Abstract
Background
We aimed to develop a gene expression–based prognostic signature for isocitrate dehydrogenase (IDH) wild-type glioblastoma using clinical trial datasets representative of glioblastoma clinical trial populations.
Methods
Samples were collected from newly diagnosed patients with IDH wild-type glioblastoma in the ARTE, TAMIGA, EORTC 26101 (referred to as “ATE”), AVAglio, and GLARIUS trials, or treated at UCLA. Transcriptional profiling was achieved with the NanoString gene expression platform. To identify genes prognostic for overall survival (OS), we built an elastic net penalized Cox proportional hazards regression model using the discovery ATE dataset. For validation in independent datasets (AVAglio, GLARIUS, UCLA), we combined elastic net–selected genes into a robust z-score signature (ATE score) to overcome gene expression platform differences between discovery and validation cohorts.
Results
NanoString data were available from 512 patients in the ATE dataset. Elastic net identified a prognostic signature of 9 genes (CHEK1, GPR17, IGF2BP3, MGMT, MTHFD1L, PTRH2, SOX11, S100A9, and TFRC). Translating weighted elastic net scores to the ATE score conserved the prognostic value of the genes. The ATE score was prognostic for OS in the ATE dataset (P < 0.0001), as expected, and in the validation cohorts (AVAglio, P < 0.0001; GLARIUS, P = 0.02; UCLA, P = 0.004). The ATE score remained prognostic following adjustment for O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation status and corticosteroid use at baseline. A positive correlation between ATE score and proneural/proliferative subtypes was observed in patients with MGMT non-methylated promoter status.
Conclusions
The ATE score showed prognostic value and may enable clinical trial stratification for IDH wild-type glioblastoma.
Keywords: gene expression, glioblastoma, NanoString, overall survival, prognostic signature
Key Points.
We identified a new RNA signature predictive of OS in IDH wild-type glioblastoma.
Prognostic value was maintained after adjustment for several clinical variables.
The signature may enable trial stratification for IDH wild-type glioblastoma.
Importance of the Study.
There has been limited success in identifying prognostic molecular markers for IDH wild-type glioblastoma, or in the development of new therapies to extend OS for these patients. We pooled samples from clinical trial datasets representative of glioblastoma clinical trial populations, and used transcriptional profiling and elastic net penalized Cox proportional hazards regression to develop a prognostic expression signature. We identified a signature of 9 genes as prognostic for OS in the discovery dataset (P < 0.0001) and validated the prognostic association in independent cohorts (AVAglio, P < 0.0001; GLARIUS, P = 0.02; UCLA, P = 0.004). This novel signature can be measured in routine diagnostic formalin-fixed paraffin-embedded samples. Prognostic value was maintained after adjustment for MGMT promoter methylation status and corticosteroid use status at baseline, suggesting that the signature may be used in trial stratification for patients with IDH wild-type glioblastoma. Future studies are required to further validate the signature’s prognostic and/or predictive effects.
Diagnosis of brain tumors has evolved from using purely histologic criteria to incorporating both histologic and molecular features.1 The current classification system recognizes glioblastoma (GBM) with or without mutations in the isocitrate dehydrogenase genes (IDH1 or IDH2) as distinct and prognostically separate entities.1 Recently, the introduction of global methylation profiles led to further refinement and improvement of the classification.2 Methylation of the O6-methylguanine-DNA methyltransferase gene (MGMT) promoter is associated with prolonged overall survival (OS) in GBM treated with alkylating agents,3 although routine testing remains challenging due to variations in detection methods and cutoff definitions.4,5
The past decade has seen little progress in the development of new therapeutic modalities that extend OS for patients with GBM, or in the identification of methods to predict which patients will fare better than others with current standard of care. For newly diagnosed GBM, the current standard of care, comprising surgical resection followed by temozolomide and radiotherapy, was established in 2005 in a randomized phase III trial.6 Results from the trial also showed an association between hypermethylation of the MGMT promoter and improved benefit from alkylating chemotherapy,7 yet MGMT promoter methylation testing is still not routinely performed.
Understanding of molecular features of tumors associated with poor outcome may aid efforts to develop new pharmacologic therapies. While large-scale sequencing approaches have revealed mutations that are likely drivers of oncogenesis in discrete subsets of GBM cases,8–10 with the exception of mutations in IDH1/IDH2, driver mutations associated with these tumor subtypes have no established prognostic value.
Studies seeking to identify prognostic molecular markers for IDH wild-type GBM have yielded limited results.11–15MGMT promoter methylation has been shown to predict outcome of patients with GBM undergoing radiotherapy combined with temozolomide or temozolomide alone.7,16,17 RNA expression subtypes of GBM (proneural, proliferative, and mesenchymal) have been well described18; however, prognostic value for these subtypes has not been validated within the IDH wild-type tumor population. Similarly, studies reporting prognostic expression signatures derived from analysis of The Cancer Genome Atlas (TCGA) GBM dataset have not demonstrated prognostic value in sample sets confined to IDH wild-type cases.9,19–23 Recent trials have accrued patients according to MGMT promoter methylation or epidermal growth factor receptor amplification status.24,25
To develop a gene expression–based prognostic signature for IDH wild-type GBM with markers that can be assessed in formalin-fixed paraffin-embedded (FFPE) tumor samples, we integrated several large datasets from archival samples obtained from GBM clinical trial patients with well-defined treatments, comprehensive annotation, and gene expression data from a common platform.
Patients and Methods
Study Design and Sample Collection
This project was reviewed and agreed in collaboration with Genentech and the investigators (representing their own institutions and trials). Baseline FFPE tumor samples were collected from patients with newly diagnosed IDH wild-type GBM who provided written informed consent to participate in exploratory translational research within the ARTE,26 TAMIGA,27 EORTC 2610128 (hereafter collectively termed “ATE”), AVAglio,29 and GLARIUS30 trials. Additional samples from patients with newly diagnosed IDH wild-type GBM treated in the upfront setting with standard of care, with or without experimental agents, were obtained from the University of California, Los Angeles (UCLA). All samples were taken from patients at initial surgical resection before they were treated with any other therapeutic intervention. Patient baseline characteristics are summarized in Table 1.
Table 1.
