Abstract
Numerous models are available for the preclinical study of sepsis, and they fall into one of three general categories: (1) administration of exogenous toxins (e.g., lipopolysaccharide, zymosan), (2) virulent bacterial or viral challenge, and (3) host barrier disruption, e.g., cecal ligation and puncture (CLP) or colon ascendens stent peritonitis (CASP). Of the murine models used to study the pathophysiology of sepsis, CLP combines tissue necrosis and polymicrobial sepsis secondary to autologous fecal leakage, as well as hemodynamic and biochemical responses similar to those seen in septic humans. Further, a transient numerical reduction of multiple immune cell types, followed by development of prolonged immunoparalysis, occurs in CLP-induced sepsis just as in humans. Use of the CLP model has led to a vast expansion in knowledge regarding the intricate physiological and cellular changes that occur during and after a septic event. This updated article details the steps necessary to perform this survival surgical technique, as well as some of the obstacles that may arise when evaluating the sepsis-induced changes within the immune system. It also provides representative monoclonal antibody (mAb) panels for multiparameter flow cytometric analysis of the murine immune system in the septic host.
Basic Protocol:
Cecal ligation and puncture in the mouse
Keywords: cecal ligation and puncture, CLP, sepsis, septic shock, surgical murine model, systemic inflammatory response syndrome (SIRS)
CECAL LIGATION AND PUNCTURE IN THE MOUSE
BASIC PROTOCOL
The following protocol describes the method for performing the murine cecal ligation and puncture (CLP) model of polymicrobial sepsis. CLP is a well-established experimental model with >3800 published articles representing the cumulative efforts of >12,000 individual authors (Fig. 1A). Initially described as a porcine model of sepsis (Imamura & Clowes, 1975), CLP was rapidly adapted to other animal model systems. The first report using CLP in mice was published in 1983 (Baker, Chaudry, Gaines, & Baue, 1983), and the mouse is now the predominant CLP animal model used to study sepsis, accounting for >65% of all CLP publication in the last 10 years (Fig. 1A–D). Interestingly, CLP has also been employed to address questions regarding acute lung and brain injury (Singer et al., 2016; Wang et al., 2019; Ye et al., 2019; Yehya et al., 2015), demonstrating that CLP can be used as a tool to address a diverse array of experimental questions. Since the previous publication of the CLP methodology in Current Protocols in Immunology in 2010 (Cuenca, Delano, Kelly-Scumpia, Moldawer, & Efron, 2010), the number of papers published using CLP has doubled (Fig. 1B), with the number of murine CLP papers increasing 2.4-fold (Fig. 1C). Notably, this increase has come from the efforts of ~7700 authors using CLP that had not done so prior to 2010 (Fig. 1A). This surge in utilization necessitates some reflection on the current state and flexibility of the technique. Thus, we herein describe strategies to manipulate the severity of sepsis induced by CLP, possible methodologic hurdles one may encounter when performing the CLP surgery, troubleshooting of those problems, and incorporation of images/data to aid in the proper utilization of this technique.
Figure 1.
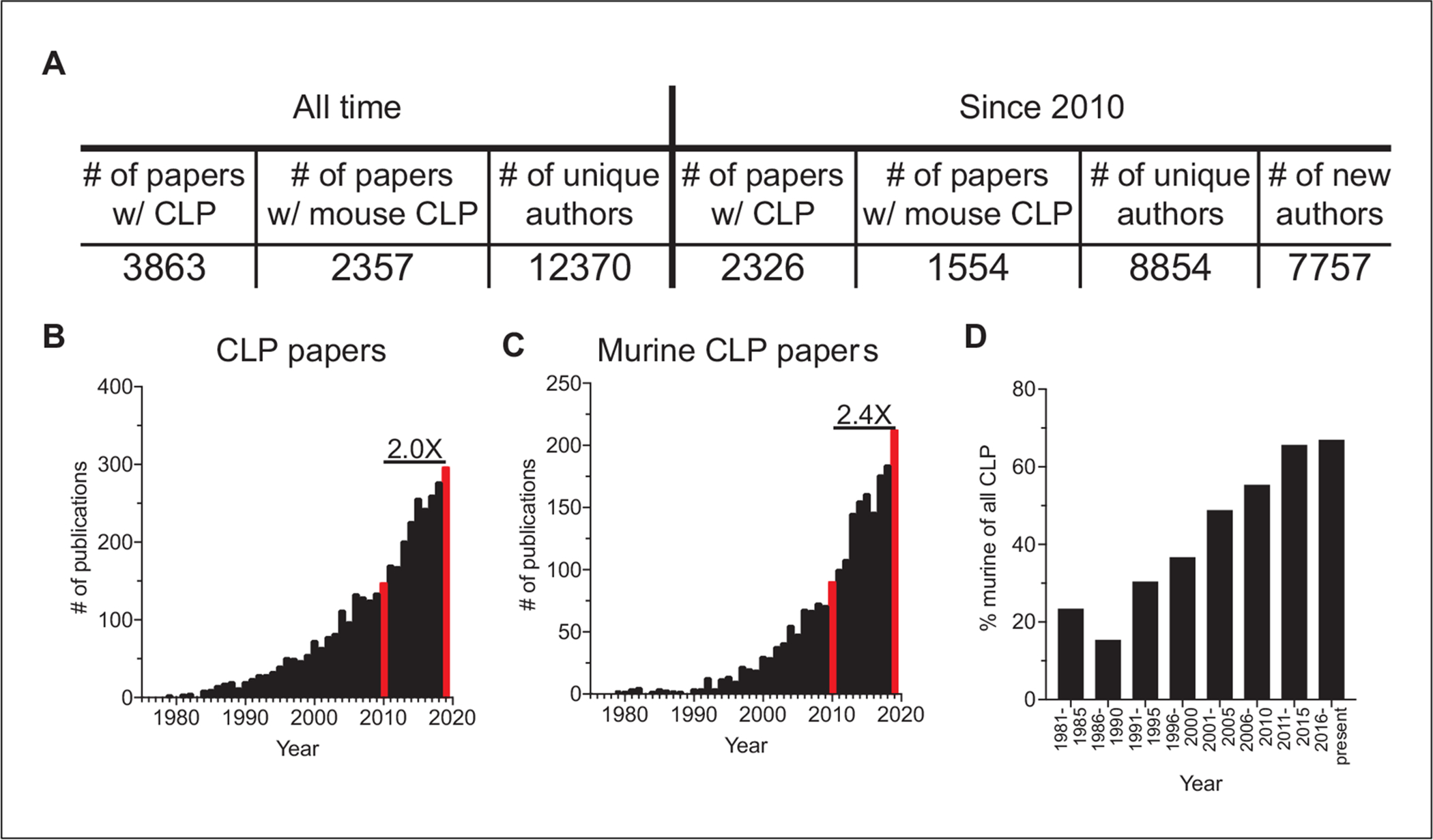
Utilization of the cecal ligation and puncture (CLP) model over time based on publications. Total and murine CLP results were generated by PubMed searches of “cecal ligation and puncture” or “mouse cecal ligation and puncture,” respectively, performed on June 25, 2020. Indexing information was downloaded and used for evaluation. Total CLP results include 50 review articles, 26 published since 2010. Murine CLP results include 21 review articles, 10 published since 2010. (A) Synopsis of CLP publications, murine CLP publications, and number of unique authors, in total and since 2010. The number of unique authors that have only published a CLP paper since 2010 is also included. (B,C) Numbers of total and murine CLP publications per year from 1975 to 2019. (D) Representation of murine CLP publications among all CLP publications in 5-year periods from 1981 to present.
NOTE:
Protocols involving survival surgeries on live animals must be reviewed and approved by the investigator’s Institutional Animal Care and Use Committee (IACUC). Appropriate personal protective equipment (e.g., gloves, surgical mask, hair net, lab coat, protective sleeves) should be worn based on institutional guidance and regulations. In addition, use of any controlled substances (e.g., ketamine) requires appropriate licensure and storage.
