Key Points
Question
What is the effect of integrated palliative and oncology care (IPC) on patient-reported and end-of-life (EOL) outcomes for patients with acute myeloid leukemia (AML)?
Findings
In this randomized clinical trial of 160 adults with AML, IPC improved patient-reported quality of life, as well as depression, anxiety, and posttraumatic stress symptoms during intensive chemotherapy and for up to 24 weeks. Among patients who died, those receiving IPC were more likely to have reported discussing their EOL care preferences and less likely to have received chemotherapy near the EOL.
Meaning
For patients with AML, IPC led to substantial improvements in quality of life, psychological distress, and EOL care, and should be considered a new standard of care for this population.
This randomized clinical trial assesses the effect of integrated palliative and oncology care on patient-reported and end-of-life outcomes in patients with acute myeloid leukemia.
Abstract
Importance
Patients with acute myeloid leukemia (AML) receiving intensive chemotherapy experience substantial decline in their quality of life (QOL) and mood during their hospitalization for induction chemotherapy and often receive aggressive care at the end of life (EOL). However, the role of specialty palliative care for improving the QOL and care for this population is currently unknown.
Objective
To assess the effect of integrated palliative and oncology care (IPC) on patient-reported and EOL outcomes in patients with AML.
Design, Setting, and Participants
We conducted a multisite randomized clinical trial of IPC (n = 86) vs usual care (UC) (n = 74) for patients with AML undergoing intensive chemotherapy. Data were collected from January 2017 through July 2019 at 4 tertiary care academic hospitals in the United States.
Interventions
Patients assigned to IPC were seen by palliative care clinicians at least twice per week during their initial and subsequent hospitalizations.
Main Outcomes and Measures
Patients completed the 44-item Functional Assessment of Cancer Therapy–Leukemia scale (score range, 0-176) to assess QOL; the 14-item Hospital Anxiety and Depression Scale (HADS), with subscales assessing symptoms of anxiety and depression (score range, 0-21); and the PTSD Checklist–Civilian version to assess posttraumatic stress disorder (PTSD) symptoms (score range, 17-85) at baseline and weeks 2, 4, 12, and 24. The primary end point was QOL at week 2. We used analysis of covariance adjusting and mixed linear effect models to evaluate patient-reported outcomes. We used Fisher exact test to compare patient-reported discussion of EOL care preferences and receipt of chemotherapy in the last 30 days of life.
Results
Of 235 eligible patients, 160 (68.1%) were enrolled; of the 160 participants, the median (range) age was 64.4 (19.7-80.1) years, and 64 (40.0%) were women. Compared with those receiving UC, IPC participants reported better QOL (adjusted mean score, 107.59 vs 116.45; P = .04), and lower depression (adjusted mean score, 7.20 vs 5.68; P = .02), anxiety (adjusted mean score, 5.94 vs 4.53; P = .02), and PTSD symptoms (adjusted mean score, 31.69 vs 27.79; P = .01) at week 2. Intervention effects were sustained to week 24 for QOL (β, 2.35; 95% CI, 0.02-4.68; P = .048), depression (β, −0.42; 95% CI, −0.82 to −0.02; P = .04), anxiety (β, −0.38; 95% CI, −0.75 to −0.01; P = .04), and PTSD symptoms (β, −1.43; 95% CI, −2.34 to −0.54; P = .002). Among patients who died, those receiving IPC were more likely than those receiving UC to report discussing EOL care preferences (21 of 28 [75.0%] vs 12 of 30 [40.0%]; P = .01) and less likely to receive chemotherapy near EOL (15 of 43 [34.9%] vs 27 of 41 [65.9%]; P = .01).
Conclusions and Relevance
In this randomized clinical trial of patients with AML, IPC led to substantial improvements in QOL, psychological distress, and EOL care. Palliative care should be considered a new standard of care for patients with AML.
Trial Registration
ClinicalTrials.gov Identifier: NCT02975869
Introduction
Patients with acute myeloid leukemia (AML) receiving intensive induction chemotherapy face an abrupt onset of a life-threatening illness that necessitates urgent initiation of treatment and a prolonged hospitalization for recovery.1,2,3,4,5 During this hospitalization, patients experience marked physical symptoms due to effects of intensive chemotherapy, which negatively effects their quality of life (QOL).6,7,8,9,10,11,12 The majority of patients also experience psychological distress as they struggle with uncertainty regarding their prognosis, the isolation they experience during the hospital stay, and the loss of their independence.6,7,8,9,10,11,12,13,14,15,16,17,18,19 In addition to their physical and psychosocial symptom burden, there is a critical need to optimize end-of-life (EOL) care for patients with AML.20,21,22,23,24 These patients rarely discuss their EOL care preferences, are often hospitalized, and receive chemotherapy in the last weeks of life.18,21,25,26 Yet, interventions to improve QOL, reduce psychological distress, and optimize EOL care for this population are lacking.
Specialty palliative care has been shown to improve QOL, reduce symptom burden and psychological distress, and enhance EOL outcomes for patients with advanced solid tumors.27,28,29,30,31 Additionally, palliative care integrated with transplant care has been shown to improve QOL and reduce psychological distress for patients with hematologic cancers undergoing stem cell transplantation.32,33 However, oncologists rarely consult palliative care for patients with AML, in part because of the lack of evidence for the role of early palliative care in this population.21,22,34,35 Clinical trials are needed to determine if involvement of palliative care can improve the experience and outcomes of patients with AML.
We conducted a multisite, nonblinded randomized clinical trial to assess the effect of integrated palliative and oncology care (IPC) vs usual care (UC) on QOL, mood, symptom burden, posttraumatic stress symptoms, and EOL outcomes for hospitalized patients with AML receiving intensive chemotherapy. We hypothesized that patients receiving IPC would have (1) better QOL, (2) lower psychological distress, (3) reduced symptom burden, (4) higher rates of discussing their EOL care preferences with their clinicians, and (5) lower rates of hospitalization and chemotherapy administration near the EOL.
