Key Points
Question
What is the 10-year cumulative incidence of glaucoma and glaucoma suspect after removal of unilateral cataract in infancy in the Infant Aphakic Treatment Study, and how is the retinal nerve fiber layer and optic nerve head appearance affected?
Findings
In this secondary analysis of a randomized clinical trial, risk of glaucoma and glaucoma plus glaucoma suspect diagnosis at 10 years rose to 22% and 40%, respectively, with no difference between treatment groups (primary intraocular lens vs aphakia). Eyes with glaucoma (vs glaucoma suspects or neither) had relatively preserved retinal nerve fiber layer, similar optic nerve head appearance, and visual acuity.
Meaning
The results of this study support the need for lifelong surveillance for glaucoma and glaucoma-related events following cataract removal in infancy.
Abstract
Importance
Glaucoma-related adverse events constitute serious complications of cataract removal in infancy, yet long-term data on incidence and visual outcome remain lacking.
Objective
To identify and characterize incident cases of glaucoma and glaucoma-related adverse events (glaucoma + glaucoma suspect) among children in the Infant Aphakia Treatment Study (IATS) by the age of 10.5 years and to determine whether these diagnoses are associated with optic nerve head (ONH) and peripapillary retinal nerve fiber layer (RNFL) assessment.
Design, Setting, and Participants
Analysis of a multicenter randomized clinical trial of 114 infants with unilateral congenital cataract who were aged 1 to 6 months at surgery. Data on long-term glaucoma-related status and outcomes were collected when children were 10.5 years old (July 14, 2015, to July 12, 2019) and analyzed from March 30, 2019, to August 6, 2019.
Interventions
Participants were randomized at cataract surgery to either primary intraocular lens (IOL), or aphakia (contact lens [CL]). Standardized definitions of glaucoma and glaucoma suspect were created for IATS and applied for surveillance and diagnosis.
Main Outcomes and Measures
Development of glaucoma and glaucoma + glaucoma suspect in operated-on eyes up to age 10.5 years, plus intraocular pressure, axial length, RNFL (by optical coherence tomography), and ONH photographs.
Results
In Kaplan-Meier analysis, for all study eyes combined (n = 114), risk of glaucoma after cataract removal rose from 9% (95% CI, 5%-16%) at 1 year, to 17% (95% CI, 11%-25%) at 5 years, to 22% (95% CI, 16%-31%) at 10 years. The risk of glaucoma plus glaucoma suspect diagnosis after cataract removal rose from 12% (95% CI, 7%-20%) at 1 year, to 31% (95% CI, 24%-41%) at 5 years, to 40% (95% CI, 32%-50%) at 10 years. Risk of glaucoma and glaucoma plus glaucoma suspect diagnosis at 10 years was not significantly different between treatment groups. Eyes with glaucoma (compared with eyes with glaucoma suspect or neither) had longer axial length but relatively preserved RNFL and similar ONH appearance and visual acuity at age 10 years.
Conclusions and Relevance
Risk of glaucoma-related adverse events continues to increase with longer follow-up of children following unilateral cataract removal in infancy and is not associated with primary IOL implantation. Development of glaucoma (or glaucoma suspect) after removal of unilateral congenital cataract was not associated with worse visual acuity outcomes at 10 years.
Trial Registration
ClinicalTrials.gov Identifier: NCT00212134
This secondary analysis of a randomized clinical trial identifies and characterizes incident cases of glaucoma and glaucoma-related adverse events among children by the age of 10.5 years and associates these diagnoses with optic nerve head and peripapillary retinal nerve fiber layer assessment.
Introduction
Glaucoma is a well-documented and serious complication after childhood cataract removal, with reported frequency and risk factors for glaucoma and glaucoma suspect varying by study population, definition, and follow-up time, in a variety of retrospective1,2,3,4,5,6,7,8,9,10,11,12 and several prospective studies.13,14,15,16,17,18 The Infant Aphakia Treatment Study (IATS), a multicenter, randomized, controlled clinical trial sponsored by the National Eye Institute, compared outcomes of surgery for unilateral cataract with or without primary intraocular lens (IOL) implantation in infants between ages 1 and 6 months.17,18,19,20,21,22,23
The effect of primary IOL implantation at cataract surgery in very young children has been difficult to evaluate, owing largely to the paucity of randomized trials. Five-year results of IATS18 found similar rates of glaucoma-related adverse events with and without primary IOL implantation, as did a randomized trial of primary IOL vs aphakia for bilateral cataracts operated on in children younger than 2 years.14 To our knowledge, longer-term rates and outcomes of glaucoma-related adverse events following cataract surgery in infants randomized to primary IOL or aphakia are lacking.
Optic nerve head (ONH) photographs are an established means of evaluating for glaucomatous damage.24 Spectral-domain optical coherence tomography (SD-OCT) has proven invaluable for evaluating and treating adults with glaucoma and can even predict among those with glaucoma suspect those eyes at higher risk for developing visual field loss.25,26 In children, peripapillary retinal nerve fiber layer thickness (RNFL) assessed by SD-OCT27 has been shown to be effective in differentiating between normal and glaucomatous eyes.28,29
In this study, we report 10-year results of the development of glaucoma-related adverse events in IATS participants, considering original treatment group (primary IOL vs contact lens [CL]) and previously identified risk factors, as well as ONH health (RNFL by SD-OCT and masked reading of ONH photographs).
Methods
The IATS design, eligibility criteria, surgical technique, follow-up schedule, patching and optical correction regimens, evaluation methods, and patient characteristics at baseline have been previously reported.19 This study was approved by each participating site’s institutional review board, and written informed consent was obtained for each participating child. The definitions for glaucoma, glaucoma suspect, and glaucoma-related adverse events (glaucoma + glaucoma suspect) were established and applied, as previously reported (eTable 1 in the Supplement),17,18 and management was at treating physician’s discretion. The originally designed IATS study concluded 5 years following cataract removal. No IATS visits occurred between ages 5 years and 10 years, other than standard of care determined by the treating physicians.
Funding and approval were obtained to recall children enrolled into IATS for a detailed follow-up examination at a mean (SD) age of 10.5 years (3 months) (hereafter referred to as the 10-year examination, meaning approximately 10 years after cataract removal). Spectral-domain OCT RNFL imaging and ONH photography were performed; all ONH photographs were graded in a standardized fashion by 3 expert examiners (eAppendix; eFigures 5 and 6 in the Supplement). Information on statistical analysis can also be found in the eAppendix in the Supplement. A significance level of .05 was used in statistical inference-making, except where otherwise stated, and hypothesis tests were 2-sided.
Results
Development of Glaucoma
The IATS randomized 114 infants (57 each to CL and IOL): 110 (96.5%) completed clinical examination at age 10.5 years (mean [SD] age 10.6 [0.3] years; n = 55 for each group), with mean postsurgical follow-up of 10.4 years (range, 9.3-11.5); 2 participants in each group were lost to follow-up. Details of the 10.5-year testing times, CONSORT diagram for recruitment, and visual acuity results19 have been published. Glaucoma status at the 10-year examination was available for 106 participants (55 IOL and 51 CL).
