Abstract
Stroke mortality and morbidity is expected to rise. Despite considerable recent advances within acute ischemic stroke treatment, scope remains for development of widely applicable neuroprotective agents. Glucagon-like peptide-1 receptor agonists (GLP-1RAs), originally licensed for the management of Type 2 Diabetes Mellitus, have demonstrated pre-clinical neuroprotective efficacy in a range of neurodegenerative conditions. This systematic scoping review reports the pre-clinical basis of GLP-1RAs as neuroprotective agents in acute ischemic stroke and their translation into clinical trials. We included 35 pre-clinical studies, 11 retrospective database studies, 7 cardiovascular outcome trials and 4 prospective clinical studies. Pre-clinical neuroprotection was demonstrated in normoglycemic models when administration was delayed by up to 24 h following stroke induction. Outcomes included reduced infarct volume, apoptosis, oxidative stress and inflammation alongside increased neurogenesis, angiogenesis and cerebral blood flow. Improved neurological function and a trend towards increased survival were also reported. Cardiovascular outcomes trials reported a significant reduction in stroke incidence with semaglutide and dulaglutide. Retrospective database studies show a trend towards neuroprotection. Prospective interventional clinical trials are on-going, but initial indicators of safety and tolerability are favourable. Ultimately, we propose that repurposing GLP-1RAs is potentially advantageous but appropriately designed trials are needed to determine clinical efficacy and cost-effectiveness.
Keywords: Acute stroke, neuroprotection, reperfusion, clinical trials, animal models
Introduction
Stroke accounts for 6.5 million deaths per year globally and by 2030 will result in an annual loss of over 200 million disability-adjusted life years.1,2 With an increasing number of strokes occurring in younger patients, alongside an increased number of stroke survivors, the cost of post-stroke care is rising. There is, therefore, significant scope to improve upon the current position.
Considerable advances have been made in acute ischemic stroke (AIS) treatment, notably reperfusion therapies, but these are limited to 10–20% of total stroke patients following careful clinical and radiological selection.3 Even when intravenous thrombolysis and/or endovascular thrombectomy are administered, reduction in disability is highly time dependent.4,5 Scope remains for further improvement, especially for patients who are unsuitable for reperfusion therapies or those within remote environments.
Using simpler clinical selection processes, neuroprotective therapies could bring benefits to a wider patient group. Neuroprotectants could also enhance the benefits of reperfusion therapies by preservation of the ischemic penumbra and reduction in ischemic reperfusion injury. Despite many demonstrating pre-clinical potential, a suitable agent has not yet been identified by translational studies.6 There remains a multitude of factors affecting the translation from bench-to-bedside. Namely, animal models are not perfect in their representation of the heterogeneity of clinical stroke.7 Stroke in humans occurs in the context of ageing, co-morbidity (hypertension, diabetes mellitus, atrial fibrillation, pre-existing cerebrovascular disease) and concomitant medication use.8 Furthermore, factors such as gender, cerebral blood flow, body temperature and glycemic status may influence stroke mechanism and outcomes associated with therapy.6,9–11
Glucagon-Like Peptide-1 (GLP-1) receptor agonists are gaining increasing momentum as possible neuroprotective agents in AIS. GLP-1 is an incretin hormone. Alongside its role in insulin secretion from the pancreas and glucagon suppression, it also crosses the blood-brain barrier (BBB) and promotes synaptic function, enhances neurogenesis, reduces apoptosis and protects neurons from oxidative stress.12 GLP-1 is produced in the brain and receptors are distributed throughout the central nervous system.13 GLP-1 Receptor Agonists (GLP-1RAs), licensed for Type 2 Diabetes Mellitus (T2DM) have already demonstrated pre-clinical neuroprotective efficacy in Alzheimer’s Disease and clinical trials in neurodegenerative conditions are ongoing.12,14
The aim of this systematic scoping review is to report the pre-clinical basis of GLP-1RAs as neuroprotective agents in AIS and their translation into clinical trials. In addition to describing the characteristics and quality of studies, the objectives are to specifically consider timing of administration, association with glycemic status, neuroprotective outcomes and application to clinical care.
Materials and methods
Eligibility criteria
Pre-clinical: We included pre-clinical in vivo studies which administered naturally occurring GLP-1, a mimetic or analogue, before, during or after stroke induction. Normoglycemic, hyperglycemic and induced T2DM models were included.
Studies were excluded if their only focus was hemorrhagic stroke as this does not reflect the proposed mechanism for how GLP-1 is involved in ischemic tissue injury. Those studies which reported incidence of hemorrhagic transformation as a complication of AIS were included as these reflect post-stroke complications.
Clinical: We included all prospective clinical trials which administered GLP-1RAs before, during or after stroke onset with outcome measures defined to identify neuroprotective efficacy by way of stroke volume reduction or improvement in post-stroke function or mortality. We also included any potential feasibility or safety-based studies in this area.
Our scoping searches identified that very few prospective clinical trials measuring stroke outcomes were available. Pragmatically, we therefore also included all retrospective database analyses of stroke incidence or composite cardiovascular outcomes in patients treated with GLP-1RAs. Furthermore, we included cardiovascular outcome trials (CVOTs) of GLP-1RAs to evaluate the incidence of stroke in this relatively higher risk cohort.
Studies were excluded if their full-text was not available or not published in English. Efforts were made to contact authors directly to obtain any missing articles or data.
Database search strategy
After several initial scoping searches,15 we accessed Web of Science on 19 March 2020 to search MEDLINE, Web of Science core collection, BIOSIS and SciELO from 1 January 2000. Keywords were EITHER ‘GLP(-)1, glucagon like peptide(-)1, exenatide, liraglutide, lixisenatide, albiglutide, dulaglutide, semaglutide’ AND EITHER ‘stroke, CVA, cerebrovascular, h(a)emorrhage, small vessel disease’. Articles were cross-referenced and references were searched to identify further studies of interest.
All articles/studies were screened independently in an unblinded, standardised manner by MM and HE by way of title and abstract to identify those suitable for full-text review. Queries and disagreements were resolved by discussion. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were applied. Pre-clinical studies were appraised according to Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines,16 and the updated Stroke Therapy Academic Industry Roundtable Preclinical Recommendations (STAIR) guidelines.7 Data supporting the findings of this review are available from the corresponding author upon reasonable request.
Results
Study selection
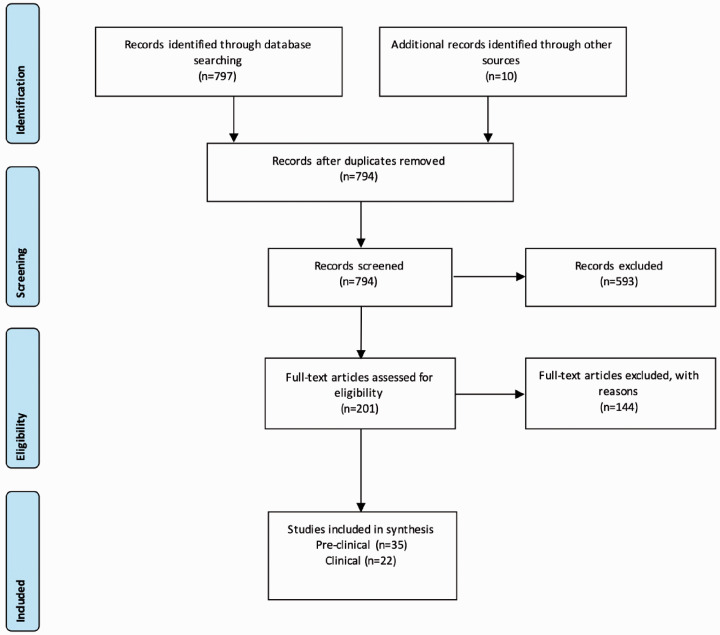
The literature search identified 797 results (see Figure 1) alongside 10 from other sources. After removal of duplicates, this left 794 for screening. We excluded 593 articles based upon title and review of abstract leaving 201 full text articles to review. In total, 35 preclinical studies, 11 retrospective database studies, 7 cardiovascular outcome trials and 4 prospective clinical studies met the inclusion criteria.
Figure 1.
PRISMA flow chart demonstrating the selection of studies.
Pre-clinical studies
Characteristics of included studies
As shown in Table 1, 35 pre-clinical studies were included within this review. Studies were completed between 2009 and 2020. Studies were predominantly based upon mouse and rat models of stroke; however, one study utilised a gerbil model.17 Stroke induction was either via transient (range 30–120 min) or permanent common carotid (CCAO) or middle cerebral artery occlusion (MCAO). Most studies induced unilateral occlusion in keeping with spontaneously occurring stroke onset in humans, but six studies utilised a bilateral occlusion model. Cerebral ischemia was induced by either ligation, filament occlusion or ablation of the relevant artery.
Table 1.