Methods
Participants
Hospitalized patients 18 years and older with high-risk AML receiving intensive chemotherapy were eligible to participate. We defined patients with high-risk AML as (1) newly diagnosed patients 60 years and older with an antecedent hematologic disorder or therapy-related disease, or (2) patients with relapsed or primary refractory AML. We considered intensive chemotherapy as a combination of anthracycline and cytarabine (ie, the 7 + 3 regimen) or a modification of this regimen on a clinical trial with additional drug(s) added or other similar intensive chemotherapy regimens requiring a 3- to 6-week hospitalization. We excluded patients with a diagnosis of acute promyelocytic leukemia and those receiving nonintensive chemotherapy. We also excluded patients already receiving palliative care and those with major psychiatric or comorbid conditions that would prohibit their adherence to study procedures, as determined by the treating oncologist.
Study Procedures
This study was approved by the institutional review boards at all participating sites (Massachusetts General Hospital, Duke University Medical Center, University of Pennsylvania, and Ohio State University) and followed Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines. From January 2017 to July 2019, we enrolled 160 patients in a multisite, nonblinded randomized trial of IPC as compared with UC. We identified consecutive eligible hospitalized patients with AML by screening the hospital admission census at participating institutions. A research assistant obtained permission by email from the treating oncologist to approach eligible patients within 72 hours of initiating chemotherapy. Willing participants provided written informed consent and completed baseline study questionnaires. Participants were not blinded to the intervention. Patients were registered and randomized by the Dana-Farber/Harvard Cancer Center Quality Assurance Office for Clinical Trials to receive IPC or UC. We used computer-generated 1:1 randomization stratified by study site and disease status (newly diagnosed vs relapsed/refractory). Participants completed subsequent study questionnaires at weeks 2, 4, 12, and 24 after enrollment.
Integrated Palliative and Oncology Care Intervention
Patients randomized to IPC met with an inpatient palliative care physician, advance practice nurse, or physician assistant within 72 hours of randomization. The palliative care clinician conducted at least 2 visits per week throughout the patient’s hospitalization for intensive chemotherapy and all subsequent hospitalizations up to 1 year after randomization. Patients and the palliative care clinician were permitted to initiate additional visits during hospitalizations as needed. Palliative care clinicians did not see patients in the outpatient setting.
We developed the IPC intervention based on prior work developing and evaluating the effect of palliative care for patients with solid tumors, and also those with hematologic cancers undergoing stem cell transplantation.27,28,32,33 Palliative care clinicians initially focused on establishing rapport, assessing palliative care needs, and developing a relationship with the patient. Throughout hospitalization, clinicians addressed patients’ symptoms, assessed their illness understanding, ascertained their goals and expectations, and assisted with their treatment decision-making. Palliative care clinicians documented the elements of care that they addressed after each visit by using a structured questionnaire in the Research Electronic Data Capture (REDCap) system.
Usual Care
Patients assigned to UC received supportive care measures as per their oncology team. They were permitted to receive palliative care at their request or at the request of their oncologist.
Study Measures
Participant-Reported Measures
We used the 44-item Functional Assessment of Cancer Therapy–Leukemia scale that includes 5 subscales assessing physical, functional, emotional, social well-being, and leukemia-specific concerns during the past week (score range, 0-176), with higher scores indicating better QOL.36 We measured patients’ anxiety and depression with the 14-item Hospital Anxiety and Depression Scale (HADS). The HADS consists of 2 subscales assessing symptoms of anxiety and depression, with subscale scores ranging from 0 (no distress) to 21 (maximum distress) and cut-off scores of more than 7 indicating clinically significant symptoms.37 We also assessed depression with the 9-item Patient Health Questionnaire (PHQ-9), a measure that detects symptoms of major depressive disorder, according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, and can be evaluated continuously with higher scores indicating worse mood.38 We used the revised 10-item Edmonton Symptom Assessment Scale, which uses a 0-10 scale (score range, 0-100), with higher scores indicating greater symptom burden.39 We used the 17-item PTSD Checklist–Civilian version to evaluate the severity of posttraumatic stress disorder (PTSD) symptoms (score range, 17-85), with higher scores indicating worse PTSD symptoms.40
We used 1 item to assess patient-reported discussion of EOL care preferences with their clinicians, as per prior studies.41 Specifically, patients were asked, “Have you and your doctors discussed any particular wishes you have about the care you want to receive if you were dying?” Response items were yes or no. Although patients completed this measure at all study time points, we used the assessment prior to death or at 6-month follow-up, as defined in the study protocol (Supplement 1).
EOL Outcomes
The research assistant collected data on hospitalization, chemotherapy administration based on chemotherapy administration flow sheets, and hospice referrals (if applicable) from patient electronic medical records (EMRs). The research assistant obtained date of death from the patient EMRs or from obituaries. All EOL outcomes were obtained by January 2020, at a minimum of 6-month follow-up for all study participants.
Statistical Analysis
We performed statistical analyses using Stata, version 9.3 (StataCorp). We summarized participants’ baseline characteristics between randomized groups, using frequency and percentage for categorical variables and median (SD) range for continuous variables. A 2-sided P value less than .05 was considered statistically significant.
The primary end point of the study was a comparison of QOL at week 2 between study groups using analysis of covariance and controlling for baseline criterion score. We chose the second week of hospitalization as the primary end point because it is the most symptomatic phase of the intensive chemotherapy hospitalization.10,42 We also compared symptom burden using the Edmonton Symptom Assessment Scale, depression symptoms using PHQ-9 and the depression subscale of HADS, anxiety symptoms using the anxiety subscale of HADS, and PTSD symptoms using the PTSD Checklist–Civilian at week 2 between the study groups using analysis of covariance and controlling for baseline criterion score. We also dichotomized the HADS depression and anxiety subscale scores as described above to compare frequencies of depression and anxiety symptoms between the study groups at week 2 using Fisher exact test.
