Abstract
Background
Clinicians caring for patients with Multiple Sclerosis (MS) need improved biomarkers to aid them in disease management.
Objective
We assessed the predictive value of the candidate biomarker CXCL13 index in comparison to oligoclonal bands (OCBs) and CSF neurofilament light (NfL) concentration, examining the ability of each biomarker to predict future disease activity in clinically and radiologically isolated syndromes, relapsing-remitting MS, and progressive MS.
Methods
Matched serum and CSF samples were obtained from 67 non-inflammatory neurologic disease patients and 67 MS patients. CSF and serum CXCL13 and CSF NfL were analyzed by Luminex and ELISA, respectively. CXCL13 data were also analyzed as CSF/serum ratios and indices. Electronic medical records were accessed to determine diagnosis, CSF profiles, and disease activity after the lumbar puncture.
Results
Among CXCL13 measures, CXCL13 index was the best predictor of future disease activity in MS patients (AUC = 0.82; CI = 0.69–0.95; p = 0.0002). CXCL13 index values were significantly elevated in activity-positive MS patients compared to activity-negative patients (p < 0.0001). As a single predictor, CXCL13 index outperformed both OCBs and CSF NfL in sensitivity, specificity, and positive and negative predictive value, for future disease activity in MS patients. Moreover, combining CXCL13 index and CSF NfL status improved sensitivity and predictive values for disease activity in MS patients.
Conclusions
The CXCL13 index is an excellent candidate prognostic biomarker for disease activity in patients with MS.
Keywords: Cerebrospinal fluid, multiple sclerosis, CXCL13, biomarkers
Introduction
Clinically isolated syndrome (CIS) may be an isolated neuroinflammatory event or the first occurrence of relapsing-remitting multiple sclerosis (RRMS).1 Early treatment may be considered to lower the risks of future episodes in CIS patients, delaying conversion to clinically definite MS. Conversely, there appears to be no benefit to treating CIS patients who will remain monophasic. Thus, the challenge is to identify those CIS patients at higher risk of future disease activity.2 In other forms of disease within the MS spectrum, such as primary-progressive MS (PPMS), secondary-progressive MS (SPMS), or radiologically isolated syndrome (RIS), the ability to predict future disease activity would also be helpful in management. Ideally, a molecular biomarker could assist in addressing this challenge.
A logical candidate biomarker would be a molecule(s) involved in B lymphocyte biology since B cells are highly involved in MS pathogenesis.3 CSF oligoclonal bands (OCBs) have been successfully used in initial diagnosis,4 but their predictive value for future neuroinflammation is suboptimal.5 An alternative candidate may be the conventional lymphoid chemokine CXCL13, as CXCL13 is aberrantly elevated in CSF from MS patients and its intrathecal production was recently shown to provide diagnostic and prognostic value in MS patients.6 In contrast to measuring only CSF CXCL13, quantifying intrathecal synthesis of CXCL13 (CXCL13 index -ICXCL13), similar to use of the IgG index in MS patients, corrects for differences in both serum CXCL13 and blood-CSF barrier (BCSFB) integrity between MS patients7–9 which can influence passive transfer of serum CXCL13 into the CSF. In non-inflammatory neurological diseases and normals, CXCL13 may be produced in the periphery, but not intrathecally.
In the current study, we aimed to test the hypothesis that ICXCL13 is a powerful predictor of future disease activity in MS patients. We sought to compare ICXCL13’s predictive ability to other biomarkers, including various CXCL13 measures (serum, CSF, CSF/serum ratio), the diagnostic biomarker OCB, and the emerging biomarker neurofilament light (NfL) in patients with MS.
Methods
Specimens
Patients at Dartmouth-Hitchcock Medical Center (DHMC) underwent diagnostic lumbar puncture (LP) during routine care. Matched sera were obtained within a few hours of the LP. All patients included in the study underwent a full diagnostic work-up, including history, examination, routine CSF/serum analyses (DHMC), and OCB determination and immunoglobulin G (IgG) index (Mayo Clinic; Rochester, MN).
Standard protocol approvals, registrations, and patient consents
Written informed consent was obtained from all study participants for the inclusion of their CSF and serum into the DHMC’s Department of Neurology CSF biobank. The study adhered to the Declaration of Helsinki and was approved by the ethical standards committee at DHMC (STUDY00029241).
Patient selection
A provisional diagnosis was given at the time of the LP and later confirmed at the time of analysis by verifying the patient’s diagnosis in DHMC’s electronic medical record system. Demyelinating diseases within the MS spectrum based on the revised 2017 McDonald Criteria10 -CIS, RRMS, RIS, PPMS, and SPMS- were generally considered as “MS”. For patients with an initial demyelinating event, the diagnosis at the time of the LP prior to imaging and OCB determination was used; thus, if a patient presented with an initial demyelinating event and had a lumbar puncture performed at this time, they would be diagnosed with CIS10 regardless of future MRI and OCB findings near the time of presentation, even if MRI and OCB findings near the time of the lumbar puncture fulfilled McDonald 2017 criteria for RRMS.10 27 of the 41 CIS patients met McDonald 2017 criteria for RRMS after evaluation of their CIS episode, the majority meeting dissemination in time criteria by the presence of oligoclonal bands in the CSF.
Inclusion criteria for this study included age 18-75 years, no corticosteroid therapy 30 days before the LP, and determination of OCBs and IgG index.
Sixty-seven patients within the MS spectrum met the inclusion criteria (Table 1). Five patients had a follow-up of less than 0.5 years and were excluded from activity-bases analyses, including the only patient in the study on an immunomodulatory treatment at the LP.
Table 1.