Materials
Mice
General anesthetic: isoflurane (2.5% in oxygen gas) or ketamine/xylazine
Ophthalmic lubricant ointment (Akorn Animal Health, 9399-162-35)
Local antiseptic: 5% povidone-iodine
Local anesthetic: bupivacaine (6 mg/kg s.c. in 100 μl) or lidocaine
Tissue adhesive/surgical glue (e.g., Vetbond, 3M, 1469SB)
Sterile injectable normal saline (0.9%)
Post-operative analgesia: Meloxicam (2 mg/kg s.c. in 1 ml 0.9% saline) or flunixin meglumine
Biological safety cabinet
Autoclavable instrument packs
Small animal scale
Veterinary anesthesia machine with isoflurane vaporizer, flow meter, and nose cone
Plexiglas induction chamber
Sterile surgical pad/drape
Heated surgical mat
Electric razor
Sterile surgical sponge or swab
Stainless steel, straight, blunt tipped surgical scissors
Stainless steel, blunt tipped forceps
Stainless steel, serrated forceps (2)
4–0 silk suture
25-G hollow-bore needle (or other size depending on desired severity of sepsis)
4–0 absorbable polyfilament sutures
Heating pad
Surgical instrument micro bead sterilizer
Prepare for surgery
-
1
Place surgical tools in sterilization pouches and sterilize by autoclaving.
-
2
Record mouse weights on the day of surgery.
Anesthetize and prepare first mouse
-
3
Set up the veterinary anesthesia machine and place the animal into the induction chamber. Administer an isoflurane concentration of 2.5% with O2 flow at 2 L/min.
A injected cocktail of ketamine and xylazine is another commonly used anesthetic that can also be used for this procedure. Dosing amounts are based on mouse weight: generally 87.5 and 5–12.5 mg/kg, respectively, although this may vary based on IACUC guidelines.
-
4
Determine depth of anesthesia by firmly pinching the foot pad while the mouse lies on its back.
When properly anesthetized, the mouse should be nonresponsive to foot pad pinching. An over-anesthetized mouse will display gasping respiration.
-
5
Once the mouse is fully anesthetized, remove it from the induction chamber, place it on a heated surgical mat, and secure the nose cone (Fig. 2A). Apply ophthalmic ointment.
-
6
Shave the lower abdomen and clean the skin with 5% povidone-iodine antiseptic using a surgical sponge (Fig. 2B). If needed, clear residual fur from the surgical area using a piece of gauze dipped in 70% ethanol.
Removing fur will aid in making and closing the incision, but care should be taken not to use too much ethanol, as this could increase the chance of hypothermia.
-
7
Administer bupivacaine (6 mg/kg s.c.) to the site where the paramidline laparotomy will be performed.
Figure 2.
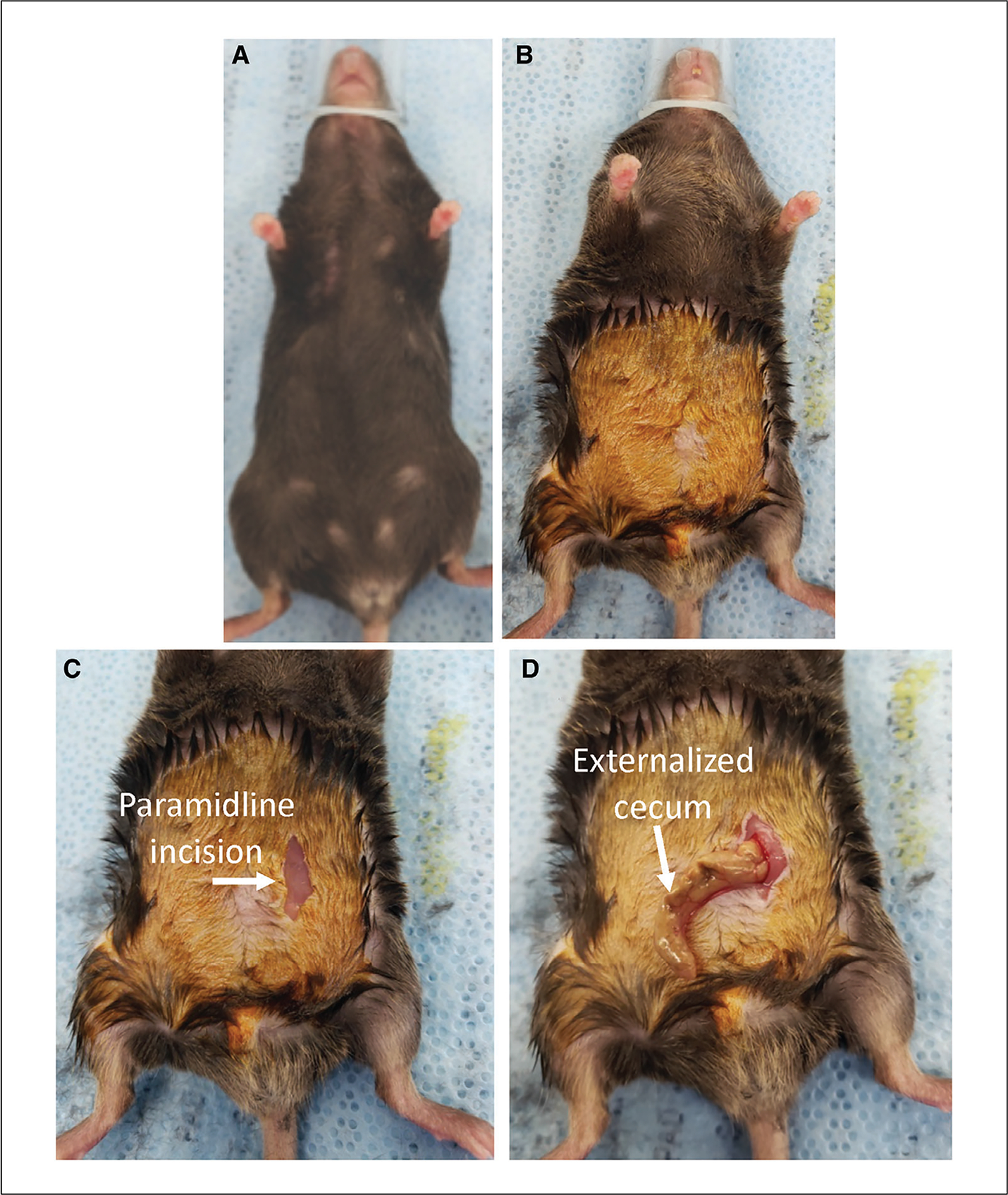
Prepping the mouse and exposing the cecum. (A) The mouse is positioned on heated surgical mat and the nose cone secured. (B) The abdominal fur is shaved and 5% povidone-iodine antiseptic is applied. (C) A small (~1 cm) paramidline incision is made through the skin. (D) The cecum is externalized.
Perform CLP
-
8
Make a small paramidline incision ~1 cm in length though the skin, being careful to avoid cutting the abdominal wall (Fig. 2C). Once the skin has been cut and the abdominal wall identified, make an incision through the abdominal wall.
To avoid cutting the intestines, use scissors with blunt tips, grasp the abdominal wall firmly with forceps, and pull it away from the mouse prior to cutting.
-
9
Use forceps to locate and externalize the cecum (Fig. 2D).
The cecum is commonly located on the animal’s left side (or right when the mouse is on its back), but this location can vary, particularly in larger mice.
-
10
Using forceps and a 4–0 silk suture, tie one knot to ligate the distal third (~1 cm) of the cecum (Fig. 3A).
This step is important in governing the severity of the CLP. Ligating a smaller portion of the cecum will cause less-severe sepsis and lower mortality, whereas ligating a larger portion will result in a more-severe sepsis, heightened inflammatory response, and higher mortality rate.
-
11
Using a 25-G hollow-bore needle, puncture the ligated portion of the cecum from the basolateral side into the lumen (Fig. 3B). Carefully verify puncture placement by extruding a small amount of cecal material though the puncture with forceps (Fig. 3C).
Extrusion of cecal material should be done slowly and with minimal pressure to avoid tearing and further perforation of the cecum. In conjunction with the ligation step, the amount of cecal material released into the peritoneum will determine the severity of the sepsis and subsequent response. Variation in the number and size of the puncture(s) can be used to modulate sepsis severity, inflammatory response, and mortality rate.
