Abstract
This study examines the representations of human gene patents in Chinese newspapers. We conducted a qualitative content analysis of news articles published between 2006 and 2017 to identify the major themes in media coverage, ethical considerations, perceptions of risks and benefits, and attitudes towards the patentability of human genes. The results show that two key ethical concerns were expressed by journalists: (1) that it is morally wrong to own or patent human genes and (2) that gene patents could potentially impede patients’ access to healthcare services. Nonetheless, the press coverage has tended to be largely favorable (57.8%), rather than opposed (17.8%) to human gene patenting. There were no normative claims that human genes should not be patentable in China, which indicates a generally positive attitude towards patentability in media discourse. Most articles that expressed criticism toward gene patenting discussed challenges in other countries, with significant attention given to the United States Supreme Court’s ruling in the Myriad case that invalidated Myriad Genetics’ patents on the BRCA1 and BRCA2 genes. Overall, the newspapers were uncritical of the Chinese gene patenting regime. News reporting on the issue was highly suggestive of a strong pro-commercialization stance, although some discussions emphasized potential risks over benefits. Our analysis highlights the need for balanced media reporting on human gene patents in China and a top-down approach to engage the public in substantive discussions on the ethical and societal implications of the existing patent regime.
Keywords: Chinese newspapers, Content analysis, Human gene patents, Patentability
Introduction
In 2016, the Government of China launched a key national project to advance genomics research and the development of precision medicine (the Ministry of Science and Technology of the P.R. China 2016). The program invests in innovation that supports the expansion of precision medicine technologies, including gene sequencing and genetic testing research, with the objective of translating relevant research into clinical practice (Cyranoski 2016). In the field of biotechnology research and development, the current patent regime is considered an effective legal measure in China for protecting biological innovations and a vehicle for promoting the commercialization of genetic research and applications (Salter 2009). The issue of human gene patents has not been treated differently from other areas of biotechnology innovation. Rather, social and ethical concerns that arise from granting patents over human genes and human genetic alterations occurring naturally tend to be overshadowed by the overall instrumentalism of the Chinese IP regime (Du 2018). While controversies over the patenting of human genes and their media coverage in other countries have been extensively studied (Caulfield et al. 2007; Kamenova et al. 2014; Du et al. 2015), the role that Chinese news media have played in shaping the public debate on this issue has not yet been established. There are no previous studies of how Chinese newspapers have covered the gene patent controversy and what key issues have been framed for public debate. Here, we undertake the first systematic analysis of the nature of media discourse on the ethical and policy issues related to human gene patenting in China. In order to shed light on the news coverage of human gene patents in China, we analyze news articles on the issue published in Chinese newspapers over a 12-year period, from 1 January 2006 to 31 December 2017, that discuss any aspect related to the patenting of human genes. Our analysis focuses on how risks and benefits were represented, what ethical concerns were highlighted, what issues pertaining to China’s gene patents regime were identified, and the overall attitude towards the patentability of human genes. We contextualize our findings with a broader discussion of gene patenting law in China and international jurisdictions and highlight the potential implications of a strong IP regime for patients’ access to healthcare services and quality of genetic testing services. We conclude with a brief consideration of strategies for public communication that can increase the public understanding and engagement with this complex issue.
The Legal Context of Gene Patents in China and Internationally
The development of Chinese modern patent law started in 1979, and the current Patent Law of People’s Republic of China (the Patent Law) was adopted by the Standing Committee of the National People Congress in March 1984. After the entering into force in April 1985, the law has been amended in 1992, 2000, and 2008, respectively. The objective of the Patent Law is clearly defined in Article 1, which states that the law is “to protect the legitimate rights and interests of the patentee, encourage invention and creation, promote the application of invention and creation, improve the ability of innovation, and promote the progress of science and technology and economic and social development” (The Patent Law 2008). Based on the authorization of the Patent Law, the patent administration department under the State Council has the authority to accept patent applications, conduct patent examinations, and authorize patents. Under the current policies encouraging the development of a strong biotech-based economy, the number of applications for patents in the field of biotechnology has gradually increased. Between 2006 and 2015, 22,193 applications were made for biotechnology patents, within which 10,394 applications were granted for patent protections (Wang et al. 2018). Nonetheless, the complexity and rapid development of biotechnology have consistently challenged the existing patent regime and, in many cases, emerging ethical and social controversies associated with innovations in biotechnology have made it very difficult for judges to interpret whether a patent claim falls within the scope of objects protected by the patent law. For instance, the patentability of the separation of DNA fragments remains controversial in many jurisdictions (Gold and Carbone 2010; Kamenova et al. 2014; Du et al. 2015).
In an international context, there is no consensus on the patentability and patent protection scope of human genes. There is a significant divergence in patent law internationally, especially on the eligibility of patent claims relating to BRCA 1/2 genetic sequences, as illustrated in decisions of the highest courts in the United States and Australia (Nicol et al. 2019). Lawsuits against the patentability of human genes have been brought to courts and court battles have often persisted for decades. The most notable case is the legal challenge in the United States against Myriad Genetics’ gene patents for two genes associated with breast and ovarian cancer, i.e., BRCA1 and BRCA2. In 2009, the American Civil Liberties Union (ACLU) and the Public Patent Foundation, along with 20 doctors, patients, professional medical associations, and women’s health groups filed a lawsuit in the US District Court for the Southern District of New York against Myriad Genetics, the US Patent and Trademark Office, and the University of Utah Research Foundation, alleging that patents on BRCA1 and BRCA2 are invalid (Simoncelli and Park 2015). This started a prolonged legal battle, which culminated in 2013 when the US Supreme Court invalidated the company’s patents for “natural” human genes (Association for Molecular Pathology v Myriad Genetics 2013). Specifically, the Court concluded that a naturally occurring DNA segment in the human body, as well as the information encoded in it is a product of nature and, therefore, a patent should not be granted merely because such a segment has been isolated. Nonetheless, the Justices unanimously decided that cDNA (an edited version of the original gene that omits non-coding portions) is patent eligible because it is not naturally occurring. Subsequent gene patenting controversies revolved around non-invasive prenatal testing (NIPT), and specifically on the issue of whether the fetus cell-free DNA and a test method that detects it from a pregnant female is patentable. In the Ariosa v Sequenom (2015), the Federal Circuit denied the patentability of the discovery of naturally occurring cell-free fetus DNA and the well-known method for detecting its presence in maternal circulation. However, in a very recent decision in the Illumina v Ariosa (2020), the Federal Circuit upheld the patentability of a method for preparing an extracellular cell-free DNA fraction and using it for analyzing genetic alternations involved in fetal chromosomal aberrations. The Court held that the method increases the relative amount of cell-free fetus DNA in the processed sample that has not naturally occurred in maternal blood. This method, according to the Court, is different from observing naturally occurring cell-free fetus DNA or detecting the presence of the phenomenon and, hence, is patent-eligible (Illumina v Ariosa 2020).