Patient demographics and CSF profiles.
| NIND | CIS | PPMS | RIS | RRMS | SPMS | |
|---|---|---|---|---|---|---|
| n | 67 | 41 | 13 | 4 | 8 | 1 |
| Gender, F/M | 51/16 | 29/12 | 9/4 | 4/0 | 5/3 | 0/1 |
| Age (mean ± SD) | 45 ± 9.4 | 40 ± 12.9 | 50 ± 14.1 | 39 ± 8.9 | 34 ± 8.3 | 68 |
| Disease duration prior to LP in years (mean ± SD) | N/A | 1.8 (3.55) | 5.1 (5.9) | 0.0 (N/A) | 1.3 (2.0) | 15 |
| # patients with follow-up ≥0.5 yr | N/A | 36 | 13 | 3 | 8 | 1 |
| Follow-up (yrs)* (mean ± SD) | N/A | 2.6 ± 1.5 | 2.4 ± 1.2 | 2.8 ± 0.9 | 2.8 ± 1.8 | 0.5 |
| Treatment type during follow-up:None; Type I; Type II;Type I and Type II | N/A | 20; 9; 5; 2 | 8; 0; 4; 1 | 2; 0; 0; 1 | 1; 1; 0; 6 | 1; 0; 0; 0 |
| Activity during follow- up >0.5 years; Y/N | N/A | 11/24 | 4/7 | 2/1 | 8/0 | 0 |
| OCB positive/negative | 0/63 | 28/13 | 10/3 | 3/1 | 7/1 | 1/0 |
| Qalbumin | 4.0 ± 1.1 | 4.4 ± 1.8 | 5.6 ± 2.6 | 3.6 ± 1.4 | 4.3 ± 1.8 | 8.6 |
| CSF total nucleated cell count per µl (mean ± SD) | 1.0 ± 3.3 | 5.0 ± 7.6 | 3.0 ± 3.4 | 24 ± 45.3 | 7.0 ± 6.7 | 2 |
| CSF protein mg/dL (mean ± SD) | 30.0 ± 6.5 | 33.0 ± 12.5 | 38.0 ± 16.2 | 27.0 ± 4.8 | 35.0 ± 12.2 | 54 |
Age, disease duration prior to LP, follow-up length (years), Qalbumin (CSF albumin/serum albumin), CSF total nucleated cells, and CSF protein. SD = standard deviation. * indicates only patients with a follow-up of ≥0.5 years were included. Treatment types: Type I = interferons and glatiramer acetate; Type II= natalizumab, fingolimod, rituximab, ocrelizumab, teriflunomide, dimethyl fumarate; Type I and Type II treatment indicates patient was on a Type I treatment and shifted to a Type II treatment during the follow-up period.
Sixty-seven NIND patients were considered as controls. NIND diagnoses included: headache syndromes (n = 28), non-inflammatory neuropathies (n = 16), cognitive dysfunction (n = 6), epilepsy (n = 3), and other non-inflammatory neurological illnesses (n = 14) including Arnold-Chiari deformity (n = 1), dizziness (n = 1), Horner syndrome (n = 1), facial numbness (n = 2), fasciculation (n = 1), leg weakness (n = 3), movement disorders (n = 2), post-concussive syndrome (n = 1), and ischemic stroke (n = 2). All NIND patients had non-inflammatory CSF profiles (Table 1).
Determination of inflammatory activity
Clinicians identifying clinical and radiographic disease activity were blinded to the biomarker data. The following definition was used to determine activity after LP: the presence of clinical relapses or new gadolinium-enhancing lesions, or new or unequivocally enlarging T2 lesions.2 Activity status was defined as a binary outcome i.e. activity positive or activity negative, based on the presence or absence of one or more of the above described activity parameters.
In pMS patients, none of the patients experienced progression during the course of the follow-up and activity in these patients was similarly based on clinical or MRI activity.
Disease activity during the follow-up period was present in both untreated patients and patients treated with immunomodulatory or immunosuppressive therapies. Treatment types during the follow-up period are described in Table 1.
Determination of CXCL13 index
Fifty-five MS patients had matched CSF and serum available, while 12 MS patients only had CSF. Sixty-five NIND patients had matched CSF and serum available and were tested for CXCL13. CXCL13 concentrations were determined in undiluted CSF and serum diluted 1:4 by Luminex technology, utilizing a commercial CXCL13 assay (#171BK12MR2; Bio-Rad, Hercules, CA) and following the manufacturer’s directions (lower limit of quantitation 0.7 pg/ml).
CSF/serum ratios were expressed as QCXCL13. ICXCL13 was calculated as (CSFCXC13/serumCXCL13)/(CSFalbumin/serumalbumin) = QCXCL13/Qalbumin.
Determination of CSF NfL levels
CSF NfL levels were analyzed using a commercial sandwich ELISA (Uman Diagnostics, Umea, Sweden) according to the manufacturer’s instructions (n = 64 MS; n = 15 NIND).
Statistical analysis
Non-parametric analyses were used for data analysis, including Mann-Whitney U test, Kruskal Wallis one–way analysis of variance and posthoc corrected Dunn’s multiple comparisons test, and Spearman’s rank-order correlation. Predictive discriminating values were calculated by receiver operating curve (ROC) analysis. Kaplan-Meier survival analysis utilized log-rank (Mantel-Cox) tests to compare survival curves and logrank methods for hazard ratio calculations.
All statistical analyses were performed using GraphPad Prism version 7.00 (GraphPad, San Diego, CA). P-values < 0.05 were deemed to be statistically significant.
Results
Serum and CSF CXCL13 in NIND and MS patients
In MS patients, both serum CXCL13 and CSF CXCL13 concentrations were significantly elevated compared to NIND controls (p < 0.0001; Figure 1; Supplemental Table 1). CSF CXCL13 levels were positively correlated with serum CXCL13 levels in NIND (r = 0.52, p < 0.0001) and MS patients (r = 0.33, p = 0.01). Moreover, CSF levels positively correlated with Qalbumin in MS patients (r = 0.32; p = 0.01). CXCL13 QCXCL13 (CSF/serum ratio) values were also significantly increased in MS patients compared to NIND controls (p < 0.0001). ICXCL13 values were calculated to adjust for Qalbumin variability (Table 1) among patients. ICXCL13 values were significantly higher in MS compared to NIND patients (p < 0.0001), indicating intrathecal CXCL13 synthesis in MS.
Figure 1.
