Abstract
Multiple endocrine neoplasia type 1 (MEN-1) is an autosomal dominant syndrome consisting of endocrine tumors of the parathyroid gland, pituitary gland, and pancreas. MEN-1 is caused by loss of function mutations in the MEN1 gene, which encodes the tumor suppressor protein menin. Cutaneous collagenomas and facial angiofibromas also have been associated with MEN-1 and may serve as diagnostic clues to the diagnosis. We present a case of amelanotic melanoma resembling a large angiofibroma in a young man with MEN-1.
Report of a Case |
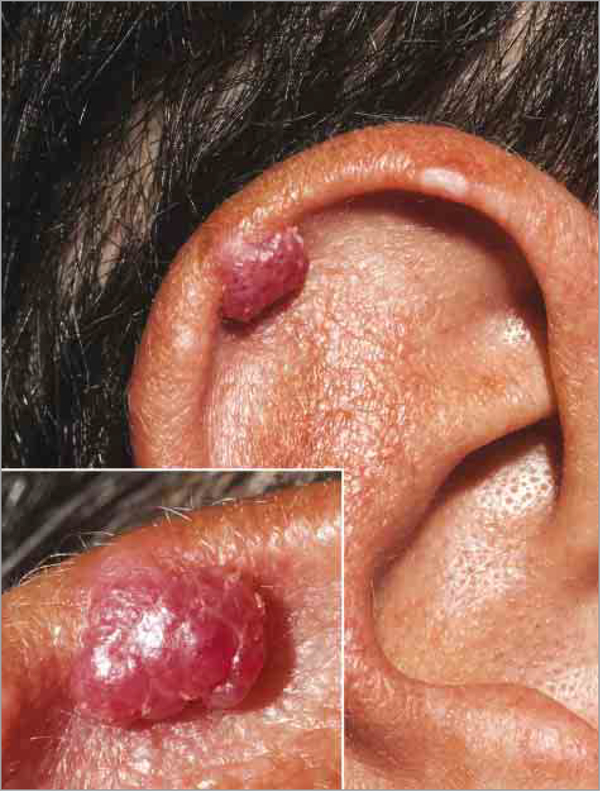
A man in his 20s with MEN-1 since his teens manifesting as hyperparathyroidism, status post parathyroidectomy, presented with a possible angiofibroma on the helix of the right ear (Figure 1) of 1 year’s duration. The lesion had grown and bled with irritation and/or trauma over the preceding months. The patient’s history included multiple facial lesions of the nose and cheeks, several of which had been previously removed and histologically confirmed to be angiofibromas.
Figure 1. Amelanotic Melanoma.
Raised, red lesion on the right helical ear. The close-up inset photograph reveals an exophytic vascular-appearing papule with a lobulated surface.
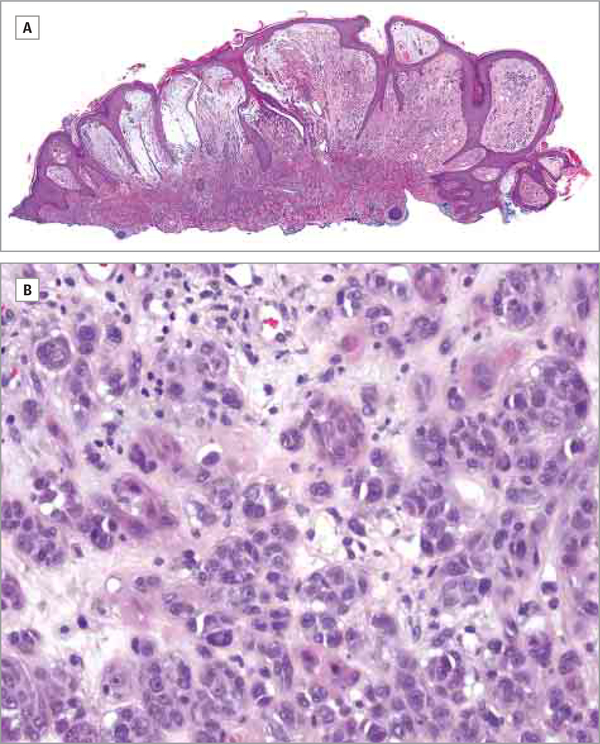
On examination, an 8-mm, red, telangiectatic, shiny exophytic lobulated papule was present on the helix of the right ear. A shave biopsy of the helical lesion was performed. Histologic analysis showed a nodular proliferation of atypical melanocytes with epithelioid morphology in a vertical growth pattern. The lesional cells showed markedly atypical to pleomorphic nuclei with occasional multinucleated forms, mitotic figures, and abundant cytoplasm. Immunohistochemical stains showed that the tumor cells were positive for S100, MART-1, and tyrosinase, supporting the diagnosis of melanoma. The atypical cells extended into the dermis consistent with malignant melanoma, nodular type, with at least Clark level IV invasion (Figure 2). The margins were involved, and the tumor thickness was at least 2.8 mm. There was also prominent papillary dermal edema with superficial dermal vascular and fibroblastic proliferation representing a possible angiofibroma in proximity to the tumor cells. He subsequently underwent reexcision of the lesion, and sentinel node biopsy findings were negative.