The United States was not the only country where patent claims in relation to genes had been challenged in courts. In D’Arcy v Myriad Genetics (2015), the High Court of Australia reversed the Federal Court’s decision on Myriad’s patent claims on isolated BRCA1 DNA and invalidated the patentability of gene-related patents. The Court’s decision was mainly based on public policy concerns, considering that granting a monopoly for Myriad’s patent claims would “inhibit other researchers and medical practitioners from diagnostically testing the BRCA1 gene for an entirely different purpose” (D’Arcy v Myriad Genetics 2015). Indeed, societal considerations, particularly the impact of patents on patients’ access to health care, have been important when courts were deciding whether to uphold patent rights. For example, in Canada, in 2014, the Children’s Hospital of Eastern Ontario (CHEO) challenged Transgenomic’s patents for five genes associated with long QT syndrome (LQTS) — a rare disorder of the heart’s electrical activity that may cause sudden, uncontrollable and dangerous arrhythmias. The case led to a settlement that allowed the CHEO and all Canadian public health institutions royalty free use of long QT syndrome gene patents on a not-for-profit basis (Bonter et al. 2018).
In the European Union, the legal context for patentability of genetic sequences is more favorable than the present IP regime in the United States. Under the EU Directive 98/44/EC on the legal protection of biotechnological inventions and European Patent Office’s Guidelines for Examination, the sequence or partial sequence of a gene “may constitute a patentable invention, even if the structure of that element is identical to that of a natural element” (Directive 98/44/EC 1998). European Parliament resolution of 10 May 2012 on patents for biotechnological inventions required the purpose-bound patent protection for gene sequences. According to this requirement, an applicant should claim the specific application of the patent when filing a patent application for a DNA sequence. Consequently, an isolated gene sequence with a concrete application description is patentable under the existing EU legislation. Therefore, patents on genes that are invalid in the US may still be valid in EU (Hawkins et al. 2019).
In China, the controversy over human gene patents has been subjected to scrutiny within the academic community. A recent study indicates that Chinese scholars have tended to regard the existing patent regime as an important legal tool for protecting the genetic resources of China and the proprietary interests of inventors (Du 2018). Their supportive attitudes towards human gene patents did not change significantly after the US Supreme Court’s ruling in the Myriad case (Du 2018). Under the Patent Law, Article 25 excludes the patentability on scientific discovery and diagnostic methods. In terms of human genes, the issue of whether human DNA segments constitute a scientific discovery or an invention that warrants patenting remained unclear until the release of Guidelines for Patent Examination by the State Intellectual Property Office of the P.R. China in 2010. The 2010 Guidelines stipulated that a human gene or a DNA segment was patentable when “1) it is isolated or extracted from the natural sequence for the first time, and 2) the application value of the gene for industry is accurately expressed” (State Intellectual Property Office of the P.R. China 2010; Jamison 2015). Notwithstanding, inventions that are against social ethics or harmful to public interest are not patentable according to Article 5 of the Patent Law. In addition, if an invention is based on genetic resources, while the acquisition of the genetic resources is illegal, the invention cannot be patented. In practice, genetic testing services have been widely available in the market, but no patent infringement claims have been recorded so far.
Gene Patenting Controversy and the Role of News Media
In most jurisdictions, public opposition to human gene patents stems from their negative implications for patients’ access to healthcare services such as predictive genetic testing and diagnosis (Simoncelli and Park 2015). Patent regimes often increase the cost of genetic tests and can lead to higher insurance premiums (Chandrasekharan and Fiffer 2010). Other than impacts on healthcare access and test costs, diagnostic monopolies due to exclusive rights of genetic patent holders can diminish the quality of genetic testing (Andrews and Paradise 2005). Patients and doctors are unable to obtain results of same tests from other laboratories, and thus, they cannot have the testing results verified by independent providers (Evans and Watson 2015).
Understanding intricacies involved in the gene patenting controversy requires expertise that may not be available to the general public and, therefore, coverage on the issue in popular media outlets has the potential to open up a wider public debate, especially on practical and ethical concerns that can have an impact on people’s lives. Although there is no convincing evidence to assert that media messages exert a direct influence on peoples’ beliefs, decisions, and behavior, two interrelated processes in news media production—“framing” and “agenda-setting”—provide an analytical framework to assess how media content can influence audiences and shape the public discourse on specific issues (Gauntlett 2004). The concept of framing describes the process of selective presentation in news coverage of specific topics, facts, controversies, actors, and assertions in news stories (Entman 1993; Scheufele 1999). To frame an issue, according to Entman, “is to select some aspects of a perceived reality and make them more salient in a communicating text, in such a way as to promote a particular problem definition, causal interpretation, moral evaluation, and/or treatment recommendation” (Entman 1993). Media outlets routinely deploy frames in news reporting to call attention to some aspects of reality while obscuring other elements. Framing can have a lasting influence, and once news reports have framed an issue in a particular light, public perceptions remain stable over time (Entman 1993; Nisbet et al. 2003).
