Abstract
B lymphocytes have a central role in autoimmune diseases, which are often defined by specific autoantibody patterns and feature a loss of B cell tolerance. A prototypic disease associated with B cell hyperactivity is systemic lupus erythematosus (SLE). In patients with SLE, the loss of B cell tolerance to autoantigens is controlled in a cell-intrinsic manner by Toll-like receptors (TLRs), which sense nucleic acids in endosomes. TLR7 drives the extrafollicular B cell response and the germinal centre reaction that are involved in autoantibody production and disease pathogenesis. Surprisingly, TLR9 seems to protect against SLE, even though it is required for the production of autoantibodies recognizing double-stranded DNA-associated antigens, which are abundant in SLE and are a hallmark of this disease. The protective function of TLR9 is at least partly mediated by its capacity to limit the stimulatory activity of TLR7. The roles of TLR7 and TLR9 in the effector function of B cells in lupus-like disease and in patients with SLE, and the unique features of TLR signalling in B cells, suggest that targeting TLR signalling in SLE might be therapeutically beneficial.
Subject terms: Immunology, Autoimmune diseases
Loss of B cell tolerance to autoantigens in systemic lupus erythematosus (SLE) is driven by TLR7, whereas TLR9 appears to protect against SLE by limiting the stimulatory activity of TLR7. The unique features of Toll-like receptor signalling in B cells implicate it as a therapeutic target in SLE.
Key points
Intrinsic TLR7 and TLR9 signalling in B cells plays an important role in the development and pathogenesis of systemic lupus erythematosus (SLE).
In patients with SLE, effector plasma cells are generated via the extrafollicular response and via the formation of spontaneous germinal centres.
TLR7 plays key roles in the extrafollicular response and the response mediated by germinal centres.
Some plasma cells produce IL-10 and can have protective roles in lupus-like disease.
Introduction
The diagnosis of autoimmune diseases often relies on the identification of characteristic autoantibody profiles, emphasizing their association with the activation of autoreactive B cells. Furthermore, B cell depletion therapy can have beneficial effects in patients with these disorders1, highlighting the importance of B cells in the pathogenesis of autoimmune diseases. In autoimmune diseases B cells have been regarded almost exclusively for their role in autoantibody production, although we now know that they also mediate deleterious functions through antibody-independent activities, including: the presentation of antigen to T cells, co-stimulatory functions via the expression of accessory molecules engaging stimulatory receptors on T cells and the production of cytokines2. These findings highlight the need to extend the repertoire of effector B cell subsets studied with regard to autoimmune diseases beyond antibody-secreting plasmablasts and plasma cells.
Identifying the signalling pathways controlling the differentiation of effector B cell subsets might shed light on the pathophysiological mechanisms at play during autoimmune diseases. B cell activation is controlled by four classes of receptors, namely B cell receptors (BCRs) that bind autoantigens, cytokine receptors, receptors implicated in cognate T cell–B cell interactions (including checkpoint molecules), and innate immune receptors including Toll-like receptors (TLRs). The implication of TLRs in some autoimmune diseases is underlined by their association with polymorphisms in TLR genes (for example, TLR4 and TLR7)3,4. TLR signalling promotes three key activities through which B cells can contribute to autoimmune diseases: the production of antibodies, the presentation of antigens to T cells and the production of cytokines5–7. The importance of both B cells and TLRs in autoimmune diseases suggests a role for intrinsic TLR signalling in B cells in these disorders.
In this Review we discuss how intrinsic TLR signalling controls the differentiation of effector B cells during autoimmune diseases, with a particular focus on systemic lupus erythematosus (SLE). First, we document the importance of intrinsic TLR signalling in B cells in the development of SLE. Second, we consider the contribution of intrinsic TLR signalling to the generation of pathogenic effector B cell subsets. Third, we highlight features of TLR signalling that are specific to B cells, some of which regulate their anti-inflammatory functions. Fourth, we discuss current therapeutic opportunities and perspectives for targeting TLR signalling in autoimmune diseases.
TLR signalling in B cells drives SLE
TLR7 predisposes humans to SLE
Genetic association studies implicate TLR signalling in SLE8–10. In particular, polymorphisms resulting in increased expression of TLR7 (the ligand for which is single-stranded RNA) are associated with an increased risk of developing SLE11–13. TLR7 expression is higher in women than in men owing to the localization of TLR7 on the X chromosome14. One X chromosome is normally inactivated in women; yet, some genes on the X chromosome, including TLR7, always seem to escape inactivation14. As a result, TLR7 is biallelically expressed in plasmacytoid dendritic cells (pDCs), monocytes and B cells, and TLR7 is thus present at a higher level in these cells in women than in men14. In line with this finding, B cells from women exposed to TLR7 agonist in vitro differentiate more efficiently into CD27hi plasmablasts than B cells from men; this gender difference is not observed upon the addition of agonists of TLR9, the gene encoding which is on chromosome 3 (ref.14). This observation is consistent with a higher prevalence of SLE in women than in men15. Further documenting how X chromosome number affects susceptibility to SLE, the presence of two X chromosomes in men with Klinefelter’s syndrome is associated with a higher predisposition to SLE than in men with a single X chromosome16, and women with a single X chromosome (for example, those with Turner syndrome) are less prone to SLE than women with two X chromosomes15. A reduction in TLR7 activity might thus reduce the development of SLE. TLR7 expression is also modulated by metabolic parameters (for example, it is increased by a high-fat diet, which exacerbates SLE)17, and by cytokines such as type I interferons, which augment the expression of TLR7 but not TLR9 in pDCs18. It might thus also be possible to reduce the symptoms of SLE by modulating TLR7 function.
TLR7 predisposes mice to lupus-like disease
TLR7 expression similarly modulates predisposition to lupus-like disease in mice. Overexpression of TLR7 induces systemic autoimmunity in mouse strains not prone to lupus10,19,20, and the deletion of Tlr7 reduces lupus development in strains that spontaneously develop such diseases21,22. Genetic analysis of the cell types implicated in this reduction underlined the importance of intrinsic TLR7 signalling in B cells in the pathogenesis of lupus-like disease in mice23. Specifically, mice that are genetically predisposed to lupus-like disease but have a B cell-specific Tlr7 deletion displayed reduced disease, lower levels of autoantibodies against RNA-associated and apoptosis-related autoantigens and diminished immune activity, as indicated by a lower number of germinal centre B cells, T follicular helper (TFH) cells, macrophages and neutrophils, including in kidneys; kidneys in these mice had no sign of glomerulonephritis, in contrast to control mice, which were genetically predisposed to lupus-like disease without deletion of Tlr7 (ref.23).
TLR8 and TLR9 protect mice from lupus-like disease
In addition to TLR7, intracellular nucleic acids are detected by TLR8, which also senses single-stranded RNA, and by TLR9, which is a receptor for DNA sequences containing unmethylated cytosine-phosphate-guanosine motifs24. Different roles have been identified for these TLRs in distinct models of lupus-like disease. In some models both TLR8 and TLR9 exerted protective effects25,26. Specifically, Tlr8-null mice and Tlr9-null mice displayed more severe lupus than controls, with increased deposition of immunoglobulins and more severe lupus nephritis. These mice also displayed enhanced immune activity and had more germinal centres and antibody-secreting cells, as well as increased autoantibody titres, than controls26. Disease exacerbation was abrogated when Tlr7 was also deleted from Tlr8- or Tlr9-null mice, indicating that TLR8 and TLR9 might limit the pathogenesis of lupus by limiting the deleterious effects of TLR7 (ref.26). Indeed, TLR8 and TLR9 restricted TLR7 activity in dendritic cells and B cells respectively26,27. As expected, disease in Tlr8−/− Tlr9−/− double-knockout mice was worse than disease in mice with a single gene defect, reflecting the additive effect of these two abnormalities26. Of note, TLR8 does not always act protectively in lupus-like disease in mice because it facilitated the production of anti-RNA antibodies in the absence of Tlr7 in a model of lupus-like disease in which mice carry a transgenic autoreactive BCR28. The cell type responsible for this TLR8-mediated effect was not formally identified in this model, in which TLR7 was the main TLR driving anti-RNA autoantibody production by B cells and TLR9 acted protectively. There is thus no direct evidence that TLR8 signalling can inhibit or increase TLR7 activity in B cells; it might act in other cell types, for instance in neutrophils to increase their secretion of type I interferons28.