Overview of the pre-clinical studies.
| Author | Year | Animal model | Co-morb. | Drug | Admin route | Sub-studies | Stroke induction (time/min) |
Administration time (min) |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-ischemia | Post-ischemia | Main outcomes (NP – Neuroprotective) | |||||||||
| GLP-1RA administered prior to stroke onset (including chronic pre-treatment) | |||||||||||
| Li et al.18 | 2009 | Rat | Ex-4 | i.c.v. | u.l tMCAO (60) | 15 | NP – ↓ infarct volume and improves functional outcome | ||||
| Briyal et al.19 | 2012 | Rat | Ex-4 | i.p. | BQ123 | u.l. pMCAO | BD – 7 days | NP – ↓ infarct volume, ↓ motor deficit, ↓ oxidative stress parameters (↓ MDA, ↑ GSH, ↑ SOD) NP via ↓ oxidative stress and independent of endothelin receptor. | |||
| Briyal et al.29 | 2014 | Rat | DM | Liraglutide | s.c. | Non-diabetic, insulin | u.l. pMCAO | OD – 14 days | NP – ↓ infarct volume, ↓ motor deficit, ↓ oxidative stress parameters (↓ MDA, ↑ GSH, ↑ SOD), ↓ apoptosis (↑ Bcl-2, ↓ Bax). NP in both non-diabetic & DM models with similar infarct volume reduction, insulin treatment did not reduce infarct volume. | ||
| Chien et al.44 | 2015 | Rat | DM | PEx-4 | s.c. | Non-diabetic, Ex-4 | b.l tCCAO (10) & hypotension | OD – 14 days | NP – ↓ brain oedema, ↓ cognitive deficit, ↓ oxidative stress, ↑ cerebral blood flow. PEx-4 more NP than Ex-4 in DM. | ||
| Zhang et al.45 | 2015 | Mouse | Pro-GLP-1 | i.p. | shRNA | u.l. tMCAO (90) | OD – 7 days | NP – ↓infarct volume, ↓ neurological deficit, ↓ apoptosis (↑ Bcl-2, ↓ Bax). NP not glucose dependent. NP blocked by shRNA (suggesting GLP-1R mediated NP) | |||
| Jiang et al.38 | 2016 | Rat | DM | rhGLP-1 | i.p. | nimodipine, insulin | u.l. tMCAO (90) | TDS – 14 days | NP in DM– ↓ infarct volume (more than insulin group), ↓ neurological deficit, ↓ brain injury markers (↓ S100B, ↓ NSE, ↓ MBP) | ||
| Zhang et al.46 | 2016 | Mouse | DMB | p.o. | Ex-4, Ex-9-39, shRNA | u.l. tMCAO (60) | 30 | NP given orally– ↓ infarct volume, ↓ neurological deficit, ↓ apoptosis (↑ Bcl-2, ↓ Bax). No impact on plasma insulin and glucose levels in non-diabetic mice. DMB activation of GLP-1R different to Ex-4. NP blocked by shRNA but not by Ex-9-39. | |||
| Zhang et al.22 | 2016 | Mouse | Ex-4 | i.n. | Intraperitoneal Ex-4, shRNA | u.l. tMCAO (90) | OD – 7 days | NP given intranasally – ↓ infarct volume, ↓ neurological deficit, ↓ apoptosis (↓ caspase-3) Intranasal route produced ↑ CNS concentration than intraperitoneal No impact on plasma insulin and glucose levels and NP was blocked by shRNA suggesting NP is GLP-1R mediated. | |||
| Kim et al.23 | 2017 | Rat | Ex-4 | i.c.v | Ex-9-39 | u.l. MCAO (60) | 30 | NP – ↓ infarct volume (by up to 75%), ↓ neurological deficit, ↓ oxidative damage/inflammation (↓ COX-2, ↓ PE2) | |||
| Li et al.48 | 2017 | Rat | OXM | i.c.v. | u.l. tMCAO (60) | 15 | NP – ↓ infarct volume, ↑ locomotive activity | ||||
| Abdel-latif et al.42 | 2018 | Rat | DM | Lixisenatide | i.p. | glimepiride | b.l. tCCAO (30) | OD – 14 days | NP – ↓ infarct volume, ↓ neurological deficit, ↓ oxidative stress (↓ MDA, ↑ GSH, ↑ catalase), ↓ inflammation/apoptosis (↓ caspase-3, ↓ TNF-α). Lixisenatide more NP than glimepiride. | ||
| Fang et al.39 | 2018 | Rat | DM | rhGLP-1 | i.p. | nimodipine | u.l. tMCAO (90) | TDS – 14 days | NP – ↓ infarct volume, ↓ neurological deficit, ↓ oxidative stress parameters (↓ MDA,↑ GSH, ↑ SOD), ↓ apoptosis (↑ Bcl-2, ↓ Bax, ↓ caspase-3), ↑EAAT2, | ||
| Filchenko et al.31 | 2018 | Rat | DM | Liraglutide | s.c. | Non-diabetic, metformin | u.l. tMCAO (30) | OD – 7 days | NP – ↓ infarct volume, ↓ neurological deficit only in non-DM model, NP not associated with glycemic control amelioration (Metformin not NP) | ||
| Gad et al.51 | 2020 | Rat | Lixisenatide | i.p. | 0.7 and 7 nmol/kg lixisenatide | b.l. tCCAO (60) | OD – 14 days | NP – ↓ oxidative stress parameters (↓ MDA, ↑ GSH, ↑ SOD), ↓ apoptosis (↑ Bcl-2, ↓ Bax, ↓ caspase-3), ↓ inflammatory markers (↓ MPO, ↓ IL-1β, ↓ TNF-α), ↑ viable hippocampal neurons on histological staining. Higher dose Lixisenatide ↑ NP | |||
| GLP-1RA administered prior to and following stroke onset | |||||||||||
| Hyun Lee et al.17 | 2011 | Gerbil | Ex-4 | i.p. | b.l. tCCAO (5) | 120 | 60 | NP – ↓ neurological deficit, ↑ GLP-1R immunoreactivity, ↓ microglial activation | |||
| Darsalia et al.20 | 2012 | Rat | DM | Ex-4 | i.p. | 0.1, 2 or 5µg/kg of Ex-4 | u.l. tMCAO (90) | BD – 4 weeks | BD –2–4 weeks | NP – ↓ infarct volume (no difference between 2 & 4 weeks post MCAO), ↓ microglial infiltration but marginal effect on activation, ↑ stem cell proliferation & neuroblast formation. NP is dose dependent. | |
| Jia et al.21 | 2015 | Rat | Ex-4/Catapol | i.c.v. | Ex-9-39 | u.l. tMCAO (60) | 15 | 0 | NP – ↓ infarct volume, ↓ neurological deficit, ↑ hippocampal β-endorphin expression, NP blocked by Ex-9-39, β-endorphin anti-serum and naloxone | ||
| Deng et al.30 | 2018 | Rat | DM | Liraglutide | i.p. | Non-DM | u.l. pMCAO | BD – 7 days | BD – 7 days | NP- ↓ infarct volume, ↓ neurological deficit, ↓ oxidative stress (↑ SOD), ↓ inflammation (↓ MPO), ↑ Nrf2, ↑ HO-1 (antioxidative stress signalling pathway) No significant difference in NP between DM & non-DM | |
| GLP-1RA administered following stroke onset (including delayed administration) | |||||||||||
| Teramoto et al.24 | 2011 | Mouse | Ex-4 | t.v. | u.l tMCAO (60) | 0,60,180 | NP – ↓ infarct volume, ↓ neurological deficit, ↓ oxidative stress, ↓ inflammation (↓ microglial activation), ↑ intracellular cAMP levels (due to GLP-1R activation). NP probably mediated via raised intracellular cAMP levels | ||||
| Sato et al.32 | 2013 | Rat | Liraglutide | i.p. | u.l. tMCAO (90) | 60 | NP – ↓ infarct volume, ↓ neurological deficit, ↓ oxidative stress, ↑ VEGF in cortex but not in the striatum. | ||||
| Darsalia et al.25 | 2014 | Mouse | DM | Ex-4 | i.p. | 2 month old & 14 month obese/DM mice | u.l. tMCAO (30) | 90, 180 or 270 then OD 1 week | NP – ↑ neuronal survival, ↑ M2 microglial markers Non statistically significant reduction in pro-inflammatory markers. NP in non-DM/DM at 50µg/kg at 90/180 min and at 90 min for 5µg/kg. Stroke volume not affected by Ex-4 administration. Not NP when Ex-4 administered 4.5hrs after stroke onset. NP in both young and old animal models | ||
| Zhao et al.40 | 2015 | Rat | DM | rhGLP-1 | i.p. | nimodipine | u.l. tMCAO (120) | 0 | NP – ↓ infarct volume, ↑ neuronal survival, ↓ oxidative stress (↓ MDA, ↑ GSH, ↑ SOD), ↓ nitric oxide damage (iNOS, eNOS) Statistically significant reduction in blood glucose in high dose group (no hypoglycemia) | ||
| Han et al.47 | 2016 | Rat | GLP-1/GIP (DA) | i.p. | GLP-1RA (SA) | u.l. tMCAO (60) | 60 | NP – ↓ infarct volume, ↓ neurological deficit, ↓ apoptosis (↑ Bcl-2, ↓ Bax), ↓ nitric oxide damage (iNOS). DA more NP than SA. | |||
| Chen et al.26 | 2016 | Mouse | Warfarin & Ex-4 | t.v. | P13K inhibitor | u.l. tMCAO (45) | 0 | NP – ↓ infarct volume, ↓ neurological deficit, ↑ BBB integrity, ↓ oxidative stress (↓ 8-OHdG, ↓ HHE), ↓ inflammation, NP blocked by P13K inhibitor. Ameliorated warfarin associated hemorrhagic transformation. | |||
| Kuroki et al.27 | 2016 | Mouse | DM | Ex-4 | i.p. | insulin | u.l. tMCAO (60) | 60 | Hyperglycemia associated with ↑ infarct volume, ↑ cerebral oedema, ↑ hemorrhagic transformation. Ex-4 NP – ↓ infarct volume, ↓ cerebral oedema, ↓ oxidative stress, ↓ inflammation (↓ TNF-α), ↓ MMP-9 activation, ↓ Iba-1 positive microglia, NP in hyperglycemic model. Insulin not NP. | ||