We then used mixed linear effect models using maximum likelihood to account for missing data to examine the effect of IPC on patient-reported outcomes longitudinally across all time points (baseline, week 2, week 4, week 12, and week 24). For all these analyses, we report the beta estimated coefficient (β). A positive β coefficient indicates a positive association between the intervention and the outcome of interest. A negative β coefficient indicates a negative association between the intervention and the outcome of interest.
We compared rates of patient-reported EOL discussions, hospitalizations in the last week of life, chemotherapy administration in the last 30 days of life, and hospice use between the 2 study groups by using Fisher exact test. We used Poisson regression to compare hospice length of stay between the study groups.
We powered this study based on prior experience integrating palliative care for patients undergoing stem cell transplantation. With a sample size of 160 patients, we ensured greater than 90% power to detect a 6.9-point difference in patient-reported QOL at week 2, with a 2-sided .05 significant level and assuming 10% missing data at week 2. To address the issue of multiple tests, we used the false discovery rate (FDR) control method. For secondary outcomes, we selected an FDR of 15%, which denotes the acceptable percentage of results that potentially represent false positives.43
Results
Patient Participants
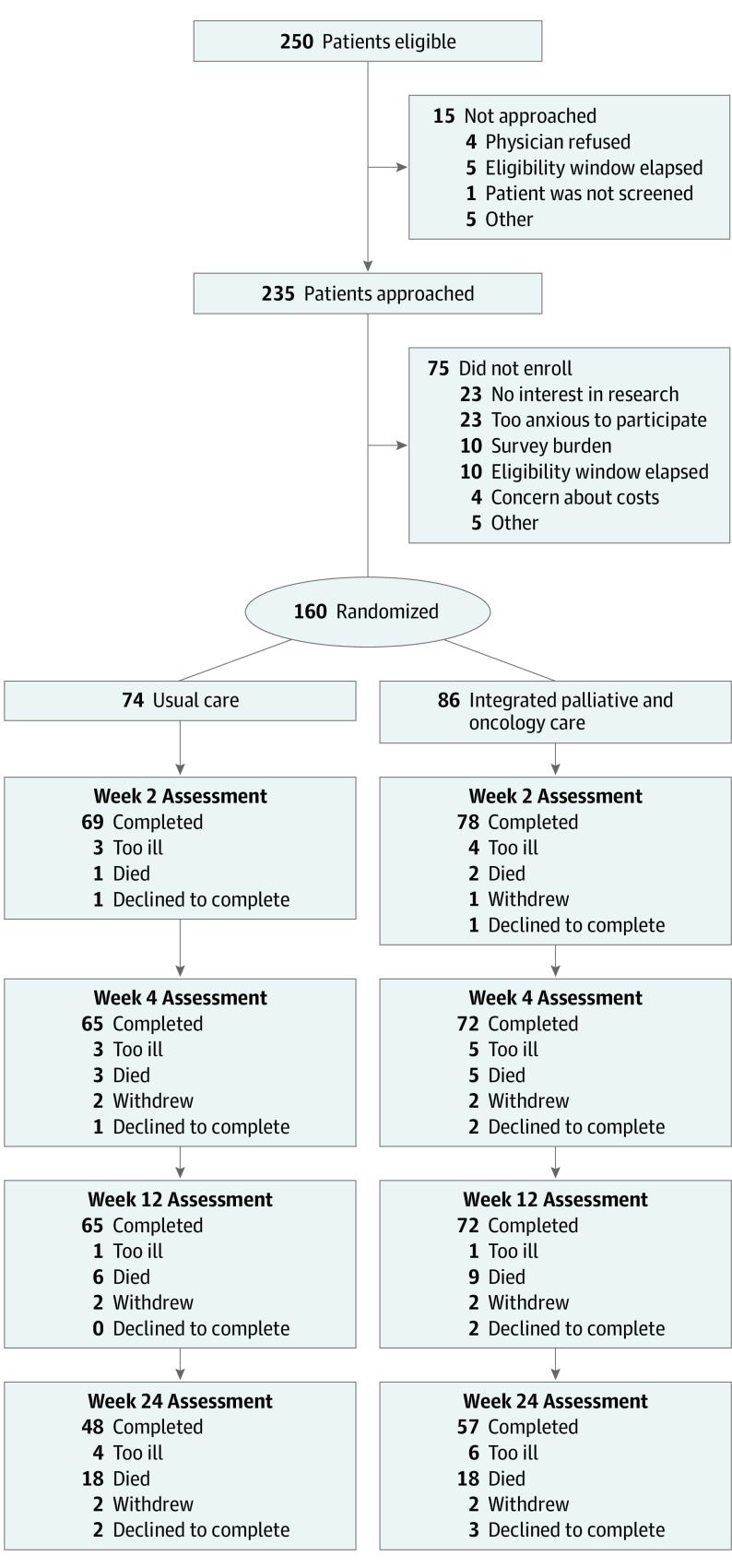
A total of 250 patients were screened for eligibility (Figure 1). We approached 235 eligible patients and enrolled 160 (68.1%) participants. Because of the use of stratification and 1:1 randomization, 86 patients were assigned to IPC, and 74 patients were assigned to UC. Of the 160 enrolled patients, 138 were White (86.2%), and the median (range) age was 64.4 (19.7-80.1) years (eTable 1 in Supplement 2). Overall, 109 (68.1%) patients had newly diagnosed AML. There were no meaningful differences in clinical characteristics between the 2 study groups. At weeks 2, 4, 12, and 24, data were missing for 13 (8.1%), 23 (14.4%), 23 (14.4%), and 55 (34.4%) patients, respectively.
Figure 1. Consort Diagram.
Palliative Care Visits
Patients assigned to IPC had a mean (range) of 2.2 (2-5) visits per week during their hospitalization for intensive chemotherapy. Only 6 patients assigned to UC received a palliative care consultation during their initial hospitalization, and 24 of 74 (32.4%) patients had a palliative care consultation during subsequent hospitalizations. During hospitalization for intensive chemotherapy, the palliative care clinicians most commonly reported establishing rapport (64.9%), addressing symptoms (64.7%), and coping (63.6%) with patients receiving the intervention (eFigure 1 in Supplement 2); eFigure 2 in Supplement 2 depicts topics covered by palliative care clinicians during subsequent hospitalizations.
