Abstract
Maintenance of lipid asymmetry across the two leaflets of the plasma membrane (PM) bilayer is a ubiquitous feature of eukaryotic cells. Loss of this asymmetry has been widely associated with cell death. However, increasing evidence points to the physiological importance of non-apoptotic, transient changes in PM asymmetry. Such transient scrambling events are associated with a range of biological functions, including intercellular communication and intracellular signaling. Thus, regulation of the PM’s interleaflet lipid distribution is a broadly important but underappreciated cellular process with key physiological and structural consequences. Here, we compile the mounting evidence revealing multifaceted, functional roles of PM asymmetry and transient loss thereof. We discuss the ensuing consequences of reversible asymmetry on PM structure, biophysical properties, and interleaflet coupling. We argue that despite widespread recognition of broad aspects of membrane asymmetry, its importance in cell biology demands more in-depth investigation of its features, regulation, as well as physiological and pathological implications.
INTRODUCTION
The PMs of eukaryotic cells are complex mixtures of phospholipids, glycolipids, sterols, and proteins. Classical models of their organization considered a somewhat passive, solvating role for the lipid molecules surrounding functional proteins9. However, nearly concurrent studies proved that almost all major classes of phospholipids are asymmetrically distributed across the two PM leaflets in human red blood cells (RBCs)10. Shortly thereafter, regulated loss of this asymmetry was shown to mediate myoblast fusion11 and platelet activation12, implying important functional roles for membrane lipids and their distribution.
Asymmetric transbilayer composition of the mammalian PM.
Understanding the structural and functional roles of PM lipid organization requires detailed knowledge of the lipid compositions of the two bilayer leaflets. Modern mass spectroscopy allows unprecedented quantitation of complex lipidomes, and this technology was recently used to measure the comprehensive lipid complements of the two PM leaflets in human RBCs1,13 (Fig 1A). The results agree broadly with classical estimates of PM asymmetry (Table 1) with an exoplasmic (outer) leaflet composed primarily of the choline-headgroup phospholipids phosphatidylcholine (PC) and sphingomyelin (SM) while the cytoplasmic (inner) leaflet contains larger phospholipid complexity, including phosphatidylethanolamine (PE) and the charged lipids phosphatidylserine (PS) and phosphatidylinositol (PI). In addition to this classically recognized headgroup asymmetry, lipidomics revealed novel interleaflet differences in acyl chain structure, with inner leaflet lipids bearing ~2-fold more unsaturations1 (Fig 1A). A surprising and interesting detail was that PC lipids go counter to this trend, with more saturated species tending to accumulate in the inner leaflet1 (Fig 1A).
Figure 1. The interleaflet asymmetry of the PM bilayer is complex and dynamic.
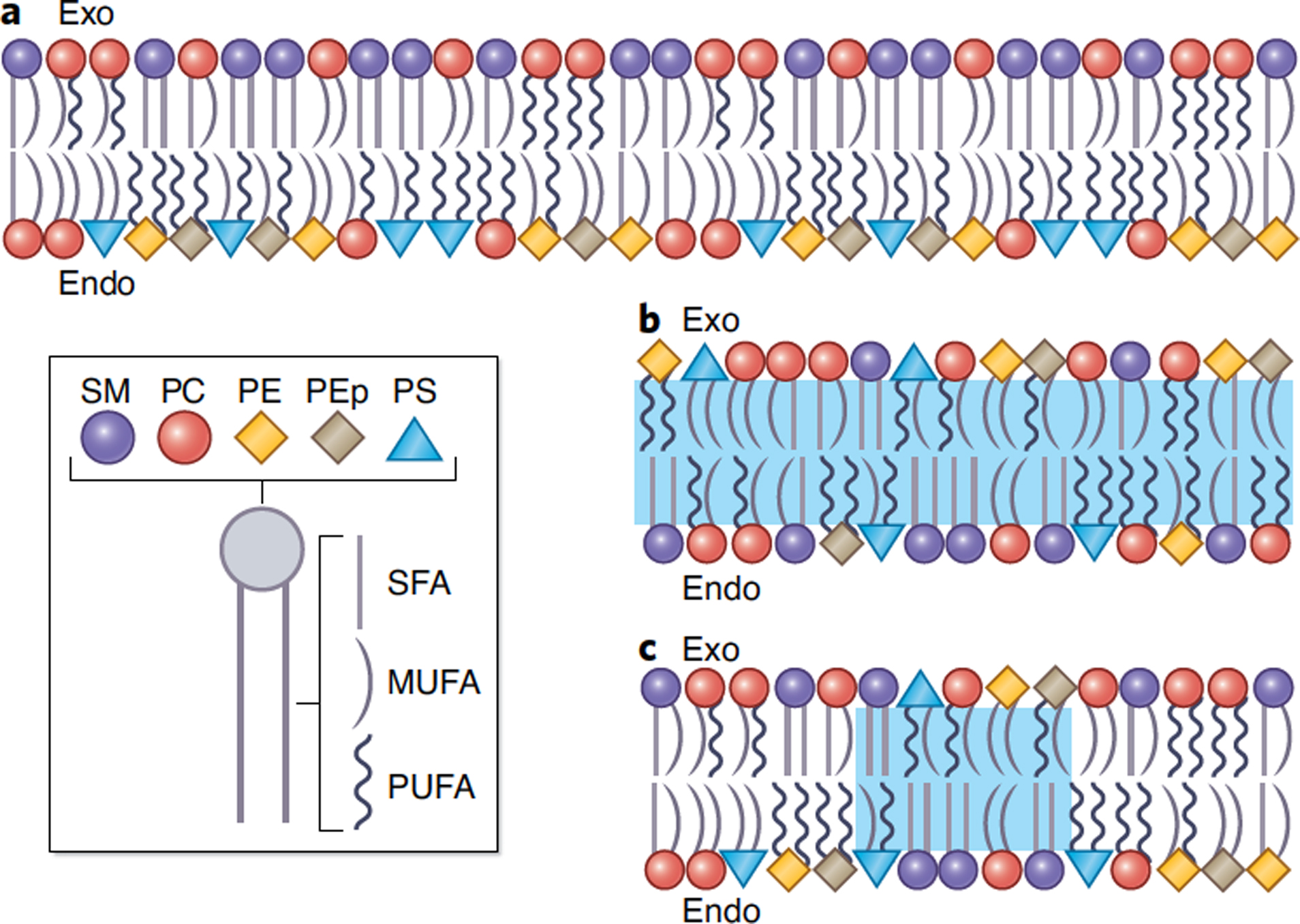
(A) Data-driven1 schematic illustration of the phospholipid asymmetry of the RBC PM. Lipid asymmetry can be released by lipid scrambling (i.e. interleaflet lipid mixing, blue regions) that occurs either (B) globally or (C) at specialized local sites. Legend on the bottom left applies to all panels; SFA denotes saturated, MFA monounsaturated, and PUFA polyunsaturated fatty acids.
Table 1.
Overall leaflet PL class compositions of RBC PMs. Shown are mol% of various classes per leaflet.
| Group | Year | Technique | Leaflet | SM | PC | PE | PI | |
|---|---|---|---|---|---|---|---|---|
| Verkleij et al10 | 1973 | enzyme digestion* + TLC | Human | exo- | 41% | 48% | 11% | 0% |
| endo- | 10% | 17% | 49% | 0% | ||||
| Renooij16 | 1974 | enzyme digestion* + TLC | Rat | exo- | 27% | 59% | 11% | |
| endo- | 0% | 31% | 39% | |||||
| Rawyler et al17 | 1985 | PLA digestion + PC transfer protein + fluorescamine labeling + TLC | Mouse | exo- | 33% | 45% | 16% | 6% |
| endo- | 3% | 48% | 29% | 4% | ||||
| Van der Schaft et al18 | 1987 | PLA digestion + PC transfer protein + fluorescamine labeling + TLC | Monkey | exo- | 42% | 53% | 5% | 0% |
| endo- | 4% | 34% | 39% | 8% | ||||
| Lorent et al1 | 2020 | enzyme digestion + shotgun lipidomics | Human | exo- | 48% | 45% | 3% | 0% |
| endo- | 3% | 25% | 42% | 2% |
RBCs provide the optimal platform to study transbilayer lipid distributions because they lack internal membranes. However, broad inferences about PM lipid asymmetry in nucleated cells have been made based on their exoplasmic lipid contents. Such studies are qualitatively consistent with conclusions from RBCs, with outer leaflets containing high levels of SM and PC and negligibly low amounts of charged lipids14. Comparison of exoplasmic leaflet lipidomes to those of the overall compositions of PMs isolated from nucleated cells15 clearly reveals that the broad details of PM asymmetry are preserved across mammalian cell types.
Hallmarks of steady-state PM asymmetry.
A hallmark of PM asymmetry common to nearly all eukaryotic organisms is the near-exclusive confinement of charged lipids to the cytosolic PM leaflet. Structurally, this segregation of charge contributes to significant electrostatic potential across cell membranes19,20 and very different surface charge densities for the two membrane faces. These effects likely have direct functional implications for the insertion and orientation of transmembrane (TM) proteins. Positively charged residues on such proteins are highly over-represented in cytosolic juxtamembrane regions, likely because this orientation facilitates their proximity to the negatively charged cytosolic leaflet21. This so-called “positive-inside rule” points to a key role for membrane asymmetry in determining TM protein topology and has been essential for inferring topology of uncharacterized proteins21. Remarkably, dynamic lipid-dependent changes in protein topology have been observed in bacterial proteins prompting a proposed extension of the positive-inside rule to a “charge-balance rule”22. Whether analogous “protein flip-flop” occurs in eukaryotic organisms remains to be shown.