TLR7 and TLR9 functionally interact in B cells
Understanding the functional interaction between TLR7 and TLR9 in B cells relies on understanding how these TLRs are engaged. These TLRs are intracellular and as, unlike dendritic cells, B cells do not internalize extracellular material through micropinocytosis or endocytosis, in B cells they are not directly accessible to natural extracellular nucleic acids29. Instead, in B cells, the main portal of antigen entry into cells is through the BCR, which, after engagement, is internalized with the bound antigen and delivered to intracellular compartments, including late endosomes in which TLR7 and TLR9 are present29–31. The arrival of BCR–antigen complexes in late endosomes activates these TLRs and triggers the co-stimulation of B cells29. This co-stimulation is crucial for autoreactive B cell activation in mouse models of lupus-like disease because deletion of Tlr7 or Tlr9 results in the loss of autoantibodies against RNA- or DNA-containing antigens respectively21,32. Thus, no pathway appears to be able to compensate for the absence of Tlr7 or Tlr9 in the development of lupus-like disease in mice.
Mechanisms of antagonism between TLR7 and TLR9 in B cells
Antagonism between TLR7 and TLR9 can occur within a single B cell if this B cell expresses a BCR that recognizes autoantigens comprising both TLR7 and TLR9 agonists. In this scenario, it was found that TLR9 engagement restrained the differentiation of B cells instructed by TLR7 in vitro33. In fact, B cells did not differentiate into CD138hi antibody-secreting cells unless Tlr9 was deleted or TLR9 was pharmacologically inhibited upon antigen stimulation33. In agreement with this observation, antigens engaging both BCR and TLR7 (but not TLR9) could induce antigen-specific B cell differentiation into CD138hi antibody-secreting cells; this differentiation was not observed for antigens co-engaging the BCR and TLR9, or with synthetic agonists of TLR7 or TLR9 that did not trigger the BCR33. Although these in vitro findings do not perfectly recapitulate what is happening in vivo (where the engagement of TLR9 in B cells contributes positively to the production of anti-DNA autoantibodies27), they underline functional differences between TLR7 and TLR9 during B cell activation, and the unique response induced upon co-engagement of BCR and TLR7 that is likely relevant to the development of lupus in mice. In keeping with a role for TLR7 in the development of lupus, TLR7 signalling (but not TLR9, TLR2, TLR3 or TLR4 signalling) is strictly required for the formation of spontaneous germinal centres in vivo in mice34.
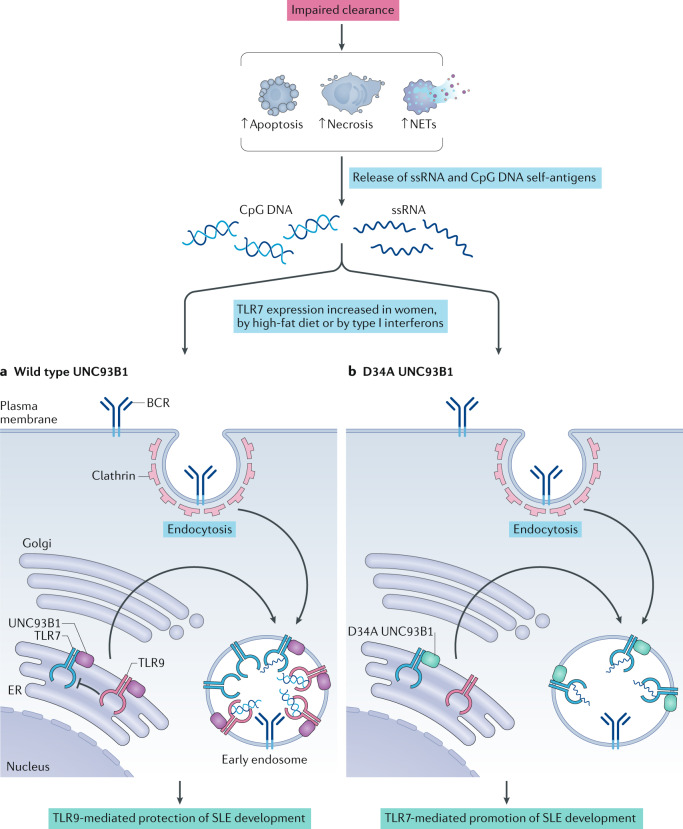
The antagonistic interaction between TLR7 and TLR9 within one B cell is underscored by the competition of these TLRs for the intracellular protein UNC93B1, which promotes their trafficking to endosomal compartments35,36 (Fig. 1). Different amino acids within UNC93B1 bind to TLR7 and TLR9, and UNC93B1 harbouring a N-terminal D34A mutation interacts normally with TLR9 but more strongly with TLR7 than wild type UNC93B1; enhanced UNC93B1–TLR7 binding increases the export of TLR7 from the endoplasmic reticulum to the endosomal compartment, favouring TLR7 signalling over TLR9 signalling. Mice carrying this mutation in Unc93b1 develop a fatal systemic inflammatory syndrome35. In the endolysosomal compartment, the duration of TLR7 signalling is controlled by the interaction of UNC93B1 with Syntenin-1 (also known as syndecan-binding protein (SDCBP)), which can terminate transmembrane receptor signalling by promoting their transport to intralumenal vesicles of multivesicular bodies37. Mice with a mutation in Unc93b1 that negatively affects the interaction of UNC93B1 with Syntenin-1 develop a systemic inflammatory disease similar to TLR7-overexpressing mice37. These findings underscore the importance of intracellular TLR7 trafficking in regulating TLR7 signalling. These processes can be altered via naturally occurring mutations; for instance, dogs with a mutation in the C-terminal domain of UNC93B1 that reduces the interaction of UNC93B1 with Syntenin-1 spontaneously develop cutaneous lupus38.
Fig. 1. Opposing roles of TLR7 and TLR9 in SLE.
Systemic lupus erythematosus (SLE) is characterized by the presence of autoreactive B cells that recognize DNA-associated antigens (such as unmethylated cytosine-phosphate-guanosine (CpG) motifs) and RNA-associated antigens (such as the single-stranded RNA (ssRNA) small nuclear RNA, U11). These self-antigens are thought to be released owing to dysregulated processes that increase the abundance of neutrophil extracellular traps (NETs), necrotic cells or apoptotic cells. These autoantigens can be recognized at the surface of B cells by the B cell receptor (BCR), which initiates their cellular internalization. Once in endosomes, self-antigens can trigger Toll-like receptor 7 (TLR7) and/or TLR9 in B cells. Genetic or environmental signals leading to the overexpression of TLR7 (such as gender, diet and the cytokine environment, including the level of type I interferons) increase the susceptibility of individuals to SLE. Under physiological conditions, TLR7 signalling is restrained by TLR9, which protects individuals from the development of SLE. By contrast, the disruption of TLR9 function can favour TLR7 signalling and facilitate the development of SLE. Such disruption can involve the intracellular protein UNC93B1, which drives TLR7 and TLR9 trafficking to endosomal compartments (a). D34A mutation in transmembrane UNC93B1 favours its interaction with TLR7 (b), which can raise the abundance of TLR7 in endosomes, intensify TLR7 signalling and initiate a fatal systemic inflammatory syndrome in an experimental model. ER, endoplasmic reticulum.
TLR7 and TLR9 signalling in B cells might also exert opposite effects on SLE via cell-extrinsic mechanisms that condition the immune environment. Little is known about such possible cell-extrinsic effects of B cells in SLE, which might involve B cell-mediated production of cytokines (such as IL-10) that can increase protection against autoimmune diseases including SLE (see below).
Understanding TLR autoantigens in SLE
A complete understanding of the role of these TLRs (TLR7, TLR8 and TLR9) in SLE pathogenesis requires the source and biochemical properties of the relevant autoantigens to be elucidated. Extracellular nucleic acids are involved in SLE pathogenesis, and mutations in DNASE1 (encoding deoxyribonuclease-1) have been associated with the development of SLE in humans and lupus-like disease in mice39,40. These self-antigens are thought to be released owing to dysregulated cellular processes resulting in an increase in abundance of neutrophil extracellular traps, necrotic cells or apoptotic cells (Fig. 1). Many intracellular autoantigens are redistributed from intracellular sites to the plasma membrane during apoptosis41, and the impaired clearance of apoptotic cells has been associated with SLE pathogenesis42. The small nuclear RNA U11 is also an endogenous agonist of TLR7 that drives immune pathogenesis43. Thus, abundantly available nucleic acids in patients with SLE contribute to active and chronic disease, likely by triggering persistent T cell-dependent and T cell-independent B cell activation. Autoreactive lymphocytes might also be activated through ‘epitope mimicry’ with the microbiota, as documented for autoreactive T cells targeting the SLE autoantigen Ro60 (a protein element of small cytoplasmic ribonucleoprotein hY-RNA complexes) from the host and microbiota44. Furthermore, rearrangements of the heavy-chain variable (VH) gene VH4-34, which are preferentially employed in autoimmunity, especially the idiotype 9G4 (a particular group of VH4-34-containing antibodies45) in SLE46, which contributes to the autoantibody repertoire against RNA and dsDNA47, cross-react with the gut microbiota48. Notably, impaired B cell selection of 9G4+ B cells has been observed in various autoimmune conditions including SLE46. Identifying where, when and how these TLR antigens drive effector B cell differentiation will provide important insights into the pathophysiology of SLE.