While media framing theory emphasizes how “frames” as rhetorical and organizing structures influence people to process information and construct meanings in shared contexts, the related agenda-setting theory provides insight into how media can make people focus their attention on some topics, while overlooking others. The major tenet of this perspective is that even though media cannot make people think or behave in a certain way, they can still have a profound impact on what people think about by highlighting certain issues and excluding others from coverage (McCombs and Shaw 1972). Mass media therefore play a considerable role in public opinion formation and policy making by heavily influencing the salience of certain issues on the public agenda. Historically, media coverage of advances in genetics and genomics across countries has been largely unbalanced, focusing either on the negative consequences of genetic advances (e.g., the risk of insurance or employment discrimination and the possibility of human genetic modification), or exaggerating the potential of genetic technologies for medicine and the health benefits of genetic testing—a phenomenon known as “genohype” (Geller et al. 2002; Bubela and Caulfield 2004; Caulfield 2004).
Previous studies have shown that news media have played an important role in framing the gene patenting controversy and closely related health policy issues (Kamenova et al. 2014; Chapman et al. 2014; Zarzeczny et al. 2010). In Canada, for example, the CHEO’s lawsuit against the American holder of gene patents related to long QT syndrome attracted extensive media attention. The lawsuit was reported by all major Canadian news media outlets and coverage tended to emphasize negative attitudes towards the patenting of human genes (Bonter et al. 2018). Additionally, news stories on this legal controversy were widely disseminated on the social network sites, including Twitter, raising public concerns about gene patenting such as hurdles for access to genetic testing and the morality of commercializing human genes. A Canadian study analyzing Twitter users’ responses to the CHEO’s lawsuit indicated that most tweets supported the Hospital’s position and argued against the patentability of human genes in principle (Du et al. 2015).
Given that the Chinese patent law allows human genes to be patented, China may face potential backlash, where patent holders would seek to strictly enforce patent rights and maintain the high cost of genetic testing and diagnostic services. We have already seen this scenario plays out in the United States, Canada, and many EU countries, where it generated a strong public opposition to biotech and gene patents.
Methods
We collected newspaper articles using the Chinese National Knowledge Infrastructure (CNKI) Core Newspaper Full-Text Database, the most comprehensive database in China that collects more than 500 Chinese language newspaper publications (Information of the Database 2019). The dataset for this study was collected in two stages. During the first stage, we searched for articles published between 1 January 2006, and 31 December 2017 that included both search terms: “基因” (genes) and “专利” (patent). As the Chinese characters for “gene” (基因) and “genetically modified organism” (转基因) are only one Chinese character different, we conducted another search that excluded articles containing the term “转基因” (genetically modified organisms). This search strategy generated 272 newspaper articles.
During the second stage, we completed a thorough review of all the newspaper articles and found out that many gene patents reported in these articles were not human gene patents. Rather, they were patents on genes of animals, plants, and microorganisms. Since our research focused exclusively on representations of human gene patents, we labeled these articles as irrelevant and removed them from the dataset. It was important to conduct a thorough review of all irrelevant articles since news stories often did not use the adjective “human” (in Chinese “人类”) before genes or gene patents when discussing human gene patenting, including issues concerning BRCA1/2 genes. Relevant news reports would have been overlooked and excluded from the analysis should we had added “human” as a keyword search term to filter the articles directly in the database. Following this preliminary screening of all articles collected during the first round, 45 articles qualified for inclusion in the study. Detailed information about the news articles in our study, e.g. newspapers, publication dates and the articles’ headlines, is available online, in Zenodo (10.5281/zenodo.2595240).
The content analysis we conducted was based on the following questions: (1) when and where was the article published? (2) Which genes does the article mention? (3) Does the newspaper article discuss or mention any concerns that are against gene patents? (4) If it does, what are they? (5) Do the newspaper articles mention any benefits that support gene patents? (6) If it does, what are the benefits? (7) What is the attitude towards gene patents, i.e., negative, positive, or neutral? (8) Does the newspaper article mention the need for improvement of the IP rights system for gene patents in China? (9) If this is mentioned, what are the proposed strategies/measures? These questions were organized in structured codes and included in a coding book accessible on Zenodo. In order to assess the overall attitude of each news article towards the patenting of human genes (question #7), we adopted a three-part categorization, commonly used in studies deploying qualitative content analysis, which aim to assess the tone of media coverage on a particular issue: (1) articles were coded as “positive” when there was a strong emphasis on the expected benefits of gene patents, rather than their potential negative impacts; (2) articles were categorized as “negative” when the discussion was mostly focused on risks and potential negative societal impacts of human gene patenting; and (3) they were categorized as “neutral,” if the tone of the coverage was largely descriptive, i.e., the news articles reported specific patent registrations or other relevant information, without further analysis of social and ethical issues.
The second author coded the articles in the dataset and completed the statistical analysis, which was verified by the first author. After completing the data analysis, we tasked an independent researcher with coding a random sample of 30% of the dataset (n = 14), which was sufficient for establishing reliability, and conducted an inter-coder reliability assessment using Cohen’s kappa (κ). The extent of agreement between the coders was interpreted based on the benchmark scale proposed by Landis and Koch (1977), which is one of the most widely used benchmark scales to estimate the degree of inter-rater agreement in qualitative research. This scale has suggested the following kappa values as guidelines for assessing the strength of inter-rater agreement: < 0 = poor; .01–.20 = slight; .21–.40 = fair; .41–.60 = moderate; .61–.80 = substantial; and .81–1.00 = almost perfect. Kappa scores on the coding categories in our study ranged from .76 to 1.00, which indicates substantial or almost perfect inter-rater agreement between the coders.