A new needle should be used after every ten mice to ensure consistent puncture of the cecum and reduce the chance for luminal tearing during verification. Needles should also be exchanged between different experimental groups to prevent potential effects of distinct microbiota.
-
12
Return the ligated cecum to the abdomen.
It is important to return the cecum well into the abdominal cavity, as cecal tissue necrosis near the abdominal incision can lead to degradation of the sutures and cause the incision to heal improperly or even reopen.
-
13
Close the peritoneum using serrated forceps to tie two or three 4–0 absorbable polyfilament uninterrupted sutures (Fig. 3D).
Alternatively, sutures can be hand-tied.
-
14
Close the skin using tissue adhesive (Fig. 3E).
An autoclip wound-closing system can be used to close the skin, if preferred. Clips will need to be removed as the skin heals (following institutional requirements).
With practice, one CLP procedure can be completed in 5 min or less. Depending on protocols for instrument sterilization, the total length of a procedure and prep time per animal is 10–15 min.
Figure 3.
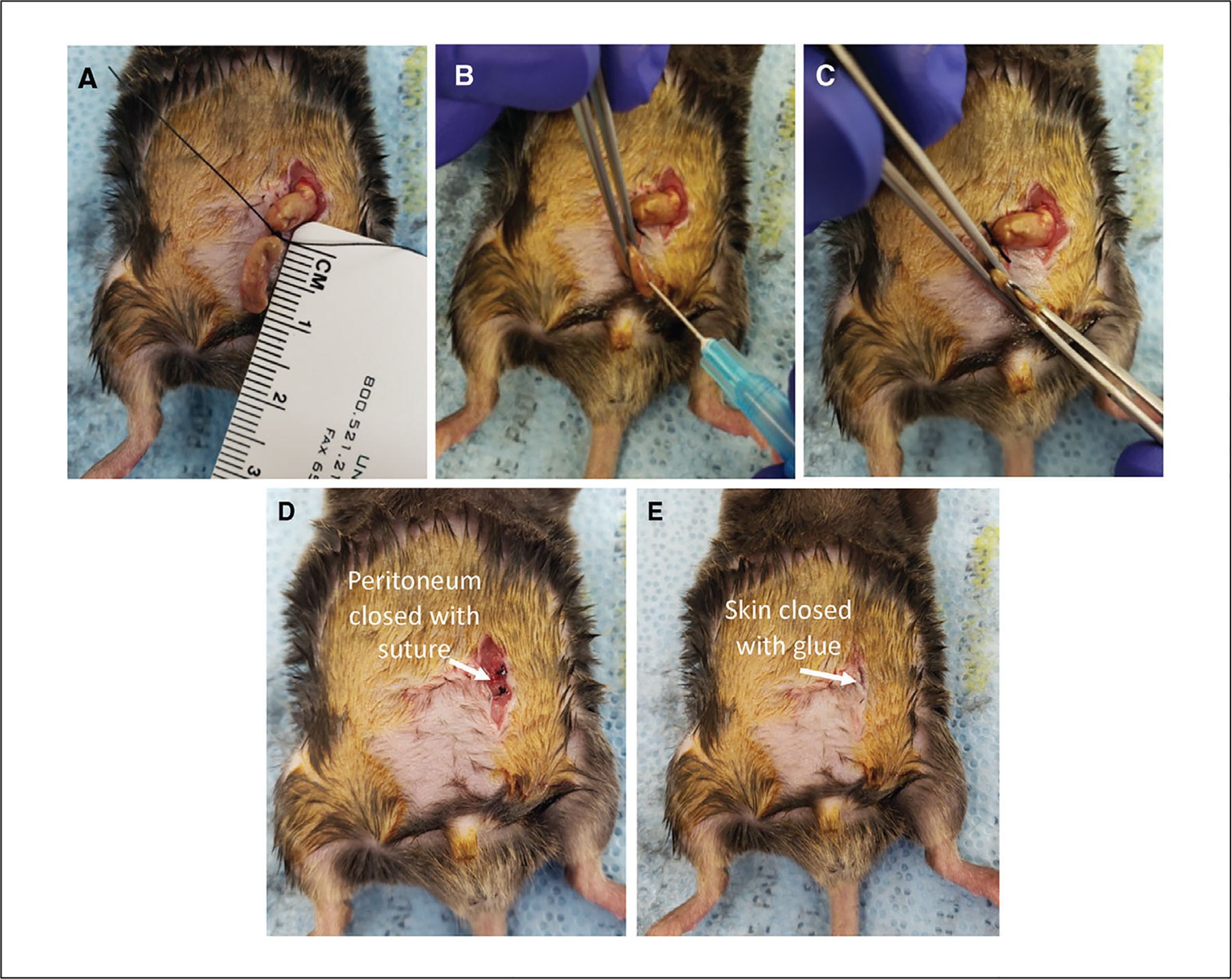
Performing CLP. (A) The cecum is ligated using a ruler as a guide. (B) The cecum is punctured from the basolateral side into the lumen using a 25-G needle. (C) To verify puncture placement, a small amount of cecal material is extruded though the puncture using forceps. (D) The peritoneum is closed using two or three 4–0 absorbable polyfilament uninterrupted sutures. (E) The skin is closed using Vetbond tissue adhesive.
Perform post-operative care
-
15
Inject post-operative analgesia (e.g., meloxicam, 2 mg/kg) in 1 ml saline subcutaneously into the scruff of the neck or flank.
Other common analgesic agents include opioids (such as buprenorphine) or nonsteroidal anti-inflammatory drugs (NSAIDs). Recommended and approved analgesics may vary by IACUC.
Post-operative analgesia and fluid resuscitation are typically given at the conclusion of surgery and for multiple days following the procedure. The length of time that analgesia/fluids are given will depend on individual institutional requirements.
-
16
Place the mouse on its back on sterile paper towel in a clean cage. Position the cage on a heating pad set on low, such that half of the cage is heated by the mat. Monitor the mouse until it has regained consciousness and is able to move around the cage.
Depending on the anesthesia method, the recovery time may be quite short (using isoflurane) or long (using ketamine/xylazine). Animals should be monitored at regular intervals until they are alert and ambulatory.
Perform additional CLPs
-
17
Before beginning the next CLP, sterilize the surgical tools using a bead sterilizer or alcohol.
-
18
Repeat for the desired number of animals, including sham (control) animals that are processed through all the above steps except steps 10–11 (cecal ligation and puncture).
-
19
Monitor animals every 24 hr for 5 days for weight loss, signs of pain or distress, or changes in grooming, posture, and mobility.
Signs of pain and distress include vocalization when touched and labored breathing. If mice are showing signs of pain or distress, changes in grooming, posture, or mobility, further analgesia or euthanasia should be considered.
Post-operative monitoring is done to avoid or minimize discomfort, distress, and pain and prevent animals from spontaneous death. Check with your IACUC for guidelines on post-operative monitoring and euthanasia. Moist food can be given to help to keep the mice hydrated.
COMMENTARY
Background Information
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated immune response that occurs as the result of an infection (Shankar-Hari et al., 2016). Sepsis causes thousands of deaths annually (Rudd et al., 2020). Improvements in intensive care over the last 40 years have reduced mortality from an initial infection from 80% to 20%−30% (Angus & van der Poll, 2013; Angus, Pereira, & Silva, 2006). Despite these gains, survivors of the initial infection suffer from long-term impairments in immunity and increased susceptibility to common secondary infections (Hotchkiss, Monneret, & Payen, 2013). In fact, ~70% of sepsis-related deaths occur after the patient has recovered from the initial septic event as a result of immunosuppression, with many deaths occurring weeks or months later (Donnelly, Hohmann, & Wang, 2015; Hotchkiss et al., 2013).