| Li et al.50 | 2016 | Mouse | DM | Ex-4 or liraglutide | i.p. | u.l. tMCAO (60) | 0, 3, 6 or 12hrs | NP – ↓ neurological deficit including ↓ bladder dysfunction, ↑ cerebral microcirculation. ↓ cerebral oedema, ↓ oxidative stress (↓ DHE, ↓ ROS, ↑ SOD), ↓ apoptosis (↓ caspase-3, ↓ TUNEL, ↓ Bax, ↓ PARP, ↑ Bcl-2), ↓ inflammation (↓ ICAM-1, NF-2κB, p50, p65). | |||
| Yang et al.28 | 2016 | Rat | Ex-4 | t.v. | u.l. tMCAO & b.l. tCCAO (60) | 0 & 24hrs | NP – ↓ DNA damage (↑ APE1, ↓ TUNEL) | ||||
| Zhu et al.33 | 2016 | Rat | Liraglutide | s.c. | u.l pMCAO | 1hr, then OD for 1, 3 and 7 days | NP – ↓ infarct volume, ↓ neurological deficit, ↓ apoptosis (↓ ROS, ↓ caspase-3, -8, -9, ↓ PARP, ↑ Bcl-2, ↓ Bax), ↓ DNA damage (↓ TUNEL), ↓ stress related hyperglycemia without causing hypoglycemia. | ||||
| Dong et al.34 | 2017 | Rat | Liraglutide | s.c. | u.l. MCAO (90) | 24hrs, then OD for 4 weeks | NP – ↓ neurological deficit, ↑ GLP-1R expression, ↑ glucose metabolism in ischemic penumbra (18-FDG-PET) ↑ neurovascular remodelling (↑ NeuN, ↑ GFAP, ↑ vWF). NP when first dose delayed by 1 day | ||||
| Abdel-latif et al.41 | 2018 | Rat | Lixisenatide | i.p. | Ex-9-39, pentoxyphylline | b.l. tCCAO (30) | 1 & 24hrs | NP – ↓ infarct volume, ↓ neurological deficit, ↓ apoptosis (caspase-3), ↓ oxidative stress (↓ GSH, ↓ MDA, ↓ catalase, ↓ NO), ↓ inflammation (TNF-α) – these effects not inhibited by GLP-1R antagonist Ex-39 suggesting GLP-1R independent pathway. | |||
| Chen et al.35 | 2018 | Mouse | Liraglutide | i.p. | u.l. pMCAO | 24hrs, then OD for 14 days | NP – ↓ infarct volume, ↓ neurological deficit, ↑ angiogenesis (↑VEGF) No significant difference in blood glucose levels between liraglutide and controls (as expected, stimulated insulin release is in a blood-glucose dependent manner). NP when first dose delayed by 1 day | ||||
| Wang et al.49 | 2018 | Mouse | P7C3 | t.v. | Ex-9-39 | u.l. tMCAO (40) | 120 | NP – ↓ neurological deficit, ↑ neurogenesis (↑ doublecortin, ↑ β-tub3, ↑ adam11, ↑ adamts20, ↓ GSK-3) NP is GLP-1R dependent. | |||
| Yang et al.43 | 2019 | Rat | Semaglutide | i.p. | Blood glucose monitoring | u.l. pMCAO | 2hrs, then alternate days 1,7,14 or 21 days | NP – ↓ infarct volume, ↓ neurological deficit, ↓ neuronal loss, ↓ apoptosis (↑ Bcl-2, ↓ Bax, ↓ caspase-3), ↑ neurogenesis, improved growth factor signalling (↑ ERK1, ↑ ERS-1), No hypoglycemia in normoglycemic model. | |||
| He et al.36 | 2019 | Mice | Liraglutide | i.p. | u.l. pCCAO | OD for 7 days | NP – ↓ neurological deficit, ↓ oxidative stress | ||||
| Zeng et al.37 | 2020 | Rat | Liraglutide | i.p. | P13k inhibitor | u.l pMCAO | 0 then OD | NP – ↓ infarct volume, ↓ cerebral oedema, ↓ apoptosis ( ↑ Bcl-2, ↓ caspase-3, ↓ TUNEL), ↓ inflammation (↓ TNF-α, IL-18, IL-1β, IL-6, COX-2) P13k inhibitor partially reversed NP effects. | |||
SA: single agonist; DA: dual agonist; Ex-4- exendin-4; PEx-4: Exendin-4 loaded poly-microspheres; Ex-9-39: GLP-1R antagonist; pro-GLP-1: long acting GLP-1RA; rhGLP-1: recombinant human GLP-1; DMB: 6,7-dichloro-2-methyl-sulfonyl-3-N-tert-butylaminoquinoxaline; OXM: oxyntomodulin; shRNA: small hairpin RNA (targets GLP-1R); BQ123- endothelin receptor antagonist; P7C3- aminopropyl carbazole compound; i.p.: intraperitoneal; s.c.: subcutaneous; p.o.: oral; i.n.: intranasal; t.v.: transvenous; i.c.v.: intracerebroventricular; OD/BD/TDS: once/twice/three times daily; DM: diabetes mellitus; Non-DM: non diabetes mellitus (healthy); u.l.: unilateral; b.l.: bilateral; MCAO: middle cerebral artery occlusion; CCAO: common carotid artery occlusion; (t/p)MCAO: transient/permanent; SOD: superoxide dismutase; MDA: malondialdehyde; GSH: glutathione; 8-OHdG: 8-hydroxy-2-deoxyguanosine; HHE: 4-hydroxyhexenal; DHE: dihydroethidium; ROS: reactive oxygen species; Bcl-2: B cell lymphoma 2; Bax: Bcl-2-like protein 4; Caspase-3; PARP: poly-ADP ribose polymerase; TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling; S100b: s100- calcium binding protein B; NSE: neuron specific enolase; MBP: myelin basic protein; COX-2: cyclo-oxygenase-2; PE2: Prostaglandin E2; EAAT2: Excitatory amino acid transporter-2; MPO: myeloperoxidase; HO-1: heme oxygenase-1; Nrf2: nuclear factor erythroid-2; NOS: nitric oxide synthase; BBB: blood brain barrier; MMP-9: matrix metalloproteinase-9; ICAM-1: intracellular adhesion molecule-1; NeuN: neuronal nuclear protein (neuronal marker); GFAP: Glial fibrillary acidic protein (glial marker); vWF: von Willebrand factor (endothelial marker); β-tub3: beta tubulin III; GSK-3: glycogen synthase kinase 3; IL: interleukin.
Twelve studies administered exendin-4,17–28 nine used liraglutide,29–37 three used rhGLP-1 (recombinant human GLP-1),38–40 three used lixisenatide41–43 and one study each reported the utility of semaglutide,43 PEx-4 (exendin-4 loaded poly-microspheres),44 proGLP-1 (long acting GLP-1RA),45 DMB (GLP-1R agonist/modulator),46 dual GLP-1/Glucose-dependent Insulinotropic Peptide (GIP) agonist (GLP-1/GIP DA),47 oxyntomodulin (co-activates GLP-1R and glucagon receptor),48 P7C3 (aminopropyl carbazole compound)49 and one study directly compared exendin-4 with liraglutide.50 In eight studies, GLP-1R antagonists, such as Ex-9-39, were administered to study the role of the GLP-1R in neuroprotective mechanisms.19,21–23,41,45,46,49
Two studies investigated multiple doses of GLP-1RAs to compare neuroprotective efficacy and concluded that neuroprotection was dose-dependent.20,51
Most studies administered GLP-1RAs via intraperitoneal, subcutaneous or transvenous routes. However, Zhang et al. reported neuroprotection with both oral DMB46 and intranasal exendin-4.22
Some 14 studies administered GLP-1RAs chronically prior to the onset of stroke. Clinically, this would represent those patients who receive GLP-1RAs as part of routine T2DM management and then go on to experience AIS.18,19,22,23,29,31,38, 39,42,44–46,48,51 Chronic pre-treatment occurred between 14 days prior and 15 minutes prior to stroke onset, for one to three times daily.
A further four studies administered GLP-1RAs prior to and following stroke onset for between 0 and four weeks – with dosing schedules of up to twice daily.17,20,21,30
Most closely aligned with the proposed clinical application of administering treatment to GLP-1RA naïve patients in the hyper-acute AIS setting, 17 studies only administered the medication following stroke onset.24–28,32–37,40,41,43,47,49,50 First dose was delayed between 0 min and 24 h post-onset and continued for up to four weeks.
Alterations in physiological parameters such as body temperature have the potential to impact the outcome of stroke. Li et al. reported no significant change in body temperature when measured before and after treatment with exendin-4.18 We did not identify any GLP-1RA study of AIS which varied temperature between groups. Most studies regulated body temperature within normal physiological parameters during MCAO surgery and animals were housed within a temperature-controlled environment.
Quality of included studies
Pre-clinical study methodology was appraised according to the STAIR 2009 criteria, with a maximum available score of 7. Median score was 3 (range 2–7). Inclusion/exclusion criteria and reporting of potential conflicts of interest/funding were consistently reported in 100% and 94% of studies, respectively. Fewer studies commented on randomisation (54%), allocation concealment (17%) and blinded assessment of outcome (43%). Only four studies (11%) reported performing a sample size calculation.