Baseline patient demographics and clinical characteristics
| Characteristic | ARTE (N = 75) Wirsching et al 201826 | TAMIGA (N = 296) Brandes et al 201927 | EORTC 26101 (N = 437) Wick et al 201728 | AVAglio (N = 921) Chinot et al 201429 | GLARIUS (N = 182) Herrlinger et al 201630 | UCLA (N = 161) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients randomized, n | Arm A 50 | Arm B 25 | Arm A 61 | Arm B 62 | Arm A 288 | Arm B 149 | Arm A 458 | Arm B 463 | Arm A 122 | Arm B 60 | 161 |
| Samples with gene expression available, n | 50 | 99 | 279 | 339 | 123 | 154 | |||||
| Treatment | RT + BEV | RT | First line: RT + TMZ + BEV → 6 cycles of TMZ + BEV → BEV | Lomustine + BEV | Lomustine | BEV + RT + TMZ | Placebo + RT +TMZ | BEV + RT→ BEV + irinotecan | TMZ + RT→ TMZ | RT + TMZ | |
| Second line: lomustine + BEV | Second line: lomustine + placebo | ||||||||||
| Age, y | |||||||||||
| Median | 70 | 70 | 56 | 59 | 57 | 60 | 57 | 56 | 56 | 56 | 59 |
| Range | 65–87 | 65–79 | 30–74 | 36–74 | 23–82 | 21–79 | 20–84 | 18–79 | 25–78 | 26–78 | 22–84 |
| Sex | |||||||||||
| Male | 32 (64) | 16 (64) | 44 (72) | 45 (73) | 174 (60) | 91 (61) | 282 (62) | 298 (64) | 80 (69) | 34 (63) | 94 (58) |
| Female | 18 (36) | 9 (36) | 17 (28) | 17 (27) | 114 (40) | 58 (39) | 176 (38) | 165 (36) | 36 (31) | 20 (37) | 67 (42) |
| Glucocorticoids at baseline | |||||||||||
| Yes | 22 (44) | 12 (44) | 20 (33) | 19 (31) | 144 (50) | 71 (48) | 187 (41) | 208 (45) | NA | NA | NA |
| No | 27 (54) | 13 (56) | 41 (67) | 43 (69) | 144 (50) | 78 (52) | 269 (59) | 253 (55) | |||
| Missing | 1 (2) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Karnofsky Performance Status (KPS) | |||||||||||
| 100 | 25 (50) | 16 (64) | 16 (26) | 11 (18) | NA | NA | 308 (67) | 322 (70) | 90 (78) | 44 (82) | 19 (12) |
| 90 | (90–100)a | (90–100)a | 17 (28) | 21 (34) | (90–100) a | (90–100) a | (90– 100) a | (90–100) a | 102 (63) | ||
| 80 | 21 (42) | 6 (24) | 12 (20) | 16 (26) | 149 (33) | 140 (30) | 25 (22) | 9 (17) | 23 (14) | ||
| 70 | (70–80)a | (70–80)a | 11 (18) | 8 (13) | (50–80) a | (50–80) a | (70–80) a | (70–80) a | 11 (7) | ||
| 60 | 4 (8) | 3 (12) | 5 (8) | 5 (8) | 3 (2) | ||||||
| 50 | 0 | 0 | 0 | 1 (2) | 3 (2) | ||||||
| Surgical status | |||||||||||
| Open biopsy | NA | NA | NA | NA | 60 (13) | 44 (10) | 0 | 2 (4) | 13 (8) | ||
| Partial resection | 210 (46) | 223 (48) | 58 (50) | 27 (50) | 52 (32) | ||||||
| Complete resection | 188 (41) | 196 (42) | 58 (50) | 25 (46) | 83 (52) | ||||||
| Missing | 0 | 0 | 0 | 0 | 13 (8) | ||||||
| WHO PS | |||||||||||
| 0 | NA | NA | 100 (35) | 49 (33) | 227 (50) | 238 (52) | NA | NA | NA | ||
| 1 | 160 (56) | 81 (54) | 231 (50) | 224 (49) | |||||||
| 2 | 28 (10) | 19 (13) | (1 or 2) | ||||||||
| MMSE score | |||||||||||
| <27 | 26 (52) | 3 (12) | 16 (26) | 12 (19) | NA | NA | 106 (24) | 108 (24) | 19 (16) | 9 (17) | NA |
| ≥27 | 19 (38) | 17 (68) | 24 (39) | 25 (40) | 345 (77) | 351 (77) | 95 (82) | 43 (80) | |||
| Missing | 5 (10) | 5 (20) | 21 (34) | 25 (40) | NA | NA | 2 (2) | 2 (4) | |||
| MGMT promoter status | |||||||||||
| Methylated | 10 (20) | 6 (24) | 11 | 12 | 67 (23) | 37 (25) | 117 (26) | 120 (26) | 0 | 0 | 24 (15) |
| Unmethylated | 37 (74) | 18 (72) | 26 | 25 | 87 (30) | 38 (26) | 225 (49) | 236 (51) | 116 (100) | 54 (100) | 45 (28) |
| No data | 3 (6) | 1 (4) | NA | NA | 111 (39) | 61 (41) | 116 (25) | 107 (23) | 0 | 0 | NA |
| RPA class | |||||||||||
| III | NA | 5 (8) | 12 (20) | NA | NA | 76 (17) | 75 (16) | NA | NA | NA | |
| IV | 14 (23) | 8 (13) | 261 (57) | 279 (60) | |||||||
| V | 9 (15) | 9 (15) | 121 (26) | 108 (23) | |||||||
| Missing | 34 (55) | 32 (52) | 0 | 0 | |||||||
Data are n (%) of patients, unless otherwise stated.
aRange of KPS scores.
Abbreviations: BEV, bevacizumab. MMSE, Mini Mental State Exam. NA, not available. RPA, recursive partitioning analysis. RT, radiotherapy. TMZ, temozolomide. WHO, World Health Organization.
NanoString Gene Expression Data Generation
RNA was extracted from 1068 FFPE patient samples and run on a customized GBM panel comprising 814 features, as previously described,31 on the NanoString gene expression platform. As the 512 samples from the ATE trials were all analyzed in the same laboratory on a single NanoString code set and processed using a shared control specimen for normalization, data from these samples could be readily pooled. Samples from AVAglio, GLARIUS, and UCLA were processed with a new NanoString code set targeting the same 814 features.
The raw probe intensities were corrected for background using blanks (water) and then normalized using the NanoStringQCPro package in R.32 Raw data were preprocessed using functions from the NanoStringQCPro R package: positive control normalization, background correction (using water blanks), and RNA content normalization (median normalization). Prior to prognostic signature analysis, sample-wise normalization was performed by transforming counts to the log2 scale and subtracting the sample-wise mean expression of housekeeping genes. Genes were subsequently centered and scaled across the complete dataset (ATE, AVAglio, GLARIUS, and UCLA) to convert gene expression to z-scores.
Gene Expression Analysis and Subtype Classification
Quantile normalized gene expression data for 441 TCGA GBM samples10 processed on an Affymetrix Human Exon 1.0 ST Array were downloaded from Firebrowse (firebrowse.org; version 2016 01 28). Gene ontology (GO) term enrichment was performed with the “goanna” function implemented in the limma R package (version 3.83.3).33 Hierarchical clustering was performed using the R ward.D2 method. The dendrogram was visualized using a custom R script. The Phillips subtypes were assigned to the validation and discovery datasets using the Partitioning Around Medoids classifier described previously.34,35
Prognostic Signature Analysis Using Elastic Net Regression
Using OS data, we built an elastic net penalized Cox proportional hazards regression model using the glmnet R package with alpha fixed at 0.5 (and lambda selected via cross-validation using the “1 se” approach). For validation of the signature in independent datasets, we used only the signs of the elastic net fitted coefficients, rather than the actual coefficient values, to calculate an unweighted averaged z-score signature (ATE score) per patient. This averaged z-score approach was intended to minimize the impact of batch differences between the patient cohorts.