The negative charge of many PM lipids and the surface charge density consequent to their accumulation on cytosolic leaflets also serve a key organizing role for peripheral membrane proteins with surface-exposed polybasic stretches23–25, providing strong electrostatic attractions for their localization to the PM. This functional localization can then be regulated by either tuning lipid composition or phosphorylating relevant protein motifs to disrupt the electrostatic attractions26.
The functional roles of the charged inner leaflet are complemented by the versatile roles of the mostly neutral exoplasmic leaflet. Enriched in tightly packing sphingolipids, the outer PM leaflet is highly ordered and rigid1,27,28. This composition and structure are likely related to the essential barrier function of the PM, imparting low passive permeability to most solutes29. The unique properties of the outer PM leaflet may also serve as a recognition feature preventing healthy cells from becoming targets of the body’s own immune system. An example is perforin, a protein secreted by cytotoxic T cells to form pores in target cells. Perforin binding and activity depend on relatively loose lipid packing, which would not be the case in most healthy outer PM leaflets30. In addition, multiple reports have indicated the presence of specialized PM signaling platforms, or rafts, which are believed to originate in the exoplasmic leaflet and participate in a multitude of functional contexts31.
Finally, the asymmetric lipid distribution and high cholesterol content (~40 mol%; ~25% w/w or v/v) combine to impart unique properties to the mammalian PM: relatively thick32 and structurally asymmetric, with a more tightly packed outer half. These features create the potential for ‘sorting signals’ that facilitate protein sorting by optimizing the structural “fit” between a protein and the hydrophobic membrane core (see1,33 and references therein).
Maintenance and disruption of PM asymmetry.
The importance of the complex PM asymmetry is in part illustrated by the ubiquitous abundance of proteins responsible for shuttling lipids between leaflets. Energy-consuming asymmetry-driving enzymes known as either flippases, moving lipids “in” (i.e. to the cytoplasmic leaflet), or floppases, pushing lipids “out” (into the exoplasmic leaflet)34,35, and energy-independent scramblases facilitating bi-directional lipid flow, all participate in the regulated maintenance of specific lipid distributions in the PM. In comparison to flippases/floppases, scramblases are less headgroup selective and have much higher lipid fluxes, meaning that lipid asymmetry is much faster to break than to re-establish. For many years, the single-pass TM protein PLSCR1 was implicated as the only known scramblase; however, more recent insights have identified a family of TMEM16 proteins as bona fide lipid channels (see36 and references therein). Most notable among these is TMEM16F (also referred to as Ano6), a calcium-gated scramblase playing a major role in thrombosis among other processes (see below), whose loss-of-function leads to a human bleeding disorder called Scott Syndrome36. Recent insights into the mechanism of lipid scrambling by TMEM16F imply lack of lipid headgroup specificity and rapid flux, making them efficient disrupters of PM asymmetry37. Ultimately, the opposing actions of slow-but-constitutive flippases/floppases versus fast-but-regulated scramblases combine to dynamically regulate transbilayer membrane lipid distributions.
Loss of PM asymmetry.
Given the multifaceted roles of the PM, it is not surprising that loss of PM asymmetry can have profound consequences for the cell. During apoptosis, the simultaneous activation of Xkr8, a caspase-mediated scramblase, and inactivation of aminophospholipid flippases leads to rapid exposure of PS throughout the outer PM leaflet12,38 (Fig 1B). Although most widely studied in the context of apoptosis, PS exposure can also be observed during non-apoptotic forms of cell death, including necrosis, ferroptosis, and necroptosis39.
While lipid scrambling is typically associated with cell death, recent findings have definitely established that transient, reversible, and localized disruption of PM asymmetry in living cells (Fig 1C) is implicated in a variety of physiological contexts. In the next section, we summarize examples of functional transbilayer lipid redistribution that demonstrate the involvement of PM asymmetry and scrambling in intracellular signaling and intercellular communication. We then turn to the structural consequences of lipid asymmetry in cell membranes, including the complex issue of coupling and communication between the two lipid leaflets. We conclude with a discussion of PM cholesterol, whose distribution is poorly understood and likely to have massive implications on PM structure and function.
FUNCTIONAL ROLES OF REVERSIBLE LIPID ASYMMETRY IN LIVING CELLS
An early clue that changes in membrane asymmetry are not restricted to terminal stages of cell death was the discovery that cells can expose PS very rapidly in response to apoptotic stimuli, but more importantly, that this exposure is reversible, i.e. preceding commitment to cell death40. This result was significant in demonstrating that loss of PM asymmetry could potentially be a regulated and transient cellular response to a stimulus. This effect has been subsequently confirmed in a wide range of physiological contexts that involve transient changes in PM asymmetry that affect intercellular communication, the structure of the PM, and interactions with membrane-associated proteins (Fig 2). Below, we discuss several examples to illustrate the ubiquity of the phenomenon; as this discussion is not intended to be comprehensive, we refer the reader to other excellent reviews on the topic6,12. It is worth noting that PS exposure is often induced by elevation of cytosolic calcium, likely via activation of Ca2+-gated scramblases like TMEM16 family members38 (Fig 2). However, the precise mechanisms underlying transient translocation of PS to the outer PM leaflet remain largely unexplored.
Figure 2. Functional roles of reversible PM lipid asymmetry.
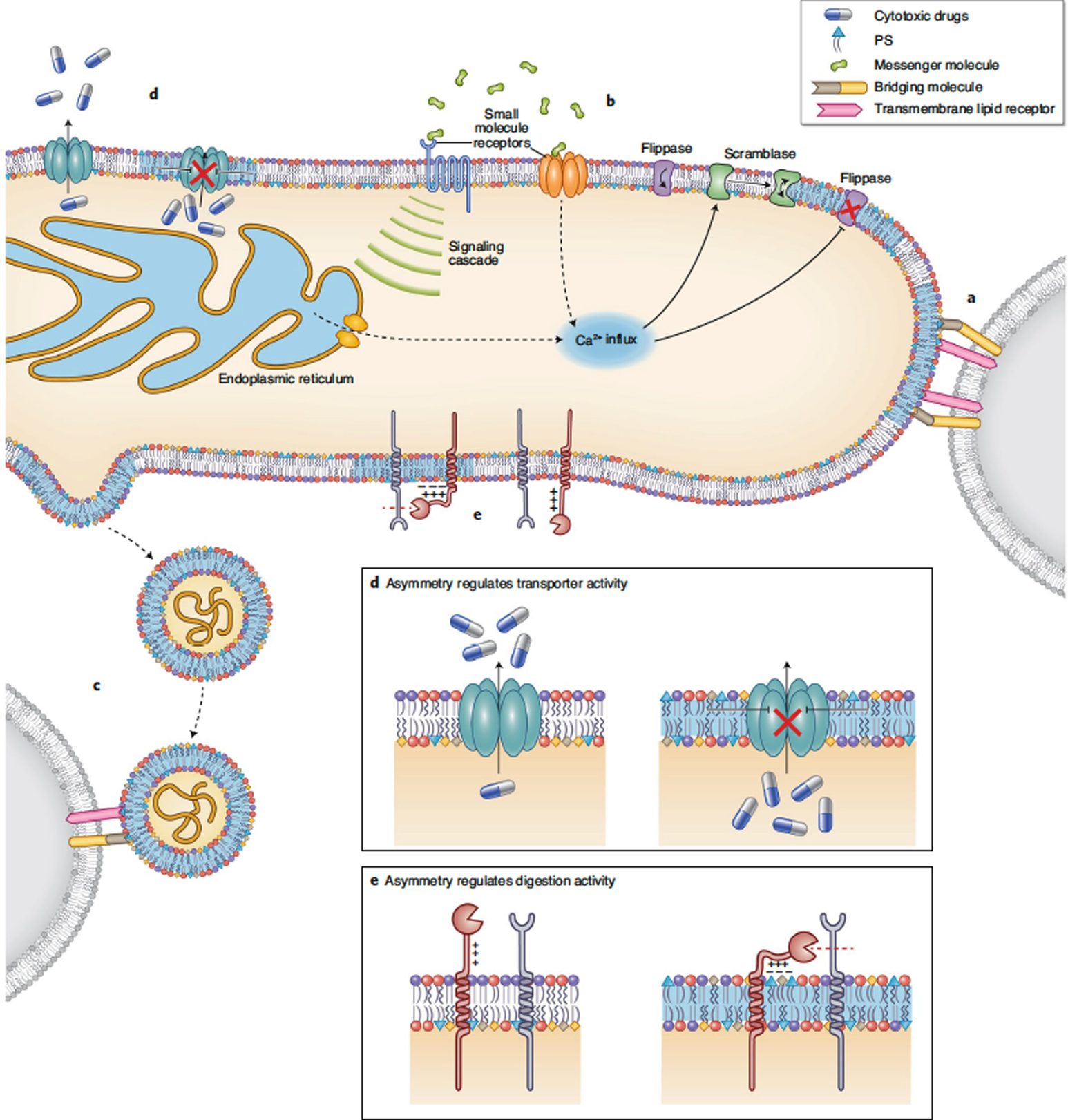
Transient, non-apoptotic changes in PM lipid asymmetry have been implicated in a variety of biological processes including (A-C) cell-cell communication and contact and (D-E) intracellular signaling. (A) Surface exposed PS facilitates intercellular contact and fusion by interacting either directly (with PS receptors) or indirectly (through bridging molecules) with PM components on neighboring cells. (B) Small molecule signals can lead to changes in PM asymmetry, e.g. ATP released at sites of cell damage, peroxynitrate secreted by microglia to facilitate engulfment of viable neurons, bicarbonate that induces sperm capacitation, and immune antigens. These signals either open calcium channels on the PM or lead to release of ER calcium stores. Increased cytosolic calcium inhibits flippases and activates scramblases, leading to loss of asymmetry6. (C) Microvesicles can bud out of regions of altered PM asymmetry. These vesicles can be sensed by their exposed PS via bridging molecules or lipid-binding proteins on other cells. (D) Loss of PM asymmetry can affect the function of transmembrane proteins. The P-glycoprotein responsible for export of cytotoxic drugs has reduced efflux activity upon PM scrambling. (E) Relatedly, proteases of the ADAM family (10 and 17) contain polybasic motifs whose conformational changes upon PS exposure may putatively promote their sheddase function7,8.
