Abstract
Using sex-sorted semen to produce offspring of desired sex is associated with reduced developmental competence in vitro and lower fertility rates in vivo. The objectives of the present study were to investigate the effects of exogenous follistatin supplementation on the developmental competence of bovine embryos produced with sex-sorted semen and possible link between TGF-β regulated pathways and embryotrophic actions of follistatin. Effects of follistatin on expression of cell lineage markers (CDX2 and Nanog) and downstream targets of SMAD signaling (CTGF, ID1, ID2 and ID3) and AKT phosphorylation were investigated. Follistatin was supplemented during the initial 72 h of embryo culture. Exogenous follistatin restored the in vitro developmental competence of embryos produced with sex-sorted semen to the levels of control embryos produced with unsorted semen, and comparable results were obtained using sorted semen from three different bulls. The mRNA abundance for SMAD signaling downstream target genes, CTGF (SMAD 2/3 pathway) and ID2 (SMAD 1/5 pathway), was lower in blastocysts produced using sex-sorted versus unsorted semen, but mRNA levels for CDX2, NANOG, ID1 and ID3 were similar in both groups. Follistatin supplementation restored CTGF and ID2 mRNA in blastocysts produced using sex-sorted semen to levels of control embryos. Moreover, levels of phosphorylated (p)AKT (Ser-473 and Thr-308) were similar in embryos derived from sex-sorted and unsorted semen, but follistatin treatment increased pAKT levels in both groups. Taken together, results demonstrated that follistatin improves in vitro development of embryos produced with sex-sorted semen and such effects are associated with enhanced indices of SMAD signaling.
Keywords: Follistatin, TGF-β, AKT, SMAD, Oocytes quality, Bovine, Sorted semen
1. Introduction
Use of sex-sorted semen in combination with in vitro embryo production is an effective means to obtain offspring of known sex [1,2], which can increase the profitability of livestock operations [3–5]. Frozen, sorted bovine semen has been effectively utilized in artificial insemination and in vitro fertilization (IVF) systems in cattle. The major drawbacks of using sex-sorted semen in such assisted reproductive technology (ART) applications is lower fertility rates in animals upon artificial insemination [6–8] and abnormal embryo development in IVF systems [9]. The reduced fertility rates of sex-sorted semen are attributed to the damage of the spermatozoa caused by the sorting procedure [6,10] that impairs the sperm quality [11].
Numerous studies indicated using sex-sorted semen diminishes not only the indices of early embryonic development in vitro but also the quality of embryos produced. Significant reduction in cleavage [12,13] and blastocyst formation rates [14–17] after IVF were reported for sex-sorted compared to unsorted semen. Morphological alterations were also observed for blastocysts produced with sorted semen and these alterations contribute to low developmental potential of such embryos [18]. Moreover, embryos produced with sex-sorted semen displayed lower expression of some developmentally important genes; glucose-3 transporter (Glut-3), glucose-6-phosphate dehydrogenase (G6PD), and Heat shock protein 70.1 (Hsp) [19]. Several approaches have been utilized to enhance the fertilization rate and quality of embryos produced using sex-sorted semen including different sperm gradient separation techniques [20], optimization of heparin and sperm concentrations for each bull [21,22], or by means of new sperm cryopreservation procedures [23]. However, most of the previous methods used to alleviate the effects of sorted semen require optimization for each single bull and the outcomes are not universally positive. Therefore, we hypothesized that using an exogenous embryotrophic factor, follistatin, could improve the in vitro developmental competence of bovine embryos produced with sex-sorted semen.
Follistatin, a binding protein for specific members of transforming growth factor β (TGF-β) superfamily has been established as an trophic factor for cattle and non-human primate embryos [24,25]. Exogenous supplementation of follistatin during the initial 72 h of in vitro embryo culture improved many indices of early embryonic progression including, early cleavage at 30-hour post insemination (hpi), development to 8–16 cell stage at 72 hpi and d 7 blastocyst formation rates [24]. Trophectoderm cell number per blastocyst and CDX2 expression, markers that are associated with increased pregnancy rates after transfer [26], were also increased in response to follistatin treatment of bovine embryos [24]. Moreover, exogenous follistatin treatment rescued the developmental competence of poor quality oocytes identified by brilliant cresyl blue staining [27]. Furthermore, we previously demonstrated multiple TGF-β regulated signaling pathways are linked to embryotrophic actions of follistatin [28–30]. Follistatin supplementation increased AKT phosphorylation at 24 h post treatment and rescued the effects of AKT signaling inhibition on cleavage (early, total), 8–16 cells and blastocyst rates [30]. Exogenous follistatin treatment however didn’t rescue the effects of SMAD2/3 or SMAD4 knockdown on blastocyst development [28,29], suggesting that the embryotrophic effects of follistatin are potentially SMAD signaling dependent. Therefore, we hypothesized that follistatin supplementation will restore in vitro development rates in embryos produced with sex-sorted semen and that the increase in developmental capacity will be associated with changes in follistatin regulated pathways; AKT, SMAD2/3 or SMAD1/5. To test these hypotheses, we analyzed the effects of exogenous follistatin supplementation, during the initial 72 h of in vitro embryo culture, on the development of early embryos produced using sex-sorted semen. Effects of follistatin on expression of cell lineage marker genes (CXD2 and Nanog) and downstream targets of SMAD signaling pathways (CTGF: SMAD2/3 and ID1, ID2 and ID3: SMAD1/5) and AKT phosphorylation in early embryos produced with sex-sorted semen were also investigated.
2. Materials and methods
All chemicals and reagents used were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless stated otherwise. Ovaries, used in this study, were collected from local slaughter house in the state of Michigan. Michigan State university institutional animal care and use committee (IACUC) doesn’t require approval for this study.
