Abstract
Opioid use disorder imposes great societal harm in the United States and in countries worldwide. Animal models that accurately capture motivational changes that occur in opioid dependence are critical to studying this disorder. The present study used a model of opioid vapor self-administration combined with a behavioral economics approach to determine whether rats would be more motivated to “work” to defend their baseline intake of fentanyl (i.e., more inelastic demand) following sufficiently frequent, intense, and chronic exposure to self-administered vaporized fentanyl. Male rats were allowed to respond for deliveries of 1.5-s of vaporized 10 mg/ml fentanyl solution. Following 15 sessions of short access (ShA; 1 h) vs. long access (LgA; 12 h) to self-administration, we conducted a between-sessions demand curve procedure, and observed significantly more inelastic demand for fentanyl (Essential Value; EV), and increased maximal response output (Omax) in LgA compared with ShA rats. In a subsequent phase, the unit-dose was doubled to 3 s of fentanyl vaporization. After seven ShA vs. LgA sessions, we assessed demand again and found that LgA rats, contrasted to ShA rats, demonstrated significantly higher baseline intake or “hedonic setpoint” (Q0), in addition to significantly increased EV and Omax. These results demonstrate that extended access to self-administration of a vaporized opioid causes changes in behavioral economic metrics consistent with development of an addiction-like state in rats. The combination of the vapor model with a translationally relevant behavioral economics framework opens new avenues to study dysregulated motivational processes in substance use disorders.
Keywords: behavioral economics, motivation, addiction, opioid dependence, opioid use disorder
1. Introduction
Opioid use disorder (OUD) is a significant problem worldwide. The prevalence of OUD globally was estimated to be 510 people per 100,000 (United Nations Office on Drugs and Crime 2019; Degenhardt et al. 2019). Rates of OUD are estimated to be the highest in the United States, with a prevalence of 1,347 per 100,000 (Global Burden of Diseases 2017). Canada, the Middle East, and East Asia are also experiencing a high prevalence of OUD (Degenhardt et al. 2019; Global Burden of Diseases 2017). Rates of fatal opioid overdose are increasing in the United States, the United Kingdom, Canada, Australia, and across Europe, but the situation is most dire in the United States. Opioid overdose deaths have dramatically increased in the United States over the past 10 years, first with prescription opioids, then with heroin and now with synthetic opioids, such as fentanyl and its analogs. Synthetic opioids were involved in the majority of opioid overdose deaths in 2017 (United Nations Office on Drugs and Crime 2019). Of the estimated 109,500 global opioid deaths in 2017, approximately 43% occurred in the United States (Global Burden of Diseases 2017). Fentanyl has a higher potency and lower production cost compared with heroin and is often consumed, either knowingly or unknowingly, after being synthesized in clandestine laboratories and added to heroin in an effort by illegal drug traffickers to increase the potency of the opioid combination at a lower cost (Kuczyńska et al. 2018). The inhalation (smoking/vaping) of opioids is one of the most common routes of administration, together with oral and intravenous. Opioid inhalation results in a rapid onset of pharmacological effects and is associated with high addiction liability (Alambyan et al. 2018; Gasior et al. 2016).
Currently, intravenous self-administration (IVSA) is considered the “gold-standard” for modeling drug abuse in rats (Belin-Rauscent et al. 2016). However, this model has limitations, such as tethering the animal during self-administration sessions and maintaining catheter patency when the rats are allowed extended periods of access to self-administration. Vapor self-administration avoids these concerns. Recent rodent studies employed this route of administration for a wide range of inhaled substances, including sufentanil (Vendruscolo et al. 2018), heroin (Gutierrez et al. 2020), methadone (Gutierrez et al. 2020), nicotine (Smith et al., 2020; Cooper et al., 2020 PMID), cannabis (Freels et al. 2020), and alcohol (De Guglielmo et al. 2017). These studies have validated that vaporized drugs serve as reinforcers and that rats will voluntarily self-administer vaporized drugs to the point of intoxication, tolerance, and dependence (e.g., Vendruscolo et al. 2018; De Guglielmo et al. 2017). This suggests that drug vapor self-administration is useful for modeling motivational changes that occur in humans following sufficiently frequent, intense, and chronic drug exposure.
Behavioral economics allows for sensitive assessment of the “elasticity” of demand for a reinforcer by measuring consumption across a range of prices. Demand is more elastic when consumption is more sensitive to increases in price (i.e., it decreases more rapidly) and less elastic when consumption is more resistant across increases in price (i.e., it decreases less rapidly). Because demand is measured as a function of price and normalized to baseline intake, the measure is not determined by the response requirement that is selected in the experiment nor scalar properties of the reinforcer (e.g., the particular drug dose that is used in the study). Thus, broad comparisons of the elasticity of demand can be made across experimental groups, reinforcer types (including drug and nondrug reinforcers), and subjects, including mice, rats, nonhuman primates, and humans (Hursh, 2014; Hursh and Silberberg, 2008; Christensen et al. 2008b; Bickel et al. 1995). Critical for behavioral economic analysis are parameters that estimate both baseline reinforcer consumption and the elasticity of demand. Q0 reflects the estimated reinforcer consumption in a theoretical situation where no cost is associated with consumption. This parameter has been described as the “hedonic setpoint” for reinforcement. Changes in this variable would be expected to reflect changes in sensitivity to reinforcement, such as the development of tolerance to a drug (Bentzley et al. 2013). The essential value (EV) of a reinforcer reflects the elasticity of demand for a reinforcer. Behavioral economic analyses can also be used to estimate Pmax and Omax. Pmax refers to the “price” at which the response output or amount of effort that is “paid” for a single reinforcer is maximal. Omax refers to the maximal response output that is found at Pmax. Several researchers have proposed that behavioral economics could be an especially useful tool for understanding disorders of reinforcement and motivation, such as drug addiction (e.g., Bickel et al. 2014).
Rodent studies have shown that measures of demand elasticity for drugs are predictive of choices between drug and nondrug reinforcers (Kearns et al. 2017), the severity of addiction-like behaviors (e.g., drug taking despite punishment and greater drug seeking during reinstatement), and the effects of putative treatments (Bentzley et al. 2014; Hammerslag et al. 2020). In humans, the elasticity of demand for drug reinforcement correlated with clinical indices of alcohol and nicotine addiction severity and treatment outcome (Bentzley et al. 2013). These findings indicate that behavioral economics provide a powerful translational tool for understanding reinforcement and motivation in drug abuse and for developing effective interventions for substance use disorders.
The present study used a behavioral economics approach to measure motivational changes that are hypothesized to occur with sufficiently frequent, intense, and chronic drug exposure in a vapor model of fentanyl self-administration. We allowed rats to self-administer fentanyl vapor in either 1 h short-access (ShA) or 12 h long-access (LgA) sessions three times per week and increased the unit dose across two experimental phases. We assessed whether extended exposure to fentanyl vapor results in the development of more inelastic fentanyl demand, similar to observations in OUD.
