Abstract
Objectives
To evaluate the diagnostic performance of real-time elastography (RTE) combined with fine-needle aspiration (FNA) biopsy in identifying malignant thyroid nodules.
Methods
This was a single-centre, retrospective study and involved patients who had underogone partial or total thyroidectomy from 01 January 2014 to 31 December 2018 at our centre. Eligible patients were at least18 years of age, had reliable grayscale ultrasound imaging results, a RTE evaluation and had undergone a FNA biopsy.
Results
Data were available from 437 patients. A high RTE score was a significant independent risk factors for malignancy. RTE plus FNA biopsy increased diagnostic accuracy compared with either method alone and the sensitivity and specificity of the combined model were 86% and 78%, respectively.
Conclusions
The combination of RTE imaging with FNA biopsy improves the diagnostic performance in differentiating benign and malignant thyroid nodules.
Keywords: Thyroid nodule, real-time elastography, fine-needle aspiration biopsy, grayscale ultrasound, diagnostic accuracy
Introduction
Thyroid nodules can be detected by imaging in more than 60% of the general population.1 Moreover, the incidence is increasing mainly due to the more frequent use of sensitive ultrasound imaging and fine-needle aspiration (FNA) of thyroid nodules.1,2 Although most thyroid nodules are asymptomatic and benign, the main challenge for their management is to rule out malignancy, which is present in approximately 5% of all nodules.3
Grayscale ultrasound imaging is often used to identify thyroid nodules and based on the American Thyroid Association (ATA) guidelines nodules can be categorized into five suspicion patterns according to their grayscale features.4 These features include: hypoechoic component; irregular margins; microcalcification; taller than wide shape; extrathyroidal extension; interrupted rim calcifications with soft tissue extrusion.4 However, ultrasound patterns show diagnostic sensitivity for only 27–63% of differentiating malignant nodules.5 FNA biopsy is recommended for nodules with an intermediate suspicion of malignancy on ultrasound imaging. 3 Although a classification scheme for reporting thyroid FNAs has been proposed (i.e., Bethesda System for Reporting Thyroid Cytopathology),6 its accuracy has been challenged.7,8 Therefore, there is a need for a definitive evaluation in the clinical workup for thyroid nodules.
Real-time elastography (RTE) is a non-invasive and inexpensive diagnostic technique that measures tissue stiffness and has been widely used in the detection of superficial tissues including thyroid, prostate and breast.9–11 The sensitivity and specificity of elastography have been reported to be better in differentiating between benign and malignant thyroid nodules than conventional technologies.12 Furthermore, studies have demonstrated the value of RTE in improving diagnostic accuracy by complementing grayscale ultrasound results.13–15
Therefore, the purpose of this retrospective study was to evaluate the diagnostic potential of RTE in combination with FNA biopsy by comparing results with those from either method alone, in patients who had undergone surgical resection and had final histopathological diagnosis of thyroid nodules.
Patients and methods
Study population
In this single-centre, retrospective study, consecutive patients who underwent partial or total thyroidectomy from 01 January 2014 to 31 December 2018 at our centre were selected for the analysis. Eligible patients were at least 18 years of age, had reliable grayscale ultrasound imaging results, a RTE evaluation and had undergone a FNA biopsy. Patients without a RTE or who had unreliable results were excluded from the analysis. The FNA cytology results were graded using the Bethesda system for reporting cytopathology.6 Histopathology of the resected nodules was confirmed by two experienced independent pathologists. For patients with multiple nodules, a FNA had been performed on the most suspicious thyroid nodules based on Thyroid Imaging Reporting and Data System (TI-RADS) grading of their ultrasound features.16 The study was approved by the Medical Ethics Committee of Lishui Municipal Central Hospital, Lishui, China (July 31, 2018) and written informed consent was obtained from each patient.
