Abstract
Background
Standard treatment for glioblastoma is radiation with concomitant and adjuvant temozolomide for 6 cycles, although the optimal number of cycles of adjuvant temozolomide has long been a subject of debate. We performed a phase II randomized trial investigating whether extending adjuvant temozolomide for more than 6 cycles improved outcome.
Methods
Glioblastoma patients treated at 20 Spanish hospitals who had not progressed after 6 cycles of adjuvant temozolomide were centrally randomized to stop (control arm) or continue (experimental arm) temozolomide up to a total of 12 cycles at the same doses they were receiving in cycle 6. Patients were stratified by MGMT methylation and measurable disease. The primary endpoint was differences in 6-month progression-free survival (PFS). Secondary endpoints were PFS, overall survival (OS), and safety (Clinicaltrials.gov NCT02209948).
Results
From August 2014 to November 2018, 166 patients were screened, 7 of whom were ineligible. Seventy-nine patients were included in the stop arm and 80 in the experimental arm. All patients were included in the analyses of outcomes and of safety. There were no differences in 6-month PFS (control 55.7%; experimental 61.3%), PFS, or OS between arms. MGMT methylation and absence of measurable disease were independent factors of better outcome. Patients in the experimental arm had more lymphopenia (P < 0.001), thrombocytopenia (P < 0.001), and nausea and vomiting (P = 0.001).
Conclusions
Continuing temozolomide after 6 adjuvant cycles is associated with greater toxicity but confers no additional benefit in 6-month PFS.
Key Points
1. Extending adjuvant temozolomide to 12 cycles did not improve 6-month PFS.
2. Extending adjuvant temozolomide did not improve PFS or OS in any patient subset.
3. Extending adjuvant temozolomide was linked to increased toxicities.
Keywords: extended adjuvant temozolomide, glioblastoma, MGMT methylation, prognosis
Importance of the Study.
Standard treatment for glioblastoma is surgery followed by radiation therapy with concomitant and adjuvant temozolomide. However, the optimal number of cycles of adjuvant temozolomide has long been a matter of debate. The only evidence that could help to clarify this issue comes from retrospective trials and cohort studies and indicates the futility of continuing temozolomide beyond 6 cycles. The present study is the only prospective trial to date to explore this issue. We have randomized uniformly treated, progression-free patients to stop temozolomide after 6 or continue until 12 cycles. In fact, less than 50% of patients who start standard treatment are able to receive all 6 cycles of adjuvant temozolomide without progression, making it nearly impossible for a randomized phase III trial to be launched. Therefore, we believe that the findings of our phase II study will be the best evidence that we will ever have on this question.
The undisputed optimal treatment for glioblastoma is maximal surgery followed by radiation therapy with concomitant and adjuvant temozolomide (TMZ), which confers benefit in both progression-free survival (PFS) and overall survival (OS).1,2 Although this treatment regimen was initially designed arbitrarily with 6 cycles of adjuvant TMZ, the optimal number of adjuvant cycles has been a matter of debate over the last years,3,4 since if patients do not relapse, continuation of TMZ beyond 6 cycles could intuitively be thought to confer additional benefit. This would seem to be especially true for patients with methylation of the promoter of the O6-methylguanine-DNA methyltransferase (MGMT) gene, who have been found to receive the greatest benefit from TMZ,5 as well as for those with measurable disease detected by MRI at the end of the 6 cycles of adjuvant TMZ. Consequently, due to the easy oral administration of TMZ, its low toxicity profile, and the lack of clearly effective therapies after relapse, adjuvant TMZ has often been extended for longer than 6 months both in the clinical setting6,7 and in several large clinical trials.2,8–10
Nevertheless, there are several negative aspects of continuing adjuvant TMZ. For example, attributing a longer PFS to the effects of TMZ continuation may cause us to overlook other prognostic factors that may be impacting the evolution of the disease. In addition, the extra cost of maintenance treatment with TMZ can negatively affect cost effectiveness11 if survival is not increased. Finally, continuation of TMZ increases the risk of toxicity and the likelihood of creating resistance to later treatments that will be administered at recurrence, which are frequently based on alkylating agents.12 To date, however, in spite of these caveats, no prospective randomized trial has successfully analyzed the impact of more than 6 cycles of adjuvant TMZ in terms of outcome and toxicity.
The Spanish Group of Research in Neuro-Oncology (GEINO) carried out a phase II randomized trial (GEINO 14–01) investigating whether extending adjuvant TMZ for more than 6 cycles improved outcome in patients with glioblastoma who had completed the planned 6 cycles of adjuvant TMZ and had not progressed (Clinicaltrials.gov NCT02209948).
Patients and Methods
Subjects and Study Design
This was an academic, randomized, open-label, multicenter trial conducted by GEINO with the participation of 20 Spanish hospitals. The trial was conducted in accordance with applicable regulatory requirements and the principles of the Declaration of Helsinki. The protocol was approved by the ethics committees of all participating centers. All patients provided their signed informed consent.
