Abstract
Thymosin alpha 1 is a peptide naturally occurring in the thymus that has long been recognized for modifying, enhancing, and restoring immune function. Thymosin alpha 1 has been utilized in the treatment of immunocompromised states and malignancies, as an enhancer of vaccine response, and as a means of curbing morbidity and mortality in sepsis and numerous infections. Studies have postulated that thymosin alpha 1 could help improve the outcome in severely ill corona virus disease 2019 patients by repairing damage caused by overactivation of lymphocytic immunity and how thymosin alpha 1 could prevent the excessive activation of T cells. In this review, we discuss key literature on the background knowledge and current clinical uses of thymosin alpha 1. Considering the known biochemical properties including antibacterial and antiviral properties, time-honored applications, and the new promising findings regarding the use of thymosin, we believe that thymosin alpha 1 deserves further investigation into its antiviral properties and possible repurposing as a treatment against severe acute respiratory syndrome coronavirus-2.
Keywords: Thymosin alpha 1, Thymalfasin, Immunomodulating, T lymphocytes, Infectious diseases, Immune deficiency, Oxidative damage
Core Tip: Thymosin alpha 1 is a naturally occurring peptide in the human thymus, which has long been recognized for its immune-modulating properties. The synthetic analog of thymosin alpha 1 has various clinical applications, such as in infectious diseases, malignancies and in immunocompromised states. There is emerging data postulating that this peptide could be of benefit in the treatment of severe acute respiratory syndrome coronavirus-2 infection. We herein discuss the underlying knowledge, current clinical uses and results of recent studies of thymosin alpha 1.
INTRODUCTION
Thymosin alpha 1 is a 28 amino acid peptide originally isolated from the thymus[1], which has been extensively studied in terms of its functions in the immune system. Thymosin alpha 1 has long been recognized as an immune enhancing, immune modulating, as well as an immune restoring agent[2], and as such it has been utilized in several clinical and research settings. The synthetic form of thymosin alpha 1, thymalfasin, is approved in more than 35 countries for the treatment of hepatitis B and C and as an immune enhancer in several other diseases[1]. More specifically, it has been of benefit as a means of augmenting immune response in immune deficiencies[3], psoriatic arthritis[4], aging[3], as well as in increasing response to vaccines[3] and decreasing chemotherapy-induced toxicity[5]. It has additionally been of value in treating oncologic patients, especially those with hepatocellular carcinoma, renal cell carcinoma and non-small cell lung cancer[5]. Last but not least, it has been used in the fight of numerous infections, such as human immunodeficiency virus (HIV)[6], pseudomonas[1], and mold toxicity[7], as well as sepsis[8], and recently in severely ill coronavirus disease 2019 (COVID-19) patients[9]. In light of the current pandemic situation, efforts are being made worldwide to understand the impact of infection caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) on the immune system in the hopes of getting closer to an effective treatment. To this end, it would be worthwhile to further investigate thymosin alpha 1 through the relevant published literature. In this review, we aim to understand the characteristics of thymosin alpha 1, from its chemical structure and biological properties all the way to its clinical applications, their safety and efficacy which would provide an insight on whether it could be used as a therapeutic option to help curb mortality and improve outcomes in severely ill COVID-19 patients.
BIOCHEMISTRY
Thymosin proteins are short, positively charged, and inherently unregulated peptides. Induction of thymosin protein configuration via organic reagents, such as trifluoroethanol, hexafluoroisopropanol, dodecyl trimethylammonium bromide, and Zn2+ ions, charges the proteins neutralizing them at a low PH to potentiate their absolute effects[10]. The nuclear magnetic resonance structure of thymosin alpha 1 has been determined by mixing in 40% trifluoroethanol/60% water (v/v) solvent. The study has determined 800 MHz of a polypeptide chain consisting of 28 residues. To comprehend its distinct structure, multiple molecular trials with solvent composed of 40% trifluoroethanol/60% transferable intermolecular potential with 3 points water (v/v) were utilized to create a three-dimensional configuration of the peptide. Ultimately, it was able to depict a distorted helical configuration with two stable regions: Alpha-helix site from 14-26 residues and two double turns in the β region in the N-terminal site consisting of 12 residues[11].
Thymalfasin, the synthetic analog of thymosin alpha 1, induces interleukin (IL)-2 production, differentiation of immature cord blood lymphocytes, production of B cell growth factors, and increased macrophage antigen presentation efficiency. It is used to treat chemotherapy-induced immunosuppression and to enhance the efficacy of influenza and hepatitis B vaccines in immunocompromised patients[12]. Thymosin alpha 1 therapy modulates and partially normalizes T-lymphocyte numbers and function. T cell rosette percentages have been shown to increase in patients with T cell lymphopenia. Thymalfasin may benefit conditions such as T cell lymphopenia, immunosuppression, and immune dysregulation seen in COVID-19 due to SARS-CoV-2 induced cytokine storm and the immunosuppressive effects of its viral envelope proteins[13]. This may be why Thymalfasin has been used in China for general treatment of COVID-19 patients since April 2020[14].
EXTRACTION AND ANALYSIS
Thymosin alpha 1 is a peptide hormone that is endogenously produced by the thymus gland and potentiates T cell-mediated immune responses via differentiation and maturation of T-cell progenitor cells, activation of dendritic and natural killer cells, and stimulation of cytokine-mediated inflammation[15]. Since first isolated from a preparation of bovine thymuses, named the thymosin fraction 5 in 1977, thymosin alpha 1 has been widely recognized for its immune-enhancing properties. Therefore, various efforts have been made towards finding the most efficient method for its production and purification. There are currently three distinct ways bioactive thymosin alpha 1 can be obtained. The first method of extraction is via isolation from calf thymuses. It can also be extracted from thymosin fraction 5, which was first isolated from calf thymuses using the technique described in 1975[12]. The second method is through solid-phase synthesis, which is a purely chemical way of peptide synthesis and is nowadays the only method accepted for production of thymosin alpha 1 for clinical use. Lastly, genetic engineering expression makes use of the advances in biotechnology to produce purified recombinant thymosin alpha 1 from either prokaryotic organisms such as Escherichia coli, or eukaryotic organisms such as yeast, plants or Pichia Pastoris. Regarding thymosin alpha 1 expression in Escherichia coli, numerous expression systems have been developed based on the insertion of the recombinant gene for human thymosin alpha 1 in different vectors, such as pGEX-2T, pThioHis B, pBV222. According to Antachopoulos et al[7] (2012), the most promising results came from the BL21/pET-28a system, with thymosin alpha 1 being 70% of total bacterial protein production. The protein can then be analyzed via sodium dodecyl sulfate-polyacrylamide gel electrophoresis or by measuring ultraviolet light absorbance at 215 nm. For purification, the primary methods proposed are nickel affinity chromatography, thermal denaturation, and high-performance liquid chromatography. Thymosin alpha 1 expression in yeast is an attractive alternative because post-translational modifications and the development of stable cell lines are made possible[6].