Grayscale ultrasonography and RTE
Results from the Grayscale ultrasound were categorized using Kwak-TIRADS parameters.16 According to these guidelines, standard, suspicious features of malignant thyroid nodules included: those with a solid structure, hypo-echogenicity, irregular margins, microcalcifications and taller than wide shape (aspect ratio >1). TI-RADS categories were determined as follows: TI-RADS 3, no suspicious features; TI-RADS 4a, 1 suspicious feature; TI-RADS 4b, 2 suspicious features; TI-RADS 4c, 3 or 4 suspicious features; TI-RADS 5, 5 suspicious features.
The same radiologist performed RTE using an Hitachi HiVision Preirus ultrasound machine and graded the results according to previously established criteria for elastography.17,18 The grades were: ES1, green nodules with the same elasticity as the adjacent tissue; ES2, 50–90% of the nodule were green, and evenly distributed elasticity; ES3, blue-green nodules, that showed uneven elasticity distribution; ES4, nodules >90% blue, representing hard thyroid tissue with little elasticity. While ES1 and ES2 suggested benign nodules, ES3 and ES4 suggested malignant nodules.
Ultrasound-guided FNA and surgical management
Diagnosed or suspicious malignancies had been treated by surgical resection. These included nodules with intermediate or malignant cytology (Bethesda 3–6) and nodules with at least two suspicious ultrasound patterns (TI-RADS 4b or higher). Nodules causing compression or symptoms of hyperthyroidism and retrosternal nodules also had undergone surgical resection. The surgical procedures used (i.e., lobectomy, sub-total or total thyroidectomy with or without regional lymph node dissection) were based on multifocality, nodule size and results of frozen section examination.
Statistical analyses
All analyses were performed using Statistical Package for Social Sciences (SPSS®) for Windows® release 25.0 (IBM Corp. Armonk, NY Released 2017). The data were expressed as the mean ± standard deviation (SD). Differences between groups were analysed using a χ2 test. The odds ratio (OR) and 95% confidence interval (CI)s for relationships between each variable and malignant thyroid nodules were calculated using uni- and multivariate binary logistic regression methods. Diagnostic potential of different models was evaluated using receiver operating characteristic (ROC) curves. A P-value <0.05 was considered to indicate statistical significance.
Results
Patient Characteristics
In total, 3103 patients who underwent partial or total thyroidectomy and had confirmed pathology results were included in the study. Of these, 512 patients had FNA cytology results and grayscale ultrasound images. Following exclusion of patients without evaluable RTE results, the final sample size was 437 patients (Figure 1). Most patients were female (332, 76%). The mean age ± SD was 47.2 ± 10.7 years (range, 14–80 years) and 323 (74%) patients were younger than 55 years. Most patients (297, 68%) had nodules no larger than 1cm (Table 1). Malignant nodules confirmed by post-operative histopathology were found in 397 (91%) patients, while 40 (9.2%) patients had benign disease. Seven patients had hyperthyroidism, compression or retrosternal lesions.
Figure 1.
Flowchart of patient selection.
Table 1.
Patient characteristics, ultrasound and fine needle aspiration findings (n = 437).