Patients were eligible if they were 18 years old or older and had a confirmed diagnosis of glioblastoma previously treated with radiation therapy with concomitant and adjuvant TMZ for 6 cycles without progression. Although the protocol was initially designed to accept patients treated with bevacizumab, since the expected results of 2 ongoing randomized trials could potentially have supported the addition of bevacizumab in the first-line setting, none of the patients included in the trial received first-line bevacizumab. Other inclusion criteria were: availability of MGMT methylation status or sufficient tissue for MGMT methylation analysis; KPS ≥60; stable doses of glucocorticoids and adequate hematologic, renal, and hepatic function. The MRI showing lack of progression had to be performed no more than 6 weeks prior to enrollment, in order to allow for tissue samples or slides to be shipped for the centralized confirmation of the diagnosis of glioblastoma and analysis of MGMT methylation if not previously performed, and in order not to delay the administration of cycle 7 of TMZ (the first cycle of the present trial). Toxicities related to any previous treatments had to be resolved to grade 1 (according to the National Cancer Institute Common Terminology Criteria for Adverse Events [NCI CTCAE], version 4.0) before randomization.
As there were no data on the outcome of patients after the first 6 cycles of maintenance treatment, we relied on reported differences in PFS with the standard treatment according to MGMT methylation status and/or the presence or absence of measurable disease.1,5 In order to balance for these potential prognostic factors between the 2 arms, patients were randomized 1:1 with 4 blocked randomization lists to stop TMZ after 6 cycles (control arm) or to continue for 6 additional cycles (cycles 7 to 12) (experimental arm). Patients were stratified by MGMT methylation status and the presence or absence of measurable disease on MRI.
Shipment of tumor samples, randomization, stratification, and monitoring were conducted by the external contract research organization. The trial is registered with Clinicaltrials.gov (NCT02209948) and is now closed.
Treatment
In the experimental arm, cycle 7 of TMZ was initiated no more than 6 weeks after day 1 of cycle 6. The TMZ dose for cycles 7 to 12 was the same as the dose administered at cycle 6 for each patient; doses ranged from 125 to 200 mg/m2/day for 5 days. Clinical evaluation, toxicity monitoring, and hematology and biochemistry assessments were performed in both arms every 28 days (on day 1 of each TMZ cycle in the experimental arm and on the day of the scheduled patient visit in the control arm). MRI was performed every 3 months for all patients until progression. Progression was defined according to Response Assessment in Neuro-Oncology criteria, which specified progression as irreversible neurological deterioration even in the absence of radiological deterioration and/or increasing doses of corticosteroids for more than 2 weeks to prevent neurological deterioration.13 At progression, subsequent therapies were administered at the discretion of the investigator and all patients were followed until death or last control visit.
Endpoints
The primary outcome of the study was to detect differences between the 2 arms in 6-month PFS. Secondary outcomes were median PFS, OS, and toxicity by treatment arm and according to stratification factors. Toxicities were assessed according to the NCI CTCAE v4.0.
In a preplanned translational substudy in patients with sufficient available tumor tissue, we assessed the isocitrate dehydrogenase (IDH) gene and the mismatch repair (MMR) deficiency proteins (MLH1, MSH2, MSH6, PMS2) that could be related to TMZ resistance. A tissue microarray was built for the study of IDH1-R132 by immunohistochemistry (IHC) and of the MMR proteins. Samples from patients younger than 56 years old with a negative IHC for IDH1-R132 were sequenced. Tumor samples obtained at second surgery at progression were requested for the study of the MMR system by IHC (see Supplementary Material).
Statistical Analyses
To detect meaningful differences in 6-month PFS between arms, we balanced prognostic factors (MGMT methylation and measurable disease) according to historical data.1,5 According to point estimates on PFS curves, we expected that 59.3% of patients with MGMT methylation and 28.5% of those without MGMT methylation would be progression free at 9 months after surgery. In addition, we expected that 40% of patients would have had gross total resection and 60% partial resection or biopsy. These data were used to stratify patients between arms. The study was not powered to detect significant differences in median PFS or median OS.
Efficacy was analyzed in the intention-to-treat population and safety in all patients. PFS was calculated from the time of inclusion to progression or death from any cause, whichever occurred first. OS was defined as the time from inclusion to death from any cause. Those patients who did not have an event were censored at the time of the last known contact. The Kaplan–Meier method was used to estimate median PFS and OS with 95% confidence intervals (CIs). Treatment groups were compared with the log-rank test. Cox regression models were used to estimate the treatment effect, reported as a hazard ratio (HR) with a 95% CI. Proportional hazard assumption was checked with a test based on the scaled Schoenfeld residuals for all models presented. All reported P-values are two-sided. Statistical analyses were performed with the SPSS package v24 and R software v3.6.2.