Effector B cell subsets driven by TLR7
SLE is associated with alterations in B cell homeostasis. Disease flares are marked by expansions in plasmablasts that are discernible in blood and correlate in magnitude with disease exacerbation49. These antibody-secreting cells have a more diverse repertoire of BCRs than antibody-secreting cells generated after vaccination with tetanus or influenza50 and contain cells of irrelevant antigen specificity, with up to 1% of IgG-antibody-secreting cells producing antibodies against influenza virus or tetanus toxin50,51. Antibody-secreting cells in these waves also contain few expanded clones that are autoreactive, indicating the presence of an autoantigen-specific response and of cells activated in a bystander manner50,51.
Differentiation of antibody-secreting B cells
The trajectory of effector B cell subsets leading to these antibody-secreting cells was reconstructed by combining the phenotyping of the peripheral blood B cell compartment by flow cytometry with analysis of the immunoglobulin gene repertoire. This reconstruction identified activated naive B cells (CD11c+IgD+CD27−CD21−MTG+CD23−) and the DN2 subset of IgD−CD27−double negative B cells (DN2 B cells; IgD−CD27−CD11c+T-Bet+CD69+CD21−CD24−CD38−CXCR5−FCRL4−FCRL5+)52, both of which are abundant in patients with SLE but rare in healthy individuals, as having a role in the production of antibody-secreting cells. Activated naive B cells have been considered to be the precursors of DN2 B cells, which are prone to differentiating into antibody-secreting cells52. Interestingly, activated naive B cells and DN2 B cells have a similar transcriptional profile, which differs by the expression of only 42 genes52,53. The transcriptome of DN2 B cells is consistent with their status as precursors to antibody-secreting cells: they express higher levels of BLIMP-1 and IRF4, master transcription factors of plasmablast and plasma cell differentiation54, as well as of SLAMF7, which is encoded by an IRF4 target gene and also found at higher levels in plasma cells than in other B cell subsets52. Furthermore, they express less ETS1, a transcription factor that inhibits antibody-secreting cell formation, than other B cell subsets52. As expected based on these observations, DN2 B cells rapidly secrete antibodies after polyclonal stimulation, including anti-Smith and anti-ribonucleoprotein (RNP), indicating that they express autoreactive BCRs that recognize RNA-associated autoantigens. Supporting the notion that these cells play a notable role in the production of such antibodies, the frequency of DN2 B cells correlates with the levels of anti-Smith and anti-RNP autoantibodies, the two RNA-associated antigens investigated in this study, in patients with SLE52. Furthermore, accumulation of CD11c+T-Bet+CD21−CD38− B cells resembling DN2 B cells, which were autoreactive and correlated with clinical manifestations, was similarly reported in a different cohort of patients with SLE55.
Roles of TLR7 in B cell differentiation
TLR7 can instruct successive steps in the differentiation of resting naive B cells to activated naive B cells, DN2 B cells and, subsequently, to antibody-secreting cells, which defines a pathway of extrafollicular B cell differentiation52. TLR7 has a stimulatory activity in all of these effector B cell subsets and can promote the differentiation of DN2 B cells, which are hyperresponsive to TLR7 agonists, into antibody-secreting cells in the presence of IL-21 and IFNγ52. By contrast, DN2 B cells are less responsive to CD40 engagement than activated naive B cells52, indicating a change in the responsiveness of B cells to external stimuli during differentiation, perhaps owing to a progressive reduction in the expression of TRAF5, a factor that is required for CD40 signalling and inhibitory for TLR signalling56,57.
The role of TLR7 in the differentiation of effector B cells in patients with SLE has been further documented through molecular studies in an in vitro culture system that generates DN2-like B cells with a transcriptome similar to that of DN2 B cells from patients with SLE58. This culture system relies on the stimulation of naive human B cells with agonists of TLR7 and BCR as well as IFNγ, IL-2, IL-21 and B cell activating factor, and generates IgD−CD27−CD11c+T-BethiCD21−CXCR5−IRF4intFcRL5+ B cells resembling DN2 B cells found in patients with SLE58. TLR7 signalling was crucial for the subsequent differentiation of these cells into antibody-secreting cells58. The sensitivity of B cells to TLR7 agonists is augmented by IFNγ, which confers them with the capacity to respond productively to amounts of TLR7 agonist that are otherwise insufficient58. Of note, the concentration of IFNγ is increased in the serum of some patients with SLE59,60. By contrast, B cells from patients with SLE are hyporesponsive to TLR9 agonists61,62, suggesting that the balance between these two opposing TLR signalling pathways is distorted in patients with SLE.
TLR7 also plays a key role in the differentiation of naive B cells into CD11c+T-Bet+CD21− B cells (which would comprise activated naive and DN2 B cells in humans) in animal models of lupus, because these cells are absent in mice when Tlr7 is deleted63. Furthermore, the repeated administration of TLR7 agonists (but not of TLR3, TLR4 or TLR9 agonists) promotes the accumulation of these cells in mice via a mechanism involving intrinsic TLR7 signalling in B cells63. Remarkably, mice with B cell-specific deletion of Tlr9 display a higher number of CD11c+CD11b+ activated B cells than their counterpart with functional Tlr9, underlining the correlation between the abundance of these cells and the development of lupus-like disease and providing another example of the opposing roles of TLR7 and TLR9 (ref.23). Of note, TLR7 also facilitates the formation of spontaneous germinal centres in mice which, in addition to the extrafollicular plasma cell response involving DN2 B cells, can lead to autoantibody production in patients with SLE64 (Fig. 2). As in patients with SLE, IFNγ is also important for the pathogenic functions of B cells in animal models of lupus, underlining the conserved role of these pathways in this disease across species65–68.
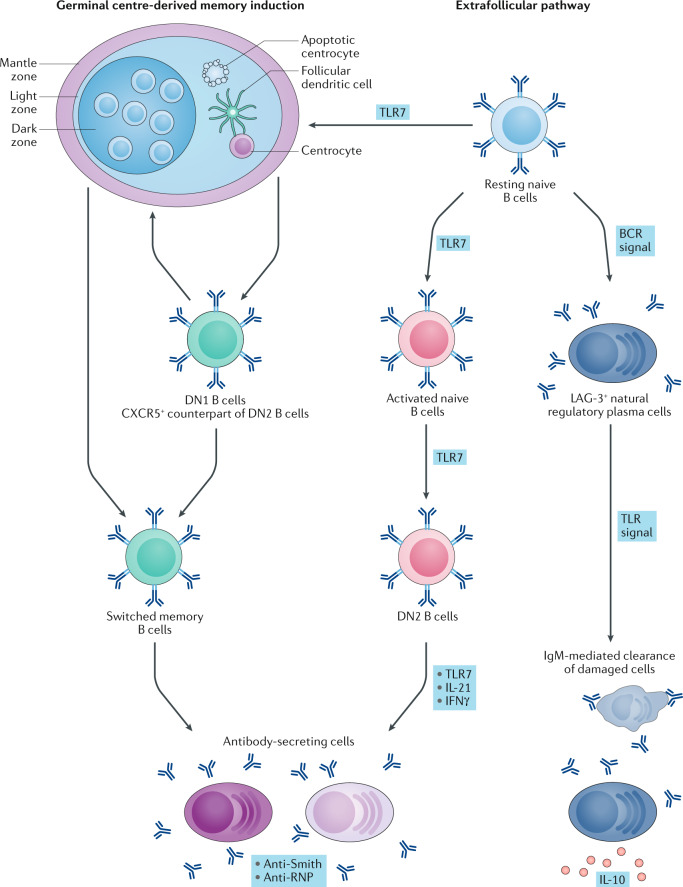
Fig. 2. TLRs drive plasma cell differentiation in SLE via different pathways.