Patient-Reported Outcomes at Week 2
Patients assigned to IPC, when compared with patients assigned to UC, reported better QOL (adjusted mean score, 116.45 vs 107.59; P = .04), lower depression (HADS depression subscale: adjusted mean score, 5.68 vs 7.20; P = .02; and PHQ-9: adjusted mean score, 6.34 vs 8.00; P = .04), anxiety (adjusted mean score, 4.53 vs 5.94; P = .02), and PTSD symptoms (adjusted mean score, 27.79 vs 31.69; P = .01) at week 2 (Table). There were no differences in symptom burden between the 2 groups. The findings remain statistically significant when using FDR to correct for multiple testing (eTable 2 in Supplement 2). At week 2, patients assigned to IPC, when compared with those receiving UC, also reported lower rates of clinically significant symptoms of depression (22 of 78 [28.2%] vs 31 of 69 [44.9%]; P = .04) and anxiety (17 of 78 [21.8%] vs 26 of 96 [37.7%]; P = .046).
Table. Effect of Integrated Palliative and Oncology Care on Patient-Reported Outcomes at Week 2.
| Measure (scale) | Sample size | Group assignment | Adjusted mean score (95% CI) | Standardized mean differencea | P value |
|---|---|---|---|---|---|
| Quality of life (FACT-Leukemia) | 139 | Usual care | 107.59 (101.45-113.74) | 0.30 | .04 |
| Integrated palliative and oncology care | 116.45 (110.69-122.21) | ||||
| Anxiety symptoms (HADS) | 147 | Usual care | 5.94 (5.10-6.79) | 0.31 | .02 |
| Integrated palliative and oncology care | 4.53 (3.74-5.33) | ||||
| Depression symptoms (HADS) | 147 | Usual care | 7.20 (6.26-8.14) | 0.34 | .02 |
| Integrated palliative and oncology care | 5.68 (4.80-6.56) | ||||
| Depression syndrome (PHQ-9) | 144 | Usual care | 8.00 (6.83-9.17) | 0.31 | .04 |
| Integrated palliative and oncology care | 6.34 (5.23-7.44) | ||||
| Symptom burden (ESAS) | 146 | Usual care | 32.82 (28.58-37.06) | 0.23 | .12 |
| Integrated palliative and oncology care | 28.24 (24.23-32.25) | ||||
| PTSD symptoms (PTSD Checklist–Civilian) | 146 | Usual care | 31.69 (29.56-33.82) | 0.30 | .01 |
| Integrated palliative and oncology care | 27.79 (25.78-29.80) |
Abbreviations: ESAS, Edmonton Symptom Assessment Scale; FACT–Leukemia, Functional Assessment of Cancer Therapy–Leukemia; HADS, Hospital Anxiety and Depression Scale; PHQ-9, 9-item Patient-Health Questionnaire; PTSD, posttraumatic stress disorder.
Standardized mean difference indicates the difference in mean outcome between groups and standard deviation of all participants.
Longitudinal Assessment of Patient-Reported Outcomes
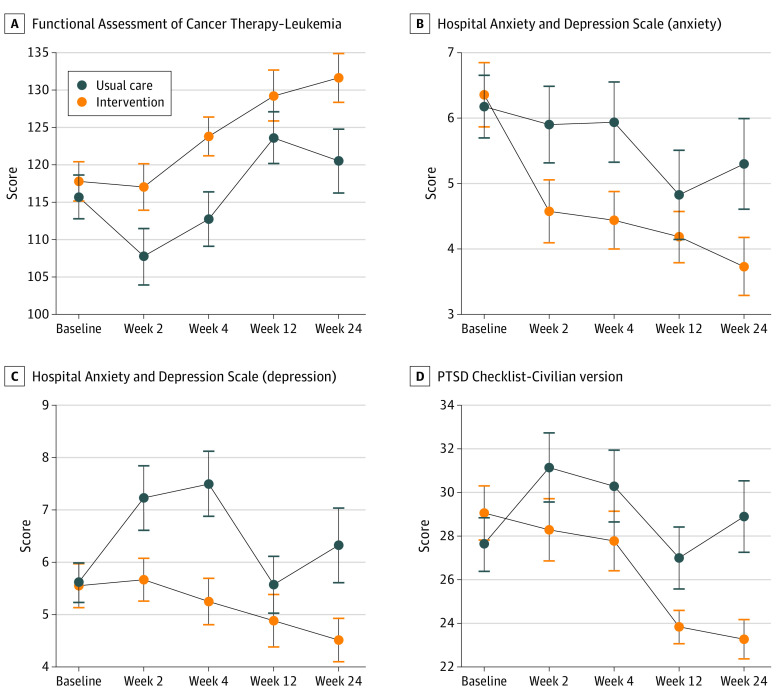
Using mixed linear effects models with maximum likelihood to impute missing data, when compared to patients receiving UC, patients assigned to IPC reported better QOL (β, 2.35; 95% CI, 0.02-4.68; P = .048), lower depression (HADS depression subscale: β, −0.42; 95% CI, −0.82 to −0.02; P = .04), anxiety (β, −0.38; 95% CI, −0.75 to −0.01; P = .04), and PTSD symptoms (β, −1.43; 95% CI, −2.34 to −0.54; P = .002) longitudinally (Figure 2). There were no differences in symptom burden or PHQ-9 scores longitudinally. The findings remained statistically significant when using FDR to correct for multiple testing (eTable 2 in Supplement 2).
Figure 2. Effect of Integrated Palliative and Oncology Care on Patient-Reported Quality of Life and Psychological Distress by Scale.