Results
Patients and Treatment
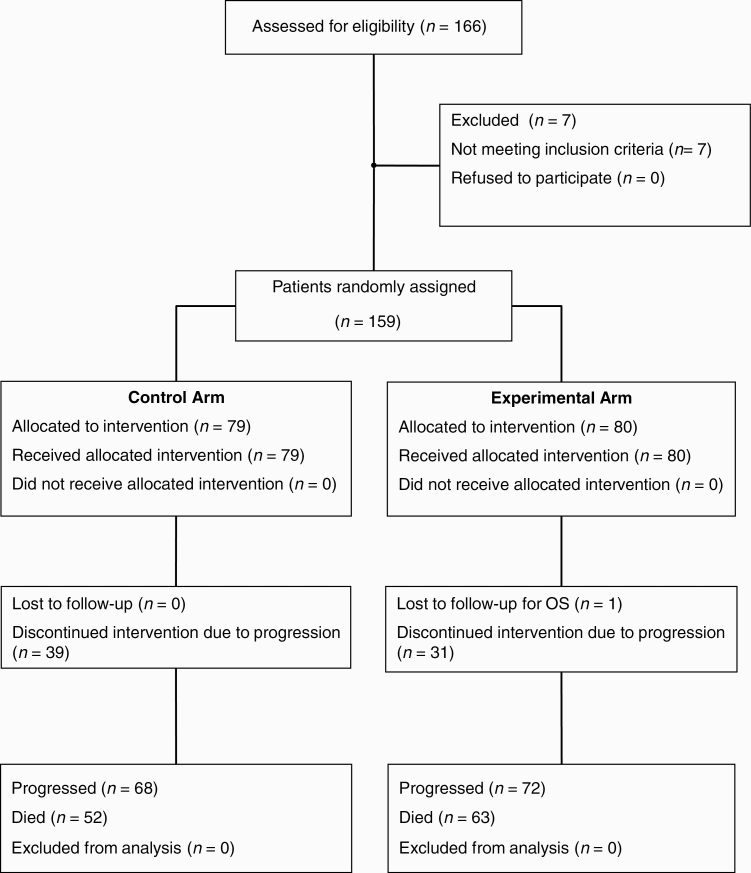
From August 22, 2014 to November 27, 2018, one hundred sixty-six patients were screened, 7 of whom were deemed to be ineligible (Fig. 1). Seventy-nine patients were randomized to stop TMZ after cycle 6 (control arm) and 80 to continue for up to 6 additional cycles (cycles 7 to 12) (experimental arm). Characteristics were similar across arms (Table 1 and Supplementary Table 1). There were slightly more patients with IDH mutations in the control arm (P = 0.195). At the time of inclusion in the study, 86.2% of patients were not receiving corticosteroids.
Fig. 1.
CONSORT diagram showing flow of patients through the trial (TMZ, temozolomide).
Table 1.
Patient characteristics at inclusion
| Characteristic | All Patients, N = 159 | Control Arm, n = 79 (49.7%) | Experimental Arm, n = 80 (50.3%) |
|---|---|---|---|
| Age, y (range) | 60.4 (29–83) | 60.4 (29–83) | 60.7 (31–79) |
| n (%) | n (%) | n (%) | |
| Sex | |||
| Female | 76 (47.8) | 38 (48.1) | 38 (47.5) |
| Male | 83 (52.2) | 41 (51.9) | 42 (52.5) |
| Measurable disease >10 mm | |||
| No | 76 (47.8) | 37 (46.8) | 39 (48.8) |
| Yes | 83 (52.2) | 42 (53.2) | 41 (51.2) |
| Bevacizumab added to first-line treatment | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| KPS | |||
| <70% | 4 (2.52) | 2 (2.53) | 2 (2.50) |
| ≥70% | 155 (97.5) | 77 (97.5) | 78 (97.5) |
| Established neurologic symptoms | |||
| No | 131 (83.0) | 71 (89.9) | 60 (75.9) |
| Yes | 27 (17.0) | 8 (10.1) | 19 (24.1) |
| Dexamethasone dose at inclusion | |||
| 0 mg | 137 (86.2) | 70 (88.6) | 67 (83.7) |
| 0.5–2 mg | 15 (9.4) | 6 (7.6) | 9 (11.3) |
| >2 mg | 7 (4.4) | 3 (3.8) | 4 (5.0) |
| Barthel index | |||
| 0 | 21 (13.2) | 9 (11.4) | 12 (15.0) |
| 1 | 138 (86.8) | 70 (88.6) | 68 (85.0) |
| Mini-Mental State Examination score | |||
| <27 | 26 (16.3) | 10 (12.7) | 16 (20.0) |
| ≥27 | 111 (69.8) | 60 (75.9) | 51 (63.7) |
| Unknown/not possible | 24 (13.9) | 9 (11.4) | 13 (16.3) |
| Anticonvulsant therapy | |||
| No | 84 (52.8) | 41 (51.9) | 43 (53.8) |
| Yes | 75 (47.2) | 38 (48.1) | 37 (46.2) |
| Initial surgery at diagnosis | |||
| Biopsy | 17 (10.7) | 10 (12.7) | 7 (8.75) |
| Complete resection (confirmed by postop MRI) | 63 (39.6) | 35 (44.3) | 28 (35.0) |
| Complete resection (without postop MRI) | 34 (21.4) | 14 (17.7) | 20 (25.0) |
| Partial resection | 45 (28.3) | 20 (25.3) | 25 (31.2) |
| MGMT methylation status | |||
| Methylated | 97 (61) | 48 (60.8) | 49 (61.2) |
| Unmethylated | 62 (39) | 31 (39.2) | 31 (38.8) |
| IDH mutation status | |||
| IDH1-R132 mutated by IHC | 8 (5) | 7 (8.9) | 1 (1.3) |
| IDH1-R132 wild-type by IHC | 137 (86.2) | 66 (83.5) | 71 (88.8) |
| IDH1-R132 mutated by sequencing | 1 | ‒ | 1 |
| IDH2-R172 mutated by sequencing | 0 | 0 | 0 |
| Total IDH mutations detected by IHC or sequencinga | 9 (5.7) | 7 (8.9) | 2 (2.5) |
| Patients ≤55 y who were not assesseda | 15 (9.4) | 6 (7.6) | 9 (11.3) |
a IDH mutations were not assessed in patients without available tissue or in those who did not give their consent for molecular analyses.