| Characteristics | Number of patients | Benignn (%) | Malignantn (%) | Statistical significance |
|---|---|---|---|---|
| Sex | ||||
| Male | 105 | 9 (9) | 96 (91) | ns |
| Female | 332 | 31 (9) | 301 (91) | |
| Age | ||||
| <55 | 323 | 25 (8) | 298 (92) | ns |
| ≥55 | 114 | 15 (13) | 99 (87) | |
| Nodule size | ||||
| ≤1 cm | 297 | 20 (7) | 277 (93) | P = 0.011 |
| >1 cm | 140 | 20 (14) | 120 (86) | |
| Internal components | ||||
| Solid | 407 | 33 (8) | 374 (92) | P = 0.005 |
| Mixed | 30 | 7 (23) | 23 (77) | |
| Hypo-echogenicity | ||||
| Yes | 429 | 35 (8) | 394 (92) | <0.001 |
| No | 8 | 5 (63) | 3 (38) | |
| Margins | ||||
| Irregular | 351 | 24 (7) | 327 (93) | P = 0.001 |
| Regular | 86 | 16 (19) | 70 (81) | |
| Calcification | ||||
| Micro | 252 | 17 (7) | 235 (93) | P = 0.042 |
| Macro or no | 185 | 23 (12) | 162 (88) | |
| Aspect ratio | ||||
| >1 | 130 | 6 (5) | 124 (95) | P = 0.032 |
| ≤1 | 307 | 34 (11) | 273 (89) | |
| Elastography (ES) scores | ||||
| 1 | 17 | 4 (24) | 13 (77) | P < 0.001 |
| 2 | 126 | 29 (23) | 97 (77) | |
| 3 | 250 | 7 (3) | 243 (97) | |
| 4 | 44 | 0 | 44 (100) | |
| Benign (1–2) | 143 | 33 (23) | 110 (77) | P < 0.001 |
| Malignant (3–4) | 294 | 7 (2) | 287 (98) | |
| TI-RADS | ||||
| 3 | 1 | 1 (100) | 0 | P < 0.001 |
| 4a | 10 | 6 (60) | 4 (40) | |
| 4b | 33 | 1 (3) | 32 (97) | |
| 4c | 330 | 32 (10) | 298 (90) | |
| 5 | 63 | 0 | 63 (100) | |
| FNA biopsy | ||||
| Benign (1–2) | 67 | 23 (34) | 44 (66) | P < 0.001 |
| Intermediate (3–4) | 62 | 10 (16) | 52 (84) | |
| Malignant (5–6) | 308 | 7 (2%) | 301 (98) | |
Abbreviations: TI-RADS, Thyroid Imaging Reporting and Data System assessment categories; FNA, fine needle aspiration; ns, not statistically significant.
Ultrasound and cytologic characteristics of the thyroid nodules
Of the 437 patients, 407 (93%) had solid nodules, the remaining 30 patients had nodules with mixed components. Most nodules were hypoechogenic (429, 98%) and the majority had irregular margins (351, 80%), microcalcification (252, 58%) and aspect ratio ≤1 (307, 70%) (Table 1). The prevalence of malignancy in nodules categorized as TI-RADS 4a, 4b, 4c were 40%, 97% and 90%, respectively. All TI-RADS 5 nodules were confirmed as malignant in a post-operative histopathological diagnosis. Solidity, hypo-echogenicity, irregular margin, microcalcification and aspect ratio >1 were ultrasound features significantly associated with malignancy.
From FNA biopsies, 67 (15%) patients were diagnosed as having benign nodules (Bethesda 1–2), 62 (14%) intermediate (Bethesda 3–4), and 308 (71%) malignant (Bethesda 5–6). The rates of histology confirmed malignancy were 66%, 84%, 98% in each Bethesda cytological category (Table 1).
Diagnostic performance of RTE in differentiating malignant thyroid nodules
Representative images of each of the four RTE grades (i.e., ES1, 2, 3 and 4) are shown in Figure 2. There were significantly more malignancies in the high elastography grades (ES 3–4) (P < 0.001; Table 1). The number of patients in each ES category and the prevalence of malignant nodules are shown in Figure 3.
Figure 2.
Representative images of the four real-time elastography (RTE) scores. Higher scores correspond to a greater probability of malignancy.17,18
(a) ES1, green nodules with the same elasticity as the adjacent tissue; (b) ES2, 50–90% of the nodule were green, and evenly distributed elasticity; (c) ES3, blue-green nodules, that showed uneven elasticity distribution; (d) ES4, nodules >90% blue, representing hard thyroid tissue with little elasticity.
Figure 3.
Prevalence of thyroid cancer stratified by real-time elastography (RTE). The columns represent the total number of patients and the line represents the percentage of malignancies for each respective group.