By the 10-year examination, 25 eyes (23%) had developed glaucoma, and 21 eyes (20%) had glaucoma suspect (46 eyes total [43%] glaucoma + glaucoma suspect). The CL group had 13 eyes (25%) with glaucoma and 13 (25%) were glaucoma suspects. The IOL group had 12 eyes (22%) with glaucoma, and 8 (15%) were glaucoma suspect (Figure 1).
Figure 1. Number of IATS Participants Diagnosed as Having Glaucoma, Glaucoma Suspect, or Normal at 1, 5, and 10 Years After Surgery in the Intraocular Lens (IOL) and Contact Lens (CL) Groups.
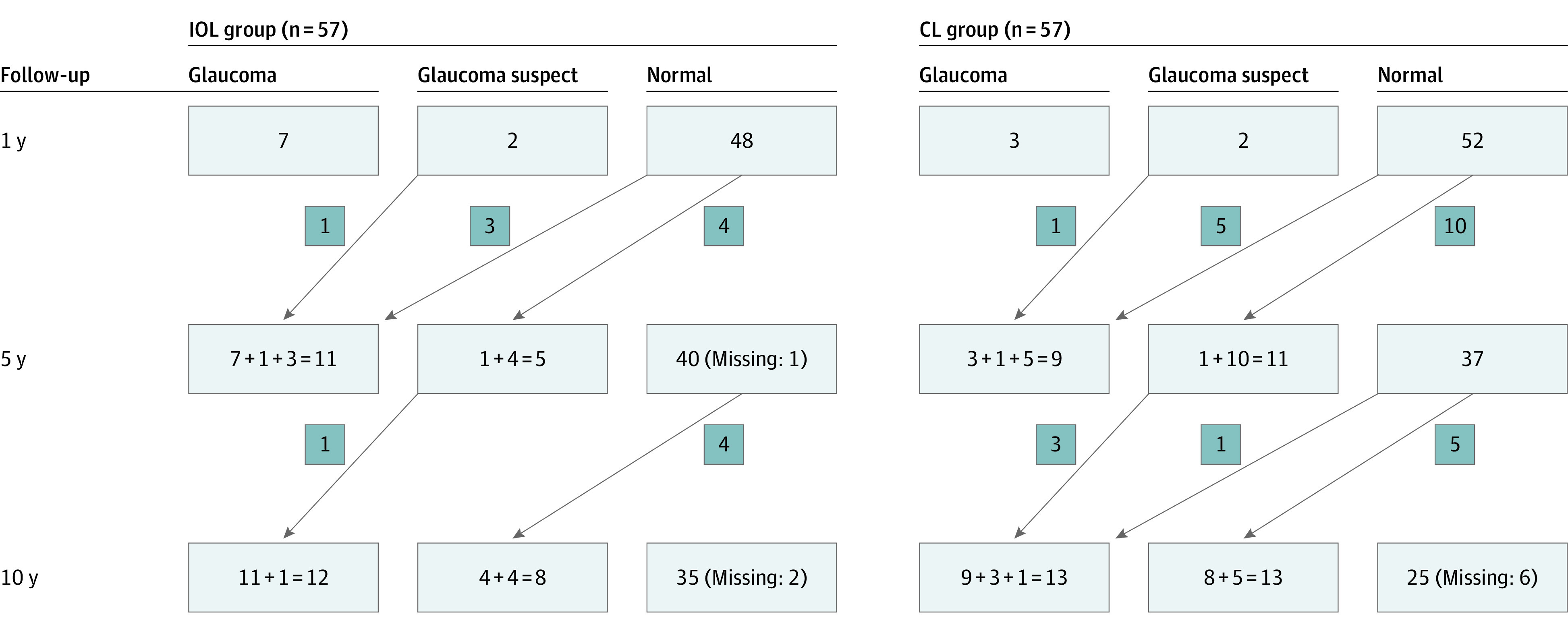
Arrows indicate the number of participants with changes in status from 1 point to the next. Where there is more than 1 number in a box, the leftmost number is the number of participants whose status did not change from the previous point, and number that is added represents changes in status from the previous point to the current point.
Of the 5 new glaucoma cases that were detected between the 5-year and the 10-year examinations, 4 were previously glaucoma suspect, and 1 was normal. There were 9 new glaucoma suspect cases at the 10-year follow-up (Figure 1).
In Kaplan-Meier analysis, for all study eyes combined, risk of glaucoma after cataract removal rose from 9% (95% CI, 5%-16%) at 1 year to 17% (95% CI, 11%-25%) at 5 years to 22% (95% CI, 16%-31%) at 10 years (Figure 2A). The risk of glaucoma + glaucoma suspect diagnosis after cataract removal rose from 12% (95% CI, 7%-20%) at 1 year to 31% (95% CI, 24%-41%) at 5 years to 40% (95% CI, 32%-50%) at 10 years (Figure 2B).
Figure 2. Kaplan-Meier Curves Showing Cumulative Probability of Glaucoma (A) and Glaucoma + Glaucoma Suspect (B) vs Time (in Years) Since Cataract Surgery for 114 Study Participants.
