Key Points
Question
What are the changes in treatment-naive pigment epithelial detachments (PEDs) associated with the initial anti–vascular endothelial growth factor injection in the Phase III, Double-masked, Multicenter, Randomized, Active Treatment-controlled Study of the Efficacy and Safety of 0.5 mg and 2.0 mg Ranibizumab Administered Monthly or on an As-needed Basis in Patients With Subfoveal Neovascular Age-Related Macular Degeneration randomized clinical trial?
Findings
In this post hoc analysis of a randomized clinical trial, approximately one-third of patients had a flattened PED 1 month following the initial ranibizumab injection, and lower baseline PED height was associated with PED flattening. Flattening was not associated with vision outcomes at 2 years, although patients treated as needed whose PED flattened at 1 month were more likely to receive fewer injections by month 24.
Meaning
These findings suggest flattening at 1 month may be a marker for less intensive as-needed injection frequency.
Abstract
Importance
Pigment epithelial detachment (PED) is a feature commonly associated with neovascular age-related macular degeneration (nAMD) and may be perceived as being difficult to treat. Therefore, this investigation explored changes in PEDs and visual acuity outcomes following an initial anti–vascular endothelial growth factor (VEGF) injection and identified factors associated with positive response.
Objective
To describe changes in treatment-naive pigment epithelial detachments associated with the initial anti-VEGF injection.
Design, Setting, and Participants
Post hoc analysis of patients from the Phase III, Double-masked, Multicenter, Randomized, Active Treatment-controlled Study of the Efficacy and Safety of 0.5 mg and 2.0 mg Ranibizumab Administered Monthly or on an As-needed Basis in Patients With Subfoveal Neovascular Age-Related Macular Degeneration (HARBOR) trial (NCT00891735) with PED at baseline. The HARBOR trial was a phase 3, randomized, multicenter, double-masked, active treatment–controlled trial. Participants included treatment-naive patients with subfoveal nAMD and PEDs at baseline; intervention arms were pooled for analysis (n = 586). The HARBOR study began in July 2009 and was completed in August 2012, and the post hoc analyses were conducted between October 2016 and May 2018.
Interventions
Intravitreal injections of ranibizumab, 0.5 mg and 2.0 mg, administered monthly or on an as-needed basis over 24 months.
Main Outcomes and Measures
Post hoc analyses of flattened PED frequency at month 1, univariate and multivariable analysis of patient and ocular characteristics at baseline and PED status at month 1, and total number of ranibizumab injections received stratified by PED status at month 1.
Results
A total of 35.5% of patients (208 of 586) with PED at baseline achieved a flattened PED after a single ranibizumab injection. An additional 17.3% subsequently achieved a flattened PED at month 2. Univariate analysis identified an association between older age, lower PED height, and lower subretinal fluid thickness with PED flattening after a single injection. Multivariable analysis identified PED height as a factor associated with this anatomical outcome. Best-corrected visual acuity scores were not superior based on PED flattening at month 1. On average, patients in the as-needed arm who achieved a flattened PED after a single ranibizumab injection required fewer injections by month 24 vs patients whose PED remained present at month 1 (11.0 vs 14.2; difference, 3.3; 95% CI, 1.9-4.6; P < .001).
Conclusions and Relevance
In this group of treatment-naive patients with PED from nAMD, after the initial ranibizumab injection approximately one-third and after the second injection approximately one-half had flattened PEDS, although visual outcomes were not superior among those that did vs did not have flattening. The findings suggest flattening may serve as a marker for less intensive as-needed injection frequencies.
Trial Registration
ClinicalTrials.gov Identifier: NCT00891735
This post hoc analysis of a randomized clinical trial investigates changes in treatment-naive pigment epithelial detachments associated with the initial anti–vascular endothelial growth factor injection.