When compared with patients assigned to usual care, patients assigned to integrated palliative and oncology care reported better quality of life (β, 2.35; 95% CI, 0.02-4.68; P = .048) (A), lower anxiety (β, −0.38; 95% CI, −0.75 to −0.01; P = .04) (B), lower depression (β, −0.42; 95% CI, −0.82 to −0.02; P = .04) (C), and fewer posttraumatic stress disorder (PTSD) symptoms (β, −1.43; 95% CI, −2.34 to −0.54; P = .002) (D).
End-of-Life Outcomes
Among patients who died (n = 87; 44 of 73 in the UC group and 43 of 84 in the IPC group), those receiving IPC vs UC were more likely to report discussing their EOL care preferences with their clinicians (21 of 28 [75.0%] vs 12 of 30 [40.0%]; P = .01) and less likely to receive chemotherapy in the last 30 days of life (15 of 43 [34.9%] vs 27 of 41 [65.9%]; P = .01). Among those receiving IPC vs UC, there was no difference in hospice use (15 of 41 [34.9%] vs 15 of 42 [36.6%]; P = .999), hospice length of stay (β, −0.80; 95% CI, −1.85 to 0.25; P = .14), and hospitalization in the last week of life (35 of 42 [83.3%] vs 32 of 43 [74.4%]; P = .43). The findings remain statistically significant when using FDR to correct for multiple testing (eTable 2 in Supplement 2).
Discussion
Results of this multisite randomized clinical trial demonstrate that IPC improves QOL, depression and anxiety symptoms, and posttraumatic stress symptoms for patients with AML receiving intensive chemotherapy compared with usual care. The intervention led to clinically meaningful and sustained improvements in QOL and psychological distress for 6 months after initiating chemotherapy in this population at high risk for long-term QOL impairments and psychological morbidity. Although many oncologists question palliative care clinicians’ ability to meet the specialized needs of patients with AML, the present findings provide compelling evidence to the contrary.22,26,34,35,44,45,46 Although prior randomized trials of IPC care models have traditionally excluded patients with hematologic cancers,27,28,29,31 this study establishes the role of palliative care for improving the QOL and care in patients with AML. While we cannot generalize these findings to all patients with hematologic cancers, the salient benefits of early palliative care in patients hospitalized with AML are consistent to what we see in patients with hematologic cancers undergoing stem cell transplantation.32,33 In contrast with the prior randomized trial of integrated palliative care for patients undergoing stem cell transplant,32,33 this study did not demonstrate a statistically significant difference in symptom burden between the 2 groups. The extent of symptom burden for patients receiving induction chemotherapy in this trial was high and comparable with those seen in prior studies of this population and those undergoing myeloablative stem cell transplantation.11,47,48,49 Nonetheless, findings of this trial provide compelling evidence to support palliative care integration into routine clinical care for patients with high-risk hematologic cancers, especially those enduring prolonged hospitalizations.
Psychological distress during intensive hospitalizations for patients with hematologic cancers is associated with long-term sequalae, including psychiatric morbidity, medical complications, and even mortality.18,19,50,51,52,53,54 Involvement of palliative care clinicians in the care of patients with AML led to notable improvement in all psychological outcomes, including depression, anxiety, and posttraumatic stress symptoms. Strikingly, these improvements were sustained up to 6 months after initiating therapy in this population. Given the growing literature on traumatic stress and PTSD in patients with AML,6,13,55,56 these findings are especially encouraging. The mechanism by which palliative care reduces psychological distress remains unclear. In patients with solid tumors, palliative care has been shown to enhance adaptive coping strategies.57 Future work should examine whether patients’ coping skills mediate the effect of the palliative care intervention on psychological distress in patients with AML.
Patients receiving the palliative care intervention also experienced improvements in critical EOL outcomes. Specifically, patients receiving the intervention were more likely to discuss their EOL care preferences with their clinicians and less likely to use chemotherapy in the last month of life. More than half of the study cohort died during the study period, with the majority of patients receiving chemotherapy in the last 30 days of life and hospitalized in the last week of life, which highlights the poor prognosis and intensity of EOL care that has been described in this population.18,21,25,26,58,59,60,61 While there were no differences in hospitalizations near the EOL or hospice use between study groups, this likely reflects the fact that hospice services are not well equipped to meet the EOL needs of this population, including palliative transfusions.20,22,26,59,60,61,62 In fact, the American Society of Hematology has released a statement recommending that hospice agencies and payers work collaboratively to ensure the availability of palliative transfusions to optimize EOL care for patients with hematologic cancers.63 Future studies examining innovative EOL care delivery models are needed to minimize the need for hospitalizations in this population.
Limitations
This study has several limitations. First, it was performed only at tertiary care academic centers. While this is the predominant setting where intensive chemotherapy is given, these findings may not be generalizable to other care settings. Second, the sample lacked racial and ethnic diversity, and thus we are unable to assess the effect of these important factors on study outcomes. Third, study staff, patients, and clinicians could not be blinded to the intervention, which may have introduced bias. Although prior studies have suggested low risk of bias with self-reported assessments and objective EOL metrics obtained from patient EMRs,64,65,66 this is nonetheless an important study limitation. Fourth, the intervention did not include the full interdisciplinary palliative care team, which may have led to more profound benefits for this population. Fifth, similar to prior palliative care studies,27,28,29,30,31,32,33 we did not use an attention control group to adjust for the potential benefits of the time palliative care clinicians spent with patients. Finally, the involvement of palliative care in the care of patients randomized to the intervention on the same leukemia hospital floor may have altered clinicians and nursing behaviors in the control group, which may have diluted the findings. Additionally, a substantial proportion of patients in the control group received palliative care during their illness course, which may have also diluted the findings.
Conclusions
The American Society of Clinical Oncology recommends concurrent palliative care from the time of diagnosis for all patients with metastatic cancer and/or high symptom burden.67,68 However, the role of palliative care in the care of patients with AML has remained uncertain given the lack of evidence of benefit for this population. Results of this randomized clinical trial demonstrate that early IPC for hospitalized patients with AML receiving intensive chemotherapy can substantially enhance their QOL and reduce their depression, anxiety, and posttraumatic stress symptoms during hospitalization for intensive chemotherapy and up to 6 months after diagnosis. Patients receiving the IPC model were also more likely to experience improvements in their EOL care. Importantly, induction chemotherapy is offered mostly at large academic hospitals with access to inpatient palliative care services, which allows for potential implementation and dissemination of this care model for patients with AML. As these patients spend the majority of their time in the hospital and clinical settings,10 there are numerous opportunities to engage palliative care clinicians early and longitudinally in their care. Thus, early palliative care at the time of diagnosis for patients with AML should become standard of care to improve the QOL and care for this population.