Survival Analysis
Univariate and multivariate survival analyses were performed using OS data for samples from each trial separately (ATE, AVAglio, and UCLA datasets) to identify potential treatment effects. Although EORTC 26101, in contrast to the other trials, recruited patients in the second-line setting, the survival times were calculated from the primary diagnosis. Since no treatment effect was observed in the trials of the validation (AVAglio, GLARIUS, and UCLA) or discovery (ATE) cohorts, we pooled all samples from the discovery datasets for elastic net penalized Cox proportional hazards regression analysis. Factors prognostic in univariate analyses of the ATE discovery dataset (age, sex, corticosteroid use, surgical status, Karnofsky performance status score, and MGMT status) were added as covariates to ATE status (defined as either a high or low ATE score split by the median) in multivariate models of OS in each cohort. Each of the models was fitted using Cox proportional hazards and visualized by Kaplan–Meier plots. To assess whether the prognostic effect of the ATE score was independent of Phillips subtype, a multivariate Cox proportional hazards analysis was conducted for each cohort; this model included ATE status, Phillips subtype, and other variables found to be significant in univariate analyses (Supplementary Table 1). All data were used for the survival analysis; however, for visualization purposes, we limited the horizontal axis to 100 months.
Results
Identification of a Prognostic Signature
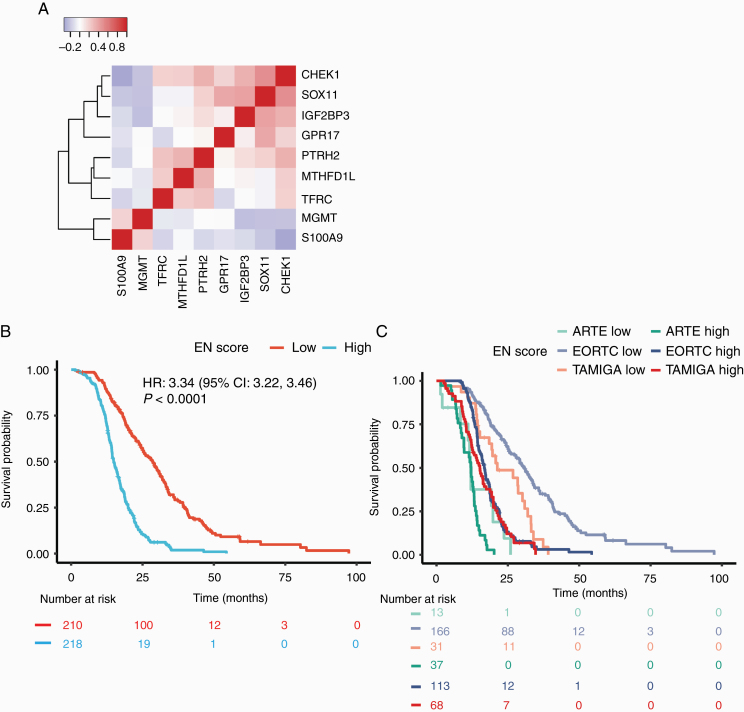
NanoString gene expression data available from patients enrolled in 3 of 6 cohorts (ARTE, n = 50; TAMIGA, n = 99; and EORTC 26101, n = 279) were used as the discovery dataset to identify a prognostic signature for OS. Elastic net penalized Cox proportional hazards regression analysis revealed a prognostic signature in the ATE dataset composed of 9 genes (CHEK1, GPR17, IGF2BP3, MGMT, MTHFD1L, PTRH2, SOX11, S100A9, and TFRC, referred to as “the elastic net” score; Fig. 1A). We also looked for additional genes for which expression was highly correlated (Pearson correlation r|>|0.65) with that of one or more of the signature genes, to provide better insight into the underlying biology. We did this first using NanoString gene expression data for the ATE cohort (Supplementary Figure 1A), and next using microarray data for the GBM cohort of TCGA, which, unlike NanoString, represents the full transcriptome (Supplementary Figure 1B). Within the NanoString gene expression data, 3 elastic net signature markers, GPR17, IGF2BP3, and SOX11, were correlated with 2 previously identified markers of proneural subtype GBM (DLL3 and KLRC3; Supplementary Figure 1A).18 In addition, expression of checkpoint kinase 1 (CHEK1) correlated with that of the proliferative subtype marker CENPK (centromere protein K). The major GO terms associated with the expanded gene set from the whole-transcriptome TCGA data included mitotic cell cycle, nucleic acid binding, myelination, mitochondrion, negative regulation of the c-Jun N-terminal kinase (JNK) cascade, and immune response. Results for the expanded gene set from the ATE data were similar, though lacking the JNK cascade GO term, possibly due to the reduced transcriptome coverage of the NanoString panel. As expected, the elastic net score was prognostic for OS in the combined ATE dataset (P < 0.0001; Fig. 1B), with the effect size mainly driven by the TAMIGA and EORTC 26101 cohorts (Fig. 1C).
Fig. 1.
Elastic net (EN) penalized Cox proportional hazards regression from ATE datasets. (A) Pearson correlation between z-scored expression of each gene in the signature in the combined ATE dataset; (B, C) Kaplan–Meier plots for overall survival in the biomarker-evaluable population for the EN signature stratified by median, for all ATE cohorts together (B), and per trial (C). P-value corresponds to Cox proportional hazards model.
Generalizing the Signature to Enable Application in Other Settings
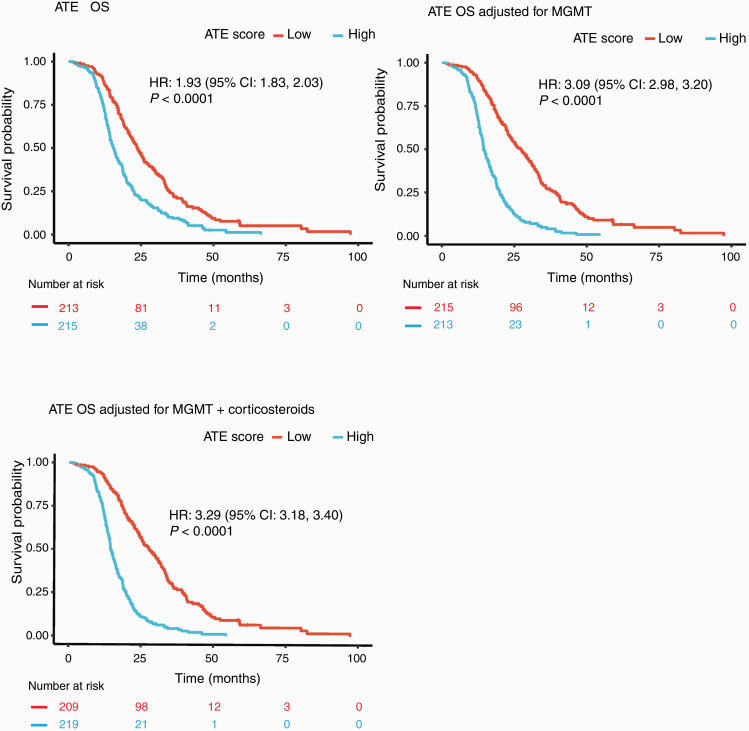
Although all studies employed NanoString gene expression platforms, batch effects across the studies presented challenges for direct application of a signature learned from studies employing one code set to those employing a different one. To address this, we calculated z-scores per gene for the 9 elastic net signature genes and averaged the results to make a new, more robust signature (hereafter known as the “ATE score”). This new ATE score correlated with the elastic net score (Supplementary Figure 2) and was prognostic in the ATE cohort (P < 0.0001; Fig. 2A). Moreover, evaluating the ATE score after adjusting for MGMT promoter methylation status (hazard ratio [HR], 3.09; Fig. 2B) or for MGMT promoter methylation status and corticosteroid use at study entry, which are known prognostic factors35 (HR, 3.29; Fig. 2C, Supplementary Table 1), achieved a similar effect size as the original elastic net score (HR, 3.34; Fig. 1B).