Introduction
Pigment epithelial detachment (PED) is characterized by retinal pigment epithelium separation from the Bruch membrane and can be found in 63% to 80% of eyes with neovascular age-related macular degeneration (nAMD).1,2,3,4 The HARBOR trial of ranibizumab in nAMD enrolled treatment-naive patients with subfoveal choroidal neovascularization. At baseline, 54.5% of patients had PED.5 The HARBOR trial was the largest study to our knowledge to use higher-resolution spectral-domain optical coherence tomography (OCT). The objective of this post hoc analysis was to describe changes in treatment-naive PEDs following first anti–vascular endothelial growth factor (VEGF) injection and investigate whether early response is associated with treatment needs/vision outcomes at 2 years.
Methods
The HARBOR trial’s full methodologic details have been reported previously.6 The HARBOR trial was conducted in accordance with Good Clinical Practice (International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use E6), applicable US Food and Drug Administration regulations, and the Health Insurance Portability and Accountability Act. Institutional review boards approved HARBOR, and all patients provided written informed consent, which included use of patient data for further analyses. Patient compensation was allowed based on visit completion and was approved by each ethics committee/institutional review board.
One thousand eighty-nine eligible treatment-naive patients with subfoveal nAMD were randomized to ranibizumab, 0.5 mg or 2.0 mg, intravitreal injections, administered monthly or as needed (PRN), following 3 monthly loading doses.6,7 This study analyzed data following the first loading dose at month 1 in the subgroup of 586 eyes with PED at baseline. The PED status (presence/absence) was assessed at month 1/month 2. The PEDs were stratified into small (35.0-164.0 μm), medium (164.5-233.0 μm), large (233.3-351.0 μm), or extra large (352.0-1395.5 μm) groups based on baseline PED height quartiles.5 Data from all treatment arms were pooled for analysis, although some analyses were stratified by dose/treatment regimen. Retinal pigment epithelium rips/PED recurrences were not analyzed. Statistical methods include univariate analysis reporting means/frequencies for baseline patient/ocular characteristics by PED status at month 1 following a single ranibizumab injection. Multivariable analysis included baseline variables that were significant in the univariate analysis at the P less than .05 level (2-sided test) and used logistic regression with backward selection to identify significant PED flattening predictors at month 1. Total number of ranibizumab injections in PRN arms received during HARBOR, and best-corrected visual acuity (BCVA) over time by PED status at month 1, are reported. Observed data were used, and there was no imputation for missing data. All analyses were performed using SAS, version 9.4 (SAS Institute Inc).
Results
After the first anti-VEGF injection, 208 patients (35.5%) with PEDs at baseline had a flattened PED (Figure 1A). Of these patients, 191 of 207 patients’ PEDs (92.3%; 1 patient did not have OCT data at month 2) remained flat at month 2 (Figure 1B). Of 378 patients (64.5%) with PEDs at baseline that remained present after a single ranibizumab injection (Figure 1A), 64 of 371 (17.3%; 7 patients did not have OCT data at month 2) had a flattened PED after their second ranibizumab injection (Figure 1C).
Figure 1. Outcomes of Pigment Epithelial Detachments (PEDs) After Ranibizumab Injection.