Reporting of pre-clinical studies was also assessed using the ARRIVE guidelines, with a maximum score of 36. Median score was 22 (range 14–29). Methods, statistical analysis, outcomes and confidence intervals were well reported, as were ethical and funding statements, but the justification for animal models, translation to human biology, limitations and adverse events were frequently not reported.
Impact of hyperglycemia in stroke models
Twelve studies were based on hyperglycemic rodents, with or without a normoglycemic control group.
Kuroki et al. demonstrated that hyperglycemia was associated with an increase in infarct volume, cerebral edema and hemorrhagic transformation in a mouse model that underwent transient, unilateral occlusion of the MCAO for 60 min.27 They reported that intraperitoneal administration of exendin-4 60 min after stroke onset was associated with a reduction in these parameters which was not replicated by insulin monotherapy.
Briyal et al. reported that liraglutide reduced infarct volume by a similar amount in both diabetic and normoglyemic models, but this neuroprotection was again not reproduced in the insulin treatment arm despite resolution of hyperglycemia.29 Deng et al. further confirmed neuroprotection was independent of glycemic status prior to stroke onset.30 Jiang et al. reported that stroke infarct volume in the rhGLP-1 group was significantly reduced when compared to insulin treatment.38 Metformin was also shown to ameliorate hyperglycemia but did not confer the additional neuroprotective outcomes associated with liraglutide.31
One study demonstrated similar neuroprotective outcomes between 2-month-old healthy mice and 14-month-old overweight, diabetic mice treated with exendin-4.25
Whilst GLP-1RAs were associated with a reduction in blood glucose in hyperglycemic models,40 no study reported hypoglycemia when GLP-1RAs were administered to normoglycemic models. This is to be expected as GLP-1RA-associated insulin secretion from the pancreas is glucose dependent.35 Indeed, Zhang et al. concluded that neuroprotection was not glucose dependent.45
Yang et al. monitored blood glucose in rats which underwent intraperitoneal administration of semaglutide starting 2 h following unilateral permanent MCAO occlusion, further demonstrating neuroprotection in the absence of hypoglycemic episodes.43
Infarct volume and neuronal survival
Administration of GLP-1RAs prior to, at the point of, or delayed following stroke onset were associated with reduction in infarct volume. In total, nine studies demonstrated a reduction in infarct volume with exendin-4,18–24,26,27 eight with liraglutide,29–33,35–37 three with rhGLP-1, three with lixisenatide and one with each of PEx-4, proGLP-1, DMB, OXM, GLP-1/GIP DA and semaglutide. Liraglutide was associated with a reduction in infarct volume when the first dose was delayed by up to 1 day.35 Kim et al. reported a reduction in infarct volume by up to 75% in a rat model of transient MCAO. Darsalia et al. reported that exendin-4 administration was associated with a reduction in stroke volume when administered for four weeks prior to, and two to four weeks following stroke onset.20 However, in a later study, whereby they only administered exendin-4 following stroke onset, it did not significantly reduce infarct volume but did reduce overall neuronal loss.25 Reduction in neuronal loss was also reported with semaglutide,43 rhGLP-140 and lixisenatide.51
Cellular function
Apoptosis represents a chain of enzymatic events resulting in programmed cell death.52 Whilst controlled apoptosis is essential for the maintenance of homeostasis, dysregulated apoptosis, for example in within the ischemic penumbra, can result in increased cell death.53 Bcl-2 is an anti-apoptotic protein which functions at least in part by reducing cytochrome C release from the mitochondria. Conversely, Bax is a pro-apoptotic protein and increases cytochrome C levels.52 Increased Bcl-2 and reduced Bax levels, contributing to an increased Bcl-2:Bax ratio and representing reduced levels of apoptosis, have been reported in three studies of liraglutide29,33,37 and one study for each pro-GLP-1,45 rhGLP-1,39 DMB,22 exendin-4,50 lixisenatide,51 GLP-1/GIP DA47 and semaglutide.43 Critically, this reduction of apoptosis was reproduced in normoglycemic models when administered after stroke onset.33,37,43,47
Caspase proteins are also pro-apoptotic and increased levels are associated with higher rates of cell death.54 Liraglutide, exendin-4, lixisenatide, rhGLP-1 and semaglutide have been shown to reduce levels of caspase-3 in pre-clinical models of stroke.33,37,39,41–43,46,50,51 Zhu et al. have also demonstrated reduced levels of caspase 8 and 9 in models administered liraglutide.33
Cleavage of PARP by caspases is a signal of apoptosis and has been implicated in cerebral ischemia.53 Reduction in PARP has been demonstrated with both liraglutide and exendin-4 treatment.33,50
TUNEL assays detect DNA degradation during the later stages of apoptosis and have demonstrated reduced apoptotic activity with liraglutide and exendin-4.37,50,55
GLP-1RA administration following stroke induction has been associated with an anti-inflammatory effect.26 Tumor necrosis factor alpha (TNF-α) is a cytokine synthesised by many cell lines, but particularly by macrophages and microglia.56 TNF-α is involved with the inflammatory response following stroke onset.56 Five studies reported a reduction in TNF-α when compared to controls, three with lixisenatide41,42,51 and one with each of liraglutide37 and exendin-4.27 Indeed, two studies reported reduced microglial activation17,24 whilst a further study by Darsalia et al. reported reduced microglial infiltration, but a marginal effect on activation.20 Reduced levels of other markers of inflammation, such as myeloperoxidase and interleukin, have also been reported.30,37,51
One study reported a non-statistically significant reduction in pro-inflammatory markers.25
There is also deterioration in markers of oxidative stress following AIS. Twelve studies reported an improvement in oxidative stress parameters following GLP-1RA administration in AIS – five studies of exendin-4,19,24,26,27,50 four studies of liraglutide,29,30,32,36 two of lixisenatide41,42 and one of rhGLP.40 Predominantly, studies reported reduced levels of malondialdehyde and increased concentrations of glutathione and superoxide dismutase.
Dong et al. performed 18-FDG PET imaging in a rat model of unilateral transient MCAO. In the animals treated with subcutaneous liraglutide, there was radiological evidence of increased glucose metabolism within the ischemic penumbra.34
Neurovascular function
The neurovascular unit incorporates both cellular and extracellular components involved in the regulation of cerebral blood flow and blood-brain barrier function – it is involved with the maintenance of cerebral homeostasis and control of cerebral blood flow.57
GLP-1RAs have been shown to increase cerebral blood flow following AIS when compared with controls.44 Li et al. reported that cerebral microcirculation is reduced following AIS, but improved to a similar degree within 4–12 h after MCAO in diabetic mice treated with either liraglutide or exendin-4 after stroke induction.50 Blood–brain barrier integrity has also been shown to improve with GLP-1RA treatment.26
Vascular endothelial growth factor (VEGF) is known to promote angiogenesis and protect ischemic neurons from injury, demonstrating a crucial role in the neurovascular remodelling post-AIS.58 Chen et al. demonstrated that intraperitoneal liraglutide therapy, administered 24 h following stroke induction and once daily for 14 days, was associated with increased VEGF expression when compared with normal saline treatment at days 7 and 14 post-AIS in a normoglycemic model.35 Similarly, Sato et al. demonstrated upregulation of VEGF in the cerebral cortex of liraglutide-treated, normoglycemic rats; however, this was not seen within the striatum.32
Markers of neuronal, glial and endothelial function (NeuN, GFAP and vWF respectively) were used by Dong et al. to investigate for evidence of neurovascular remodelling following AIS. They reported that liraglutide, administered to normoglycemic mice at 1 and 24 h following bilateral, transient CCAO, was associated with significantly higher levels of each marker – indicating increased remodelling associated with GLP-1RAs following AIS.34
Jiang et al. reported that levels of brain injury markers (S100B, NSE and MBP) were reduced with both rhGLP-1 and nimodipine treatments.38
Wang et al. investigated for evidence of increased neurogenesis associated with GLP-1R activation. They reported that doublecortin (marker of newborn neuroblasts) and β-tubulin III (marker of neurogenesis and neural progenitor activity) levels were significantly upregulated with P7C3 treatment when compared to controls. They also reported increased levels of cell proliferation markers (ki67, BrdU) and neurodevelopmental markers (adam11, adamts20) alongside markers of increased neurogenesis (GSK-3 inhibition).49
Darsalia et al. also counted ki67 expressing cells to evaluate stem cell proliferation and reported a two-fold greater number of proliferating cells in the exendin-4 treatment group compared with controls at two weeks following stroke induction; however, there was no detectable difference at four weeks. Doublecortin levels were also increased at two weeks in the exendin-4 treatment group, with no difference from controls at four weeks. When using Neun and BrdU expressing neurons to identify new, mature neurons generated after stroke onset, there was no difference between the treatment and control groups.
Doublecortin levels were also shown to be increased with semaglutide treatment.43
Neurological outcomes
Assessment of neurological outcomes was heterogeneous amongst studies, with many utilising a range of different techniques or modified adaptions. Overall, selected neurological assessments examined both cognitive and locomotive neurological function.