For many years, sepsis was considered a hyperinflammatory response, driven by proinflammatory cytokines and chemokines in the presence of a disseminated infection (van der Poll, van de Veerdonk, Scicluna, & Netea, 2017). Although it is clear that proinflammatory signals dominate the initial response, sepsis consists of both excessive inflammatory and immune suppressive responses that occur simultaneously (van der Poll et al., 2017). During a septic event, patients experience a sharp reduction in the number of immune cells as the result of apoptotic lymphocyte death. Lymphopenia contributes to decreased responses to new or secondary infections and reactivation of latent viruses (Hotchkiss et al., 1999, 2001, 2002; Kollef et al., 2008; Limaye et al., 2008; Luyt et al., 2007; Otto et al., 2011; Unsinger et al., 2010). This sepsis-induced lymphopenia is transient, and the immunoparalysis that develops after a septic event extends beyond the point of the recovery of absolute lymphocyte numbers (Cabrera-Perez, Condotta, Badovinac, & Griffith, 2014; Condotta, Cabrera-Perez, Badovinac, & Griffith, 2013; Danahy, Strother, Badovinac, & Griffith, 2016; Hotchkiss et al., 2013; Jensen, Sjaastad, Griffith, & Badovinac, 2018a). Persistent immune suppression following a septic event is now considered a leading reason for extended increased susceptibility to pathogens that are normally cleared in healthy individuals.
With the goal of identifying novel and effective therapies for the treatment of sepsis, researchers have developed numerous animal models to study the disease and test potential therapies. These include administration of exogenous toxins such as lipopolysaccha-ride and zymosan, virulent bacterial or viral challenges, and host barrier disruption as in the CLP and CASP models. CLP is the most commonly used sepsis model, in part because it replicates many factors and complexities of human sepsis through the combination of necrosis, tissue damage, and initiation of a polymicrobial infection. Following induction of sepsis in the CLP model, levels of proinflammatory cytokines (including IL-1β, IL-6, IFNγ, and TNF) in the blood increase substantially, peaking at ~6 hr post-induction (Fig. 4). Closely following the rise of proinflammatory cytokines, levels of anti-inflammatory cytokines (e.g., IL-10 and IL-4) increase. Similarly, increased serum levels of TNF, IL-6, IL-1β, IL-10 are commonly observed in human septic patients (Chaudhry et al., 2013), and high serum levels of TNF and IL-6 are associated with poor outcomes in human sepsis (Damas et al., 1992; Hu, Chen, Pang, & Chen, 2019; Neilson, Kavanagh, & Rao, 1996; Remick, Bolgos, Siddiqui, Shin, & Nemzek, 2002; Retsas et al., 2018; Song et al., 2019).
Figure 4.
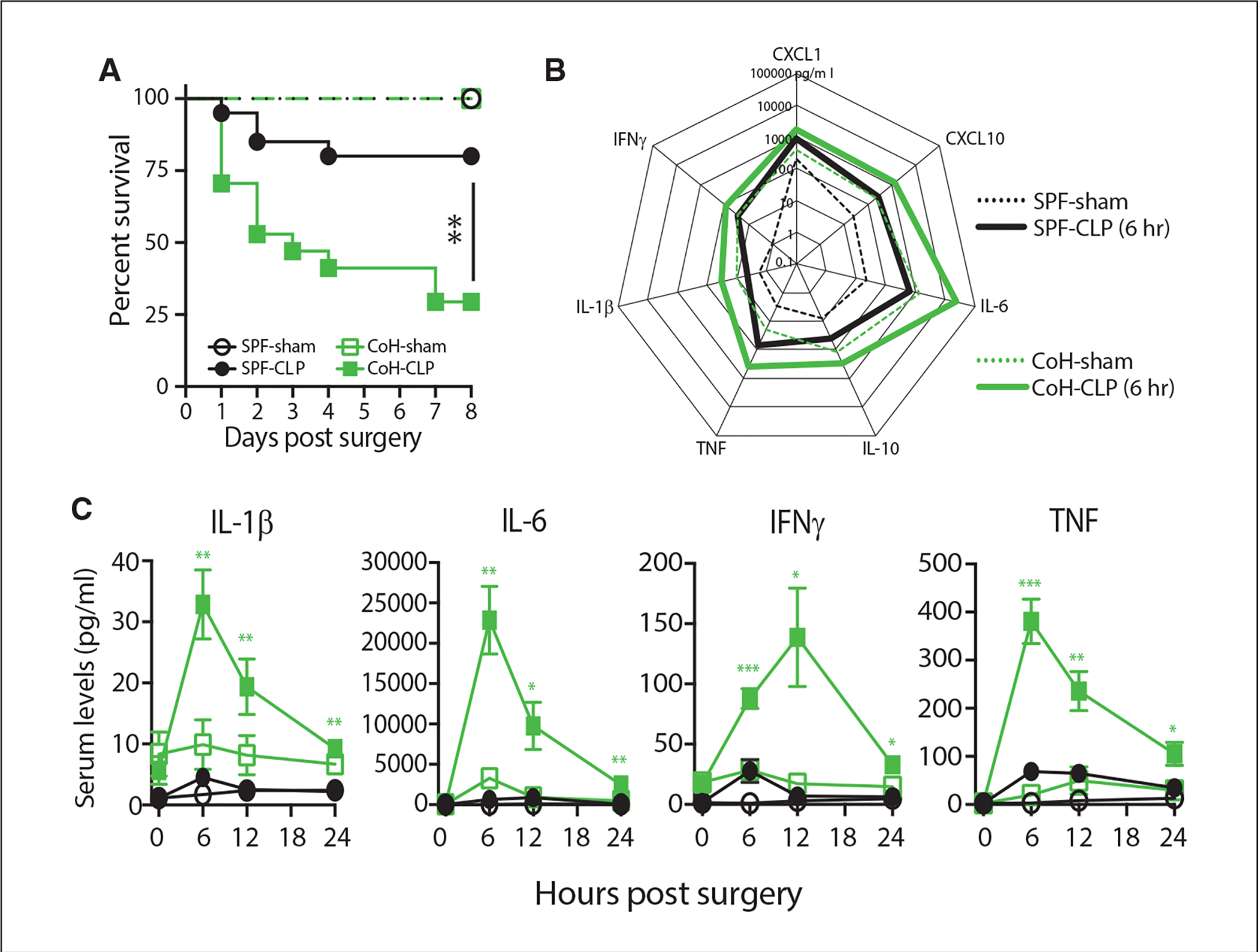
Increased morbidity/mortality in CLP-treated microbially experienced (dirty) mice correlates with an exacerbated cytokine storm. Specific pathogen–free (SPF) and dirty co-housed (CoH) B6 mice underwent sham or CLP surgery. (A) Survival was monitored over time (n = 8–17 mice/group; ** p ≤ .01). (B) Serum samples were obtained 6 hr after surgery and the amount of CXCL1, CXCL10, IL-1β, IL-6, IL-10, IFNγ, and TNF was determined by BioPlex. (C) Additional samples were collected at 12 and 24 hr post-surgery to quantify changes in IL-1β, IL-6, IFNγ, and TNF over time (n = 4–7 mice/group/time point; *p ≤ .05, ** p ≤ .01, *** p ≤ .005 for SPF-CLP vs. CoH-CLP at the indicated time points). Data are adapted from Huggins et al. (2019b).
Critical Parameters and Troubleshooting
Mice
As with other research techniques that use the mouse for in vivo experimentation, properly controlling for strain, age, and sex is important for reproducibility in the CLP model. Factors in animal source and care have also received attention recently, as differences in CLP outcomes have been reported based on animal vendor (Hilbert et al., 2017; Wilmore et al., 2018), housing conditions (Hamilton et al., 2020; Huggins, Jameson, & Hamilton, 2019a), and diet (Zhang, Dong, Xie, & Yu, 2018). In many cases, these differences are related to the composition of the gut microbiome. In addition, the maturation level of the immune system of the mouse can influence the magnitude of the septic response following CLP. Most (if not all) preclinical sepsis research done to date has used specific pathogen–free (SPF) mice, but the immune systems of SPF-housed mice are equivalent to those of neonatal humans (Beura et al., 2016). Environmental pathogen exposure is one important difference between basic human and laboratory mouse biology that must be considered when using mice to evaluate CLP-induced sepsis changes to the immune system composition and fitness (Hamilton et al., 2020; Huggins et al., 2019a; Masopust, Sivula, & Jameson, 2017). Humans are exposed to a multitude of microbes (both commensal and pathogenic) daily from birth. Consequently, the immune system of adult humans has been trained and shaped by each infection and vaccination experienced. While SPF housing of laboratory mice has been instrumental in increasing experimental reproducibility, it has simultaneously further distanced the mouse as a model from humans, largely because SPF mice live their lives with limited microbial exposure (Foster, 1959). It is important to emphasize that the use of SPF-housed laboratory mice in the CLP sepsis model has and continues to reveal a wealth of information into the pathophysiology and immunoparalysis seen during sepsis, but the use of microbially experienced (“dirty”) mice can provide sepsis researchers with another important preclinical tool for interrogating sepsis. For example, there is a significant increase in CLP-induced mortality in dirty mice compared to SPF mice that correlates with an increased systemic cytokine response (Fig. 4).