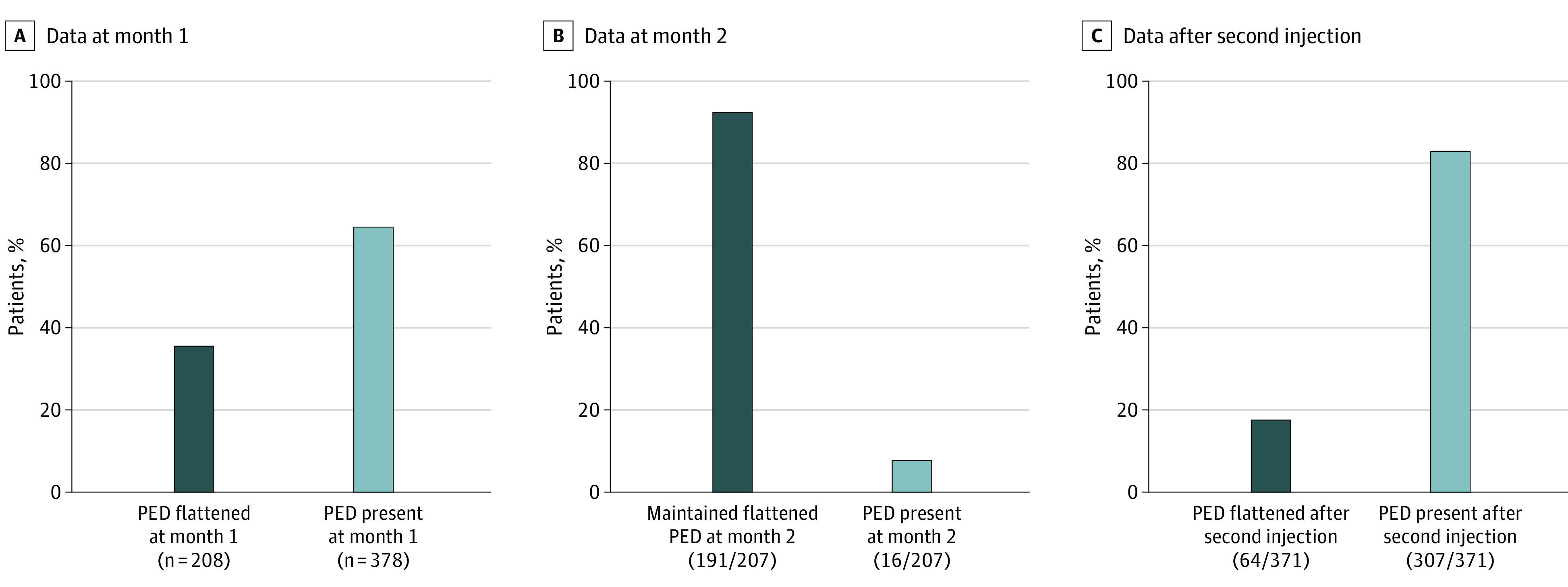
A, Proportion of patients with PEDs that flattened after a single ranibizumab injection. B, Proportion of patients whose PEDs flattened at month 1 (n = 207) and remained flattened at month 2 with continued ranibizumab treatment. C, Proportion of patients whose PEDs were present after a single injection at month 1 (n = 371) but flattened at month 2 after a second injection.
On average, patients with flat PEDs at month 1 had smaller PEDs at baseline than those with PEDs present at month 1 (206.0 μm; 95% CI, 191.7-220.4 vs 319.9 μm; 95% CI, 301.4-338.5; P < .001). More eyes with flat PEDs at month 1 were small or medium at baseline (81 of 208 [38.9%] and 66 of 208 [31.7%], respectively) vs large or extra large (40 of 208 [19.2%] and 21 of 208 [10.1%], respectively). More than half of eyes with PEDs present at month 1 had large or extra large PEDs at baseline (105 of 378 [27.8%] and 124 of 378 [32.8%], respectively). A greater proportion of patients with flat PEDs at month 1 were in the higher-dose (2.0-mg) ranibizumab treatment arm vs patients with PEDs present at month 1 (57.7% [n = 120 of 208] vs 45.5% [n = 172 of 378]).
Univariate analysis identified older age, lower PED height, and lower subretinal fluid thickness as associated with PED flattening after a single ranibizumab injection (Table). Multivariable analysis identified PED height as a highly significant factor associated with PED flattening after a single ranibizumab injection; for every 10-μm increase in PED height, the odds of having a flat PED decreased by 6.1% (odds ratio, 0.94; 95% CI, 0.92-0.96).