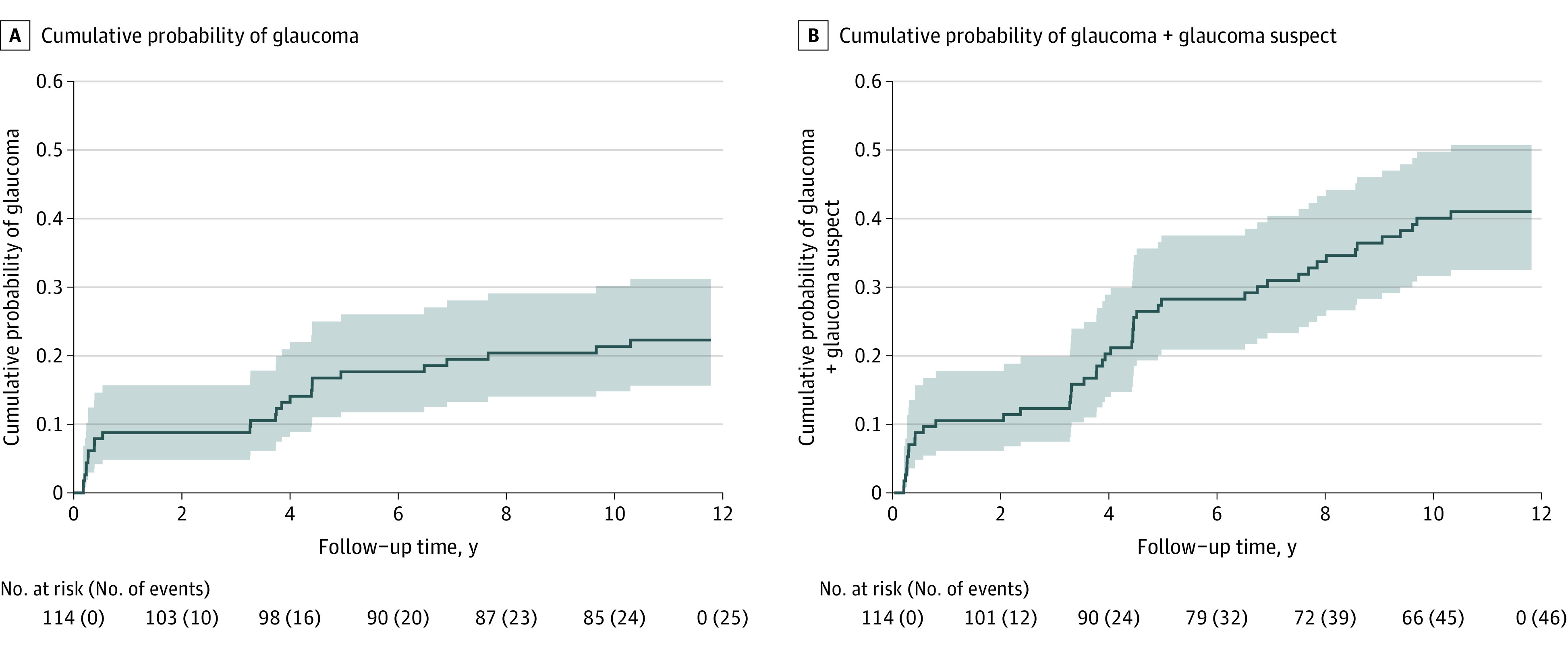
The shading denotes 95% confidence intervals for the cumulative probability. The numbers of participants at risk and the cumulative numbers of events are shown below the x-axis. The 10-year cumulative probability was 22% (95% CI, 16%-31%) for glaucoma and 40% (95% CI, 32%-50%) for glaucoma + glaucoma suspect.
Kaplan-Meier curves for developing glaucoma after cataract removal were not significantly different between the CL and IOL treatment groups (Figure 3A) (hazard ratio [HR], 1.0; 95% CI, 0.5-2.3; P = .94), with the 10-year risk in the CL and IOL groups at 23% (95% CI, 14%-37%) and 21% (95% CI, 13%-35%), respectively. Kaplan-Meier curves for developing glaucoma + glaucoma suspect diagnosis after cataract removal were also not significantly different between the treatment groups (Figure 3B) (HR, 1.3; 95% CI, 0.7-2.4; P= .36), with the 10-year risk in the CL and IOL groups at 46% (95% CI, 34%-60%) and 36% (95% CI, 25%-50%), respectively.
Figure 3. Kaplan-Meier Curves Showing the Cumulative Probability of Glaucoma (A) and Glaucoma + Glaucoma Suspect (B) vs Time (in Years) Since Cataract Surgery for the Contact Lens (CL) and Intraocular Lens (IOL) Groups (57 Participants in Each Group).
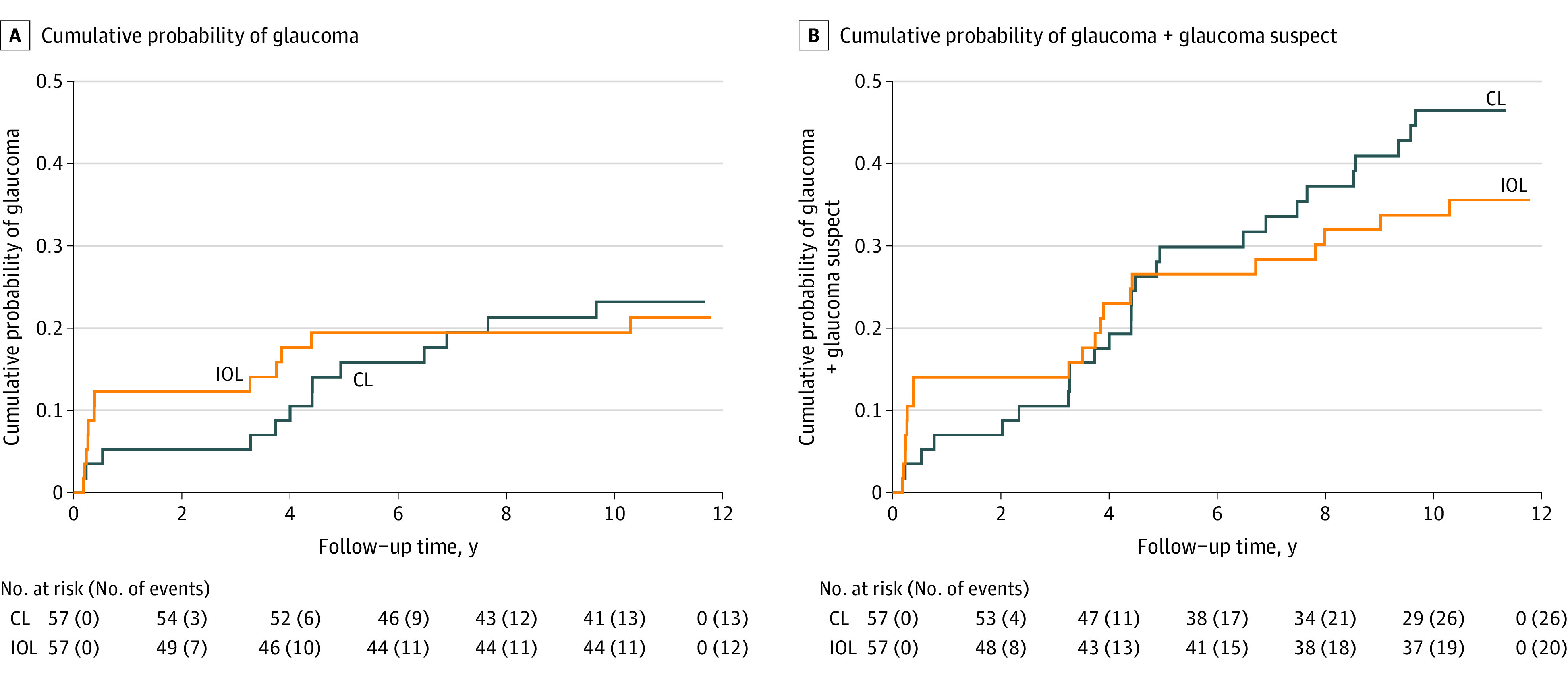
The numbers of participants at risk and the cumulative numbers of events are shown below the x-axis. At 10.5 years, the cumulative probability of glaucoma was 23% (95% CI, 14%-37%) for the CL group and 21% (95% CI, 13%-35%) for the IOL group. The 10-year cumulative probability of glaucoma + glaucoma suspect was 46% (95% CI, 34%-60%) for the CL group and 36% (95% CI, 25%-50%) for the IOL group.