Surgery
One of the strengths of the CLP model is the ability to tune the severity of sepsis through small quantifiable modifications to the procedure. Parameters commonly modulated to adjust sepsis severity include the amount of cecum ligated, amount of cecal contents introduced into the peritoneum (dependent on the number/size of punctures and quantity of cecal material extruded), skill and experience of the surgeon, time of day the procedure is performed, and mouse strain, age, and sex. Interestingly, these attributes can simultaneously be considered weaknesses of the technique, as some researchers—particularly those with little CLP experience—may find it difficult to control the severity of the septic event.
Critical variables in the CLP procedure include the size of the cecal ligation and quantity of cecal contents introduced into the peritoneum. Using a ruler for reference while making the ligation is recommended for controlling ligation size (Fig. 3A). Controlling the amount of cecal material introduced into the peritoneum can be more difficult to standardize. The best practice is to hold a small portion of the ligated cecum taut and puncture the cecum as desired, maintaining consistency in puncture number and size. Then, with a second set of forceps, slowly apply minimal pressure until the desired amount of cecal material is extruded.
Although rarely reported, another surgical parameter important in determining severity CLP-induced sepsis is the time of day the procedure is carried out (Heipertz et al., 2018). C57BL/6 mice develop sepsis more rapidly and experience worse outcomes when the disease is induced during nighttime compared to daytime (Heipertz et al., 2018). Performing CLP later in the afternoon compared to early morning can also result in higher mortality rates (F. Sjaastad, unpublished observations). Furthermore, variations in the quantity and consistency of cecal contents can vary based on the time of day (F. Sjaastad and I. Jensen, unpublished observations).
Lastly, sepsis severity and survival in the CLP model is highly dependent on the experience of the researcher. Long procedure times, large incisions, and increased trauma to intestinal tissue while locating the cecum are common when first learning the procedure and will increase sepsis severity and variability. Practicing the technique before using it on a set of experimental animals will improve the reproducibility of experimental outcomes.
Post-operative care
Immediately following induction of sepsis, it is standard to give fluid resuscitation (e.g., 1 ml of 0.9% saline) in combination with an analgesic. Common analgesic agents include opioids (such as buprenorphine) or nonsteroidal anti-inflammatory drugs (such as meloxicam). Many IACUCs recommend giving analgesics following CLP, but there is evidence that opioids and NSAIDs can alter sepsis-induced inflammation and lethality, making their use in the CLP model controversial. For example, the NSAID flurbiprofen has been reported to improve hypotension, reduce organ damage, and prolong survival (Anuar, Whiteman, Bhatia, & Moore, 2006). Others have found that buprenorphine, when given post-CLP, can increase production of inflammatory cytokines and reduce survival (Cotroneo et al., 2012; Chen et al., 2019b). Additionally, opioids may make it difficult to assess signs of morbidity.
The use of antibiotics in the CLP model is also variable (Table 1), and may not significantly change sepsis severity or outcome (Iskander, Vaickus, Duffy, & Remick, 2016). However, in the effort to improve translation of preclinical sepsis research into clinical practice, there is a growing push to reproduce the treatment septic patients receive in mouse sepsis models, including the use of antibiotics, fluid resuscitation, and monitoring of vital processes (Guillon et al., 2019).
Table 1.
Antibiotic Usage in the CLP Model
| Antibiotic (dose) | Administration schedule | Reference |
|---|---|---|
| Imipenem (25 mg/kg) | 1 hr before CLP (subcutaneous) | Halbach et al. (2017) |
| Imipenem (25 mg/kg) | 1 hr before and 3 & 6 hr after CLP (subcutaneous) | Halbach et al. (2017) |
| Imipenem (25 mg/kg) | 1 hr before and 4, 24 & 48 hr after CLP (subcutaneous) | Halbach et al. (2017) |
| Imipenem (25 mg/kg) | During CLP (intracecal injection) and 2 or 4 hr after CLP (subcutaneous) | Halbach et al. (2017) |
| Ceftriaxone (50 mg/kg), metronidazole (35 mg/kg) | 0, 12, 24 & 36 hr after CLP (subcutaneous) | Lyons et al. (2016) |
| Ertapenem (75 mg/kg) | 6 hr after and every 24 hr thereafter until day 3 (intraperitoneal) | Molinaro et al. (2015) |
| Imipenem/cilastatin (25 mg/kg) | 1 hr after and every 12 hr thereafter until day 4 (subcutaneous) | Standage, Caldwell, Zingarelli, & Wong (2012) |
| Metronidazole (12.5 mg/kg), ceftriaxone (25 mg/kg) | Every 12 hr for 5 d in 1 ml saline (intraperitoneal) | Laudanski et al. (2017) |
| Ceftriaxone (50 mg/kg), metronidazole (35 mg/kg) | 0, 12, 24 & 36 hr after CLP (subcutaneous) | Chen et al. (2019a) |
| Ceftriaxone (25 mg/kg), metronidazole (12.5 mg/kg) | 10 and 24 hr after (intraperitoneal) | Vandewalle et al. (2019) |
| No antibiotic given | Xiong et al. (2020) |
Flow cytometry of immune cells
It is becoming more evident from both preclinical and clinical investigation that sepsis establishes a state of chronic immunoparalysis, characterized by severe but transient lymphopenia and prolonged lymphocyte dysfunction. Consequently, many investigators incorporate flow cytometry into their experiments to examine how CLP-induced sepsis affects the cellular composition and function of the immune system, especially when testing ways to therapeutically restore the immune system to a more pre-sepsis state.
With the increasing capacity of modern flow cytometers, scientists now use a wide assortment of fluorophores to generate complex and comprehensive mAb panels to detect and extensively phenotype a range of immune cells in individual samples. One way to extend the range of fluorophores available is through the use of tandem dyes (for example, by conjugating Cy7 to allophycocyanin to yield APC-Cy7; Gerstner et al., 2002). When excited by a laser, the primary fluorophore transfers energy to the secondary conjugate via Förster resonance energy transfer (FRET), altering the emission spectrum of the primary fluorophore (Gerstner et al., 2002). One drawback of these tandem dyes is that they can degrade and lose their capacity transfer energy, leading to emission in the spectrum of the primary fluorophore. Tandem dye degradation is a well-established phenomenon, making it increasingly critical to include proper instrument compensation to dealing with dye degradation and overlap in fluorescence channels. Yet, compensation also assumes equivalent conditions between samples and experimental groups.
We recently identified increased production of reactive oxygen species (ROS) during CLP-induced sepsis as another potential confounder of flow cytometric analyses. Specifically, the ROS produced after CLP can facilitate the degradation of tandem dyes, leading to production of a phantom signal (Jensen et al., 2020). Subsequently, we have identified several modifications to sample preparation and staining protocols that can help with detecting and/or reducing the problem of tandem dye degradation. First, although this will reduce the number of additional channels, the extent of tandem dye degradation can be determined by including an empty channel for the primary fluorophore that is part of the tandem dye (e.g., leave an empty APC channel when staining with APC-Cy7). Second, a reducing agent such as 2-mercaptoethanol can be added to the staining buffer. Third, antibodies that recognize highly expressed targets with a clearly defined population can be used for the primary fluorophore. For example, use APC-labeled anti-Thy1.1 mAb an exceptionally bright antibody with a clear positive population, when staining with APC-Cy7. Moreover, it is important to note that accurate determination of the level of protein expression (gMFI, MFI, etc.) cannot be done, as degrading fluorophores may influence this assessment unequally. Fourth, the cells can be fixed prior to staining with tandem dye-conjugated mAb, as only living cells can produce ROS. Fixation can, however, introduce some new complications to the staining, since fixation can alter target antigens and affect mAb binding (e.g., the anti-NK1.1 mAb PK136 does not bind to fixed cells). Lastly, samples can be depleted of granulocytes (the predominant ROS-producing cells) prior to staining to reduce the amount of ROS present in the sample. Based on the above parameters, some potential mAb panels to identify different immune cell populations are provided in Table 2 (Jensen et al., 2018b; Kotov & Jenkins, 2019; Sjaastad et al., 2018).