Results
Frequencies in News Coverage
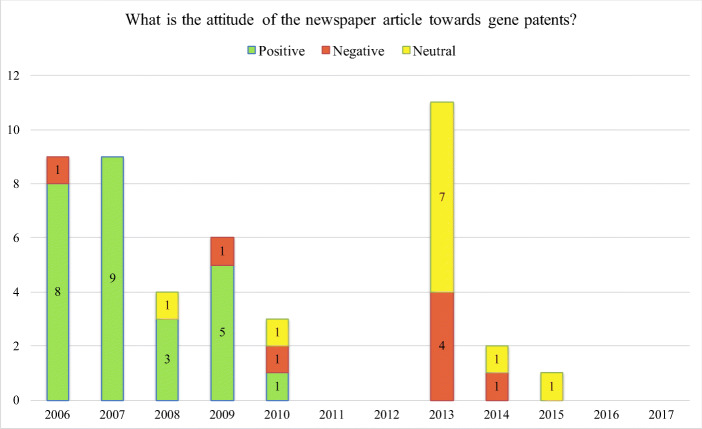
Figure 1 shows how media coverage on the topic of human gene patenting has varied over the years. We established that interest peaked in 2013 when 11 articles were published. This was the year when the U.S. Supreme Court made the landmark ruling on the patentability of human genes in the Myriad case. Patenting human genes received relatively high attention in 2006 and 2007, with 9 articles published each year. The issue continued to be covered by the media between 2008 and 2010, although interest was diminishing, e.g., only 4 articles were published in 2008, 6 articles in 2009, and 3 articles in 2010. There was very minimal reporting between 2014 and 2015, with just 2 news stories published in 2014 and 1 article in 2015. No newspaper articles that directly discussed or reported on human gene patents were published in 2011, 2012, 2016, and 2017.
Fig. 1.
What is the attitude of the newspaper article towards human gene patents?
In addition to the frequency in reporting, we tracked the news sources where the articles appeared. Specifically, we looked at whether controversies around human gene patents attracted attention from leading Chinese publications such as the state-run newspapers People’s Daily or China Daily and other prestigious or popular newspapers. The analysis established that only one news article on patenting of human genes appeared in an influential national newspaper such as Guangming Daily in 2006. The focus of this article was the progress that Chinese researchers had made in the field of biotechnology and the number of gene patents was perceived as one of the indicators for evaluating achievements in biotechnology research.
Genes, Gene Patents, and Litigation Cases Mentioned
We established that 57.8% of the newspaper articles (n = 26) reported specific genes or disease-related genes. The articles mentioned 26 specific genes. BRCA 1/2 genes were the most discussed genes and were mentioned in 14 newspaper articles. The U.S. Supreme Court case of Association for Molecular Pathology v. Myriad Genetics, Inc was the most reported gene patent infringement lawsuit and was referenced in 14 newspaper articles. Other disease-related human genes were mentioned in 5 newspaper articles as outlined in Table 1. All news reports on patent infringement lawsuits were about cases in the foreign countries. We did not identify any reports of gene patent infringement claims in China or cases that have can be traced to companies or institutions in China, e.g., Chinese gene patent holders launching lawsuits in other countries.
Table 1.
Specific or disease-related genes mentioned
| Genes | Mentions |
|---|---|
| BRCA-1, BRCA-2 | 14 |
| Hereditary opalescent dentin DSPP | 2 |
| NAIL protein gene | 2 |
| Others (EPO genes, Human rhoC full-chain genes, snoRNA, SIR2, BTF3, MDM2, HDM2, CED-3, CED-4, P62, PCT, NARC8, 68, 10, 16, Human functional genes CDNA, Diabetes-related genes, Leber hereditary optic neurology genes, Psoriasis-related LEC genes, hemophilia-related genes, long-QT genes, VANGL2) | 1 |
Ethical Concerns and Risks
Seven distinct arguments against gene patents were raised in 17 newspaper articles as outlined in Table 2, which presents a range of concerns about the ethics of human gene patents and perceptions of their potential risks and benefits. It is worth noting that there was a certain level of ambiguity in the media texts as to whether opposition to gene patents was based on moral grounds or framed around practical concerns about risks and harms associated with the IP regime. Separate categories for “ethical issue” and “risk” were adopted to distinguish between arguments made purely on ethical grounds and objections to patentability that were based on practical considerations, rather than morality in principle. Among these arguments, two expressed ethical concerns and four emphasized risks associated with patenting human genes. The most frequently cited or discussed concern was that the discovery of a human gene should not be considered an invention (n = 12). Other concerns, such as the possibility that gene patents may hinder innovations in the genomics-related research (n = 11), that allowing human genes to be patentable would increase the cost of test services (n = 11), and that patenting or owning human genes is morally wrong (n = 8), were also commonly discussed or mentioned in the newspaper articles. All reasons to oppose or support gene patents mentioned in the newspaper articles and the frequency of their mentions are outlined in Table 2.
Table 2.
Ethical concerns, risks, and benefits associated with human gene patents
| Key issues identified by the press | Mentions |
|---|---|
| Ethical concerns | |
| It is morally wrong to own/patent human genes | 8 |
| Negative impact on patients’ access to healthcare | 7 |
| Risks associated with gene patents | |
| Discovery of gene is not invention | 12 |
| Increasing costs of test services | 11 |
| Hindering research and innovation | 11 |
| Genetic test monopoly | 6 |
| Benefits associated with gene patents | |
| The patenting of human genes is an important aspect of biotechnology commercialization | 10 |
| The patent system protects innovation in biotechnology | 2 |
| The patenting of human genes helps to prevent diseases and deaths. | 2 |
Benefits
We identified only three types of benefits that were discussed in 13 newspaper articles (Table 2). The argument that the patenting of human genes is an important component of biotechnology commercialization was the most frequently discussed or mentioned benefit, which was reported in 10 articles. The argument that patenting protects innovation in biotechnology was offered in 2 articles, while another 2 emphasized the positive health implications of gene patents in preventing diseases and deaths.
Attitudes Towards Gene Patents
In general, 57.8% of newspaper articles (n = 26) showed positive attitudes towards gene patents, while 17.8% articles (n = 8) opposed gene patenting and 24.4% articles (n = 11) were neutral in tone (Fig. 1). Out of the 11 newspaper articles with a neutral tone, eight were news reports about the Myriad case reviewed by the United States Supreme Court. The authors of these reports, however, did not express any value judgments towards human gene patents in principle or as pertaining to the decision about the patentability of human genes in the Myriad case. For instance, a news article by Xinhua Daily Telegraph published on 17 April 2013 reported that the Supreme Court of the US heard the Myriad patent case on 15 April. The article described briefly the timeline of the lawsuit of BRCA gene patent infringement and simply noted that the final decision made by the Supreme Court would have a far-reaching influence on the US gene patent regime (Lin and Ren 2013). The reporter did not express either a positive or a negative attitude towards gene patent holders and gene patent issues as a whole. Therefore, articles written in this style were classified as neutral in tone and attitudes.
