (DN)/MBEN in the German HIT-SKK protocol, with survivals exceeding 90%.7,12 The survival for infants with DN/MBEN in the Head Start 3 studies was also close to 90% albeit this study is limited by a lack of biological correlates.29 Unfortunately, both strategies have significant disadvantages, with autologous transplant not being feasible or available in the majority of the world, and intraventricular MTX requiring the insertion of an Ommaya reservoir with significant risk of leukoencephalopathy and long-term neurocognitive toxicity. Indeed, the insertion of Ommaya with intraventricular administration poses difficulties in much of the developing world, and even in North America has posed challenging pertaining to the associated complications, while high-dose chemotherapy with autologous stem cell transplant harbors significant systemic risks. Our de-escalation regimen with focal radiation is an attractive option for both SHH subtypes, considering this regimen is feasible and available in 95% of the world, including low and middle low income countries, which results in superior outcomes to the ACNS1221/SJYC07 approach, avoiding complications associated with an intraventricular access device, as well as high-dose chemotherapy with stem cell rescue toxicities. Although the North American cooperative groups have previously evaluated posterior fossa irradiation in infant medulloblastoma (POG-9934), the lack of any molecular correlates renders this study almost impossible to interpret in the context of modern risk stratification.30 A major difference between POG-9934 and our strategy is the inclusion of concurrent temozolomide during radiotherapy, which prevented children from being exposed to a 10–12 week gap in chemotherapy, which is a feature of other protocols incorporating focal irradiation. Our results also suggest that this approach may be promising for Group 3 medulloblastoma, where we observe significantly improved survival compared with SJYC07, where survival was below 10%, suggesting that this may be a feasible approach across all infant medulloblastoma. Our results in Group 3 are comparable to treatment with high-dose chemotherapy as per CCG-99703, where PFS was 49.1%, and intraventricular MTX as per the German HIT cohort, where 3 year of infant Group 3 was approximately 25%).7
Our approach does carry a significant risk to long-term neurocognitive outcomes. A specific concern is our use of whole posterior fossa radiation compared with the conformal tumor bed irradiation used in posterior fossa ependymoma.31–34 Indeed, the use of conformal tumor bed irradiation in infants with posterior fossa ependymoma results in IQ reductions of 5 points over 5 years, which is significantly better than our results. Ideally a very conformal approach such as with protons could play a role, although the lack of proton irradiation and even photon conformal radiation (intensity modulated radiotherapy) in the developing world precludes this approach globally. It previously has been demonstrated that both hydrocephalus requiring CSF diversion and mutism are associated with poor intellectual functioning with distinctive trajectory of decline and mechanism of injury.31,35 Our recent observation that cerebellar mutism is almost nonexistent in SHH medulloblastoma would suggest that undocumented cerebellar mutism would not account for the observed poor neurocognitive outcomes.36 However, in our cohort, we observe no clear association between hydrocephalus at diagnosis and poor neurocognitive outcomes, suggesting that hydrocephalus alone cannot account for the significant neurocognitive decline. One limitation of our analysis is the likely bias toward formal neuropsychological testing in lower functioning survivors, which will need to be addressed in future prospective studies. As such, any further attempts to incorporate focal irradiation into the management of SHH medulloblastoma, particularly the SHHγ group with an excellent outcome, will need to carefully determine the risk ratio of impaired neurocognitive deficits of radiation versus the neurocognitive deficits of intraventricular MTX.