Serum and CSF CXCL13 measures are elevated in MS patients. CXCL13 levels in the serum (a) and CSF (b) and calculated QCXCL13 (c) and ICXCL13 (d) values for NIND or MS patients. MS patients include CIS, RIS, RRMS, and PMS subtypes. Bars indicate median values. Comparisons were made using a Mann Whitney U test. **** P < 0.0001. QCXCL13= (CSF CXCL13/serum CXCL13); ICXCL13 = (CSF CXCL13/serum CXCL13)/(CSF albumin/serum albumin); NIND = non-inflammatory neurologic diseases; MS = Multiple Sclerosis.
CXCL13 performance in discriminating future disease activity
CXCL13 measures were evaluated in MS patients with or without disease activity during the follow-up period (Figure 2(a); Supplemental Table 1). For all activity-based analyses, only patients with a follow-up period of greater than 0.5 years were included (Table 1; Supplemental Table 1). Serum CXCL13 was significantly decreased in activity-positive vs. activity-negative MS patients (p = 0.005). Conversely, CSF CXCL13 (p = 0.02), QCXCL13 (p = 0.0005), and ICXCL13 (p < 0.0001) were all increased in the activity-positive compared to the activity-negative group.
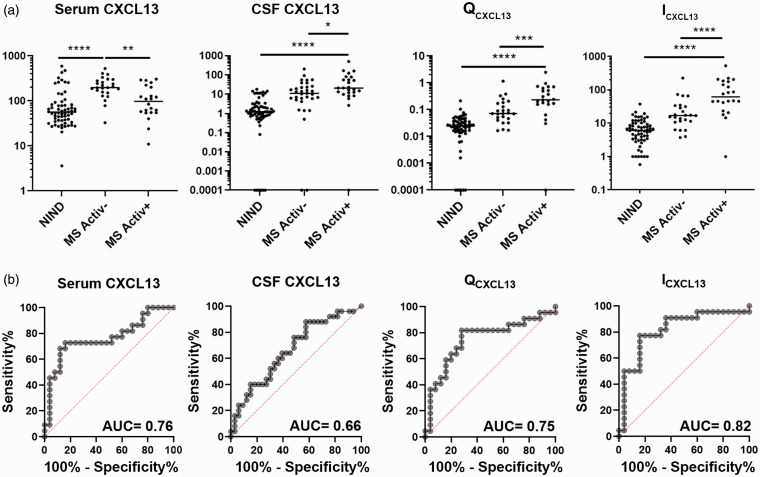
Figure 2.
Elevated ICXCL13 is a strong predictor of disease activity in MS patients. (a) CXCL13 levels in the serum (pg/ml) or CSF (pg/ml) and calculated QCXCL13 and ICXCL13 values for NIND or MS activity-negative (MS Activ-) or activity-positive (MS Activ+) patients. MS patients include CIS, RIS, RRMS, and PMS subtypes. Comparisons of different patient groups were made using a Kruskal-Wallis one-way analysis of variance and Dunn’s multiple comparisons test. Adjusted p values are indicated as *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001. (b) ROC analyses comparing serum CXCL13, CSF CXCL13, QCXCL13, or ICXCL13 values from MS activity-negative and MS activity-positive patients. AUC = Area under the curve.
In MS patients, serum and CSF CXCL13 concentrations, QCXCL13, and ICXCL13 were all tested for their individual ability to discriminate future disease activity by ROC analysis (Figure 2(b)). Among all CXCL13 measures, ICXCL13 was the best predictor of activity,11 generating the highest area under the curve (AUC = 0.82; CI = 0.69–0.95; p = 0.0002) compared to serum CXCL13 (AUC = 0.76; p = 0.002; CI = 0.62–0.90), CSF CXCL13 (AUC = 0.66; p = 0.03; CI = 0.52–0.80), and QCXCL13 (AUC = 0.75; p = 0.004; CI = 0.60-0.89).
ICXCL13 cut-off values in determining future activity
ROC analysis (Figure 2(b)) was also used to determine an optimal cut-off to identify elevated ICXCL13 values in MS patients. A cut-off value of 18.06 (Figure 3(a)) was chosen to maximize the sensitivity (91%; CI = 72–98%) with a minimal reduction in specificity (64%; CI = 44–80%).
Figure 3.
Intrathecal CXCL13 production is elevated in MS patients with evident disease activity. (a) ICXCL13 values among NIND controls and MS patient groups. Optimal cut-off (>18.06; sensitivity 91%, specificity 64%) for determining ICXCL13 elevation was determined by ROC analysis (red line). Disease activity status is indicated by activity-negative (black dots), activity-positive (red dots), or data not available due to a follow-up < 0.5 years (black x). (b) The table displays P-values from a Kruskal-Wallis one-way analysis of variance and Dunn’s multiple comparisons test comparing the different patient groups as indicated. Adjusted P-values < 0.05 were considered significant and are indicated in red. CIS = clinically isolated syndrome with no activity (CIS Act-) or with activity (CIS Act+); RIS = radiologically isolated syndrome; RRMS = relapsing-remitting MS; PMS = progressive MS (n = 13 primary progressive MS; n = 1 secondary progressive MS). C) Kaplan-Meier curve displaying probability of event free survival and time to first activity in months comparing all MS patients (CIS, RIS, RRMS, and PMS) (black), ICXCL13- MS patients (blue) and ICXCL13+ patients (red) with a follow up greater than 0.5 years.
ICXCL13 values were then compared between NIND and MS patient subtypes (Figure 3(a) and (b)). Considering the entire MS population, both CIS and RRMS patients displayed significantly increased ICXCL13 values compared to NIND patients (both p < 0.0001) (Figure 3(b)). All 8 RRMS patients exhibited disease activity during the follow-up period (mean = 3.1 years) and elevated ICXCL13 values. Among the 11 PPMS patients, 3 experienced disease activity after LP (mean follow-up = 2.4 years) and 2 of these patients had elevated ICXCL13. All RIS patients with a follow-up >0.5 years had future disease activity and elevated ICXCL13 values. In CIS patients (mean follow-up = 2.6 years), 9/10 patients who experienced future disease activity had elevated ICXCL13 values. Further analysis of the 28 CIS patients divided by activity status revealed CIS activity-positive (n = 10) patients show significantly increased ICXCL13 values compared to activity-negative CIS patients (n = 18) (p = 0.035) and NIND controls (p < 0.0001) (Figure 3(b)).