Influence of Baseline Characteristics of Enrolled Children/Study Eyes
The associations between glaucoma at the 10-year examination and treatment group (CL vs IOL), age at surgery (age ≤48 days vs >48 days), persistent fetal vasculature, corneal diameter (≤10 mm vs >10 mm), and intraocular pressure (IOP) (≥12 mm Hg vs <12 mm Hg) at baseline were examined using a multivariable proportional hazards regression model. After controlling for age, the other terms were not significant. The risk of glaucoma was greater for younger age at surgery (adjusted HR, 3.2; 95% CI, 1.2-8.2) (eTable 2 in the Supplement). The risk of glaucoma + glaucoma suspect was greater for smaller corneal diameter at surgery (adjusted HR, 2.0; 95% CI, 1.1-3.8) (eTable 2 in the Supplement).
Glaucoma Characteristics, Treatment, and Outcomes
Diagnostic Criteria for Diagnosis
The glaucoma and glaucoma suspect diagnoses at 10 years were PI determined via application of rigorous definitions. Among the 5 eyes newly diagnosed as having glaucoma between the 5-year and the 10-year examinations, 3 showed IOP greater than 21 mm Hg with optic nerve head changes, and 2 had glaucoma surgery. Among the 9 eyes diagnosed as glaucoma suspect between the 5-year and 10-year examinations, all reported IOP greater than 21 mm Hg and 2 were receiving glaucoma medications.
Glaucoma Treatment
At the 10-year examination, 12 of 25 (48%) eyes with glaucoma were in patients reportedly taking glaucoma medications, as were 9 eyes with glaucoma suspect diagnosis (of 18 with available information). Between the 5-year and 10-year examinations, 4 eyes had glaucoma surgery. Of 25 eyes with glaucoma by the 10-year examination, 11 eyes underwent glaucoma surgery, cumulatively; 7 (64%) required a single surgery. Glaucoma surgical procedures performed (eTable 3 in the Supplement) included trabeculotomy, glaucoma drainage implant, endocyclophotocoagulation, trabeculectomy, and peripheral iridectomy. A single eye with presumed angle-closure glaucoma (after surgical iridectomy) developed retinal detachment and ultimately phthisis bulbi prior to the 5-year examination.18
Glaucoma and Ocular Parameters at the 10-Year Follow-up
There were no significant differences in mean (SD) IOP of eyes diagnosed as having glaucoma (n = 25; 19.0 [5.9] mm Hg), glaucoma suspect (n = 20; 19.5 [4.2] mm Hg), and with neither diagnosis (n = 60; 16.9 [3.7] mm Hg) (eFigure 1 in the Supplement). Mean (SD) axial length for eyes with glaucoma (n = 22), glaucoma suspect (n = 19), and neither diagnosis (n = 60) was 24.8 (2.4), 23.3 (1.9), and 22.8 (2.2) mm, respectively. Eyes with glaucoma were significantly longer than those with neither diagnosis (95% Tukey-Kramer–adjusted CI, 0.7-3.3 mm); mean axial lengths were similar for glaucoma vs glaucoma suspect and glaucoma suspect vs neither diagnosis (eFigure 2 in the Supplement).
Glaucoma Status and Secondary IOL
There was no association between secondary IOL placement and glaucoma status (eAppendix in the Supplement).
Glaucoma Status and Visual Acuity and Refraction
Median visual acuity at the 10-year examination in eyes with glaucoma was 0.9 logMAR (Snellen equivalent 20/160; n = 25, interquartile range [IQR], 0.1-2.9); in eyes with glaucoma suspect was 0.9 (n = 19; IQR, 0.2-1.7); and in normal eyes was also 0.9 (n = 60; IQR, 0-2.7). For the entire treated cohort at 10 years, median visual acuity was 0.55 logMAR (Snellen equivalent 20/70; n = 50; IQR, 0.2-1.5) when age at surgery was 28 to 48 days vs 1.1 logMAR (Snellen equivalent 20/250; n = 58; IQR, 0.7-1.4) for older age at surgery (P = .03). Mean refraction was similar between eyes with glaucoma, glaucoma suspect, or neither (eAppendix and eFigure 3 in the Supplement).
Optic Nerve Head Imaging
Optical coherence tomography peripapillary RNFL imaging was attempted for every patient at the 10-year examination, obtained in 97 of 110 (88.2%), and adequate for reading in 75 treated and 85 fellow eyes. Among treated eyes with readable images, there was no significant difference (P = .08 in overall mean RNFL for eyes with glaucoma [n = 15], glaucoma suspect [n = 13], or neither [n = 44]: mean [SD] 93.3 [18.1] μm; range, 55-128; 104.2 [12.7] μm; range, 87-126; and 103.5 [15.4] μm; range, 63-145; respectively) (Table); quadrant and sectoral RNFL thickness were not significantly different. Paired (fellow vs treated eyes) differences in OCT RNFL thickness were examined and stratified by glaucoma, glaucoma suspect, and neither categories; no statistically significant differences were found (eTable 4 in the Supplement). There were 12 cases where the glaucomatous eye had thinner RNFL compared with the fellow eye (individual data not shown); one representative case is detailed (eFigure 4A and B in the Supplement).
Table. Retinal Nerve Fiber Layer Optical Coherence Tomography Quality and Thickness Measurements vs 10-Year Glaucoma Status.
| RNFL (quality and thickness) | P value comparing group meansa | Mean (SD) [range] | ||
|---|---|---|---|---|
| Glaucoma (n = 15) | Glaucoma suspect (n = 13) | Neither (n = 44) | ||
| Image quality, 1-40 dB | .77 | 28.0 (5.9) [20.0-40.0] | 27.0 (6.9) [15.0-37.0] | 26.7 (5.9) [14.0-37.0] |
| Overall, μm | .08 | 93.3 (18.1) [55.0-128.0] | 104.2 (12.7) [87.0-126.0] | 103.5 (15.4) [63.0-145.0] |
| Nasal, μm | .80 | 74.8 (18.4) [36.0-103.0] | 76.4 (22.6) [45.0-113.0] | 79.1 (20.4) [42.0-129.0] |
| Nasal inferior, μm | .06 | 96.3 (27.5) [56.0-153.0] | 105.7 (32.3) [55.0-161.0] | 121.8 (41.1) [63.0-274.0] |
| Nasal superior, μm | .36 | 100.1 (31.9) [39.0-159.0] | 111.8 (29.8) [71.0-150.0] | 113.2 (30.3) [56.0-195.0] |
| Temporal, μm | .08 | 75.1 (16.6) [52.0-107.0] | 87.8 (15.4) [68.0-115.0] | 78.7 (14.9) [55.0-118.0] |
| Temporal inferior, μm | .20 | 133.1 (33.1) [68.0-191.0] | 147.5 (28.8) [66.0-192.0] | 147.1 (23.5) [100.0-206.0] |
| Temporal superior, μm | .08 | 117.2 (33.4) [43.0-177.0] | 136.5 (23.3) [82.0-169.0] | 134.1 (24.8) [83.0-187.0] |
Abbreviation: RNFL, retinal nerve fiber layer.