Of the 80 patients in the experimental arm, 49 (61.2%) received all 6 additional planned cycles without progression. The TMZ dose at cycle 7 was 200 mg/m2 for 54 patients (67.5%), 150 mg/m2 for 10 patients (12.5%), and 125–150 mg/m2 for 16 patients (20%).
Treatment at progression was similar across treatment arms and included TMZ rechallenge, nitrosoureas, bevacizumab-based therapies, second surgery, and re-irradiation. More patients in the experimental arm than in the control arm received only palliative care (17 [23.3%] vs 5 [7.2%]; P = 0.02) (Supplementary Table 2).
Outcomes
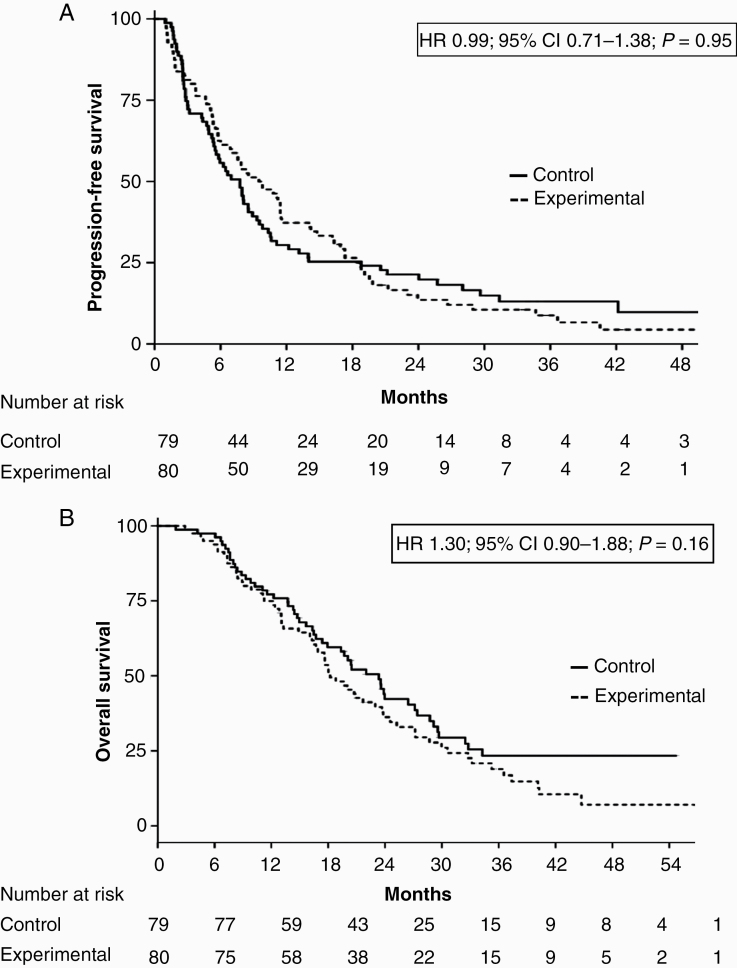
At the time of analysis (September 2019), with a median follow-up of 33.4 months, 19 of the 159 patients (11.9%) were progression free. The 6-month PFS was 55.7% (95% CI: 45.8–67.8%) for the control arm and 61.3% (95% CI: 51.5–72.9%) for the experimental arm. Median PFS was 7.9 months (95% CI: 6.2–9.6) for all patients, 7.7 months (95% CI: 5.7–9.8) for the control arm, and 9.5 months (95% CI: 5.9–13.0) for the experimental arm (hazard ratio [HR], 0.99; 95% CI: 0.71–1.38; P = 0.95) (Fig. 2A). Death occurred in 115 patients (72.3%). Median follow-up for alive patients was 23.7 months. Median OS was 20.4 months (95% CI: 16.5–24.2) for all patients, 23.3 months (95% CI: 17.9–28.7 for the control arm, and 18.2 months (95% CI: 16.7–23.8) for the experimental arm (HR, 1.30; 95% CI: 0.90–1.88; P = 0.16) (Fig. 2B).
Fig. 2.
(A) PFS and (B) OS for all patients according to treatment arm.