Fig. 2.
Kaplan–Meier plots for overall survival (OS) using the ATE score in the biomarker-evaluable population, stratified by median. (A), ATE score only; (B) adjusted for MGMT promoter methylation status; and (C) adjusted for MGMT promoter methylation status and corticosteroid use at study entry. P-values calculated from log-rank test.
In addition to the residual analysis approach in Fig. 2B, C, we also used a nested-models approach to show that the prognostic information provided by the ATE score was not redundant with that of MGMT status and/or corticosteroid use. When comparing a model with the ATE score to a model with the ATE score plus MGMT promoter methylation status, we found significantly enhanced prognostic value from the addition of MGMT promoter methylation status (chi-squared P < 0.0001). The same was true for the addition of corticosteroid use (on or off) to a model with the ATE score plus MGMT promoter methylation status (P < 0.001). We also tested a model with MGMT status only against a model with MGMT status and the ATE score; we found that addition of the signature significantly improved prognostic value (P < 0.0001). The same was true for the addition of the ATE score to a model with MGMT promoter methylation status and corticosteroid use (P < 0.0001). Thus, the ATE score provides a statistically significant increase in explanatory power beyond that of established prognostic factors, and conventional prognostic factors also add value to the ATE score.
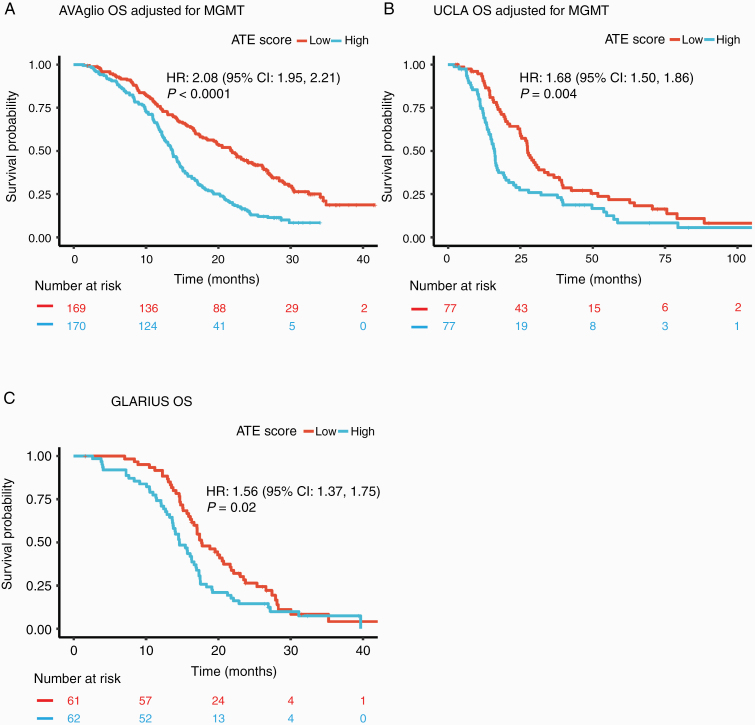
Validation of the Prognostic Signature in Independent Datasets
Our results were validated in 3 independent cohorts: the AVAglio trial (n = 339), the UCLA GBM collection (n = 154), and the GLARIUS MGMT non-methylated trial (n = 123). The ATE score was significantly prognostic for OS in each of these cohorts, both with (P < 0.0001, P = 0.004, and P = 0.02, respectively; Fig. 3) and without (P = 0.002 for AVAglio, P = 0.03 for UCLA; Supplementary Figure 3) adjustment for MGMT promoter methylation status.