Table. Patient Characteristics at Baseline, Stratified by PED Status at Month 1a.
| Characteristic | No. (%) | |
|---|---|---|
| PED present at month 1 (n = 378) | PED absent at month 1 (n = 208) | |
| Age, mean (SD), y | 78.9 (8.0) | 80.3 (7.6) |
| Sex | ||
| Female | 233 (61.6) | 138 (66.3) |
| Male | 145 (38.4) | 70 (33.7) |
| Race/ethnicity | ||
| American Indian or Alaska Native | 1 (0.3) | 0 |
| Asian | 7 (1.9) | 1 (0.5) |
| Black or African American | 2 (0.5) | 0 |
| Multiracial | 0 | 1 (0.5) |
| Native Hawaiian or other Pacific Islander | 1 (0.3) | 1 (0.5) |
| White | 363 (97.1) | 203 (98.5) |
| Ethnicity | ||
| Hispanic or Latino | 10 (2.6) | 9 (4.3) |
| Not Hispanic or Latino | 368 (97.4) | 199 (95.7) |
| PED height, mean (SD), μm | 319.9 (183.5) | 206.0 (104.9) |
| Presence of drusen | ||
| No | 368 (98.7) | 202 (98.5) |
| Yes | 5 (1.3) | 3 (1.5) |
| Soft drusen | ||
| No | 45 (12.1) | 20 (9.8) |
| Yes | 328 (87.9) | 185 (90.2) |
| Hard drusen | ||
| No | 9 (2.4) | 4 (2.0) |
| Yes | 364 (97.6) | 201 (98.0) |
| No. of intermediate/large drusen | ||
| 0-5 | 199 (53.2) | 98 (48.5) |
| ≥6 | 175 (46.8) | 104 (51.5) |
| Time from diagnosis to first RBZ injection, mean (SD), d | 40.6 (252.2) | 26.0 (61.5) |
| Subretinal fluid thickness, mean (SD), μm | 155.7 (108.8) | 111.2 (91.9) |
| Central retinal/lesion thickness, mean (SD), μm | 417.1 (138.2) | 432.1 (132.3) |
| Central subfield thickness, mean (SD), μm | 358.1 (112.3) | 372.1 (96.5) |
| CNV thickness, mean (SD), μm | 153.1 (104.4) | 148.9 (83.5) |
| Central foveal thickness, mean (SD), μm | 324.2 (133.5) | 337.3 (127.0) |
Abbreviations: CNV, choroidal neovascularization; PED, pigment epithelial detachment; RBZ, ranibizumab.
Data limited to patients with PEDs present at baseline.
In PRN arms, patients whose PEDs flattened after a single ranibizumab injection required fewer injections by month 24 vs patients whose PEDs remained present after a single ranibizumab injection (11.0 vs 14.2; difference, 3.3; 95% CI, 1.9-4.6; P < .001; Figure 2A). A small difference in mean BCVA scores was observed at baseline (53.4; 95% CI, 51.6-55.2 vs 57.0; 95% CI, 55.8-58.2; P < .001; Snellen equivalent, 20/100 vs 20/80) and month 1 (59.6; 95% CI, 57.7-61.5 vs 62.2; 95% CI, 60.8-63.5; P = .03; 20/63 vs 20/63; +6.2 vs +5.2 letters from baseline) between patients with flattened or present PEDs at month 1, respectively (Figure 2B). Despite observing approximately 10% more flattening with the higher dose, differences in mean BCVA scores observed between the 0.5-mg and 2.0-mg arms were not superior. In patients with PEDs present at month 1, the 0.5-mg and 2.0-mg arms had mean BCVA scores of 57.1 (Snellen equivalent, 20/80) and 56.9 (20/80) at baseline, 62.5 (20/63; +5.4 letters from baseline) and 61.8 (20/63; +4.9) at month 1, and 66.3 (20/50; +9.2) and 62.6 (20/63; +5.7) at month 24, respectively. In patients with flattened PEDs at month 1, the 0.5-mg and 2.0-mg arms had mean BCVA scores of 53.5 (20/80) and 53.3 (20/80) at baseline, 59.4 (20/63; +5.9) and 59.8 (20/63; +6.5) at month 1, and 63.5 (20/63; +10) and 64.4 (20/50; 11.1) at month 24, respectively.
Figure 2. Mean Number of Ranibizumab Injections and Mean Best-Corrected Visual Acuity (BCVA) Score.