Univariate logistic regression showed that high RTE values, nodule size >1 cm, solidity, hypo-echogenicity, irregular margins, microcalcification and aspect ratio >1 were significant predictors of malignancy (Table 2). These risk factors were included in a multivariate logistic regression analysis to determine if they independently predicted malignancy. Results showed that high RTE values (P < 0.001), hypo-echogenicity (P = 0.018) and irregular margins (P = 0.043) were independent risk factors associated with malignant thyroid nodules (Table 2).
Table 2.
Univariate and multivariate logistic regression analysis of clinical and ultrasound predictors for malignant thyroid nodules (n = 437).
| Risk Factors | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| Odds Ratio (95% CI) | Statistical significance | Oddds Ratio (95% CI) | Statistical significance | |
| Sex (male vs. female) | 0.91 (0.42, 1.98) | ns | – | – |
| Age (<55 vs. ≥55 years) | 0.55 (0.28, 1.09) | ns | – | – |
| Nodule size (≤1 vs. >1 cm) | 0.43 (0.23, 0.84) | P = 0.012 | 0.47 (0.22, 1.01) | ns |
| Internal components (mixed vs. solid) | 3.45 (1.38, 8.64) | P = 0.008 | 1.00 (0.31, 3.23) | ns |
| Hypo echogenicity (No vs. Yes) | 18.8 (4.30, 81.8) | P < 0.001 | 8.85 (1.46, 53.7) | P = 0.018 |
| Margins (regular vs. irregular) | 3.11 (1.57, 6.17) | P = 0.001 | 2.35 (1.03, 5.39) | P = 0.043 |
| Calcification (macro or no vs. micro) | 1.96 (1.02, 3.79) | P = 0.045 | 1.37 (0.63, 2.97) | ns |
| Aspect ratio (≤1 vs. >1 cm) | 2.57 (1.05, 6.29) | P = 0.038 | 2.14 (0.76, 6.00) | ns |
| Elastography scores (Benign [1–2] vs. Malignant [3–4]) | 12.3 (5.29, 28.6) | P <0.001 | 5.02 (2.81, 8.98) | P < 0.001 |
Abbreviations: ns, not statistically significant.
Diagnostic performance of RTE and FNA biopsy in differentiating malignant thyroid nodules
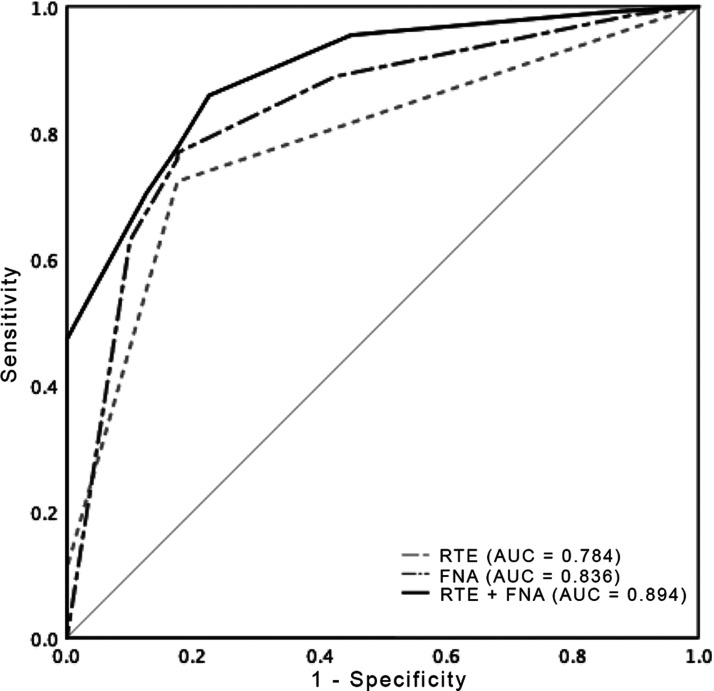
Combination model of RTE and FNA biopsy (RTE plus FNA) was scored by the sum of ES and Bethesda grades. The ROC area under curve values for RTE, FNA and RTE plus FNA were 0.78, 0.84 and 0.89, respectively (Figure 4). The cut-off value of RTE plus FNA was chosen as 5.5 for best performance (2–5 as negative; 6–10 as positive). Based on this cut-off point, the sensitivities of RTE, FNA and RTE plus FNA were, 72%, 77% and 86%, respectively. The specificities of RTE, FNA and RTE plus FNA were, 83%, 83% and 78%, respectively. The negative predictive values for RTE, FNA and RTE plus FNA were 23%, 26% and 36%, respectively (Table 3). These results suggested that the combination of RTE and FNA cytology increased the diagnostic accuracy.
