Abstract
Objective
To investigate the value of a notched unipolar electrogram (N-uniEGM) in confirming the origin of premature ventricular contractions originating from the ventricular outflow tract (VOT-PVC) during mapping and ablation procedures.
Methods
This retrospective study enrolled consecutive patients with symptomatic idiopathic frequent VOT-PVCs that underwent radiofrequency ablation. The characteristics of the uniEGM of the successful ablation targets were analysed. N-uniEGM was defined as the uniEGM presenting a QS morphology with ≥1 steep notches on the downstroke deflection. All patients were followed-up for 3 months post-ablation.
Results
The study enrolled 190 patients with a mean ± SD age of 49.0 ± 15.3 years. N-uniEGMs were recorded in 124 of 190 (65.3%) patients. The N-uniEGM distribution area was limited to a mean ± SD of 0.8 ± 0.4 cm2. N-uniEGM showed consistency with the outcomes of activation mapping and pace mapping. Patients with an N-uniEGM had an ablation success rate of 98.4% (122 of 124) and their ablation times were significantly shorter than those without an N-uniEGM (7.6 ± 3.8 s versus 15.8 ± 8.8 s, respectively). The sensitivity and specificity of N-uniEGM in predicting successful ablation of VOT-PVCs were 72.6% and 91.7%, respectively.
Conclusion
N-uniEGM was a highly specific and moderately sensitive predictor of successful radiofrequency ablation in patients with VOT-PVCs.
Keywords: Premature ventricular contractions, outflow tract, catheter ablation, unipolar mapping
Introduction
Radiofrequency catheter ablation is an effective treatment for frequent premature ventricular contractions originating from the ventricular outflow tract (VOT-PVCs).1 Previous studies have shown that in most cases, the origin of VOT-PVCs could be detected by the characteristics of surface electrocardiograms (ECG), as well as activation mapping and pace mapping.2–4 Compared with bipolar electrograms, unipolar electrograms (uniEGM) can reflect the duration and conduction direction of the local myocardium excitation with more accuracy.5 In most cases, a monophasic QS morphology with a steep initial part can be recorded at the origin of the ventricular arrhythmia by the recording electrode.6 However, as VOTs represent the highest part of both ventricles, QS configuration on a uniEGM can be recorded in a large area outside the focal source of PVC,7–9 which in turn limits the specificity of the QS-morphology uniEGM in predicting the origin of VOT-PVCs.10–12 According to previous observations, uniEGMs at the successful targets had a common distinctive ‘notched’ pattern, namely, with one or more steep notches on the initial downstroke deflection.7,8 This current study defined this type of uniEGM pattern as a ‘notched uniEGM’ (N-uniEGM). The purpose of this current study was to evaluate the value of an N-uniEGM in confirming the origin of VOT-PVCs during catheter ablation.
Patients and methods
Study population
This retrospective study enrolled consecutive patients with symptomatic idiopathic frequent VOT-PVCs that underwent radiofrequency ablation in the Heart Centre, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China between May 2011 and December 2017. In all patients, the morphology of the documented PVCs on the 12-lead surface ECG suggested a VOT origin. The inclusion criteria were as follows: (i) age ≥ 18 years; (ii) symptomatic idiopathic frequent VOT-PVCs; (iii) PVC burden ≥10% on a 24-h dynamic ECG (Holter) recording; (iv) antiarrhythmic drugs were ineffective or contraindicated. The exclusion criteria were as follows: (i) structural heart disease detected by noninvasive imaging (echocardiography, cardiac magnetic resonance imaging and coronary artery or left heart catheterization if necessary; (ii) low PVC burden (<10%) on a 24-h Holter recording; (iii) previous ablation history; (iv) other severe systemic diseases. The study protocol was approved by the Institutional Review Board Committee of Beijing Chaoyang Hospital, Capital Medical University, Beijing, China (no. 2015-3-64). All patients provided written informed consent.