ICXCL13 status and time to disease activity
We used Kaplan-Meier survival analysis to assess the time to disease activity among ICXCL13-negative or ICXCL13-positive MS patients. Results showed a significant difference in the survival curves (p = 0.001), indicating a 20-month event free survival of 27% in ICXCL13-positive patients compared to 82% in ICXCL13-negative patients (Figure 3(c)). ICXCL13-positive patients had a significantly higher hazard ratio of developing disease activity over the follow-up period (HR 7.5; CI = 3.2-17.5), demonstrating ICXCL13-positive patients were on average 7.5-times more likely to develop disease activity over the follow-up period.
Association of CSF NfL levels with disease activity and ICXCL13 status
CSF NfL measurements confirmed previous findings of elevated NfL in MS patients compared to NIND controls (p < 0.01) (Figure 4(a)). In our MS patient cohort (mean age = 41.8 ± 1.7) NfL did not correlate with age (r = 0.04; p = 0.73) or activity latency (r =−0.12; p = 0.39). Further analysis of MS patients by OCBs (Figure 4(b)) revealed that OCB-positive MS patients had increased CSF NfL compared to NIND (p = 0.0048), although there was no significant difference between OCB-negative and OCB-positive MS patients. Conversely, MS patients divided by their activity status (Figure 4(c)) or ICXCL13 value (Figure 4(d)) revealed CSF NfL was significantly increased in activity-positive (p < 0.0001) and ICXCL13-positive (p = 0.001) MS compared to NIND patients. CSF NfL levels were also significantly increased in activity-positive vs. activity-negative (p = 0.013) and ICXCL13-positive vs. ICXCL13-negative (p = 0.011) MS patients. A spearman’s correlation of CSF NfL and ICXCL13 values identified a significant positive correlation between ICXCL13 and CSF NfL in MS patients (r = 0.42; CI =0.16-0.62; p = 0.002).
Figure 4.
CSF NfL levels are elevated in ICXCL13+ MS patients. NfL concentrations (pg/ml) in CSF of NIND controls and total MS patients (a) and MS patients analyzed by OCB (b), activity determined by follow-up of ≥ 0.5 years (c), and ICXCL13 (d) negativity or positivity. MS patient subtypes indicated by the following symbols: RIS (○), CIS (▲), RRMS (◊), and PMS (×). Bars indicate median values. Line indicates optimal cut-off of >1105 pg/ml (sensitivity 67%, specificity 69%) for determining elevated NfL levels as determined by ROC analysis comparing MS activity-negative and MS activity-positive patients. P values were determined using a Mann Whitney U test (A) comparing NIND vs total MS (** p < 0.01), RRMS (## p < 0.01), or PMS (^ p < 0.05) and a Kruskal Wallis one-way analysis of variance and Dunn’s multiple comparison test (b–d) comparing NIND, MS activity-negative (MS Activ-), and MS activity-positive (MS Activ+) patient groups (Adjusted p values indicated as * p < 0.05; ** p < 0.01; **** p < 0.0001).
Evaluating the predictive value of individual biomarkers in determining future disease activity
The individual predictive value of ICXCL13, OCBs, and CSF NfL in discriminating disease activity was evaluated by sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for each measure in MS patients (Table 2) and CIS patients only (Table 3). The ICXCL13 cut-off 18.06 was used to determine ICXCL13 positivity.
Table 2.
Sensitivity, specificity, positive (PPV), and negative (NPV) predictive value, false-positive and false-negative in percent (exact 95% confidence interval in brackets) for disease activity in MS patients (CIS, RIS, RRMS, PMS) utilizing CSF ICXCL13, OCB, or NFL status alone, or in combination as indicated. All patients included had a minimum follow-up of ≥0.5 years.
| ICXCL13 (n = 47) | OCB (n = 57) | NfL (n = 56) | NfL+OCB (n = 25) | ICXCL13 + OCB (n = 32) | ICXCL13 + NfL (n = 30) | ICXCL13 + OCB + NfL (n = 19) | |
|---|---|---|---|---|---|---|---|
| Sensitivity | 91 (69–98) | 81 (60–93) | 67 (45–84) | 100 (68–100) | 94 (69–100) | 100 (60–100) | 100 (60–100) |
| Specificity | 64 (43–81) | 30 (16–49) | 69 (50–83) | 50 (24–76) | 47 (22–73) | 72 (46–89) | 55 (25–82) |
| PPV | 69 (49–84) | 48 (33–63) | 62 (41–79) | 61 (36–82) | 67 (45–84) | 71 (44–89) | 62 (32–85) |
| False-positive | 31 (16–51) | 52 (37–67) | 38 (21–59) | 39 (18–64) | 33 (16–55) | 29 (11–56) | 38 (15–68) |
| NPV | 89 (64–98) | 67 (39–87) | 73 (54–87) | 100 (56–100) | 88 (47–99) | 100 (72–100) | 100 (52–100) |
| False-negative | 11 (2–36) | 33 (13–61) | 27 (13–46) | 0 (0–44) | 13 (1–53) | 0 (0–28) | 0 (0–48) |
Table 3.