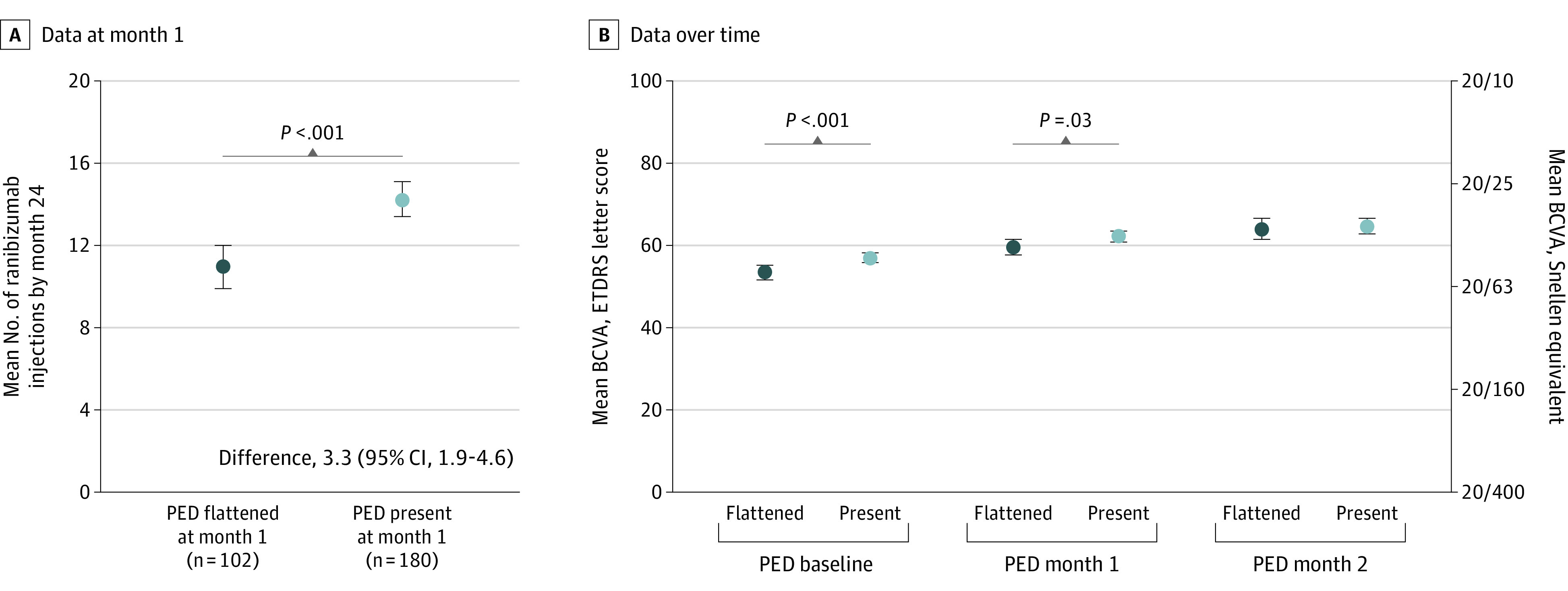
A. Mean number of ranibizumab injections by month 24 administered to patients in the as-needed (PRN) arms (pooled data from the ranibizumab, 0.5 mg and 2.0 mg PRN treatment arms) by month 1 pigment epithelial detachment (PED) status. B. Mean BCVA score (Early Treatment Diabetic Retinopathy Study [ETDRS] letters) at baseline, month 1, and month 24 by month 1 PED status. Vertical bars represent 95% CI.
Discussion
A substantial proportion of HARBOR patients with PEDs at baseline derived clinical benefit from ranibizumab. This was particularly evident because more than one-third of patients displayed flattened PEDs after a single ranibizumab injection (35.5%), and an additional 17.3% of patients attained this response by month 2, representing about 50% of all patients with PEDs at baseline. Nearly all patients with flattened PEDs after a single ranibizumab injection maintained their flattened PED through month 2. These findings appear to oppose the perception that PEDs may be difficult to treat.8 Furthermore, our findings suggest that small or medium PEDs (ie, ≤233 μm) are more likely to flatten after a single ranibizumab injection.