Trial Protocol
eTable 1. Participants Characteristics
eTable 2. Secondary Outcomes Adjusted for Multiple Testing Using False Discovery Rate
eFigure 1. Palliative Care Visit Content
eFigure 2. Focus of PC Visits During Subsequent Hospitalizations
Data Sharing Statement
References
- 1.Alibhai SM, Leach M, Kermalli H, et al. The impact of acute myeloid leukemia and its treatment on quality of life and functional status in older adults. Crit Rev Oncol Hematol. 2007;64(1):19-30. doi: 10.1016/j.critrevonc.2007.07.003 [DOI] [PubMed] [Google Scholar]
- 2.Alibhai SM, Breunis H, Timilshina N, et al. Quality of life and physical function in adults treated with intensive chemotherapy for acute myeloid leukemia improve over time independent of age. J Geriatr Oncol. 2015;6(4):262-271. doi: 10.1016/j.jgo.2015.04.002 [DOI] [PubMed] [Google Scholar]
- 3.Burnett AK, Hills RK, Milligan DW, et al. Attempts to optimize induction and consolidation treatment in acute myeloid leukemia: results of the MRC AML12 trial. J Clin Oncol. 2010;28(4):586-595. doi: 10.1200/JCO.2009.22.9088 [DOI] [PubMed] [Google Scholar]
- 4.Walter RB, Estey EH. Selection of initial therapy for newly-diagnosed adult acute myeloid leukemia: limitations of predictive models. Blood Rev. 2020;100679. doi: 10.1016/j.blre.2020.100679 [DOI] [PubMed] [Google Scholar]
- 5.Vey N Low-intensity regimens versus standard-intensity induction strategies in acute myeloid leukemia. Ther Adv Hematol. 2020;11:2040620720913010. doi: 10.1177/2040620720913010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodin G, Yuen D, Mischitelle A, et al. Traumatic stress in acute leukemia. Psychooncology. 2013;22(2):299-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zimmermann C, Yuen D, Mischitelle A, et al. Symptom burden and supportive care in patients with acute leukemia. Leuk Res. 2013;37(7):731-736. doi: 10.1016/j.leukres.2013.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zittoun R, Achard S, Ruszniewski M. Assessment of quality of life during intensive chemotherapy or bone marrow transplantation. Psychooncology. 1999;8(1):64-73. doi: [DOI] [PubMed] [Google Scholar]
- 9.El-Jawahri AR, Abel GA, Steensma DP, et al. Health care utilization and end-of-life care for older patients with acute myeloid leukemia. Cancer. 2015;121(16):2840-2848. doi: 10.1002/cncr.29430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Jawahri A, Abel GA, Traeger L, et al. Quality of life and mood of older patients with acute myeloid leukemia (AML) receiving intensive and non-intensive chemotherapy. Leukemia. 2019;33(10):2393-2402. doi: 10.1038/s41375-019-0449-1 [DOI] [PubMed] [Google Scholar]
- 11.Loh KP, Abdallah M, Kumar AJ, Neuendorff NR, Dahiya S, Klepin HD. Health-related quality of life and treatment of older adults with acute myeloid leukemia: a Young International Society of Geriatric Oncology review paper. Curr Hematol Malig Rep. 2019;14(6):523-535. doi: 10.1007/s11899-019-00552-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiese M, Daver N. Unmet clinical needs and economic burden of disease in the treatment landscape of acute myeloid leukemia. Am J Manag Care. 2018;24(16)(suppl):S347-S355. [PubMed] [Google Scholar]
- 13.Nissim R, Zimmermann C, Minden M, et al. Abducted by the illness: a qualitative study of traumatic stress in individuals with acute leukemia. Leuk Res. 2013;37(5):496-502. doi: 10.1016/j.leukres.2012.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papadopoulou C, Johnston B, Themessl-Huber M. The experience of acute leukaemia in adult patients: a qualitative thematic synthesis. Eur J Oncol Nurs. 2013;17(5):640-648. doi: 10.1016/j.ejon.2013.06.009 [DOI] [PubMed] [Google Scholar]
- 15.Farsi Z, Nayeri ND, Negarandeh R. The coping process in adults with acute leukemia undergoing hematopoietic stem cell transplantation. J Nurs Res. 2012;20(2):99-109. doi: 10.1097/jnr.0b013e318257b5e0 [DOI] [PubMed] [Google Scholar]
- 16.Nissim R, Rodin G, Schimmer A, et al. Finding new bearings: a qualitative study on the transition from inpatient to ambulatory care of patients with acute myeloid leukemia. Support Care Cancer. 2014;22(9):2435-2443. doi: 10.1007/s00520-014-2230-3 [DOI] [PubMed] [Google Scholar]
- 17.Gheihman G, Zimmermann C, Deckert A, et al. Depression and hopelessness in patients with acute leukemia: the psychological impact of an acute and life-threatening disorder. Psychooncology. 2016;25(8):979-989. doi: 10.1002/pon.3940 [DOI] [PubMed] [Google Scholar]
- 18.Ghodraty-Jabloo V, Alibhai SMH, Breunis H, Puts MTE. Keep your mind off negative things: coping with long-term effects of acute myeloid leukemia (AML). Support Care Cancer. 2016;24(5):2035-2045. doi: 10.1007/s00520-015-3002-4 [DOI] [PubMed] [Google Scholar]
- 19.Ghodraty-Jabloo V, Alibhai SM, Breunis H, Puts MT. One day at a time: improving the patient experience during and after intensive chemotherapy for younger and older AML patients. Leuk Res. 2015;39(2):192-197. doi: 10.1016/j.leukres.2014.11.028 [DOI] [PubMed] [Google Scholar]