Maintenance and pruning of neuronal connections.
The development and function of the nervous system involves pruning of supernumerary neuronal synapses, proper response to injury and inflammation, and ultimate regeneration of neuronal circuits. Localized, reversible PS exposure has been observed in each of these processes. One example is phagoptosis, a process in which microglia, the macrophages of the nervous system, phagocytose non-apoptotic neurons41. Upon inflammation (e.g. caused by bacterial components) the glial cells produce messenger signals necessary and sufficient to cause reversible PM scrambling on the surface of nearby neurons (Fig 2B). Exposed PS is then recognized by lactadherin, a PS-binding protein secreted by the activated microglia, facilitating the phagocytosis of the live neuron42 (Fig 2A). Blocking either PS exposure or lactadherin binding rescues neurons from phagoptosis despite ongoing neuroinflammation. PS scrambling may also be important for re-establishing connections in the nervous system, via triggering of axon regeneration and/or axon repair after injury43,44.
In addition to mediating inflammatory responses and axonal regeneration, dynamic PM scrambling (i.e. typically reported via exposure of PS, see Box 1) has also been shown to be important for neurodevelopment from flies to mammals (see45 and references therein), via pruning of neuronal synapses. Synaptic pruning consists of removal of underutilized synapses to fine-tune neural connectivity. This process appears to involve highly localized PS exposure on the external leaflet of synaptic connections, which are thereby marked for phagocytic clearance by microglia46. Removal of healthy membrane in the nervous tissue also occurs on a regular basis in the rod cells in the retina. Under circadian control, the photosensitive tips of these cells (i.e. photoreceptor outer segments, POS) are shed, then phagocytosed by adjacent retinal pigment epithelium (RPE). This POS shedding and phagocytosis are coincident with highly localized exposure of PS on the POS tips47. Surprisingly, this change in PM asymmetry in the rod cells seems to be induced by signals from the adjacent RPE cells47. Similar local, rapid, and reversible PS externalization was observed in the sensory hair cells in the mammalian inner ear48. These observations provide examples of evolutionarily conserved and tightly controlled intercellular communication mediated by regulation of PM asymmetry (Fig 2A–B).
BOX 1. PS exposure versus PM scrambling.
It is important to emphasize that functional loss of PM asymmetry has been almost exclusively assayed via the exposure of PS on the outer PM leaflet. Such assays are methodologically convenient due to both the near-absolute confinement of PS on the cytosolic leaflet in most cell states (e.g. Table 1) and the availability of high-affinity, PS-specific fluorescent probes (e.g. Annexin V). However, ‘PS exposure’ is a relatively limited readout of PM asymmetry because it does not report on the proportion of PS exposed nor obviously on the (re)distribution of the other PM lipids. Furthermore, Annexin V is a very high-affinity PS ligand that may interfere with the dynamics of PS redistribution. Thus, PS exposure should not necessarily be equated with complete PM lipid scrambling. In fact, PS exposure can differ drastically between various treatments in its abundance, duration, and distribution, implying important and often-overlooked regulatory roles of not only the presence, but also the extent, of lipid asymmetry in cell biology.
Muscle development.
Functional PM scrambling is associated with promoting cell-cell contact and fusion across several functional settings (Fig 2A). This effect is particularly notable in skeletal muscle development (myogenesis), which requires fusion of mononucleated myoblasts into multinucleated myotubes. Direct imaging of non-apoptotic and transient PS exposure (via binding of Annexin V, a high-affinity PS-binding protein) on the surface of differentiating myoblasts in both mouse embryos and cultured cells provided evidence that lipid redistribution actively contributes to myotube formation49. The functional role of PS exposure in primary myoblast fusion was confirmed by showing that masking of external PS inhibited, while exogenously introduced PS promoted, formation of myotubes50. The mechanism for this effect likely involves recognition of exposed PS by a receptor on a neighboring cell49 (Fig 2A), with proteins like BAI1 and Stabilin-251 proposed for this function. A potentially parallel or complementary mechanism for the fusogenic role of lipid scrambling is deactivation of PIEZO1 by surface exposed PS. PIEZO1 is a mechanosensitive PM Ca2+ channel, which when activated, has an inhibitory role in myotube formation52. Altogether, these findings reveal that transient interleaflet redistribution of PS (and likely other PM lipids11) promotes cell-cell contact/fusion and myotube formation/regeneration by regulating both extracellular and intracellular processes.
Mammalian fertilization.
Non-apoptotic exposure of inner leaflet aminophospholipids was observed in the head region of mammalian sperm cells primed to fuse with oocytes53. The role of PM scrambling in sperm-egg fusion was later confirmed by observations that PS receptors (BAI1) on the oocyte PM bind PS lipids exposed on the sperm head to initiate signaling events that ultimately lead to the fusion of the two cells54. A truly remarkable observation was that PS-exposing mammalian sperm can also fuse with skeletal myoblasts, presumably through the BAI1-mediated pathway54. This result and the ubiquity and diversity of PS receptors55 suggest that PS externalization and recognition may be a generic mechanism for cell-cell fusion that is involved in a broad palette of cellular functions (Fig 2A).
Immune signaling.
Immune cells have evolved a plethora of responses to defend the body from infections. Excitingly, many of these responses are associated with PM scrambling. For example, rapid and reversible exposure of PS on the outer leaflet of mast cells is observable after antigen-mediated activation through the Fc receptors56 (Fig 2B). Although these cells bind annexin V (primary evidence for exposed PS), no other apoptotic markers are observable, confirming cell viability. There was also a notable mechanistic and temporal correlation between PS exposure and the release of secretory granules (Fig 2C). This correlation is likely due to the key role that intracellular calcium plays in activation of both immune responses and PM scramblases57. Similar PM lipid redistribution also occurs during Ca-regulated exocytosis in neuroendocrine cells, specifically in the vicinity of secretory granule fusion sites58.
Purinergic signaling is an important mechanism for immune activation, wherein extracellular ATP or nucleotides escaping from dying cells activate receptors on immune cells to signal tissue damage. Loss of PM asymmetry is key for this mode of activation, particularly for secretion of the cytokine interleukin-1β (IL-1β) from monocytes59 (Fig 2C). Activation of the P2X7 ATP receptor in the monocyte PM leads to the rapid (within seconds), reversible exposure of PS and subsequent membrane blebbing, which may be responsible for the release of cytokine-loaded microvesicles59. These microvesicles also have PS on the outside, suggesting they are formed from scrambled regions of the PM (Fig 2C). Such rapid release of cytokine-carrying PS-decorated particles may be a mechanism for intercellular communication with other immune system cells via their PS receptors. Analogous processes are likely responsible for ATP-activated PS exposure in T-cells (Fig 2B), which causes both a decrease in the efflux activity of P-glycoprotein (Fig 2D) and shedding of the homing receptor L-selectin60. L-selectin is one of the major substrates of ADAM17, a membrane metalloproteinase, and PS exposure appears to strongly regulate its protease activity, as well as that of other members of the ADAM family7,8(Fig 2E). Finally, transient PS exposure has been implicated in the formation of contacts between cytotoxic T-cells and antigen-presenting cells61 as well as phagocytosis of non-apoptotic neutrophils62. These results present illustrations of the ubiquity of regulated PM scrambling in both inter- and intra-cellular processes.
STRUCTURAL CONSEQUENCES OF LIPID ASYMMETRY AND ITS LOSS
PM structure:
Directly studying the physical structure of the cellular PM has been technically challenging because of its complexity and plasticity63. Insights are generally based on indirect measurements of exogenous reporter molecules to infer properties such as lipid packing, order and diffusion64,65. Such methods rarely access, or even consider, asymmetry of physical properties between the two PM leaflets. To probe biophysical asymmetry in the PM, previous studies have relied on extracellular quenchers to isolate inner leaflet signal or headgroup-specific labeling to take advantage of the cell’s mechanisms for interleaflet lipid sorting. However, these methods often yielded contradictory conclusions, with some reporting more fluid inner leaflets66,67 and others fluid outer68,69. More recently, two parallel studies converged on very similar conclusions using two completely distinct, well validated approaches, suggesting a densely packed, less fluid exoplasmic leaflet and a loosely packed, relatively fluid cytosolic leaflet (Fig 3A)1,28. This trend is fully consistent with the general phospholipid compositions of the two leaflets (Table 1) as well as the acyl chain asymmetries1 (Fig 1A), which suggest that >60% of the lipids in the inner leaflet bear highly disordered polyunsaturated fatty acids while a similar fraction (~60%) of outer leaflet lipids have 1 or fewer cis double bonds and are therefore more tightly packed. Interestingly, it was recently suggested that in E. coli, the inner leaflet of the cytoplasmic membrane is the more ordered one70.
Figure 3. Effects of lipid scrambling on PM physical properties.