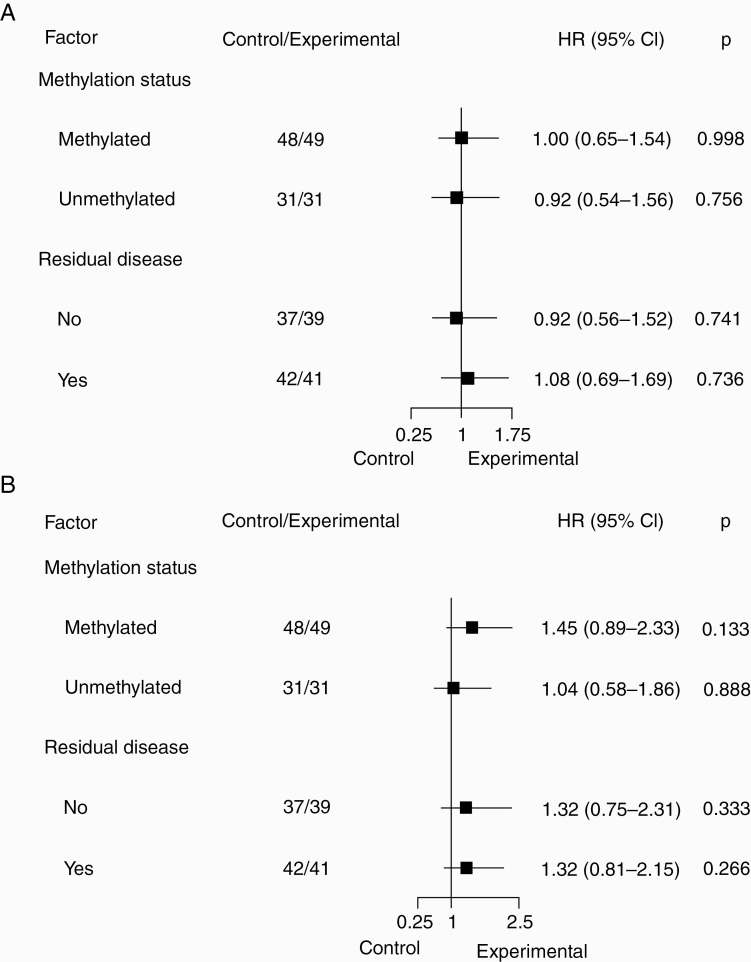
Among all 159 patients, MGMT methylation and absence of measurable disease were associated with longer PFS and OS in the univariate analyses and were independent markers for longer PFS and OS in the multivariate analyses, but treatment arm was not. These results were maintained even when including IDH mutation status in the analyses (Supplementary Table 3).
In the stratified analyses, neither patients with MGMT methylation nor those with measurable disease seemed to benefit from continuing TMZ (Fig. 3A, B).
Fig. 3.
Hazard ratios with forest plot for (A) PFS and (B) OS according to stratification factors and treatment arm.
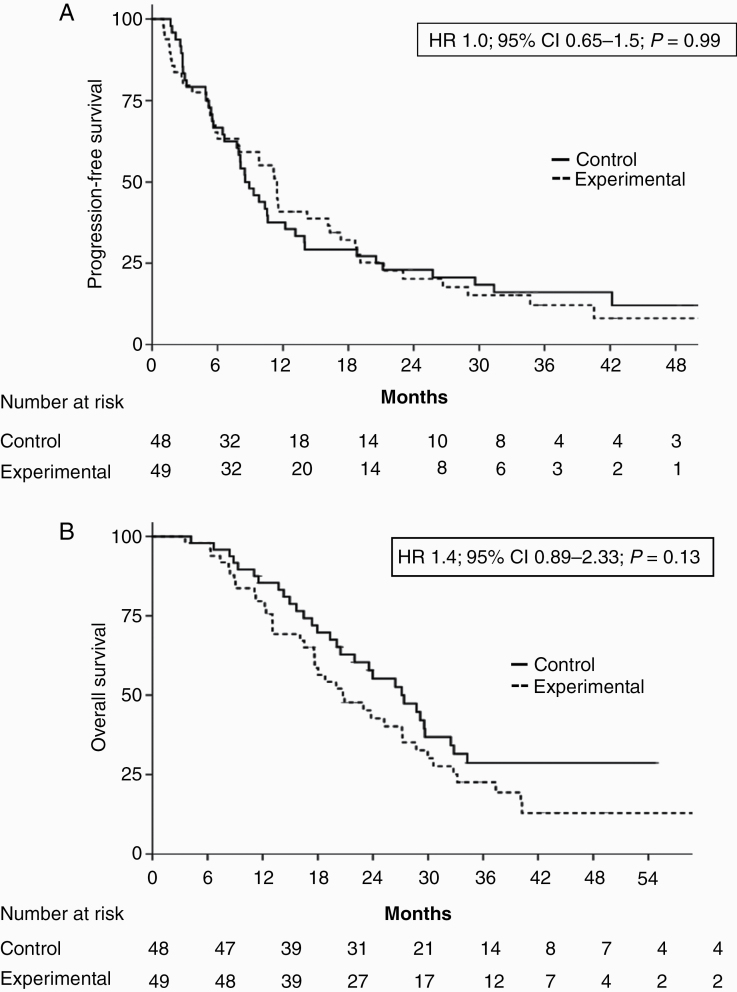
Among the 97 patients with MGMT methylation, median PFS was 8.5 months (95% CI: 6.5–10.4) for the 48 in the control arm and 11.4 months (95% CI: 9.2–13.6) for the 49 in the experimental arm (HR, 1.0; 95% CI: 0.65–1.5; P = 0.99) (Fig. 4A). However, median OS for these patients was 27.1 months (95% CI: 20.3–33.9) in the control arm and 20.7 months (95% CI: 14.7–26.7) in the experimental arm (HR, 1.45; 95% CI: 0.89–2.33; P = 0.13) (Fig. 4B). There were no differences between arms for patients with unmethylated MGMT (Supplementary Figure 1A, B).
Fig. 4.
(A) PFS and (B) OS for patients with MGMT methylation in the control and experimental arms.
Median PFS for the 83 patients with measurable disease was 7.7 months (95% CI: 4.5–10.7), compared with 8.3 months (95% CI: 7.2–9.4) for the 76 without measurable disease (HR, 0.7; 95% CI: 0.50–0.99; P = 0.04). Median OS was 17.9 months (95% CI, 13.8–22.0) and 23.8 months (95% CI, 19.5–28.0), respectively (HR, 0.6; 95% CI: 0.42–0.90; P = 0.01).