- 20.Hui D, Didwaniya N, Vidal M, et al. Quality of end-of-life care in patients with hematologic malignancies: a retrospective cohort study. Cancer. 2014;120(10):1572-1578. doi: 10.1002/cncr.28614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Jawahri AR, Abel GA, Steensma DP, et al. Health care utilization and end-of-life care for older patients with acute myeloid leukemia. Cancer. 2015;121(16):2840-2848. doi: 10.1002/cncr.29430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Jawahri A, Nelson AM, Gray TF, Lee SJ, LeBlanc TW. Palliative and end-of-life care for patients with hematologic malignancies. J Clin Oncol. 2020;38(9):944-953. doi: 10.1200/JCO.18.02386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bosshard R, O’Reilly K, Ralston S, Chadda S, Cork D. Systematic reviews of economic burden and health-related quality of life in patients with acute myeloid leukemia. Cancer Treat Rev. 2018;69:224-232. doi: 10.1016/j.ctrv.2018.07.005 [DOI] [PubMed] [Google Scholar]
- 24.Klepin HD, Rao AV, Pardee TS. Acute myeloid leukemia and myelodysplastic syndromes in older adults. J Clin Oncol. 2014;32(24):2541-2552. doi: 10.1200/JCO.2014.55.1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LeBlanc TW, Erba HP. Shifting paradigms in the treatment of older adults with AML. Semin Hematol. 2019;56(2):110-117. doi: 10.1053/j.seminhematol.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 26.LeBlanc TW, Egan PC, Olszewski AJ. Transfusion dependence, use of hospice services, and quality of end-of-life care in leukemia. Blood. 2018;132(7):717-726. doi: 10.1182/blood-2018-03-842575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742. doi: 10.1056/NEJMoa1000678 [DOI] [PubMed] [Google Scholar]
- 28.Temel JS, Greer JA, El-Jawahri A, et al. Effects of early integrated palliative care in patients with lung and GI cancer: a randomized clinical trial. J Clin Oncol. 2017;35(8):834-841. doi: 10.1200/JCO.2016.70.5046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741-749. doi: 10.1001/jama.2009.1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakitas MA, Tosteson TD, Li Z, et al. Early versus delayed initiation of concurrent palliative oncology care: patient outcomes in the ENABLE III randomized controlled trial. J Clin Oncol. 2015;33(13):1438-1445. doi: 10.1200/JCO.2014.58.6362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383(9930):1721-1730. doi: 10.1016/S0140-6736(13)62416-2 [DOI] [PubMed] [Google Scholar]
- 32.El-Jawahri A, LeBlanc T, VanDusen H, et al. Effect of inpatient palliative care on quality of life 2 weeks after hematopoietic stem cell transplantation: a randomized clinical trial. JAMA. 2016;316(20):2094-2103. doi: 10.1001/jama.2016.16786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Jawahri A, Traeger L, Greer JA, et al. Effect of inpatient palliative care during hematopoietic stem-cell transplant on psychological distress 6 months after transplant: results of a randomized clinical trial. J Clin Oncol. 2017;35(32):3714-3721. doi: 10.1200/JCO.2017.73.2800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LeBlanc TW Advance care planning and palliative care specialists in malignant hematology and stem-cell transplantation: on why it takes a village. J Oncol Pract. 2018;14(1):3-5. doi: 10.1200/JOP.2017.026930 [DOI] [PubMed] [Google Scholar]
- 35.LeBlanc TW, El-Jawahri A. When and why should patients with hematologic malignancies see a palliative care specialist? Hematology Am Soc Hematol Educ Program. 2015;2015(1):471-478. doi: 10.1182/asheducation-2015.1.471 [DOI] [PubMed] [Google Scholar]
- 36.McQuellon RP, Russell GB, Cella DF, et al. Quality of life measurement in bone marrow transplantation: development of the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) scale. Bone Marrow Transplant. 1997;19(4):357-368. doi: 10.1038/sj.bmt.1700672 [DOI] [PubMed] [Google Scholar]
- 37.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361-370. doi: 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 38.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watanabe SM, Nekolaichuk CL, Beaumont C. The Edmonton Symptom Assessment System, a proposed tool for distress screening in cancer patients: development and refinement. Psychooncology. 2012;21(9):977-985. doi: 10.1002/pon.1996 [DOI] [PubMed] [Google Scholar]
- 40.Smith MY, Redd W, DuHamel K, Vickberg SJ, Ricketts P. Validation of the PTSD Checklist-Civilian Version in survivors of bone marrow transplantation. J Trauma Stress. 1999;12(3):485-499. doi: 10.1023/A:1024719104351 [DOI] [PubMed] [Google Scholar]
- 41.Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673. doi: 10.1001/jama.300.14.1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sekeres MA, Stone RM, Zahrieh D, et al. Decision-making and quality of life in older adults with acute myeloid leukemia or advanced myelodysplastic syndrome. Leukemia. 2004;18(4):809-816. doi: 10.1038/sj.leu.2403289 [DOI] [PubMed] [Google Scholar]
- 43.Glickman ME, Rao SR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol. 2014;67(8):850-857. doi: 10.1016/j.jclinepi.2014.03.012 [DOI] [PubMed] [Google Scholar]
- 44.LeBlanc TW, El-Jawahri A. Hemato-oncology and palliative care teams: is it time for an integrated approach to patient care? Curr Opin Support Palliat Care. 2018;12(4):530-537. doi: 10.1097/SPC.0000000000000385 [DOI] [PubMed] [Google Scholar]
- 45.LeBlanc TW, O’Donnell JD, Crowley-Matoka M, et al. Perceptions of palliative care among hematologic malignancy specialists: a mixed-methods study. J Oncol Pract. 2015;11(2):e230-e238. doi: 10.1200/JOP.2014.001859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.LeBlanc TW, Roeland EJ, El-Jawahri A. Early palliative care for patients with hematologic malignancies: is it really so difficult to achieve? Curr Hematol Malig Rep. 2017;12(4):300-308. doi: 10.1007/s11899-017-0392-z [DOI] [PubMed] [Google Scholar]