Sensitivity, specificity, positive (PPV), and negative (NPV) predictive value, false-positive and false-negative in percent (exact 95% confidence interval in brackets) for disease activity in CIS patients utilizing CSF ICXCL13, OCB, or NFL status alone, or in combination as indicated. All patients included had a minimum follow-up of ≥0.5 years.
| ICXCL13 (n = 28) | OCB (n = 35) | NfL (n = 34) | NfL +OCB (n = 18) | ICXCL13 + OCB (n = 18) | ICXCL13 + NFL(n = 17) | ICXCL13 + OCB + NfL (n = 9) | |
|---|---|---|---|---|---|---|---|
| Sensitivity | 90 (54–99) | 64 (32–88) | 55 (25–82) | 100 (20–100) | 80 (30–99) | 100 (40–100) | 100 (50–100) |
| Specificity | 56 (31–78) | 29 (13–51) | 74 (51–89) | 56 (23–85) | 46 (20–74) | 69 (39–90) | 50 (17–100) |
| PPV | 53 (29–76) | 29 (13–51) | 50 (22–78) | 33 (6–76) | 36 (12–68) | 50 (17–83) | 20 (1–70) |
| False-positive | 47 (24–71) | 71 (49–87) | 50 (22–78) | 67 (24–94) | 64 (32–88) | 50 (17–83) | 80 (30–99) |
| NPV | 91 (57–100) | 64 (32–88) | 77 (54–91) | 100 (46–100) | 86 (42–99) | 100 (56–100) | 100 (40–100) |
| False-negative | 9 (0–43) | 36 (12–68) | 23 (9–46) | 0 (0–54) | 14 (1–58) | 0 (0–44) | 0 (0–60) |
An optimal cut-off value for elevated CSF NfL was determined by ROC analysis. A value >1105 pg/ml was established as cut-off (AUC = 0.73; CI = 0.59-0.86; p = 0.003), providing the highest sensitivity (67%) while minimizing reductions in specificity (69%) for discrimination11 of activity-negative vs. -positive individuals.
In MS patients, ICXCL13 outperformed both OCBs and CSF NfL values in predictive ability (Table 2). For ICXCL13, the PPV was 69% and the NPV was 89%, outperforming both OCBs (PPV = 48%; NPV = 67%) and CSF NfL (PPV = 62%; NPV = 73%), with higher sensitivity and specificity for predicting future disease activity. Also, with only two exceptions, MS patients who developed activity had ICXCL13 values >18.06 cut-off (Figure 3(a)), confirming ICXCL13’s strong NPV.
In analyzing only CIS patients, ICXCL13 also proved to be a better predictor of disease activity, with a sensitivity of 90%, a specificity of 56%, PPV of 53% and NPV of 91% (Table 3). OCBs had a lower sensitivity (64%), specificity (29%), PPV (29%) and NPV (64%). CSF NfL only showed better specificity (74%), with lower sensitivity (55%), PPV (50%) and NPV (77%).
ICXCL13 false-positives
The ICXCL13 cut-off 18.06 generated high sensitivity (91%) for identifying patients with future disease activity, although specificity was sacrificed (64%). The cut-off value with higher sensitivity resulted in a slight increase in the percentage of false-positives (31%; Table 2; Figure 3(a)) compared to selecting a cut-off with a more balanced sensitivity (77%) and specificity (72%). To further discern whether an ICXCL13-positive/activity-negative status (false-positive) was due to an insufficient follow-up period, we examined the average follow-up length compared to true-positive MS patients. We also assessed OCBs and CSF NfL profiles of true-positives, true-negatives, false-positives, and false-negatives, to determine if false-positives had a pattern similar to the true-positive or true-negative group (Table 4). ICXCL13 true-positive patients (mean follow-up length = 2.4 years; median = 2.2 years), with 80% OCB-positive, and 60% CSF NfL-positive. The ICXCL13 true-negative patients (mean follow-up = 1.4 years) were also mostly OCB-positive (56%), but in contrast to the true-positives, patients were mainly CSF NfL-negative (87%). In comparison, false-positive patients had a relatively short follow-up period (mean = 2.2 years; median =1.8 years), were mostly OCB positive (88%), and CSF NfL-positive (55%), similar to true-positive patients. Further analysis revealed 62% of ICXCL13-positive patients were also CSF NfL-positive.
Table 4.
Profiles of ICXCL13 true positive, true negative, false positive, and false negative MS patients.
| True positive (n = 20) | True negative (n = 16) | False Positive (n = 9) | False negative (n = 2) | |
|---|---|---|---|---|
| Follow-up (yrs) | 2.4 (0.5–4.8) | 1.4 (0.5–3.2) | 2.2 (0.7–3.3) | 2.7 (2.1–3.2) |
| OCB + | 16/20(80%) | 9/16(56%) | 8/9(88%) | 1/2(50%) |
| CSF NfL + | 12/20 (60%) | 2/16 (13%) | 5/9 (55%) | 1/1 (*) |
Follow-up is expressed as the mean value with the minimum to maximum value range in parentheses. True positive = ICXCL13+Activity+; True negative = ICXCL13−Activity−; False positive = ICXCL13+Activity−; False negative = ICXCL13−Activity+. * indicates only 1 of 2 false-negative patients were tested for CSF NfL concentration. MS patients include CIS, RIS, RRMS, and PMS.
Combined biomarkers in predicting future disease activity
The high number of ICXCL13-positive MS patients who were also CSF NfL-positive, the increase of both CSF NfL and ICXCL13 in activity-positive patients, and the significant positive correlation of ICXCL13 with CSF NfL led us to examine if combining ICXCL13, NfL, and OCB status, would enhance the predictive value for discriminating future disease activity.
In total MS patients, ICXCL13 combined with NfL (Table 2), proved to enhance sensitivity (100%), specificity (72%), PPV (71%), and NPV (100%) compared to either ICXCL13 or NfL alone. ICXCL13+NfL predictive values outperformed NfL+OCBs, ICXCL13+OCBs, and ICXCL13+OCBs+NfL combined predictive analyses. Notably, the addition of OCBs decreased the specificity and PPV for discriminating future disease activity.
In CIS patients, ICXCL13+NfL predictive values enhanced sensitivity (100%) and NPV (100%) (Table 3). PPV was modestly lower in ICXCL13+NfL (50%) vs. ICXCL13 (53%) alone. Specificity was also lower in ICXCL13+NfL (69%) compared to NfL alone (74%). Inclusion of OCBs, i.e., NfL+OCBs, ICXCL13+OCBs, and ICXCL13+OCBs+NfL, reduced PPV for discriminating future disease activity in CIS patients.