Figure 4.
The receiver operating characteristic (ROC) curve and area under the curve (AUC) for real-time elastography (RTE), fine needle aspiration (FNA) and combination model of RTE plus FNA.
Table 3.
Comparison of the diagnostic performance of real-time elastography (RTE), fine needle aspiration (FNA) and a combination model of RTE plus FNA for imaging of thyroid nodules.
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | |
|---|---|---|---|---|
| RTE scores (3,4 vs 1,2) | 72.3% | 82.5% | 97.6% | 23.1% |
| FNA (Bethesda scores 4,5,6 vs 1,2,3) | 76.8% | 82.5% | 97.8% | 26.4% |
| RTE + FNA | 85.9% | 77.5% | 97.4% | 35.6% |
Abbreviations: RTE, real-time elastography; FNA, fine needle aspiration.
Discussion
Results from this retrospective study in a cohort 437 patients with thyroid nodules who had undergone surgical resection, suggest that the combination of RTE with FNA biopsy results may improve the diagnostic effectiveness in identifying malignancy. We found 91% patients who had undergone thyroid surgery had histology-proven malignant nodules. These findings are greater than those reported previously in studies evaluating the diagnostic performance of elastography in thyroid tissues.17,19 The difference in results is probably because although we included patients with confirmed pathology, surgery had been performed in cases with intermediate/malignant cytology grading (i.e., Bethesda 3-6/ TI-RADS ≥4b) and those patients with hyperthyroidism, compression or retrosternal lesions.
Although FNA biopsy is recommended as a routine diagnostic method for suspicious thyroid nodules, its precision has been questioned.7,8 Problems in accuracy are thought to have occurred primarily because the diagnostic criteria of individual international society guidelines vary.20 Indeed, studies have shown that the sensitivity of FNA biopsy ranges from 54–90% and its specificity ranges from 60–98%.21 One study showed that there was no difference in malignancy rates among patients who went directly to surgery after a single intermediate FNA diagnosis, patients who had two successive intermediate FNA results and patients who had a benign aspirate following an intermediate result. 7At our centre we use the TI-RADS grading system and for cases with two or more ultrasound malignant features (i.e., TI-RADS 4b or higher) we recommend surgical biopsy even if FNA cytology is benign. Therefore, because of our protocol, we had included 40 (9%) patients with benign disease which yielded a negative predictive value of 26%. We found the sensitivity and specificity of FNA biopsy to be 77% and 83% respectively.
As a non-invasive and inexpensive imaging technique, RTE, which examines the stiffness and hardness of tissues, has been suggested as a useful, alternative tool in the examination of malignant thyroid nodules. The diagnostic performance of RTE in differentiating between benign and malignant thyroid nodules has been found to be comparable with shear wave elastography (SWE) another ultrasound-based technique, but with a statistically higher specificity.12 Furthermore, the addition of RTE had been shown to improve the diagnostic accuracy of TI-RADS.22 In suspected prostate cancer, RTE has been found to be useful in combination with Prostate Cancer Gene 3 (PCG3) scores as a pre-biopsy diagnostic indicator to reduce the number of prostate biopsies.23 In addition, RTE in combination with magnetic resonance imaging (MRI) has been found to increase precision of localizing prostate cancer lesions compared with MRI alone.24
We found that a high RTE score (i.e., thyroid nodules with poor elasticity) as well as hypo-echogenicity and irregular margins were significant independent risk factors associated with malignant thyroid nodules. Although higher than in two previous studies,12,25 the sensitivity and specificity of RTE in this study were 72% and 83% respectively. Interestingly, we observed that while the combination of RTE high score and FNA cytology had a higher sensitivity compared with cytology alone (86% vs. 77%), specificity was lower (78% vs. 83%). However, the ROC area under curve values were greatest for the combination of RTE and FNA biopsy compared with either method alone which indicated better diagnostic accuracy.