Electrophysiology study and radiofrequency ablation
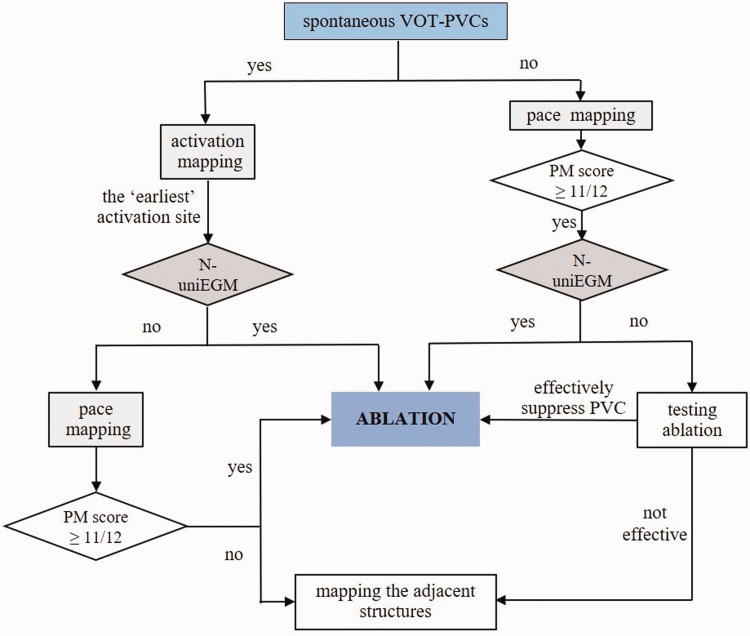
Radiofrequency catheter ablation was conducted under a 3-dimensional mapping system (CARTO® 3 System; Biosense Webster, Diamond Bar, CA, USA) after all antiarrhythmic medications were suspended for at least five half-lives prior to the procedure. The procedure was performed under conscious sedation. VOT mapping was performed using a 3.5-mm open irrigated-tip ablation catheter (NAVISTAR® THERMOCOOL® Catheter; Biosense Webster). Activation mapping or pace mapping was conducted based on the frequency of the spontaneous PVC. Following the activation mapping, all sites with the earliest local activation time would be checked for morphology of unipolar electrogram. Targets presenting with an N-uniEGM would undergo ablation. If clinical PVC only appeared sporadically, pace mapping was chosen as the initial mapping approach and sites with ideal characteristics in pace mapping would also be checked for unipolar mapping. If an N-uniEGM was revealed, then direct ablation would be conducted. The detailed workflow of VOT-PVC ablation is shown in Figure 1.
Figure 1.
The workflow used in the current study for premature ventricular contractions originating from the ventricular outflow tract (VOT-PVC). PM, pace mapping; N-uniEGM, notched unipolar electrogram.