2. Materials and Methods
2.1. Subjects
Sixteen male Wistar rats, weighing 275–350 g at the start of experiments, were purchased from Charles River (Kingston, NY, USA). The rats were group housed and kept on a 12 h/12 h light/dark cycle (lights were turned on between 5:00 AM and 7:00 AM and turned off between 5:00 PM and 7:00 PM). Food and water were provided ad libitum except where noted otherwise. Body weights were recorded before each self-administration session. One rat died from overdose during the 3-s vapor delivery differential access phase and was excluded from the data analysis for that phase. All of the procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the National Institute on Drug Abuse, Intramural Research Program, Animal Care and Use Committee.
2.2. Apparatus
Operant vapor self-administration was conducted in eight airtight 26 cm × 26 cm × 38 cm chambers (Allentown, LLC, Allentown, NJ, USA) that were housed in a dark Plexiglas cabinet as previously reported (Vendruscolo et al. 2018). Each chamber was equipped with two nosepoke holes, two white cue lights, an air outlet, and a drug delivery system that was connected to a vapor inlet port. The nosepoke holes (2.5 cm diameter) were mounted 4.5 cm above the floor on the back wall. The cue lights were mounted 6 cm above the floor to the side of each nosepoke hole. The drug delivery system consisted of the drug solution in a TFV8 Big Baby atomizer tank with a V8 Baby Q2 Core (SMOK, Shenzhen, China) that was activated by a custom e-cigarette device (La Jolla Alcohol Research, La Jolla, CA, USA) and connected by tubing to an airflow gauge and the vapor inlet port (7 cm above the floor on the left front side of the chamber). The air outlet was located in the opposite corner from the vapor inlet port 23 cm above the floor on the right side of the back wall. A constant airflow (1.5 L/min) was generated by vacuum using two sets of two Hakko HK-25L linear air pumps (Matala, Laguna Hills, CA, USA), each set connected by tubing to four of the chambers. The exhaust tubing was connected to a Whatman 6702–9500 Hepa-Cap 150 In-Line Venting Filter (Cytiva, Marlborough, MA, USA) to scavenge the remaining drug. Med Associates software and an interface (St. Albans, VT, USA) recorded nosepokes, controlled presentation of the cue light, and controlled activation of the drug delivery system.
2.3. Drugs
Fentanyl citrate (National Institute on Drug Abuse, Intramural Research Program Pharmacy, Baltimore, MD, USA) was added to a small volume of water (final water content: 6%, v/v) before dissolving in an 80/20 mixture of propylene glycol/vegetable glycerol with sonication to achieve a final fentanyl concentration of 10 mg/ml. Nosepoke responses in the active (left) nosepoke hole resulted in 1.5- or 3-s fentanyl vapor delivery. The inactive (right) nosepoke had no programmed consequences. The left cue light was activated for 60 s after an active nosepoke response, signaling a timeout period during which additional nosepokes did not lead to drug delivery. Fentanyl intake was normalized by bodyweight for each rat by dividing the number of drug deliveries by bodyweight (in kilograms).
2.4. Acquisition of fentanyl vapor self-administration
In the initial acquisition phase, the rats were allowed to self-administer 1.5-s fentanyl vapor delivery in the operant chambers for 1 h per day for 5 days on a fixed-ratio 1 (FR1) schedule (i.e., one nosepoke response earned one drug delivery) during the light cycle. The rats were then split into two groups that were matched for responding during acquisition before beginning the differential access phase.
2.5. Differential access to 1.5-s fentanyl vapor self-administration
Half of the rats were assigned to ShA sessions (1 h/day), and the other half were assigned to LgA sessions (12 h/day). During the sessions, the rats had access to 1.5-s fentanyl vapor delivery in the operant chambers on an FR1 schedule. We tested the rats for fentanyl vapor self-administration intermittently as previously reported, with both groups undergoing three sessions per week (Monday, Wednesday, and Friday). Body weight loss and self-directed behavior caused by stereotyped behavior (e.g., gnawing) in animals are long-known concerns in the study of opioid dependence (Spragg, 1940; Chen et al., 2006, Vendruscolo et al., 2011). We have observed that with this intermittent schedule of testing, rats significantly escalate drug intake over the course of a few weeks while not significantly impacting their overall health (Carmack et al., 2019). Sessions began at approximately 4:00 PM. The ShA rats underwent their 1-h session first, immediately followed by the 12-h session for LgA rats (this schedule was used for the remainder of the study). Drug delivery systems were tested for functionality before and after each session throughout the study. If equipment malfunction was suspected, then the data were imputed using the average of the previous and subsequent sessions, or the session was repeated. After 15 sessions, we assessed the rats’ demand for 1.5-s fentanyl vapor delivery using an economic demand paradigm.
2.6. Economic demand for 1.5-s fentanyl vapor delivery
After differential access to 1.5-s fentanyl vapor delivery, both groups of rats were tested for their demand for 1.5-s fentanyl vapor delivery by increasing the “price,” in which the number of nosepokes that were required increased for each delivery across sessions. Baseline FR1 consumption was calculated as the average of the last three differential access sessions. The FR doubled in each subsequent session, resulting in the following order: 2, 4, 8, 16, 32, etc. Demand was assessed in the first hour of testing for both groups, after which the LgA rats returned to an FR1 schedule for the remaining 11 h of the session to maintain opioid dependence.
2.7. Differential access to 3-s fentanyl vapor self-administration
After assessing the demand for 1.5-s fentanyl vapor delivery, a differential access phase using 3-s fentanyl vapor delivery on an FR1 schedule was used to assess the effects of a higher unit dose of fentanyl. After seven sessions, the rats were assessed for their demand for 3-s fentanyl vapor delivery using the economic demand paradigm.
2.8. Economic demand for 3-s fentanyl vapor delivery
After differential access to 3-s fentanyl vapor delivery, both groups of rats were tested for their demand for 3-s fentanyl vapor delivery in the same manner as described for the previous demand test.