The study had some limitations. Firstly, it was a retrospective and in a single-centre and so the results require validation in a prospective study involving a large cohort. Secondly, RTE imaging is highly operator-dependent, and so some measurement bias may have existed. Thirdly, we only included patients who underwent surgery. Therefore, selection bias may have affected the evaluation of accuracy of the different diagnostic tools. Finally, we did not evaluate the ultrasound features or RTE of nodules that did not undergo biopsy and so a controlled study is warranted.
To our knowledge, this is the first study to evaluate RTE with FNA biopsy in the identification of malignancy in thyroid nodules. Our results suggest the complement of RTE to FNA biopsy could improve diagnostic effectiveness in identifying malignancy in thyroid nodules. The algorithm used in this study is a simple summation of the ES grade plus the Bethesda grade. We believe that this model is easily applicable and will effectively augment diagnostic accuracy compared with either technique alone. We suggest that the model may be made more accurate by applying an optimized weighting and cut-off value for both parameters.
Acknowledgements
The authors thank all members of the Department of Thyroid and Breast Surgery, Lishui Municipal Central Hospital for assistance with surgeries and data collection.
Footnotes
Declaration of conflicting interests: The authors declare that there are no conflicts of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Lishui Critical Research Project (grant number: 2015zdxk08).
ORCID iD: Feng Cheng https://orcid.org/0000-0002-1977-9910
References
- 1.Singh Ospina N, Iñiguez-Ariza NM, Castro MR. Thyroid nodules: diagnostic evaluation based on thyroid cancer risk assessment. BMJ 2020; 368: l6670. [DOI] [PubMed] [Google Scholar]
- 2.Raue F, Frank-Raue K. Thyroid Cancer: Risk-Stratified Management and Individualized Therapy. Clin Cancer Res 2016; 22: 5012–5021. [DOI] [PubMed] [Google Scholar]
- 3.Floridi C, Cellina M, Buccimazza G, et al. Ultrasound imaging classifications of thyroid nodules for malignancy risk stratification and clinical management: state of the art. Gland Surg. 2019; 8(Suppl 3):S233–S244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016; 26: 1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Remonti LR, Kramer CK, Leitao CB, et al. Thyroid ultrasound features and risk of carcinoma: a systematic review and meta-analysis of observational studies. Thyroid 2015; 25: 538–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cibas ES, Ali SZ. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2017; 27: 1341–1346. [DOI] [PubMed] [Google Scholar]
- 7.VanderLaan PA, Marqusee E, Krane JF. Clinical outcome for atypia of undetermined significance in thyroid fine-needle aspirations: should repeated FNA be the preferred initial approach? Am J Clin Pathol 2011; 135: 770–775. [DOI] [PubMed] [Google Scholar]
- 8.Erivwo P, Ghosh C. Atypia of Undetermined Significance in Thyroid Fine-Needle Aspirations: Follow-Up and Outcome Experience in Newfoundland, Canada. Acta Cytol 2018; 62: 85–92. [DOI] [PubMed] [Google Scholar]
- 9.Magri F, Chytiris S, Chiovato L. The role of elastography in thyroid ultrasonography. Curr Opin Endocrinol Diabetes Obes 2016; 23: 416–422. [DOI] [PubMed] [Google Scholar]
- 10.Thomas A, Fischer T, Frey H, et al. Real-time elastography–an advanced method of ultrasound: First results in 108 patients with breast lesions. Ultrasound Obstet Gynecol 2006; 28: 335–340. [DOI] [PubMed] [Google Scholar]