Fig. 3.
Kaplan–Meier plots for overall survival (OS) using the ATE score in the biomarker-evaluable population, stratified by median, adjusted for MGMT promoter methylation status when relevant. (A) AVAglio; (B) UCLA; and (C) GLARIUS (which enrolled patients with MGMT promoter non-methylated status only). P-values calculated from log-rank test.
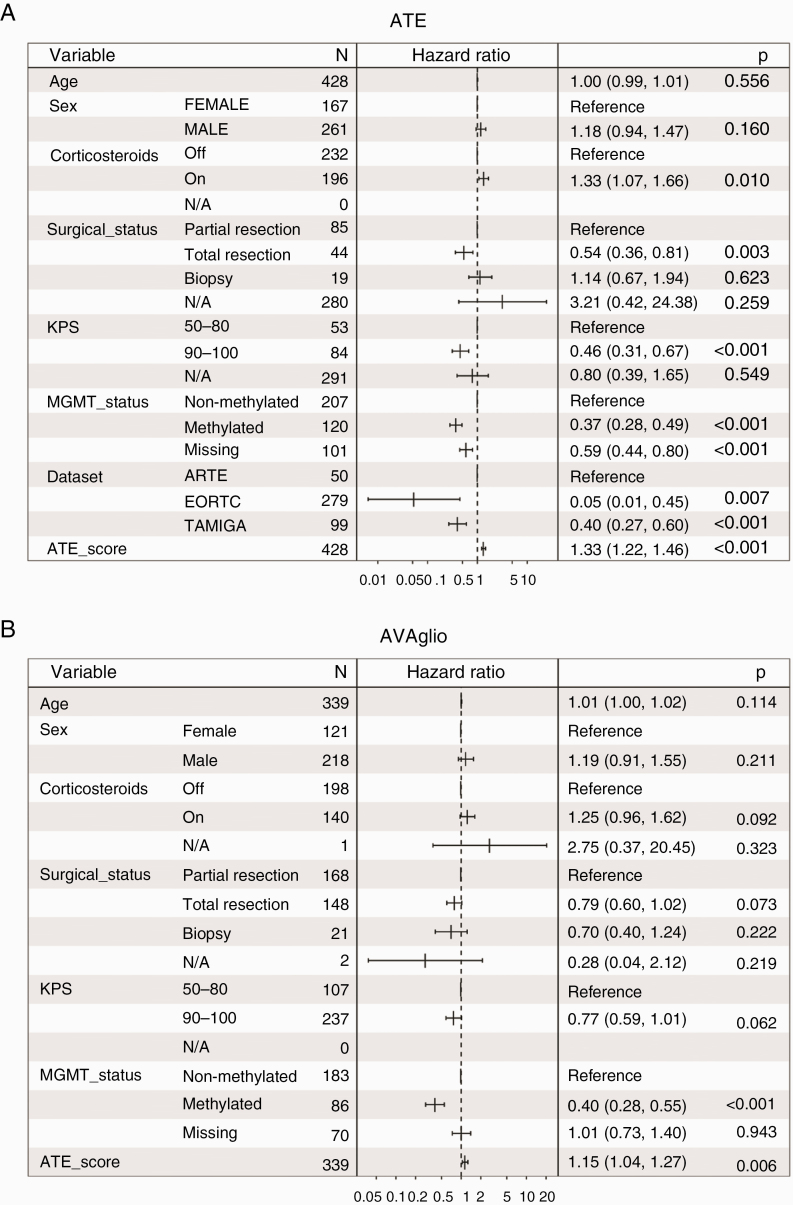
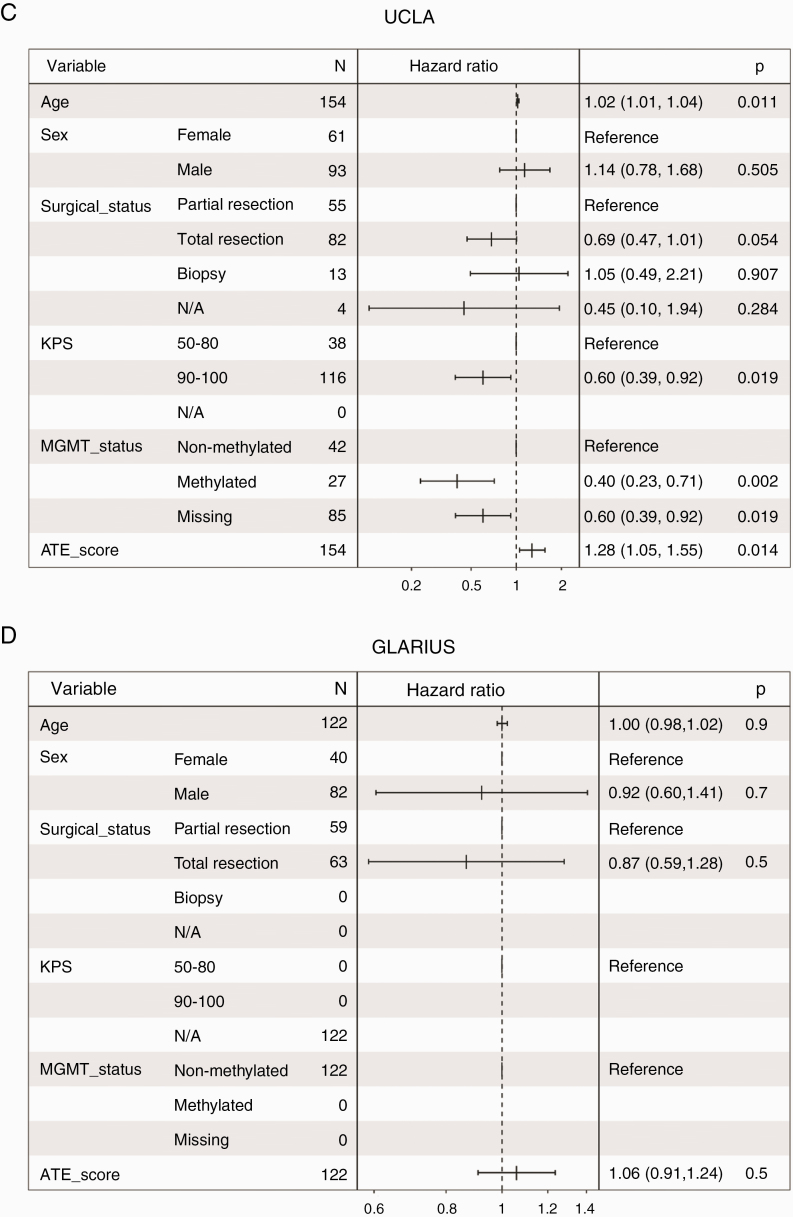
When including a full set of clinical variables that showed significant association with OS in univariate analyses of the ATE dataset (age, sex, surgical status, MGMT promoter methylation status, and, for ATE and AVAglio cohorts, corticosteroid use), the ATE score remained significantly prognostic for ATE, AVAglio, and UCLA, though not for GLARIUS (Fig. 4).
Fig. 4.
Forest plots showing multivariate Cox proportional hazard ratios with 95% confidence intervals and P-values for age, sex, surgical status, MGMT promoter methylation status, and ATE score in: (A) ATE, (B) AVAglio, (C) UCLA, and (D) GLARIUS. Corticosteroid use was also included for the ATE and AVAglio cohorts.
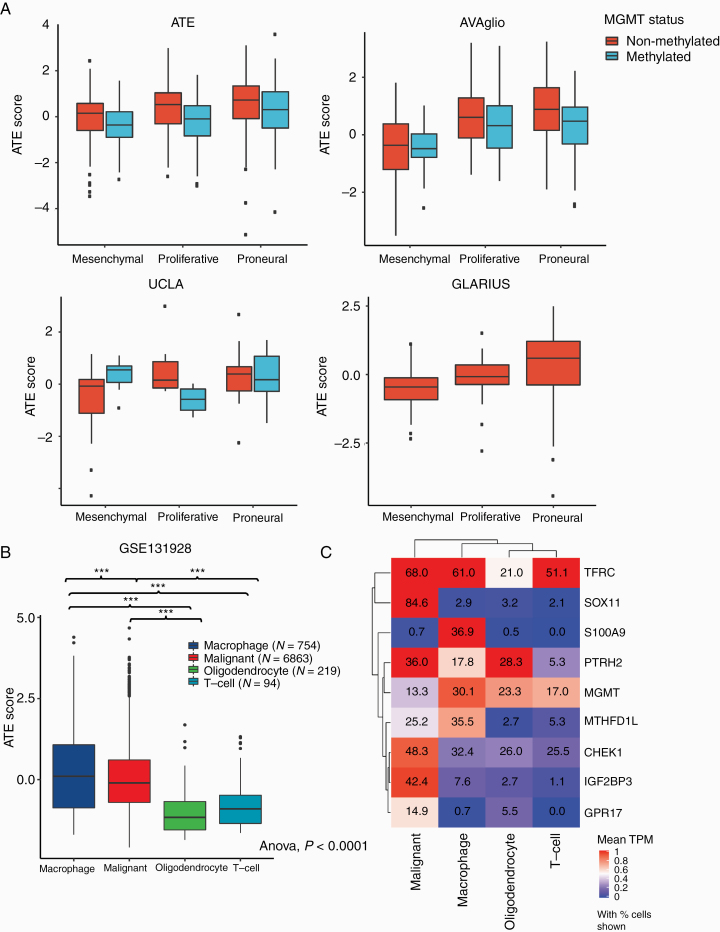
Higher median signature scores were observed in tumors of the proliferative and proneural Phillips subtypes in patients with MGMT non-methylated promoter status across all cohorts (Fig. 5). This trend was also observed for MGMT methylated samples for ATE and AVAglio cohorts. Importantly, average ATE score did not simply recapitulate prognostic effects attributable to the Phillips subtypes. In multivariate modeling considering Phillips subtype together with variables found to be significant in univariate analyses of each cohort, the signature remained significantly prognostic for ATE, AVAglio, and UCLA (P < 0.001, P = 0.002, and P = 0.019 respectively; Supplementary Figure 4).