Discussion
The course of MS is highly unpredictable, and molecular biomarkers are urgently needed to assist neurologists in caring for these patients,12 especially at the time of the first neurological event. Currently, the primary clinically useful molecular biomarker has been the presence of OCBs, which identifies intrathecally produced IgG, a manifestation of abnormal B cell biology in the CNS.13 Because of the value of OCBs in predicting the development of future neuroinflammatory events,14 OCBs have been incorporated into the McDonald 2017 criteria for the diagnosis of RRMS in patients who would have been classified as CIS with previous criteria. Although the inclusion of OCBs in the revised criteria significantly increases the overall sensitivity, specificity is sacrificed, and the PPV for the conversion of CIS to RRMS using these new criteria is suboptimal.15–17 Thus, there is a clear need for better prognostic biomarkers in MS, both in CIS and other forms of MS.
In the current study, we compared 67 NIND and 67 MS patients to identify an alternative biomarker capable of outperforming both OCBs and CSF NfL in detecting future disease activity. CXCL13, a molecule implicated in B cell biology, is a logical candidate biomarker for predicting disease activity, as B cells are highly involved in MS pathogenesis,18 and depletion therapies targeting B cells, including the CD20-depleting antibodies rituximab, ocrelizumab, and ofatumumab, are effective treatments for MS.3
Multiple laboratories have demonstrated elevations of CXCL13 in the CSF of MS patients relative to controls. However, few studies have calculated the CSF indices for this protein, i.e., the most reliable measure of intrathecal production of an analyte,6,7,19 or tested ICXCL13 as a prognostic biomarker. Neurologists utilize the IgG index as a measure of intrathecal production of IgG; similarly, the CXCL13 index is a measure of intrathecal production of CXCL13. Both measures correct for the diffusion of the analyte in the serum across a variably intact BCSFB as approximated by Qalbumin. In our study CSF CXCL13 levels correlated with serum CXCL13 and Qalbumin, suggesting serum CXCL13 and BCSFB integrity may both impact CSF CXCL13 levels. Additionally, in our analysis, ICXCL13 shows stronger sensitivity, specificity, and predictive values for future disease activity than CSF levels alone.5 The calculation of indices improves the prognostic potential of CXCL13 by taking into consideration the serum CXCL13 concentration and the movement of CXCL13 across the BCSFB. Since MS patients can present varying Qalbumin values,7–9 a correction for it is crucial.
Results in this study demonstrate that in MS patients, an elevated ICXCL13 is predictive of future disease activity as assessed by MRI measures and/or clinical relapses. Conversely, a normal ICXCL13 is highly predictive of the absence of future events. Overall, our present study identifies ICXCL13 as a single biomarker superior to both OCBs and CSF NfL in the prediction of future neuroinflammatory activity in MS patients. Elevated ICXCL13 also outperforms OCBs and CSF NfL in predicting the conversion from CIS to MS.
Although both ICXCL13 and OCBs have good sensitivities, ICXCL13 presents much better specificity and predictive values. Our findings, similar to previous studies,15–17 confirmed that OCBs result in low specificity and PPV for future disease activity. The higher performance of ICXCL13 would remedy the poor specificity and low PPV for the conversion of CIS to MS in the 2017 McDonald criteria throughout the diagnostic process of MS.5,15–17
CXCL13 may also serve as a more relevant biomarker for future disease activity as compared to NfL. Nonetheless, CXCL13 and NfL may have differing, but complementary roles for assessing MS disease course. In our study, we found considering ICXCL13 and NfL status together increased sensitivity, specificity, PPV, and NPV for future disease activity in MS patients. Increased CSF NfL correlates with the conversion from CIS to MS,20 increased clinical disease activity,21 worse brain atrophy,22 and disability progression.22,23 However, it remains unclear if multiple neuroinflammatory events are required to detect elevations in NfL. In MS, CSF NfL analysis may be best utilized as a sensitive measure of ongoing CNS damage and as a predictor of disease progression. Unlike NfL, CXCL13 is not typically produced in the CNS in the absence of inflammatory processes24 and may therefore be a more reliable indicator of neuroinflammation. Although ICXCL13 was the best single predictor of disease activity, improved sensitivity and predictive values utilizing both ICXCL13 and CSF NfL status indicates future studies should examine the utility of combining these two emerging biomarkers to predict disease activity.
Beyond ICXCL13’s predictive value for neuroinflammatory activity, it is interesting to note that ICXCL13 positive patients had significantly higher NfL levels than ICXCL13 negative patients, another indication that elevated intrathecal CXCL13 relates to disease activity and potential CNS damage. Accordingly, in MS patients, elevated CSF CXCL13 is associated with more severe cortical thinning.25 CXCL13 is produced in actively demyelinating MS lesions, but not in chronic inactive lesions or in the CNS of subjects who had no neurological disease.26 Moreover, meningeal B cell aggregates containing CXCL13 have been associated with subpial cortical lesions in progressive MS patients.27
CXCL13, which can be produced by peripherally-derived immune cells or CNS resident cells during neuroinflammation,24,28,29 is crucial for the recruitment, and possibly maintenance, of CXCR5+ immune cells, including B cells, memory T cells26 and follicular helper T cells,30 to sites of inflammation. CXCL13 may also be essential for establishing and maintaining structures resembling ectopic lymphoid follicles within the CNS associated with cortical demyelination, neuronal injury, and worse disease progression.27,31 Our finding that intrathecal production of CXCL13 occurs very early in MS and predicts future disease activity may indicate that the development of these structures arises early in the disease, and not just in the later progressive forms. Thus, the absence of an elevated ICXCL13 early in the disease may predict a low risk of neuroinflammatory events and a lower likelihood of disability progression.32
Our finding, that serum CXCL13 was lower in those patients who experienced future MS activity relative to those patients who did not, was interesting, but difficult to interpret and caution is indicated regarding the use of serum CXCL13 elevations as a readout for disease activity in MS. In the periphery, CXCL13 is expressed on high endothelial venules and is important for recruiting B cells into secondary lymphoid tissue.33 Furthermore, CXCL13 is expressed in germinal center (GC) reactions aiding in GC organization and production of antibody secreting cells.34 Elevations in plasma CXCL13 are linked to germinal center induction in secondary lymphoid tissue in both mouse and macaque studies.35 If serum CXCL13 elevations are indicative of GC responses, this may explain the disparity among findings in the literature as GC are transient structures and elevations in blood will be highly dependent on unpredictable variables. Since it is possible that intercurrent infection, even subclinical infection,36 can elevate serum CXCL13 levels, the role of infections in serum CXCL13 levels and thus ICXCL13 calculations in MS patients needs further study.