Eight studies reported a reduction in post-stroke neurological deficit when treated with liraglutide,29,31–36,50 six with exendin-4,17–19,22,23,26 two with rhGLP-1,38,39 two with lixisenatide41,42 and one study for each of PEx-4,44 proGLP-1,45 DMB,46 OXM,50 GLP-1/GIP DA,47 P7C349 and semaglutide.43
Nine studies reported a reduction in neurological deficit when GLP-1RAs were administered to normoglycemic models at least 1 h after induction of AIS.32–36,41,43,47,49
Notably, Filchenko et al. reported that whilst reduction in neurological deficit was observed in the non-diabetic rat model treated with liraglutide, this trend was not observed in the diabetic groups.31
Potential mechanisms
GLP-1RAs stimulate insulin secretion from the pancreas in a glucose-dependent manner. Neuroprotective efficacy has been demonstrated in both diabetic and normoglycemic models; their neuroprotection is therefore not glucose-dependent.27,31,45
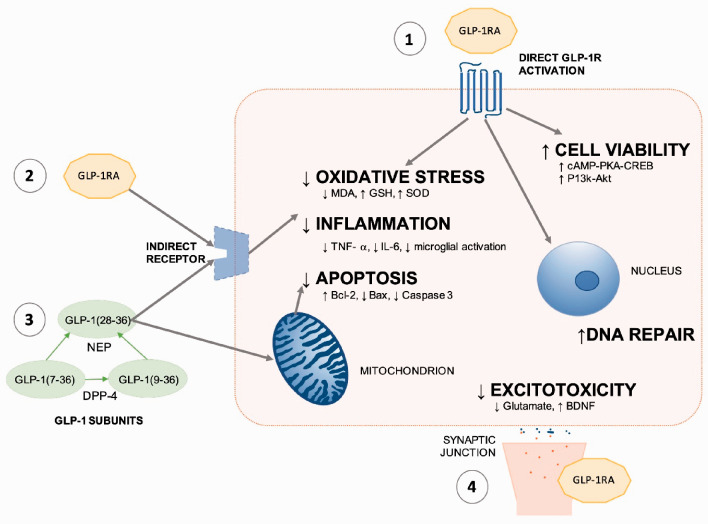
Whilst it is well documented that GLP-1R levels increase following AIS, the precise mechanism of neuroprotection is not completely understood. These have been broadly summarised in Figure 2, reflecting the direct and indirect potential mechanisms. To further understand the role of the GLP-1R, studies have utilised targeted GLP-1R shRNA and the GLP-1R antagonist, Ex-9-39. GLP-1R targeted shRNA has been reported to block the neuroprotective efficacy of GLP-1RAs.22,45,46 However, whilst there are some reports of Ex-3-9 administration blocking neuroprotection,21 suggestive of GLP-1R dependence, other studies did not demonstrated this trend, highlighting the possibility of a GLP-1R independent mechanism.41,46 For example, GLP-1[28–36] is cleaved from endogenous GLP-1; research has shown this cell-permeable nonapeptide to have antioxidant, anti-apoptotic and mitochondrial regulatory properties in pancreatic cells – further research is required to establish its role in neural tissue.59
Figure 2.
Proposed neuroprotective mechanism of action for GLP-1RAs in AIS.1. Direct GLP-1R activation by GLP-1RA. 2. Potential indirect receptor activation by GLP-1RA. 3. GLP-1 sub-units are cell permeable and may affect mitochondrial function, or act on extra-cellular receptors. 4. GLP-1RAs reduce excitotoxicity.
The reduction in apoptosis, oxidative stress and inflammation, alongside increased neurogenesis, angiogenesis and cerebral blood flow is likely to contribute to the neuroprotective outcomes. Multiple signalling cascades have been studied, with P-Akt/p-eNOS,44,50 cAMP/PKA22,45,46,49 and P13k/Akt22,26,33,37,45 pathways implicated in neuroprotection.
Clinical studies
Stroke risk in cardiovascular outcome (safety) trials
All six licensed GLP-1RAs (Table 2) have reported Cardiovascular Outcome Trials (CVOTs). SUSTAIN-6 (semaglutide) and REWIND (dulaglutide) demonstrated a significant reduction in stroke incidence, HR 0.61 (95% CI 0.38–0.99, p = 0.04) and HR 0.76 (95% CI 0.61–0.94, p = 0.017), respectively. LEADER (liraglutide) reported a non-statistically significant reduction in non-fatal stroke incidence, HR 0.89 (95% CI 0.72–1.11, p = 0.30). This was not reported in ELIXA (lixisenatide), EXSCEL (exenatide), HARMONY (albiglutide) and notably not in the oral semaglutide study, PIONEER-6.
Table 2.
GLP-1RA characteristics and stroke incidence in CVOTs.
| ELIXA75 | LEADER76 | SUSTAIN-677 | EXSCEL78 | HARMONY79 | REWIND80 | PIONEER-681 | |
|---|---|---|---|---|---|---|---|
| GLP-1RA | Lixisenatide | Liraglutide | Semaglutide | Exenatide (extended release) | Albiglutide | Dulaglutide | Semaglutide |
| Participants | 6 068 | 9 340 | 3 297 | 14 752 | 9 463 | 9 901 | 3 183 |
| Median follow-up (years) | 2.1 | 3.8 | 2.1 | 3.2 | 1.6 | 5.4 | 1.3 |
| Characteristics | |||||||
| Administration | Subcutaneous | Subcutaneous | Subcutaneous | Subcutaneous | Subcutaneous | Subcutaneous | Oral |
| Dose | 10–20 µg | 1.8 mg | 0.5–1.0mg | 2 mg | 30–50 mg | 1.5 mg | 14 mg |
| Frequency | once-daily | once-daily | once-weekly | once-weeklya | once-weekly | once-weekly | Once-daily |
| Non-fatal stroke incidence | |||||||
| Treatment group – no. (%) | 54 (1.8) | 159 (3.4) | 27 (1.6) | 169 (2.3) | 76 (1.6) | 135 (2.7) | 12 (0.8) |
| Placebo – no. (%) | 49 (1.6) | 177 (3.8) | 44 (2.7) | 193 (2.6) | 91 (1.9) | 175 (3.5) | 16 (1.0) |
| Hazard ratio (95% CI) | 1.10 (0.75–1.63) | 0.89 (0.72–1.11) p = 0.30 | 0.61 (0.38–0.99) p = 0.04 | 0.88 (0.72–1.08) | 0.84 (0.62–1.13) | 0.76 (0.61–0.94) p = 0.017 | 0.74 (0.35–1.57) |
aExenatide standard release requires twice-daily administration.
Gerstein et al. have recently further analysed the REWIND trial. They report that participants who experienced stroke during follow-up were categorised as either ischemic, hemorrhagic, or undetermined and a modified Rankin scale (mRS) was recorded to assess severity. Whilst dulaglutide reduced the incidence of ischemic stroke, it did not affect stroke severity.60
Post-hoc analysis of CVOT trial data has identified sub-groups which may potentially derive greater cardiovascular protection. Mann et al. identified enhanced benefits in patients with chronic kidney disease,61 whilst Kang et al. reported increased benefit in Asian participants.62
Meta-analysis by Barkas et al.63 demonstrated a 13% reduction in the risk of total stroke associated with GLP-1RA treatment versus placebo (RR 0.87, 95% CI 0.78–0.98, p = 0.021).
GLP-1RAs and stroke in real-world datasets
We identified eleven published retrospective studies analysing cardiovascular outcomes of T2DM patients who had been treated with GLP-1RAs (Table 3). These were conducted using data extracted from insurance/national databases.
Table 3.
Retrospective database studies.
| Author | Years studied | Participants included | Groups | Participants on GLP-1RA | GLP-1RA CVD risk (composite) -HR (95% CI) unless otherwise specified | GLP-1RA Stroke Risk -HR(95% CI) | HR Compared to |
|---|---|---|---|---|---|---|---|
| Best et al.82 | 2005–2009 | 383 525 | Exenatide, non-exenatide | 21 754 | 0.80 (0.74–0.86) | NR | Non-exenatide |
| Paul et al.65 | 2005–2009 | 39 225 | Exenatide, exenatide & insulin, insulin | 10 674 | Exenatide 0.50 (0.32–0.79) Exenatide and insulin 0.44 (0.34–0.57) |
Exenatide 0.50(0.28–0.84) Exenatide & insulin 0.38 (0.27–0.54) |
Insulin |
| Gejl et al.83 | 1977–2011 | 10 073 | Liraglutide, exenatide, insulin, biguianides, glitazones, DPP-4i, SFU | 1 749 | Liraglutide OR 0.48 (0.38–0.62) Exenatide OR 1.72 (1.20–2.48) |
NR | N/A |
| Patorno et al.84 | 2005–2013 | 219 810 | GLP-1RA, DPP-4i, SFU, insulin | 29,542 | i) 1.20 (0.76–1.89) ii) 1.05 (0.63–1.74) iii) 1.01 (0.73–1.41) |
NR | i) DPP-4i ii) SFU iii) insulin |
| Ekström et al.85 | 2005–2012 | 20 422 | GLP-1RA, acarbose, insulin, DPP-4i, meglitinide, SU, TZD | 219 | 0.26 (0.10–0.67) | NR | Sulphonylurea |
| Zimmerman et al.66 | 2005–2014 | 105 862 | GLP-1RA, non-GLP-1RA | 8 362 | 0.82 (0.74–0.91) | 0.82 (0.74–0.91) | Non-GLP-1RA |
| Anyanwagu et al.86 | 2006–2014 | 3 586 | Insulin & GLP-1RA, insulin | 1793 | 0.76 (0.41–1.42) | 0.93 (0.54–1.61) | Insulin |
| O’Brien et al.64 | 2011–2015 | 132 737 | GLP-1RA, DPP-4i, SGLT-2i, TZD, SFU, insulin | 11 351 | 0.78 (0.63–0.96) | 0.65 (0.44–0.97) | DPP-4i |
| Svanstrom et al.67 | 2010–2016 | 46 804 | Liraglutide, DPP-4i | 23 402 | 0.90 (0.83–0.98) | 0.88 (0.77–1.01) | DPP-4i |
| Raparelli et al.69 | 2011–2017 | 167 254 | GLP-1RA, DPP-4i, SGLT-2i, SFU | 14 697 | 0.70 (0.62–0.78) Women only: 0.57 (0.48–0.68) |
NR | SFU |
Note: Frison et al.87 excluded as too small to calculate HR. NR: Not recorded; DPP-4i: dipeptidyl peptidase-4 inhibitor; SFU: sulphonylurea; SGLT-2i: sodium-glucose cotransporter-2 inhibitor; TZD: thiazolidinedione.