2.1. Oocytes retrieval and in vitro embryo production
Ovary collection, oocyte retrieval, selection, in vitro maturation (IVM), in vitro fertilization (IVF) and in vitro embryo culture were performed as previously described [27]. IVF was done using frozen-thawed X-sorted bovine spermatozoa from three different bulls (bull A, B or C), control group was fertilized with unsorted frozen-thawed semen from different bulls. X-sorted semen was purchased from NorthStar Cooperative, Inc. Lansing, MI USA
2.2. Effects of follistatin supplementation on developmental potential of early embryos produced with sex-sorted semen
Presumptive zygotes produced with sex-sorted and unsorted semen were cultured with or without 10 ng/ml recombinant human follistatin (the maximally effective dose as previously described [24]) during the initial 72 h of in vitro embryo culture (IVC1), then 8–16-cell embryos were isolated and cultured in fresh media without exogenous follistatin (IVC2) until d 7 (25–30 presumptive zygotes/group, n = 4 replicates). Effects of follistatin supplementation on early cleavage at 30 hpi, total cleavage at 48 hpi, the development to 8–16 cell at 72 h and blastocyst stage at d7 were recorded.
2.3. Effects of follistatin supplementation on expression of cell lineage markers in blastocysts produced with sex-sorted semen
Effects of follistatin supplementation (10 ng/ml) during IVC1 on the transcript abundance for the trophectoderm (TE) marker CDX2, and inner cell mass (ICM) marker NANOG were determined by quantitative real time PCR in d 7 blastocysts (10 blastocyst/group. n = 4 replicates). Because similar responsiveness to follistatin was observed for in vitro produced embryos in above described experiments using sex-sorted semen from three different bulls, sex-sorted semen from single bull (C) was utilized for subsequent molecular analyses.
2.4. Effects of follistatin supplementation on expression of SMAD signaling pathway downstream targets in early embryos produced with sex-sorted semen
Effects of follistatin on expression of mRNA for downstream target genes of SMAD2/3; connective tissue growth factor (CTGF), and SMAD1/5 signaling pathway; Inhibitor of DNA Binding-1 (ID1), ID2 and ID3 were examined as described above for cell lineage markers in d 7 blastocysts (10 blastocyst/group. n = 4 replicates).
2.5. RNA Isolation, cDNA synthesis and real time PCR analysis
RNA Isolation, cDNA synthesis and real time PCR analysis were performed as previously described [24,27,29]. Primer sequences and GenBank accession numbers for all targets are listed in Supplemental Table (1).
2.6. Effects of follistatin supplementation on AKT phosphorylation in early embryos produced with sex-sorted semen
Activation of AKT requires phosphorylation on two amino acid residues: Thr308 and Ser473 [31]. To investigate the effect of follistatin supplementation during IVC1 on AKT phosphorylation in early embryos, samples were collected 10 h after follistatin supplementation [30], and subjected to Western blot analysis for phosphorylated (p) AKT-Ser473, pAKT-Thr308, total (t) AKT and actin as a loading control (20 embryo/group, n = 6 replicates/phosphorylation site).
2.7. Western blot analysis
Protein isolation, SDS-PAGE electrophoresis and membrane blotting procedures were performed as described before [30,32]. Membranes were probed sequentially starting with the primary antibody of either pAKT-Ser473 or pAKT-Thr308. After detection of pAKT, membranes were striped (10 min/room temp) with restore Western blot stripping buffer (Thermo Scientific, Waltham, MA, USA) and re-probed with tAKT antibody. After detection of tAKT, membranes were stripped and re-probed with anti-actin antibody. HRP-conjugated anti-rabbit-IgG, and anti-mouse-IgG were used as secondary antibodies. The sources and concentrations of antibodies used in this study are listed in Supplemental Table (2).
2.8. Statistical analysis
Two-way ANOVA, with main effects of semen and follistatin treatment and their interaction, was used to determine the differences in mRNA expression and protein abundance/phosphorylation levels using the general linear model’s procedure of SAS (SAS Institute Inc., Cary, NC). For experiments studying the effects of follistatin supplementation on early embryonic development, percentage data were arcsine transformed, then analyzed by mixed linear model’s analysis procedures in SAS. Differences among treatment means were compared using Fisher’s protected least significant difference (PLSD) test. Data are presented as untransformed mean ( ± ) standard error of mean (SEM). Differences with P ≤ 0.05 were considered significant.