2.9. Statistical Analysis
All of the data are expressed as means and SEM. Significant differences in body weight emerged between the ShA and LgA groups over the course of the study. This was expected because LgA rats developed opioid dependence and gained less weight. Thus, the analysis of fentanyl intake during differential access and demand was normalized to body weight, in which the number of vapor deliveries was divided by bodyweight (in kilograms). All of the data, with the exception of body weight and demand measures (Q0, EV, Pmax, and Omax) were analyzed using one-, two-, or three-way repeated-measures analysis of variance (ANOVA). Differences in body weight were analyzed using unpaired equal-variance t-tests. Demand curves, EV, and Q0 were computed based on body weight-normalized intake according to the exponential demand model of Hursh and Silberberg (2008):
| (Eq. 1) |
where Q is the quantity consumed, Q0 is consumption as the price approaches 0, k is a constant that defines the consumption range in log units (k = 1.8 was used here), α is the rate of decline in consumption, and C is the cost (i.e., FR requirement). The primary measure of interest from the demand phase was EV, which is inversely related to α and reflects the inelasticity of demand. The EV was calculated for each subject according to Hursh (2014):
| (Eq. 2) |
Pmax and Omax were computed based on individual subjects’ Q0 and α from Equation 1 according to Kaplan and Reed (2014). EV, Q0, Pmax, and Omax were compared between LgA and ShA rats using unpaired equal-variance t-tests. While assessing demand for 1.5-s fentanyl vapor self-administration, one ShA rat exhibited especially high metrics of fentanyl motivation (EV, Omax, and Pmax). The Grubb’s test determined that this rat was a significant outlier in these measures (critical z values 2.13–2.14, observed z values 2.13–2.36, p’s < 0.05). We excluded the data of this rat from all statistical analyses and it is not presented in any figures. We have however included the data of this rat along with the individual data of all rats in the supplemental file. The exclusion of this rat changed statistical conclusions pertaining to demand assessment for 1.5-s fentanyl vapor self-administration (i.e., LgA > ShA in EV, Omax, and Pmax) but did not change statistical conclusions pertaining to demand assessment for 3-s fentanyl vapor self-administration.
3. Results
3.1. Acquisition of fentanyl vapor self-administration
After five 1-h sessions of access to 1.5-s fentanyl vapor deliveries, the rats were split into two groups that were matched by active and inactive nosepoke hole responses. Retroactive analyses of the acquisition data confirmed that these two groups were not significantly different for active (F1,13 = 0.20, p = 0.67) and inactive (F1,13 = 0.012, p = 0.91) nosepokes. No significant main effect of Session on active responses was found, in which the rats maintained stable responding during this phase (F4,52 = 1.12, p = 0.36). A significant main effect of Session on inactive responses was found (F4,52 = 4.4, p = 0.004). Although the rats maintained stable inactive nosepoke responding during this phase, rats in both groups exhibited an increase in inactive nosepoke responding in session 4.
3.2. Differential access to 1.5-s fentanyl vapor self-administration
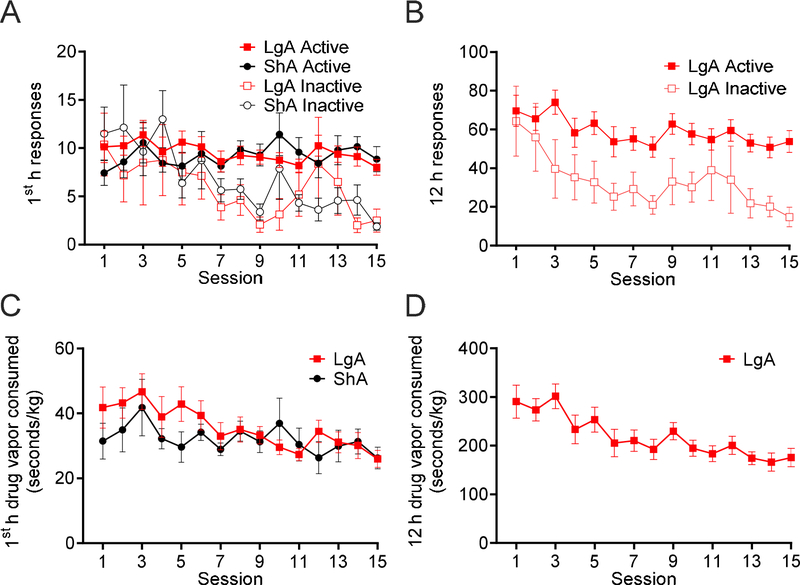
The analysis of active nosepoke responses compared with inactive nosepoke responses during the first hour of self-administration in the ShA and LgA groups (Fig. 1A) and across the entire 12 h session in the LgA group (Fig. 1B) indicated that rats in both groups learned to discriminate between active and inactive nosepoke holes by the end of this phase. A significant main effect of Session on first-hour responding was found (F14,182 = 2.96, p = 0.0004), with a significant main effect of Nosepoke Hole (F1,13 = 4.80, p = 0.047), and a significant Session × Nosepoke Hole interaction (F14,182 = 3.93, p < 0.0001). No significant main effect of Group (F1,13 < 0.01, p = 0.89) on first-hour responding was found, with no Session × Group interaction (F14,182 = 1.18, p = 0.30), Group × Nosepoke Hole interaction (F1,13 = 0.04, p = 0.85), or Session × Group × Nosepoke Hole interaction (F14,182 = 0.76, p = 0.71). Significant main effects of Session (F14,196 = 5.46, p < 0.0001) and Nosepoke Hole (F1,14 = 5.67, p = 0.032) on 12-h responding in the LgA rats were found, with no Session × Nosepoke Hole interaction (F14,196 = 1.24, p = 0.25). The consumption of 1.5-s fentanyl vapor deliveries (seconds of vapor per kilogram) during the first hour of self-administration decreased across sessions in both LgA and ShA rats (F14,182 = 2.78, p = 0.0009; Fig. 1C), with no significant main effect of Group (F1,13 = 0.82, p = 0.38), or Group × Session interaction (F14,182 = 1.00, p = 0.46). Considering drug intake over the entire 12-h sessions in the LgA group, a significant decrease in drug intake was found across sessions (F14,98 = 5.19, p < 0.0001; Fig. 1D).
Figure 1.
Rats learned to discriminate active and inactive nosepoke holes over 15 sessions of differential access to 1.5-s deliveries of 10 mg/ml fentanyl vapor. Both the LgA (n = 8) and ShA (n = 7) groups exhibited a decrease in fentanyl vapor consumption over sessions (seconds of vapor per kilogram). (A) First-hour active and inactive nosepoke responses in LgA and ShA rats during 1.5-s 10 mg/ml fentanyl vapor self-administration. The analysis revealed a significant main effect of Session (F14,182 = 2.96, p = 0.0004), a significant main effect of Nosepoke Hole (F1,13 = 4.80, p = 0.047), and a significant Session × Nosepoke Hole interaction (F14,182 = 3.93, p < 0.0001) on first-hour responding. (B) Active and inactive nosepoke responses in LgA rats during the entire 12 h session of 1.5-s 10 mg/ml fentanyl vapor self-administration. The analysis revealed significant main effects of Session (F14,196 = 5.46, p < 0.0001) and Nosepoke Hole (F1,14 = 5.67, p = 0.032) on nospoke responses. (C) First-hour consumption of 1.5-s, 10 mg/ml fentanyl vapor deliveries in LgA and ShA rats. The analysis revealed a significant main effect of Session on responses (F14,182 = 2.78, p = 0.0009) but no main effect of Group (F1,13 = 0.8167, p = 0.38) on fentanyl consumed (seconds of vapor per kilogram). (D) Consumption of 1.5-s, 10 mg/ml fentanyl vapor deliveries during the entire 12 h session in LgA rats. The analysis revealed a significant main effect of Session on fentanyl consumed (seconds of vapor per kilogram; F14,98 = 5.19, p < 0.0001). Individual subject-level data for responses made, reinforcers earned, and bodyweight are included in the accompanying supplemental material.