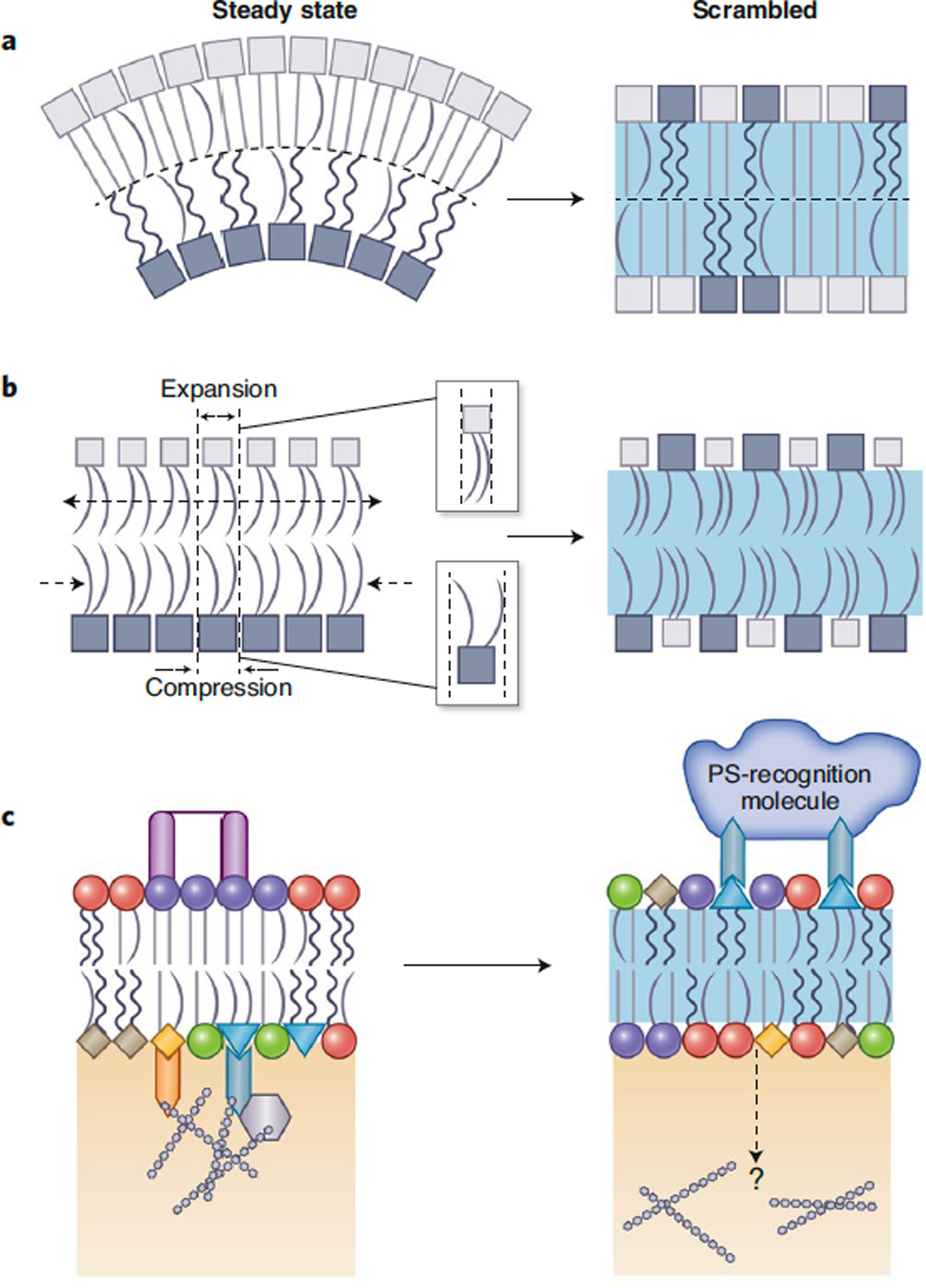
(A) Asymmetric PMs can exhibit unique structural and mechanical characteristics, including higher lipid packing and lower fluidity in the exoplasmic leaflet. Also, due to the asymmetric phospholipid distribution, such membranes likely have non-zero spontaneous curvatures. In contrast, fully scrambled membranes have identical packing, thickness and fluidity in the two leaflets, and no spontaneous curvature. (B) Depending on the interleaflet abundances of phospholipids, an asymmetric membrane may be subject to intrinsic leaflet stresses arising from suboptimal packing densities of the two leaflets. Symmetrizing the lipid compositions of the leaflets would restore optimal lipid packing and eliminate differential stress. (C) Communication between membrane leaflets can be achieved through interleaflet coupling. Clustering of glycolipids or GPI-anchored proteins in the exoplasmic leaflet, or charged lipids interacting with the cytoskeleton in the cytosolic leaflet, can transmit signals across the membrane and promote lateral reorganization of the opposing leaflet. Changes in PM asymmetry leading to surface exposure of PS may result in local detachment of the cytoskeleton and/or promote pinning from the outside via PS-binding molecules.
Generally, lipid packing is positively correlated with membrane thickness. Inferences about the overall thickness of the PM have been made from the inter-organellar sorting of single-pass transmembrane (TM) proteins. Both experimental and bioinformatic analyses reveal that PM proteins have longer TM segments than those of inner membranes, implying that the PM is the thickest membrane in the cell (see32,71 and references therein). Further, PM proteins tend to have asymmetric TM segments, with relatively larger surface areas facing inner leaflet lipids, consistent with the relatively loose lipid packing therein1. Measurements of individual leaflet thicknesses have not been attempted yet, though recent electron microscopy quantification of membrane thickness imply that such measurements are possible72,73. The trends in lipid packing prompt the expectation that the exoplasmic leaflet is thicker than the cytosolic (Fig 3A).
Complete PM scrambling is expected to result in wholesale mixing of the lipid species of the two leaflets, producing a compositionally and biophysically symmetric membrane (Fig 1B and Fig 3). A more complicated question concerns the physical state of the bilayer during transient, localized loss of PM asymmetry, as in the many cases described above. In those, PS-binding probes are not homogeneously distributed, but rather highly localized into specific regions or small clusters over the cell surface49,53,57,58,74,75 (Fig 1C). This is a rather surprising distribution, as scrambled lipids would be expected to rapidly diffuse over the entire cell surface. The mechanisms responsible for maintaining this confined distribution are currently unknown, though possible contributing factors include non-uniform distribution of scramblases, presence of diffusion barriers at the PM in the form of the actin cytoskeleton meshwork57, or clustering of PS by lipid binding proteins (including experimental PS probes; see Box 1). If such domains constitute regions of fully scrambled PM, they may have structural properties similar to completely scrambled PMs (e.g. in apoptosis)1,76. On the other hand, they may present environments entirely distinct from either the asymmetric or fully scrambled state.
PM mechanics:
The structural properties discussed above refer mainly to the membrane’s static conformation. In contrast, PM mechanical properties, e.g. bending rigidity, stretch modulus, and resting tension, describe its deformations in response to mechanical stresses77. As with static parameters, quantification of these mechanical quantities in living systems is extremely challenging due to the chemical complexity of the membrane and its intrinsic integration with other cellular components, most notably the cortical cytoskeleton. The rigidity of the PM is strongly influenced by active processes in the cell78 and both the resting tension and elastic modulus are non-homogeneously distributed across the cell surface79,80. However, while it is difficult to characterize the mechanical properties of the asymmetric PM in a living cell, several points can be made about the relevant effects of lipid scrambling. First, changes in PM asymmetry would be expected to change the spontaneous curvature of the membrane (Fig 3A). Such spontaneous, or intrinsic, curvature originates from the chemical structures of the component lipids; in a symmetric bilayer, it is by definition zero because any intrinsic curvature of one leaflet is exactly offset by identical, but reversely oriented, apposing leaflet. However, in a bilayer like the PM, whose asymmetrically distributed phospholipids have very different spontaneous curvatures, there is likely a non-zero energetic cost to keeping the bilayer flat that would be released upon membrane scrambling (Fig 3A).
Another concept specifically relevant for asymmetric membranes is leaflet intrinsic stress, which arises from differential packing of the two leaflets81 (Fig 3B). Symmetric bilayers at equilibrium are tensionless. In the asymmetric case, one leaflet may have a different optimal packing density and consequently, a different preferred overall area, than the one imposed by the closed geometry of the membrane. An example of such a configuration is a leaflet of tightly packing SM lipids (area/molecule ~ 55 A2) coupled to a leaflet composed of an identical number of loosely packing unsaturated PS lipids (area/molecule ~ 70 A2). Because both leaflets must have identical areas in a flat bilayer, the SM leaflet becomes stretched from its optimal configuration while the PS leaflet is compressed, leading to intrinsic resting stress and suboptimal leaflet thicknesses (Fig 3B). Intrinsic stress can also be generated if identically composed leaflets have different number of lipids between them, or can be absent from an asymmetric bilayer in which the number and types of lipids are balanced appropriately between leaflets82. Neither of these scenarios are likely in a living asymmetric cell PM. Instead, in the limiting cases of an asymmetric PM and its fully scrambled equilibrated counterpart, intrinsic stresses are likely to significantly affect the properties of the former and be irrelevant for the latter (Fig 3B). Notably, even though differential stresses result from suboptimal leaflet packing densities, such configurations are not necessarily unstable. Rather, intrinsic stresses may naturally characterize the preferred configuration of a bilayer with actively maintained membrane asymmetry.
An important development for understanding the properties of cellular PMs in isolation from the overwhelming complexity of living cells are Giant Plasma Membrane Vesicles (GPMVs). GPMVs preserve the chemical complexity of the native PM, but are separated from the cell, lack assembled actin/tubulin cytoskeleton, and are amenable to biophysical approaches developed for model membranes83. However, these vesicles are not strictly asymmetric as illustrated by the exposure of some endoplasmic leaflet lipids on their external surface84,85. Although technical difficulties preclude facile analysis of individual leaflet compositions86, it is likely that these vesicles are better models of a scrambled, symmetric PM rather than its asymmetric counterpart. Despite no direct evidence for complete scrambling, GPMVs have been shown to have pores of varying sizes86, priming them for rapid interleaflet lipid diffusion and likely precluding any potential maintenance of asymmetry. Thus, great caution must be exercised in relating the properties of isolated plasma membrane vesicles to those of intact cell PMs.