3. Results
3.1. Follistatin supplementation rescued the poor developmental potential of early bovine embryos produced with sex-sorted semen
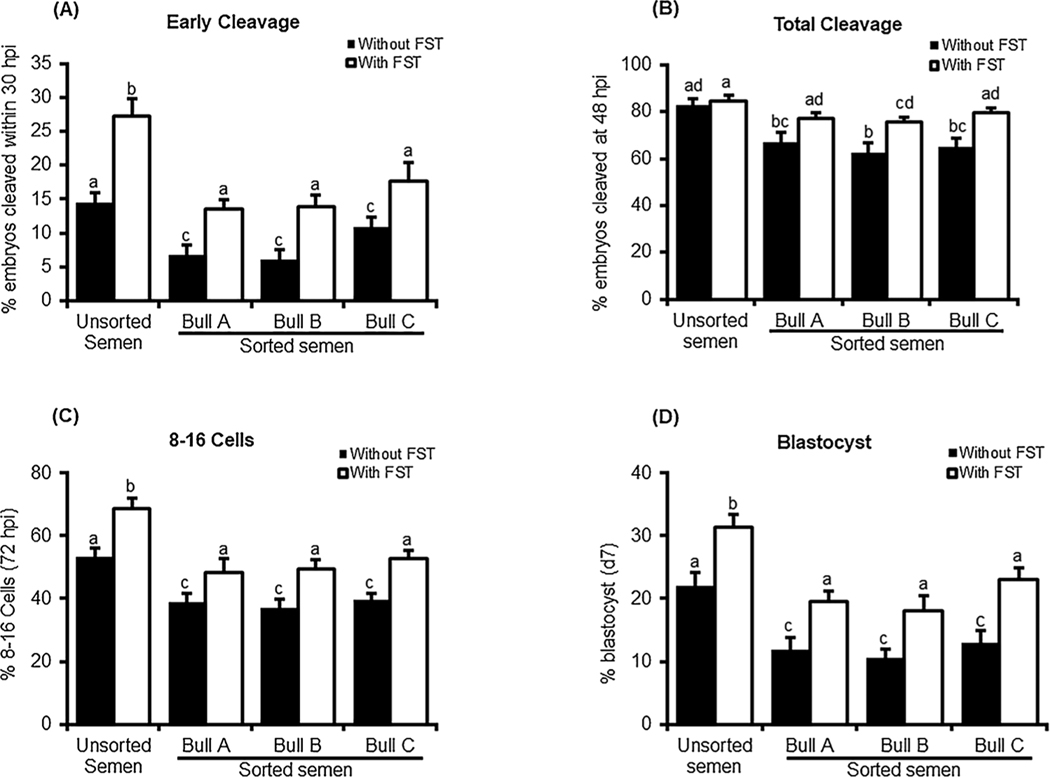
As expected, in vitro fertilization with sex-sorted semen versus unsorted semen resulted in significant reduction in all indices of early embryonic progression including early cleavage at 30 hpi, total cleavage at 48 hpi, the development to 8–16 cell at 72 hpi and d7 blastocyst formation rate (Fig. 1A–D). Exogenous follistatin supplementation (10 ng/ml) during IVC1 resulted in a significant improvement in developmental progression of bovine embryos produced in vitro using sex-sorted semen. The number of embryos cleaved within 30 hpi was increased to a level similar to the non-treated controls (embryos produced with unsorted semen) although still lower than follistatin treated control embryos. Similar patterns were observed for the rates of development to 8–16 cell and blastocyst stages. Follistatin supplementation completely restored total cleavage of embryos produced with sex-sorted semen to the levels of follistatin treated embryos produced with unsorted semen and comparable results were obtained using X sorted semen from three different bulls.
Fig. 1.
Effects of follistatin supplementation during IVC1 on developmental progression of early bovine embryos produced with sex-sorted semen. Presumptive zygotes produced with unsorted or sorted semen (bull A, B and C) were cultured with or without 10 ng/ml recombinant human follistatin for 72 h, then 8–16- cell embryos were isolated, washed and cultured in fresh media without follistatin until d7 (n = 4 replicates, n = 25–30 zygote/group). Effects of follistatin on (A) early cleavage, (B) total cleavage, (C) development to 8–16 cell stage and (D) d7 blastocyst rate were determined. Data are expressed as mean ± SEM. Values with different superscripts across indicate treatment significant differences (P < 0.05).
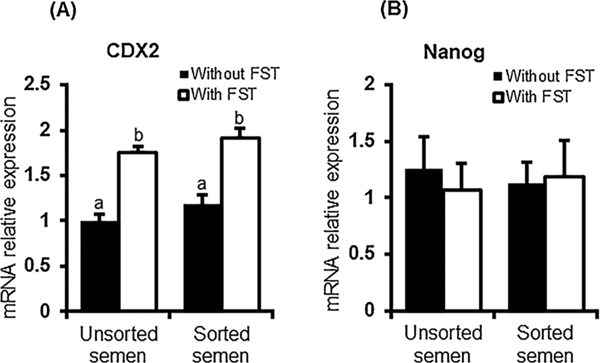
3.2. Using sex-sorted semen for IVF didn’t impact the expression of cell lineage specific genes, CDX2 or Nanog in early embryos
Above results showed that using sorted semen decreased the developmental competence of early embryos. To investigate the effects of using sorted semen at the molecular level, we measured the blastocyst expression level of cell lineage markers CDX2 and NANOG. Quantitative real time PCR revealed that the mRNA abundance for CDX2 and NANOG was similar in day 7 blastocysts produced with X- sorted and unsorted semen. Follistatin treatment (10 ng/ml) similarly increased the mRNA abundance of CDX2 in both groups (Fig. 2A). No significant differences in the mRNA transcript abundance for NANOG, were observed between the blastocysts produced with sex-sorted or unsorted semen in presence or absence of exogenous follistatin supplementation (Fig. 2B).
Fig. 2.
Expression of cell lineage markers in bovine blastocysts produced with sex-sorted semen. The mRNA abundance for CDX2 (A) and Nanog (B) in d7 blastocyst produced with sorted or unsorted semen (n = 4 replicates, n = 10 blastocysts/pool) was determined by qRT−PCR. Expression was normalized relative to the abundance of RPS18 as a housekeeping gene. Data are expressed as mean ± SEM. Values with different superscripts across treatment indicate significant differences (P < 0.05).