Fig. 5.
(A) ATE score by MGMT promoter methylation status and Phillips subtype in: ATE, AVAglio, UCLA, and GLARIUS; (B) ATE score calculated in individual cells isolated from adult glioblastoma tumors (n = 20) from study GSE13192836; (C) hierarchical clustering of average gene expression levels for the 9 ATE signature genes in the 4 cell types identified in study GSE131928.36 Values indicate fraction of cells with detectable expression.
To determine whether the signature is heterogeneously expressed within a single tumor, we looked at the expression of the 9 signature genes and the ATE score in recently published single-cell data from Neftel et al, obtained from adult IDH wild-type tumors, including both tumor cells and other cell types from the tumor microenvironment.36 Neftel et al grouped their data into 4 major cell types, and we found that the fraction of cells with detectable expression, as well as median expression, was highest in the macrophage and/or malignant (ie, tumor) cell groups relative to the other cell types captured in their study (Fig. 5B, C).
Discussion
We aimed to determine whether a prognostic expression signature could be developed for newly diagnosed IDH wild-type GBM using samples from several datasets that are representative of existing GBM clinical trial patient populations.26–30 We identified a z-score signature (ATE score) predictive of OS in a large dataset from 3 pooled patient populations, and validated the prognostic value of the signature in 3 additional independent datasets. The newly described signature comprised 9 gene expression markers (CHEK1, GPR17, IGF2BP3, MGMT, MTHFD1L, PTRH2, SOX11, S100A9, and TFRC) that could be measured in routine FFPE diagnostic samples. Prognostic value was maintained after adjustment for MGMT promoter methylation status and corticosteroid use status at baseline, as well as other potentially prognostic clinical variables, suggesting that the signature could be used as an aid in trial design for patients with IDH wild-type GBM. For example, the ATE score could be used to balance poor- and good-prognosis patients in trials of IDH wild-type GBM, avoiding molecular bias, or as a stratification factor in larger trials. Depending on the scope of the trial, such stratification could be done prospectively at inclusion or post hoc.
Association was seen between the signature and previously described expression subtypes,9,18 especially within MGMT non-methylated samples. Specifically, a positive association was observed between high signature scores and both proliferative and proneural subtypes. Of the 9 genes in the signature, only CHEK1 and GPR17 showed significant differential subtype expression (upregulated in proliferative and proneural, respectively) in the original dataset used to define Phillips subtypes.18 Consistent with this, these 2 signature genes plus IGF2BP3 and SOX11 correlated with expression of previously identified Phillips subtype markers. However, the majority of the genes in the signature were not related to previously described GBM expression subtypes. Furthermore, the signature showed a significant association with outcome across multiple validation datasets in multivariate models that included previously described subtypes. Thus, there is significant prognostic value in the signature that is independent of previously described subtypes, and the signature represents a novel and complementary prognostic molecular classifier of outcomes in newly diagnosed IDH wild-type GBM.
Interestingly, in multivariate models, the signature association with OS was significant in AVAglio and UCLA datasets, but not in GLARIUS. The direction of effect in GLARIUS was consistent with that seen in the other cohorts, but statistical significance was not achieved. This may be a consequence of the smaller sample size in GLARIUS, or the fact that GLARIUS was unique in enrolling only patients with MGMT non-methylated promoter status.
The ATE score showed statistically significant, though relatively modest, effect size (HR, 1.15–1.31 in the 3 significant multivariate models) compared with the effect size seen for MGMT promoter methylation status. Importantly, in all datasets examined, the Kaplan–Meier curves obtained in signature-only analyses separated early and remained separate throughout their course, suggesting that the ATE score does more than identify small subsets of patients who are likely to have especially poor or good outcome.
As the majority of patients in our investigation received standard of care in the upfront setting, we cannot say definitively whether the ATE score predicts survival outcomes in the absence of standard treatment with temozolomide and radiation. No significant interactions were observed between the signature and treatment arm in any of the trials included in the analysis. Thus, there is no evidence to suggest that the ATE score is predictive of response to any of the experimental interventions tested in these trials, such as bevacizumab.
Consistent with the expectation that subsets of genes identified by elastic net each contribute to the prognostic value of the ATE score independently, examination of the described functions of the proteins derived from these genes revealed a pattern of non-overlapping biologies. MGMT promoter methylation has been extensively studied as a marker in GBM, and has been reported to predict both response to temozolomide and prolonged OS in patients receiving alkylating chemotherapy with or without irradiation.6,17,31MGMT and CHEK1 are both involved in DNA damage repair, but while MGMT plays a direct role in repair of alkylated DNA, CHEK1 plays a role in response to radiation-induced damage.37,38 It is thus conceivable that enhanced expression of these 2 genes confers escape from current standard of care, promoting inferior survival. Both SOX11 and GPR17 are key regulators of differentiation within the central nervous system, but the role of SOX11 is confined to neurogenesis,39–41 and that of GPR17 to gliogenesis.42–44 The metabolic proteins identified in the ATE score (TFRC, MTHFD1L, and PTRH2) influence separate metabolic processes and appear to be critical at distinct points in development of the nervous system. Specifically, GBM cancer stem cells preferentially require TFRC to propagate and form tumors in vivo.45MTHFD1L and PTRH2 are mitochondrial enzymes; while a role for these enzymes in GBM biology has not been previously described, it has been reported that MTHFD1L confers metabolic advantages in hepatocellular carcinoma through its role in folate metabolism.46S100A9 secreted protein has a pro-inflammatory function,47 and S100A9 is part of a 4-gene predictor of disease progression in human muscle invasive bladder cancer.48 While IGF2BP3 is an RNA-binding protein, evidence implicates it as an important mediator of tumor-stromal interactions, and expression of IGF2BP3 has been reported as a marker of poor survival and metastasis in multiple human malignancies.49–51
Further evidence for the contribution of multiple biological processes to the predictive value of the ATE score comes from GO enrichment analysis of the extended set of genes that are highly correlated with signature genes in the GBM cohort of TCGA (Supplementary Figure 1). This analysis revealed 6 distinct biological terms: mitotic cell cycle, nucleic acid binding, regulation of JNK signaling, mitochondria, myelination, and immune response. While mitotic cell cycle and nucleic acid binding are biologies readily relatable to tumor cell proliferation and response to current standard treatment, the other terms identified point to potential roles for additional independent biological processes. Of note, identification of the GO term “immune response” is consistent with strong expression of the ATE score in GBM-associated macrophages (Fig. 5B). In fact, expression of S100A9 appears to occur almost exclusively in macrophages associated with IDH wild-type GBMs. This contrasts sharply with expression of other ATE markers, including SOX11 and IGF2BP3, both of which appear to be largely confined to GBM cells. Thus, it seems likely that both biological processes intrinsic to tumor cells and actions of stromal and immune components contribute to the predictive power of the ATE score. Further study is needed to determine which of the ATE marker genes are related to intrinsic properties of tumor aggressiveness and which might reflect response to the current standard of care or the role of other cell types in the tumor microenvironment.