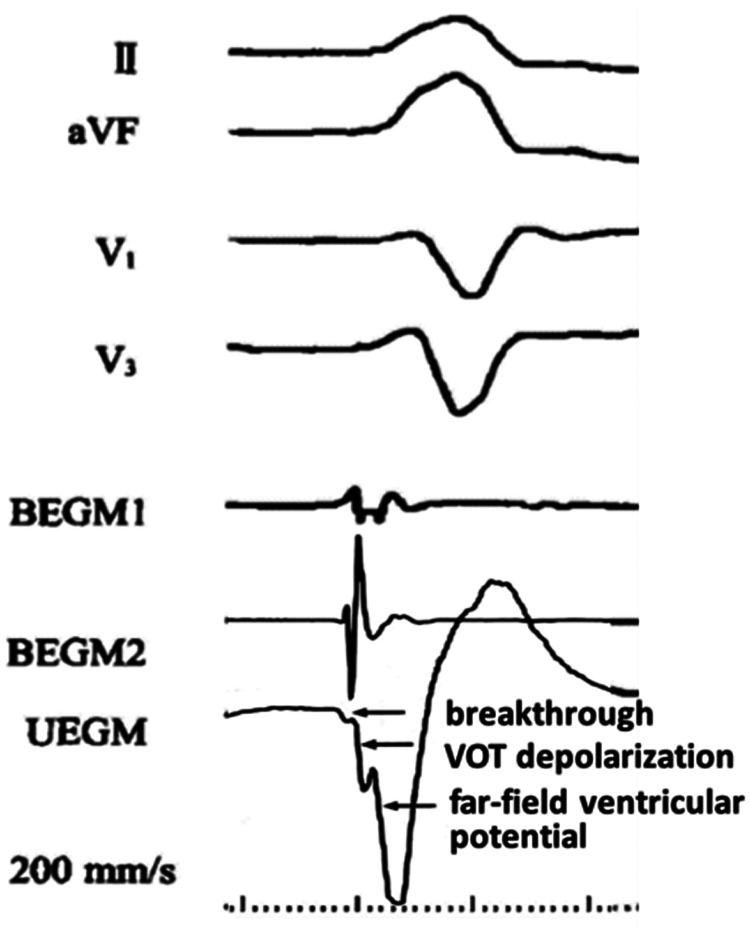
Radiofrequency energy was delivered at 25–30 W with a pre-set temperature of 43°C and the flow rate of saline during radiofrequency delivery was 17–20 ml/min. At each targeted mapping site, the location of the catheter tip was tagged. If PVCs were abolished within 10 s, the energy application would be continued for a total of 90–120 s and the site would be tagged as a successful site. The ablation endpoint was defined as the absence of clinical PVCs within 30 min after the last radiofrequency delivery, which was confirmed by non-inductility during isoproterenol infusion (2–4 µg/min) and the Valsalva manoeuvre (per 10–15 s). An N-uniEGM was defined as a uniEGM with a ‘QS’ morphology and one or more steep notches on the downstroke deflection (filtration: 0.1–250 Hz) (Figure 2). The distribution area was defined as the assembly of all sites presenting with an N-uniEGM around the successful site, which was automatically calculated by the area measurement function of the CARTO® 3 System.
Figure 2.
An example of a notched unipolar electrogram (UEGM) and a deduction of its mechanism. The notches in the unipolar electrogram represent local myocardial activation at the breakthrough of the premature ventricular contraction, depolarization of the ventricular outflow tract (VOT) and far field ventricular potential, respectively. BEGM1, distal bipolar electrogram; BEGM2, proximal bipolar electrogram.
Clinical follow-up
All patients were followed-up for 3 months after the procedure. The monthly-checked ECG and 24-h Holter were documented. Acute procedural success was defined as the elimination of clinical PVCs or the reduction of PVC burden by ≥90% within 24 h post-ablation. Long-term success was achieved if PVC burden was reduced by ≥90% compared with the pre-ablation level without taking any antiarrhythmic drugs.
Statistical analyses
All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 19.0 (IBM Corp., Armonk, NY, USA). Continuous variables are expressed as mean ± SD and were compared using Student’s t-test. Categorical variables are expressed as number (%) and compared using χ2-test. A P-value < 0.05 was considered statistically significant.
Results
A total of 190 consecutive patients (83 males; mean ± SD age, 49.0 ± 15.3 years) with symptomatic idiopathic frequent VOT-PVCs underwent radiofrequency ablation and were enrolled in the present study. The mean ± SD PVC burden within 24 h before the procedure was 20.4 ± 11.2%. The baseline demographic and clinical characteristics of the patients are summarized in Table 1. Of the 190 patients, 180 had frequent PVCs and 10 patients had both PVCs and ventricular tachycardia.
Table 1.