Table 2.
Representative mAb Panels for Flow Cytometry
| Cell (marker) type | Color | Marker | Clone |
|---|---|---|---|
| CD4 T cells (surface) | Red 780 | Live/Dead | 13-0865-T100, Tonbo |
| APC-Cy7 | CD11b (dump) | M1/70 | |
| APC-Cy7 | CD11c (dump) | N418 | |
| APC-Cy7 | B220 (dump) | RA3–6B2 | |
| APC-Cy7 | F4/80 (dump) | BM8.1 | |
| BV510 | CD44 | IM7 | |
| BV711 | CD8α | 53–6.7 | |
| BUV395 | Thy1.2 | 53–2.1 | |
| BUV496 | CD4 | GK1.5 | |
| APC | Tetramer | ||
| BV650 | CXCR5 | L138D7 | |
| CD4 T cells (intracellular) | AF488 | Foxp3 | FJK-16S |
| AF700 | Ki-67 | 16A8 | |
| PE | Bcl-6 | K112–91 | |
| PE-Cy7 | T-bet | 4B10 | |
| CD8 T cells | FITC | CD27 | LG.3A10 |
| rf710 | CD62L | MEL-14 | |
| Red 780 | Live/Dead | 13-0865-T100, Tonbo | |
| APC-Cy7 | CD11b (dump) | M1/70 | |
| APC-Cy7 | CD11c (dump) | N418 | |
| APC-Cy7 | B220 (dump) | RA3–6B2 | |
| APC-Cy7 | F4/80 (dump) | BM8.1 | |
| BV421 | CD127 | A7R34 | |
| BV510 | CD44 | IM7 | |
| BV711 | CD8α | 53–6.7 | |
| APC | Tetramer | ||
| PE-Cy7 | KLRG1 | 2F1 | |
| BUV395 | Thy1.2 | 53–2.1 | |
| BUV496 | CD4 | GK1.5 | |
| PE | CXCR3 | CXCR3–173 | |
| T cell cytokines (surface) | Red 780 | Live/Dead | 13-0865-T100, Tonbo |
| APC-Cy7 | CD11b (dump) | M1/70 | |
| APC-Cy7 | CD11c (dump) | N418 | |
| APC-Cy7 | B220 (dump) | RA3–6B2 | |
| APC-Cy7 | F4/80 (dump) | BM8.1 | |
| BV510 | CD44 | IM7 | |
| BV711 | CD8α | 53–6.7 | |
| BUV395 | Thy1.2 | 53–2.1 | |
| BUV496 | CD4 | GK1.5 | |
| APC | Tetramer | ||
| T cell cytokines (intracellular) | PE | TNF | MP6-XT22 |
| BV650 | IFN-γ | XMG1.2 | |
| PE-Cy7 | IL-2 | JES6–5H4 | |
| Antigen-experienced T cells | Red 780 | Live/Dead | 13-0865-T100, Tonbo |
| APC-Cy7 | CD11b (dump) | M1/70 | |
| APC-Cy7 | CD11c (dump) | N418 | |
| APC-Cy7 | B220 (dump) | RA3–6B2 | |
| APC-Cy7 | F4/80 (dump) | BM8.1 | |
| BV510 | CD44 | IM7 | |
| BUV395 | Thy1.2 | 53–2.1 | |
| AF647 | CD49d | R1–2 | |
| BV650 | CD8α | 53–6.7 | |
| FITC | CD4 | GK1.5 | |
| PE-Cy7 | CD11a | M17/4 | |
| B cells | FITC | IgA | mA-6E1 |
| PerCP-ef710 | IgM | II/41 | |
| PE | B-PE | PB71, Prozyme | |
| AF594 | IgG3 | A21155 | |
| AF647 | IgG2b | A21242 | |
| AF700 | CD38 | 90 | |
| APC-eF780 | CD90.2 (dump) | 53–2.1 | |
| APC-eF780 | CD11c (dump) | N418 | |
| APC-eF780 | F4–80 (dump) | BM8 | |
| APC-eF780 | Gr-1 (dump) | RB6–8C5 | |
| eF450 | GL7 | GL-7 | |
| BV510 | IgE | R35–72 | |
| BV605 | IgG1 | A85–1 | |
| BV711 | IgG2c | IgG2a [b]; 5.7 | |
| BV786 | IgD | 11–26c.2a | |
| BUV395 | B220 | RA3–6B2 | |
| NK cells (surface) | APC | NK1.1 | PK136 |
| APC-Cy7 | CD3e (dump) | 145–2C11 | |
| APC-Cy7 | CD19 (dump) | 1D3 | |
| BV605 | CD27 | LG.3A10 | |
| BV711 | CD11b | M1/70 | |
| PE-Cy7 | KLRG1 | 14C2A07 | |
| BV786 | Ly6C | HK1.4 | |
| BV421 | Ly49H | 3D10 | |
| BV650 | NKp46 | 29A1.4 | |
| FITC | Ly49D | 4E5 | |
| PE-Dazzle | NKG2D | 1D11 | |
| PE | TREM-1 | MA5–28221 | |
| NK cells (intracellular) | PerCP | IFN-γ | XMG1.2 |
| AF700 | GzmB | QA16A02 | |
| BV510 | TNF | MP6-XT22 | |
| Dendritic cells | BUV395 | CD8α | 53–6.7 |
| BV711 | CD11c | N418 | |
| BV650 | MHCII | M5/114.15.2 | |
| Violet 510 | Live/Dead | 13-0870-T100, Tonbo | |
| BV510 | CD19 (dump) | 6D5 | |
| BV510 | NK1.1 (dump) | PK136 | |
| BV510 | CD3e (dump) | 145–2C11 | |
| BV421 | XCR1 | ZET | |
| PerCP-Cy5.5 | Ly6C | HK1.4 | |
| AF488 | CD11b | M1/70 | |
| PE-Cy7 | CD172a (SIRPa) | P84 | |
| PE | CD40, CD80, or CD86 | 1C10/16–10A1/GL1 | |
| APC-Cy7 | F4/80 | BM8.1 | |
| AF700 | Ly6G | 1A8 | |
| APC | Siglec H | 551 |
Understanding the Results
The CLP model induces intra-abdominal peritonitis and a chronic septic state characterized by the loss of appetite and body weight, ruffled hair, shivering, diarrhea, and/or peri-orbital exudates. After CLP, the amount of pro- and anti-inflammatory cytokines and chemokines will increase quickly, peaking 6–12 hr after sepsis induction. The major adverse post-procedural event that could occur in this model is death. Animals that die from CLP surgery most often do so within the first 48 hr. Mortality rates can be influenced by differences in mouse size, weight, age, vendor, and housing conditions, and there are no methods to predict which mice will survive the surgery short of actual survival. In cases where a milder sepsis is induced, the mice will become lethargic and lose weight over the initial 2–3 days after surgery but will recover. Sham mice are expected to survive, with minimal increases in serum cytokines and chemokines. At 24–48 hr post-surgery, a numerical reduction of several immune cell types in the blood and various lymphoid tissues can be observed. Using the technique described in this protocol (cecal ligation of 1 cm and a single puncture with a 25-G needle), a survival rate of 80%−90% is typically observed. Increasing the ligation size, number/size of punctures, and amount of cecal content extruded will reduce the survival rate.
Acknowledgments
This work was supported by National Institutes of Health grants R01GM115462 (T.S.G.), R01AIGM113961 (V.P.B.), R35GM 134880 (V.P.B.), T32CA009138 (F.V.S.), and T32AI007313 (F.V.S.), as well as Veterans Administration Merit Review Award I01BX001324 (T.S.G.).