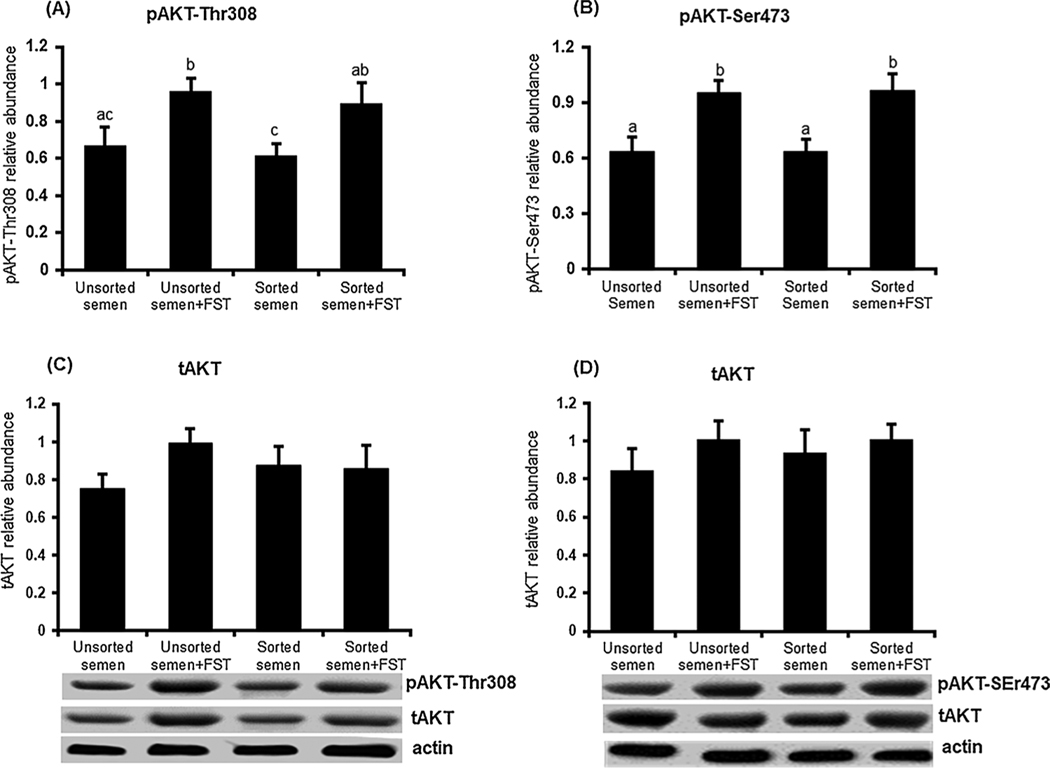
3.3. Early bovine embryos produced with sex-sorted semen show no change in AKT signaling
To examine effects of using sex-sorted semen on AKT signaling activity in early embryos, we analyzed AKT-Ser473 and Thr308 phosphorylation in early embryos 10 h post treatment. Immunoblot analysis revealed that using sex-sorted semen did not alter AKT phosphorylation as no differences in either pAKT-Ser473 or Thr308 were observed between embryos produced with sex-sorted and unsorted semen (Fig. 3A–B). Exogenous follistatin supplementation significantly (p < 0.05) increased pAKT-Ser473 and pAKT-Thr308 in embryos produced with both sorted and unsorted semen compared to untreated control embryos. No semen or follistatin effects were observed on tAKT abundance (Fig. 3C–D).
Fig. 3.
Effects of follistatin treatment on AKT phosphorylation in early bovine embryos produced with sex-sorted semen. Presumptive zygotes produced with sorted or unsorted semen were cultured with or without 10 ng/ml follistatin for 10 h, then subjected to Western blot analysis for pAKT-Thr308, pAKT-Ser473, tAKT and actin (n = 6 replicates/phosphorylation site, n = 20 embryos/group). Data were normalized relative to the abundance of actin and are expressed as mean ± SEM. Values with different superscripts across treatments indicate significant differences (P < 0.05). Representative Western blot images are shown.
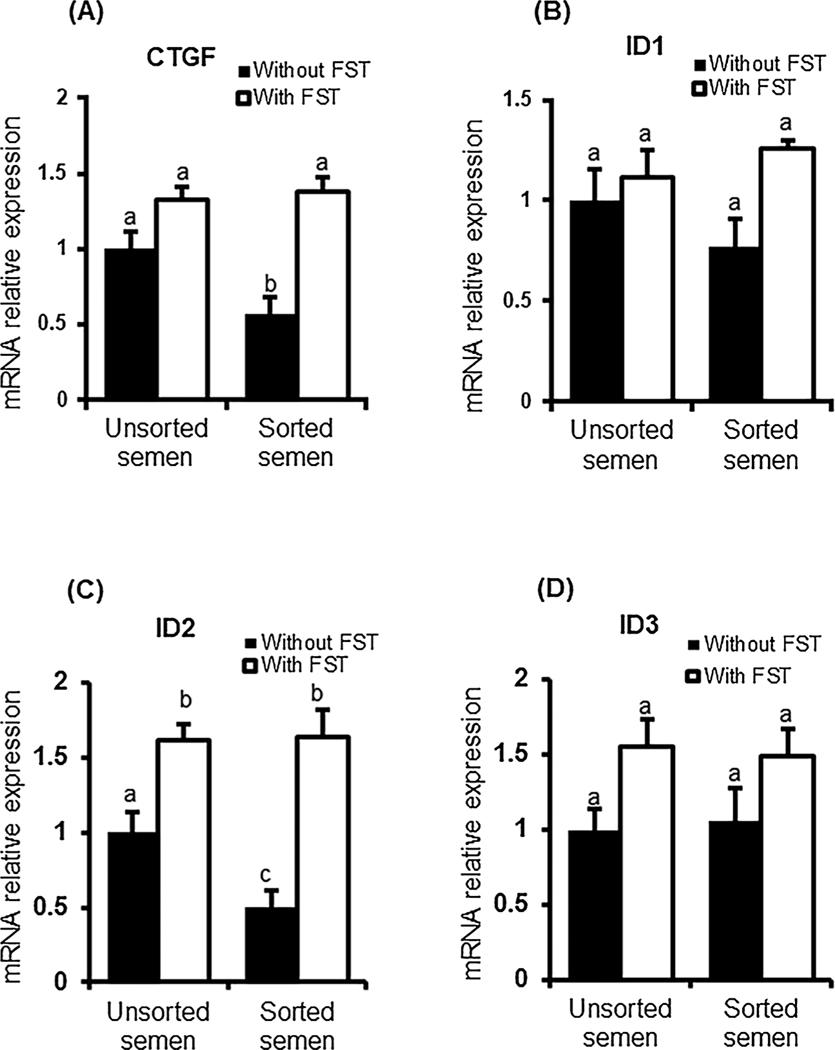
3.4. Follistatin supplementation restored the effects of using sex-sorted semen on the expression of SMAD downstream target genes
Above results suggest that using sex-sorted semen has no impact on either expression of cell lineage marker genes, CDX2 and NANOG, or AKT phosphorylation in early embryos. Whereas, follistatin supplementation similarly increased CDX2 expression and AKT phosphorylation in embryos produced with sorted and unsorted semen. To further elucidate the possible role of follistatin in modulating TGF-β signaling pathways in early embryos produced with sex-sorted semen, we examined the expression of SMAD signaling downstream target genes in the presence or absence of follistatin. Transcript analysis revealed that expression of CTGF (SMAD2/3 downstream target) was significantly (p < 0.05) reduced in embryos produced with sex-sorted semen compared to control embryos produced with unsorted semen. Follistatin supplementation restored CTGF expression in embryos derived from sex-sorted semen to the level of follistatin treated controls (Fig. 4A). For SMAD 1/5 downstream targets, using sex-sorted semen didn’t impact the expression of ID1 or ID3 but significantly (P < 0.05) decreased the mRNA transcript abundance for ID2 compared to control embryos produced with unsorted semen. Follistatin supplementation restored ID2 expression in embryos produced with sorted semen to levels seen in control embryos. Follistatin treatment increased the expression of ID1 and ID3 (main effect P < 0.05) but there was no effect of semen type or interaction between follistatin and semen type (Fig. 4B–D).
Fig. 4.
Expression of downstream targets of SMAD signaling in bovine blastocysts produced with sex-sorted semen. The mRNA abundance for CTGF (A), ID1 (B), ID2 (C) and ID3 (D) in d7 blastocyst produced with sorted or unsorted semen (n = 4 replicates, n = 10 blastocysts/pool) was determined by qRT-PCR. Expression was normalized relative to the abundance of RPS18 as a housekeeping gene. Data are expressed as mean ± SEM. Values with different superscripts across treatment indicate significant differences (P < 0.05).
4. Discussion