Two distinct pathways generate pathogenic antibody-secreting cells in patients with systemic lupus erythematosus (SLE): germinal centre reactions and the extrafollicular pathway, both of which engage resting naive B cells. The germinal centre pathway generates the DN1 subset of double negative B cells (DN1 B cells; that is, IgD− CD27− cells that are CXCR5+) and the memory B cells produced in germinal centres can re-enter the germinal centre reaction or differentiate into antibody-secreting cells that produce isotype switched anti-Smith and anti-RNP. The spontaneous generation of the germinal centre is dependent on TLR7. TLR7 also drives the extrafollicular pathway, in which resting naive B cells become activated naive B cells (CD11c+IgD+CD27−CD21−MTG+CD23−) and, subsequently, the DN2 subset of IgD− CD27− double-negative B cells (DN2 B cells; IgD− CD27− CD11c+ Tbet+ CD69+CD21−CD24−CD38−CXCR5−FCRL4−FCRL5+). DN2 B cells are precursors of pathogenic antibody-secreting cells in patients with SLE, the differentiation into which is promoted by TLR7, IL-21 and IFNγ. Of note, resting naive B cells can also generate, in a manner dependent on the B cell receptor (BCR), regulatory plasma cells that are characterized by the cell surface expression of lymphocyte activation gene 3 protein (LAG-3). These regulatory plasma cells produce a uniquely high level of IL-10 in response to TLR signalling. At steady state, they also secrete IgM with reactivity against antigens expressed by damaged cells, suggesting that they might be involved in the clearance of damaged cells.
In conclusion, and as previously discussed69suggests that two pathways of B cell activation lead to the formation of autoreactive antibody-secreting cells in patients with SLE: the extrafollicular response (supported by activated naive B cells and DN2 B cells) and germinal centre reactions47. Remarkably, intrinsic TLR7 signalling in B cells has emerged as a key player in both responses (Fig. 2). These findings provide a framework in which to carry out the biochemical analyses of the molecular mechanisms implicated in these cellular processes. Interestingly, these pathways are not uniquely confined to autoimmunity as they have been reported to be active in patients with COVID-19 (ref.70).
Mechanisms of TLR signalling in B cells
B cells uniquely respond to TLR agonists
B cells are defined by the expression of a cell surface BCR and, as they also express multiple TLRs, these cells are thus at the intersection of adaptive and innate immunity. Intrinsic innate signalling in B cells is essential for the development of lupus-like disease in mice, as lupus nephritis was absent in mice with a B cell-type specific ablation of Myd88 (Myd88 encodes the signalling adaptor protein MyD88, which acts downstream of TLRs and IL-1 receptors)71–73. The response of B cells to TLR agonists is unique compared with other cell types of the immune system. B cells proliferate intensively and produce large amounts of IL-10 upon TLR stimulation, a combination not observed in myeloid cells7. Underlining the complex role of intrinsic TLR signalling in B cells during disease, B cell-derived IL-10 can be protective in autoimmune diseases74, including in mouse models of lupus-like disease75. Thus, in mice deficient in Lyn, which encodes a non-receptor tyrosine-protein kinase that regulates innate and adaptive immune responses, IL-10 production by B cells inhibits the progression of lupus-like disease even when no other cell types can produce this cytokine75.
Interplay between TLR signalling and BCR signalling
These unique features of TLR-driven cellular activation are related to the distinctive expression of BCR on B cells; B cells in which BCR has been genetically ablated fail to proliferate upon TLR stimulation76,77. At the intracellular level, TLR-stimulated B cell proliferation involves the adaptor protein Src tyrosine kinase SYK, which promotes BCR signalling by phosphorylating immunoreceptor tyrosine-based activation motifs in the cytoplasmic domains of the Igα and Igβ substructures of the BCR78,79. Thus, Syk-deficient B cells show impaired proliferation and IL-10 secretion upon activation with TLR agonists76. This fact is related to the defective activation of AKT and ERK in the absence of SYK. Remarkably, this TLR–SYK–AKT–ERK pathway is independent of MyD88 as it is still active in Myd88-deficient mice76. Finally, PI3K also has a key role in this pathway as over-expression of this kinase restored a proliferative response in BCR-deficient B cells stimulated with TLR agonists77.
The recruitment of the BCR signalling cascade downstream of TLR engagement in B cells underscores the interconnection between the innate and cognate functions of these cells. Along these lines, the engagement of the BCR upregulates TLR expression in human B cells, endowing these cells with the capacity to respond to TLR agonists5,80. In this context, genome-wide association studies identified the BCR signalling pathway as the biological process most affected by genetic polymorphisms facilitating the development of SLE81. These polymorphisms might not only facilitate BCR signalling but also increase TLR signalling in B cells, which might enable the growth of autoreactive B cell clones and the release of cytokines by them. Interestingly, the two B cell subsets with the highest capacity to produce IL-10 upon TLR stimulation in the mouse, namely LAG-3+CD138hi natural regulatory plasma cells and CD1dhi B cells, develop in a BCR-dependent manner82,83 (Fig. 2).
TLR signalling also comprises a SYK-independent pathway because the activation of NF-κB and the secretion of IL-6 (events downstream of TLR signalling) occurred normally in Syk-deficient B cells stimulated via TLR76. IL-6 production by B cells is directly relevant to the pathogenesis of SLE, as IL-6 expression is increased in patients with active SLE84 and correlates with disease activity in patients with lupus nephritis85. IL-6 expression can be induced in B cells by TLR7 agonists86,87, and this expression is further enhanced by IFNγ88. Remarkably, the ablation of IL-6 production specifically from B cells abrogated the spontaneous formation of germinal centres in lupus-prone mice and inhibited disease development88. Thus, elimination of the arm of intrinsic TLR signalling in B cells that results in IL-6 production, which seems to be independent of SYK, might be more beneficial in patients with SLE.
From a therapeutic standpoint, the fact that BCR signalling adaptors such as SYK have been implicated in TLR-mediated cell function suggests that inhibitors of BCR signalling might block the TLR-driven functions of B cells. Accordingly, the SYK inhibitor entospletinib reduced human B cell responses to TLR9 agonist89, and SYK expression was increased in activated CD21low B cells90.
Targeting TLR signalling to treat SLE
Inhibiting signalling adaptors
There is much interest in inhibiting signalling adaptors implicated in the pro-inflammatory functions of TLRs to treat inflammatory diseases. Although TLRs are prototypic pathogen recognition receptors, humans with loss of function mutations in MYD88, which transduces signals via all TLRs except TLR3, display a narrow susceptibility to pyogenic bacterial infections by Streptococcus pneumoniae, Staphylococcus aureus and Pseudomonas aeruginosa, but are resistant to other common microbial pathogens91. Remarkably, their susceptibility to these pathogens decreases with age. Similarly, children with a deficiency in IRAK4 (which encodes IL-1 receptor-associated kinase 4, a component of the TLR signalling pathways important for TLR7 and TLR9, as well as other TLRs) display an increased susceptibility to pyogenic bacterial infections during their first 10 years of life, which improves with age92. The improvement of pathogen control in both of these groups of individuals is likely related to the emergence of compensatory adaptive mechanisms involving T lymphocytes or B lymphocytes93. The inhibition of key molecules of the TLR signalling pathway, such as MYD88 or IRAK4, might thus allow inflammation to be tapered without compromising host defence against pathogens.
Inhibiting TLR activation in endosomes
The inhibition of endosomal TLR activation appears to be the most pertinent for treating patients with SLE. Existing treatments already target this pathway; notably, hydroxychloroquine and bafilomycin inhibit endosome acidification and/or maturation, thereby inhibiting both TLR7 and TLR9 signalling94. Hydroxychloroquine only moderately inhibits TLR signalling, and does not result in a surge of infection, consistent with the concept that TLR inhibition modulates, rather than suppresses, the immune system95. It inhibits the inflammatory response of human memory B cells, including their TLR-stimulated production of IL-6 (ref.96). Several other inhibitors of endosomal TLRs are under clinical evaluation for use in rheumatic diseases, including SLE and psoriasis, that also involve TLR7 signalling. TLR7, TLR8 and TLR9 signal through IRAK4, the inhibition of which has been studied in various assays and showed superior effects compared with hydroxychloroquine on the inhibition of cytokine production and inflammatory gene expression in peripheral blood mononuclear cells97. An early trial using the IRAK4 inhibitor PF06650833 provided promising phase I data in healthy individuals, showing a favourable safety and pharmacokinetic profile as well as evidence of pharmacological effect98. Additional inhibitors of IRAK1 and IRAK4 or of TAK1, a signalling molecule involved in TLR signalling, are under development99. Of note, these approaches do not differentiate between different endosomal TLRs95 and they affect multiple cell types in addition to B cells. The safety of these inhibitors thus needs to be considered carefully.