Baseline demographic and clinical characteristics of the patients (n = 190) with premature ventricular contractions originating from the ventricular outflow tract that were included in the current study.
| Characteristic | Value |
|---|---|
| Age, years | 49.0 ± 15.3 |
| Sex, male | 83 (43.7%) |
| Hypertension | 66 (34.7%) |
| Diabetes mellitus | 30 (15.8%) |
| LVEDD, mm | 47.7 ± 5.5 |
| LVEF, % | 65.9 ± 7.3 |
| PVC burden, % | 20.4 ± 11.2 |
| AADs | |
| Class I | 88 (46.3%) |
| Class II | 22 (11.6%) |
| Class III | 35 (18.4%) |
| ≥2 | 45 (23.7%) |
Data presented as mean ± SD or n of patients (%).
LVEDD, left ventricular diameter at end diastole; LVEF, left ventricular ejection fraction; PVC, premature ventricular contraction; AADs, antiarrhythmic drugs.
Of the 190 patients with frequent VOT-PVCs, 184 patients (96.8%) achieved acute procedural success; in whom 168 patients (88.4%) achieved total elimination of PVCs and 16 patients (8.4%) had a marked reduction (≥90%) in PVC frequency. Only six of 190 patients (3.2%) failed to achieve a significant reduction in PVC frequency and resumed antiarrhythmic drugs. Among the 168 patients that achieved complete elimination of PVCs, 138 patients had PVC originating from the right ventricular outflow tract (RVOT), 25 from the pulmonary sinus cusp, 25 from the aortic cusp (including eight from the left cusp, eight from the right cusp and nine from the left/right cusp junction) and five from sites under the aortic valve. No recurrence was observed in the patients that achieved acute procedural success. No major complications occurred in this study cohort.
An N-uniEGM was recorded in 124 of 190 patients (65.3%), of whom 97 patients had PVC origins in the RVOT (including 25 cases with origins above the pulmonary valve), 22 patients had an aortic cusp origin (including six patients from the left cusp, seven patients from the right cusp and nine patients from the left/right cusp junction) and five patients had origins under the aortic cusp. The sites where an N-uniEGM was recorded were highly overlapped with sites either with the earliest local activation time on the activation map or with perfect similarity during pace mapping (Figure 3). Among the 124 patients that displayed an N-uniEGM, activation mapping was also performed in 120 (96.8%) patients and pace mapping was necessary in another four (3.2%) patients that lacked a sufficient intraprocedural quantity of PVCs for activation mapping. In the 120 patients that underwent both N-uniEGM mapping and activation mapping, the targets identified by the former were always consistent with those identified by the latter. However, in the four patients that underwent both N-uniEGM mapping and pace mapping, adequate pace maps were obtained in only two (50.0%) patients when pacing was performed at the sites that presented with an N-uniEGM. In the other two patients, pacing at the sites that presented with an N-uniEGM either produced inadequate pace maps or failed to capture the ventricle. Ablation at the target without the characteristic N-uniEGM often failed to abolish the PVCs (Figure 4). The distribution area of an N-uniEGM was limited to a mean ± SD of 0.8 ± 0.4 cm2 and the mean ± SD number of notches of an N-uniEGMs recorded at the RVOT, aortic sinus and under the aortic cusp was 1.2 ± 0.5, 1.4 ± 0.8 and 1.7 ± 0.6, respectively. Patients with recorded N-uniEGMs generally showed a higher success rate than those patients without N-uniEGMs (122 of 124 [98.4%] versus 62 of 66 [93.9%], respectively), although the difference was not significant. The shortest ablation time required to suppress the PVCs (also referred to as valid ablation time) was significantly shorter in patients with an N-uniEGM than in those without (7.6 ± 3.8 s versus 15.8 ± 8.8 s, respectively; P < 0.05); and a significant difference was also observed in the total ablation time (178 ± 36 s versus 260 ± 114 s, respectively; P < 0.05). The sensitivity and specificity of N-uniEGM in predicting successful ablation of VOT-PVCs were 72.6% and 91.7%, respectively.