Using sex-sorted semen for IVF is associated with poor developmental competence of the resulting embryos. Results of the present study demonstrated that exogenous follistatin supplementation during the initial 72 h of in vitro embryo culture reversed the negative effects of using sex-sorted semen on early embryo development. Previous studies demonstrated that bovine embryos produced with sorted semen showed significant reduction in cleavage rate compared to those produced with unsorted semen [13,17,33]. In the present study, follistatin supplementation restored cleavage rates to a similar level as observed for control embryos produced using unsorted semen. Follistatin reversed the effects of using sex-sorted semen on total cleavage, possibly by accelerating the first cleavage. Using sorted semen may have delayed the first cell cycle, therefore the number of embryos reaching 2 cell-stage at 30 hpi was reduced and consequently the total cleavage rate at 48 hpi was also reduced. Previous studies demonstrated that damage to sperm DNA during sorting process impacts the subsequent development of the produced embryos rather the fertilization process itself [17,34]. Moreover, residues from the DNA labelling dye Hoechst 33,342 used during semen sorting process are retained in the spermatozoa and may affect post fertilization events including delay of timing of first embryonic cleavage [35]. Exogenous follistatin supplementation promotes the cell cycle progression and accelerates the first cleavage [24] and subsequently increased the number of embryos cleaved at 48 hpi in sex-sorted embryos. Furthermore, AKT is implicated in regulation of early cleavage, inhibition of AKT signaling during IVC1 significantly decreased early and total cleavage rates, and follistatin supplementation increased AKT phosphorylation and rescued the effects of AKT inhibition on cleavage rates [30]. Hence, we hypothesized that AKT signaling may be altered in embryos produced with sex-sorted semen. However, AKT phosphorylation levels were not impacted by using sorted semen, at the examined timepoint. Follistatin did increase AKT phosphorylation in embryos produced with sex sorted and unsorted semen, allowing for the possibility that activation of AKT signaling is promoting cleavage in early embryos produced with sex sorted semen.
Follistatin treatment restored in vitro developmental potential of IVF embryos derived using sorted semen from three different bulls, suggesting that the embryotropic actions of follistatin on in vitro developmental potential of embryos derived from sex-sorted semen are not bull dependent. However, only bulls that showed measurable blastocyst rates greater than 5% for sex-sorted semen were used in this study. Therefore, it would be relevant to determine if follistatin can improve in vitro embryo development when sorted semen from bulls yielding even lower developmental rates is utilized.
Previous studies have found that male bovine IVF embryos grow faster than female counterparts [14,36], however other studies have demonstrated similar development rates for embryos derived from X and Y sorted semen [4,13,16,37]. Moreover, embryo sex did not affect total cell number or number TE cell number in bovine blastocysts [38]. Our results demonstrated that follistatin improved the development rate of X-sorted embryos to levels of unsorted embryos, but we cannot discount the possibility that the embryotrophic effects of follistatin are due to the sex of the embryos produced. However, as follistatin has a similar effect on early embryonic development in embryos produced with unsorted semen we believe it is likely not greatly influenced by the sex of the embryo.