Opinions About Chinese Gene-Related Patent Applications
Five articles pointed out problems with Chinese gene-related patent applications and raised suggestions for improving the patenting process for genetic research in China. Among these five news reports, four indicated that the number of gene patent acquisitions had increased quickly, while progress in patenting genetic technology and its commercialization had been slow and weak. For example, a news report by Chinese Business News published on 3 June 2009 included an interview with Mr. Zhang Qin, Deputy Director of the State Intellectual Property Office (Ma 2009). According to this news article, Mr. Zhang indicated that the Chinese businesses were weak in genetic technology innovation and development. In terms of gene patent applications, the number of filed gene patent applications by domestic businesses had decreased dramatically. In particular, from 2004 to 2008, no commercial enterprise entered the top ten gene patent holders in China. All ten leaders were academic organizations, including eight universities and two research institutes. In the western countries, by contrast, the main holders of gene-related patents are usually corporations and other for-profit organizations (Ma 2009). Another article published in 2009 argued that more professional patent agencies should be developed to facilitate the application for gene-related patents and provide inventors with better patent protections (Wang 2009). All these articles were generally supportive of the patentability of human genes.
Discussion
In recent years, gene sequencing and genetic testing industry in China has grown at an exponential rate. At the same time, the number of genetic diagnosis-related patents, especially those involving genetic diagnosis for cancers, has increased alongside the booming gene sequencing industry. For instance, according to a recent study, patent applications in the field of cancer gene diagnosis-related patents, such as diagnostic method patents and patents on genetic engineered DNA used in cancer diagnosis, have increased significantly from 1993 to 2011, with the highest growth rate observed in 2008. For instance, compared to the number of patent applications in 2007, 151 more patent claims were filed in 2008 (Li et al. 2016). However, the overall trend of gene patent applications did not present a corresponding increase. Previous research has indicated that Chinese applications for gene patents dropped from a peak in 2000 and had kept a stable low-level from 2001 to 2010 (Kers et al. 2014). Our analysis of the Chinese press reveals that there has been a limited media coverage on human gene patens and patenting controversies over the past decade, with only 45 newspaper articles reporting on these topics. Additionally, the issue has not received extended and nuanced coverage in nationwide and highly influential Chinese newspapers. There was only one news article on patenting human genes that was printed in an influential national newspaper such as Guangming Daily in 2006. This finding indicates that there has been marginal interest towards the issue of gene-related patents and their societal implications in China’s mainstream media.
Gene patent regimes can affect people’s lives in tangible ways, especially in cases when granting patents to biotech companies leads to the increased cost of diagnosis and poor quality of test results. Given the highly technical aspects of legal debates over patentability, news media can play an important role in knowledge dissemination and can increase the public understanding of gene patents and their potential impact on patient access to diagnostic tests and innovation. Most articles in our dataset that discussed the patentability of human genes were news reports on the U.S. Supreme Court’s Myriad case, while substantive and focused discussions on the Chinese patent regime for human genes were rarely seen. Moreover, some important national patent regulation updates, such as the 2010 Guidelines, did not trigger much attention in the newspaper coverage. This finding is consistent with an earlier study on the media coverage of gene patents, which investigated how the Myriad case was portrayed by the print media in the United Kingdom, Canada, and the United States (Caulfield et al. 2007). This study has shown that the Myriad case attracted more attention than any other issue and health policy related to gene patents (Caulfield et al. 2007).
Overall, our analysis has shown that positive attitudes towards the patentability of human genes were prevalent in the news reports. Although there was a greater emphasis in the news articles on ethical concerns about and arguments against human gene patents highlighting risks, media coverage has been largely characterized by favorable attitudes towards gene patenting. Additionally, 17 out of the 26 news reports showing a positive attitude towards gene patents did not state any specific reasons for supporting the patenting of human genes. Rather, these news stories expressed an overall favorable attitude without further in-depth discussions. This was especially the case with news publications prior to 2010. However, articles published after 2010 have clearly dispensed with the overall supportive attitude towards gene patenting, which suggests a gradual shift towards neutral and potentially negative perceptions. This change in tone is likely due to the Myriad case, which was highly publicized in the Chinese Press. The lawsuit, which was filed by the American Civil Liberties Union (ACLU) and the Public Patent Foundation on behalf of scientific associations, genetic counselors, women patients, cancer survivors, and breast cancer, and women’s health groups, brought into light the negative impact gene patents have on patients’ access to life-saving genetic testing.
Media coverage on the issue of human gene patents in China lacks depth in terms of the information presented and substantive concerns identified. Except for the Myriad case, specific gene patents and lawsuits were rarely reported or discussed in detail in the news reports. In general, news articles published before 2013 just mentioned the number of gene patents, while the rest of the content was mainly about the progress and problems in biotechnology research and development. On the one hand, the patent regime has been framed as an important part of biotechnology commercialization and gene patents have been used as an indicator for evaluating the innovation capability in the field of biotechnology. On the other hand, since 2009, news articles have paid more attention to the quality of genetic innovations patented and the industrialization level of genetic technology. One example is a news report published in Medical Economics News on 8 June 2009. The report praised the increased numbers of genetic patent applications and, at the same time, highlighted the importance of the innovation quality underlying the genetic patents and their applications in gene and pharmacy-related research fields (Li 2009).