Conclusions
The ATE score showed value in predicting prognosis within an IDH wild-type GBM patient population representative of patients entering clinical trials in the United States and Europe. The signature is novel and can be measured in routine FFPE samples from diagnosis, making it easy to implement in clinical trials. As our discovery approach selects subsets of markers with independent effects by design, multiple biological processes are associated with the 9 signature genes, including response to DNA damage, tumor metabolism, regulation of neural differentiation, and tumor-stromal interactions. Future studies are required to further validate the prognostic and/or predictive effects of the signature.
Funding
This research was supported by Roche/Genentech.
Supplementary Material
Acknowledgments
We acknowledge the help of Fios Genomics Ltd, Edinburgh, UK, for conducting univariate and multivariate survival analyses. Third-party medical writing assistance, under the direction of the authors, was provided by Fiona Fernando, PhD, contract medical writer at Gardiner-Caldwell Communications, and was funded by F. Hoffmann-La Roche Ltd.
Conflict of interest statement. RMJ is an employee and stockholder in Roche/Genentech. HSP was a paid consultant to Roche/Genentech at the time of these analyses, and is a shareholder of NanoString stock. CB is an employee of Roche/Genentech. CWB has no conflicts of interest. TFC reports a pending patent (U.S. Provisional Application No: 62/819,322); honoraria for consulting services from Roche, Trizel, Medscape, Bayer, Amgen, Odonate Therapeutics, Pascal Biosciences, Bayer, Del Mar Pharmaceuticals, Tocagen, Karyopharm, GW Pharma, Kiyatec, Abbvie, Boehringer Ingelheim, VBI, Deciphera, VBL, Agios, Merck, Genocea, Celgene, Puma, Lilly, BMS, Cortice, Wellcome Trust, Novocure, Novogen, Boston Biomedical, Sunovion, Human Longevity, Insys, ProNai, Pfizer, Notable labs, and Medqi; contracts with UCLA for Brain tumor program; stockholder in Notable Labs; and member of the board for the 501c3 Global Coalition for Adaptive Research. AD is an employee of ORIC Pharmaceuticals and was an employee of Genentech at the time of these analyses, she holds stock in ORIC Pharmaceuticals and Genentech, and has patent or intellectual property interest in Genentech. UH reports grants and personal fees from Roche, personal fees and non-financial support from Medac and Bristol-Myers Squibb, and personal fees from Novocure, Novartis, Daiichi-Sankyo, Noxxon, AbbVie, Bayer and Jansen. RBJ has no potential conflicts of interest. AL has received honoraria from Merck, Genentech, Abbvie, and Optune. MW has received research grants from Abbvie, Adastra, BMS, Dracen, MSD, Merck, Novocure, Piqur, and Roche, and honoraria for lectures or advisory board participation or consulting from Abbvie, Basilea, BMS, Celgene, MSD, Merck, Novocure, Orbus, Roche, and Tocagen. WW has received study drug support from Apogenix, Pfizer, and Roche. CM, RB, and JG are employees of Roche and hold Roche stock.
Authorship statement. Conception and design: RB, RMJ. Provision of study material or patients: UH, MW. Collection and assembly of data: UH, CM, MW. Data analysis and interpretation: RB, RMJ, CWB, AD, HSP, MW. Manuscript writing: RMJ, HSP, CB, CWB, TFC, AD, UH, RBJ, AL, CM, MW, WW, RB, JG. Final approval of manuscript: RMJ, HSP, CB, CWB, TFC, AD, UH, RBJ, AL, CM, MW, WW, RB, JG.
Data Sharing
Researchers have access to raw and processed NanoString data and associated patient-level covariates through the Gene Expression Omnibus (GEO) database, access number GSE150615, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE150615. For further details on Roche’s Global Policy on the Sharing of Clinical Information, and how to request access to related clinical study documents, see https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
References
- 1. Louis DN, Perry A, Reifenberger G, et al. . The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 2. Capper D, Jones DTW, Sill M, et al. . DNA methylation-based classification of central nervous system tumours. Nature. 2018;555(7697):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weller M, van den Bent M, Tonn JC, et al. ; European Association for Neuro-Oncology (EANO) Task Force on Gliomas European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18(6):e315–e329. [DOI] [PubMed] [Google Scholar]
- 4. Hegi ME, Genbrugge E, Gorlia T, et al. . MGMT promoter methylation cutoff with safety margin for selecting glioblastoma patients into trials omitting temozolomide: a pooled analysis of four clinical trials. Clin Cancer Res. 2019;25(6):1809–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mansouri A, Hachem LD, Mansouri S, et al. . MGMT promoter methylation status testing to guide therapy for glioblastoma: refining the approach based on emerging evidence and current challenges. Neuro Oncol. 2019;21(2):167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups ; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 7. Hegi ME, Diserens AC, Gorlia T, et al. . MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 8. Parsons DW, Jones S, Zhang X, et al. . An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verhaak RG, Hoadley KA, Purdom E, et al. ; Cancer Genome Atlas Research Network Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brennan CW, Verhaak RG, McKenna A, et al. ; TCGA Research Network The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Labussière M, Boisselier B, Mokhtari K, et al. . Combined analysis of TERT, EGFR, and IDH status defines distinct prognostic glioblastoma classes. Neurology. 2014;83(13):1200–1206. [DOI] [PubMed] [Google Scholar]
- 12. Simon M, Hosen I, Gousias K, et al. . TERT promoter mutations: a novel independent prognostic factor in primary glioblastomas. Neuro Oncol. 2015;17(1):45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mosrati MA, Malmström A, Lysiak M, et al. . TERT promoter mutations and polymorphisms as prognostic factors in primary glioblastoma. Oncotarget. 2015;6(18):16663–16673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spiegl-Kreinecker S, Lötsch D, Ghanim B, et al. . Prognostic quality of activating TERT promoter mutations in glioblastoma: interaction with the rs2853669 polymorphism and patient age at diagnosis. Neuro Oncol. 2015;17(9):1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arita H, Yamasaki K, Matsushita Y, et al. . A combination of TERT promoter mutation and MGMT methylation status predicts clinically relevant subgroups of newly diagnosed glioblastomas. Acta Neuropathol Commun. 2016;4(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Malmström A, Grønberg BH, Marosi C, et al. ; Nordic Clinical Brain Tumour Study Group (NCBTSG) Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. [DOI] [PubMed] [Google Scholar]
- 17. Wick W, Platten M, Meisner C, et al. ; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–715. [DOI] [PubMed] [Google Scholar]
- 18. Phillips HS, Kharbanda S, Chen R, et al. . Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. [DOI] [PubMed] [Google Scholar]