Chen et al[16] describe their own yeast-based expression system for thymosin alpha 1, which proved to be effective in producing thymosin alpha 1 capable of increasing CD8+ counts in mice pre-treated with cyclophosphamide. An example of thymosin alpha 1 expression in plants (transgenic tomato Solanum Lycopersicum) is described by Chen et al[17]. As promising as it may seem, the genetic engineering method for thymosin alpha 1 production has not yet been introduced into clinical practice, primarily due to difficulties pertaining to extraction and purification. Other than direct extraction from calf thymus, which can only produce trace amounts of the peptide, thymosin alpha 1 used for therapeutic purposes comes from chemical synthesis.
Enzyme-linked immunosorbent assay and radioimmunoassay are the most commonly used methods for quantitative analysis of the peptide. Tuthill et al[18] also suggest liquid chromatography with tandem mass spectrometry, which has proven to be accurate, precise, and sensitive for measurement of thymosin alpha 1 in the serum[18].
STORAGE OF THYMOSIN ALPHA 1
Thymosin alpha 1 should be stored at -20 degrees Celsius. Lyophilized thymosin alpha 1 may remain stable for up to three weeks at room temperature; however, for long term storage, it should be kept below -180 degrees Celsius and stored in the desiccated form. When ready to use, it may be reconstituted and subsequently stored at 40 degrees Celsius for a period of two to seven days. If the intention is to store thymosin alpha 1 for a longer period of time, then it is advised to store it in combination with a carrier protein such as 0.1% human serum albumin or bovine serum albumin. It is recommended to avoid repeated freezing and thawing[19].
BIOLOGICAL ACTIVITIES AND HEALTH BENEFITS
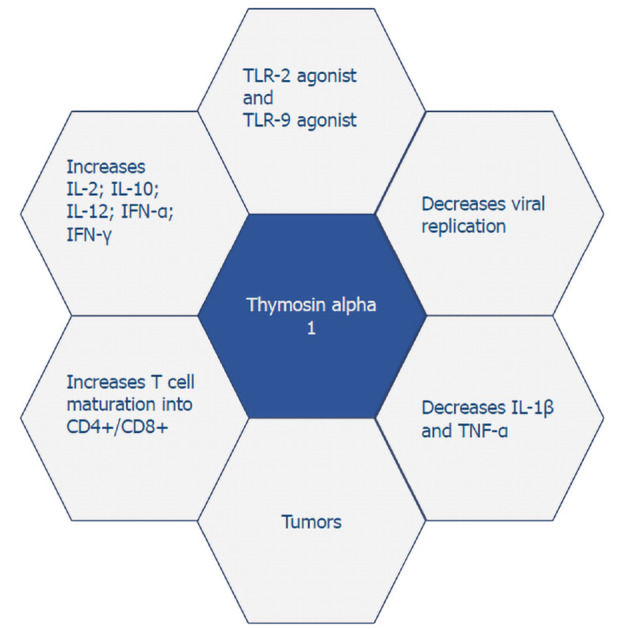
Thymosin alpha 1 functions as a toll-like receptor (TLR)-9 and TLR-2 agonist in both myeloid and dendritic cells, the professional antigen-presenting cells[20]. By targeting TLRs, thymosin alpha 1 can stimulate the adaptive immune response, which is essential for fighting viral, bacterial, and fungal infections and cancers, as well as stimulation of posterior humoral immunity[20-22]. Additionally, thymosin alpha 1 can increase levels of IL-2, IL-10, IL-12, interferon (IFN)-α, and IFN-γ[23]. The role of thymosin alpha 1 in stimulating T-cell dependent antibody production is also the reason why it has been considered as a vaccine adjuvant for enhancing response to vaccines[24].
Thymosin alpha 1 has a wide range of biological activities that range from anti-tumor to immune-modulating properties (Figure 1). The immune response of thymosin alpha 1 is due to its action in elevating the activity of T cell maturation into CD4+/CD8+ T cells. It works to directly activate natural killer cells as well as CD8+ T cells through which it kills virally infected cells. Thymosin alpha 1 has a negative effect on IL-1β and tumor necrosis factor-α, which in turn leads to a decreased inflammatory response and is quite beneficial in conditions such as chronic hepatitis and acute pancreatitis. Not only does it play a role in enhancing cytokine expression, but it also increases the prominence of major histocompatibility complex I/viral antigens on their respective target infected cells and decreases viral replication[6]. Naylor and his associates pointed out that thymosin alpha 1 does not only have one but rather a varied range of targets for its immune-enhancing activity[25].
Figure 1.
Thymosin alpha 1 has a wide range of biological activities. IL: Interleukin; IFN: Interferon; TLR: Toll-like receptors.
Thymosin alpha 1 has exhibited the ability to restrain tumor growth, hence its use in the treatment of various cancers. It has anti-proliferative properties which have been exhibited in lung and liver tumor metastases. According to studies conducted by Moody et al[25], the anti-tumor activity of thymosin alpha 1 worked best with small tumor size. Overall, thymosin alpha 1 works via two main mechanisms: Either stimulating the immune system or employing its anti-proliferative activities on tumor cells. The protective action of thymosin alpha 1 against oxidative damage as a result of its effect on liver superoxide dismutase and glutathione peroxidase has been explored by Armutcu et al[26].