For each RNFL measurement, a 1-way fixed-effects analysis of variance F test was performed to compare the 3 group means.
Optic nerve head photographs were obtained in 104 of 110 treated eyes (95%); 94 were adequate for grading (3 of 3 graders agreed in 91 cases). Of ONH photographs graded, 82 of 94 (87%) were classified as “normal,” with agreement in 3 of 3 readers for 79 of these 82 eyes (96%); 7 of 94 (7%) were classified as glaucoma suspect (3 of 3 graders agreed in all 7 cases); and 5 of 94 (5%) were classified as glaucoma (3 graders agreed on 4 eyes; 1 grader found the remaining eye imaging inadequate).
There was slight/fair agreement between the clinical diagnoses of glaucoma/glaucoma suspect/neither by IATS criteria vs the diagnosis made based on ONH reading (κ, 0.29; 95% CI, 0.12-0.46) (eTable 5 in the Supplement). While 58 of 60 eyes (97%) that were normal (neither glaucoma nor glaucoma suspect by IATS clinical criteria) had ONH photographs graded normal, only 2 of 15 eyes (13%) with clinical glaucoma suspect diagnosis had matching ONH grading (the rest being read as normal), and 4 of 19 eyes (21%) with clinical glaucoma diagnosis had matching ONH grading (with 4 of 19 suspect and 11 of 19 normal). Because ONH photographs were not available prior to the 10-year examination, information on ONH cupping reversal in eyes whose elevated IOP was successfully controlled is unavailable.
Among 20 eyes with glaucoma (by IATS criteria) and adequate ONH photographs, RNFL imaging was available for 13. There was no difference in RNFL parameters for ONH photographs graded as glaucoma vs glaucoma suspect vs neither (eTable 6 in the Supplement; representative case Figure 4A and B).
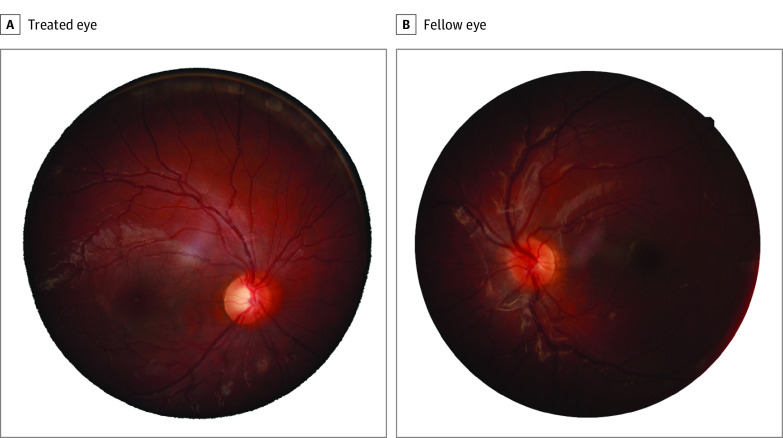
Figure 4. Examples of Optic Nerve Head (ONH) Images for the Treated (A) and Fellow Eye (B) of the Same Infant Aphakia Treatment Study (IATS) Participants Whose ONH Optical Coherence Tomography Images Are Shown in eFigure 4A and B in the Supplement.
The treated eye was diagnosed as having glaucoma based both on the IATS criteria (eTable 1 in the Supplement) and ONH imaging, while the fellow eye was graded as normal (neither glaucomatous nor glaucoma suspect). The peripapillary retinal nerve fiber layer for the ONH of the treated eye (eFigure 4A in the Supplement) was 105 μm; the peripapillary RNFL for the ONH of the fellow eye (eFigure 4B in the Supplement) was 117 μm.
Discussion
Glaucoma developed in the operated-on eyes of 25 of 110 infants (23%) with unilateral cataract enrolled in the IATS by age 10.5 years; an additional 21 eyes were glaucoma suspects, for a total 46 operated-on eyes (40%) with glaucoma + glaucoma suspect diagnosis. The risk of developing glaucoma rose similarly in both CL and IOL groups between 5 and 10 years after cataract surgery, as did the risk of glaucoma + glaucoma suspect diagnosis. Because the IATS protocol required IOP assessment only at the 1-year examination under anesthesia and the 4-year, 4.5-year, or 5-year visits and again at the 10-year visit, the IATS protocol precludes any comment on the annualized rate of conversion to glaucoma suspect and glaucoma among treated eyes.
Five-year follow-up from the only other randomized clinical trial of primary IOL vs aphakia, in this case for bilateral congenital cataracts removed before age 2 years, using similar diagnostic criteria,14 found similar rates of glaucoma between the pseudophakic and aphakic eyes (16% vs 14%, respectively), with most having cataract surgery at younger than 6 months, and most cases occurring in the first year following surgery. We are not aware of any other prospective study with 10-year follow-up that also used uniform definitions of glaucoma and glaucoma suspect and that randomized eyes receiving primary IOL implantation vs remaining aphakic.
The question of whether primary IOL implantation affects the risk of glaucoma or glaucoma suspect development following cataract removal in infancy has been addressed by many retrospective studies1,2,3,4,5,6,7,8,9,10,11,12,30,31,32,33,34 and some prospective but nonrandomized studies,13,15,16 all subject to some degree of selection bias. The IATS, by virtue of its random assignment, prospective design, and excellent participant retention rate, lacks the corresponding bias of prior nonrandomized studies.19 As we reported at 1-year and 5-year follow-up times,17,18 we now report similar but rising rates of glaucoma and glaucoma + glaucoma suspect diagnoses in each treatment group (IOL vs CL) at 10 years following surgery. In their prospective randomized study of primary IOL placement vs aphakia (for bilateral congenital cataracts), Vasavada et al14 similarly noted no difference in glaucoma cases between groups, at 1 year or 5 years after cataract removal.
Standard definitions of glaucoma and glaucoma suspect were developed and uniformly applied for surveillance and diagnosis of eyes within the IATS, with the former requiring not only elevated IOP but also associated structural changes in the infant eye or need for glaucoma surgery.17,18 The definitions of glaucoma and glaucoma suspect developed for IATS17 were adopted by the international consensus group.35
Among various risk factors previously evaluated for the development of glaucoma following cataract surgery in children, young age at surgery has been frequently identified.11,12,14,15,32,36 Younger patient age at surgery (ages 28-48 days vs 49-210 days) was also noted as a risk factor for the development of a glaucoma-related adverse event in the IATS at 1 year and 5 years following cataract removal17,18; in multivariate analysis, it remains the strongest and only independent risk factor for glaucoma 10 years after surgery. For development of any glaucoma-related adverse event at 10 years, small corneal diameter at surgery, closely related to age at surgery,18 emerged as the only risk factor. Secondary IOL was not significantly associated with glaucoma-related adverse events in the CL group at 10 years, although numbers were small, and there may be selection bias favoring the group of eyes that did receive secondary IOL surgery.