ICXCL13 may serve a role in routine clinical practice to assist clinicians in making treatment decisions in patients with MS including selecting optimal responders to therapy, determining therapeutic response, and identifying CIS or MS patients at a higher risk of inflammatory attacks who would benefit from immunomodulators. In a previous study, Alvarez et. al demonstrated ICXCL13 was crucial in identifying “optimal responders” to rituximab.37 Moreover, reductions in ICXCL13 following rituximab treatment associated with decreased biomarkers of tissue destruction including CSF myelin basic protein and NfL, implying ICXCL13 may be useful as an indicator of treatment response. Although further studies are necessary to evaluate the role of ICXCL13 in treatment decisions, these findings provide promising results that ICXCL13 may be an optimal biomarker for monitoring CNS inflammation.
Limitations
Our present study has a few weaknesses: a limited follow-up period after LP, a possible selection bias toward a restricted MS patient population, and findings of elevated ICXCL13 in other inflammatory, although not demyelinating, neurological diseases.6
The average follow-up for all MS patients was 2.6 years, a time window that may be insufficient to effectively monitor the course of MS and which may increase the reported ICXCL13 false-positives (median follow-up = 1.8 years). However, in a recent study, more than 80% of CIS patients who had developed activity defined by either MRI or clinical evidence of relapse had done so by two years.38 Similarly, in our study the majority of MS patients developed activity within the first 20 months of the follow-up period.
Since this was not a controlled study in which all MS patients underwent an LP and were enrolled, there may have been a selection bias toward only some types of MS patients undergoing an LP in routine clinical practice primarily for diagnostic purposes, including those experiencing an initial neurological event or seeking confirmation of diagnosis. For instance, most patients in the CIS group underwent LPs within a few months after the CIS event. However, also in the CIS group, 7 patients underwent LP not for an initial evaluation of a CIS event, but for determination of whether a CSF profile consistent with MS still existed after a long period, an average of 6 years after the CIS, with no activity. The inclusion of these patients with a long disease duration prior to the LP elevated the mean disease duration after the LP, and also increased the proportion of patients without activity. Predictive values generated therefore best apply to patient populations undergoing diagnostic LPs as a whole, and might not be relevant to all subpopulations within the MS umbrella. Additionally, international differences in lumbar puncture practice also might influence predictive values of ICXCL13. Future studies should address both limitations by utilizing more extended clinical follow-up periods and randomized clinical trial methodology to include more PPMS, RRMS and SPMS patients at various disease stages.
Finally, elevated intrathecal CXCL13 synthesis may occur in other neuroinflammatory diseases, e.g., Lyme neuroborreliosis and viral meningoencephalitis.6 Generally, MS is clinically distinguishable from other neuroinflammatory and non-demyelinating diseases. In patients where this distinction is difficult, further analysis of CSF and serum biomarkers using Luminex assays for cytokines and immunoglobulins can be helpful in diagnosis.6
Conclusions
ICXCL13 is an excellent molecular biomarker for the prediction of future disease activity in MS patients. As a single predictor, ICXCL13 outperforms both CSF OCBs and CSF NfL in identifying future disease activity. This study lays the groundwork for future studies examining the utility of ICXCL13 in the management of MS patients.
Supplemental Material
Supplemental material, sj-pdf-1-mso-10.1177_2055217320981396 for Intrathecally produced CXCL13: A predictive biomarker in multiple sclerosis by Krista D DiSano, Francesca Gilli and Andrew R Pachner in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Footnotes
Authors’ contributions: All authors contributed to the study concept and design, to data acquisition and analysis, and to drafting the manuscript and figures.
Conflict of Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. DiSano reports no disclosures. Dr. Gilli has received research support from Biogen, Sanofi-Genzyme, and Hitchcock Foundation. Dr. Pachner has received research support from EMD-Serono, Sanofi-Genzyme, Roche, Novartis, Bornstein Research Fund, and Biogen.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by research grants from EMD-Serono, Bornstein Research Fund, Diamond endowment, Edgerton Fund, and the Hitchcock Foundation.
ORCID iD: Krista D DiSano https://orcid.org/0000-0001-7407-8327
Supplemental material: Supplemental material for this article is available online.