Recently, O’Brien et al. reported a retrospective cohort study of 132 737 insured adults with T2DM commenced on a second line diabetes medication between 2011 and 2015.64 Adjusting for confounding variables, GLP-1RAs were associated with a reduction in stroke risk (HR 0.65, 95% CI 0.44-0.97) when compared to treatment with Dipeptidyl Peptidase-4 (DPP-4) inhibitors. Reduction in stroke risk was reported in a further two studies65,66; however, both Svantstrom et al. and Anyanwagu et al. did not report this trend.67,68
Raparelli et al. reported an increased GLP-1RA associated reduction in cardiovascular risk in females compared to males.69
Prospective interventional studies
Daly et al. report the first pilot, non-randomised interventional study administering exenatide to eleven patients within 12 h of stroke onset followed by twice-daily injections until discharge.70 Glucose levels were monitored, followed by a three-month modified Rankin score. Although mild nausea and vomiting were common, they reported no serious adverse events nor associated hypoglycemic episodes. They concluded that exenatide was safe and tolerable in AIS patients and did not worsen functional or neurological outcome. This study was not sufficiently powered to assess for improvement in stroke outcomes.
Larsson et al. report on PROLOGUES, a randomised controlled trial comparing pre-hospital administration of exenatide with standard care for hyperglycemia in patients with suspected stroke.71 Nineteen patients were randomised, of an intended forty two, with eight of them receiving exenatide. The trial was stopped early due to slow inclusion—due to baseline glucose criteria and obtaining informed consent. No associated adverse events were reported.
Results are awaited for two prospective studies. The Short-Term EXenatide in Acute ischemic Stroke (STEXAS) trial72 is a randomised, open-label, parallel-group pilot study to investigate the efficacy of exenatide in lowering blood glucose levels in patients with AIS and hyperglycemia. They plan to recruit 30 patients with AIS to either insulin or exenatide for up to 72 h. They will assess feasibility of administration, incidence of hypoglycemia and functional outcomes at three months.
The Treatment with EXenatide in Acute Ischemic Stroke (TEXAIS) trial73 is a three-year, phase 2, multi-centre, prospective, randomised, open-label, blinded end-point trial comparing exenatide to standard care and aims to be powered to detect a change in neurological outcome. Primary end-point is defined as major neurological improvement at seven days (≥8 point improvement in NIHSS score, or score 0–1). In contrast to STEXAS, this study will include patients independent of their glycemic status.
Discussion
As survival following stroke increases, so does the impact of post-stroke disability. Alongside the ongoing development in reperfusion therapies as part of the gold standard of stroke care, a safe, widely applicable, well-tolerated and cost-effective neuroprotective agent becomes increasingly attractive.
We report a systematic review of the pre-clinical research to support the neuroprotective properties of GLP-1RAs targeting the ischemic-reperfusion injury in animal models of AIS. These have been shown to be safe and effective in normoglycemic models and demonstrate neuroprotective outcomes by way of reduced infarct volume, apoptosis, oxidative stress, inflammation and increased neurogenesis, angiogenesis and cerebral blood flow. Crucially, they have demonstrated an improvement in post-stroke functional outcomes, including within both locomotive and cognitive domains.
In keeping with the real-world potential of GLP-1RAs as a neuroprotectant in the management of AIS, pre-clinical trials have now demonstrated reproducible benefits in normoglycemic models with delayed administration up to 24 h after stroke induction.
Their neuroprotective mechanism is not fully understood, with further research required. However, emerging trends towards both GLP-1R-dependent, and independent, pathways have been reported. Following direct comparison studies with metformin and insulin, their effects are due to more than restoration of glucose homeostasis. Additional research is required to fully understand the role of cleaved GLP-1 subunits, including GLP-1[28–36] and could go some way to explain the difference in stroke reduction between GLP-1RAs in CVOTs.
The economic benefits of repurposing existing treatments should not be overlooked, especially as the CVOTs have demonstrated their cardiovascular safety, alongside a reduced incidence of non-fatal stroke. Retrospective database studies show a trend towards a reduction in cardiovascular events, providing an important perspective from ‘real-world’ usage.
Prospective clinical trials of GLP-1RAs in stroke are ongoing, with initial outcomes suggestive of safety, tolerability and that GLP-1RAs are feasibly administered in the AIS cohort.
The comparative effectiveness of reduction in overall cardiovascular risk with newer antidiabetic treatments, such as GLP-1RAs, sodium-glucose co-transporter-2 (SGLT-2) inhibitors and DPP-4 inhibitors is unclear. However, a recent network meta-analysis has shown that only GLP-1RAs reduced non-fatal stroke incidence (OR 0.88, 95% CI 0.77–0.99).74
We acknowledge the limitations of this study. Pre-clinical studies were generally conducted on a homogenous population of young animals without representing the co-morbidities often afflicting the human stroke population. However, one study did demonstrate comparable neuroprotective outcomes in both young mice and older, obese, diabetic models.25
Further research into the impact of factors such as age, hypertension and gender is required. In addition, studies relied on a uniform etiology of stroke onset, vascular territory and duration of occlusion – constants which are not representative of the diverse, heterogenous clinical manifestation of AIS. However, the inter-study differences in stroke induction protocols (e.g. unilateral vs. bilateral, duration and vessel occlusion) do provide some mitigation to this. Furthermore, some studies utilized simultaneous bilateral occlusion of the carotid arteries to cause global ischemia which would not normally occur in human stroke; however, this does still provide a valuable model of ischemic injury.
We also acknowledge the effect of publication bias favouring positive outcome and the limitations presented by the results of our STAIR and ARRIVE assessments. The potential for under-powered research is appreciated.
Overall, pre-clinical studies are restricted by small numbers, limited standardisation, disparity between study dose and the licensed clinical dose and having a limited ability to replicate the numerous confounding variables encountered in real-world stroke practice.
Of course, CVOTs report stroke incidence rather than stroke outcome. Other limitations include short trial duration, complex multi-morbid patient characteristics, and the potential for pharmacological interactions with concomitant medications affecting outcomes. GLP-1RAs are known to reduce blood pressure, body weight and cholesterol, all potentially mitigating stroke risk. Analysis of strokes occurring within the REWIND trial demonstrated a reduction in stroke incidence, but no impact on stroke severity as measured by mRS.
Retrospective studies generally included large numbers and are based upon real-world use and adherence. They reported encouraging results, often demonstrating a reduction in cardiovascular risk and/or a modest reduction in stroke risk. Eloquent attempts were often made to adjust for confounding variables, but these do remain a significant limitation, as does incomplete extraction of events from databases.66 These studies are also limited by heterogeneity in treatment duration, dose, and by the grouping together of GLP-1RAs.
There remains an unfulfilled requirement for neuroprotective agents in the management of AIS. Ultimately, many agents have previously failed to translate from bench to bedside.
However, we propose that further research into the repurposing GLP-1RAs – already licensed, with established side-effect profiles, cardiovascular safety record and clear indicators from pre-clinical research of their functional benefit – is both potentially clinically and economically advantageous. Further research is required to establish optimal dosing, cost-effectiveness, interaction with reperfusion therapies, timing of administration, and to fully understand the underlying mechanism of action – with consideration of age, gender and co-morbidity – in order to guide larger clinical trial development.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: CH is a named inventor on several patent applications that cover the use of GLP-1RAs for the treatment of neurodegenerative disorders. WDS has received speaker honoraria, conference sponsorship by Astra-Zeneca, Boehringer Ingelheim, Bristol Myers Squibb, Colgate Palmolive, Eli Lilly, GlaxoSmithKline, Lundbeck, Menarini, Merck, Novartis, Novo Nordisk, Pfizer, Sanofi Aventis, Servier, and Takeda. He holds research grants from Astra-Zeneca, Novo Nordisk and Novartis.
Authors’ contributions: All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MM and HE. The first draft of the manuscript was written by MM and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Ethical approval: This systematic review does not contain any new studies with human participants or animal performed by any of the authors.