Since thymosin alpha 1 is a polypeptide naturally present in the thymus, it plays a fundamental role in the control of inflammation, immunity, and tolerance. Thymosin alpha 1 has an immune-modulating action through its interaction with toll-like receptors. Due to the action of thymosin alpha 1 on other cell types, it is used as a therapeutic agent for diseases with evident immune dysfunction[4]. Clinical trials with thymosin alpha 1 for diseases like DiGeorge syndrome, non-small cell lung cancer, hepatocellular carcinoma, hepatitis B and C, HIV, and melanoma have been conducted and yielded promising results[27,28]. FDA approved the orphan drug thymalfasin (Zadaxin) for treatment of malignant melanoma, chronic active hepatitis B, DiGeorge anomaly with immune defects, and hepatocellular carcinoma due to its immunomodulatory and anti-tumor effect.
CLINICAL AND COMMERCIAL APPLICATIONS
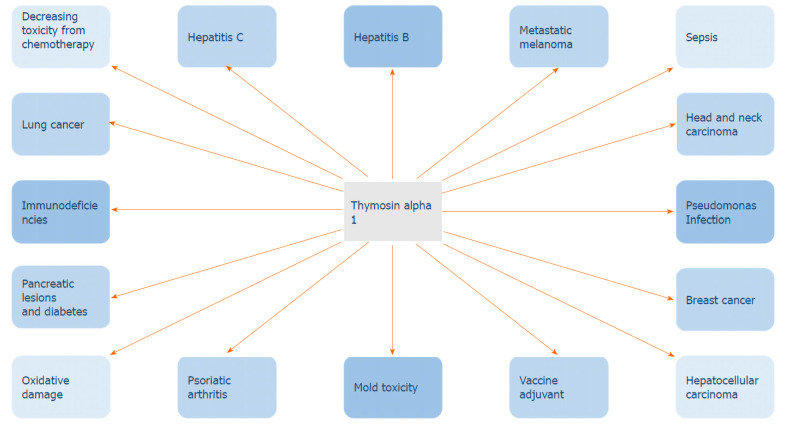
Thymosin alpha 1 has been extensively tested and its synthetic form, thymalfasin, is widely used in the clinical field (Figure 2). Some of its applications are as follows.
Figure 2.
Clinical applications of thymosin alpha 1.
Hepatitis B
The safety and efficacy of thymosin alpha 1 in patients with chronic hepatitis B have been tested through clinical trials. Thymosin alpha 1 has been tested as monotherapy as well as in combination with interferon-alpha and other nucleoside analogs. There has been found a complete virological response rate [clearance of serum hepatitis B virus deoxyribonucleic acid and hepatitis B e antigen] of 40.6% in patients given 1.6 mg subcutaneous injection twice a week and 26.5% in patients given the same regimen for 52 wk[29]. However, it is important to note that treatment of Hepatitis B using thymosin alpha 1 was only used in the era of interferon and is now obsolete in the era post-discovery of direct antiviral agents.
Hepatitis C
Thymosin alpha 1 as a monotherapy does not seem to be useful in treating hepatitis C infection. However, combination therapy of thymosin alpha 1 and pegylated interferon alpha 2a could effectively repress viral replication in hepatitis C patients. Thymosin alpha 1, in combination with interferon-alpha 1 has also been tested for treatment in patients with chronic hepatitis C. Moreover, thymosin alpha 1 is well tolerated, with no significant adverse effects observed. A meta-analysis conducted by Sherman, included many trials showing the superiority of the combination of thymosin alpha 1 and interferon alpha 1 compared to interferon alpha mono-therapy[30]. It remains important to note that similar to Hepatitis B, the treatment of Hepatitis C with thymosin alpha 1 has been discontinued in favor of direct antiviral agents.
Sepsis
The use of thymosin alpha 1 in patients with sepsis has shown a significant decrease in mortality due to multiple-organ failure, which is the primary cause of death in sepsis[8].
HIV infection
Thymosin alpha 1, interferon alpha 1, and zidovudine combination therapy has been well-tolerated in HIV patients. Thymosin alpha 1 enhances the function and increases the number of CD4+ T cells, while it also decreases viral load. Thymosin alpha 1 influences thymic T-cell output. The safety and efficacy of thymosin alpha 1 in combination with highly-active antiretroviral therapy in stimulating immune reconstitution has been proven[6]. It has been shown that thymosin alpha 1 is well tolerated, and could dramatically increase the levels of signal joint T cell receptor excision circles in patients with advanced HIV disease. Prolonged use of high-dose thymosin alpha 1 is more effective[2].
Pseudomonas - bone marrow transplant patients
Thymosin alpha 1 is also used in other infections like pseudomonas or infections following bone marrow transplant[1].
Mold toxicity
This thymic peptide has the ability to prime dendritic cells and to enhance Th1 and Treg cells so that inflammation is balanced, and an antifungal response is generated. Th1 response will activate the production of Th2 cytokines (IFN-γ, IL-2, IL-12, IL-18), stimulating phagocytic activity. Therefore, cytotoxic CD4+, CD8+, and T cells and opsonizing antibodies will be produced, generating a protective effect against fungal pathogens[7].
Immune deficiency
Treatment with thymosin alpha 1 serves as a stimulus for IL-2 receptor expression and IL-2 internalization. It also has a restoring effect on patients with a suppressed lymphokine-activated killer cell activity and with immunodeficiency[29]. Acting through Toll-like receptors in both myeloid and plasmacytoid dendritic cells, thymosin alpha 1 stimulates the signaling pathways and initiates the production of immune-related cytokines. Thus, thymosin alpha 1 is anticipated to bring about encouraging results in the treatment of immunocompromised patients. Overall, it improves immune system function without causing adverse events[13].
Psoriatic arthritis
Thymosin alpha 1 is a potent modulator of immunity and inflammation. Evidence is growing that diseases characterized by deregulation of the immune system and inflammation, such as psoriatic arthritis, are associated with serum levels of thymosin alpha 1 significantly lower than those of healthy individuals. The data is consistent with the role of thymosin alpha 1 as a regulator of immunity, tolerance and inflammation in patients with psoriatic arthritis[4].
Vaccine adjuvant
The use of thymosin alpha 1 as an adjuvant to the influenza vaccine has shown promising results, especially among elderly and immunocompromised patients[31]. Thymosin alpha 1 has also been shown to improve the immunogenicity of the influenza vaccine[3].