Literature Cited
- Angus DC, Pereira CA, & Silva E (2006). Epidemiology of severe sepsis around the world. Endocrine, Metabolic and Immune Disorders-Drug Targets, 6, 207–212. doi: 10.2174/187153006777442332. [DOI] [PubMed] [Google Scholar]
- Angus DC, & van der Poll T (2013). Severe sepsis and septic shock. New England Journal of Medicine, 369, 840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- Anuar F, Whiteman M, Bhatia M, & Moore PK (2006). Flurbiprofen and its nitric oxide-releasing derivative protect against septic shock in rats. Inflammation Research, 55, 498–503. 10.1007/s00011-006-5150-7. [DOI] [PubMed] [Google Scholar]
- Baker CC, Chaudry IH, Gaines HO, & Baue AE (1983). Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery, 94, 331–335. [PubMed] [Google Scholar]
- Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, … Masopust D (2016). Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature, 532, 512–516. doi: 10.1038/nature17655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Perez J, Condotta SA, Badovinac VP, & Griffith TS (2014). Impact of sepsis on CD4 T cell immunity. Journal of Leukocyte Biology, 96, 767–777. doi: 10.1189/jlb.5MR0114-067R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry H, Zhou J, Zhong Y, Ali MM, McGuire F, Nagarkatti PS, & Nagarkatti M (2013). Role of cytokines as a double-edged sword in sepsis. In Vivo, 27(6), 669–684. [PMC free article] [PubMed] [Google Scholar]
- Chen CW, Xue M, Zhang W, Xie J, Coopersmith CM, & Ford ML (2019a). 2B4 but not PD-1 blockade improves mortality in septic animals with preexisting malignancy. JCI Insight, 4, e127867. doi: 10.1172/jci.insight.127867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Brenner M, Aziz M, Chavan SS, Deutschman CS, Diamond B, … Wang H (2019b). Buprenorphine markedly elevates a panel of surrogate markers in a murine model of sepsis. Shock, 52(5), 550–553. 10.1097/shk.0000000000001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condotta SA, Cabrera-Perez J, Badovinac VP, & Griffith TS (2013). T-cell-mediated immunity and the role of TRAIL in sepsis-induced immunosuppression. Critical Reviews in Immunology, 33, 23–40. doi: 10.1615/CritRevImmunol.2013006721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotroneo TM, Hugunin KM, Shuster KA, Hwang HJ, Kakaraparthi BN, & Nemzek-Hamlin JA (2012). Effects of buprenorphine on a cecal ligation and puncture model in C57BL/6 mice. Journal of the American Association for Laboratory Animal Science, 51(3), 357–365. [PMC free article] [PubMed] [Google Scholar]
- Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moldawer LL, & Efron PA (2010). Cecal ligation and puncture. Current Protocols in Immunology, 91, 19.13.1–19.13.11. doi: 10.1002/0471142735.im1913s91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damas P, Ledoux D, Nys M, Vrindts Y, De Groote D, Franchimont P, & Lamy M (1992). Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Annals of Surgery, 215, 356–362. doi: 10.1097/00000658-199204000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danahy DB, Strother RK, Badovinac VP, & Griffith TS (2016). Clinical and experimental sepsis impairs CD8 T-cell-mediated immunity. Critical Reviews in Immunology, 36, 57–74. doi: 10.1615/CritRevImmunol.2016017098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly JP, Hohmann SF, & Wang HE (2015). Unplanned readmissions after hospitalization for severe sepsis at academic medical center-affiliated hospitals. Critical Care Medicine, 43, 1916–1927. doi: 10.1097/CCM.0000000000001147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster HL (1959). Housing of disease-free vertebrates. Annals of the New York Academy of Sciences, 78, 80–88. doi: 10.1111/j.1749-6632.1959.tb53096.x. [DOI] [PubMed] [Google Scholar]
- Gerstner AO, Lenz D, Laffers W, Hoffman RA, Steinbrecher M, Bootz F, & Tarnok A (2002). Near-infrared dyes for six-color immunophenotyping by laser scanning cytometry. Cytometry, 48, 115–123. doi: 10.1002/cyto.10119. [DOI] [PubMed] [Google Scholar]
- Guillon A, Preau S, Aboab J, Azabou E, Jung B, Silva S, … Translational Research Committee of the French Intensive Care Society. (2019). Preclinical septic shock research: Why we need an animal ICU. Annals of Intensive Care, 9, 66 10.1186/s13613-019-0543-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbach JL, Wang AW, Hawisher D, Cauvi DM, Lizardo RE, Rosas J, … De Maio A (2017). Why antibiotic treatment is not enough for sepsis resolution: An evaluation in an experimental animal model. Infection and Immunity, 85, e00664–00617. doi: 10.1128/IAI.00664-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SE, Badovinac VP, Beura LK, Pierson M, Jameson SC, Masopust D, & Griffith TS (2020). New insights into the immune system using dirty mice. Journal of Immunology, 205, 3–11. doi: 10.4049/jimmunol.2000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heipertz EL, Harper J, Lopez CA, Fikrig E, Hughes ME, & Walker WE (2018). Circa-dian rhythms influence the severity of sepsis in mice via a TLR2-dependent, leukocyte-intrinsic mechanism. Journal of Immunology, 201, 193–201. doi: 10.4049/jimmunol.1701677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbert T, Steinhagen F, Senzig S, Cramer N, Bekeredjian-Ding I, Parcina M, … Klaschik S (2017). Vendor effects on murine gut microbiota influence experimental abdominal sepsis. Journal of Surgical Research, 211, 126–136. doi: 10.1016/j.jss.2016.12.008. [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Monneret G, & Payen D (2013). Immunosuppression in sepsis: A novel understanding of the disorder and a new therapeutic approach. The Lancet Infectious Diseases, 13, 260–268. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, … Karl IE (1999). Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Critical Care Medicine, 27, 1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Tinsley KW, Swanson PE, Grayson MH, Osborne DF, Wagner TH, … Karl IE (2002). Depletion of dendritic cells, but not macrophages, in patients with sepsis. Journal of Immunology, 168, 2493–2500. doi: 10.4049/jimmunol.168.5.2493. [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE Jr., Hui JJ, Chang KC, … Karl IE (2001). Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. Journal of Immunology, 166, 6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- Hu P, Chen Y, Pang J, & Chen X (2019). Association between IL-6 polymorphisms and sepsis. Innate Immunity, 25, 465–472. doi: 10.1177/1753425919872818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins MA, Jameson SC, & Hamilton SE (2019a). Embracing microbial exposure in mouse research. Journal of Leukocyte Biology, 105, 73–79. doi: 10.1002/JLB.4RI0718-273R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins MA, Sjaastad FV, Pierson M, Kucaba TA, Swanson W, Staley C, … Hamilton SE (2019b). Microbial exposure enhances immunity to pathogens recognized by TLR2 but increases susceptibility to cytokine storm through TLR4 sensitization. Cell Reports, 28(7), 1729–1743. doi: 10.1016/j.celrep.2019.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura M, & Clowes GH Jr. (1975). Hepatic blood flow and oxygen consumption in starvation, sepsis and septic shock. Surgery, Gynecology and Obstetrics, 141, 27–34. [PubMed] [Google Scholar]
- Iskander KN, Vaickus M, Duffy ER, & Remick DG (2016). Shorter duration of post-operative antibiotics for cecal ligation and puncture does not increase inflammation or mortality. PLoS One, 11, e0163005. doi: 10.1371/journal.pone.0163005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen IJ, McGonagill PW, Lefebvre MN, Griffith TS, Harty JT, & Badovinac VP (2020). Worry and FRET: ROS production leads to fluorochrome tandem degradation and impairs interpretation of flow cytometric results. Immunity, 52, 419–421. doi: 10.1016/j.immuni.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen IJ, Sjaastad FV, Griffith TS, & Badovinac VP (2018a). Sepsis-induced T cell immunoparalysis: The ins and outs of impaired T cell immunity. Journal of Immunology, 200, 1543–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen IJ, Winborn CS, Fosdick MG, Shao P, Tremblay MM, Shan Q, … Badovinac VP (2018b). Polymicrobial sepsis influences NK-cell-mediated immunity by diminishing NK-cell-intrinsic receptor-mediated effector responses to viral ligands or infections. PLoS Pathogens, 14, e1007405. doi: 10.1371/journal.ppat.1007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollef KE, Schramm GE, Wills AR, Reich-ley RM, Micek ST, & Kollef MH (2008). Predictors of 30-day mortality and hospital costs in patients with ventilator-associated pneumonia attributed to potentially antibiotic-resistant Gram-negative bacteria. Chest, 134, 281–287. doi: 10.1378/chest.08-1116. [DOI] [PubMed] [Google Scholar]