ORCID iDs
Mark P Maskery https://orcid.org/0000-0001-5661-6267
Christian Holscher https://orcid.org/0000-0002-8159-3260
References
- 1.Feigin VL, Norrving B, Mensah GA.Global burden of stroke. Circ Res 2017; 120: 439–448. [DOI] [PubMed] [Google Scholar]
- 2.Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 2014; 383: 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMeekin P, White P, James MA, et al. Estimating the number of UK stroke patients eligible for endovascular thrombectomy. Eur Stroke J 2017; 2: 319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 5.Wardlaw JM, Murray V, Berge E, et al. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev 2014; 7: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neuhaus AA, Couch Y, Hadley G, et al. Neuroprotection in stroke: the importance of collaboration and reproducibility. Brain 2017; 140: 2079–2092. [DOI] [PubMed] [Google Scholar]
- 7.Fisher M, Feuerstein G, Howells DW, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 2009; 40: 2244–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi S, Mörike K, Klotz U.The clinical implications of ageing for rational drug therapy. Eur J Clin Pharmacol 2008; 64: 183–199. [DOI] [PubMed] [Google Scholar]
- 9.Hurn PD, Vannucci SJ, Hagberg H.Adult or perinatal brain injury: does sex matter? Stroke 2005; 36: 193–195. [DOI] [PubMed] [Google Scholar]
- 10.O’Collins VE, Macleod MR, Donnan GA, et al. 1,026 Experimental treatments in acute stroke. Ann Neurol 2006; 59: 467–477. [DOI] [PubMed] [Google Scholar]
- 11.Sutherland BA, Minnerup J, Balami JS, et al. Neuroprotection for ischaemic stroke: translation from the bench to the bedside. Int J Stroke 2012; 7: 407–418. [DOI] [PubMed] [Google Scholar]
- 12.Hölscher C.Potential role of glucagon-like peptide-1 (GLP-1) in neuroprotection. CNS Drugs 2012; 26: 871–882. [DOI] [PubMed] [Google Scholar]
- 13.Gallwitz B.Extra-pancreatic effects of incretin-based therapies. Endocrine 2014; 47: 360–371. [DOI] [PubMed] [Google Scholar]
- 14.Hölscher C.Central effects of GLP-1: new opportunities for treatments of neurodegenerative diseases. J Endocrinol 2014; 221: T31–T41. [DOI] [PubMed] [Google Scholar]
- 15.Boland A, Cherry G, Dickson R.Doing a systematic review: a student’s guide. 1st ed. London: Sage Publications, 2013. [Google Scholar]
- 16.Kilkenny C, Browne WJ, Cuthill IC, et al. Improving bioscience research reporting: the ARRIVE Guidelines for Reporting Animal Research. PLoS Biol 2010; 8: e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyun Lee C, Yan B, Yoo K-Y, et al. Ischemia-induced changes in glucagon-like peptide-1 receptor and neuroprotective effect of its agonist, exendin-4, in experimental transient cerebral ischemia. J Neurosci Res 2011; 89: 1103–1113. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Perry T, Kindy MS, et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci 2009; 106: 1285–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Briyal S, Gulati K, Gulati A.Repeated administration of exendin-4 reduces focal cerebral ischemia-induced infarction in rats. Brain Res 2012; 1427: 23–34. [DOI] [PubMed] [Google Scholar]
- 20.Darsalia V, Mansouri S, Ortsäter H, et al. Glucagon-like peptide-1 receptor activation reduces ischaemic brain damage following stroke in Type 2 diabetic rats. Clin Sci 2012; 122: 473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jia Y, Gong N, Li T-F, et al. Peptidic exenatide and herbal catalpol mediate neuroprotection via the hippocampal GLP-1 receptor/β-endorphin pathway. Pharmacol Res 2015; 102: 276–285. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H, Meng J, Zhou S, et al. Intranasal delivery of exendin-4 confers neuroprotective effect against cerebral ischemia in mice. AAPS J 2016; 18: 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim S, Jeong J, Jung H-S, et al. Anti-inflammatory effect of glucagon like peptide-1 receptor agonist, exendin-4, through modulation of IB1/JIP1 expression and JNK signaling in stroke. Exp Neurobiol 2017; 26: 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teramoto S, Miyamoto N, Yatomi K, et al. Exendin-4, a glucagon-like peptide-1 receptor agonist, provides neuroprotection in mice transient focal cerebral ischemia. J Cereb Blood Flow Metab 2011; 31: 1696–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darsalia V, Hua S, Larsson M, et al. Exendin-4 reduces ischemic brain injury in normal and aged type 2 diabetic mice and promotes microglial M2 polarization. PLoS ONE 2014; 9: e103114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen F, Wang W, Ding H, et al. The glucagon-like peptide-1 receptor agonist exendin-4 ameliorates warfarin-associated hemorrhagic transformation after cerebral ischemia. J Neuroinflammation 2016; 13: 204. DOI:10.1186/s12974-016-0661-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuroki T, Tanaka R, Shimada Y, et al. Exendin-4 inhibits matrix metalloproteinase-9 activation and reduces infarct growth after focal cerebral ischemia in hyperglycemic mice. Stroke 2016; 47: 1328–1335. [DOI] [PubMed] [Google Scholar]
- 28.Yang J-L, Chen W-Y, Chen Y-P, et al. Activation of GLP-1 receptor enhances neuronal base excision repair via PI3K-AKT-induced expression of apurinic/apyrimidinic endonuclease 1. Theranostics 2016; 6: 2015–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Briyal S, Shah S, Gulati A.Neuroprotective and anti-apoptotic effects of liraglutide in the rat brain following focal cerebral ischemia. Neuroscience 2014; 281: 269–281. [DOI] [PubMed] [Google Scholar]
- 30.Deng C, Cao J, Han J, et al. Liraglutide activates the Nrf2/HO-1 antioxidant pathway and protects brain nerve cells against cerebral ischemia in diabetic rats. Comput Intell Neurosci 2018; 2018: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Filchenko I, Simanenkova A, Chefu S, et al. Neuroprotective effect of glucagon-like peptide-1 receptor agonist is independent of glycaemia normalization in type two diabetic rats. Diab Vasc Dis Res 2018; 15: 567–570. [DOI] [PubMed] [Google Scholar]
- 32.Sato K, Kameda M, Yasuhara T, et al. Neuroprotective effects of liraglutide for stroke model of rats. Int J Mol Sci 2013; 14: 21513–21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu H, Zhang Y, Shi Z, et al. The neuroprotection of liraglutide against ischaemia-induced apoptosis through the activation of the PI3K/AKT and MAPK pathways. Sci Rep 2016; 6: 26859. DOI:10.1038/srep26859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong W, Miao Y, Chen A, et al. Delayed administration of the GLP-1 receptor agonist liraglutide improves metabolic and functional recovery after cerebral ischemia in rats. Neurosci Lett 2017; 641: 1–7. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Zhang X, He J, et al. Delayed administration of the glucagon-like peptide 1 analog liraglutide promoting angiogenesis after focal cerebral ischemia in mice. J Stroke Cerebrovasc Dis 2018; 27: 1318–1325. [DOI] [PubMed] [Google Scholar]
- 36.He W, Wang H, Zhao C, et al. Role of liraglutide in brain repair promotion through Sirt1‐mediated mitochondrial improvement in stroke. J Cell Physiol 2020; 235: 2986–3001. [DOI] [PubMed] [Google Scholar]
- 37.Zeng S, Bai J, Jiang H, et al. Treatment with liraglutide exerts neuroprotection after hypoxic–ischemic brain injury in neonatal rats via the PI3K/AKT/GSK3β pathway. Front Cell Neurosci 2020; 13: 585. DOI:10.3389/fncel.2019.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang D, Wang Y, Zang Y, et al. Neuroprotective Effects of rhGLP-1 in diabetic rats with cerebral ischemia/reperfusion injury: rhGLP-1 neuroprotective benefits. Drug Dev Res 2016; 77: 124–133. [DOI] [PubMed] [Google Scholar]
- 39.Fang Y, Jiang D, Wang Y, et al. Neuroprotection of rhGLP-1 in diabetic rats with cerebral ischemia/reperfusion injury via regulation of oxidative stress, EAAT2, and apoptosis. Drug Dev Res 2018; 79: 249–259. [DOI] [PubMed] [Google Scholar]
- 40.Zhao L, Xu J, Wang Q, et al. Protective effect of rhGLP-1 (7–36) on brain ischemia/reperfusion damage in diabetic rats. Brain Res 2015; 1602: 153–159. [DOI] [PubMed] [Google Scholar]
- 41.Abdel-latif RG, Heeba GH, Taye A, et al. Lixisenatide ameliorates cerebral ischemia-reperfusion injury via GLP-1 receptor dependent/independent pathways. Eur J Pharmacol 2018; 833: 145–154. [DOI] [PubMed] [Google Scholar]
- 42.Abdel-latif RG, Heeba GH, Taye A, et al. Lixisenatide, a novel GLP-1 analog, protects against cerebral ischemia/reperfusion injury in diabetic rats. Naunyn Schmiedebergs Arch Pharmacol 2018; 391: 705–717. [DOI] [PubMed] [Google Scholar]
- 43.Yang X, Feng P, Zhang X, et al. The diabetes drug semaglutide reduces infarct size, inflammation, and apoptosis, and normalizes neurogenesis in a rat model of stroke. Neuropharmacology 2019; 158: 107748. [DOI] [PubMed] [Google Scholar]
- 44.Chien C-T, Jou M-J, Cheng T-Y, et al. Exendin-4-loaded PLGA microspheres relieve cerebral ischemia/reperfusion injury and neurologic deficits through long-lasting bioactivity-mediated phosphorylated Akt/eNOS signaling in rats. J Cereb Blood Flow Metab 2015; 35: 1790–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang H, Meng J, Li X, et al. Pro-GLP-1, a Pro-drug of GLP-1, is neuroprotective in cerebral ischemia. Eur J Pharm Sci 2015; 70: 82–91. [DOI] [PubMed] [Google Scholar]