Past research has already criticized the instrumentalism of the patent regime in China. In his research examining patent protection for Chinese biotechnology inventions, Liu (2005) indicated that the patent regime was regarded as an economic tool by the Chinese government, while the societal concerns associated with the patent system did not attract enough attention and were not well addressed. The strong protection of innovation was considered an effective way to further the biotechnology industry’s development and promote the welfare of the society. Our study shows that news media coverage in China is much in line with this tendency of overlooking complexities involved in the patenting of human genes. Furthermore, the findings are consistent with a previous analysis of the media portrayal of genetically modified organisms (GMOs), which have established that Chinese print media tend to depict genetic research and biotechnology innovation in a positive light (Du and Rachul 2012). Although we established that there were more mentions of ethical concerns and risks than discussions of specific benefits associated with gene patents, an overall positive attitude towards gene patents and the patent regime at a large was prevalent in the news coverage.
News articles sometimes contained inconsistencies and inaccuracies when reporting genetics-related stories. For example, during our data collection process, we noticed a 2016 news report that confused gene patents with gene-editing tool patents. The article used the phrase “gene patent” in the title (i.e., “The owner of a gene patent is taken, the winner is the king in the field of quantum – Nature magazine’s expectation for 2017”), but its contents were actually about the patenting of the CRISPR-Cas9 technology rather than gene patents. This article was subsequently excluded from the final data set. Previous studies have shown that accurate and adequate reporting is essential for educating the public about cutting-edge biotechnology and shaping an informed public debate (Zhao et al. 2014). Our analysis of the media discourse on human gene patenting also suggests that investing in and cultivating science journalism in China will be beneficial, especially as the field of biotechnology research and development in China continues to advance rapidly (Zhao et al. 2014; Catalan-Matamoros and Peñafiel-Saiz 2017).
As mentioned earlier, there have been no patent infringement claims reported so far in China. This is likely the reason, as we hypothesized, that the topic of gene patents had not attracted significant attention by Chinese news media outlets. There are indications that this lack of patent infringement claims might be due to weak enforcement by current IP right holders. Nonetheless, there is no reason to expect that the patent owners will continue to ignore infringements, especially in cases where a certain type of genetic tests are mainly prescribed and implemented by public health clinics, while the relevant patents are held by private companies (Hawkins et al. 2019). The situation with the CHEO’s lawsuit in Canada, which we discussed earlier, is a good example of a potential conflict that may arise. Patent infringement challenges over NIPT services in the United Kingdom can provide additional explanatory insight relevant to the current context in China. In 2015, Illumina, a gene and genetic testing patent holder, started challenging unauthorized genetic testing services relating to the use of cell-free fetal DNA for NIPT in the UK. The infringement lawsuits were not expected by most researchers and genetic testing providers, who had ignored the company’s ownership of the gene and genetic diagnosis patents and did not consider infringement to be a real problem (Hawkins 2011; Montgomery 2017). In March 2015, Illumina filed a patent infringement suit against Premaitha over NIPT patents. Later in January 2016, Illumina filed another two patent infringement lawsuits against the Doctors Laboratory, TDL Genetics, and Ariosa Diagnostics over its NIPT patents. The UK High Court of Justice heard the lawsuits in a combined way in 2017 and made decisions that largely favored Illumina, ruling that the detection method is patentable (Hawkins et al. 2019; Genomeweb 2019). Predictably, gene-related patent infringement disputes will not just be a theoretical possibility in the continually growing market in China, but will likely present real legal and ethical challenges to public health. It will be beneficial to have a more balanced and extensive media coverage of gene patenting issues to support an informed public debate should a high-profile patent infringement case is brought by an IP owner in China.
Conclusion
Overall, our analysis has established that there was limited coverage of human gene patenting in the Chinese newspapers. Reporting on the issue frequently lacked depth and nuance in terms of the information presented and substantive concerns discussed. Newspaper articles have tended to favor gene patenting, which has been viewed as an important driver for the growth of the genetic testing industry in China, although there were some informative discussions highlighting potential risks over benefits. Most articles that were critical of gene patenting were news reports on the United States Supreme Court Myriad case, which invalidated the company’s patents on BRCA1 and BRCA2 genes in 2013. Critical perspectives on the Chinese gene patent regime were rarely found. Nonetheless, the experiences of other jurisdictions we discussed throughout the article suggest that the current situation in China can change if patent infringement cases are filed or legal challenges are mounted to improve access to genetic tests and diagnosis. As we have seen in the past, gene patents can have tangible effects on people’s lives and can garner significant media attention, especially in cases of strong public opposition to patenting and patient activism to improve access to health care innovation. There is a need for a more balanced and nuanced media reporting on the issue in China, as well as placing a greater emphasis on the broader ethical and societal implications of the patent system.
We suggest that media and science journalism can play a larger role in fostering a more critical debate on human gene patents and increasing public engagement with the IP regime. While it is unrealistic to expect strong grassroots activism around gene patents in China, similar to the Myriad case in the United States, leadership from government and academia can be effective in shaping a more nuanced media narrative. Public engagement informed by the principles of deliberative democratic theory is not an entirely new concept in China. For example, a top-down approach to encourage public participation in policymaking has been previously utilized in the long process of reforming the country’s health care system, which was completed in 2009 (Kornreich et al. 2012). Although the participation forums introduced by the government, in this case, took the form of consultation — an approach that is mainly used for soliciting feedback from the public and stakeholders — the initiative created some space for limited deliberation (Kornreich et al. 2012). A similar top-down initiative may provide a more effective mechanism for elevating the public discourse on gene patenting, especially as the issue presently does not seem to attract significant attention from Chinese news media.
Abbreviations
- BRCA 1
Breast cancer gene 1
- BRCA2
Breast cancer gene 2
- cDNA
Complementary deoxyribonucleic acid
- DNA
Deoxyribonucleic acid
- CHEO
Children’s Hospital of Eastern Ontario
- LQTS
Long QT syndrome
- ACLU
American Civil Liberties Union
Authors’ Contributions
LD designed the study and wrote the manuscript. SJL collected and analyzed the data. KK wrote and substantially revised the manuscript. All authors read and approved the final manuscript.
Funding Information
This research was funded by the University of Macau multi-year research grant–MYRG2018-00074-FLL and the Ministry of Science and Technology of the People’s Republic of China under the project: Research on Precision Medicine Ethics, Policy, and Legal Framework (Grant: 2017YFC0910100).