References
- 1.Miller D, Barkhof F, Montalban X, et al. Clinically isolated syndromes suggestive of multiple sclerosis, part I: natural history, pathogenesis, diagnosis, and prognosis. Lancet Neurol 2005; 4: 281–288. [DOI] [PubMed] [Google Scholar]
- 2.Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 2014; 83: 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Negron A, Robinson RR, Stuve O, et al. The role of B cells in multiple sclerosis: current and future therapies. Cell Immunol 2019; 339: 10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arrambide G, Tintore M, Espejo C, et al. The value of oligoclonal bands in the multiple sclerosis diagnostic criteria. Brain 2018; 141: 1075–1084. [DOI] [PubMed] [Google Scholar]
- 5.Brettschneider J, Czerwoniak A, Senel M, et al. The chemokine CXCL13 is a prognostic marker in clinically isolated syndrome (CIS). PLoS One 2010; 5: e11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pachner AR, DiSano K, Royce DB, et al. Clinical utility of a molecular signature in inflammatory demyelinating disease. Neurol Neuroimmunol Neuroinflamm 2019; 6: e520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reiber H, Peter JB. Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J Neurol Sci 2001; 184: 101–122. [DOI] [PubMed] [Google Scholar]
- 8.Liebsch R, Kornhuber ME, Dietl D, et al. Blood-CSF barrier integrity in multiple sclerosis. Acta Neurol Scand 1996; 94: 404–410. [DOI] [PubMed] [Google Scholar]
- 9.Castellazzi M, Morotti A, Tamborino C, et al. Increased age and male sex are independently associated with higher frequency of blood-cerebrospinal fluid barrier dysfunction using the albumin quotient. Fluids Barriers CNS 2020; 17: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–173. [DOI] [PubMed] [Google Scholar]
- 11.Unal I. Defining an optimal cut-point value in ROC analysis: an alternative approach. Comput Math Methods Med 2017; 2017: 3762651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziemssen T, Akgun K, Bruck W. Molecular biomarkers in multiple sclerosis. J Neuroinflammation 2019; 16: 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Disanto G, Morahan JM, Barnett MH, et al. The evidence for a role of B cells in multiple sclerosis. Neurology 2012; 78: 823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tintore M, Rovira A, Rio J, et al. Do oligoclonal bands add information to MRI in first attacks of multiple sclerosis? Neurology 2008; 70: 1079–1083. [DOI] [PubMed] [Google Scholar]
- 15.Lee DH, Peschke M, Utz KS, et al. Diagnostic value of the 2017 McDonald criteria in patients with a first demyelinating event suggestive of relapsing-remitting multiple sclerosis. Eur J Neurol 2019; 26: 540–545. [DOI] [PubMed] [Google Scholar]
- 16.Gobbin F, Zanoni M, Marangi A, et al. 2017 McDonald criteria for multiple sclerosis: earlier diagnosis with reduced specificity? Mult Scler Relat Disord 2019; 29: 23–25. [DOI] [PubMed] [Google Scholar]
- 17.van der Vuurst de Vries RM, Mescheriakova JY, Wong YYM, et al. Application of the 2017 revised McDonald criteria for multiple sclerosis to patients with a typical clinically isolated syndrome. JAMA Neurol 2018; 75: 1392–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker D, Marta M, Pryce G, et al. Memory B cells are major targets for effective immunotherapy in relapsing multiple sclerosis. EBioMedicine 2017; 16: 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reiber H. Cerebrospinal fluid–physiology, analysis and interpretation of protein patterns for diagnosis of neurological diseases. Multiple Sclerosis (Houndmills, Basingstoke, England) 1998; 4: 99–107. [DOI] [PubMed] [Google Scholar]
- 20.Arrambide G, Espejo C, Eixarch H, et al. Neurofilament light chain level is a weak risk factor for the development of MS. Neurology 2016; 87: 1076–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lycke JN, Karlsson JE, Andersen O, et al. Neurofilament protein in cerebrospinal fluid: a potential marker of activity in multiple sclerosis. J Neurol Neurosurg Psychiatry 1998; 64: 402–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hakansson I, Tisell A, Cassel P, et al. Neurofilament levels, disease activity and brain volume during follow-up in multiple sclerosis. J Neuroinflammation 2018; 15: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhan A, Jacobsen C, Myhr KM, et al. Neurofilaments and 10-year follow-up in multiple sclerosis. Mult Scler 2018; 24: 1301–1307. [DOI] [PubMed] [Google Scholar]
- 24.Lalor SJ, Segal BM. Lymphoid chemokines in the CNS. J Neuroimmunol 2010; 224: 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magliozzi R, Scalfari A, Pisani AI, et al. The CSF profile linked to cortical damage predicts multiple sclerosis activity. Ann Neurol 2020; 88: 562–573. [DOI] [PubMed] [Google Scholar]
- 26.Krumbholz M, Theil D, Cepok S, et al. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain 2006; 129: 200–211. [DOI] [PubMed] [Google Scholar]
- 27.Magliozzi R, Howell O, Vora A, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 2007; 130: 1089–1104. [DOI] [PubMed] [Google Scholar]
- 28.Phares TW, DiSano KD, Stohlman SA, et al. CXCL13 promotes isotype-switched B cell accumulation to the central nervous system during viral encephalomyelitis. Brain Behav Immun 2016; 54: 128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meinl E, Krumbholz M, Hohlfeld R. B lineage cells in the inflammatory central nervous system environment: migration, maintenance, local antibody production, and therapeutic modulation. Ann Neurol 2006; 59: 880–892. [DOI] [PubMed] [Google Scholar]
- 30.Cunill V, Massot M, Clemente A, et al. Relapsing-remitting multiple sclerosis is characterized by a T follicular cell pro-inflammatory shift, reverted by dimethyl fumarate treatment. Front Immunol 2018; 9: 1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serafini B, Rosicarelli B, Magliozzi R, et al. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol 2004; 14: 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khademi M, Kockum I, Andersson ML, et al. Cerebrospinal fluid CXCL13 in multiple sclerosis: a suggestive prognostic marker for the disease course. Mult Scler 2011; 17: 335–343. [DOI] [PubMed] [Google Scholar]
- 33.Girard JP, Moussion C, Forster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol 2012; 12: 762–773. [DOI] [PubMed] [Google Scholar]
- 34.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol 2012; 30: 429–457. [DOI] [PubMed] [Google Scholar]
- 35.Havenar-Daughton C, Lindqvist M, Heit A, et al. CXCL13 is a plasma biomarker of germinal center activity. Proc Natl Acad Sci U S A 2016; 113: 2702–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galanti M, Birger R, Ud-Dean M, et al. Rates of asymptomatic respiratory virus infection across age groups. Epidemiol Infect 2019; 147: e176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alvarez E, Piccio L, Mikesell RJ, et al. Predicting optimal response to B-cell depletion with rituximab in multiple sclerosis using CXCL13 index, magnetic resonance imaging and clinical measures. Mult Scler J Exp Transl Clin 2015; 1: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rotstein DL, Healy BC, Malik MT, et al. Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA Neurol 2015; 72: 152–158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-mso-10.1177_2055217320981396 for Intrathecally produced CXCL13: A predictive biomarker in multiple sclerosis by Krista D DiSano, Francesca Gilli and Andrew R Pachner in Multiple Sclerosis Journal – Experimental, Translational and Clinical