Decreasing toxicity from chemotherapy
Clinical studies show that thymosin alpha 1 has been utilized in patients with different malignancies, reducing the toxicity of chemotherapy, and improving the quality of life. An increase in the numbers and functions of immune cells and the decrease of toxicity from chemotherapy was also an effect of utilizing this medication. In general, fewer infections occurred during chemotherapy, neurotoxicity decreased, and the quality of life improved[5].
Oxidative damage, pancreatic lesions and diabetes
Many studies have shown that thymosin alpha 1 has protective effects against oxidative damage. By remarkably amplifying the activity of catalase, superoxide dismutase, and glutathione peroxidase, thymosin alpha 1 reduces the production of reactive oxygen species and prevents oxidative damage to hepatic tissue. Thymosin alpha 1 has well-established antiproliferative properties seen with various human malignancies and this is a result of its capacity to decrease oxidative stress[6]. It also helps ameliorate pancreatic damage and the resulting diabetes by reducing the production of malondialdehyde and by improving the function of superoxide dismutase and catalase. The antioxidant properties of thymosin alpha 1 are considered to be of great benefit in the treatment of pancreatic lesions[32].
Applications in oncologic patients
Multiple studies have shown promising results for the use of thymosin alpha 1 in patients with metastatic melanoma, head and neck carcinoma, lung cancer, breast cancer, and hepatocellular carcinoma[33]. Thymosin alpha 1 is indicated as adjuvant for chemotherapy-induced immune depression, immune insufficiency, and immune suppression in patients[5]. In addition, it has been shown that thymosin alpha 1 in combination with chemotherapy or radiation improves survival rate in patients with non-small cell lung cancer, which accounts for 85% of all lung cancers and is known for its low responsiveness to chemotherapy[5].
SAFETY AND DOSES
Thymosin alpha 1 is usually found in an injection form and is commonly prescribed by a primary care physician. Thymosin alpha 1 is usually administered twice a week via a subcutaneous route. The standard single dosage ranges from 0.8 to 6.4 mg, while multiple doses range from 1.6 to 16 mg for five to seven days. Utilized in various illnesses such as liver disease, cancer, and autoimmune diseases, thymosin alpha 1 has been shown to be well-tolerated and safe[34].
ADVERSE EFFECTS AND CONTRAINDICATIONS
Thymalfasin, the synthetic form of thymosin alpha 1, is usually well tolerated. The most common adverse effects include local irritation, redness, or discomfort at the site of injection. In clinical trials, the combination of thymalfasin with interferon 2b was reported to have rare side effects such as fever, fatigue, muscle aches, nausea, vomiting, and neutropenia when compared to interferon-alpha 2b alone or with placebo[34]. Thymalfasin is contraindicated in patients with hypersensitivity to thymosin alpha 1 or any of the components of the injection. Due to the immunomodulatory action of thymalfasin, it is also contraindicated in immu-nosuppressed patients, such as organ transplant recipients, unless the benefits of the treatment exceed the risks[35].
EVIDENCE FROM PREVIOUS HUMAN CLINICAL STUDIES
Thymosin alpha 1 has been utilized in various cases to enhance cell-mediated immunity and for the treatment of a multitude of different diseases (Table 1). A study was performed to demonstrate the effect of thymosin alpha 1 in the human breast cancer lines ZR-75-1, MCF-7, MDA-MB-231, MCF-10A and BT-549. For this experiment, thymosin alpha 1 was dissolved in sterile water and stored in 2 mL plastic tubes at -20 °C. Results showed that thymosin alpha 1 inhibited cell proliferation and induced apoptosis in human leukemia, non-small cell lung cancer, melanoma, and other cancers. Apoptosis was significantly induced in human breast cancer and leukemia cell lines with a thymosin alpha 1 concentration of 100 to 160 IM. Additionally, data exhibited that ZR-75-1 and MCF-7 cells display different sensitivities to thymosin alpha 1. In general, the study revealed that thymosin alpha 1 could be a possible approach to breast cancer treatment[36]. Other studies demonstrate that thymosin alpha 1 could be a promising therapy for severe sepsis. Various small-scale studies as well as a large-scale, multicenter, single-blinded, and randomized control trial were conducted in six tertiary teaching hospitals in China, with the purpose of demonstrating the vital role thymosin alpha 1 plays in sepsis therapy. Patients admitted to the intensive care unit with severe sepsis were distributed randomly among the control group and the thymosin alpha 1 group. Hypodermic injections of 1.6 mg of thymosin alpha-1 or normal saline were distributed to all individuals two times a day for five days; afterwards, the dose was reduced to once per day. Results showed that the thymosin alpha 1 group was 9.0% lower in mortality rate than the control group[21].
Table 1.
Summarizing pre-clinical and clinical studies
|
Pre-clinical studies
| ||
|
Ref.