Follistatin supplementation during in vitro culture has been previously shown to increase CDX2 mRNA (trophectoderm cell marker) and trophectoderm cell numbers in resulting blastocysts [24]. Hence, we analyzed the transcript abundance of cell lineage marker genes CDX2 and NANOG to determine whether sorted semen impacts the expression of these genes and if the stimulatory effect of follistatin on CDX2 expression is still observed in embryos derived from sex-sorted semen. CDX2 and NANOG transcript abundance was similar in bovine blastocysts derived from sorted versus unsorted semen, and follistatin increased CDX2 expression in both groups. Previous studies reported that embryos produced with sex-sorted semen exhibited lower total cell number compared to those produced with unsorted semen derived from same bull, however the TE/ICM ratio was similar in both groups [33]. Another study reported no differences in total cell number between blastocysts from sorted versus unsorted semen [39]. Although we did not examine the cell number in these studies, we found that using sex-sorted semen for IVF has no impact on the expression of examined cell lineage expressed genes in early bovine embryos.
Previous studies demonstrated the essential requirement of SMAD signaling for the embryotrophic actions of follistatin, as knockdown of SMAD4 or SMAD2/3 abolished the embryotrophic effects of follistatin on embryos produced with unsorted semen [28,29]. To elucidate whether SMAD signaling is impacted by using sex sorted semen and the stimulatory effects of follistatin are linked to SMAD signaling in embryos derived from sex-sorted semen, we analyzed the mRNA expression of SMAD downstream target genes; CTGF (SMAD2/3), ID1, ID2 and ID3 (SMAD1/5). Using sex-sorted semen for IVF resulted in decreased expression of CTGF and ID2 suggesting that SMAD signaling activity is impaired in embryos produced with sex-sorted semen.
Inhibition of SMAD2 or SMAD3 diminishes the TGF-β1-induced up-regulation of CTGF [40]. CTGF is a downstream target of TGF-β signaling that regulates multiple biological processes including follicular development and ovulation [41]. CTGF is a member of CCN cysteinerich protein family [42], and in bovine blastocysts is mainly expressed in TE cells [43]. Follistatin supplementation restored the expression of CTGF (SMAD2/3 downstream target) to level of embryos produced with unsorted semen, implying that SMAD2/3 signaling maybe involved in the positive effects of follistatin on embryos produced with sex sorted semen.
Follistatin also restored the expression of ID2 to levels seen in control embryos. ID genes are downstream targets of SMAD1/5 pathway regulated by TGF-β members in a cell-type-specific manner and therefore play diverse physiological roles [44–46]. ID2 regulates trophoblast maintenance and differentiation in bovine embryos [47] and elevated levels of mRNA for ID2 were observed in mouse blastocysts before implantation [48] suggesting its importance in embryonic development.
Taken together results demonstrate that using sorted semen affects the expression of specific downstream target genes of SMAD signaling pathways. Follistatin supplementation restored the expression of CTGF and ID2 to the levels observed in embryos produced with unsorted semen, suggesting a potential role for both SMAD2/3 and SMAD1/5 signaling in mediating embryotrophic actions of follistatin in early embryos produced by sorted semen. However, further experiments will be needed to confirm the role of SMAD signaling in follistatin actions on embryos produced with sex-sorted semen and to determine further developmental potential of the resulting blastocysts.
5. Conclusions
Results of the present study demonstrate that follistatin supplementation during the initial 72 h of in vitro embryo culture increased the number of cleaving embryos at 48 hpi and restored the in vitro developmental competence of bovine IVF embryos produced with sex-sorted semen to the levels of control embryos produced with unsorted semen. Embryos produced with sex-sorted semen had reduced expression of some downstream target genes of SMAD signaling pathways (CTGF and ID2), and exogenous follistatin was able to restore expression of these genes to levels seen in control embryos, suggesting a potential role for SMAD signaling in mediating embryotrophic actions of follistatin in early embryos produced by sorted semen. Collectively, the results provide novel information about the use of follistatin to improve development of embryos produced with sex-sorted semen.
Supplementary Material
Acknowledgements
This work was supported by the National Institute of Child Health and Human Development of the National Institutes of Health [grant number R01HD072972]; and Michigan State University AgBioResearch. The funding agencies had no role neither in study design, data collection, analysis, or interpretation nor in the writing of the report; or in the decision to submit the paper for publication
Footnotes
Conflict of interest statement
None of the authors has any financial or other potential conflict of interest related to this manuscript.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.repbio.2018.06.004.