Previously, POG-9934 has reported acceptable neurocognitive declines with induction chemotherapy and posterior fossa irradiation, using a telephone-based abbreviated assessment at multiple timepoints up to 48 months postdiagnosis. However, median functional quotients in the POG-9934 study was 72.4, which is consistent with our results; significantly below the population mean, it would constitute mild-moderate intellectual disability. Considering that the long-term declines with radiotherapy continue through adolescence, we would interpret our results with a guarded approach and would advocate for continued radiation sparing approaches which limit treatment-related toxicity.32 Within the developed world, conformal proton radiotherapy could represent an attractive alternative, albeit not feasible in most of the world.37,38 However, compared with autologous transplant or intraventricular MTX, our strategy with conformal fields may be an attractive, effective, low-cost option in much of the developing world. Indeed at Hospital Garrahan in Buenos Aires we have moved to tumor bed boosts based on intensity-modulated radiotherapy, which highlights the feasibility of this approach in South America, avoiding high-dose MTX with the goal to minimize neurocognitive decline.31
The major limitations of this study are the retrospective design and lack of formal evaluation of ototoxicity and neurocognition, where a clear selection of testing in the more impaired population may exist. Another major limitation of our study is the lack of germline material, which precludes germline analysis, and genome sequencing. Indeed, the 2 late deaths in the SHHγ group were likely radiation-induced second malignancies, and germline predisposition syndromes could not be assessed. Properly controlled clinical trials for infant medulloblastoma with appropriate functional follow-up specific to low to middle income countries is urgently required for this patient population. Moreover, with respect to ototoxicity, otoprotective agents such as sodium thiosulfate hold tremendous promise for this patient population and warrant urgent evaluation as part of these clinical trials.39
We report a novel and effective treatment strategy for infant medulloblastoma which warrants further investigation in larger molecularly defined cohorts. The routine application of DNA methylation–based tumor profiling has the potential to enable rapid centralized molecular classification in future clinical trials. Moving forward, international cooperation and innovative trial design will be required to develop molecular risk adapted approaches to therapy for all infants worldwide with medulloblastoma.
Funding
This work was funded by Meagan’s Walk Foundation Fellowship in Pediatric Neuro-Oncology and The Terry Fox Foundation International Fellowship to LB, Canadian Institutes for Health Research, Garron Family Cancer Centre, the C.R. Younger Foundation, Meagan’s Walk and b.r.a.i.n.child to VR. This study was also partially funded with support provided by the Government of Ontario, Ministry of Research, Innovation and Science, and the Princess Margaret Cancer Foundation and the Princess Margaret Cancer Centre–OICR Translational Genomics Laboratory.
Conflict of interest statement. The authors have no conflicts of interest.
Authorship statement. Conceptualization: LB, DA, VR. Methodology: LB, DA, VR. Investigation: LB, DA, UB, EB, VR. Data curation: LB, DA, CS, AG, FL, GL, CR, AH. Formal analysis: LB, AH, VR. Writing, review, and editing: LB, EB, VR. Resources: LB, DA, CS, AG, FL, GL, CR. Project administration: LB, VR. Supervision: VR. Funding acquisition: LB, VR.
Supplementary Material
References
- 1. Rutkowski S, Cohen B, Finlay J, et al. Medulloblastoma in young children. Pediatr Blood Cancer. 2010;54(4):635–637. [DOI] [PubMed] [Google Scholar]
- 2. Lafay-Cousin L, Bouffet E, Hawkins C, Amid A, Huang A, Mabbott DJ. Impact of radiation avoidance on survival and neurocognitive outcome in infant medulloblastoma. Curr Oncol. 2009;16(6):21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Duffner PK, Horowitz ME, Krischer JP, et al. Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med. 1993;328(24):1725–1731. [DOI] [PubMed] [Google Scholar]