Data Availability
The dataset used and/or analyzed during the current study are available in Zenodo. (doi: 10.5281/zenodo.2595240).
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Code Availability
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Li Du, Email: stephendu@um.edu.mo.
Kalina Kamenova, Email: kamenova@genomicsandsociety.com.
References
- Andrews, Lori B., and Jordan Paradise. 2005. Gene patents: the need for bioethics scrutiny and legal change. Yale Journal of Health Policy, Law, and Ethics 5 (1): 403–412. Accessed 10 July 2020. https://digitayjhple/yjhple/vol5/iss1/13. [PubMed]
- Ariosa Diagnostics v Sequenom. 2015. 788 F.3d 1371.
- Association for Molecular Pathology v Myriad Genetics. 2013. 569 US 12–398.
- Bonter, Katherine L., Carmela De Luca, and Christi J. Guerrini. 2018. Gene patents in Canada: Is there a new legal landscape? Molecular Diagnosis & Therapy 22: 149–155. 10.1007/s40291-017-0313-9. [DOI] [PubMed]
- Bubela, Tania, and Tim Caulfield. 2004. Do the print media “hype” genetic research? A comparison of newspaper stories and peer-reviewed research papers. Canadian Medical Association Journal 170 (9): 1399–1407. 10.1503/cmaj.1030762. [DOI] [PMC free article] [PubMed]
- Catalan-Matamoros, Daniel, and Carmen Peñafiel-Saiz. 2017. The use of traditional media for public communication about medicines: a systematic review of characteristics and outcomes. Health Communication 34: 415–423. 10.1080/10410236.2017.1405485. [DOI] [PubMed]
- Caulfield, Timothy. 2004. Biotechnology and the popular press: hype and the selling of science. Trends in Biotechnology 22 (7): 337–339. 10.1016/.tibtech.2004.03.014. [DOI] [PubMed]
- Caulfield, Timothy, Tania Bubela, and C.J. Murdoch. 2007. Myriad and the mass media: the covering of a gene patent controversy. Genetics in Medicine 9 (12): 850–855. 10.1097/GIM.0b013e31815bf965. [DOI] [PubMed]
- Chandrasekharan, Subhashini, and Melissa Fiffer. 2010. Impact of gene patents and licensing practices on access to genetic testing for hearing loss. Genetics in Medicine 12: S171–S193. 10.1097/GIM.0b013e3181d7b053. [DOI] [PMC free article] [PubMed]
- Chapman, Simon, Abby Haynes, Gemma Derrick, Heidi Sturk, Wayne D. Hall, and Alexis St. George. 2014. Reaching “an audience that you would never dream of speaking to”: influential public health researchers’ views on the role of news media in influencing policy and public understanding. Journal of Health Communication 19 (2): 260–273. 10.1080/10810730.2013.811327. [DOI] [PubMed]
- Cyranoski, David. 2016. China embraces precision medicine on a massive scale. Nature 529 (7584): 9–10. 10.1038/529009a. [DOI] [PubMed]
- D’Arcy v Myriad Genetics. 2015. HCA: 35.
- Directive 98/44/EC of the European Parliament and of the Council of 6 July 1998 on the legal protection of biotechnological inventions. 30.7.1998, L213/13.
- Du, Li. 2018. Patenting human genes: Chinese academic articles’ portrayal of gene patents. BMC Medical Ethics 19: 29. 10.1186/s1290-018-0271-8. [DOI] [PMC free article] [PubMed]
- Du, Li, and Christan Rachul. 2012. Chinese newspaper coverage of genetically modified organisms. BMC Public Health 12: 326. 10.1186/1471-2458-12-326. [DOI] [PMC free article] [PubMed]
- Du, Li, Kalina Kamenova, and Timothy Caulfield. 2015. The gene patent controversy on Twitter: a case study of Twitter users’ responses to the CHEO lawsuit against long QT gene patents. BMC Medical Ethics 16: 55. 10.1186/s12910-015-0049-1. [DOI] [PMC free article] [PubMed]
- Entman, Robert M. 1993. Framing: toward clarification of a fractured paradigm. Journal of Communication 43 (4): 51–58. 10.1111/j.1460-2466.1993.tb01304.x.
- Evans, James P., and Michael S. Watson. 2015. Genetic testing and FDA regulation: overregulation threatens the emergence of genomic medicine. JAMA 313 (7): 669–670. 10.1001/jama.2014.18145. [DOI] [PubMed]
- Gauntlett, D. 2004. Ten things wrong with the “Effects Model.”. In Media studies: the essential resource,ed. P. Rayner, P. Wall, and S. Kruger, 112. London: Routledge.
- Geller, Gaul, Barbara A. Bernhardt, and Neil A. Holtzman. 2002. The media and public reaction to genetic research. JAMA 287 (6): 773. 10.1001/jama.287.6.773-JMS0213-3-1. [PubMed]
- Genomeweb. 2019. Illumina wins NIPT infringement suit against Roche’s Arisoa Diagnostics. GenomeWeb, 17 June 2019. https://www.genomeweb.com/sequencing/illumina-wins-nipt-infringement-suit-against-roches-ariosa-diagnostics#.XtZ_Y54zb6A. Accessed 29 June 2020.
- Gold, E. Richard, and Julia Carbone. 2010. Myriad genetics: In the eye of the policy storm. Genetics in Medicine. 10.2139/ssrn.1260098. [DOI] [PMC free article] [PubMed]
- Hawkins, Naomi. 2011. The impact of human gene patents on genetic testing in the United Kingdom. Genetics in Medicine 13 (4): 320–324. 10.1097/GIM.0b013e3181fc50bc. [DOI] [PMC free article] [PubMed]
- Hawkins, Naomi, Dianne Nicol, Subhashini Chandrasekaran, and Robert Cook-Deegan. 2019. The continuing saga of patents and non-invasive prenatal testing. Prenatal Diagnosis 39 (6): 441–447. 10.1002/pd.5450. [DOI] [PMC free article] [PubMed]
- Illumina v Ariosa Diagnostics. 2020. No. 2019–1419 (Fed. Cir. Mar. 17, 2020).