|
Year
|
Application of thymosin alpha 1
|
| Guo et al[36] | 2015 | The anti-tumor effect of thymosin alpha 1 was studied on human cancer cell lines. The study concluded that thymosin alpha 1 can decrease proliferation and induce apoptosis in human leukemia, non-small cell lung cancer, melanoma, and other cancers. The study concluded that thymosin alpha 1 could be an approach to breast cancer treatment |
| Clinical studies | ||
| Sherman et al[29] | 2010 | Thymosin alpha 1 was tested as monotherapy and in combination with interferon-alpha for the treatment of chronic hepatitis B. It was also shown to stimulate IL-2 receptor expression and IL-2 internalization and to enhance immune response in patients with immunodeficiency |
| Eckert et al[30] | 1994 | Combination therapy of thymosin alpha 1 and pegylated interferon alpha 2a preferred over interferon monotherapy for the treatment of chronic hepatitis C |
| Li et al[8] | 2015 | Significant decrease in mortality due to multiple organ failure in patients with sepsis |
| Li et al[6] | 2010 | Thymosin alpha 1 can be safely used as an adjuvant to antiretroviral therapy in HIV patients. It helps increase CD4+ count, stimulates the function of CD4+ cells, and helps decrease viral load. By amplifying the activity of catalase, superoxide dismutase, and glutathione peroxidase, it decreases oxidative damage to tissues. Thymosin alpha 1 reduces tumor cell proliferation in human malignancies by decreasing oxidative stress |
| Matteucci et al[2] | 2017 | Thymosin alpha 1 significantly increases levels of sjTREC in patients with advanced HIV disease |
| Camerini et al[1] | 2015 | Thymosin alpha 1 can be used in pseudomonas infections or infections following bone marrow transplant |
| Antachopoulos et al[7] | 2012 | Thymosin alpha 1 might be effective against mold toxicity |
| King et al[13] | 2016 | Thymosin alpha 1 increases cytokine production and is expected to be beneficial in immunocompromised patients |
| Pica et al[4] | 2018 | It has been postulated that thymosin alpha 1 can help regulate immunity and reduce inflammation in patients with psoriatic arthritis |
| Panatto et al[31] | 2011 | Thymosin alpha 1 has shown promising results as an adjuvant to the influenza vaccine |
| Carraro et al[3] | 2012 | Thymosin alpha 1 improves immunogenicity of the influenza vaccine |
| Qin et al[32] | 2009 | Thymosin alpha 1 can reduce oxidative damage to the pancreas and mitigate the risk of resulting diabetes |
| Costantini et al[33] | 2019 | Thymosin alpha 1 has shown promising results in patients with malignancies, such as metastatic melanoma, head and neck carcinoma, lung cancer, breast cancer, and hepatocellular carcinoma |
| Romani et al[21] | 2007 | A single-blind randomized control trial was conducted in six tertiary hospitals in China to study the beneficial effects of thymosin alpha 1 on patients with sepsis. The results showed 9% lower mortality in the treatment group compared to the control group |
| Sugahara et al[37] | 2002 | Patients with chronic hepatitis B who were treated with thymosin alpha 1 showed an overall improvement in serum ALT levels. ALT levels were reduced to normal in 42.9%. A total disappearance of serum HBV DNA was noted in 28.6% of patients |
IL: Interleukin; sjTREC: Signal joint T cell receptor excision circles; HIV: Human immunodeficiency virus; ALT: Glutamic-pyruvic transaminase; HBV: Hepatitis B virus; DNA: Deoxyribonucleic acid.
As discussed above, one of the strongest properties of thymosin alpha 1 is its role in the activation of T cell responses in the body. A study in seven patients with chronic hepatitis caused by hepatitis B virus tried to identify the immunomodulatory properties of thymosin alpha 1. Each individual was treated for a total of 24 wk with a hypodermic injection at a dose of 1.29/0.4 mg/body/day six times weekly for the first 2 wk and then twice weekly for an additional 22 wk. Subsequently, liver biopsies were performed to gather data. The serum alanine transaminase levels improved to 47.39/17.0 IU/L and normalized in 42.9% of patients after 48 wk of treatment. However, complete disappearance of serum hepatitis B virus deoxyribonucleic acid was seen in 28.6%. Thymosin alpha 1 also affected maturation of T-cells, demonstrating its high immunomodulatory properties. Overall, it has been reported that combination therapy with thymosin alpha 1 and IFN-α has demonstrable biological activity in patients with viral hepatitis[37].
THYMOSIN ALPHA 1/THYMALFASIN VS THYMOSIN BETA 4/TIMBETASIN
Thymosin alpha 1 and thymosin beta 4 are two hormone peptides that are secreted from the thymus and have vastly different chemical compositions and immunological actions. These proteins are separated from thymosin fraction 5 and have the potential to change a variety of immune functions in mammals. Thymosin alpha 1 is thought to be responsible for rebuilding the immune system by enhancing cell-mediated immunity in animals without a thymus gland. Thymosin beta 4 is in the family of actin monomer-sequestering proteins which essentially regulate unpolymerized actin and have an active role in maintaining the free G-actin monomers in the cytoplasm. Thymosin alpha 1 is clinically relevant in various types of cancer, specifically hepatocellular carcinoma, lung cancer and melanomas. Thymosin beta 4, has a strong response to virally infected cells. It is currently being tested as a possible therapy against influenza, HIV, and acquired immune deficiency syndrome[38-41].
COULD THYMOSIN ALPHA 1 IMPROVE THE OUTCOMES IN COVID-19 PATIENTS?
The COVID-19 pandemic has had a worldwide impact and multiple studies have shown the immunological effects of this disease. All countries affected by SARS-CoV-2 are focused on searching for an effective treatment. Thymosin alpha 1 has a very prominent role in both immunity control and inflammation (Table 2). So far, it has been used in various pathologic conditions: Infections, sepsis, immune deficiencies and malignancies, just to name a few. It has also been found to curb mortality in several of them, such as sepsis and HIV infection. Although clinical studies on the efficiency of thymosin alpha 1 in treating COVID-19 are still limited, it would be of great value to further explore the potential benefits that this drug can bring about in mitigating the devastating effects of the current pandemic.
Table 2.
Summary of ongoing clinical trials of thymosin and coronavirus disease 2019
|
Clinical trial number
|
Location
|
Status
|
Condition
|
Intervention
|
Results
|
| NCT04428008 | United States | Not yet recruiting | COVID-19 | Drug: Thymalfasin | Not yet available |
| NCT04487444 | United States | Recruiting | COVID-19 | Drug: Thymalfasin | Not yet available |
| NCT04268537 | China | Not yet recruiting | COVID-19 | Drug: PD-1 blocking antibody + standard treatment; Drug: Thymosin + Standard treatment; Other: Standard treatment | Not yet available |
| NCT04320238 | China | Recruiting | COVID-19 | Drug: Recombinant human interferon Alpha-1b; Drug: Thymosin alpha 1 | Not yet available |
Data retrieved from clinicaltrials.gov on October 5th, 2020. COVID-19: Coronavirus disease 2019.
A recent study in COVID-19 patients demonstrated how thymosin alpha 1 significantly promoted the proliferation of activated T cells and this led to a critical prevention of lymphopenia in infected patients. In total, there were 25 severely and critically ill patients who participated in the study. Eleven of them received daily treatment of thymosin alpha 1 for one week, while the rest of the patients remained untreated. Data illustrates that patients in the thymosin alpha 1 treatment group had a higher number of lymphocytes than patients without treatment[42]. In another retrospective study conducted in China, patients in the treatment group received subcutaneous injections of 10 mg thymosin alpha 1 once per day for at least seven consecutive days. Thymosin alpha 1 supplementation showed improvement and restoration of T cell counts in COVID-19 patients with severe lymphocytopenia and, in the end, thymosin alpha 1 supplementation reduced mortality in patients severely ill with COVID-19[30].