Safety
Toxicity among patients in the experimental arm was mild, with few grade 3–4 episodes. However, lymphopenia (P < 0.001), thrombocytopenia (P < 0.001), and nausea and vomiting (P = 0.001) were more frequent in the experimental than in the control arm (Table 2 and Supplementary Table 4). Four patients (5%) needed a dose reduction and 4 (5%) needed a cycle delay. Three patients (3.7%) had to discontinue TMZ treatment due to an adverse event.
Table 2.
Adverse events with different frequencies across treatment arms
| Adverse Event | Control Arm N = 79 N (%) | Experimental Arm N = 80 N (%) | P | ||||
|---|---|---|---|---|---|---|---|
| Any | Grades 1–2 | Grades 3–4 | Any | Grades 1–2 | Grades 3–4 | ||
| Lymphopenia | 33 (41.7) | 33 (41.7) | 0 | 55 (68.7) | 52 (65.0) | 3 (3.7) | <0.001 |
| Thrombocytopenia | 17 (21.5) | 17 (21.5) | 0 | 38 (47.5) | 36 (45.0) | 2 (2.5) | <0.001 |
| Nausea and vomiting | 10 (12.6) | 10 (12.6) | 0 | 30 (37.5) | 30 (37.5) | ‒ | 0.001 |
| Fatigue | 21 (26.6) | 21 (26.6) | 0 | 35(43.7) | 35(43.7) | ‒ | 0.050 |
| Leukopenia | 20 (25.3) | 20 (25.3) | 0 | 30 (37.5) | 29 (36.2) | 1 (1.3) | 0.098 |
| Hospitalization due to adverse event | 5 (5.0) | 10 (10.0) | 0.233 | ||||
| Second neoplasia | 0 | 1 (breast cancer) | NA |
NA, not applicable.
P-values for “any” grade toxicity.
Hematological toxicities were analyzed from the second follow-up visit (at cycle 2, which detected the toxicity arising from cycle 1) until the final follow-up visit (30 days after the last temozolomide cycle or the last control visit). As grade 1 lymphopenia and thrombocytopenia were not exclusion criteria, some patients presented residual low values at screening and at cycle 1, as a result of the previous 6 cycles of adjuvant temozolomide. A complete list of adverse events is included in Supplementary Table 4.
Translational Substudy
In tumor samples obtained at first surgery, 6 patients in the control arm had partial immunoreactivity of MSH6, while 5 in the experimental arm had partial immunoreactivity14 (1 MSH2/MSH6, 3 MSH6, and 1 MLH1) (Supplementary Table 5). We detected no differences in loss of the MMR immunoreactive patterns between the 2 arms of the study in 7 samples from second surgeries. In 2 samples (1 in each arm), there was a drop in the expression of MLH1. Second surgeries for these 2 patients were performed 6 months after the last TMZ cycle in the control arm (cycle 6) and 10 months after the last TMZ cycle in the experimental arm (cycle 12). No significant changes were detected in other MMR proteins.
Discussion
This randomized trial compared 6-month PFS in glioblastoma patients who stopped adjuvant TMZ after 6 cycles and those who continued for up to 6 additional cycles. Our finding of no difference between the 2 groups in 6-month PFS was reflected in a similar lack of differences in PFS and OS, suggesting that patients do not receive extra benefit from continuing adjuvant TMZ beyond 6 cycles. In contrast, continuing for longer than 6 cycles was associated with more cases of thrombocytopenia, lymphocytopenia, and nausea and vomiting.
Three retrospective analyses have also addressed this question. Blumenthal and colleagues3 performed a pooled analysis of 624 glioblastoma patients treated with either 6 or 12 cycles of TMZ in 4 studies. The authors excluded the immortal time bias15 by calculating residual PFS and OS as the time elapsed from the potential cycle 7, defined as 28 days after cycle 6. After adjusting for prognostic factors, they found improved residual PFS (HR, 0.80; P = 0.03) for patients who continued on TMZ, especially for those with methylated MGMT (n = 342; HR, 0.65; P < 0.01) but no differences in residual OS.3 Similar results were reported by the German Glioma Network with a series of 142 patients.4MGMT methylation status was known in 93% of their patients and the presence/absence of measurable disease in 61.2%.15 They corrected the immortal time bias with a 7-month landmark analysis to estimate PFS and OS after the end of the first 6 TMZ cycles. Median landmark PFS was longer for patients continuing TMZ (13.5 vs 10.2 mo; P = 0.03) but there were no differences in landmark OS (25.6 vs 26.2 mo; P = 0.12). A third retrospective trial found no differences in either PFS or OS.16
In contrast to these previous studies, our study prospectively randomized patients once they had completed 6 cycles of TMZ without progression, and we have reported the patient characteristics at the time of randomization. Our patients were younger than the average glioblastoma patient and neurologically stable without needing corticosteroids; they had a preserved MMR system; and the frequency of IDH-mutated tumors was similar to that previously reported in glioblastoma (5%).17 Nevertheless, we detected no differences between arms in 6-month PFS, median PFS, or median OS.