Boosting TLR signalling
Although the dominant rationale for targeting TLRs to treat autoimmune diseases is to inhibit TLR signalling, the data outlined above indicate that some TLR signalling is protective in inflammatory diseases, including in lupus-like disease; thus, boosting TLR signalling to strengthen its regulatory function might be an alternative treatment strategy. Indeed, there is already some insight into the use of TLR agonists in the clinic, which shows the feasibility and safety of such approaches. Cancer therapy has undertaken approaches to target TLRs with FDA-approved agonists, including: the locally administered Bacillus Calmette–Guérin vaccine (comprising live attenuated Mycobacterium bovis) for bladder cancer, which can stimulate TLR2 and TLR4 (microbial cell wall) and TLR9 (bacterial DNA); topical imiquimod for pre-malignant actinic keratosis, which targets TLR7 in basal cell carcinoma; and monophosphoryl lipid A, which is a bioactive part of a lipopolysaccharide targeting TLR4 in human papillomavirus-associated cervical cancer100. Further TLR agonists and antagonists are in clinical development for cancer. In this context, TLR agonists are expected to facilitate programmed cell death and enhance immune surveillance101. TLR antagonists are used to limit the TLR-driven growth of tumour cells and it should be considered if some of these strategies could be beneficial in autoimmune diseases.
Conclusions
There is increasing evidence that TLR7 has an important role in SLE pathogenesis, with functions conserved in humans and in mice. TLR7 is critical for the extrafollicular and germinal centre responses associated with the activation of autoreactive B cells that is implicated in this disorder. Genetic studies have shown that TLR7 signalling in B cells is particularly important in orchestrating disease. It is remarkable that the different endosomal TLRs that act as nucleic acid sensors, namely TLR7, TLR8 and TLR9, have distinct roles in patients with SLE. In fact, TLR8 and TLR9 might even have beneficial functions in patients with SLE. Uncovering the biochemistry of these molecular processes is thus important and might lead to the identification of novel targets for drug development.
The ability of TLR signalling to activate and inhibit immune signalling, which is not completely understood, suggests that several strategies could target this pathway to treat disease. Although current clinical developments for targeting TLR signalling are still limited compared with treatments for other biological targets102,103, a number of molecules are currently in development for targeting TLR signalling in inflammatory diseases104 (Table 1). It will be of great interest to follow their clinical development and possible application. Beyond the currently developed approaches, it will also be of interest to identify strategies to rebalance the TLR7 and TLR9 pathways and thus readjust immune homeostasis. It is possible that B cell depletion therapy could reset these pathways by replacing B cells in which this dysregulation might be epigenetically imprinted by novel naive B cells. By contrast, this defect might persist in B cells, including memory B cells that have resisted depletion, thus favouring the restart of disease. Finally, it is important to consider that the inhibition of BCR signalling might interrupt some functions of TLR signalling in B cells76. This interruption might be pertinent for the use of the SYK inhibitor fostamatinib, which is approved for treating the autoimmune disease chronic immune thrombocytopenia, a disease in which the role of intrinsic TLR signalling in B cells is not defined105.
Table 1.
TLR modulators in clinical development for inflammatory diseases
| TLR target | Compound | Target disease | Mechanism of action | Development phase (NCT number) | Refs |
|---|---|---|---|---|---|
| TLR7 | Imiquimod | Actinic keratosis | Immune-stimulator | Phase I (NCT01151956); phase IV (NCT00777127, NCT01453179) | 108–112 |
| GSK2245035 | Rhinitis | Induces type I IFN; immune-stimulator | Phase II (NCT01788813, NCT02446613, NCT01607372); phase I (NCT01480271) | 113,114 | |
| Asthma | Induces type I IFN; immune-stimulator | Phase II (NCT03707678); phase I/II (NCT02833974) | 115 | ||
| TLR9 | CYT003-QbG10 | Asthma | Induces a TH1 cell-mediated immune response | Phase II (NCT02087644, NCT00890734) | 116,117 |
| AZD1419 | Asthma | Induces TH1-type IFN response | Phase II (NCT02898662) | 118,119 | |
| Hydroxychloroquine | Sjögren’s syndrome | Immune modulator | Phase III (NCT00632866, NCT01601028) | 120,121 | |
| IRAK4 | ND-2158 | Rodent models of: lipopolysaccharide-induced TNF production; collagen-induced arthritis; gout; activated B cell like-diffuse large B cell lymphoma; chronic lymphocytic leukaemia | Small molecule inhibitors of inflammatory pathways | Preclinical | 122,123 |
| BMS-986126 | Systemic lupus erythematosus | Inhibitor | Preclinical | 124 | |
| PF-06650833 | Rheumatic autoimmune diseases | Inhibitor | Phase I (NCT02224651, NCT02485769); phase II (NCT02996500) | 98 | |
| BAY1834845 | Psoriasis; pelvic inflammatory disease | Small-molecule inhibitor | Phase I (NCT03493269, NCT03054402) | 125 | |
| IRAK1, IRAK4 and TAK1 | HS-243 | Autoimmune diseases | Inhibitor | Preclinical | 99 |
IRAK-, IL-1 receptor-associated kinase; TAK1, transforming growth factor-β-activated kinase 1; TH1, T helper 1; TLR, Toll-like receptor.
It remains incompletely understood why TLR7 might be deleterious and TLR9 might be protective in SLE, although it is possible that this phenomenon is related to the distinct types of autoantigens that these TLRs recognize. Some immune complexes containing RNP induced TLR7-mediated production of TNF by macrophages and type I IFN by pDCs106,107. However, it is unclear if immune complexes containing TLR9 agonists have similar immunological properties to those containing TLR7 agonists as there has been no systematic comparison of the myeloid cell response to immune complexes associated with RNA (and thus TLR7) versus DNA (and thus TLR9) moieties. There is also some evidence that TLR7 and TLR9 signalling might have opposing roles in SLE given that they distinctively impact B cell activation and differentiation, as mentioned above33. However, the relevance of this difference in patients with SLE has not been tested experimentally. Of note, we still have a limited knowledge of the differences in signalling and cellular responses driven by distinct TLRs; these differences might be a fruitful area for future drug development, especially considering that TLR signalling in B cells can have potent anti-inflammatory functions by eliciting the production of IL-10. The distinct molecular mechanisms associated with the control of immunity, including B cell responses, by TLRs thus seems directly relevant for the development of novel therapeutic strategies for autoimmune diseases.
Acknowledgements
S.F.’s lab is supported for research in this area by ERC PREG-LAB 647696, and an AXA Chair in Translational Immunology. T.D.’s lab is supported by DFG (491/7-5, 10-2, 11-1, and TRR130 project 24).