References
- [1].Wheeler MB, Rutledge JJ, Fischer-Brown A, VanEtten T, Malusky S, Beebe DJ. Application of sexed semen technology to in vitro embryo production in cattle. Theriogenology 2006;65:219–27. [DOI] [PubMed] [Google Scholar]
- [2].Ashry M, Smith GW. Application of embryo transfer using in vitro produced embryos: intrinsic factors affecting efficiency. Cattle Pract 2015;23:8. [PMC free article] [PubMed] [Google Scholar]
- [3].Hossein-Zadeh NG, Nejati-Javaremi A, Miraei-Ashtiani SR, Kohram H. Bio-economic evaluation of the use of sexed semen at different conception rates and herd sizes in Holstein populations. Anim Reprod Sci 2010;121:17–23. [PubMed] [Google Scholar]
- [4].Trigal B, Gomez E, Caamano JN, Munoz M, Moreno J, Carrocera S, et al. In vitro and in vivo quality of bovine embryos in vitro produced with sex-sorted sperm. Theriogenology 2012;78:1465–75. [DOI] [PubMed] [Google Scholar]
- [5].Xu J, Chaubal SA, Du F. Optimizing IVF with sexed sperm in cattle. Theriogenology 2009;71:39–47. [DOI] [PubMed] [Google Scholar]
- [6].Bodmer M, Janett F, Hassig M, den Daas N, Reichert P, Thun R. Fertility in heifers and cows after low dose insemination with sex-sorted and non-sorted sperm under field conditions. Theriogenology 2005;64:1647–55. [DOI] [PubMed] [Google Scholar]
- [7].Soares JG, Martins CM, Carvalho NA, Nicacio AC, Abreu-Silva AL, Campos Filho EP, et al. Timing of insemination using sex-sorted sperm in embryo production with Bos indicus and Bos taurus superovulated donors. Anim Reprod Sci 2011;127:148–53. [DOI] [PubMed] [Google Scholar]
- [8].Beilby KH, de Graaf SP, Evans G, Maxwell WM, Wilkening S, Wrenzycki C, et al. Quantitative mRNA expression in ovine blastocysts produced from X- and Y-chromosome bearing sperm, both in vitro and in vivo. Theriogenology 2011;76:471–81. [DOI] [PubMed] [Google Scholar]
- [9].Maxwell WM, Evans G, Hollinshead FK, Bathgate R, De Graaf SP, Eriksson BM, et al. Integration of sperm sexing technology into the ART toolbox. Anim Reprod Sci 2004;82–83:79–95. [DOI] [PubMed] [Google Scholar]
- [10].Seidel GE Jr., Garner DL. Current status of sexing mammalian spermatozoa. Reproduction 2002;124:733–43. [DOI] [PubMed] [Google Scholar]
- [11].Underwood SL, Bathgate R, Pereira DC, Castro A, Thomson PC, Maxwell WM, et al. Embryo production after in vitro fertilization with frozen-thawed, sex-sorted, re-frozen-thawed bull sperm. Theriogenology 2010;73:97–102. [DOI] [PubMed] [Google Scholar]
- [12].Zhang M, Lu KH, Seidel GE. Development of bovine embryos after in vitro fertilization of oocytes with flow cytometrically sorted, stained and unsorted sperm from different bulls. Theriogenology 2003;60:1657–63. [DOI] [PubMed] [Google Scholar]
- [13].Bermejo-Alvarez P, Lonergan P, Rath D, Gutierrez-Adan A, Rizos D. Developmental kinetics and gene expression in male and female bovine embryos produced in vitro with sex-sorted spermatozoa. Reprod Fertil Dev 2010;22:426–36. [DOI] [PubMed] [Google Scholar]
- [14].Lu KH, Cran DG, Seidel GE Jr.. In vitro fertilization with flow-cytometrically-sorted bovine sperm. Theriogenology 1999;52:1393–405. [DOI] [PubMed] [Google Scholar]
- [15].Merton JS, Haring RM, Stap J, Hoebe RA, Aten JA. Effect of flow cytometrically sorted frozen/thawed semen on success rate of in vitro bovine embryo production. Theriogenology 1997;47:295. [Google Scholar]
- [16].Bermejo-Alvarez P, Rizos D, Rath D, Lonergan P, Gutierrez-Adan A. Can bovine in vitro-matured oocytes selectively process X- or Y-sorted sperm differentially? Biol Reprod 2008;79:594–7. [DOI] [PubMed] [Google Scholar]
- [17].Inaba Y, Abe R, Geshi M, Matoba S, Nagai T, Somfai T. Sex-sorting of spermatozoa affects developmental competence of in vitro fertilized oocytes in a bull-dependent manner. J Reprod Dev 2016;62:451–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Palma GA, Olivier NS, Neumuller C, Sinowatz F. Effects of sex-sorted spermatozoa on the efficiency of in vitro fertilization and ultrastructure of in vitro produced bovine blastocysts. Anatom Histol Embryol 2008;37:67–73. [DOI] [PubMed] [Google Scholar]
- [19].Morton KM, Herrmann D, Sieg B, Struckmann C, Maxwell WM, Rath D, et al. Altered mRNA expression patterns in bovine blastocysts after fertilisation in vitro using flow-cytometrically sex-sorted sperm. Mol Reprod Dev 2007;74:931–40. [DOI] [PubMed] [Google Scholar]
- [20].Puglisi R, Vanni R, Galli A, Balduzzi D, Parati K, Bongioni G, et al. In vitro fertilisation with frozen-thawed bovine sperm sexed by flow cytometry and validated for accuracy by real-time PCR. Reproduction 2006;132:519–26. [DOI] [PubMed] [Google Scholar]
- [21].An L-Y, Chaubal SA, Liu Y, Chen Y, Nedambale TL, Xu J, et al. Significant heparin effect on bovine embryo development during sexed in vitro fertilization. J Reprod Dev 2017;63:175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lu KH, Seidel GE Jr.. Effects of heparin and sperm concentration on cleavage and blastocyst development rates of bovine oocytes inseminated with flow cytometrically-sorted sperm. Theriogenology 2004;62:819–30. [DOI] [PubMed] [Google Scholar]
- [23].Hu JH, Jiang ZL, Lv RK, Li QW, Zhang SS, Zan LS, et al. The advantages of low-density lipoproteins in the cryopreservation of bull semen. Cryobiology 2011;62:83–7. [DOI] [PubMed] [Google Scholar]
- [24].Lee KB, Bettegowda A, Wee G, Ireland JJ, Smith GW. Molecular determinants of oocyte competence: potential functional role for maternal (oocyte-derived) follistatin in promoting bovine early embryogenesis. Endocrinology 2009;150:2463–71. [DOI] [PubMed] [Google Scholar]