The methylguanine methyltransferase protein, encoded by the MGMT gene, repairs and eliminates the methyl adduct inserted by TMZ at O6-guanine purine.18 Aberrant promoter methylation of MGMT silences the protein, leading to better outcome to TMZ. Patients with MGMT methylation are highly sensitive to TMZ treatment and would thus be expected to obtain maximum benefit from extending treatment longer than 6 cycles. However, we found a disconcerting indication of a potential negative impact on OS in patients with MGMT methylation who continued on TMZ. Although patients with MGMT methylation who stopped after 6 cycles had a nonsignificant trend to a shorter initial PFS than those who continued (8.5 vs 11.4 mo), they ultimately attained a longer OS (27.1 vs 20.7 mo). Second-line therapies in both arms were mainly TMZ rechallenge or nitrosoureas, which share similar mechanisms of action, though more patients received only palliative care in the experimental arm (23.3% vs 7.2%). We could speculate that the trend to shorter OS in patients who continued TMZ might be related to an acquired resistance that was induced by a deficient MMR system under continued TMZ exposure19,20 and that later worsened at recurrence.21–23 This interpretation would suggest that accumulation of mutations due to lack of repair could lead to a hypermutator phenotype that is resistant to further therapy.19,24,25 However, our findings do not support this hypothesis. In fact, all our patients had a slightly better preserved MMR system at diagnosis than that reported at diagnosis in unselected cohorts,14,26 and we did not detect a drop in the IHC expression of the MMR proteins in 5 of the 7 samples from second surgeries, while 1 patient in each arm showed only partial immunoreactivity of MLH1.
Our trial has limitations, including the fact that it was not powered to detect significant differences in PFS or OS. To reach this power, we would have had to know the survival distribution for patients who complete the standard 6 cycles without progression from the last cycle to death, but this information was not available at the time the trial was designed. Alternatively, in order to launch a non-inferiority trial for PFS, we would have needed to include nearly 500 patients to stratify for the same factors. As patients who complete the first 6 cycles of TMZ represent less than 50% of all patients who start treatment, such a trial was not feasible within the setting of the funding grant. Secondly, results might have been affected by the small, nonsignificant imbalance in the distribution of IDH-mutated tumors between arms, mainly derived from the 15 patients younger than 56 years old who could not be properly assessed. Nevertheless, when IDH mutation status was included in the multivariate models, treatment arm was still not identified as a prognostic factor. Finally, the trial would have ideally been run with a placebo as a blinded control, but at the time the study was launched, there was no generic TMZ available and all capsules were marked with the trademark of Temodal and the dosage, making it impossible to blind these capsules by fabricating empty capsules in order to create a blinded placebo.
In summary, our findings in this prospective trial, which confirm previous retrospective data, suggest that continuing TMZ beyond 6 cycles confers no benefit in outcome for any subset of patients who have already received the first 6 standard cycles of adjuvant treatment, leading us to recommend that discontinuing TMZ after 6 cycles be considered a feasible option in patients who are progression free.
Funding
This work was supported by a grant from the Spanish Institute Carlos III (ISCIII: PI13/01751 to C.M.).
Supplementary Material
Acknowledgments
We thank our patients and their relatives for their willingness to participate in this study. We also thank all the sites and their teams, including data managers and data coordinators for their contribution to the study. Furthermore, we thank Francisco Fernandez, Cristina Bueno Paredes, and all the team at MFAR for their valuable help in the conduct of the trial and their availability and support; Jose Maria Velarde from the Institut Investigació en Ciències de la Salut Germans Trias i Pujol for his support in managing the databases; David Lopez, at the time coordinator of Pharmacy at the Institut Català d’Oncologia, Badalona, for his help in investigating how to create a placebo; and Renée Grupp for her assistance in style correction. This study was presented in part at the 2019 ASCO Annual Meeting (June 2019) as an oral presentation and at the ESMO and EANO 2019 Congresses (September 2019) as a poster discussion.
Conflict of interest statement
CB reports a grant from Instituto de Salud Carlos III, during the conduct of the study; advisory role from Celgene, Karyopharm, AbbVie, and Lipopharma and travel and accommodation expenses from Pharmamar and AbbVie. MAV reports a grant from Pfizer and advisory role for Pharmamar and Lilly. JMS reports a grant from Pfizer and Catalysis and advisory role for GW Pharma, AbbVie and Celgene. SdB reports advisory role for Novartis and Elisai. EP reports personal fees and non-financial support from Amgen, Roche, Sanofi, Celgene and Tocagen. LMN reports non-financial support from Novartis, Ipsen, and Roche. MG-G reports personal fees from Genentech, Novartis, Pfizer, and Daiichi. MA reports personal fees from Roche, Bristol, and Lilly. AH reports personal fees from Tesaro, Clovis, Roche Astra Zeneca, and MSD. CO reports nonfinancial support from Pfizer, Novartis, Eisai, and Teva. MM-G reports personal fees from Roche, Celgene, and Pfizer. AB reports personal fees from Roche, Pfizer, Novartis, MSD, Bristol, Pierre Fabre, and Merck and travel support from Merck and Novartis. CC reports advisory role for AbbVie. All other authors declare no competing interests.