- Kotov JA, & Jenkins MK (2019). Cutting edge: T cell-dependent plasmablasts form in the absence of single differentiated CD4(+) T cell subsets. Journal of Immunology, 202, 401–405. doi: 10.4049/jimmunol.1801349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudanski K, Lapko N, Zawadka M, Zhou BX, Danet-Desnoyers G, & Worthen GS (2017). The clinical and immunological performance of 28 days survival model of cecal ligation and puncture in humanized mice. PLoS One, 12, e0180377. doi: 10.1371/journal.pone.0180377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limaye AP, Kirby KA, Rubenfeld GD, Leisenring WM, Bulger EM, Neff MJ, … Boeckh M (2008). Cytomegalovirus reactivation in critically ill immunocompetent patients. Journal of the American Medical Association, 300, 413–422. doi: 10.1001/jama.2008.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyt CE, Combes A, Deback C, Aubriot-Lorton MH, Nieszkowska A, Trouillet JL, … Chastre J (2007). Herpes simplex virus lung infection in patients undergoing prolonged mechanical ventilation. American Journal of Respiratory and Critical Care Medicine, 175, 935–942. doi: 10.1164/rccm.200609-1322OC. [DOI] [PubMed] [Google Scholar]
- Lyons JD, Mittal R, Fay KT, Chen CW, Liang Z, Margoles LM, … Coopersmith CM (2016). Murine lung cancer increases CD4+ T cell apoptosis and decreases gut proliferative capacity in sepsis. PLoS One, 11, e0149069. doi: 10.1371/journal.pone.0149069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Sivula CP, & Jameson SC (2017). Of mice, dirty mice, and men: Using mice to understand human immunology. Journal of Immunology, 199, 383–388. doi: 10.4049/jimmunol.1700453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinaro R, Pecli C, Guilherme RF, Alves-Filho JC, Cunha FQ, Canetti C, … Benjamim CF (2015). CCR4 controls the suppressive effects of regulatory T cells on early and late events during severe sepsis. PLoS One, 10, e0133227. doi: 10.1371/journal.pone.0133227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson D, Kavanagh JP, & Rao PN (1996). Kinetics of circulating TNF-alpha and TNF soluble receptors following surgery in a clinical model of sepsis. Cytokine, 8, 938–943. doi: 10.1006/cyto.1996.0126. [DOI] [PubMed] [Google Scholar]
- Otto GP, Sossdorf M, Claus RA, Rodel J, Menge K, Reinhart K, … Riedemann NC (2011). The late phase of sepsis is characterized by an increased microbiological burden and death rate. Critical Care, 15, R183. doi: 10.1186/cc10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remick DG, Bolgos GR, Siddiqui J, Shin J, & Nemzek JA (2002). Six at six: Interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock, 17, 463–467. doi: 10.1097/00024382-200206000-00004. [DOI] [PubMed] [Google Scholar]
- Retsas T, Huse K, Lazaridis LD, Karampela N, Bauer M, Platzer M, … Dimopoulos G (2018). Haplotypes composed of minor frequency single nucleotide polymorphisms of the TNF gene protect from progression into sepsis: A study using the new sepsis classification. International Journal of Infectious Diseases, 67, 102–106. doi: 10.1016/j.ijid.2017.12.008. [DOI] [PubMed] [Google Scholar]
- Rudd KE, Johnson SC, Agesa KM, Shack-elford KA, Tsoi D, Kievlan DR, … Naghavi M (2020). Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet, 395, 200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, … Sepsis Definitions Task F (2016). Developing a new definition and assessing new clinical criteria for septic shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). Journal of the American Medical Association, 315, 775–787. doi: 10.1001/jama.2016.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer BH, Newstead MW, Zeng X, Cooke CL, Thompson RC, Singer K, … Stan-diford TJ (2016). Cecal ligation and puncture results in long-term central nervous system myeloid inflammation. PLoS One, 11, e0149136. doi: 10.1371/journal.pone.0149136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjaastad FV, Condotta SA, Kotov JA, Pape KA, Dail C, Danahy DB, … Griffith TS (2018). Polymicrobial sepsis chronic immunoparalysis is defined by diminished Agspecific T cell-dependent B cell responses. Frontiers in Immunology, 9, 2532. doi: 10.3389/fimmu.2018.02532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Park DW, Moon S, Cho HJ, Park JH, Seok H, & Choi WS (2019). Diagnostic and prognostic value of interleukin-6, pentraxin 3, and procalcitonin levels among sepsis and septic shock patients: A prospective controlled study according to the Sepsis-3 definitions. BMC Infectious Diseases, 19, 968. doi: 10.1186/s12879-019-4618-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standage SW, Caldwell CC, Zingarelli B, & Wong HR (2012). Reduced peroxi-some proliferator-activated receptor alpha expression is associated with decreased survival and increased tissue bacterial load in sepsis. Shock, 37, 164–169. doi: 10.1097/SHK.0b013e31823f1a00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsinger J, Kazama H, McDonough JS, Griffith TS, Hotchkiss RS, & Ferguson TA (2010). Sepsis-induced apoptosis leads to active suppression of delayed-type hypersensitivity by CD8+ regulatory T cells through a TRAIL-dependent mechanism. Journal of Immunology, 184, 6766–6772. doi: 10.4049/jimmunol.0904054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Poll T, van de Veerdonk FL, Scicluna BP, & Netea MG (2017). The immunopathology of sepsis and potential therapeutic targets. Nature Reviews Immunology, 17, 407–420. doi: 10.1038/nri.2017.36. [DOI] [PubMed] [Google Scholar]
- Vandewalle J, Steeland S, Van Ryckeghem S, Eggermont M, Van Wonterghem E, Vandenbroucke RE, & Libert C (2019). A study of cecal ligation and puncture-induced sepsis in tissue-specific tumor necrosis factor receptor 1-deficient mice. Frontiers in Immunology, 10, 2574. doi: 10.3389/fimmu.2019.02574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, An X, Wang X, Hu X, Bi J, Tong L, … Bai C (2019). Peroxiredoxin 6 knockout aggravates cecal ligation and puncture-induced acute lung injury. International Immunopharmacology, 68, 252–258. doi: 10.1016/j.intimp.2018.12.053. [DOI] [PubMed] [Google Scholar]
- Wilmore JR, Gaudette BT, Gomez Atria D, Hashemi T, Jones DD, Gardner CA, … Allman D (2018). Commensal microbes induce serum IgA responses that protect against polymicrobial sepsis. Cell Host and Microbe, 23, 302–311.e303. doi: 10.1016/j.chom.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong S, Hong Z, Huang LS, Tsukasaki Y, Nepal S, Di A, … Malik AB (2020). IL-1beta suppression of VE-cadherin transcription underlies sepsis-induced inflammatory lung injury. Journal of Clinical Investigation, 130, 3684–3698. doi: 10.1172/JCI136908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye B, Tao T, Zhao A, Wen L, He X, Liu Y, … Lou J (2019). Blockade of IL-17A/IL-17R pathway protected mice from sepsis-associated encephalopathy by inhibition of microglia activation. Mediators of Inflammation, 2019, 8461725. doi: 10.1155/2019/8461725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehya N, Xin Y, Oquendo Y, Cereda M, Rizi RR, & Margulies SS (2015). Cecal ligation and puncture accelerates development of ventilator-induced lung injury. American Journal of Physiology—Lung Cellular and Molecular Physiology, 308, L443–451. doi: 10.1152/ajplung.00312.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Dong A, Xie K, & Yu Y (2018). Dietary supplementation with high fiber alleviates oxidative stress and inflammatory responses caused by severe sepsis in mice without altering microbiome diversity. Frontiers in Physiology, 9, 1929. doi: 10.3389/fphys.2018.01929. [DOI] [PMC free article] [PubMed] [Google Scholar]