3.3. Demand for 1.5-s fentanyl vapor self-administration
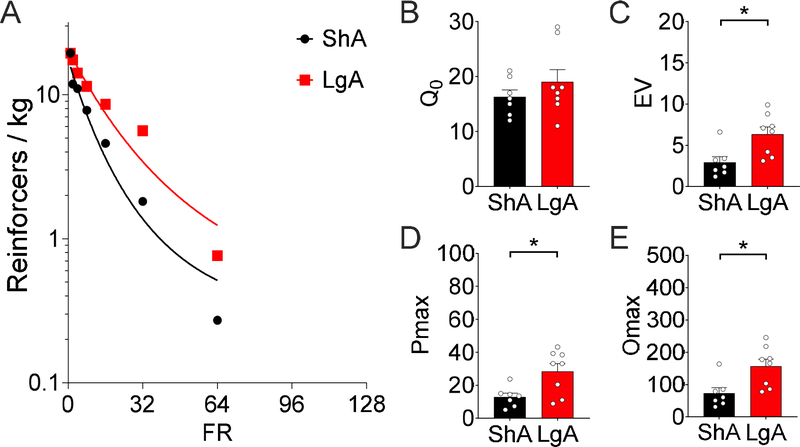
Body weight was not significantly different between LgA and ShA rats at the start of the demand assessment (LgA = 460 ± 10.2 g, ShA = 493 ± 16.5 g, t13 = 1.78, p = 0.10). Demand curve fit for intake of 1.5 s of 10 mg/ml fentanyl vapor across increasing FR requirement was excellent for both groups (k =1.8; ShA: R2 = 0.95, LgA: R2 = 0.93; Fig. 2A). No significant difference in hedonic setpoint, reflected by Q0 (t13 = 1.02, p = 0.33; Fig. 2B), was found for 1.5-s fentanyl vapor delivery. However, a difference in demand elasticity, reflected by EV was observed (t13 = 2.99, p = 0.011; Fig. 2C). Furthermore, differences were found in the FR at which responding peaked, reflected by Pmax (t13 = 2.85, p = 0.014; Fig. 2D), and maximal responding, reflected by Omax (t13 = 2.97, p = 0.011; Fig. 2E), between ShA and LgA rats for 1.5-s 10 mg/ml fentanyl vapor delivery demand.
Figure 2.
Demand inelasticity, maximal responding, and price of maximal responding significantly increased in rats in the LgA (n = 8) condition compared with the ShA (n = 7) condition in terms of their demand for 1.5-s 10 mg/ml fentanyl vapor deliveries after 15 sessions of self-administration. (A) Demand curve fit to the intake of 1.5 s of 10 mg/ml fentanyl vapor at increasing FR requirements (k =1.8, ShA R2 = 0.95, LgA R2 = 0.93) in rats in the ShA and LgA conditions. (B) Q0 after access to the self-administration of 1.5 s of 10 mg/ml fentanyl vapor. No difference was found between ShA and LgA rats (t13 = 1.02, p = 0.33). (C) The EV of fentanyl after access to the self-administration of 1.5 s of 10 mg/ml fentanyl vapor. Increased EV (i.e., more inelastic demand for fentanyl) was observed in LgA rats (t13 = 2.99, p = 0.011. (D) Pmax in rats in the ShA and LgA conditions for 1.5-s 10 mg/ml fentanyl vapor delivery. LgA rats responding peaked at a higher FR than ShA rats, reflected by Pmax (t13 = 2.85, p = 0.014. (E) Omax in the ShA and LgA groups for 1.5-s 10 mg/ml fentanyl vapor delivery. LgA rats maximal responding was higher than ShA rats, reflected by Omax (t13 = 2.97, p = 0.011). Individual subject-level data for responses made, reinforcers earned, and bodyweight are included in the accompanying supplemental material.
3.4. Differential access to 3-s fentanyl vapor self-administration
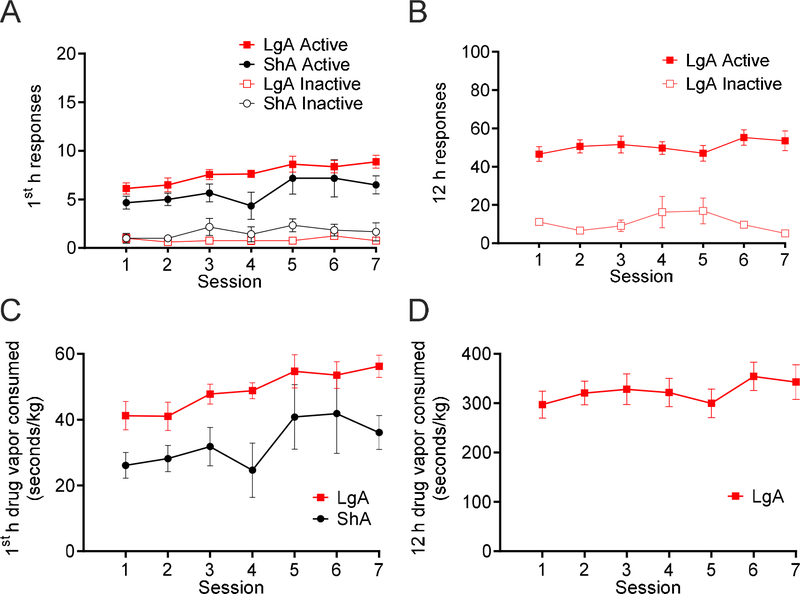
The analysis of active nosepoke hole responses compared with inactive nosepoke hole responses during the first hour of self-administration in ShA and LgA rats (Fig. 3A) and across the entire 12-h session in the LgA group (Fig. 3B) indicated that all of the rats continued to discriminate between active and inactive nosepoke holes across the entire phase. There were significant main effects of Session (F6,72 = 3.55, p = 0.0039) and Nosepoke Hole (F1,12 = 173.0, p < 0.0001) on first-hour responding, with a significant Session × Nosepoke Hole interaction (F6, 72 = 2.82, p = 0.016) and Group × Nosepoke Hole interaction (F1,12 = 10.57, p = 0.0069). No significant main effect of Group on first-hour responding was found (F1,12 = 0.97, p = 0.34), with no Session × Group interaction (F6,72 = 0.47, p = 0.83) or Session × Group × Nosepoke Hole interaction (F6,72 = 0.85, p = 0.53). A significant main effect of Nosepoke Hole on 12-h responding in the LgA group was found (F1,14 = 120.6, p < 0.0001), with no significant main effect of Session (F6,84 = 0.51, p = 0.80), and no Session × Nosepoke Hole interaction (F6,84 = 1.81, p = 0.11). The consumption of 3-s fentanyl vapor deliveries (seconds of vapor/kg) during the first hour of self-administration increased across sessions in both LgA and ShA rats (F6,72 = 4.56, p = 0.0006; Fig. 3C). Additionally, the LgA group had higher overall intake during the first hour compared with the ShA group (F1,12 = 7.87, p = 0.016; Fig. 3D). No significant Session × Group interaction was found for drug intake during the first hour (F6,72 = 0.58, p = 0.75). No main effect of Session was found on LgA intake over the entire 12-h session (F6,42 = 0.95, p = 0.47).