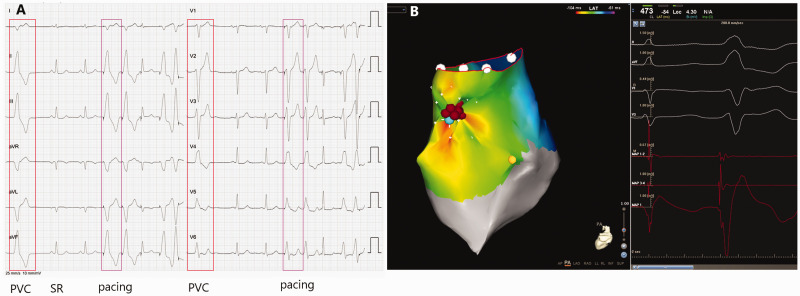
Figure 3.
Representative recordings showing the use of a notched unipolar electrogram (N-uniEGM) in the ablation of premature ventricular contractions originating from the right ventricular outflow tract (RVOT-PVC). (A) 12-lead surface electrocardiograms of sinus rhythm (SR), PVC and pacing. In this patient, the surface electrocardiogram indicated that the PVC originated from the RVOT. (B) Activation mapping of the origin of the PVC in the RVOT using the CARTO® 3 System with simultaneous recordings of surface electrocardiogram, unipolar and bipolar electrograms. The earliest ventricular activation (EVA) site was proved to be located near the anterior septum of the RVOT by 3-dimensional electroanatomic mapping. Pacing mapping morphology at this site coincided perfectly with the spontaneous PVC. An N-uniEGM was also recorded at the EVA. Radiofrequency energy delivery for 2 s at this site terminated the clinical PVC. The colour version of this figure is available at: http://imr.sagepub.com.
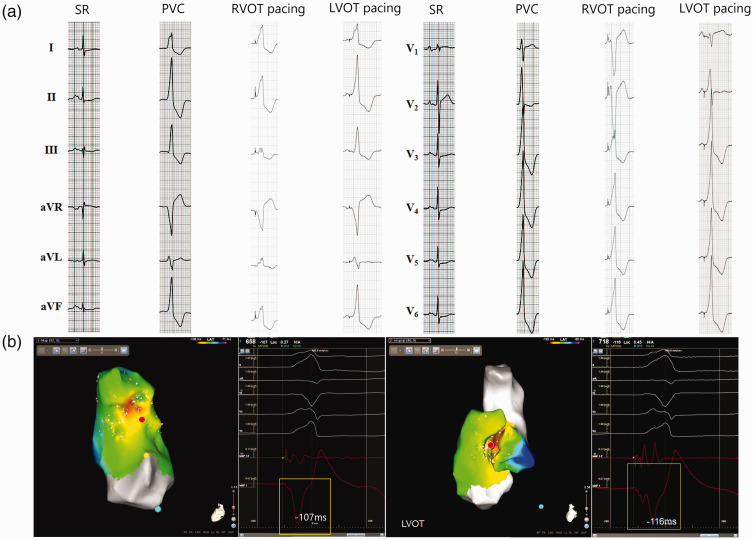
Figure 4.
Representative recordings showing the use of a notched unipolar electrogram (N-uniEGM) in the ablation of premature ventricular contractions originating from the left ventricular outflow tract (LVOT-PVC). (a) 12-lead surface electrocardiogram of sinus rhythm (SR), PVC and pacing at the right ventricular outflow tract (RVOT) and LVOT. (b) Activation mapping of the PVC target from the RVOT and LVOT using the CARTO® 3 System with simultaneous surface electrocardiogram recordings, bipolar and unipolar electrograms at the same sites and uniEGM. In this patient, the initial activation mapping at the RVOT revealed that the earliest ventricular activation site (EVA) was in the septum of the RVOT, which preceded the onset of surface QRS complex for 107 ms. Pacing mapping at this site produced a QRS morphology with a low similarity with the morphology of spontaneous PVC. The uniEGM at this site presented a QS morphology with a blunt initial part and no characteristics of N-uniEGM. Ablation at this site did not show any impact on the PVC. Activation mapping at the LVOT revealed an earlier EVA site (–116 ms) in the right coronary sinus and pacing mapping at this site produced a QRS morphology with better similarity with the spontaneous PVC. At this target site, unipolar mapping showed a characteristic N-uniEGM. PVCs disappeared after ablation at this site for 4.6 s. The colour version of this figure is available at: http://imr.sagepub.com.