While there was a wide range of visual acuity outcomes at 10 years in the IATS,23 mean visual acuity was similar among eyes with glaucoma, glaucoma suspect, and neither diagnosis. As was the case at 5 years,37 those children having earlier vs later surgery (28-48 vs 49-210 days) had better median acuity. Therefore, the desire to achieve maximum visual acuity must be counterbalanced against an increased risk of glaucoma or glaucoma suspect diagnosis in an infant with a unilateral congenital cataract.
Eyes diagnosed with glaucoma at 10 years after cataract removal in the IATS had longer axial lengths than those with glaucoma suspect or neither diagnosis (in keeping with the requirement for anatomic changes for glaucoma diagnosis), but similar IOP, refraction, and visual acuity. Ours is the only prospective study of glaucoma-related adverse events following cataract surgery in infancy that included an attempt to evaluate the optic nerve status with OCT and masked grading of ONH photographs at the 10-year examination. Despite their age, adequate OCT imaging was not achieved in all treated or fellow eyes of participants. Perhaps reassuringly, the mean global RNFL thickness was similar among eyes with glaucoma, glaucoma suspect, and neither diagnosis, suggesting adequate glaucoma treatment to date. Pairwise comparison between treated and fellow eyes by glaucoma category did not yield meaningful differences. While there was excellent agreement among 3 masked graders of ONH photographs, most OHN photographs appeared normal even in eyes with the clinical IATS definitions of glaucoma and glaucoma suspect, again suggesting (along with similar 10-year mean IOP among glaucoma, suspect, and normal eyes) either reversal of cupping in eyes with glaucoma or adequate treatment to maintain a healthy-appearing ONH by photography. While ONH cupping reversal commonly occurs following IOP control in childhood glaucoma, glaucomatous damage to RNFL does not recover; therefore, the relatively normal RNFL in most glaucomatous eyes in IATS supports good glaucoma control.38
While the IATS did not dictate clinical management of glaucoma following cataract surgery, it is interesting to note that by 10 years, glaucoma surgery had been performed in 11 of 25 eyes (48%) with glaucoma (7 requiring a single surgery by the 10-year examination). Trabeculotomy was the most commonly performed glaucoma surgery, followed by glaucoma drainage device implantation. One eye lost light perception (owing to retinal detachment prior to the 5-year report).18 Others have reported varying rates of glaucoma surgery for glaucoma following cataract surgery,14,39,40 likely influenced by patient characteristics and follow-up times. Medication use was reported in approximately 50% of eyes with glaucoma or glaucoma suspect at the 10-year examination.
Limitations
Limitations of this study include the relatively small sample size of the cohort (reducing power to adequately assess some factors), lack of study-related treatment protocols for glaucoma-related adverse events, and lack of required study follow-up between 5 and 10 years. We cannot suggest a mechanism for glaucoma-related adverse events nor make treatment recommendations. Additionally, we cannot comment about glaucoma-related adverse event risk in children older than 6 months at surgery, although a 2019 retrospective study41 addressed this issue and reported a much lower rate of glaucoma (no cases of glaucoma and 4% incidence of glaucoma suspect by 5 years).41 Similarly, while the IATS included only those with unilateral cataract, Vasavada et al14 report similar findings in a bilateral cohort with shorter follow-up.14 Finally, we can only report on the risk of developing glaucoma-related adverse events up to age 10.5 years; this risk would likely increase with longer follow-up. This study’s strengths include its prospective design with randomization to primary IOL implantation vs aphakia, standardized definitions of glaucoma and glaucoma suspect, long-term follow-up with excellent retention, and incorporation of anatomic features of ONH health including OCT and photography.
Conclusions
Risk of glaucoma-related adverse events continues to rise with longer follow-up of children following unilateral cataract removal in infancy and is not influenced by primary IOL implantation. Development of glaucoma (or glaucoma suspect) did not significantly affect visual acuity outcomes at 10 years after removal of unilateral congenital cataract. The RNFL and ONH photographic appearance (with good agreement among 3 expert masked graders) was relatively well preserved in eyes with glaucoma and glaucoma suspect diagnoses.
While it is clear that the risk of glaucoma and glaucoma + glaucoma suspect (with strict application of uniform definitions for both diagnoses) continues to rise over time after removal of unilateral cataract in infancy, we cannot predict, based on our data, whether some of these treated eyes will ultimately avoid developing glaucoma or glaucoma suspect. Lifelong surveillance for the development of glaucoma-related adverse events remains important, with the hope that early diagnosis and treatment may facilitate preservation of ONH health and maximal visual function.
eAppendix. Optic Nerve Head Photography and Retinal Nerve Fiber Layer Imaging Protocol, Statistical Methods and Supplementary Results
eTable 1. Definitions of Glaucoma, Glaucoma Suspect and Glaucoma-Related Adverse Event
eTable 2. Relationship Between Baseline Characteristics and 10-year Glaucoma and Glaucoma+Glaucoma Suspect
eTable 3. Glaucoma Surgeries for Glaucomatous Eyes
eTable 4. Means and 99.9% Confidence Intervals for Paired Differences Between Fellow and Treated Eye Retinal Nerve Fiber Layer Optical Coherence Tomography Quality and Thickness Measurements, by Glaucoma Status
eTable 5. Glaucoma Status by IATS Criteria vs. by Optic Nerve Head
eTable 6. Mean (Standard deviation) Retinal Nerve Fiber Layer (RNFL) OCT Quality and Thickness Measurements, by Optic Nerve Head Grading Category
eFigure 1. 10-year Intraocular Pressure (mm Hg) vs. Glaucoma Status
eFigure 2. 10-year Axial Length (mm) vs. Glaucoma Status
eFigure 3. 10-year Refractive Error (D) vs. Glaucoma Status
eFigure 4. Example of Retinal Nerve Fiber Layer (RNFL) Optical Coherence Tomography (OCT) Images for the treated eye with glaucoma (4A) and fellow normal eye (4B) of an IATS study subject