- Information of the Database. 2019. 中国学术期刊(光盘版). http://kns.cnki.net/kns/brief/result.aspx?dbprefix=CCND. Accessed 29 June 2020.
- Jamison, Molly. 2015. Patent harmonization in biotechnology: towards international reconciliation of the gene patent debate. Chicago Journal of International Law 15: 688–720. https://chicagounbound.uchicago.edu/cjil/vol15/iss2/9. Accessed 10 July 2020.
- Kamenova, Kalina, Amir Reshef, and Timothy Caulfield. 2014. Angelina Jolie’s faulty gene: newspaper coverage of a celebrity’s preventive bilateral mastectomy in Canada, the United States, and the United Kingdom. Genetics in Medicine 16: 522–528. 10.1038/gim.2013.199. [DOI] [PubMed]
- Kers, Jannigje, Elco Van Burg, Tom Stoop, and Martina C. Cornel. 2014. Trends in genetic patent applications: the commercialization of academic intellectual property. European Journal of Human Genetics 22: 1155–1159. 10.1038/ejhg.2013.305. [DOI] [PMC free article] [PubMed]
- Kornreich, Yoel, Ilan Vertinsky, and Pitman B. Potter. 2012. Consultation and deliberation in China: the making of China’s health-care reform. The China Journal 68: 176–203. 10.1086/666583.
- Landis, J. Richard, and Gary G. Koch. 1977. The measurement of observer agreement for categorical data. Biometrics. 33 (1): 159–174. 10.2307/2529310. [PubMed]
- Li, Y. 2009. 基因产业:好风还需凭借力 (“Gene industry: good wind depends on borrowing,” translated by authors). Medical Economics News.
- Li B, Bao H, Lan X. 基于专利地图的中美肿瘤基因诊断技术竞争力分析 (“Analysis of the competitiveness of cancer gene diagnosis technology between China and the United States based on the patent map,” translated by authors) China Medical Biotechnology. 2016;11:82–87. [Google Scholar]
- Lin, Xiaochun, and Haijun Ren. 2013. 美国最高法院再审基因专利案 (“The US supreme court reviewed the gene patent case,” translated by authors). Xinhua Daily Telegraph.
- Liu, Deming. 2005. Now the wolf has indeed come! Perspective on the patent protection of biotechnology inventions in China. American Journal of Comparative Law 53: 207–260. 10.1093/ajcl/53.1.207
- Ma, Xiaohua. 2009. 量高质低 我国基因工程遭遇专利尴尬 (“High quantity while low-quality Chinese bio-engineering is facing patent embarrassment,” translated by authors). Chinese Business News.
- McCombs, Maxwell E., and Donald L. Shaw. 1972. The agenda-setting function of mass media. Public Opinion Quarterly 36 (2): 176–187. 10.1086/267990.
- Ministry of Science and Technology of P.R. China. 2016. 科技部关于发布国家重点研发计划精准医学研究等重点专项2016年度项目申报指南的通知 (“Notice of the Ministry of Science and Technology on the issuance of 2016 annual project application guide for national key R & D plan on precision medicine research,” translated by authors). http://www.most.gov.cn/tztg/201603/t20160308_124542.htm. Accessed 29 June 2020.
- Montgomery, Rachel. 2017. Illumina wins NIPT patent case in UK High Court. https://www.bionews.org.uk/page_96274. Accessed 29 June 2020.
- Nicol, Dianne, Rochelle C. Dreyfuss, E. Richard Gold, Wei Li, John Liddicoat, and Geertrui Van Overwalle. 2019. International divergence in gene patenting. Annual Review of Genomics and Human Genetics 20: 519–541. 10.1146/annurev-genom-083118-015112. [DOI] [PubMed]
- Nisbet, Matthew C., Dominique Brossard, and Adrianne Kroepsch. 2003. Framing science: The stem cell controversy in an age of press/politics. Harvard International Journal of Press/Politics 8 (2): 36–70. 10.1177/1081180X02251047.
- Patent Law of People’s Republic of China. 2008. http://www.sipo.gov.cn/zcfg/zcfgflfg/flfgzl/fl_zl/1063508.htm. Accessed 29 June 2020.
- Salter, Brian. 2009. China, globalization and health biotechnology innovation: Venture capital and the adaptive state. East Asian Science, Technology and Society 3 (4): 401–420. 10.1215/s12280-009-9090-9.
- Scheufele, Dietram A. 1999. Framing as a theory of media effects. Journal of Communication 49 (1): 103–122. 10.1111/j.1460-2466.1999.tb02784.x.
- Simoncelli, Tania, and Sandra S. Park. 2015. Making the case against gene patents. Perspectives on Science 23 (1): 106–145. 10.1162/POSC_a_00161.
- State Intellectual Property Office of the P. R. China . Guidelines for Patent Examination 2010. Beijing: Intellectual Property Publishing House; 2010. [Google Scholar]
- Wang Z. 基因研究应瞄准市场 产业化困局待解 (“Genetic research should target market and dilemma in industrialization should be solved” translated by authors) 2009. [Google Scholar]
- Wang, Ruiyan, Qin Cao, Qiuwei Zhao, and Yin Li. 2018. Bioindustry in China: an overview and perspective. Nature Biotechnology 40 (Part A): 46–51. 10.1016/j.nbt.2017.08.002. [DOI] [PubMed]
- Zarzeczny, Amy, Christan Rachul, Matthew Nisbet, and Timothy Caulfield. 2010. Stem cell clinics in the news. Nature Biotechnology 28: 1243–1246. 10.1038/nbt1210-1243b. [DOI] [PubMed]
- Zhao, Feifei, Yan Chen, Siqi Ge, Xinwei Yu, Shuang Shao, Michael Black, et al. 2014. A quantitative analysis of the mass media coverage of genomics medicine in China: a call for science journalism in the developing world. OMICS 18 (4): 222–230. 10.1089/omi.2013.0108. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset used and/or analyzed during the current study are available in Zenodo. (doi: 10.5281/zenodo.2595240).