Interleaflet coupling:
An open question at the heart of membrane structure and asymmetry is how information about lipid organization in one leaflet gets transmitted to the opposing leaflet, otherwise known as ‘interleaflet coupling’ (Fig 3C). Obviously, transmembrane proteins are the major pathway for signal transduction and material transport across the bilayer; however, communication across the membrane is also possible without membrane spanning protein domains. For example, it has been shown that binding of extracellular bacterial toxins, antibodies, or lectins to glycolipids (which are present almost exclusively on the exoplasmic PM leaflet) is sufficient to initiate signaling cascades inside the cell87,88. Similarly, clustering of the glycosylphosphatidylinositol-anchored protein CD59 exclusively on the outer PM leaflet results in activation of mitogenic signaling inside the cell88. The mechanism of transbilayer signaling relies on recruitment of inner leaflet anchored proteins (Lyn and H-Ras) immediately underneath the outer CD59 clusters. Similar effects are observed by clustering the ganglioside GM1 on the exoplasmic side of the PM, indicating a more general mechanism of signal propagation through the lipid bilayer88. It is important to note that in these examples, communication between leaflets is believed to be mediated solely by acyl chains, either coupled to lipids or proteins. Such immobilization (or pinning) of membrane components in one leaflet appears to be a major contributor to transbilayer coupling in cells89 (Fig 3C). On the cytosolic face of the PM, pinning can be mediated by interactions of anionic lipids like PS and PIP2 with cytoskeletal filaments (see references in89). These pinned lipids can potentially communicate to sphingolipids in the opposing PM leaflet90, possibly through interdigitation of acyl chains across the bilayer mid-plane87,91,92. PM scrambling would likely decrease pinning to the cytoskeleton by translocating the main mediators of PM-actin interaction (anionic lipids) to the exoplasmic leaflet (Fig 3C). At the same time, it is conceivable that PS exposed on the cell surface could promote pinning from the outside by PS-binding proteins in the extracellular space (e.g. Annexin). In the case of complete PM scrambling, such signals could be transduced to the cytosol via long-chain sphingolipids shuffled to the inner leaflet (Fig 3C). While only speculative, such mechanistic principles could explain membrane structure and signaling changes occurring during wholesale or partial loss of PM asymmetry.
It is important to note that while interleaflet lipid coupling is likely essential for the physiology of living membranes, its underlying physical mechanisms remain poorly understood. Robust bilayer-mediated interleaflet coupling has been demonstrated in a variety of experimental and computational studies in synthetic, symmetric bilayers2,3,31,87,93. However, a critically important consideration for analysis and interpretation of both experimental and computational asymmetric model systems is the residual leaflet tension (differential stress) that arises from an imbalance of the optimal lipid packing densities in the two leaflets81. Such an imbalance could be produced by removing lipids from one side of the bilayer, creating a metastable state that could be relatively long-lived due to slow phospholipid flip-flop. While a living asymmetric PM can likely maintain non-zero differential stresses within its leaflets indefinitely, overlooking such intrinsic stresses resulting from compositional asymmetry in model membranes can severely distort structural and mechanistic insights81,82. Thus, a major challenge for membrane biophysics is the development and characterization of methods to monitor and control the intrinsic leaflet stresses in biomimetic, asymmetric bilayers.
The weird, wandering, wayward world of cholesterol
At ~40 mol%, cholesterol is the most abundant single component of the PM1, more than doubling the abundance of the most abundant phospholipid class (PC). Given that cholesterol is not a bilayer-forming lipid and flips rapidly between leaflets94, it should be viewed separately from the membrane’s relatively stable phospholipid matrix. Instead, cholesterol’s unique qualities arise from its complex, dynamic interactions with, and effects on, other PM resident molecules. Both because of its abundance and its central role in membrane structure and dynamics, it is essential to understand cholesterol’s interleaflet distribution and determinants thereof. Remarkably, existing evidence on cholesterol distribution spans almost the entire range of possible outcomes, from 80% of cholesterol residing in the inner leaflet to more than 10-fold enrichment in the outer leaflet4,5,95. This stunning lack of agreement illustrates the challenges of measuring the leaflet residence of a molecule that flips to the opposing leaflet on a microsecond timescale94, likely redistributing in response to perturbations induced by the very techniques designed to detect its distribution96,97,95.
While conclusions from direct measurements of cholesterol’s interleaflet distribution in living cell PMs remain deeply controversial, the factors that determine its preference for various membrane environments are better understood. For headgroups, cholesterol interacts poorly with PE lipids, well with sphingolipids, and surprisingly favorably with PS lipids98. For acyl chains, cholesterol is relatively averse to polyunsaturated fatty acids compared to saturated ones99, although interactions with PE remain unfavorable irrespective of chain saturation98.
The precise transverse distribution of cholesterol is likely to have profound effects on PM properties. Cholesterol concentration is positively correlated with lipid packing, order, thickness and headgroup hydration, negatively related to lipid diffusion and water penetration into the hydrophobic core, and also affects the electrostatic properties of the membrane, to name but a few structural consequences100–102. Membrane mechanical properties are also starkly sensitive to cholesterol abundance, including both compressibility and bending stiffness103,104, as well as spontaneous curvature105, and these effects are nonadditive and strongly dependent on phospholipid composition106. Importantly, cholesterol’s ability to rapidly flip between the two membrane leaflets gives it the unique capability to relax stresses in a bilayer, as elegantly demonstrated in symmetric model membranes96. However, this ability does not necessarily imply that cholesterol-containing membranes are stress-free. In asymmetric bilayers, an important additional consideration is cholesterol’s differential interaction energy with other membrane lipids, with cholesterol potentially creating stresses by partitioning strongly to one leaflet. Thus, cholesterol’s role in regulating membrane stress in complex physiological settings remains a mystery81,97.
Taking into account these factors and the detailed leaflet compositions of mammalian PM1, a naïve prediction might be that cholesterol is enriched in the PM exoplasmic leaflet due to its enrichment of saturated SM and depletion of PE and polyunsaturated PS. This scenario would result in a very tightly packed outer leaflet coupled to a relatively loosely packed inner one (Fig 4A), which is qualitatively in line with recent observations1,28. However, this naïve scenario does not take into account other potential driving factors of cholesterol distribution. One possible example is a difference in the total number of phospholipids in the two leaflets, e.g. the exoplasmic leaflet possessing many more lipids than the cytosolic one. Such a situation would cause intrinsic leaflet stresses, with outer leaflet lipids being relatively compressed compared to their equilibrium state, and vice versa in the inner leaflet (see Fig 3). Such stresses could be relieved in part by cholesterol flip-flop, via a passive enrichment in the inner leaflet (Fig 4C). It is even possible that various factors balance exactly, with the preference of cholesterol for SM on the outer leaflet being exactly balanced by the suboptimal packing of the inner leaflet lipids (Fig 4B). Ultimately, the fundamental drivers of cholesterol distribution in asymmetric bilayers are only starting to become defined and may include relative preferences of cholesterol for saturated lipids81 (Fig 4D), active pumping by transporters4 (Fig 4E) and repulsive interactions with long acyl chains pushing cholesterol down into the cytosolic leaflet5 (Fig 4F). Overall, it is becoming apparent that cholesterol’s interfleaflet distribution is likely to be dynamic and governed by the specific compositions, phospholipid abundances, and differential stresses of the two leaflets, and possibly energy-dependent terms driving the system away from physicochemical equilibrium4. These parameters will certainly be difficult to reliably measure, especially in vivo, but the reward is a potentially transformative understanding of PM structure, dynamics, and function.
Figure 4. Possible configurations for cholesterol’s interleaflet distribution in the PM.
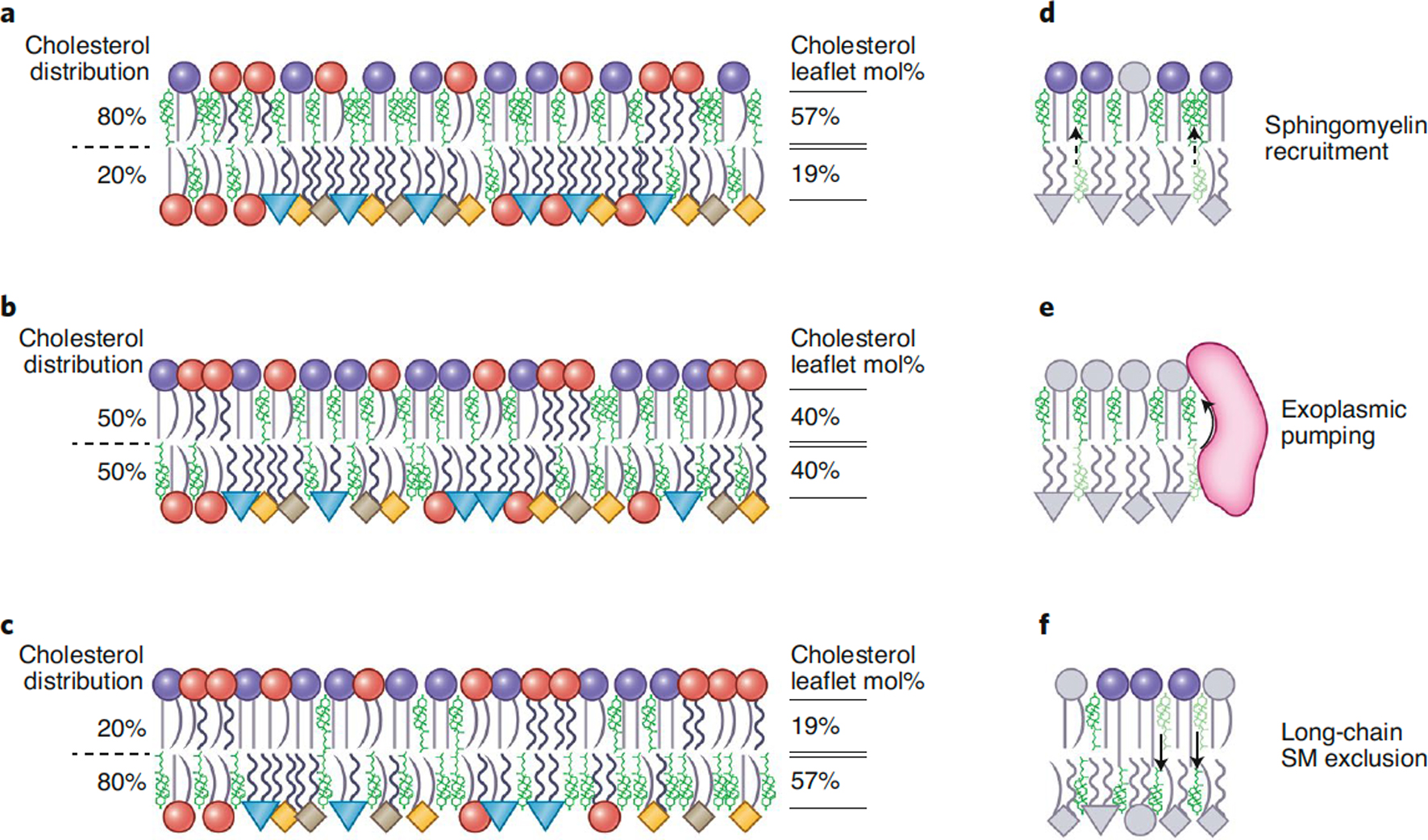
The same data-driven representation of PL asymmetry as in Fig 1, but with cholesterol (green) enriched either in the (A) top, (B) neither or (C) bottom leaflet, as indicated by the exo/endo percentages (left). In all bilayers the phospholipid compositions of the two leaflets, as well as the total cholesterol mole fraction in the membrane (40%), correspond to those measured in RBC PMs1–3, but the abundance of phospholipids between leaflets varies, resulting in the leaflet cholesterol mol% indicated on the right. An imbalance in total lipid abundance between leaflets may give rise to leaflet stresses (indicated by packing of headgroups) that remain stable as long as the phospholipid asymmetry is maintained. Potential contributors to cholesterol interleaflet distribution include: (D) recruitment of cholesterol to the exoplasmic leaflet by strong preference for saturated sphingomyelin, (E) active pumping of cholesterol to the exoplasmic leaflet by transporter proteins4, and (F) exclusion of cholesterol from the exoplasmic leaflet via long-chain sphingomyelin species5.