eFigure 5. Retinal Nerve Fiber Layer (RNFL) by Optical Coherence Tomography: Study Case Report Form
eFigure 6. Optic Nerve Head (ONH) Photography: Study Case Report Form
References
- 1.Lambert SR, Purohit A, Superak HM, Lynn MJ, Beck AD. Long-term risk of glaucoma after congenital cataract surgery. Am J Ophthalmol. 2013;156(2):355-361.e2. doi: 10.1016/j.ajo.2013.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simon JW, Mehta N, Simmons ST, Catalano RA, Lininger LL. Glaucoma after pediatric lensectomy/vitrectomy. Ophthalmology. 1991;98(5):670-674. doi: 10.1016/S0161-6420(91)32235-8 [DOI] [PubMed] [Google Scholar]
- 3.Egbert JE, Christiansen SP, Wright MM, Young TL, Summers CG. The natural history of glaucoma and ocular hypertension after pediatric cataract surgery. J AAPOS. 2006;10(1):54-57. doi: 10.1016/j.jaapos.2005.07.002 [DOI] [PubMed] [Google Scholar]
- 4.Johnson CP, Keech RV. Prevalence of glaucoma after surgery for PHPV and infantile cataracts. J Pediatr Ophthalmol Strabismus. 1996;33(1):14-17. [DOI] [PubMed] [Google Scholar]
- 5.Rabiah PK Frequency and predictors of glaucoma after pediatric cataract surgery. Am J Ophthalmol. 2004;137(1):30-37. doi: 10.1016/S0002-9394(03)00871-7 [DOI] [PubMed] [Google Scholar]
- 6.Swamy BN, Billson F, Martin F, et al. Secondary glaucoma after paediatric cataract surgery. Br J Ophthalmol. 2007;91(12):1627-1630. doi: 10.1136/bjo.2007.117887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen TC, Bhatia LS, Halpern EF, Walton DS. Risk factors for the development of aphakic glaucoma after congenital cataract surgery. J Pediatr Ophthalmol Strabismus. 2006;43(5):274-280. doi: 10.3928/01913913-20060901-01 [DOI] [PubMed] [Google Scholar]
- 8.Kuhli-Hattenbach C, Lüchtenberg M, Kohnen T, Hattenbach LO. Risk factors for complications after congenital cataract surgery without intraocular lens implantation in the first 18 months of life. Am J Ophthalmol. 2008;146(1):1-7. doi: 10.1016/j.ajo.2008.02.014 [DOI] [PubMed] [Google Scholar]
- 9.Wong IB, Sukthankar VD, Cortina-Borja M, Nischal KK. Incidence of early-onset glaucoma after infant cataract extraction with and without intraocular lens implantation. Br J Ophthalmol. 2009;93(9):1200-1203. doi: 10.1136/bjo.2008.155200 [DOI] [PubMed] [Google Scholar]
- 10.Kirwan C, Lanigan B, O’Keefe M. Glaucoma in aphakic and pseudophakic eyes following surgery for congenital cataract in the first year of life. Acta Ophthalmol. 2010;88(1):53-59. doi: 10.1111/j.1755-3768.2009.01633.x [DOI] [PubMed] [Google Scholar]
- 11.Bothun ED, Wilson ME, Vanderveen DK, et al. Outcomes of bilateral cataracts removed in infants 1 to 7 months of age using the Toddler Aphakia and Pseudophakia Treatment Study Registry. Ophthalmology. 2020;127(4):501-510. doi: 10.1016/j.ophtha.2019.10.039 [DOI] [PubMed] [Google Scholar]
- 12.Mataftsi A, Haidich AB, Kokkali S, et al. Postoperative glaucoma following infantile cataract surgery: an individual patient data meta-analysis. JAMA Ophthalmol. 2014;132(9):1059-1067. doi: 10.1001/jamaophthalmol.2014.1042 [DOI] [PubMed] [Google Scholar]
- 13.Solebo AL, Russell-Eggitt I, Cumberland PM, Rahi JS; British Isles Congenital Cataract Interest Group . Risks and outcomes associated with primary intraocular lens implantation in children under 2 years of age: the IoLunder2 cohort study. Br J Ophthalmol. 2015;99(11):1471-1476. doi: 10.1136/bjophthalmol-2014-306394 [DOI] [PubMed] [Google Scholar]
- 14.Vasavada AR, Vasavada V, Shah SK, et al. Five-year postoperative outcomes of bilateral aphakia and pseudophakia in children up to 2 years of age: a randomized clinical trial. Am J Ophthalmol. 2018;193:33-44. doi: 10.1016/j.ajo.2018.06.005 [DOI] [PubMed] [Google Scholar]
- 15.Freedman SF, Kraker RT, Repka MX, et al. ; Pediatric Eye Disease Investigator Group (PEDIG) . Incidence and management of glaucoma or glaucoma suspect in the first year after pediatric lensectomy. JAMA Ophthalmol. 2020;138(1):71-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balekudaru S, Agarkar S, Guha S, et al. Prospective analysis of the predictors of glaucoma following surgery for congenital and infantile cataract. Eye (Lond). 2019;33(5):796-803. doi: 10.1038/s41433-018-0316-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck AD, Freedman SF, Lynn MJ, Bothun E, Neely DE, Lambert SR; Infant Aphakia Treatment Study Group . Glaucoma-related adverse events in the Infant Aphakia Treatment Study: 1-year results. Arch Ophthalmol. 2012;130(3):300-305. doi: 10.1001/archophthalmol.2011.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freedman SF, Lynn MJ, Beck AD, Bothun ED, Örge FH, Lambert SR; Infant Aphakia Treatment Study Group . Glaucoma-related adverse events in the first 5 years after unilateral cataract removal in the infant aphakia treatment study. JAMA Ophthalmol. 2015;133(8):907-914. doi: 10.1001/jamaophthalmol.2015.1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambert SR, Buckley EG, Drews-Botsch C, et al. ; Infant Aphakia Treatment Study Group . The infant aphakia treatment study: design and clinical measures at enrollment. Arch Ophthalmol. 2010;128(1):21-27. doi: 10.1001/archophthalmol.2009.350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plager DA, Lynn MJ, Buckley EG, Wilson ME, Lambert SR; Infant Aphakia Treatment Study Group . Complications, adverse events, and additional intraocular surgery 1 year after cataract surgery in the infant Aphakia Treatment Study. Ophthalmology. 2011;118(12):2330-2334. doi: 10.1016/j.ophtha.2011.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plager DA, Lynn MJ, Buckley EG, Wilson ME, Lambert SR; Infant Aphakia Treatment Study Group . Complications in the first 5 years following cataract surgery in infants with and without intraocular lens implantation in the Infant Aphakia Treatment Study. Am J Ophthalmol. 2014;158(5):892-898. doi: 10.1016/j.ajo.2014.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambert SR, Lynn MJ, Hartmann EE, et al. ; Infant Aphakia Treatment Study Group . Comparison of contact lens and intraocular lens correction of monocular aphakia during infancy: a randomized clinical trial of HOTV optotype acuity at age 4.5 years and clinical findings at age 5 years. JAMA Ophthalmol. 2014;132(6):676-682. doi: 10.1001/jamaophthalmol.2014.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lambert SR, Cotsonis G, DuBois L, et al. ; Infant Aphakia Treatment Study Group . Long-term effect of intraocular lens vs contact lens correction on visual acuity after cataract surgery during infancy: a randomized clinical trial. JAMA Ophthalmol. 2020;138(4):365-372. doi: 10.1001/jamaophthalmol.2020.0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):714-720. doi: 10.1001/archopht.120.6.714 [DOI] [PubMed] [Google Scholar]