- 47.Leak Bryant A, Lee Walton A, Shaw-Kokot J, Mayer DK, Reeve BB. Patient-reported symptoms and quality of life in adults with acute leukemia: a systematic review. Oncol Nurs Forum. 2015;42(2):E91-E101. doi: 10.1188/15.ONF.E91-E101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bryant AL, Drier SW, Lee S, Bennett AV. A systematic review of patient reported outcomes in phase II or III clinical trials of myelodysplastic syndromes and acute myeloid leukemia. Leuk Res. 2018;70:106-116. doi: 10.1016/j.leukres.2018.06.006 [DOI] [PubMed] [Google Scholar]
- 49.El-Jawahri AR, Traeger LN, Kuzmuk K, et al. Quality of life and mood of patients and family caregivers during hospitalization for hematopoietic stem cell transplantation. Cancer. 2015;121(6):951-959. doi: 10.1002/cncr.29149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.El-Jawahri A,Chen YB, Brazauskas R, et al. Impact of pre-transplant depression on outcomes of allogeneic and autologous hematopoietic stem cell transplantation. Cancer. 2017;123(10):1828-1838. doi: 10.1002/cncr.30546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Antoni MH, Dhabhar FS. The impact of psychosocial stress and stress management on immune responses in patients with cancer. Cancer. 2019;125(9):1417-1431. doi: 10.1002/cncr.31943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones WC, Parry C, Devine S, Main DS, Okuyama S, Tran ZV. Prevalence and predictors of distress in posttreatment adult leukemia and lymphoma survivors. J Psychosoc Oncol. 2015;33(2):124-141. doi: 10.1080/07347332.2014.992085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gray TF, Temel JS, El-Jawahri A. Illness and prognostic understanding in patients with hematologic malignancies. Blood Rev. 2020;100692. doi: 10.1016/j.blre.2020.100692 [DOI] [PubMed] [Google Scholar]
- 54.Allart P, Soubeyran P, Cousson-Gélie F. Are psychosocial factors associated with quality of life in patients with haematological cancer? a critical review of the literature. Psychooncology. 2013;22(2):241-249. doi: 10.1002/pon.3026 [DOI] [PubMed] [Google Scholar]
- 55.Rodin G, Deckert A, Tong E, et al. Traumatic stress in patients with acute leukemia: a prospective cohort study. Psychooncology. 2018;27(2):515-523. doi: 10.1002/pon.4488 [DOI] [PubMed] [Google Scholar]
- 56.Abbey G, Thompson SB, Hickish T, Heathcote D. A meta-analysis of prevalence rates and moderating factors for cancer-related post-traumatic stress disorder. Psychooncology. 2015;24(4):371-381. doi: 10.1002/pon.3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greer JA, Jacobs JM, El-Jawahri A, et al. Role of patient coping strategies in understanding the effects of early palliative care on quality of life and mood. J Clin Oncol. 2018;36(1):53-60. doi: 10.1200/JCO.2017.73.7221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abuelgasim KA, Albuhayri B, Munshi R, et al. Impact of age and induction therapy on outcome of 180 adult patients with acute myeloid leukemia; retrospective analysis and literature review. Leuk Res Rep. 2020;14:100206. doi: 10.1016/j.lrr.2020.100206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Odejide OO, Cronin AM, Condron NB, et al. Barriers to quality end-of-life care for patients with blood cancers. J Clin Oncol. 2016;34(26):3126-3132. doi: 10.1200/JCO.2016.67.8177 [DOI] [PubMed] [Google Scholar]
- 60.Odejide OO, Steensma DP. Patients with haematological malignancies should not have to choose between transfusions and hospice care. Lancet Haematol. 2020;7(5):e418-e424. doi: 10.1016/S2352-3026(20)30042-9 [DOI] [PubMed] [Google Scholar]
- 61.Odejide OO, Cronin AM, Earle CC, Tulsky JA, Abel GA. Why are patients with blood cancers more likely to die without hospice? Cancer. 2017;123(17):3377-3384. doi: 10.1002/cncr.30735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Odejide OO, Salas Coronado DY, Watts CD, Wright AA, Abel GA. End-of-life care for blood cancers: a series of focus groups with hematologic oncologists. J Oncol Pract. 2014;10(6):e396-e403. doi: 10.1200/JOP.2014.001537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.ASH statement in support of palliative blood transfusions in hospice setting. American Society of Hematology. June 25, 2019. Accessed November 3, 2020. https://www.hematology.org/advocacy/policy-statements/2019/palliative-blood-transfusions-in-hospice
- 64.Atkinson TM, Wagner JS, Basch E. Trustworthiness of patient-reported outcomes in unblinded cancer clinical trials. JAMA Oncol. 2017;3(6):738-739. doi: 10.1001/jamaoncol.2016.3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hagger MS, Orbell S. A meta-analytic review of the common-sense model of illness representations. Psychol Health. 2003;18(2):141-184. doi: 10.1080/088704403100081321 [DOI] [Google Scholar]
- 66.Kavalieratos D, Corbelli J, Zhang D, et al. Association between palliative care and patient and caregiver outcomes: a systematic review and meta-analysis. JAMA. 2016;316(20):2104-2114. doi: 10.1001/jama.2016.16840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith TJ, Temin S, Alesi ER, et al. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol. 2012;30(8):880-887. doi: 10.1200/JCO.2011.38.5161 [DOI] [PubMed] [Google Scholar]
- 68.Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112. doi: 10.1200/JCO.2016.70.1474 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Participants Characteristics
eTable 2. Secondary Outcomes Adjusted for Multiple Testing Using False Discovery Rate
eFigure 1. Palliative Care Visit Content
eFigure 2. Focus of PC Visits During Subsequent Hospitalizations
Data Sharing Statement