CONCLUSION
The energy inputs required for active maintenance of PM asymmetry, and the deleterious effects of its loss, serve as unambiguous indicators of its functional importance. We are now beginning to understand the biophysical consequences of compositional asymmetry and how they cooperatively participate in cellular processes. The ubiquity of transient PM scrambling (Fig 2) suggests that the dynamic loss and regeneration of membrane asymmetry may underlie many aspects of membrane physiology, including fusion, bending, and signaling. As such, the mechanisms of generation and resolution of transiently scrambled PM environments, their biophysical consequences, and ultimately their functional significance, are emerging as topics of widespread relevance for cellular physiology.
This exciting time for membrane research is bringing clarity while also giving rise to intriguing new questions and hypotheses. The characterization of a chemically complete asymmetric PM lipidome highlights several understudied biophysical features of biologically relevant lipid mixtures. For the inner leaflet, these would include ether lipids (e.g. plasmalogens) and hybrid PE and PS lipids bearing one saturated and one long polyunsaturated fatty acid chain; for the outer leaflet, long-chain sphingolipids mixed with polyunsaturated phosphatidylcholines. How do these lipids mix with each other and other major membrane components, what are the resulting bilayer characteristics, and how does cholesterol fit into the picture? Answers to these questions will shed light on the properties of individual PM leaflets and potentially the mechanisms of communication between them. A critically important parameter for both cholesterol distribution and the structural/mechanical properties of all asymmetric membranes is the differential leaflet stress caused by suboptimal packing of leaflet components (see Fig 3B). Measuring and manipulating this poorly understood parameter will require development of novel tools and methods. Unraveling the effects and roles of such bilayer stress both computationally and experimentally is essential for progress in understanding the many faces of asymmetric membranes.
While PS exposure has been widely used to denote a change in PM asymmetry, more detailed protocols and probes are necessary to determine which stimuli (if any) yield lipid-selective and/or incomplete scrambling (see Fig 1 and Box 1). The behaviors of such partially or completely scrambled PM are a topic ripe with opportunities: how does a scrambled domain interact with the surrounding asymmetric membrane? How does scrambling affect physical and functional interleaflet coupling? How does changing the composition of both leaflets affect protein-membrane interactions? Can scrambled versus asymmetric domains be used to laterally organize or functionally regulate membrane proteins? Or perhaps scrambled environments exhibit strong non-ideal mixing behavior, being prone to large scale phase separation, as observed in model membranes and GPMVs? In conclusion, transient loss of PM lipid asymmetry is a widespread and currently underappreciated biological phenomenon whose fascinating structural and functional consequences are emerging as an exciting direction of membrane biology and biophysics.
ACKNOWLEDGEMENTS
Funding for IL was provided by the NIH/National Institute of General Medical Sciences (R35 GM134949, R01 GM124072, R21 AI146880), the Volkswagen Foundation (93091), and the Human Frontiers Science Program (RGP0059/2019). MD was supported by F32 GM134704. JLS was supported by T32 GM008280. Authors have no competing interests.
References:
- 1.Lorent JH et al. Plasma membranes are asymmetric in lipid unsaturation, packing and protein shape. Nat Chem Biol 16, 644–652, doi: 10.1038/s41589-020-0529-6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarmento MJ, Hof M & Sachl R Interleaflet Coupling of Lipid Nanodomains - Insights From in vitro Systems. Front Cell Dev Biol 8, 284, doi: 10.3389/fcell.2020.00284 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seo S, Murata M & Shinoda W Pivotal Role of Interdigitation in Interleaflet Interactions: Implications from Molecular Dynamics Simulations. J Phys Chem Lett 11, 5171–5176, doi: 10.1021/acs.jpclett.0c01317 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Liu SL et al. Orthogonal lipid sensors identify transbilayer asymmetry of plasma membrane cholesterol. Nat Chem Biol 13, 268–274, doi: 10.1038/nchembio.2268 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Courtney KC et al. C24 Sphingolipids Govern the Transbilayer Asymmetry of Cholesterol and Lateral Organization of Model and Live-Cell Plasma Membranes. Cell Rep 24, 1037–1049, doi: 10.1016/j.celrep.2018.06.104 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Shin HW & Takatsu H Phosphatidylserine exposure in living cells. Crit Rev Biochem Mol Biol 55, 166–178, doi: 10.1080/10409238.2020.1758624 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Bleibaum F et al. ADAM10 sheddase activation is controlled by cell membrane asymmetry. J Mol Cell Biol 11, 979–993, doi: 10.1093/jmcb/mjz008 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sommer A et al. Phosphatidylserine exposure is required for ADAM17 sheddase function. Nature communications 7, 11523, doi: 10.1038/ncomms11523 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singer SJ & Nicolson GL The fluid mosaic model of the structure of cell membranes. Science 175, 720–731 (1972). [DOI] [PubMed] [Google Scholar]
- 10.Verkleij AJ et al. The asymmetric distribution of phospholipids in the human red cell membrane. A combined study using phospholipases and freeze-etch electron microscopy. Biochimica et biophysica acta 323, 178–193 (1973). [DOI] [PubMed] [Google Scholar]
- 11.Sessions A & Horwitz AF Myoblast aminophospholipid asymmetry differs from that of fibroblasts. FEBS Lett 134, 75–78, doi: 10.1016/0014-5793(81)80554-6 (1981). [DOI] [PubMed] [Google Scholar]
- 12.Bevers EM & Williamson PL Getting to the Outer Leaflet: Physiology of Phosphatidylserine Exposure at the Plasma Membrane. Physiol Rev 96, 605–645, doi: 10.1152/physrev.00020.2015 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Makarova M & Owen DM Asymmetry across the membrane. Nat Chem Biol 16, 605–606, doi: 10.1038/s41589-020-0545-6 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Zachowski A Phospholipids in animal eukaryotic membranes: transverse asymmetry and movement. Biochem J 294 (Pt 1), 1–14, doi: 10.1042/bj2940001 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Symons JL et al. Lipidomic atlas of mammalian cell membranes reveals hierarchical variation induced by culture conditions, subcellular membranes, and cell lineages. Soft Matter, doi: 10.1039/d0sm00404a (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Renooij W, Van Golde LM, Zwaal RF, Roelofsen B & Van Deenen LL Preferential incorporation of fatty acids at the inside of human erythrocyte membranes. Biochimica et biophysica acta 363, 287–292, doi: 10.1016/0005-2736(74)90069-8 (1974). [DOI] [PubMed] [Google Scholar]
- 17.Rawyler A, van der Schaft PH, Roelofsen B & Op den Kamp JA Phospholipid localization in the plasma membrane of Friend erythroleukemic cells and mouse erythrocytes. Biochemistry 24, 1777–1783, doi: 10.1021/bi00328a031 (1985). [DOI] [PubMed] [Google Scholar]
- 18.Van der Schaft PH, Roelofsen B, Op den Kamp JA & Van Deenen LL Phospholipid asymmetry during erythropoiesis. A study on Friend erythroleukemic cells and mouse reticulocytes. Biochimica et biophysica acta 900, 103–115, doi: 10.1016/0005-2736(87)90282-3 (1987). [DOI] [PubMed] [Google Scholar]
- 19.Gurtovenko AA & Vattulainen I Lipid transmembrane asymmetry and intrinsic membrane potential: two sides of the same coin. J Am Chem Soc 129, 5358–5359, doi: 10.1021/ja070949m (2007). [DOI] [PubMed] [Google Scholar]
- 20.Ma Y et al. A FRET sensor enables quantitative measurements of membrane charges in live cells. Nat Biotechnol 35, 363–370, doi: 10.1038/nbt.3828 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Entova S, Billod JM, Swiecicki JM, Martin-Santamaria S & Imperiali B Insights into the key determinants of membrane protein topology enable the identification of new monotopic folds. Elife 7, doi: 10.7554/eLife.40889 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dowhan W, Vitrac H & Bogdanov M Lipid-Assisted Membrane Protein Folding and Topogenesis. Protein J 38, 274–288, doi: 10.1007/s10930-019-09826-7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapus A & Janmey P Plasma membrane--cortical cytoskeleton interactions: a cell biology approach with biophysical considerations. Compr Physiol 3, 1231–1281, doi: 10.1002/cphy.c120015 (2013). [DOI] [PubMed] [Google Scholar]