In COVID-19 treatment, it has been postulated to administer thymosin alpha 1 as an intramuscular injection for 7 d for patients who have CD8 cells less than 400/μL and CD4 cells less than 650/μL. This is postulated on the understanding that thymosin alpha 1 induction showed improvement in T cell number in elderly patients with comorbidities like hypertension and cardiovascular diseases. Healthy people who are older than 60 years of age should receive thymosin alpha 1 as a supplement to prevent COVID-19 infection[43]. It has also been suggested that thymosin alpha 1 taken before administration of methylprednisolone in COVID-19 patients may prevent steroid-induced death of thymocytes[44]. The National Health Commission of China included thymosin alpha 1 as an alternative treatment option for patients with lymphocytopenia or immunodeficiency.
Currently, there are various ongoing clinical trials registered on clinicaltrials.gov of thymosin in COVID-19 patients (Table 2).
CONCLUSION
Thymosin alpha 1 is a thymus peptide with recognized immune modulating capacity and biochemical properties. The synthetic analogue of thymosin alpha 1, thymalfasin, induces IL-2 and B cell growth factor production, differentiation of immature cord blood lymphocytes, raises efficiency of macrophage antigen presentation, and the modulation and partial normalization of function and number of T-lymphocytes. The effects of immune stimulation occur through TLR action in both the myeloid and plasmacytoid dendritic cells with production of cytokines. The immunosuppressive effects of the SARS-CoV-2 viral envelope in inducing cytokine storm may be modulated with thymosin alpha 1 therapy. This would be especially beneficial in preventing catastrophic events such as cytokine storm in more severe cases. Thymosin alpha 1 and its synthetic analogue thymalfasin have well-studied safety profiles and are well-tolerated with only minor side effects. Clinical studies have demonstrated the significant role of thymosin alpha 1 in immune and inflammatory responses, and extensive research has shown its effective use in a myriad of diseases ranging from hepatitis and HIV to immune deficiencies and cancers, as well as its use as a vaccine adjuvant.
Within the context of COVID-19 infection, it has been shown to reduce mortality in those with severe disease and aid in restoring some immune function through increasing thymic activity. Further study would be highly beneficial in determining if thymosin alpha 1 could serve as a therapeutic agent or in combination with other treatments to mitigate the progression and severity of the disease. For this purpose, we can conclude that further studies are mandated for using thymosin alpha 1 in these patients.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Dr. Marcos A Sanchez-Gonzalez, MD, PhD for actively providing valuable advice and suggestions during the course of the project.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Manuscript source: Unsolicited manuscript
Peer-review started: October 13, 2020
First decision: November 18, 2020
Article in press: December 2, 2020
Specialty type: Infectious diseases
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Bendary M, Scicchitano P S-Editor: Zhang L L-Editor: A P-Editor: Ma YJ
Contributor Information
Asimina Dominari, Division of Research and Academic Affairs, Larkin Health System, South Miami, FL 33143, United States.
Donald Hathaway III, Division of Research and Academic Affairs, Larkin Health System, South Miami, FL 33143, United States. donald.hathaway@larkinhospital.com.
Krunal Pandav, Division of Research and Academic Affairs, Larkin Health System, South Miami, FL 33143, United States.
Wanessa Matos, Division of Research and Academic Affairs, Larkin Health System, South Miami, FL 33143, United States.
Sharmi Biswas, Division of Research and Academic Affairs, Larkin Health System, South Miami, FL 33143, United States.
Gowry Reddy, Division of Research and Academic Affairs, Larkin Health System, South Miami, FL 33143, United States.
Sindhu Thevuthasan, Division of Research and Academic Affairs, Larkin Health System, South Miami, FL 33143, United States.
Muhammad Adnan Khan, Division of Research and Academic Affairs, Larkin Health System, South Miami, FL 33143, United States.
Anoopa Mathew, Division of Research and Academic Affairs, Larkin Health System, South Miami, FL 33143, United States.
Sarabjot Singh Makkar, Division of Research and Academic Affairs, Larkin Health System, South Miami, FL 33143, United States.
Madiha Zaidi, Division of Research and Academic Affairs, Larkin Health System, South Miami, FL 33143, United States.
Michael Maher Mourad Fahem, Division of Research and Academic Affairs, Larkin Health System, South Miami, FL 33143, United States.
Renato Beas, Division of Research and Academic Affairs, Larkin Health System, South Miami, FL 33143, United States.
Valeria Castaneda, Division of Research and Academic Affairs, Larkin Health System, South Miami, FL 33143, United States.
Trissa Paul, Division of Research and Academic Affairs, Larkin Health System, South Miami, FL 33143, United States.
John Halpern, Division of Research and Academic Affairs, Larkin Health System, South Miami, FL 33143, United States.
Diana Baralt, Division of Research and Academic Affairs, Larkin Health System, South Miami, FL 33143, United States.
References
- 1.Camerini R, Garaci E. Historical review of thymosin α 1 in infectious diseases. Expert Opin Biol Ther. 2015;15 Suppl 1:S117–S127. doi: 10.1517/14712598.2015.1033393. [DOI] [PubMed] [Google Scholar]
- 2.Matteucci C, Grelli S, Balestrieri E, Minutolo A, Argaw-Denboba A, Macchi B, Sinibaldi-Vallebona P, Perno CF, Mastino A, Garaci E. Thymosin alpha 1 and HIV-1: recent advances and future perspectives. Future Microbiol. 2017;12:141–155. doi: 10.2217/fmb-2016-0125. [DOI] [PubMed] [Google Scholar]
- 3.Carraro G, Naso A, Montomoli E, Gasparini R, Camerini R, Panatto D, Tineo MC, De Giorgi L, Piccirella S, Khadang B, Ceracchi M, De Rosa A. Thymosin-alpha 1 (Zadaxin) enhances the immunogenicity of an adjuvated pandemic H1N1v influenza vaccine (Focetria) in hemodialyzed patients: a pilot study. Vaccine. 2012;30:1170–1180. doi: 10.1016/j.vaccine.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Pica F, Gaziano R, Casalinuovo IA, Moroni G, Buè C, Limongi D, D'Agostini C, Tomino C, Perricone R, Palamara AT, Sinibaldi Vallebona P, Garaci E. Serum thymosin alpha 1 Levels in normal and pathological conditions. Expert Opin Biol Ther. 2018;18:13–21. doi: 10.1080/14712598.2018.1474197. [DOI] [PubMed] [Google Scholar]
- 5. Zadaxin (Thymalfasin): Uses, Dosage, Side Effects, Interactions, Warning [Internet]. RxList. 2009. [cited 2020 September 27]. Available from: https://www.rxlist.com/zadaxin-drug.htm#description .