Discussion
This current study demonstrated that the combination of activation/pacing mapping and N-uniEGM can enhance the accuracy in localizing the VOT-PVC origin during mapping and ablation of idiopathic frequent VOT-PVCs. In other words, if the target determined by traditional mapping modalities also presents with an N-uniEGM pattern, it is highly likely that this site is the specific origin of the VOT-PVC; and if not, it is indicated that a more precise origin is yet to be detected. N-uniEGM shows a high specificity in discriminating the successful ablation site and it is a promising supplement to the current mapping methods for catheter ablation of VOT-PVCs.
Regarding catheter ablation of VOT-PVCs, the acute success rate in published series ranged from 77% to 97%, while the long-term success rate ranged from 77% to 83%.13–15 The results of the current study were similar to the overall success of ablation for PVCs reported in the literature.13–15 In the group with acute procedural success, 16 patients still had sporadic PVCs within the first 24-h post-ablation. These patients might have deeper PVC origins than patients that achieved total elimination of PVCs, resulting in a hindered radiofrequency energy delivery to the PVC origin during catheter ablation. In the six failed patients, the sites of the PVC origin remained unknown.
To date, activation and pace mapping represent the mainstream methods in localizing the origin of frequent PVCs. Despite the value of these methods, there are some inevitable limitations: activation mapping measures the time interval between the local bipolar electrogram and the onset of the QRS complexes on the surface ECG, the longer this interval, the closer this site is to the PVC origin. The site with the longest ‘leading time’ is regarded as the origin of PVCs. However, the longest ‘leading time’ of each patient is different. Therefore, it is difficult to draw a quantitative standard that is universal in application. In addition, the ‘earliest’ activation site recorded in a single mapping attempt, i.e. RVOT, is not necessarily the authentic earliest ventricular activation site (EVA), as the recorded EVA site may just be an adjacent site to the true origin of the PVC, which lies beyond the mapping scope. In pace mapping, a target site is determined to be the origin of the PVC if the paced QRS morphology at this site coincides with the spontaneous PVC morphology in 11 out of 12 leads on the ECG. But in some cases, pace mapping at a certain area closely related to the true PVC origin can produce paced QRS morphology mimicking the morphology of PVC from ‘the authentic origin’ thus leading to in-effective ablation. For example, when mapping PVCs originating from the RVOT, a similar pacing QRS morphology can be recorded within a large nearby area; however, for PVCs originating from the LVOT, even the mapping EGM recorded at the exact PVC origin may fail to copy the morphology of spontaneous PVCs (Figure 4). In such cases, the N-uniEGM manifests its own valuable reference.
The low specificity of the traditional uniEGM characteristics in locating the focal source of VOT-PVCs is well known.16,17 Recent research explored certain uniEGM characteristics in an attempt to identify a parameter with more specificity in guiding the ablation of VOT-PVCs, including the ratio of R wave amplitude to the negative wave amplitude (R-ratio), the maximum downslope (MaxSlope) and the duration required for the downslope to reach its peak (D-Max).4 A previous study demonstrated that the smaller the R-ratio, the larger the MaxSlope, and the shorter the D-Max is, the more likely the site is the true PVC origin.4 However, these findings were not repeated by another study.18 Therefore, the value of the N-uniEGM method is yet to be examined. A previous study reported that a ‘QS morphology’ on a uniEGM can present at both the effective and non-effective targets during mapping and ablation of RVOT-PVCs.7In contrast, this current study demonstrated that N-uniEGM presents only in the effective targets. This phenomenon was also observed in another study.8 Both previous studies mentioned this characteristic in their case reports, but neither study provided systematic descriptions or explanations of this phenomenon.7,8
The mechanisms underlying N-uniEGM are yet to be elucidated. The anterior septum, pulmonary artery and aortic cusp, where most VOT-PVCs originate from, all feature highly complex myocardial structures and are innervated with extremely abundant cardiac autonomic nerves.19 It can be deduced that this anatomical substrate is associated with the formation of VOT-PVCs. Accordingly, the conduction of PVCs from its origin to its nearby area would be slowed down due to the high anisotropy. In our opinion, the N-uniEGM reflects the heterogeneity of the local activation conduction from the PVC origin to the adjacent sites (Figure 1). In addition, the muscle bundles surrounding the proximal part of the pulmonary artery and the aortic cusp have a relatively small volume, which causes a more complex and fragmented electrogram with very slow conduction, thus contributing to the formation of the local characteristic uniEGM.