Author contributions
All of the authors researched data for the article and made substantial contributions to discussion of content, writing and review/editing of the manuscript before submission.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information
Nature Reviews Rheumatology thanks S. Jackson, Z. Rahman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Crickx E, Weill JC, Reynaud CA, Mahevas M. Anti-CD20-mediated B-cell depletion in autoimmune diseases: successes, failures and future perspectives. Kidney Int. 2020;97:885–893. doi: 10.1016/j.kint.2019.12.025. [DOI] [PubMed] [Google Scholar]
- 2.Shen P, Fillatreau S. Antibody-independent functions of B cells: a focus on cytokines. Nat. Rev. Immunol. 2015;15:441–451. doi: 10.1038/nri3857. [DOI] [PubMed] [Google Scholar]
- 3.Davis MLR, et al. Associations of toll-like receptor (TLR)-4 single nucleotide polymorphisms and rheumatoid arthritis disease progression: an observational cohort study. Int. Immunopharmacol. 2015;24:346–352. doi: 10.1016/j.intimp.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 4.Alzabin S, et al. Investigation of the role of endosomal toll-like receptors in murine collagen-induced arthritis reveals a potential role for TLR7 in disease maintenance. Arthritis Res. Ther. 2012;14:R142. doi: 10.1186/ar3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruprecht CR, Lanzavecchia A. Toll-like receptor stimulation as a third signal required for activation of human naive B cells. Eur. J. Immunol. 2006;36:810–816. doi: 10.1002/eji.200535744. [DOI] [PubMed] [Google Scholar]
- 6.Jiang W, et al. TLR9 stimulation drives naive B cells to proliferate and to attain enhanced antigen presenting function. Eur. J. Immunol. 2007;37:2205–2213. doi: 10.1002/eji.200636984. [DOI] [PubMed] [Google Scholar]
- 7.Lampropoulou V, et al. TLR-activated B cells suppress T cell-mediated autoimmunity. J. Immunol. 2008;180:4763–4773. doi: 10.4049/jimmunol.180.7.4763. [DOI] [PubMed] [Google Scholar]
- 8.Lee YH, Choi SJ, Ji JD, Song GG. Association between toll-like receptor polymorphisms and systemic lupus erythematosus: a meta-analysis update. Lupus. 2016;25:593–601. doi: 10.1177/0961203315622823. [DOI] [PubMed] [Google Scholar]
- 9.Mohan C, Putterman C. Genetics and pathogenesis of systemic lupus erythematosus and lupus nephritis. Nat. Rev. Nephrol. 2015;11:329–341. doi: 10.1038/nrneph.2015.33. [DOI] [PubMed] [Google Scholar]
- 10.Pisitkun P, et al. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Ortiz H, et al. Association of TLR7 copy number variation with susceptibility to childhood-onset systemic lupus erythematosus in Mexican population. Ann. Rheum. Dis. 2010;69:1861–1865. doi: 10.1136/ard.2009.124313. [DOI] [PubMed] [Google Scholar]
- 12.Conrad DF, et al. Origins and functional impact of copy number variation in the human genome. Nature. 2010;464:704–712. doi: 10.1038/nature08516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang CM, et al. Genetic variations in toll-like receptors (TLRs 3/7/8) are associated with systemic lupus erythematosus in a Taiwanese population. Sci. Rep. 2014;4:3792. doi: 10.1038/srep03792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Souyris M, et al. TLR7 escapes X chromosome inactivation in immune cells. Sci. Immunol. 2018;3:eaap8855. doi: 10.1126/sciimmunol.aap8855. [DOI] [PubMed] [Google Scholar]
- 15.Margery-Muir AA, Bundell C, Nelson D, Groth DM, Wetherall JD. Gender balance in patients with systemic lupus erythematosus. Autoimmun. Rev. 2017;16:258–268. doi: 10.1016/j.autrev.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Scofield RH, et al. Klinefelter’s syndrome (47,XXY) in male systemic lupus erythematosus patients: support for the notion of a gene-dose effect from the X chromosome. Arthritis Rheum. 2008;58:2511–2517. doi: 10.1002/art.23701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanna Kazazian N, et al. Lupus autoimmunity and metabolic parameters are exacerbated upon high fat diet-induced obesity due to TLR7 signaling. Front. Immunol. 2019;10:2015. doi: 10.3389/fimmu.2019.02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bekeredjian-Ding IB, et al. Plasmacytoid dendritic cells control TLR7 sensitivity of naive B cells via type I IFN. J. Immunol. 2005;174:4043–4050. doi: 10.4049/jimmunol.174.7.4043. [DOI] [PubMed] [Google Scholar]
- 19.Deane JA, et al. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27:801–810. doi: 10.1016/j.immuni.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh ER, et al. Dual signaling by innate and adaptive immune receptors is required for TLR7-induced B-cell-mediated autoimmunity. Proc. Natl Acad. Sci. USA. 2012;109:16276–16281. doi: 10.1073/pnas.1209372109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christensen SR, et al. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Fairhurst AM, et al. Yaa autoimmune phenotypes are conferred by overexpression of TLR7. Eur. J. Immunol. 2008;38:1971–1978. doi: 10.1002/eji.200838138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson SW, et al. Opposing impact of B cell-intrinsic TLR7 and TLR9 signals on autoantibody repertoire and systemic inflammation. J. Immunol. 2014;192:4525–4532. doi: 10.4049/jimmunol.1400098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyake K. Nucleic acid-sensing toll-like receptors: beyond ligand search. Adv. Drug Deliv. Rev. 2008;60:782–785. doi: 10.1016/j.addr.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Tran NL, Manzin-Lorenzi C, Santiago-Raber ML. Toll-like receptor 8 deletion accelerates autoimmunity in a mouse model of lupus through a toll-like receptor 7-dependent mechanism. Immunology. 2015;145:60–70. doi: 10.1111/imm.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desnues B, et al. TLR8 on dendritic cells and TLR9 on B cells restrain TLR7-mediated spontaneous autoimmunity in C57BL/6 mice. Proc. Natl Acad. Sci. USA. 2014;111:1497–1502. doi: 10.1073/pnas.1314121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tilstra JS, et al. B cell-intrinsic TLR9 expression is protective in murine lupus. J. Clin. Invest. 2020;130:3172–3187. doi: 10.1172/JCI132328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Umiker BR, et al. Dosage of X-linked toll-like receptor 8 determines gender differences in the development of systemic lupus erythematosus. Eur. J. Immunol. 2014;44:1503–1516. doi: 10.1002/eji.201344283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eckl-Dorna J, Batista FD. BCR-mediated uptake of antigen linked to TLR9 ligand stimulates B-cell proliferation and antigen-specific plasma cell formation. Blood. 2009;113:3969–3977. doi: 10.1182/blood-2008-10-185421. [DOI] [PubMed] [Google Scholar]
- 30.Viglianti GA, et al. Activation of autoreactive B cells by CpG dsDNA. Immunity. 2003;19:837–847. doi: 10.1016/s1074-7613(03)00323-6. [DOI] [PubMed] [Google Scholar]
- 31.Lau CM, et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J. Exp. Med. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christensen SR, et al. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J. Exp. Med. 2005;202:321–331. doi: 10.1084/jem.20050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nundel K, et al. Cell-intrinsic expression of TLR9 in autoreactive B cells constrains BCR/TLR7-dependent responses. J. Immunol. 2015;194:2504–2512. doi: 10.4049/jimmunol.1402425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soni C, et al. B cell-intrinsic TLR7 signaling is essential for the development of spontaneous germinal centers. J. Immunol. 2014;193:4400–4414. doi: 10.4049/jimmunol.1401720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukui R, et al. Unc93B1 restricts systemic lethal inflammation by orchestrating toll-like receptor 7 and 9 trafficking. Immunity. 2011;35:69–81. doi: 10.1016/j.immuni.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Fukui R, et al. Unc93B1 biases toll-like receptor responses to nucleic acid in dendritic cells toward DNA- but against RNA-sensing. J. Exp. Med. 2009;206:1339–1350. doi: 10.1084/jem.20082316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Majer O, Liu B, Kreuk LSM, Krogan N, Barton GM. UNC93B1 recruits syntenin-1 to dampen TLR7 signalling and prevent autoimmunity. Nature. 2019;575:366–370. doi: 10.1038/s41586-019-1612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leeb T, et al. A missense variant affecting the C-terminal tail of UNC93B1 in dogs with exfoliative cutaneous lupus erythematosus (ECLE) Genes. 2020;11:159. doi: 10.3390/genes11020159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yasutomo K, et al. Mutation of DNASE1 in people with systemic lupus erythematosus. Nat. Genet. 2001;28:313–314. doi: 10.1038/91070. [DOI] [PubMed] [Google Scholar]
- 40.Napirei M, et al. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nat. Genet. 2000;25:177–181. doi: 10.1038/76032. [DOI] [PubMed] [Google Scholar]