- 4. Kiltie AE, Lashford LS, Gattamaneni HR. Survival and late effects in medulloblastoma patients treated with craniospinal irradiation under three years old. Med Pediatr Oncol. 1997;28(5):348–354. [DOI] [PubMed] [Google Scholar]
- 5. Duffner PK. Long-term effects of radiation therapy on cognitive and endocrine function in children with leukemia and brain tumors. Neurologist. 2004;10(6):293–310. [DOI] [PubMed] [Google Scholar]
- 6. Palmer SL, Gajjar A, Reddick WE, et al. Predicting intellectual outcome among children treated with 35–40 Gy craniospinal irradiation for medulloblastoma. Neuropsychology. 2003;17(4):548–555. [DOI] [PubMed] [Google Scholar]
- 7. Pietsch T, Schmidt R, Remke M, et al. Prognostic significance of clinical, histopathological, and molecular characteristics of medulloblastomas in the prospective HIT2000 multicenter clinical trial cohort. Acta Neuropathol. 2014;128(1):137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cohen BH, Geyer JR, Miller DC, et al. ; Children’s Oncology Group Pilot study of intensive chemotherapy with peripheral hematopoietic cell support for children less than 3 years of age with malignant brain tumors, the CCG-99703 phase I/II study. A report from the Children’s Oncology Group. Pediatr Neurol. 2015;53(1):31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Muller K, Mynarek M, Zwiener I, et al. Postponed is not canceled: role of craniospinal radiation therapy in the management of recurrent infant medulloblastoma—an experience from the HIT-REZ 1997 & 2005 studies. Int J Radiat Oncol Biol Phys. 2014;88(5):1019–1024. [DOI] [PubMed] [Google Scholar]
- 10. von Bueren AO, von Hoff K, Pietsch T, et al. Treatment of young children with localized medulloblastoma by chemotherapy alone: results of the prospective, multicenter trial HIT 2000 confirming the prognostic impact of histology. Neuro Oncol. 2011;13(6):669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rutkowski S, von Hoff K, Emser A, et al. Survival and prognostic factors of early childhood medulloblastoma: an international meta-analysis. J Clin Oncol. 2010;28(33):4961–4968. [DOI] [PubMed] [Google Scholar]
- 12. Rutkowski S, Bode U, Deinlein F, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005;352(10):978–986. [DOI] [PubMed] [Google Scholar]
- 13. Lafay-Cousin L, Smith A, Chi SN, et al. Clinical, pathological, and molecular characterization of infant medulloblastomas treated with sequential high-dose chemotherapy. Pediatr Blood Cancer. 2016;63(9):1527–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Geyer JR, Sposto R, Jennings M, et al. ; Children’s Cancer Group Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: a report from the Children’s Cancer Group. J Clin Oncol. 2005;23(30):7621–7631. [DOI] [PubMed] [Google Scholar]
- 15. Ridola V, Grill J, Doz F, et al. High-dose chemotherapy with autologous stem cell rescue followed by posterior fossa irradiation for local medulloblastoma recurrence or progression after conventional chemotherapy. Cancer. 2007;110(1):156–163. [DOI] [PubMed] [Google Scholar]
- 16. Grill J, Sainte-Rose C, Jouvet A, et al. ; French Society of Pediatric Oncology Treatment of medulloblastoma with postoperative chemotherapy alone: an SFOP prospective trial in young children. Lancet Oncol. 2005;6(8):573–580. [DOI] [PubMed] [Google Scholar]
- 17. Pompe RS, von Bueren AO, Mynarek M, et al. Intraventricular methotrexate as part of primary therapy for children with infant and/or metastatic medulloblastoma: feasibility, acute toxicity and evidence for efficacy. Eur J Cancer. 2015;51(17):2634–2642. [DOI] [PubMed] [Google Scholar]
- 18. Shih DJ, Northcott PA, Remke M, et al. Cytogenetic prognostication within medulloblastoma subgroups. J Clin Oncol. 2014;32(9):886–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Taylor MD, Northcott PA, Korshunov A, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123(4):465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Northcott PA, Jones DT, Kool M, et al. Medulloblastomics: the end of the beginning. Nat Rev Cancer. 2012;12(12):818–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Remke M, Ramaswamy V. Infant medulloblastoma—learning new lessons from old strata. Nat Rev Clin Oncol. 2018;15(11):659–660. [DOI] [PubMed] [Google Scholar]