Figure 3.
Rats discriminated between active and inactive nosepoke holes over seven sessions of differential access to 3-s deliveries of 10 mg/ml fentanyl vapor. Both the LgA (n = 8) and ShA (n = 6) groups exhibited an increase in first-hour consumption of fentanyl vapor (seconds of vapor per kilogram) over sessions. (A) First-hour active and inactive nosepoke responses during 3-s 10 mg/ml fentanyl vapor self-administration in LgA and ShA rats. The analysis revealed significant main effects of Session (F6,72 = 3.55, p = 0.0039) and Nosepoke Hole (F1,12 = 173.0, p < 0.0001) on responding, a significant Session × Nosepoke Hole interaction (F6, 72 = 2.82, p = 0.016), and a significant Group × Nosepoke Hole interaction (F1,12 = 10.57, p = 0.0069). (B) Active and inactive nosepoke responses during the entire 12 h session of 3-s 10 mg/ml fentanyl vapor self-administration in LgA rats. The analysis revealed a significant main effect of Nosepoke Hole on responding (F1,14 = 120.6, p < 0.0001). (C) First-hour consumption of 3-s, 10 mg/ml fentanyl vapor deliveries in the LgA and ShA groups. The analysis revealed significant main effects of Session (F6,72 = 4.56, p = 0.0006) and Group (F1,12 = 7.87, p = 0.016) on fentanyl consumed (seconds of vapor per kilogram). (D) 3-s, 10 mg/ml fentanyl vapor deliveries consumed during the entire 12 h session in the LgA group. The analysis revealed no significant main effect of Session on fentanyl consumed (seconds of vapor per kilogram; F6,42 = 0.95, p = 0.47). Individual subject-level data for responses made, reinforcers earned, and bodyweight are included in the accompanying supplemental material.
3.5. Demand for 3-s fentanyl vapor self-administration
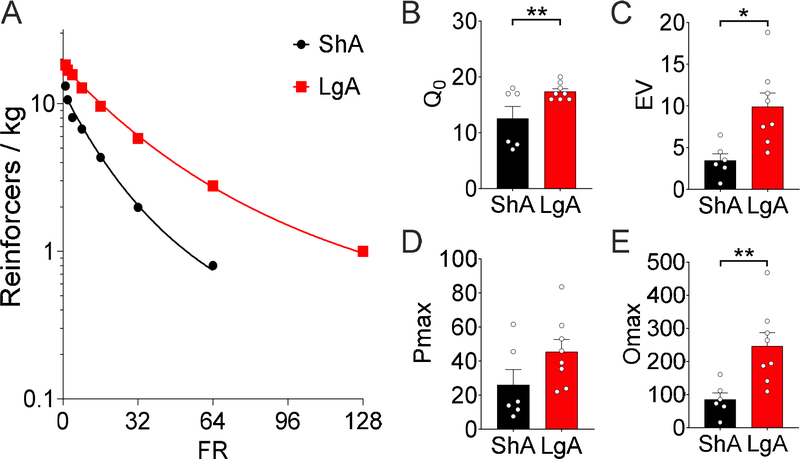
At the start of the demand assessment for 3-s fentanyl vapor delivery, the LgA rats weighed significantly less than the ShA rats (LgA = 472 ± 13 g, ShA = 539 ± 21 g; t12 = 2.87, p = 0.014). Demand curve fit for intake of 3 s of 10 mg/ml fentanyl vapor across increasing FR requirement was excellent for both groups (k =1.8; ShA: R2 = 0.99, LgA: R2 = 1.0; Fig. 4A). At this dose, LgA rats had higher hedonic setpoints, reflected by Q0 (t12 = 2.49, p = 0.029; Fig. 4B), greater demand inelasticity, reflected by EV (t12 = 3.18, p = 0.0079; Fig. 4C), and greater maximal responding, reflected by Omax (t12 = 3.20, p = 0.0077; Fig. 4D) for 3-s 10 mg/ml fentanyl vapor deliveries compared with ShA rats. No differences were found in the FR at which responding peaked, reflected by Pmax (t12 = 1.70, p = 0.11; Fig. 4E), between ShA and LgA rats. At the closest available FR to Pmax (i.e., FR32 for both groups), the LgA rats made twice as many responses as the ShA rats (ShA = 48.3 ± 9.3, LgA = 104.6 ± 14.2; t12 = 3.06, p = 0.0099).
Figure 4.
Hedonic setpoint, demand inelasticity, and maximal responding significantly increased in rats in the LgA (n = 8) condition compared with the ShA (n = 6) condition after seven sessions of the self-administration of 3-s deliveries of 10 mg/ml fentanyl vapor. (A) Demand curve fit for the ShA and LgA intake of 3-s deliveries of 10 mg/ml fentanyl vapor at increasing FR requirements (k =1.8, ShA R2 = 0.99, LgA R2 = 1.0). (B) Q0 after access to the self-administration of 3-s deliveries of 10 mg/ml fentanyl vapor. LgA rats had a significantly higher Q0 compared with ShA rats (t12 = 2.49, p = 0.029). (C) The EV of fentanyl after access to the self-administration of 3-s deliveries of 10 mg/ml fentanyl vapor. LgA rats had a significantly higher EV compared with ShA rats (t12 = 3.18, p = 0.0079). (D) Pmax of ShA and LgA rats for 3-s deliveries of 10 mg/ml fentanyl vapor. No significant difference was found between LgA and ShA rats (t12 = 1.70, p = 0.11). (E) Omax of the ShA and LgA groups for 3-s deliveries of 10 mg/ml fentanyl vapor. LgA rats had a significantly higher Omax compared with ShA rats (t12 = 3.20, p = 0.0077). Individual subject-level data for responses made, reinforcers earned, and bodyweight are included in the accompanying supplemental material.
4. Discussion
The present study employed a behavioral economics approach and found that rats developed less elastic demand for fentanyl following LgA (12 h) compared with ShA (1 h) fentanyl vapor self-administration sessions. The rats in the LgA condition were more motivated to work to maintain their intake in the face of rising “prices” (i.e., incremental increase in FR), and their hedonic setpoint was significantly higher relative to rats in the ShA condition. This raises the possibility that the present procedure might be useful for discerning critical changes in motivation that occur in opioid dependence.
Previous IVSA studies have found that rats will work to defend their intake of fentanyl and fentanyl analogs (Fragale et al. 2019; Mohammadkhani et al. 2020). Furthermore, demand elasticity has been shown to become less elastic after extended access to drugs of abuse, including fentanyl (Christensen et al. 2008a; Wade-Galuska et al. 2011; Fragale et al. 2020). The present findings extend this literature by reporting changes in behavioral economic metrics that are consistent with the development of an addiction-like phenotype following extended-access fentanyl vapor self-administration.