- 24.McLaughlin S & Murray D Plasma membrane phosphoinositide organization by protein electrostatics. Nature 438, 605–611, doi:nature04398 [pii] 10.1038/nature04398 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Platre MP & Jaillais Y Anionic lipids and the maintenance of membrane electrostatics in eukaryotes. Plant Signal Behav 12, e1282022, doi: 10.1080/15592324.2017.1282022 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L, Shi X, Guo X, Li H & Xu C Ionic protein-lipid interaction at the plasma membrane: what can the charge do? Trends Biochem Sci 39, 130–140, doi: 10.1016/j.tibs.2014.01.002 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Swamy MJ et al. Coexisting domains in the plasma membranes of live cells characterized by spin-label ESR spectroscopy. Biophys J 90, 4452–4465, doi: 10.1529/biophysj.105.070839 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta A, Korte T, Herrmann A & Wohland T Plasma membrane asymmetry of lipid organization: fluorescence lifetime microscopy and correlation spectroscopy analysis. J Lipid Res 61, 252–266, doi: 10.1194/jlr.D119000364 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill WG & Zeidel ML Reconstituting the barrier properties of a water-tight epithelial membrane by design of leaflet-specific liposomes. J Biol Chem 275, 30176–30185, doi: 10.1074/jbc.M003494200 (2000). [DOI] [PubMed] [Google Scholar]
- 30.Williamson P & Schlegel RA Back and forth: the regulation and function of transbilayer phospholipid movement in eukaryotic cells. Mol Membr Biol 11, 199–216, doi: 10.3109/09687689409160430 (1994). [DOI] [PubMed] [Google Scholar]
- 31.Levental I, Levental KR & Heberle FA Lipid Rafts: Controversies Resolved, Mysteries Remain. Trends in Cell Biology (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitra K, Ubarretxena-Belandia I, Taguchi T, Warren G & Engelman DM Modulation of the bilayer thickness of exocytic pathway membranes by membrane proteins rather than cholesterol. Proc Natl Acad Sci U S A 101, 4083–4088, doi: 10.1073/pnas.0307332101 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lorent JH et al. Structural determinants and functional consequences of protein affinity for membrane rafts. Nature communications 8, 1219, doi: 10.1038/s41467-017-01328-3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montigny C, Lyons J, Champeil P, Nissen P & Lenoir G On the molecular mechanism of flippase- and scramblase-mediated phospholipid transport. Biochimica et biophysica acta 1861, 767–783, doi: 10.1016/j.bbalip.2015.12.020 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Daleke DL Phospholipid flippases. J Biol Chem 282, 821–825, doi: 10.1074/jbc.R600035200 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Kodigepalli KM, Bowers K, Sharp A & Nanjundan M Roles and regulation of phospholipid scramblases. FEBS Lett 589, 3–14, doi: 10.1016/j.febslet.2014.11.036 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Falzone ME et al. Structural basis of Ca(2+)-dependent activation and lipid transport by a TMEM16 scramblase. Elife 8, doi: 10.7554/eLife.43229 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagata S, Suzuki J, Segawa K & Fujii T Exposure of phosphatidylserine on the cell surface. Cell Death Differ 23, 952–961, doi: 10.1038/cdd.2016.7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shlomovitz I, Speir M & Gerlic M Flipping the dogma - phosphatidylserine in non-apoptotic cell death. Cell Commun Signal 17, 139, doi: 10.1186/s12964-019-0437-0 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hammill AK, Uhr JW & Scheuermann RH Annexin V staining due to loss of membrane asymmetry can be reversible and precede commitment to apoptotic death. Exp Cell Res 251, 16–21, doi: 10.1006/excr.1999.4581 (1999). [DOI] [PubMed] [Google Scholar]
- 41.Brown GC & Neher JJ Microglial phagocytosis of live neurons. Nat Rev Neurosci 15, 209–216, doi: 10.1038/nrn3710 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Neher JJ et al. Inhibition of microglial phagocytosis is sufficient to prevent inflammatory neuronal death. J Immunol 186, 4973–4983, doi: 10.4049/jimmunol.1003600 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Neumann B et al. EFF-1-mediated regenerative axonal fusion requires components of the apoptotic pathway. Nature 517, 219–222, doi: 10.1038/nature14102 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Hisamoto N et al. Phosphatidylserine exposure mediated by ABC transporter activates the integrin signaling pathway promoting axon regeneration. Nature communications 9, 3099, doi: 10.1038/s41467-018-05478-w (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scott-Hewitt N et al. Local externalization of phosphatidylserine mediates developmental synaptic pruning by microglia. bioRxiv, 2020.2004.2024.059584, doi: 10.1101/2020.04.24.059584 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scott-Hewitt N et al. Local externalization of phosphatidylserine mediates developmental synaptic pruning by microglia. EMBO J 39, e105380, doi: 10.15252/embj.2020105380 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruggiero L, Connor MP, Chen J, Langen R & Finnemann SC Diurnal, localized exposure of phosphatidylserine by rod outer segment tips in wild-type but not Itgb5−/− or Mfge8−/− mouse retina. Proc Natl Acad Sci U S A 109, 8145–8148, doi: 10.1073/pnas.1121101109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goodyear RJ, Gale JE, Ranatunga KM, Kros CJ & Richardson GP Aminoglycoside-induced phosphatidylserine externalization in sensory hair cells is regionally restricted, rapid, and reversible. J Neurosci 28, 9939–9952, doi: 10.1523/JNEUROSCI.1124-08.2008 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van den Eijnde SM et al. Transient expression of phosphatidylserine at cell-cell contact areas is required for myotube formation. J Cell Sci 114, 3631–3642 (2001). [DOI] [PubMed] [Google Scholar]
- 50.Jeong J & Conboy IM Phosphatidylserine directly and positively regulates fusion of myoblasts into myotubes. Biochem Biophys Res Commun 414, 9–13, doi: 10.1016/j.bbrc.2011.08.128 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park SY et al. Stabilin-2 modulates the efficiency of myoblast fusion during myogenic differentiation and muscle regeneration. Nature communications 7, 10871, doi: 10.1038/ncomms10871 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsuchiya M et al. Cell surface flip-flop of phosphatidylserine is critical for PIEZO1-mediated myotube formation. Nature communications 9, 2049, doi: 10.1038/s41467-018-04436-w (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Vries KJ, Wiedmer T, Sims PJ & Gadella BM Caspase-independent exposure of aminophospholipids and tyrosine phosphorylation in bicarbonate responsive human sperm cells. Biol Reprod 68, 2122–2134, doi: 10.1095/biolreprod.102.012500 (2003). [DOI] [PubMed] [Google Scholar]
- 54.Rival CM et al. Phosphatidylserine on viable sperm and phagocytic machinery in oocytes regulate mammalian fertilization. Nature communications 10, 4456, doi: 10.1038/s41467-019-12406-z (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naeini MB, Bianconi V, Pirro M & Sahebkar A The role of phosphatidylserine recognition receptors in multiple biological functions. Cell Mol Biol Lett 25, 23, doi: 10.1186/s11658-020-00214-z (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin S et al. Immunologic stimulation of mast cells leads to the reversible exposure of phosphatidylserine in the absence of apoptosis. Int Arch Allergy Immunol 123, 249–258, doi: 10.1159/000024451 (2000). [DOI] [PubMed] [Google Scholar]
- 57.Rysavy NM et al. Beyond apoptosis: the mechanism and function of phosphatidylserine asymmetry in the membrane of activating mast cells. Bioarchitecture 4, 127–137, doi: 10.1080/19490992.2014.995516 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ory S et al. Phospholipid scramblase-1-induced lipid reorganization regulates compensatory endocytosis in neuroendocrine cells. J Neurosci 33, 3545–3556, doi: 10.1523/JNEUROSCI.3654-12.2013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.MacKenzie A et al. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity 15, 825–835, doi: 10.1016/s1074-7613(01)00229-1 (2001). [DOI] [PubMed] [Google Scholar]
- 60.Elliott JI et al. Membrane phosphatidylserine distribution as a non-apoptotic signalling mechanism in lymphocytes. Nat Cell Biol 7, 808–816, doi: 10.1038/ncb1279 (2005). [DOI] [PubMed] [Google Scholar]
- 61.Fischer K et al. Antigen recognition induces phosphatidylserine exposure on the cell surface of human CD8+ T cells. Blood 108, 4094–4101, doi: 10.1182/blood-2006-03-011742 (2006). [DOI] [PubMed] [Google Scholar]
- 62.Stowell SR et al. Galectin-1 induces reversible phosphatidylserine exposure at the plasma membrane. Mol Biol Cell 20, 1408–1418, doi: 10.1091/mbc.E08-07-0786 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Levental KR et al. Lipidomic and biophysical homeostasis of mammalian membranes counteracts dietary lipid perturbations to maintain cellular fitness. Nature communications 11, 1339, doi: 10.1038/s41467-020-15203-1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levental KR et al. Polyunsaturated lipids regulate membrane domain stability by tuning membrane order. Biophys J 110(8), 1800–1810, doi: 10.1016/j.bpj.2016.03.012 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sezgin E et al. Measuring nanoscale diffusion dynamics in cellular membranes with super-resolution STED-FCS. Nat Protoc 14, 1054–1083, doi: 10.1038/s41596-019-0127-9 (2019). [DOI] [PubMed] [Google Scholar]