This current study had several limitations. First, the retrospective nature of this analysis means that further prospective evaluation is necessary. Secondly, in some difficult cases, radiofrequency energy had to be delivered to multiple sites because more than one breakthrough of the same PVC origin was found. Thus, some ablation sites would be close to the previous ablation sites, and theoretically, the predictive value of N-uniEGM might be affected by the previous lesions. To address this issue, only the uniEGMs mapped before the first ablation attempt were utilized in this current study. Thirdly, some level of subjectivity was inevitably involved in the evaluation of the morphology of steep notches. However, patients in whom it was difficult to discriminate the steep notches were rare in this study, although this remains a limitation of this approach. Finally, in this current study, the PVC burden before the ablation procedures was based solely on 24-h Holter monitoring. This short monitoring interval may skew the interpretation of the procedural outcomes because PVC burden can vary from day to day, so 7-day Holter monitoring would be a more adequate tool for assessing the true PVC burden.
In conclusion, the combination of N-uniEGM with activation and pace mapping can enhance the accuracy in localizing the effective ablation target during mapping and ablation of idiopathic VOT-PVCs, which should reduce the ablation time. In challenging cases in which only few PVCs occur during the operation or the morphology of the electrogram for pacing mapping and spontaneous PVC morphology does not show perfect consistency, the N-uniEGM provides a useful guiding value in the ablation procedure.
Footnotes
Declaration of conflicting interest: The authors declare that there are no conflicts of interest.
Funding: This study was funded by a grant to Dr Liu from the 1351 Personal Training Plan of Beijing Chao-Yang Hospital (no. CYMY-2017-03).
ORCID iD: Ming-Yang Gao https://orcid.org/0000-0002-8304-7909
References
- 1.Lamba J, Redfearn DP, Michael KA, et al. Radiofrequency catheter ablation for the treatment of idiopathic premature ventricular contractions originating from the right ventricular outflow tract: a systematic review and meta-analysis. Pacing Clin Electrophysiol 2014; 37: 73–78. [DOI] [PubMed] [Google Scholar]
- 2.Kaneshiro T, Suzuki H, Nodera M, et al. Mapping strategy associated with QRS morphology for catheter ablation in patients with idiopathic ventricular outflow tract tachyarrhythmia. Pacing Clin Electrophysiol 2016; 39: 338–344. [DOI] [PubMed] [Google Scholar]
- 3.Bogun F, Taj M, Ting M, et al. Spatial resolution of pace mapping of idiopathic ventricular tachycardia/ectopy originating in the right ventricular outflow tract. Heart Rhythm 2008; 5: 339–344. [DOI] [PubMed] [Google Scholar]
- 4.Okumura Y, Watanabe I, Nakai T, et al. A quantitative and qualitative analysis of the virtual unipolar electrograms from non-contact mapping of right or left-sided outflow tract premature ventricular contractions/ventricular tachycardia origins. J Interv Card Electrophysiol 2011; 30: 17–25. [DOI] [PubMed] [Google Scholar]