Extended access to IVSA has been repeatedly shown to result in the escalation of intake compared with limited access for various drugs of abuse, including cocaine, amphetamine, alcohol, opioids, nicotine, and Δ9-THC, and this escalation is associated with elevations of intracranial self-stimulation thresholds between drug taking sessions (Koob, 2017). Such elevations of reward thresholds are hypothesized to reflect a hypohedonic-like state (Koob, 2017) that drives greater consumption and motivation for the drug via negative reinforcement processes (i.e., drug self-administration to remove these negative states; Jang et al. 2013). In the present study, the rats did not escalate their fentanyl intake during LgA or ShA sessions with 1.5-s 10 mg/ml fentanyl vapor delivery. However, we observed a significantly increased EV and Omax metrics in the LgA compared with ShA rats. This indicates that this level of exposure to fentanyl was sufficient to produce group differences in motivation for the drug. LgA rats also peaked their responding at a significantly higher FR (Pmax) compared to ShA rats.
It is worth noting that the absence of an escalation pattern of intake with the 1.5-s unit-dose of fentanyl vapor is somewhat incongruent with reports that lower doses of self-administered psychostimulant drugs have been reported to generate faster escalation of drug-taking (e.g., Kitamura et al., 2006). In fact, rats in both groups decreased their consumption of opioids across sessions of self-administration with 1.5-s fentanyl vapor deliveries. There are several potential reasons for this. First, we suspect that the downward trend in intake in both the LgA and ShA groups across FR1 sessions of differential access to 1.5-s fentanyl vapor delivery may be attributable to learning processes that are associated with drug taking patterns. Nosepoking is a prepotent response, and the high numbers of nosepokes that we observed early during training is unsurprising. This is particularly true for LgA animals that not only learned an operant response and discriminated between the active and inactive nosepoke holes but also learned to titrate their intake of vaporized drug to maintain intoxication over an extended period of time. Second, the 1.5-s unit dose of fentanyl vapor may not have been ideal to maintain stable behavior. Low unit doses of self-administered drug are well known to be associated with both within- and between-subject variability that can alternate between periods of high and low activity (Wise, 1987). It is also possible that escalation patterns with low doses of opioids are different from what has been reported for stimulant drugs. Note that the escalation of opioid self-administration was not observed when low (although reinforcing) doses of intravenous heroin, fentanyl, and oxycodone were self-administered (Wade et al., 2015) and escalation is also dependent on the length of the self-administration sessions (Vendruscolo et al., 2011).
Over the next seven sessions of differential access to the longer, 3-s vapor delivery of 10 mg/ml fentanyl vapor, rats in both the LgA and ShA conditions escalated their intake across sessions. Vendruscolo et al. (2018) and Moussawi et al. (2020) reported a greater escalation of intake in LgA conditions compared with ShA conditions in rats and mice that responded for vaporized opioids. The sequential design of the present study produced a confound in terms of training history and unit-dose that made it difficult to assess the escalation pattern with the 3-s unit dose of vaporized fentanyl (i.e., training with the 1.5-s fentanyl deliveries resulted in increased demand metrics in LgA rats, and thus, this training experience should be expected to facilitate subsequent responding for the 3-s dose). However, we found that LgA rats had significantly higher drug intake than ShA rats in the first hour of each session across all sessions of differential access to the 3-s fentanyl vapor deliveries. This indicates that, at least relative to ShA rats, the LgA rats exhibited escalated fentanyl consumption and suggests the emergence of tolerance and opioid dependence. This is not surprising given that the different access schedules that were employed resulted in LgA rats being exposed to approximately 10-times the amount of fentanyl relative to ShA rats in this phase. Further suggesting that rats in the LgA condition developed opioid dependence; body weight was lower in rats in the LgA condition compared with the ShA condition following differential access to 3-s fentanyl vapor deliveries. A loss of body weight is a hallmark of opioid dependence (Spragg, 1940).
Increased intake in LgA compared to ShA rats during sessions of differential access with the 3-s unit dose is expected as a result of drug tolerance (i.e., reflected by a rightward shift of the dose-response curve under an FR1 schedule) and the development of an allostatic, higher hedonic setpoint (i.e., an upward shift of the dose-response curve under an FR1 schedule; Ahmed and Koob, 1998). Demand curve testing paralleled this difference in baseline consumption, as LgA rats were significantly higher than ShA rats in terms of the Q0 measure, that has been proposed to measure hedonic setpoint. In addition to a higher hedonic setpoint, there was a significant difference between ShA and LgA groups in the EV measure, as demand for fentanyl became less elastic in LgA rats. Because the EV measure accounts for baseline intake through normalization to Q0, the differences observed in EV indicate that rats in the LgA condition, compared with the ShA condition, had greater motivation to defend their intake, independent from differences in hedonic setpoint. The independence of the EV measure from baseline consumption patterns (i.e., as were observed under the preceding FR1 schedule) highlights the utility of a behavioral economics approach. By comparison, other measures of value, such as choice tasks or progressive-ratio (PR) responding confound baseline intake and motivation and are further limited by their specificity to particular constraints of the task. For example, in a choice task, the measures depend on comparisons to other reinforcers. A PR task identifies the maximum level of responding for a particular reinforcer strength but does not describe behavior at lower price points. The present data thus support the long-held assumption that in addition to a shift in hedonic setpoint, a motivational shift occurs following sufficiently intense, frequent, or prolonged drug use that drives addiction-like consumption and pursuit of drugs (e.g., Koob 2017; Jang et al. 2013).
Further supporting the hypothesis that LgA rats developed greater motivation for fentanyl, rats in the LgA condition had a significantly higher Omax compared with ShA rats, indicating a higher maximal output during the demand curve procedure. Pmax was higher in LgA vs. ShA rats, but not significantly so, as it was in the 1.5-s unit-dose phase. It is possible that this is because rats in general completed a broader range of ratios during this test as a result of the higher unit-dose of fentanyl being a stronger reinforcer and thus maintaining a wider range of behavior. The critical feature for understanding differences in motivation is response output (i.e., Omax) rather than price of maximal output (i.e., Pmax). This is clear when comparing the raw active responses at the FR that was closest to Pmax (i.e., FR32 for each group), as LgA group rats made on average twice as many responses than the ShA group (48.3 ± 9.3 vs. 104.6 ± 14.2, respectively).
One limitation of the vapor model compared with traditional IVSA is that the precise amount of drug that each animal receives cannot be determined. However, using the same vapor apparatus as in the present study, previous work has shown that the number of vapor deliveries correlated with blood levels of sufentanil in rats (Vendruscolo et al. 2018) and fentanyl in mice (Moussawi et al. 2020). Thus, in the second phase of the present study when the duration of drug vapor delivery was doubled from 1.5 s to 3 s, the rats would be assumed to receive higher unit doses of the drug and thus higher total amounts of the drug (i.e., total seconds of drug vapor self-administered at the end of the 1.5-s unit-dose phase compared with the 3-s unit-dose phase in the first hour [compare Fig. 1C and 3C] and 12 h [compare Fig. 1D and 3D]).