- 22. Nör C, Ramaswamy V. Clinical and pre-clinical utility of genomics in medulloblastoma. Expert Rev Neurother. 2018;18(8):633–647. [DOI] [PubMed] [Google Scholar]
- 23. Korshunov A, Sahm F, Stichel D, et al. Molecular characterization of medulloblastomas with extensive nodularity (MBEN). Acta Neuropathol. 2018;136(2):303–313. [DOI] [PubMed] [Google Scholar]
- 24. Robinson GW, Rudneva VA, Buchhalter I, et al. Risk-adapted therapy for young children with medulloblastoma (SJYC07): therapeutic and molecular outcomes from a multicentre, phase 2 trial. Lancet Oncol. 2018;19(6):768–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cavalli FMG, Hübner JM, Sharma T, et al. Heterogeneity within the PF-EPN-B ependymoma subgroup. Acta Neuropathol. 2018;136(2):227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hovestadt V, Remke M, Kool M, et al. Robust molecular subgrouping and copy-number profiling of medulloblastoma from small amounts of archival tumour material using high-density DNA methylation arrays. Acta Neuropathol. 2013;125(6):913–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lafay-Cousin L, Bouffet E, Strother D, et al. Phase II study of nonmetastatic desmoplastic medulloblastoma in children younger than 4 years of age: a report of the Children’s Oncology Group (ACNS1221). J Clin Oncol. 2020;38(3):223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ramaswamy V, Remke M, Bouffet E, et al. Recurrence patterns across medulloblastoma subgroups: an integrated clinical and molecular analysis. Lancet Oncol. 2013;14(12):1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dhall G, O’Neil SH, Ji L, et al. Excellent outcome of young children with nodular desmoplastic medulloblastoma treated on “Head Start” III: a multi-institutional, prospective clinical trial. Neuro Oncol. 2020. doi: 10.1093/neuonc/noaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ashley DM, Merchant TE, Strother D, et al. Induction chemotherapy and conformal radiation therapy for very young children with nonmetastatic medulloblastoma: Children’s Oncology Group study P9934. J Clin Oncol. 2012;30(26):3181–3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moxon-Emre I, Bouffet E, Taylor MD, et al. Impact of craniospinal dose, boost volume, and neurologic complications on intellectual outcome in patients with medulloblastoma. J Clin Oncol. 2014;32(17):1760–1768. [DOI] [PubMed] [Google Scholar]
- 32. Zapotocky M, Beera K, Adamski J, et al. Survival and functional outcomes of molecularly defined childhood posterior fossa ependymoma: cure at a cost. Cancer. 2019;125(11):1867–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mulhern RK, Palmer SL, Merchant TE, et al. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol. 2005;23(24):5511–5519. [DOI] [PubMed] [Google Scholar]
- 34. Merchant TE, Kiehna EN, Li C, Xiong X, Mulhern RK. Radiation dosimetry predicts IQ after conformal radiation therapy in pediatric patients with localized ependymoma. Int J Radiat Oncol Biol Phys. 2005;63(5):1546–1554. [DOI] [PubMed] [Google Scholar]
- 35. Moxon-Emre I, Taylor MD, Bouffet E, et al. Intellectual outcome in molecular subgroups of medulloblastoma. J Clin Oncol. 2016;34(34):4161–4170. [DOI] [PubMed] [Google Scholar]
- 36. Jabarkheel R, Amayiri N, Yecies D, et al. Molecular correlates of cerebellar mutism syndrome in medulloblastoma. Neuro Oncol. 2020;22(2):290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Antonini TN, Ris MD, Grosshans DR, et al. Attention, processing speed, and executive functioning in pediatric brain tumor survivors treated with proton beam radiation therapy. Radiother Oncol. 2017;124(1):89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kahalley LS, Peterson R, Ris MD, et al. Superior intellectual outcomes after proton radiotherapy compared with photon radiotherapy for pediatric medulloblastoma. J Clin Oncol. 2020;38(5):454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brock PR, Maibach R, Childs M, et al. Sodium thiosulfate for protection from cisplatin-induced hearing loss. N Engl J Med. 2018;378(25):2376–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.