- 11.Junker D, De Zordo T, Quentin M, et al. Real-time elastography of the prostate. Biomed Res Int 2014; 2014: 180804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu X, Liu Y, Qian L. Diagnostic potential of real-time elastography (RTE) and shear wave elastography (SWE) to differentiate benign and malignant thyroid nodules. Medicine (Baltimore) 2017; 96: e8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trimboli P, Guglielmi R, Monti S, et al. Ultrasound Sensitivity for Thyroid Malignancy Is Increased by Real-Time Elastography: A Prospective Multicenter Study. J Clin Endocrinol Metab 2012; 97: 4524–4530. [DOI] [PubMed] [Google Scholar]
- 14.Mansor M, Okasha H, Esmat S, et al. Role of Ultrasound Elastography in Prediction of Malignancy in Thyroid Nodules. Endocr Res 2012; 37: 67–77. [DOI] [PubMed] [Google Scholar]
- 15.Zhang B, Tian J, Pei S, et al. Machine Learning-Assisted System for Thyroid Nodule Diagnosis. Thyroid 2019; 29: 858–867. [DOI] [PubMed] [Google Scholar]
- 16.Kwak JY, Han KH, Yoon JH, et al. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology 2011; 260: 892–899. [DOI] [PubMed] [Google Scholar]
- 17.Rivo-Vázquez Á, Rodríguez-Lorenzo Á, Rivo-Vázquez JE, et al. The use of ultrasound elastography in the assessment of malignancy risk in thyroid nodules and multinodular goitres. Clin Endocrinol (Oxf) 2013; 79: 887–891. [DOI] [PubMed] [Google Scholar]
- 18.Itoh A., Ueno E., Tohno E., et al. Breast disease: clinical application of US elastography for diagnosis. Radiology 2006; 239: 341–350. [DOI] [PubMed] [Google Scholar]
- 19.Azizi G, Keller J, Lewis M, et al. Performance of Elastography for the Evaluation of Thyroid Nodules: A Prospective Study. Thyroid 2013; 23: 734–740. [DOI] [PubMed] [Google Scholar]
- 20.Ha EJ, Na DG, Baek JH, et al. US Fine-Needle Aspiration Biopsy for Thyroid Malignancy: Diagnostic Performance of Seven Society Guidelines Applied to 2000 Thyroid Nodules. Radiology 2018; 287: 893–900. [DOI] [PubMed] [Google Scholar]
- 21.Magri F, Chytiris S, Zerbini F, et al. Maximal stiffness evaluation by real-time ultrasound elastography, an improved tool for the differential diagnosis of thyroid nodules. Endocr Pract 2015; 21: 474–481. [DOI] [PubMed] [Google Scholar]
- 22.Pei S, Zhang B, Cong S, et al. Ultrasound Real-Time Tissue Elastography Improves the Diagnostic Performance of the ACR Thyroid Imaging Reporting and Data System in Differentiating Malignant from Benign Thyroid Nodules: A Summary of 1525 Thyroid Nodules. Int J Endocrinol 2020; 2020: 1749351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nygard Y, Haukaas SA, Halvorsen OJ, et al. A positive Real-Time Elastography (RTE) combined with a Prostate Cancer Gene 3 (PCA3) score above 35 convey a high probability of intermediate- or high-risk prostate cancer in patient admitted for primary prostate biopsy. BMC Urol 2016; 16: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brock M, Loppenberg B, Roghmann F, et al. Impact of real-time elastography on magnetic resonance imaging/ultrasound fusion guided biopsy in patients with prior negative prostate biopsies. J Urol 2015; 193: 1191–1197. [DOI] [PubMed] [Google Scholar]
- 25.Tian W, Hao S, Gao B, et al. Comparing the Diagnostic Accuracy of RTE and SWE in Differentiating Malignant Thyroid Nodules from Benign Ones: a Meta-Analysis. Cell Physiol Biochem 2016; 39: 2451–2463. [DOI] [PubMed] [Google Scholar]