Limitations
It should be noted that baseline PED contents (eg, serous or fibrovascular) were not reported in this study; therefore, effect of PED contents on flattening cannot be evaluated. However, anti-VEGF treatment would likely have been the suggested treatment regardless of contents on spectral-domain OCT.9 Additionally, some cases may represent retina angiomatous proliferation or type 3 Gass lesions,10 which were not looked for in this study. Inclusion of such cases might artificially raise the number of PED cases reported in this study, and therefore, actual treatment response may be higher than observed.
We found that patients in the PRN arm with flattened PED at month 1 were associated with fewer injections by month 24. We found that patients in the PRN arm with flattened PED at month 1 required fewer injections by month 24, specifically, 11.0 vs 14.2 injections for a difference of 3.3 injections (95% CI, 1.9-4.6) over 2 years. This suggests that early treatment response may be indicative of less intensive injection frequency over 24 months. It is important to highlight that HARBOR visual acuity outcomes were similar regardless of PED status at month 1.
Conclusions
In this post hoc analysis, approximately half of treatment-naive PEDs flattened after 2 anti-VEGF injections. The PED height at baseline was identified as a significant factor associated with flattening. Flattening had no effect on vision outcomes. Thus, PED flattening early in treatment may serve as a marker of less intensive injection frequency.
References
- 1.Gass JDM. Pathogenesis of tears of the retinal pigment epithelium. Br J Ophthalmol. 1984;68(8):513-519. doi: 10.1136/bjo.68.8.513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khanani AM, Eichenbaum D, Schlottmann PG, Tuomi L, Sarraf D. Optimal management of pigment epithelial detachments in eyes with neovsacular age-related macular degeneration. Retina. 2018;38(11):2103-2117. doi: 10.1097/IAE.0000000000002195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coscas F, Coscas G, Souied E, Tick S, Soubrane G. Optical coherence tomography identification of occult choroidal neovascularization in age-related macular degeneration. Am J Ophthalmol. 2007;144(4):592-599. doi: 10.1016/j.ajo.2007.06.014 [DOI] [PubMed] [Google Scholar]
- 4.Schmidt-Erfurth U, Waldstein SM, Deak GG, Kundi M, Simader C. Pigment epithelial detachment followed by retinal cystoid degeneration leads to vision loss in treatment of neovascular age-related macular degeneration. Ophthalmology. 2015;122(4):822-832. doi: 10.1016/j.ophtha.2014.11.017 [DOI] [PubMed] [Google Scholar]
- 5.Sarraf D, London NJ, Khurana RN, et al. Ranibizumab treatment for pigment epithelial detachment secondary to neovascular age-related macular degeneration: post hoc analysis of the HARBOR study. Ophthalmology. 2016;123(10):2213-2224. doi: 10.1016/j.ophtha.2016.07.007 [DOI] [PubMed] [Google Scholar]
- 6.Busbee BG, Ho AC, Brown DM, et al. ; HARBOR Study Group . Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013;120(5):1046-1056. doi: 10.1016/j.ophtha.2012.10.014 [DOI] [PubMed] [Google Scholar]
- 7.Ho AC, Busbee BG, Regillo CD, et al. ; HARBOR Study Group . Twenty-four-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2014;121(11):2181-2192. doi: 10.1016/j.ophtha.2014.05.009 [DOI] [PubMed] [Google Scholar]
- 8.Freeman WR, Kozak I, Yuson RM, Nigam N, Cheng L, Mojana F. Prognostic implications of pigment epithelial detachment in bevacizumab (avastin)-treated eyes with age-related macular degeneration and choroidal neovascularization. Retina. 2011;31(9):1812-1818. doi: 10.1097/IAE.0b013e31821987a4 [DOI] [PubMed] [Google Scholar]
- 9.Age-Related Macular Degeneration Preferred Practice Pattern . 2019;
- 10.Freund KB, Zweifel SA, Engelbert M. Do we need a new classification for choroidal neovascularization in age-related macular degeneration? Retina. 2010;30(9):1333-1349. doi: 10.1097/IAE.0b013e3181e7976b [DOI] [PubMed] [Google Scholar]