- 5.Barlow MA, Klein GJ, Simpson CS, et al. Unipolar electrogram characteristics predictive of successful radiofrequency catheter ablation of accessory pathways. J Cardiovasc Electrophysiol 2000; 11: 146–154. [DOI] [PubMed] [Google Scholar]
- 6.Sorgente A, Epicoco G, Ali H, et al. Negative concordance pattern in bipolar and unipolar recordings: an additional mapping criterion to localize the site of origin of focal ventricular arrhythmias. Heart Rhythm 2016; 13: 519–526. [DOI] [PubMed] [Google Scholar]
- 7.Merino JL, Jiménez-Borreguero J, Peinado R, et al. Unipolar mapping and magnetic resonance imaging of “idiopathic” right ventricular outflow tract ectopy. J Cardiovasc Electrophysiol 1998; 9: 84–87.9475581 [Google Scholar]
- 8.Asso A, Pascual ED, López M, et al. Catheter ablation of repetitive monomorphic ventricular tachycardia from left ventricular outflow tract guided by unipolar mapping. J Interv Card Electrophysiol 2000; 4: 435–439. [DOI] [PubMed] [Google Scholar]
- 9.Stevenson WG, Soejima K. Recording techniques for clinical electrophysiology. J Cardiovasc Electrophysiol 2005; 16: 1017–1022. [DOI] [PubMed] [Google Scholar]
- 10.Man KC, Daoud EG, Knight BP, et al. Accuracy of the unipolar electrogram for identification of the site of origin of ventricular activation. J Cardiovasc Electrophysiol 1997; 8: 974–979. [DOI] [PubMed] [Google Scholar]
- 11.Spach MS, Barr RC, Serwer GA, et al. Extracellular potentials related to intracellular action potentials in the dog Purkinje system. Circ Res 1972; 30: 505–519. [DOI] [PubMed] [Google Scholar]
- 12.Joshi S, Wilber DJ. Ablation of idiopathic right ventricular outflow tract tachycardia: current perspectives. J Cardiovasc Electrophysiol 2005; 16: S52–S58. [DOI] [PubMed] [Google Scholar]
- 13.Latchamsetty R, Yokokawa M, Morady F, et al. Multicenter outcomes for catheter ablation of idiopathic premature ventricular complexes. JACC Clin Electrophysiol 2015; 1: 116–123. [DOI] [PubMed] [Google Scholar]
- 14.Fichtner S, Senges J, Hochadel M, et al. Safety and efficacy in ablation of premature ventricular contraction: data from the German ablation registry. Clin Res Cardiol 2017; 106: 49–57. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Shen Y, Yin R, et al. The safety of catheter ablation for premature ventricular contractions in patients without structural heart disease. BMC Cardiovasc Disord 2018; 18: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soejima Y, Aonuma K, Iesaka Y, et al. Ventricular unipolar potential in radiofrequency catheter ablation of idiopathic non‐reentrant ventricular outflow tachycardia. Jpn Heart J 2004; 45: 749–760. [DOI] [PubMed] [Google Scholar]
- 17.Ribbing M, Wasmer K, Mönnig G, et al. Endocardial mapping of right ventricular outflow tract tachycardia using noncontact activation mapping. J Cardiovasc Electrophysiol 2003; 14: 602–608. [DOI] [PubMed] [Google Scholar]
- 18.Niu GD, Feng TJ, Jiang CL, et al. Predictive value of unipolar and bipolar electrograms in idiopathic outflow tract ventricular arrhythmia mapping and ablation. J Cardiovasc Electrophysiol 2018; 29: 900–907. [DOI] [PubMed] [Google Scholar]
- 19.Gami AS, Noheria A, Lachman N, et al . Anatomical correlates relevant to ablation above the semilunar valves for the cardiac electrophysiologist: a study of 603 hearts. J Interv Card Electrophysiol 2011; 30: 5–15. [DOI] [PubMed] [Google Scholar]