- 19. Kim YW, Koul D, Kim SH, et al. . Identification of prognostic gene signatures of glioblastoma: a study based on TCGA data analysis. Neuro Oncol. 2013;15(7):829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kun S, Duan Q, Liu G, Lu JM. Prognostic value of DNA repair genes based on stratification of glioblastomas. Oncotarget. 2017;8(35): 58222–58230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tang J, He D, Yang P, He J, Zhang Y. Genome-wide expression profiling of glioblastoma using a large combined cohort. Sci Rep. 2018;8(1):15104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu P, Yang J, Liu J, et al. . Identification of glioblastoma gene prognosis modules based on weighted gene co-expression network analysis. BMC Med Genomics. 2018;11(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang Y, Xu J, Zhu X. A 63 signature genes prediction system is effective for glioblastoma prognosis. Int J Mol Med. 2018;41(4):2070–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van den Bent M, Eoli M, Sepulveda JM, et al. . INTELLANCE 2/EORTC 1410 randomized phase II study of Depatux-M alone and with temozolomide vs temozolomide or lomustine in recurrent EGFR amplified glioblastoma [published online ahead of print July 1, 2020]. Neuro Oncol. 2020. doi: 10.1093/neuonc/noaa115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Draaisma K, Chatzipli A, Taphoorn M, et al. . Molecular evolution of IDH wild-type glioblastomas treated with standard of care affects survival and design of precision medicine trials: a report from the EORTC 1542 study. J Clin Oncol. 2020;38(1):81–99. [DOI] [PubMed] [Google Scholar]
- 26. Wirsching HG, Tabatabai G, Roelcke U, et al. . Bevacizumab plus hypofractionated radiotherapy versus radiotherapy alone in elderly patients with glioblastoma: the randomized, open-label, phase II ARTE trial. Ann Oncol. 2018;29(6):1423–1430. [DOI] [PubMed] [Google Scholar]
- 27. Brandes AA, Gil-Gil M, Saran F, et al. . A randomized phase II trial (TAMIGA) evaluating the efficacy and safety of continuous bevacizumab through multiple lines of treatment for recurrent glioblastoma. Oncologist. 2019;24(4):521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wick W, Gorlia T, Bendszus M, et al. . Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954–1963. [DOI] [PubMed] [Google Scholar]
- 29. Chinot OL, Wick W, Mason W, et al. . Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 30. Herrlinger U, Schäfer N, Steinbach JP, et al. . Bevacizumab plus irinotecan versus temozolomide in newly diagnosed O6-methylguanine-DNA methyltransferase nonmethylated glioblastoma: the randomized GLARIUS trial. J Clin Oncol. 2016;34(14):1611–1619. [DOI] [PubMed] [Google Scholar]
- 31. Sandmann T, Bourgon R, Garcia J, et al. . Patients with proneural glioblastoma may derive overall survival benefit from the addition of bevacizumab to first-line radiotherapy and temozolomide: retrospective analysis of the AVAglio trial. J Clin Oncol. 2015;33(25):2735–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nickles D, Sandmann T, Ziman R, Bourgon R. NanoStringQCPro: quality metrics and data processing methods for NanoString mRNA gene expression data. R package version 1.18.0; 2019. doi: 10.18129/B9.bioc.NanoStringQCPro. [DOI] [Google Scholar]
- 33. Ritchie ME, Phipson B, Wu D, et al. . Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maechler M, Rousseeuw P, Struyf A, Hubert M, Hornik K. Cluster: cluster analysis basics and extensions. R package version 2.1.0; 2019. Available at: https://cran.r-project.org/web/packages/cluster/cluster.pdf [Google Scholar]
- 35. Pitter KL, Tamagno I, Alikhanyan K, et al. . Corticosteroids compromise survival in glioblastoma. Brain. 2016;139(Pt 5):1458–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Neftel C, Laffy J, Filbin MG, et al. . An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell. 2019;178(4):835–849.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bao S, Wu Q, McLendon RE, et al. . Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. [DOI] [PubMed] [Google Scholar]
- 38. Bai X, Wang J, Huo L, et al. . Serine/threonine kinase CHEK1-dependent transcriptional regulation of RAD54L promotes proliferation and radio resistance in glioblastoma. Transl Oncol. 2018;11(1):140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bergsland M, Werme M, Malewicz M, Perlmann T, Muhr J. The establishment of neuronal properties is controlled by Sox4 and Sox11. Genes Dev. 2006;20(24):3475–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li Y, Wang J, Zheng Y, et al. . Sox11 modulates neocortical development by regulating the proliferation and neuronal differentiation of cortical intermediate precursors. Acta Biochim Biophys Sin (Shanghai). 2012;44(8):660–668. [DOI] [PubMed] [Google Scholar]
- 41. Wang Y, Lin L, Lai H, Parada LF, Lei L. Transcription factor Sox11 is essential for both embryonic and adult neurogenesis. Dev Dyn. 2013;242(6):638–653. [DOI] [PubMed] [Google Scholar]
- 42. Chen Y, Wu H, Wang S, et al. . The oligodendrocyte-specific G protein-coupled receptor GPR17 is a cell-intrinsic timer of myelination. Nat Neurosci. 2009;12(11):1398–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Simon K, Hennen S, Merten N, et al. . The orphan G protein-coupled receptor GPR17 negatively regulates oligodendrocyte differentiation via Gαi/o and its downstream effector molecules. J Biol Chem. 2016;291(2):705–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lu C, Dong L, Zhou H, et al. . G-protein-coupled receptor GPR17 regulates oligodendrocyte differentiation in response to lysolecithin-induced demyelination. Sci Rep. 2018;8(1):4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schonberg DL, Miller TE, Wu Q, et al. . Preferential iron trafficking characterizes glioblastoma stem-like cells. Cancer Cell. 2015;28(4):441–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee D, Xu IM, Chiu DK, et al. . Folate cycle enzyme MTHFD1L confers metabolic advantages in hepatocellular carcinoma. J Clin Invest. 2017;127(5):1856–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schenten V, Plançon S, Jung N, et al. . Secretion of the phosphorylated form of S100A9 from neutrophils is essential for the proinflammatory functions of extracellular S100A8/A9. Front Immunol. 2018;9:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim WJ, Kim SK, Jeong P, et al. . A four-gene signature predicts disease progression in muscle invasive bladder cancer. Mol Med. 2011;17(5–6):478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lederer M, Bley N, Schleifer C, Hüttelmaier S. The role of the oncofetal IGF2 mRNA-binding protein 3 (IGF2BP3) in cancer. Semin Cancer Biol. 2014;29:3–12. [DOI] [PubMed] [Google Scholar]
- 50. Wang J, Qi J, Hou X. Systematically dissecting the function of RNA-binding proteins during glioma progression. Front Genet. 2019;10:1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang GH, Zhong QY, Gou XX, et al. . Seven genes for the prognostic prediction in patients with glioma. Clin Transl Oncol. 2019;21(10):1327–1335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.