- 66.Morrot G et al. Asymmetric lateral mobility of phospholipids in the human erythrocyte membrane. Proc Natl Acad Sci U S A 83, 6863–6867 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.el Hage Chahine JM, Cribier S & Devaux PF Phospholipid transmembrane domains and lateral diffusion in fibroblasts. Proc Natl Acad Sci U S A 90, 447–451 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cogan U & Schachter D Asymmetry of lipid dynamics in human erythrocyte membranes studied with impermeant fluorophores. Biochemistry 20, 6396–6403 (1981). [DOI] [PubMed] [Google Scholar]
- 69.Nikolova-Karakashian MN, Petkova H & Koumanov KS Influence of cholesterol on sphingomyelin metabolism and hemileaflet fluidity of rat liver plasma membranes. Biochimie 74, 153–159 (1992). [DOI] [PubMed] [Google Scholar]
- 70.Bogdanov M et al. Phospholipid distribution in the cytoplasmic membrane of Gram-negative bacteria is highly asymmetric, dynamic, and cell shape-dependent. Sci Adv 6, eaaz6333, doi: 10.1126/sciadv.aaz6333 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Diaz-Rohrer BB, Levental KR, Simons K & Levental I Membrane raft association is a determinant of plasma membrane localization. Proc Natl Acad Sci U S A 111, 8500–8505, doi: 10.1073/pnas.1404582111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heberle FA et al. Direct label-free imaging of nanodomains in biomimetic and biological membranes by cryogenic electron microscopy. Proc Natl Acad Sci U S A 117, 19943–19952, doi: 10.1073/pnas.2002200117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cornell CE, Mileant A, Thakkar N, Lee KK & Keller SL Direct imaging of liquid domains in membranes by cryo-electron tomography. Proc Natl Acad Sci U S A 117, 19713–19719, doi: 10.1073/pnas.2002245117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Demo SD et al. Quantitative measurement of mast cell degranulation using a novel flow cytometric annexin-V binding assay. Cytometry 36, 340–348, doi: (1999). [DOI] [PubMed] [Google Scholar]
- 75.Smrz D, Draberova L & Draber P Non-apoptotic phosphatidylserine externalization induced by engagement of glycosylphosphatidylinositol-anchored proteins. J Biol Chem 282, 10487–10497, doi: 10.1074/jbc.M611090200 (2007). [DOI] [PubMed] [Google Scholar]
- 76.Pyrshev KA, Klymchenko AS, Csucs G & Demchenko AP Apoptosis and eryptosis: Striking differences on biomembrane level. Biochim Biophys Acta Biomembr 1860, 1362–1371, doi: 10.1016/j.bbamem.2018.03.019 (2018). [DOI] [PubMed] [Google Scholar]
- 77.Le Roux AL, Quiroga X, Walani N, Arroyo M & Roca-Cusachs P The plasma membrane as a mechanochemical transducer. Philos Trans R Soc Lond B Biol Sci 374, 20180221, doi: 10.1098/rstb.2018.0221 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lacoste D & Bassereau P An Update on Active Membrane. Liposomes, Lipid Bilayers and Model Membranes: From Basic Research to Application, 271 (2014). [Google Scholar]
- 79.Shi Z, Graber ZT, Baumgart T, Stone HA & Cohen AE Cell Membranes Resist Flow. Cell 175, 1769–1779 e1713, doi: 10.1016/j.cell.2018.09.054 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dumitru AC et al. Nanoscale membrane architecture of healthy and pathological red blood cells. Nanoscale Horiz 3, 293–304, doi: 10.1039/c7nh00187h (2018). [DOI] [PubMed] [Google Scholar]
- 81.Hossein A & Deserno M Spontaneous Curvature, Differential Stress, and Bending Modulus of Asymmetric Lipid Membranes. Biophys J 118, 624–642, doi: 10.1016/j.bpj.2019.11.3398 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Doktorova M & Weinstein H Accurate in silico modeling of asymmetric bilayers based on biophysical principles. Biophys J 115, 1638–1643, doi: 10.1016/j.bpj.2018.09.008 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Steinkuhler J, Sezgin E, Urbancic I, Eggeling C & Dimova R Mechanical properties of plasma membrane vesicles correlate with lipid order, viscosity and cell density. Commun Biol 2, 337, doi: 10.1038/s42003-019-0583-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Keller H, Lorizate M & Schwille P PI(4,5)P2 degradation promotes the formation of cytoskeleton-free model membrane systems. Chemphyschem 10, 2805–2812, doi: 10.1002/cphc.200900598 (2009). [DOI] [PubMed] [Google Scholar]
- 85.Baumgart T et al. Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc Natl Acad Sci U S A 104, 3165–3170, doi: 10.1073/pnas.0611357104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Skinkle AD, Levental KR & Levental I Cell-Derived Plasma Membrane Vesicles Are Permeable to Hydrophilic Macromolecules. Biophys J 118, 1292–1300, doi: 10.1016/j.bpj.2019.12.040 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Skotland T & Sandvig K The role of PS 18:0/18:1 in membrane function. Nature communications 10, 2752, doi: 10.1038/s41467-019-10711-1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koyama-Honda I et al. High-speed single-molecule imaging reveals signal transduction by induced transbilayer raft phases. bioRxiv (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fujimoto T & Parmryd I Interleaflet Coupling, Pinning, and Leaflet Asymmetry-Major Players in Plasma Membrane Nanodomain Formation. Front Cell Dev Biol 4, 155, doi: 10.3389/fcell.2016.00155 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Abe M et al. A role for sphingomyelin-rich lipid domains in the accumulation of phosphatidylinositol-4,5-bisphosphate to the cleavage furrow during cytokinesis. Mol Cell Biol 32, 1396–1407, doi: 10.1128/mcb.06113-11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rog T et al. Interdigitation of long-chain sphingomyelin induces coupling of membrane leaflets in a cholesterol dependent manner. Biochimica et biophysica acta 1858, 281–288, doi: 10.1016/j.bbamem.2015.12.003 (2016). [DOI] [PubMed] [Google Scholar]
- 92.Gupta A, Muralidharan S, Torta F, Wenk MR & Wohland T Long acyl chain ceramides govern cholesterol and cytoskeleton dependence of membrane outer leaflet dynamics. Biochim Biophys Acta Biomembr 1862, 183153, doi: 10.1016/j.bbamem.2019.183153 (2020). [DOI] [PubMed] [Google Scholar]
- 93.Marrink SJ et al. Computational Modeling of Realistic Cell Membranes. Chem Rev 119, 6184–6226, doi: 10.1021/acs.chemrev.8b00460 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bennett WF, MacCallum JL, Hinner MJ, Marrink SJ & Tieleman DP Molecular view of cholesterol flip-flop and chemical potential in different membrane environments. J Am Chem Soc 131, 12714–12720, doi: 10.1021/ja903529f (2009). [DOI] [PubMed] [Google Scholar]
- 95.Steck TL & Lange Y Transverse distribution of plasma membrane bilayer cholesterol: Picking sides. Traffic 19, 750–760, doi: 10.1111/tra.12586 (2018). [DOI] [PubMed] [Google Scholar]
- 96.Bruckner RJ, Mansy SS, Ricardo A, Mahadevan L & Szostak JW Flip-flop-induced relaxation of bending energy: implications for membrane remodeling. Biophys J 97, 3113–3122, doi: 10.1016/j.bpj.2009.09.025 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miettinen MS & Lipowsky R Bilayer Membranes with Frequent Flip-Flops Have Tensionless Leaflets. Nano Lett 19, 5011–5016, doi: 10.1021/acs.nanolett.9b01239 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nyholm TKM, Jaikishan S, Engberg O, Hautala V & Slotte JP The Affinity of Sterols for Different Phospholipid Classes and Its Impact on Lateral Segregation. Biophys J 116, 296–307, doi: 10.1016/j.bpj.2018.11.3135 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wassall SR & Stillwell W Polyunsaturated fatty acid-cholesterol interactions: domain formation in membranes. Biochimica et biophysica acta 1788, 24–32, doi: 10.1016/j.bbamem.2008.10.011 (2009). [DOI] [PubMed] [Google Scholar]
- 100.Marsh D Handbook of Lipid Bilayers. Second Edition edn, (CRC Press, 2013). [Google Scholar]
- 101.Doktorova M et al. Cholesterol Promotes Protein Binding by Affecting Membrane Electrostatics and Solvation Properties. Biophys J 113, 2004–2015, doi: 10.1016/j.bpj.2017.08.055 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leeb F & Maibaum L Spatially Resolving the Condensing Effect of Cholesterol in Lipid Bilayers. Biophys J 115, 2179–2188, doi: 10.1016/j.bpj.2018.10.024 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Evans E, Rawicz W & Smith BA Back to the future: mechanics and thermodynamics of lipid biomembranes. Faraday Discuss 161, 591–611 (2013). [DOI] [PubMed] [Google Scholar]
- 104.Chakraborty S et al. Reassessment of membrane mechanics: How cholesterol stiffens unsaturated lipid membranes. Proc Natl Acad Sci U S A In press(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kollmitzer B, Heftberger P, Rappolt M & Pabst G Monolayer spontaneous curvature of raft-forming membrane lipids. Soft Matter 9, 10877–10884, doi: 10.1039/C3SM51829A (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sodt AJ, Venable RM, Lyman E & Pastor RW Nonadditive Compositional Curvature Energetics of Lipid Bilayers. Phys Rev Lett 117, 138104, doi: 10.1103/PhysRevLett.117.138104 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]