The sequential design that was used in the present study did not allow us to determine whether 3-s fentanyl vapor delivery alone or the cumulative effect of chronic vapor across the experiment caused the increased consumption and motivation for fentanyl in the LgA rats while testing demand for the 3-s unit-dose of fentanyl vapor (Fig. 4). In terms of the cumulative difference in intake of the two groups through the course of the study, during the 15 sessions of differential-access training with 1.5-s fentanyl vapor deliveries, and during the 7 sessions of the differential-access training with 3-s fentanyl vapor deliveries, the following results were generated. ShA rats consumed on average 479.9 (± 44.2), and 229.5 (± 43.6) seconds of vapor/kg, respectively, whereas LgA rats consumed 3,282.5 (± 196.6), and 2,264.4 (± 150.9) seconds of vapor/kg, respectively. After accounting for the fact that 1.5-s unit-dose phase was less than half the length of the 3-s unit-dose phase (7 vs. 15 sessions), it is clear that ShA rats maintained their intake across phases of the experiment, consuming on average 32.0-s (± 3.5) vapor/session in the 1.5-s unit-dose, and 32.8-s (± 6.2) vapor/session in the 3-s unit dose phase (not significantly different across phases; t5 = 0.15, p = 0.89). LgA rats, however, displayed enhanced consumption in the 3-s phase (323.5-s ±21.6 vapor/session) compared to the 1.5-s phase (218.8-s ± 13. vapor/session; t7 = 5.69, p = 0.0007). The increased drug consumption in the 3-s unit dose phase specific to the LgA group suggests that neurobiological changes underlying baseline fentanyl self-administration and motivation for fentanyl occurred during this phase that were unique to the LgA group. This likely contributed to the performance of LgA rats in the subsequent demand test for 3-s fentanyl vapor deliveries in which increased hedonic setpoint (see Q0 in Fig. 4B) and demand metrics of EV and Omax (Figs. 4C and 4E) were observed relative to ShA rats.
In addition to the observation that LgA but not ShA rats consumed more fentanyl vapor per session once the switch to the higher unit dose of 3-s was employed, LgA rats also demonstrated significantly lower bodyweight and increased hedonic setpoint (Q0) following 7 sessions of self-administration with the 3-s unit dose that were not present following 15 sessions of self-administration with the 1.5-s unit dose. This suggests that the higher unit dose of fentanyl was more effective in inducing motivational changes associated with fentanyl dependence. Regardless, in acknowledgement of the limitations of the present study, future studies should systematically manipulate the unit-dose of fentanyl and measure differences in motivation between ShA and LgA conditions. However, we believe that the main conclusion of this manuscript would not be changed by the outcome of such a study, i.e., that with sufficiently intense, frequent, and chronic fentanyl self-administration, enhanced motivation for fentanyl develops.
Another limitation of the present study was that only male rats were used. Only males were used because this was an initial study to determine whether demand curve analyses of fentanyl vapor self-administration are technically feasible. We started with male rats to directly compare behavior to previously published work (Vendruscolo et al. 2018). Future studies will seek to determine whether the present results can be extended to female subjects, an especially pertinent question given recent findings of sex differences in opioid self-administration in rodents (e.g., Towers et al. 2019). Additionally, we did not systematically vary the housing conditions of animals in this study. All animals were group housed from birth through to adulthood, including for the duration of this study, but with the exception that rats were socially isolated during self-administration sessions (i.e., three times per week, for 1-h sessions in ShA rats and 12-h sessions in LgA rats). The effects of social isolation on drug self-administration in adult animals are mild and it is unclear whether isolation would impact self-administration in adulthood (i.e., after group-housing through adolescence, as in the present study) and after self-administration behavior has already been established (in the present study, rats in both groups were trained to acquire self-administration in 1-h sessions with matched housing conditions until self-administration was acquired; see Bozarth et al., 1989 and Shaham et al., 2003 for review).
The vapor model provides several advantages over traditional IVSA, as described extensively in Vendruscolo et al. (2018) and Moussawi et al. (2020). Notably, the vapor approach avoids the necessity of catheterization, which eliminates stress that is associated with surgery and the difficulty of maintaining catheter patency in long-term studies. The vapor model also does not require a tether, thus facilitating techniques that also require attachments to the animal (e.g., intracranial self-stimulation, in vivo electrophysiology, optogenetics, and calcium imaging). Recent studies have shown that rodents will self-administer a range of vaporized drugs, including alcohol (De Guglielmo et al. 2017), cannabis extracts (a mixture of 28.4% Δ9-THC, 1.38% cannabidiol, and 1.8% cannabinol and a mixture of 1.16% Δ9-THC, 59.34% cannabidiol, 2.1% cannabichromene, 1.1% cannabigerol, and 0.01% tetrahydrocannabivarin and cannabinol; Freels et al. 2020), nicotine (Smith et al. 2020), and such opioids as sufentanil (Vendruscolo et al. 2018), fentanyl (Moussawi et al. 2020), and heroin (Gutierrez et al. 2020). These studies have reported good correspondence between the number of drug deliveries and drug concentrations that are administered using this technology and resulting levels of hypothesized intoxication (De Guglielmo et al. 2017; Vendruscolo et al. 2018; Freels et al. 2020; Gutierrez et al. 2020). They also provide evidence that opioid vapor is self-administered for its reinforcing properties (Vendruscolo et al. 2018; Gutierrez et al. 2020), such that the level of responding for opioid vapor is sensitive to changes in FR requirements and the drug concentration. Furthermore, evidence of the development of tolerance to and dependence on self-administered opioid vapor has been reported, in which rodents exhibit a greater escalation of intake over sessions in LgA conditions compared with ShA conditions (Vendruscolo et al. 2018). Additionally, Vendruscolo et al. (2018) reported higher somatic withdrawal scores and lower pain thresholds, measured by mechanical sensitivity, in LgA rats compared with ShA rats. Gutierrez et al. (2020) reported an increase in anxiety-like behavior during heroin withdrawal. More recently, Moussawi et al. (2020) reported that all of these principles of reinforcement; sensitivity to changes in FR requirement and drug concentration, the development of drug dependence, and the escalation of drug intake are replicable when using mice to study fentanyl vapor self-administration. Moussawi et al. (2020) also reported that mice with LgA to fentanyl vapor self-administration exhibited greater resistance to punishment when the drug vapor was adulterated with capsaicin, alterations of electrophysiological properties in ventral tegmental area dopamine neurons during protracted abstinence, and the reinstatement of fentanyl vapor seeking following extinction.