- [25].VandeVoort CA, Mtango NR, Lee YS, Smith GW, Latham KE. Differential effects of follistatin on nonhuman primate oocyte maturation and pre-implantation embryo development in vitro. Biol Reprod 2009;81:1139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].El-Sayed A, Hoelker M, Rings F, Salilew D, Jennen D, Tholen E, et al. Large-scale transcriptional analysis of bovine embryo biopsies in relation to pregnancy success after transfer to recipients. Physiol Genomics 2006;28:84–96. [DOI] [PubMed] [Google Scholar]
- [27].Ashry M, Lee K, Mondal M, Datta TK, Folger JK, Rajput SK, et al. Expression of TGFbeta superfamily components and other markers of oocyte quality in oocytes selected by brilliant cresyl blue staining: relevance to early embryonic development. Mol Reprod Dev 2015;82:251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lee KB, Zhang K, Folger JK, Knott JG, Smith GW. Evidence supporting a functional requirement of SMAD4 for bovine preimplantation embryonic development: a potential link to embryotrophic actions of follistatin. Biol Reprod 2014;91:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang K, Rajput SK, Lee KB, Wang D, Huang J, Folger JK, et al. Evidence supporting a role for SMAD2/3 in bovine early embryonic development: potential implications for embryotropic actions of follistatin. Biol Reprod 2015;93:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ashry M, Rajput SK, Folger JK, Knott JG, Hemeida NA, Kandil OM, et al. Functional role of AKT signaling in bovine early embryonic development: potential link to embryotrophic actions of follistatin. Reprod Biol Endocrinol 2018;16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Freudlsperger C, Burnett JR, Friedman JA, Kannabiran VR, Chen Z, Van Waes C. EGFR-PI3K-AKT-mTOR signaling in head and neck squamous cell carcinomas: attractive targets for molecular-oriented therapy. Expert Opin Ther Targets 2011;15:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hand JM, Zhang K, Wang L, Koganti PP, Mastrantoni K, Rajput SK, et al. Discovery of a novel oocyte-specific krüppel-associated box domain-containing zinc finger protein required for early embryogenesis in cattle. Mech Dev 2017;144:103–12. [DOI] [PubMed] [Google Scholar]
- [33].Liu X, Hu T, Sun W, Hao H, Liu Y, Zhao X, et al. Comparison of the developmental competence and quality of bovine embryos obtained by in vitro fertilization with sex-sorted and unsorted semen from seven bulls. Livest Sci 2015;181:263–70. [Google Scholar]
- [34].Henkel R, Hajimohammad M, Stalf T, Hoogendijk C, Mehnert C, Menkveld R, et al. Influence of deoxyribonucleic acid damage on fertilization and pregnancy. Fertil Steril 2004;81:965–72. [DOI] [PubMed] [Google Scholar]
- [35].Seidel GE Jr.. Sexing mammalian sperm - where do we go from here? J Reprod Dev 2012;58:505–9. [DOI] [PubMed] [Google Scholar]
- [36].Peippo J, Kurkilahti M, Bredbacka P. Developmental kinetics of in vitro produced bovine embryos: the effect of sex, glucose and exposure to time-lapse environment. Zygote 2001;9:105–13. [DOI] [PubMed] [Google Scholar]
- [37].Carvalho JO, Sartori R, Machado GM, Mourao GB, Dode MA. Quality assessment of bovine cryopreserved sperm after sexing by flow cytometry and their use in in vitro embryo production. Theriogenology 2010;74:1521–30. [DOI] [PubMed] [Google Scholar]
- [38].Siqueira LG, Hansen PJ. Sex differences in response of the bovine embryo to colony-stimulating factor 2. Reproduction 2016;152:645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jo HT, Bang JI, Kim SS, Choi BH, Jin JI, Kim HL, et al. Production of female bovine embryos with sex-sorted sperm using intracytoplasmic sperm injection: efficiency and in vitro developmental competence. Theriogenology 2014;81:675–82. e1. [DOI] [PubMed] [Google Scholar]
- [40].Cheng JC, Chang HM, Fang L, Sun YP, Leung PC. TGF-beta1 Up-regulates connective tissue growth factor expression in human granulosa cells through smad and ERK1/2 signaling pathways. PLoS One 2015;10:e0126532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nagashima T, Kim J, Li Q, Lydon JP, DeMayo FJ, Lyons KM, et al. Connective tissue growth factor is required for normal follicle development and ovulation. Mol Endocrinol 2011;25:1740–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Holbourn KP, Acharya KR, Perbal B. The CCN family of proteins: structure-function relationships. Trends Biochem Sci 2008;33:461–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Nagatomo H, Kagawa S, Kishi Y, Takuma T, Sada A, Yamanaka K, et al. Transcriptional wiring for establishing cell lineage specification at the blastocyst stage in cattle. Biol Reprod 2013;88:158. [DOI] [PubMed] [Google Scholar]
- [44].Kowanetz M, Valcourt U, Bergström R, Heldin C-H, Moustakas A. Id2 and Id3 define the potency of cell proliferation and differentiation responses to transforming growth factor β and bone morphogenetic protein. Mol Cell Biol 2004;24:4241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Qi X, Li TG, Hao J, Hu J, Wang J, Simmons H, et al. BMP4 supports self-renewal of embryonic stem cells by inhibiting mitogen-activated protein kinase pathways. Proc Natl Acad Sci U S A 2004;101:6027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mody AA, Wordinger RJ, Clark AF. Role of ID proteins in BMP4 inhibition of profibrotic effects of TGF-β2 in human TM cells. Invest Ophthalmol Vis Sci 2017;58:849–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Janatpour MJ, McMaster MT, Genbacev O, Zhou Y, Dong J, Cross JC, et al. Id-2 regulates critical aspects of human cytotrophoblast differentiation, invasion and migration. Development 2000;127:549–58. [DOI] [PubMed] [Google Scholar]
- [48].Xie Y, Awonuga A, Liu J, Rings E, Puscheck EE, Rappolee DA. Stress induces AMPK-dependent loss of potency factors Id2 and Cdx2 in early embryos and stem cells [corrected]. Stem Cells Dev 2013;22:1564–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.