- 6.Li J, Liu CH, Wang FS. Thymosin alpha 1: biological activities, applications and genetic engineering production. Peptides. 2010;31:2151–2158. doi: 10.1016/j.peptides.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antachopoulos C, Katragkou A, Roilides E. Immunotherapy against invasive mold infections. Immunotherapy. 2012;4:107–120. doi: 10.2217/imt.11.159. [DOI] [PubMed] [Google Scholar]
- 8.Li C, Bo L, Liu Q, Jin F. Thymosin alpha1 based immunomodulatory therapy for sepsis: a systematic review and meta-analysis. Int J Infect Dis. 2015;33:90–96. doi: 10.1016/j.ijid.2014.12.032. [DOI] [PubMed] [Google Scholar]
- 9.Romani L, Tomino C, Puccetti P, Garaci E. Off-label therapy targeting pathogenic inflammation in COVID-19. Cell Death Discov. 2020;6:49. doi: 10.1038/s41420-020-0283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoch K, Volk DE. Structures of Thymosin Proteins. Vitam Horm. Epub 2016; 102: 1-24. [DOI] [PubMed] [Google Scholar]
- 11.Elizondo-Riojas MA, Chamow SM, Tuthill CW, Gorenstein DG, Volk DE. NMR structure of human thymosin alpha-1. Biochem Biophys Res Commun. 2011;416:356–361. doi: 10.1016/j.bbrc.2011.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thymalfasin [Internet]. Pubchem.ncbi.nlm.nih.gov. [cited 2020 September 27]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Thymalfasin .
- 13.King R, Tuthill C. Immune Modulation with Thymosin Alpha 1 Treatment. Vitam Horm. 2016;102:151–178. doi: 10.1016/bs.vh.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Liu XH, Lu SH, Chen J, Xia L, Yang ZG, Charles S, Yang Y, Lin Y, Lu HZ. Clinical characteristics of foreign-imported COVID-19 cases in Shanghai, China. Emerg Microbes Infect. 2020;9:1230–1232. doi: 10.1080/22221751.2020.1766383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poo JL, Sánchez-Avila F, Kershenobich D, García-Samper X, Gongora J, Uribe M. Triple combination of thymalfasin, peginterferon alfa-2a and ribavirin in patients with chronic hepatitis C who have failed prior interferon and ribavirin treatment: 24-week interim results of a pilot study. J Gastroenterol Hepatol. 2004;19 Suppl 6:S79–S81. doi: 10.1111/j.1440-1746.2004.03634.x. [DOI] [PubMed] [Google Scholar]
- 16.Chen F, Chen XM, Chen Z, Jiang HL, Pan XP, Hu ZR, Liu RH, Chen XM. Construction and application of a yeast expression system for thymosin alpha1. Biocell. 2005;29:253–259. [PubMed] [Google Scholar]
- 17.Chen Y, Wang A, Zhao L, Shen G, Cui L, Tang K. Expression of thymosin alpha1 concatemer in transgenic tomato (Solanum lycopersicum) fruits. Biotechnol Appl Biochem. 2009;52:303–312. doi: 10.1042/BA20080054. [DOI] [PubMed] [Google Scholar]
- 18.Tuthill CW, Rudolph A, Li Y, Tan B, Fitzgerald TJ, Beck SR, Li YX. Quantitative analysis of thymosin alpha1 in human serum by LC-MS/MS. AAPS PharmSciTech. 2000;1:E11. doi: 10.1208/pt010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thymosin alpha 1 steroid [Internet]. Mybiosource.com. [cited 2020 September 27]. Available from: https://www.mybiosource.com/steroid/thymosin-alpha-1/639043 .