Figure 2. Hematoxylin-Eosin-Stained Histopathologic Sections From Shave Biopsy Specimen.
A, In this composite of 2 images, prominent papillary dermal edema is seen with superficial dermal vascular and fibroblastic proliferation; atypical melanocytes in a vertical growth pattern are also apparent (original magnification ×1).
B, Higher magnification (×20) shows a nodular proliferation of atypical melanocytes with epithelioid morphology; the melanocytes have atypical to pleomorphic nuclei with occasional multinucleated forms, mitotic figures, and abundant cytoplasm consistent with malignant melanoma.
Discussion |
Melanoma in the setting of MEN-1 appears to be a rare phenomenon. Nord et al1 reported a series 7 patients with both MEN-1 and melanoma, but in a large cohort of 220 patients with MEN-1, only 11 (0.5%) developed melanoma,2 suggesting that the rate of melanoma in patients with MEN-1 may be similar to the risk in the general population.3 Böni et al4 failed to find loss of heterozygosity in the MEN1 gene in 23 cases of primary melanoma. They conclude that while the MEN1 gene product, menin, acts as a tumor suppressor for MEN-1-related endocrine tumors, the genetic changes associated with that disease have no bearing on melanoma tumorigenesis.
The term amelanotic melanoma generally refers to melanomas either entirely devoid of pigment or with very little pigmentation. Amelanotic melanomas are relatively rare, representing approximately 1.8% to 8.1%5 of all melanomas. Diagnosis may be a challenge because the lesions are not only clinically devoid of pigment but also vary in appearance. In a series reported by Ariel,6 20 of 77 patients received inappropriate treatment for amelanotic melanoma prior to histologic confirmation.
In our case, the patient had an outdoor occupation in southern California with significant daily sun exposure. To our knowledge, this is the first case of an amelanotic melanoma in a patient with MEN-1 and also the first with overlapping histologic features of angiofibroma. This case illustrates the need for clinicians to maintain a clinical suspicion for melanoma, even in nonpigmented lesions.
Acknowledgments
Funding/Support: This study was supported in part by the National Institutes of Health (NIH).
Role of the Funder/Sponsor: The NIH had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: None reported.
Contributor Information
G. Thomas Brown, Laboratory of Pathology, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Edward W. Cowen, Dermatology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Chyi-chia Richard Lee, Laboratory of Pathology, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
References
- 1.Nord B, Platz A, Smoczynski K, et al. Malignant melanoma in patients with multiple endocrine neoplasia type 1 and involvement of the MEN1 gene in sporadic melanoma. Int J Cancer. 2000;87(4):463–467. [DOI] [PubMed] [Google Scholar]
- 2.Trump D, Farren B, Wooding C, et al. Clinical studies of multiple endocrine neoplasia type 1 (MEN1). QJM. 1996;89(9):653–669. [DOI] [PubMed] [Google Scholar]
- 3.Howlader NA, Krapcho M, Garshell J, et al. SEER Cancer Statistics Review, 1975–2011. http://seer.cancer.gov/csr/1975_2011/ Accessed June 23, 2014.
- 4.Böni R, Vortmeyer AO, Huang S, Burg G, Hofbauer G, Zhuang Z. Mutation analysis of the MEN1 tumour suppressor gene in malignant melanoma. Melanoma Res. 1999;9(3):249–252. [DOI] [PubMed] [Google Scholar]
- 5.Ariel IM. Malignant melanoma of the upper extremities. J Surg Oncol. 1981;16(2):125–143. [DOI] [PubMed] [Google Scholar]
- 6.Ariel IM. Amelanotic Malignant Melanomas. New York, NY: Appleton-Century-Crofts; 1981. [Google Scholar]