- 41.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J. Exp. Med. 1994;179:1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shao WH, Cohen PL. Disturbances of apoptotic cell clearance in systemic lupus erythematosus. Arthritis Res. Ther. 2011;13:202. doi: 10.1186/ar3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Negishi H, et al. Identification of U11snRNA as an endogenous agonist of TLR7-mediated immune pathogenesis. Proc. Natl Acad. Sci. USA. 2019;116:23653–23661. doi: 10.1073/pnas.1915326116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greiling TM, et al. Commensal orthologs of the human autoantigen Ro60 as triggers of autoimmunity in lupus. Sci. Transl. Med. 2018;10:eaan2306. doi: 10.1126/scitranslmed.aan2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richardson C, et al. Molecular basis of 9G4 B cell autoreactivity in human systemic lupus erythematosus. J. Immunol. 2013;191:4926–4939. doi: 10.4049/jimmunol.1202263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richardson CT, et al. Failure of B Cell Tolerance in CVID. Front. Immunol. 2019;10:2881. doi: 10.3389/fimmu.2019.02881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jenks SA, et al. Distinct effector B cells induced by unregulated toll-like receptor 7 contribute to pathogenic responses in systemic lupus erythematosus. Immunity. 2020;52:203. doi: 10.1016/j.immuni.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schickel JN, et al. Self-reactive VH4-34-expressing IgG B cells recognize commensal bacteria. J. Exp. Med. 2017;214:1991–2003. doi: 10.1084/jem.20160201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacobi AM, et al. Correlation between circulating CD27high plasma cells and disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2003;48:1332–1342. doi: 10.1002/art.10949. [DOI] [PubMed] [Google Scholar]
- 50.Tipton CM, et al. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat. Immunol. 2015;16:755–765. doi: 10.1038/ni.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klinman DM, Steinberg AD. Systemic autoimmune disease arises from polyclonal B cell activation. J. Exp. Med. 1987;165:1755–1760. doi: 10.1084/jem.165.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jenks SA, et al. Distinct effector B cells induced by unregulated toll-like receptor 7 contribute to pathogenic responses in systemic lupus erythematosus. Immunity. 2018;49:725–739 e726. doi: 10.1016/j.immuni.2018.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jenks SA, Cashman KS, Woodruff MC, Lee FE, Sanz I. Extrafollicular responses in humans and SLE. Immunol. Rev. 2019;288:136–148. doi: 10.1111/imr.12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM. The generation of antibody-secreting plasma cells. Nat. Rev. Immunol. 2015;15:160–171. doi: 10.1038/nri3795. [DOI] [PubMed] [Google Scholar]
- 55.Wang S, et al. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11chiT-bet+ B cells in SLE. Nat. Commun. 2018;9:1758. doi: 10.1038/s41467-018-03750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buchta CM, Bishop GA. TRAF5 negatively regulates TLR signaling in B lymphocytes. J. Immunol. 2014;192:145–150. doi: 10.4049/jimmunol.1301901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kraus ZJ, Nakano H, Bishop GA. TRAF5 is a critical mediator of in vitro signals and in vivo functions of LMP1, the viral oncogenic mimic of CD40. Proc. Natl Acad. Sci. USA. 2009;106:17140–17145. doi: 10.1073/pnas.0903786106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zumaquero E, et al. IFNγ induces epigenetic programming of human T-bethi B cells and promotes TLR7/8 and IL-21 induced differentiation. eLife. 2019;8:e41641. doi: 10.7554/eLife.41641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu R, et al. Dysregulation of innate and adaptive serum mediators precedes systemic lupus erythematosus classification and improves prognostic accuracy of autoantibodies. J. Autoimmun. 2016;74:182–193. doi: 10.1016/j.jaut.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Munroe ME, et al. Altered type II interferon precedes autoantibody accrual and elevated type I interferon activity prior to systemic lupus erythematosus classification. Ann. Rheum. Dis. 2016;75:2014–2021. doi: 10.1136/annrheumdis-2015-208140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gies V, et al. Impaired TLR9 responses in B cells from patients with systemic lupus erythematosus. JCI Insight. 2018;3:e96795. doi: 10.1172/jci.insight.96795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sieber J, et al. Active systemic lupus erythematosus is associated with a reduced cytokine production by B cells in response to TLR9 stimulation. Arthritis Res. Ther. 2014;16:477. doi: 10.1186/s13075-014-0477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rubtsov AV, et al. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c+ B-cell population is important for the development of autoimmunity. Blood. 2011;118:1305–1315. doi: 10.1182/blood-2011-01-331462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boneparth A, et al. TLR7 influences germinal center selection in murine SLE. PLoS One. 2015;10:e0119925. doi: 10.1371/journal.pone.0119925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Domeier PP, et al. IFN-γ receptor and STAT1 signaling in B cells are central to spontaneous germinal center formation and autoimmunity. J. Exp. Med. 2016;213:715–732. doi: 10.1084/jem.20151722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jackson SW, et al. B cell IFN-gamma receptor signaling promotes autoimmune germinal centers via cell-intrinsic induction of BCL-6. J. Exp. Med. 2016;213:733–750. doi: 10.1084/jem.20151724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thibault DL, et al. IRF9 and STAT1 are required for IgG autoantibody production and B cell expression of TLR7 in mice. J. Clin. Invest. 2008;118:1417–1426. doi: 10.1172/JCI30065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chodisetti SB, et al. Type II but not type I IFN signaling is indispensable for TLR7-promoted development of autoreactive B cells and systemic autoimmunity. J. Immunol. 2020;204:796–809. doi: 10.4049/jimmunol.1901175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dorner T, Lipsky PE. B cells: depletion or functional modulation in rheumatic diseases. Curr. Opin. Rheumatol. 2014;26:228–236. doi: 10.1097/BOR.0000000000000000. [DOI] [PubMed] [Google Scholar]
- 70.Woodruff M, et al. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat. Immunol. 2020;21:1506–1516. doi: 10.1038/s41590-020-00814-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Becker-Herman S, et al. WASp-deficient B cells play a critical, cell-intrinsic role in triggering autoimmunity. J. Exp. Med. 2011;208:2033–2042. doi: 10.1084/jem.20110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Teichmann LL, Schenten D, Medzhitov R, Kashgarian M, Shlomchik MJ. Signals via the adaptor MyD88 in B cells and DCs make distinct and synergistic contributions to immune activation and tissue damage in lupus. Immunity. 2013;38:528–540. doi: 10.1016/j.immuni.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hua Z, et al. Requirement for MyD88 signaling in B cells and dendritic cells for germinal center anti-nuclear antibody production in Lyn-deficient mice. J. Immunol. 2014;192:875–885. doi: 10.4049/jimmunol.1300683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 75.Scapini P, et al. B cell-derived IL-10 suppresses inflammatory disease in Lyn-deficient mice. Proc. Natl Acad. Sci. USA. 2011;108:E823–E832. doi: 10.1073/pnas.1107913108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schweighoffer E, Nys J, Vanes L, Smithers N, Tybulewicz VLJ. TLR4 signals in B lymphocytes are transduced via the B cell antigen receptor and SYK. J. Exp. Med. 2017;214:1269–1280. doi: 10.1084/jem.20161117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Otipoby KL, et al. The B-cell antigen receptor integrates adaptive and innate immune signals. Proc. Natl Acad. Sci. USA. 2015;112:12145–12150. doi: 10.1073/pnas.1516428112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reth M, Brummer T. Feedback regulation of lymphocyte signalling. Nat. Rev. Immunol. 2004;4:269–277. doi: 10.1038/nri1335. [DOI] [PubMed] [Google Scholar]
- 79.Ackermann JA, et al. Syk tyrosine kinase is critical for B cell antibody responses and memory B cell survival. J. Immunol. 2015;194:4650–4656. doi: 10.4049/jimmunol.1500461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101:4500–4504. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- 81.Julia A, et al. Genome-wide association study meta-analysis identifies five new loci for systemic lupus erythematosus. Arthritis Res. Ther. 2018;20:100. doi: 10.1186/s13075-018-1604-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lino AC, et al. LAG-3 inhibitory receptor expression identifies immunosuppressive natural regulatory plasma cells. Immunity. 2018;49:120–133 e129. doi: 10.1016/j.immuni.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J. Immunol. 2009;182:7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tackey E, Lipsky PE, Illei GG. Rationale for interleukin-6 blockade in systemic lupus erythematosus. Lupus. 2004;13:339–343. doi: 10.1191/0961203304lu1023oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abdel Galil SM, Ezzeldin N, El-Boshy ME. The role of serum IL-17 and IL-6 as biomarkers of disease activity and predictors of remission in patients with lupus nephritis. Cytokine. 2015;76:280–287. doi: 10.1016/j.cyto.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 86.Glaum MC, et al. Toll-like receptor 7-induced naive human B-cell differentiation and immunoglobulin production. J. Allergy Clin. Immunol. 2009;123:224–230 e224. doi: 10.1016/j.jaci.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 87.Hanten JA, et al. Comparison of human B cell activation by TLR7 and TLR9 agonists. BMC Immunol. 2008;9:39. doi: 10.1186/1471-2172-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arkatkar T, et al. B cell-derived IL-6 initiates spontaneous germinal center formation during systemic autoimmunity. J. Exp. Med. 2017;214:3207–3217. doi: 10.1084/jem.20170580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weissenberg SY, et al. Identification and characterization of post-activated B cells in systemic autoimmune diseases. Front. Immunol. 2019;10:2136. doi: 10.3389/fimmu.2019.02136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Keller B, et al. High SYK expression drives constitutive activation of CD21low B cells. J. Immunol. 2017;198:4285–4292. doi: 10.4049/jimmunol.1700079. [DOI] [PubMed] [Google Scholar]