- 20.Romani L, Bistoni F, Gaziano R, Bozza S, Montagnoli C, Perruccio K, Pitzurra L, Bellocchio S, Velardi A, Rasi G, Di Francesco P, Garaci E. Thymosin alpha 1 activates dendritic cells for antifungal Th1 resistance through toll-like receptor signaling. Blood. 2004;103:4232–4239. doi: 10.1182/blood-2003-11-4036. [DOI] [PubMed] [Google Scholar]
- 21.Romani L, Bistoni F, Montagnoli C, Gaziano R, Bozza S, Bonifazi P, Zelante T, Moretti S, Rasi G, Garaci E, Puccetti P. Thymosin alpha1: an endogenous regulator of inflammation, immunity, and tolerance. Ann N Y Acad Sci. 2007;1112:326–338. doi: 10.1196/annals.1415.002. [DOI] [PubMed] [Google Scholar]
- 22.Wu J, Zhou L, Liu J, Ma G, Kou Q, He Z, Chen J, Ou-Yang B, Chen M, Li Y, Wu X, Gu B, Chen L, Zou Z, Qiang X, Chen Y, Lin A, Zhang G, Guan X. The efficacy of thymosin alpha 1 for severe sepsis (ETASS): a multicenter, single-blind, randomized and controlled trial. Crit Care. 2013;17:R8. doi: 10.1186/cc11932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuthill C, Rios I, De Rosa A, Camerini R. Thymosin α1 continues to show promise as an enhancer for vaccine response. Ann N Y Acad Sci. 2012;1270:21–27. doi: 10.1111/j.1749-6632.2012.06680.x. [DOI] [PubMed] [Google Scholar]
- 24.Naylor PH, Quadrini K, Garaci E, Rasi G, Hadden JW. Immunopharmacology of thymosin alpha1 and cytokine synergy. Ann N Y Acad Sci. 2007;1112:235–244. doi: 10.1196/annals.1415.036. [DOI] [PubMed] [Google Scholar]
- 25.Moody TW. Thymosin alpha1 as a chemopreventive agent in lung and breast cancer. Ann N Y Acad Sci. 2007;1112:297–304. doi: 10.1196/annals.1415.040. [DOI] [PubMed] [Google Scholar]
- 26.Armutcu F, Coskun O, Gürel A, Kanter M, Can M, Ucar F, Unalacak M. Thymosin alpha 1 attenuates lipid peroxidation and improves fructose-induced steatohepatitis in rats. Clin Biochem. 2005;38:540–547. doi: 10.1016/j.clinbiochem.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Tuthill C, Rios I, McBeath R. Thymosin alpha 1: past clinical experience and future promise. Ann N Y Acad Sci. 2010;1194:130–135. doi: 10.1111/j.1749-6632.2010.05482.x. [DOI] [PubMed] [Google Scholar]
- 28.Chien RN, Liaw YF, Chen TC, Yeh CT, Sheen IS. Efficacy of thymosin alpha1 in patients with chronic hepatitis B: a randomized, controlled trial. Hepatology. 1998;27:1383–1387. doi: 10.1002/hep.510270527. [DOI] [PubMed] [Google Scholar]
- 29.Sherman KE. Thymosin alpha 1 for treatment of hepatitis C virus: promise and proof. Ann N Y Acad Sci. 2010;1194:136–140. doi: 10.1111/j.1749-6632.2010.05460.x. [DOI] [PubMed] [Google Scholar]
- 30.Eckert K, Schmitt M, Garbin F, Wahn U, Maurer HR. Thymosin alpha 1 effects, in vitro, on lymphokine-activated killer cells from patients with primary immunodeficiencies: preliminary results. Int J Immunopharmacol. 1994;16:1019–1025. doi: 10.1016/0192-0561(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 31.Panatto D, Amicizia D, Lai PL, Camerini R, De Rosa A, Gasparini R. Utility of thymosin alpha-1 (Zadaxin) as a co-adjuvant in influenza vaccines: a review. J Prev Med Hyg. 2011;52:111–115. [PubMed] [Google Scholar]
- 32.Qin Y, Chen FD, Zhou L, Gong XG, Han QF. Proliferative and anti-proliferative effects of thymosin alpha1 on cells are associated with manipulation of cellular ROS levels. Chem Biol Interact. 2009;180:383–388. doi: 10.1016/j.cbi.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 33.Costantini C, Bellet MM, Pariano M, Renga G, Stincardini C, Goldstein AL, Garaci E, Romani L. A Reappraisal of Thymosin Alpha1 in Cancer Therapy. Front Oncol. 2019;9:873. doi: 10.3389/fonc.2019.00873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ancell CD, Phipps J, Young L. Thymosin alpha-1. Am J Health Syst Pharm. 2001;58:879–85; quiz 886. doi: 10.1093/ajhp/58.10.886. [DOI] [PubMed] [Google Scholar]
- 35.Rekdal M, Pai A, Bs M. Experimental data of co-crystals of Etravirine and L-tartaric acid. Data Brief. 2018;16:135–140. doi: 10.1016/j.dib.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo Y, Chang H, Li J, Xu XY, Shen L, Yu ZB, Liu WC. Thymosin alpha 1 suppresses proliferation and induces apoptosis in breast cancer cells through PTEN-mediated inhibition of PI3K/Akt/mTOR signaling pathway. Apoptosis. 2015;20:1109–1121. doi: 10.1007/s10495-015-1138-9. [DOI] [PubMed] [Google Scholar]
- 37.Sugahara S, Ichida T, Yamagiwa S, Ishikawa T, Uehara K, Yoshida Y, Yang XH, Nomoto M, Watanabe H, Abo T, Asakura H. Thymosin-alpha1 increases intrahepatic NKT cells and CTLs in patients with chronic hepatitis B. Hepatol Res. 2002;24:346–354. doi: 10.1016/s1386-6346(02)00145-6. [DOI] [PubMed] [Google Scholar]
- 38.Renault L. Intrinsic, Functional, and Structural Properties of β-Thymosins and β-Thymosin/WH2 Domains in the Regulation and Coordination of Actin Self-Assembly Dynamics and Cytoskeleton Remodeling. Vitam Horm. 2016;102:25–54. doi: 10.1016/bs.vh.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Kim J, Jung Y. Thymosin Beta 4 Is a Potential Regulator of Hepatic Stellate Cells. Vitam Horm. 2016;102:121–149. doi: 10.1016/bs.vh.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 40.Xue B, Robinson RC. Actin-Induced Structure in the Beta-Thymosin Family of Intrinsically Disordered Proteins. Vitam Horm. 2016;102:55–71. doi: 10.1016/bs.vh.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 41.Hsia J, Sztein MB, Naylor PH, Simon GL, Goldstein AL, Hayden FG. Modulation of thymosin alpha 1 and thymosin beta 4 Levels and peripheral blood mononuclear cell subsets during experimental rhinovirus colds. Lymphokine Res. 1989;8:383–391. [PubMed] [Google Scholar]
- 42.Yu K, He J, Wu Y, Xie B, Liu X, Wei B, Zhou H, Lin B, Zuo Z, Wen W, Xu W, Zou B, Wei L, Huang X, Zhou P. Dysregulated adaptive immune response contributes to severe COVID-19. Cell Res. 2020;30:814–816. doi: 10.1038/s41422-020-0391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bergstrom DJ, Kotb R, Louzada ML, Sutherland HJ, Tavoularis S, Venner CP Myeloma Canada Research Network Consensus Guideline Consortium. Consensus Guidelines on the Diagnosis of Multiple Myeloma and Related Disorders: Recommendations of the Myeloma Canada Research Network Consensus Guideline Consortium. Clin Lymphoma Myeloma Leuk. 2020;20:e352–e367. doi: 10.1016/j.clml.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 44.Zhang L, Liu Y. Potential interventions for novel coronavirus in China: A systematic review. J Med Virol. 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]