- 46.Zhang H, Liu Y, Guan S, et al. An orally active allosteric GLP-1 receptor agonist is neuroprotective in cellular and rodent models of stroke. PLoS ONE 2016; 11: e0148827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han L, Holscher C, Xue G, et al. A novel dual-glucagon-like peptide 1 and glucose-dependent insulinotrophic polypeptide receptor agonist is neuroprotective in transient focal cerebral ischaemia in the rat. Neuroreport 2016; 6: 23–32. [DOI] [PubMed] [Google Scholar]
- 48.Li Y, Wu K-J, Yu S-J, et al. Neurotrophic and neuroprotective effects of oxyntomodulin in neuronal cells and a rat model of stroke. Exp Neurol 2017; 288: 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y-H, Liou K-T, Tsai K-C, et al. GSK-3 inhibition through GLP-1R allosteric activation mediates the neurogenesis promoting effect of P7C3 after cerebral ischemic/reperfusional injury in mice. Toxicol Appl Pharmacol 2018; 357: 88–105. [DOI] [PubMed] [Google Scholar]
- 50.Li P-C, Liu L-F, Jou M-J, et al. The GLP-1 receptor agonists exendin-4 and liraglutide alleviate oxidative stress and cognitive and micturition deficits induced by middle cerebral artery occlusion in diabetic mice. BMC Neurosci 2016; 17: 50. DOI: 10.1186/s12868-016-0272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gad SN, Nofal S, Raafat EM, et al. Lixisenatide reduced damage in hippocampus CA1 neurons in a rat model of cerebral ischemia-reperfusion possibly via the ERK/P38 signaling pathway. J Mol Neurosci 2020; 70: 1026–1037. DOI: 10.1007/s12031-020-01497-9. [DOI] [PubMed] [Google Scholar]
- 52.Pawlowski J, Kraft AS.Bax-induced apoptotic cell death. Proc Natl Acad Sci 2000; 97: 529–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chaitanya G, Alexander JS, Babu P.PARP-1 cleavage fragments: signatures of cell-death proteases in neurodegeneration. Cell Commun Signal 2010; 8: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Porter AG, Jänicke RU.Emerging roles of caspase-3 in apoptosis. Cell Death Differ 1999; 6: 99–104. [DOI] [PubMed] [Google Scholar]
- 55.Darzynkiewicz Z, Galkowski D, Zhao H.Analysis of apoptosis by cytometry using TUNEL assay. Methods 2008; 44: 250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilkins HM, Swerdlow RH.TNFα in cerebral ischemia: another stroke against you? J Neurochem 2015; 132: 369–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bell AH, Miller SL, Castillo-Melendez M, et al. The neurovascular unit: effects of brain insults during the perinatal period. Front Neurosci 2020; 13: 1452. DOI: 10.3389/fnins.2019.01452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma Y, Zechariah A, Qu Y, et al. Effects of vascular endothelial growth factor in ischemic stroke. J Neurosci Res 2012; 90: 1873–1882. [DOI] [PubMed] [Google Scholar]
- 59.Zhou B, Ji K, Peng A, et al. GLP-1(28-36)amide, a long ignored peptide revisited. Open Biochem J 2014; 8: 107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gerstein HC, Hart R, Colhoun HM, et al. The effect of dulaglutide on stroke: an exploratory analysis of the REWIND trial. Lancet Diab Endocrinol 2020; 8: 106–114. [DOI] [PubMed] [Google Scholar]
- 61.Mann JFE, Fonseca V, Mosenzon O, et al. Effects of liraglutide versus placebo on cardiovascular events in patients with type 2 diabetes mellitus and chronic kidney disease: results from the LEADER Trial. Circulation 2018; 138: 2908–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kang YM, Cho YK, Lee J, et al. Asian subpopulations may exhibit greater cardiovascular benefit from long-acting glucagon-like peptide 1 receptor agonists: a meta-analysis of cardiovascular outcome trials. Diabetes Metab J 2018; 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barkas F, Elisaf M, Milionis H.Protection against stroke with glucagon-like peptide 1 receptor agonists: a systematic review and meta-analysis. Eur J Neurol 2019; 26: 559–565. DOI: 10.1111/ene.13905. [DOI] [PubMed] [Google Scholar]
- 64.O’Brien MJ, Karam SL, Wallia A, et al. Association of second-line antidiabetic medications with cardiovascular events among insured adults with type 2 diabetes. JAMA Netw Open 2018; 1: e186125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paul SK, Klein K, Maggs D, et al. The association of the treatment with glucagon-like peptide-1 receptor agonist exenatide or insulin with cardiovascular outcomes in patients with type 2 diabetes: a retrospective observational study. Cardiovasc Diabetol 2015; 14: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zimmerman RS, Hobbs TM, Wells BJ, et al. Association of glucagon-like peptide-1 receptor agonist use and rates of acute myocardial infarction, stroke and overall mortality in patients with type 2 diabetes mellitus in a large integrated health system. Diabetes Obes Metab 2017; 19: 1555–1561. [DOI] [PubMed] [Google Scholar]
- 67.Svanström H, Ueda P, Melbye M, et al. Use of liraglutide and risk of major cardiovascular events: a register based cohort study in Denmark and Sweden. Lancet Diab Endocrinol 2019; 7: 106–114. [DOI] [PubMed] [Google Scholar]
- 68.Anyanwagu U, Mamza J, Mehta R, et al. Cardiovascular events and all-cause mortality with insulin versus glucagon-like peptide-1 analogue in type 2 diabetes. Heart 2016; 102: 1581–1587. [DOI] [PubMed] [Google Scholar]
- 69.Raparelli V, Elharram M, Moura CS, et al. Sex differences in cardiovascular effectiveness of newer glucose‐lowering drugs added to metformin in type 2 diabetes mellitus. J Am Heart Assoc 2020; 9: e012940. doi:10.1161/JAHA.119.012940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Daly SC, Chemmanam T, Loh P-S, et al. Exenatide in acute ischemic stroke. Int J Stroke 2013; 8: E44–E44. [DOI] [PubMed] [Google Scholar]
- 71.Larsson M, Castren M, Lindström V, et al. Prehospital exenatide in hyperglycemic stroke – a randomized trial. Acta Neurol Scand 2019; 140: 443–448. DOI: 10.1111/ane.13166. [DOI] [PubMed] [Google Scholar]
- 72.McGrath RT, Hocking SL, Priglinger M, et al. Rationale and design of Short-Term EXenatide therapy in Acute ischaemic Stroke (STEXAS): a randomised, open-label, parallel-group study. BMJ Open 2016; 6: e008203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muller C, Cheung NW, Dewey H, et al. Treatment with exenatide in acute ischemic stroke trial protocol: a prospective, randomized, open label, blinded end-point study of exenatide vs. standard care in post stroke hyperglycemia. Int J Stroke 2018; 13: 857–862. [DOI] [PubMed] [Google Scholar]
- 74.Fei Y, Tsoi M-F, Cheung BMY.Cardiovascular outcomes in trials of new antidiabetic drug classes: a network meta-analysis. Cardiovasc Diabetol 2019; 18: 112. DOI: 10.1186/s12933-019-0916-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373: 2247–2257. [DOI] [PubMed] [Google Scholar]
- 76.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375: 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375: 1834–1844. [DOI] [PubMed] [Google Scholar]
- 78.Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017; 377: 1228–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 2018; 392: 1519–1529. [DOI] [PubMed] [Google Scholar]
- 80.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019; 394: 121–130. [DOI] [PubMed] [Google Scholar]
- 81.Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2019; 381: 841–851. [DOI] [PubMed] [Google Scholar]
- 82.Best JH, Hoogwerf BJ, Herman WH, et al. Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon-like peptide 1 (GLP-1) receptor agonist exenatide twice daily or other glucose-lowering therapies: a retrospective analysis of the LifeLink database. Diab Care 2011; 34: 90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gejl M, Starup-Linde J, Scheel-Thomsen J, et al. Risk of cardiovascular disease: the effects of diabetes and anti-diabetic drugs – a nested case–control study. Int J Cardiol 2015; 178: 292–296. [DOI] [PubMed] [Google Scholar]
- 84.Patorno E, Everett BM, Goldfine AB, et al. Comparative cardiovascular safety of glucagon-like peptide-1 receptor agonists versus other antidiabetic drugs in routine care: a cohort study. Diab Obes Metab 2016; 18: 755–765. [DOI] [PubMed] [Google Scholar]
- 85.Ekström N, Svensson A-M, Miftaraj M, et al. Cardiovascular safety of glucose-lowering agents as add-on medication to metformin treatment in type 2 diabetes: report from the Swedish National Diabetes Register. Diabetes Obes Metab 2016; 18: 990–998. [DOI] [PubMed] [Google Scholar]
- 86.Anyanwagu U, Mamza J, Donnelly R, et al. Effect of adding GLP-1RA on mortality, cardiovascular events, and metabolic outcomes among insulin-treated patients with type 2 diabetes: a large retrospective UK cohort study. Am Heart J 2018; 196: 18–27. [DOI] [PubMed] [Google Scholar]
- 87.Frison V, Simioni N, Marangoni A, et al. Clinical impact of 5 years of liraglutide treatment on cardiovascular risk factors in patients with type 2 diabetes mellitus in a real-life setting in Italy: an observational study. Diabetes Ther 2018; 9: 2201–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]