- 25.Mwanza JC, Oakley JD, Budenz DL, Anderson DR; Cirrus Optical Coherence Tomography Normative Database Study Group . Ability of cirrus HD-OCT optic nerve head parameters to discriminate normal from glaucomatous eyes. Ophthalmology. 2011;118(2):241-8.e1. doi: 10.1016/j.ophtha.2010.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miki A, Medeiros FA, Weinreb RN, et al. Rates of retinal nerve fiber layer thinning in glaucoma suspect eyes. Ophthalmology. 2014;121(7):1350-1358. doi: 10.1016/j.ophtha.2014.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alasil T, Wang K, Keane PA, et al. Analysis of normal retinal nerve fiber layer thickness by age, sex, and race using spectral domain optical coherence tomography. J Glaucoma. 2013;22(7):532-541. doi: 10.1097/IJG.0b013e318255bb4a [DOI] [PubMed] [Google Scholar]
- 28.Ghasia FF, Freedman SF, Rajani A, Holgado S, Asrani S, El-Dairi M. Optical coherence tomography in paediatric glaucoma: time domain versus spectral domain. Br J Ophthalmol. 2013;97(7):837-842. doi: 10.1136/bjophthalmol-2012-302648 [DOI] [PubMed] [Google Scholar]
- 29.Lee H, Proudlock FA, Gottlob I. Pediatric optical coherence tomography in clinical practice-recent progress. Invest Ophthalmol Vis Sci. 2016;57(9):OCT69-OCT79. doi: 10.1167/iovs.15-18825 [DOI] [PubMed] [Google Scholar]
- 30.Asrani S, Freedman S, Hasselblad V, et al. Does primary intraocular lens implantation prevent “aphakic” glaucoma in children? J AAPOS. 2000;4(1):33-39. doi: 10.1016/S1091-8531(00)90009-0 [DOI] [PubMed] [Google Scholar]
- 31.Sahin A, Caça I, Cingü AK, et al. Secondary glaucoma after pediatric cataract surgery. Int J Ophthalmol. 2013;6(2):216-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trivedi RH, Wilson ME Jr, Golub RL. Incidence and risk factors for glaucoma after pediatric cataract surgery with and without intraocular lens implantation. J AAPOS. 2006;10(2):117-123. doi: 10.1016/j.jaapos.2006.01.003 [DOI] [PubMed] [Google Scholar]
- 33.Lin H, Chen W, Luo L, et al. ; Study Group of CCPMOH . Ocular hypertension after pediatric cataract surgery: baseline characteristics and first-year report. PLoS One. 2013;8(7):e69867. doi: 10.1371/journal.pone.0069867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parks MM Visual results in aphakic children. Am J Ophthalmol. 1982;94(4):441-449. doi: 10.1016/0002-9394(82)90237-9 [DOI] [PubMed] [Google Scholar]
- 35.World Glaucoma Association. Childhood Glaucoma: the 9th Consensus Report of the World Glaucoma Association. Kugler Publications; 2013. [Google Scholar]
- 36.Vishwanath M, Cheong-Leen R, Taylor D, Russell-Eggitt I, Rahi J. Is early surgery for congenital cataract a risk factor for glaucoma? Br J Ophthalmol. 2004;88(7):905-910. doi: 10.1136/bjo.2003.040378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hartmann EE, Lynn MJ, Lambert SR; Infant Aphakia Treatment Study Group . Baseline characteristics of the infant aphakia treatment study population: predicting recognition acuity at 4.5 years of age. Invest Ophthalmol Vis Sci. 2014;56(1):388-395. doi: 10.1167/iovs.14-15464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ely AL, El-Dairi MA, Freedman SF. Cupping reversal in pediatric glaucoma: evaluation of the retinal nerve fiber layer and visual field. Am J Ophthalmol. 2014;158(5):905-915. doi: 10.1016/j.ajo.2014.07.030 [DOI] [PubMed] [Google Scholar]
- 39.Chen TC, Walton DS, Bhatia LS. Aphakic glaucoma after congenital cataract surgery. Arch Ophthalmol. 2004;122(12):1819-1825. doi: 10.1001/archopht.122.12.1819 [DOI] [PubMed] [Google Scholar]
- 40.Bhola R, Keech RV, Olson RJ, Petersen DB. Long-term outcome of pediatric aphakic glaucoma. J AAPOS. 2006;10(3):243-248. doi: 10.1016/j.jaapos.2006.01.005 [DOI] [PubMed] [Google Scholar]
- 41.Bothun ED, Wilson ME, Traboulsi EI, et al. ; Toddler Aphakia and Pseudophakia Study Group (TAPS) . Outcomes of unilateral cataracts in infants and toddlers 7 to 24 months of age: Toddler Aphakia and Pseudophakia Study (TAPS). Ophthalmology. 2019;126(8):1189-1195. doi: 10.1016/j.ophtha.2019.03.011 [DOI] [PubMed] [Google Scholar]
- 42.Egbert JE, Wright MM, Dahlhauser KF, Keithahn MA, Letson RD, Summers CG. A prospective study of ocular hypertension and glaucoma after pediatric cataract surgery. Ophthalmology. 1995;102(7):1098-1101. doi: 10.1016/S0161-6420(95)30906-2 [DOI] [PubMed] [Google Scholar]
- 43.Pressman SH, Crouch ER Jr. Pediatric aphakic glaucoma. Ann Ophthalmol. 1983;15(6):568-573. [PubMed] [Google Scholar]
- 44.Phelps CD, Arafat NI. Open-angle glaucoma following surgery for congenital cataracts. Arch Ophthalmol. 1977;95(11):1985-1987. doi: 10.1001/archopht.1977.04450110079005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Optic Nerve Head Photography and Retinal Nerve Fiber Layer Imaging Protocol, Statistical Methods and Supplementary Results
eTable 1. Definitions of Glaucoma, Glaucoma Suspect and Glaucoma-Related Adverse Event
eTable 2. Relationship Between Baseline Characteristics and 10-year Glaucoma and Glaucoma+Glaucoma Suspect
eTable 3. Glaucoma Surgeries for Glaucomatous Eyes
eTable 4. Means and 99.9% Confidence Intervals for Paired Differences Between Fellow and Treated Eye Retinal Nerve Fiber Layer Optical Coherence Tomography Quality and Thickness Measurements, by Glaucoma Status
eTable 5. Glaucoma Status by IATS Criteria vs. by Optic Nerve Head
eTable 6. Mean (Standard deviation) Retinal Nerve Fiber Layer (RNFL) OCT Quality and Thickness Measurements, by Optic Nerve Head Grading Category
eFigure 1. 10-year Intraocular Pressure (mm Hg) vs. Glaucoma Status
eFigure 2. 10-year Axial Length (mm) vs. Glaucoma Status
eFigure 3. 10-year Refractive Error (D) vs. Glaucoma Status
eFigure 4. Example of Retinal Nerve Fiber Layer (RNFL) Optical Coherence Tomography (OCT) Images for the treated eye with glaucoma (4A) and fellow normal eye (4B) of an IATS study subject
eFigure 5. Retinal Nerve Fiber Layer (RNFL) by Optical Coherence Tomography: Study Case Report Form
eFigure 6. Optic Nerve Head (ONH) Photography: Study Case Report Form