- 91.von Bernuth H, et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321:691–696. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ku CL, et al. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. J. Exp. Med. 2007;204:2407–2422. doi: 10.1084/jem.20070628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Slack E, et al. Innate and adaptive immunity cooperate flexibly to maintain host-microbiota mutualism. Science. 2009;325:617–620. doi: 10.1126/science.1172747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ahmad-Nejad P, et al. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur. J. Immunol. 2002;32:1958–1968. doi: 10.1002/1521-4141(200207)32:7<1958::AID-IMMU1958>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 95.Schrezenmeier E, Dorner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat. Rev. Rheumatol. 2020;16:155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 96.Torigoe M, et al. Hydroxychloroquine efficiently suppresses inflammatory responses of human class-switched memory B cells via toll-like receptor 9 inhibition. Clin. Immunol. 2018;195:1–7. doi: 10.1016/j.clim.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 97.Hjorton K, et al. Cytokine production by activated plasmacytoid dendritic cells and natural killer cells is suppressed by an IRAK4 inhibitor. Arthritis Res. Ther. 2018;20:238. doi: 10.1186/s13075-018-1702-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Danto SI, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of PF-06650833, a selective interleukin-1 receptor-associated kinase 4 (IRAK4) inhibitor, in single and multiple ascending dose randomized phase 1 studies in healthy subjects. Arthritis Res. Ther. 2019;21:269. doi: 10.1186/s13075-019-2008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Scarneo SA, et al. A highly selective inhibitor of interleukin-1 receptor-associated kinases 1/4 (IRAK-1/4) delineates the distinct signaling roles of IRAK-1/4 and the TAK1 kinase. J. Biol. Chem. 2020;295:1565–1574. doi: 10.1074/jbc.RA119.011857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Braunstein MJ, Kucharczyk J, Adams S. Targeting Toll-like receptors for cancer therapy. Target. Oncol. 2018;13:583–598. doi: 10.1007/s11523-018-0589-7. [DOI] [PubMed] [Google Scholar]
- 101.Cen X, Liu S, Cheng K. The role of Toll-like receptor in inflammation and tumor immunity. Front. Pharmacol. 2018;9:878. doi: 10.3389/fphar.2018.00878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Felten R, et al. The 2018 pipeline of targeted therapies under clinical development for systemic lupus erythematosus: a systematic review of trials. Autoimmun. Rev. 2018;17:781–790. doi: 10.1016/j.autrev.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 103.Dorner T, Furie R. Novel paradigms in systemic lupus erythematosus. Lancet. 2019;393:2344–2358. doi: 10.1016/S0140-6736(19)30546-X. [DOI] [PubMed] [Google Scholar]
- 104.Javaid N, Yasmeen F, Choi S. Toll-like receptors and relevant emerging therapeutics with reference to delivery methods. Pharmaceutics. 2019;11:441. doi: 10.3390/pharmaceutics11090441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Roskoski R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors: a 2020 update. Pharmacol. Res. 2020;152:104609. doi: 10.1016/j.phrs.2019.104609. [DOI] [PubMed] [Google Scholar]
- 106.Berggren O, et al. B lymphocytes enhance interferon-alpha production by plasmacytoid dendritic cells. Arthritis Rheum. 2012;64:3409–3419. doi: 10.1002/art.34599. [DOI] [PubMed] [Google Scholar]
- 107.Mold C, Clos TW. C-reactive protein inhibits plasmacytoid dendritic cell interferon responses to autoantibody immune complexes. Arthritis Rheum. 2013;65:1891–1901. doi: 10.1002/art.37968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Goldenberg G, Linkner RV, Singer G, Frankel A. An investigator-initiated study to assess the safety and efficacy of imiquimod 3.75% cream when used after cryotherapy in the treatment of hypertrophic actinic keratoses on dorsal hands and forearms. J. Clin. Aesthet. Dermatol. 2013;6:36–43. [PMC free article] [PubMed] [Google Scholar]
- 109.Hadley J, et al. Results of an investigator-initiated single-blind split-face comparison of photodynamic therapy and 5% imiquimod cream for the treatment of actinic keratoses. Dermatol. Surg. 2012;38:722–727. doi: 10.1111/j.1524-4725.2012.02340.x. [DOI] [PubMed] [Google Scholar]
- 110.Serra-Guillen C, et al. A randomized pilot comparative study of topical methyl aminolevulinate photodynamic therapy versus imiquimod 5% versus sequential application of both therapies in immunocompetent patients with actinic keratosis: clinical and histologic outcomes. J. Am. Acad. Dermatol. 2012;66:e131–e137. doi: 10.1016/j.jaad.2011.11.933. [DOI] [PubMed] [Google Scholar]
- 111.Strohal R, Kerl H, Schuster L. Treatment of actinic keratoses with 5% topical imiquimod: a multicenter prospective observational study from 93 Austrian office-based dermatologists. J. Drugs Dermatol. 2012;11:574–578. [PubMed] [Google Scholar]
- 112.Gollnick H, Dirschka T, Ostendorf R, Kerl H, Kunstfeld R. Long-term clinical outcomes of imiquimod 5% cream vs. diclofenac 3% gel for actinic keratosis on the face or scalp: a pooled analysis of two randomized controlled trials. J. Eur. Acad. Dermatol. Venereol. 2020;34:82–89. doi: 10.1111/jdv.15868. [DOI] [PubMed] [Google Scholar]
- 113.Ellis AK, Tsitoura DC, Quint D, Powley W, Lee LA. Safety and pharmacodynamics of intranasal GSK2245035, a TLR7 agonist for allergic rhinitis: a randomized trial. Clin. Exp. Allergy. 2017;47:1193–1203. doi: 10.1111/cea.12974. [DOI] [PubMed] [Google Scholar]
- 114.Tsitoura D, et al. Early clinical evaluation of the intranasal TLR7 agonist GSK2245035: use of translational biomarkers to guide dosing and confirm target engagement. Clin. Pharmacol. Ther. 2015;98:369–380. doi: 10.1002/cpt.157. [DOI] [PubMed] [Google Scholar]
- 115.Biggadike K, et al. Discovery of 6-Amino-2-{[(1 S)-1-methylbutyl]oxy}-9-[5-(1-piperidinyl)pentyl]-7,9-dihydro-8H-pu rin-8-one (GSK2245035), a highly potent and selective intranasal toll-like receptor 7 agonist for the treatment of asthma. J. Med. Chem. 2016;59:1711–1726. doi: 10.1021/acs.jmedchem.5b01647. [DOI] [PubMed] [Google Scholar]
- 116.Casale TB, et al. CYT003, a TLR9 agonist, in persistent allergic asthma - a randomized placebo-controlled Phase 2b study. Allergy. 2015;70:1160–1168. doi: 10.1111/all.12663. [DOI] [PubMed] [Google Scholar]
- 117.Beeh KM, et al. The novel TLR-9 agonist QbG10 shows clinical efficacy in persistent allergic asthma. J. Allergy Clin. Immunol. 2013;131:866–874. doi: 10.1016/j.jaci.2012.12.1561. [DOI] [PubMed] [Google Scholar]
- 118.Jackson S, et al. First-in-human study with the inhaled TLR9 oligonucleotide agonist AZD1419 results in interferon responses in the lung, and is safe and well-tolerated. Clin. Pharmacol. Ther. 2018;104:335–345. doi: 10.1002/cpt.938. [DOI] [PubMed] [Google Scholar]
- 119.Psallidas I, et al. A phase 2a, double-blind, Placebo-controlled randomized trial of inhaled TLR9 agonist AZD1419 in asthma. Am. J. Respir. Crit. Care Med. 2020 doi: 10.1164/rccm.202001-0133OC. [DOI] [PubMed] [Google Scholar]
- 120.Gottenberg JE, et al. Effects of hydroxychloroquine on symptomatic improvement in primary Sjogren syndrome: the JOQUER randomized clinical trial. JAMA. 2014;312:249–258. doi: 10.1001/jama.2014.7682. [DOI] [PubMed] [Google Scholar]
- 121.Yoon CH, et al. Effect of hydroxychloroquine treatment on dry eyes in subjects with primary Sjogren’s syndrome: a double-blind randomized control study. J. Korean Med. Sci. 2016;31:1127–1135. doi: 10.3346/jkms.2016.31.7.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kelly PN, et al. Selective interleukin-1 receptor-associated kinase 4 inhibitors for the treatment of autoimmune disorders and lymphoid malignancy. J. Exp. Med. 2015;212:2189–2201. doi: 10.1084/jem.20151074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gimenez N, et al. Targeting IRAK4 disrupts inflammatory pathways and delays tumor development in chronic lymphocytic leukemia. Leukemia. 2020;34:100–114. doi: 10.1038/s41375-019-0507-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dudhgaonkar S, et al. Selective IRAK4 inhibition attenuates disease in murine lupus models and demonstrates steroid sparing activity. J. Immunol. 2017;198:1308–1319. doi: 10.4049/jimmunol.1600583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wiese MD, Manning-Bennett AT, Abuhelwa AY. Investigational IRAK-4 inhibitors for the treatment of rheumatoid arthritis. Expert Opin. Investig. Drugs. 2020;29:475–482. doi: 10.1080/13543784.2020.1752660. [DOI] [PubMed] [Google Scholar]