Authorship statement
Conception and design: CB, MAV. Data collection, analysis, and interpretation: CB, MAV, FJP-M, CS, AE, NM, CC. Provision of study material or patients: CB, MAV, JMS, CM, SdB, EP, JM-L, AE, RdlP, JF, RG, LMN, MG-G, MA, AH, SP, CO, PP-S, MC, MM-G, AB, OG, RL, MD, SV. Central histological review: CC. Immunohistochemistry analyses: CC, NM. Central MGMT methylation analyses and IDH sequencing analyses: CS. Data analysis and interpretation: CB, FJP-M, MAV, AE. Manuscript writing: CB. Final approval of manuscript: All authors.
References
- 1. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 2. Perry JR, Laperriere N, O’Callaghan CJ, et al. ; Trial Investigators Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376(11):1027–1037. [DOI] [PubMed] [Google Scholar]
- 3. Blumenthal DT, Gorlia T, Gilbert MR, et al. . Is more better? The impact of extended adjuvant temozolomide in newly diagnosed glioblastoma: a secondary analysis of EORTC and NRG Oncology/RTOG. Neuro Oncol. 2017;19(8):1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gramatzki D, Kickingereder P, Hentschel B, et al. . Limited role for extended maintenance temozolomide for newly diagnosed glioblastoma. Neurology. 2017;88(15):1422–1430. [DOI] [PubMed] [Google Scholar]
- 5. Hegi ME, Diserens AC, Gorlia T, et al. . MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 6. Roldán Urgoiti GB, Singh AD, Easaw JC. Extended adjuvant temozolomide for treatment of newly diagnosed glioblastoma multiforme. J Neurooncol. 2012;108(1):173–177. [DOI] [PubMed] [Google Scholar]
- 7. Balañá C, Vaz MA, Lopez D, et al. . Should we continue temozolomide beyond six cycles in the adjuvant treatment of glioblastoma without an evidence of clinical benefit? A cost analysis based on prescribing patterns in Spain. Clin Transl Oncol. 2014;16(3):273–279. [DOI] [PubMed] [Google Scholar]
- 8. Weller M, Butowski N, Tran DD, et al. ; ACT IV trial investigators Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18(10):1373–1385. [DOI] [PubMed] [Google Scholar]
- 9. Stupp R, Taillibert S, Kanner A, et al. . effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gilbert MR, Wang M, Aldape KD, et al. . Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Messali A, Hay JW, Villacorta R. The cost-effectiveness of temozolomide in the adjuvant treatment of newly diagnosed glioblastoma in the United States. Neuro Oncol. 2013;15(11):1532–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weller M, van den Bent M, Tonn JC, et al. ; European Association for Neuro-Oncology (EANO) Task Force on Gliomas Evidence-based management of adult patients with diffuse glioma. Authors’ reply. Lancet Oncol. 2017;18(8):e430–e431. [DOI] [PubMed] [Google Scholar]
- 13. Wen PY, Macdonald DR, Reardon DA, et al. . Updated response assessment criteria for high-grade gliomas: Response Assessment in Neuro-Oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 14. Indraccolo S, Lombardi G, Fassan M, et al. . genetic, epigenetic, and immunologic profiling of MMR-deficient relapsed glioblastoma. Clin Cancer Res. 2019;25(6):1828–1837. [DOI] [PubMed] [Google Scholar]
- 15. Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;340:b5087. [DOI] [PubMed] [Google Scholar]
- 16. Barnett A, Knusel K, Ali A, Bamashmos AS, Sagar S, Ahluwalia MS. Efficacy of extended adjuvant temozolomide cycle duration in newly diagnosed glioblastoma: four-year experience of a single major tertiary care institution (P2.6–035). Neurology. 2019;92(15 Supplement):P2.6–035. [Google Scholar]
- 17. Yan H, Parsons DW, Jin G, et al. . IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaina B, Christmann M, Naumann S, Roos WP. MGMT: key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair (Amst). 2007;6(8):1079–1099. [DOI] [PubMed] [Google Scholar]
- 19. Sa JK, Choi SW, Zhao J, et al. . Hypermutagenesis in untreated adult gliomas due to inherited mismatch mutations. Int J Cancer. 2019;144(12):3023–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McFaline-Figueroa JL, Braun CJ, Stanciu M, et al. . Minor changes in expression of the mismatch repair protein MSH2 exert a major impact on glioblastoma response to temozolomide. Cancer Res. 2015;75(15):3127–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shinsato Y, Furukawa T, Yunoue S, et al. . Reduction of MLH1 and PMS2 confers temozolomide resistance and is associated with recurrence of glioblastoma. Oncotarget. 2013;4(12):2261–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yip S, Miao J, Cahill DP, et al. . MSH6 mutations arise in glioblastomas during temozolomide therapy and mediate temozolomide resistance. Clin Cancer Res. 2009;15(14):4622–4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hunter C, Smith R, Cahill DP, et al. . A hypermutation phenotype and somatic MSH6 mutations in recurrent human malignant gliomas after alkylator chemotherapy. Cancer Res. 2006;66(8):3987–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li GM. Mechanisms and functions of DNA mismatch repair. Cell Res. 2008;18(1):85–98. [DOI] [PubMed] [Google Scholar]
- 25. Wang J, Cazzato E, Ladewig E, et al. . Clonal evolution of glioblastoma under therapy. Nat Genet. 2016;48(7):768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Felsberg J, Thon N, Eigenbrod S, et al. ; German Glioma Network Promoter methylation and expression of MGMT and the DNA mismatch repair genes MLH1, MSH2, MSH6 and PMS2 in paired primary and recurrent glioblastomas. Int J Cancer. 2011;129(3):659–670. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.