4.1. Conclusion
The present results extended the existing literature on drug vapor self-administration and behavioral economics by demonstrating that extended access to the self-administration of a vaporized opioid (i.e., fentanyl) increased metrics that reflect greater motivation for fentanyl, indicating the emergence of an addiction-like state. The present findings also validated a unique behavioral economics approach to study drug vapor self-administration. The combination of the vapor model and the highly sensitive and translatable behavioral economics framework opens new avenues to study the neurobiological mechanisms that underlie the dysregulation of motivational processes in substance use disorders.
Supplementary Material
Highlights to accompany manuscript NEUROPHARM-D-20-00546:
Translationally relevant rat models of opioid use disorder are needed
Fentanyl vapor self-administration was tested using a behavioral economic framework
Rats allowed extended access developed more inelastic demand for fentanyl
This approach can facilitate studying the neurobiology of disordered motivation
5. Acknowledgements
The authors thank Michael Arends for proofreading the manuscript.
6. Funding
This work was supported by the National Institutes on Drug Abuse Intramural Research Program. BJT received funding from National Institutes of Health (grant no. DA048530).
Abbreviations:
- (OUD)
Opioid use disorder
- (IVSA)
intravenous self-administration
- (EV)
essential value
- (ShA)
short access
- (LgA)
long access
- (FR)
fixed-ratio
- (PR)
progressive-ratio
Footnotes
Declaration of Interest: The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. References
- Ahmed SH, Koob GF (1998) Transition from moderate to excessive drug intake: change in hedonic set point. Science 282:298–300. [DOI] [PubMed] [Google Scholar]
- Alambyan V, Pace J, Miller B, et al. The Emerging Role of Inhaled Heroin in the Opioid Epidemic: A Review. JAMA Neurol 75 (11), 1423–1434 (2018). 10.1001/jamaneurol.2018.1693 [DOI] [PubMed] [Google Scholar]
- Belin-Rauscent A, Fouyssac M, Bonci A, et al. How Preclinical Models Evolved to Resemble the Diagnostic Criteria of Drug Addiction. Biol Psychiatry. 79 (1), 39–46 (2016). 10.1016/j.biopsych.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Fender KM, Aston-Jones G The behavioral economics of drug self-administration: a review and new analytical approach for within-session procedures. Psychopharmacology. 226(1), 113–125 (2013). 10.1007/s00213-012-2899-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Jhou TC, Aston-Jones G Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat. Proceedings of the National Academy of Sciences 111 (32), 11822–11827 (2014). 10.1073/pnas.1406324111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, DeGrandpre RJ Higgins ST The behavioral economics of concurrent drug reinforcers: a review and reanalysis of drug self-administration research. Psychopharmacology 118, 250–259 (1995). 10.1007/BF02245952 [DOI] [PubMed] [Google Scholar]
- Bickel WK, Johnson MW, Koffarnus MN, et al. The Behavioral Economics of Substance Use Disorders: Reinforcement Pathologies and Their Repair. Annual Review of Clinical Psychology 10(1), 641–677 (2014). 10.1146/annurev-clinpsy-032813-153724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmack SA, Keeley RJ, Vendruscolo JC, et al. Heroin addiction engages negative emotional learning brain circuits in rats. The Journal of clinical investigation, 129, 2480–2484 (2019). 10.1172/JCI125534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SA, O’Dell L, Hoefer M, et al. Unlimited access to heroin self-administration: independent motivational markers of opiate dependence. Neuropsychopharmacology, 31, 2692–2707 (2006). 10.1038/sj.npp.1301008 [DOI] [PubMed] [Google Scholar]
- Christensen CJ, Silberberg A, Hursh SR et al. Essential value of cocaine and food in rats: tests of the exponential model of demand. Psychopharmacology 198, 221–229 (2008b). 10.1007/s00213-008-1120-0 [DOI] [PubMed] [Google Scholar]
- Christensen CJ, Silberberg A, Hursh SR, et al. Demand for cocaine and food over time. Pharmacol Biochem Behav 91, 209–16 (2008a). 10.1016/j.pbb.2008.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper SY, Akers AT, Henderson BJ (2020). Flavors enhance nicotine vapor self-administration in male mice. Nicotine & Tobacco Research ntaa 165, 10.1093/ntr/ntaa165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Guglielmo G, Kallupi M, Cole MD, et al. Voluntary induction and maintenance of alcohol dependence in rats using alcohol vapor self-administration. Psychopharmacology (Berl). 234 (13), 2009–2018 (2017). 10.1007/s00213-017-4608-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Grebely J, Stone J, et al. Global patterns of opioid use and dependence: harms to populations, interventions, and future action. The Lancet. 394(10208), 1560–1579 (2019). 10.1016/S0140-6736(19)32229-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragale JE, James MH, Aston-Jones G Intermittent self-administration of fentanyl induces a multifaceted addiction state associated with persistent changes in the orexin system. Biorxiv.org (2020). doi: 10.1101/2020.04.23.055848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragale JE, Pantazis CB, James MH, et al. The role of orexin-1 receptor signaling in demand for the opioid fentanyl. Neuropsychopharmacol 44, 1690–1697 (2019). 10.1038/s41386-019-0420-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freels TG, Baxter-Potter LN, Lugo JM, et al. Vaporized Cannabis Extracts Have Reinforcing Properties and Support Conditioned Drug-Seeking Behavior in Rats. Journal of Neuroscience 40 (9), 1897–1908 (2020). 10.1523/JNEUROSCI.2416-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasior M, Bond M, Malamut R. Routes of abuse of prescription opioid analgesics: a review and assessment of the potential impact of abuse-deterrent formulations. Postgrad Med 128 (1), 85–96 (2016). 10.1080/00325481.2016.1120642 [DOI] [PubMed] [Google Scholar]
- Global Burden of Diseases. Global, regional, and national incidence, prevalence,and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1789–858 (2018). 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez A, Nguyen JD, Creehan KM, et al. Self-administration of heroin by vapor inhalation in female Wistar rats. Biorxiv.org (2020). 10.1101/2020.03.30.016725 [DOI] [Google Scholar]
- Hammerslag LR, Hofford RS, Kang Q, et al. , Changes in fentanyl demand following naltrexone, morphine, and buprenorphine in male rats. Drug and Alcohol Dependence. 207, 107804 (2020). 10.1016/j.drugalcdep.2019.107804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, & Silberberg A Economic demand and essential value. Psychological Review. 115(1), 186–198 (2008). 10.1037/0033-295X.115.1.186 [DOI] [PubMed] [Google Scholar]
- Hursh SR Behavioral economics and the analysis of consumption and choice In McSweeney FK, Murphy ES (Eds.), The Wiley Blackwell Handbook of Operant and Classical Conditioning (pp. 275–305). Hoboken, NJ: Wiley; (2014). [Google Scholar]
- Jang C, Whitfield T, Schulteis G et al. A dysphoric-like state during early withdrawal from extended access to methamphetamine self-administration in rats. Psychopharmacology 225, 753–763 (2013). 10.1007/s00213-012-2864-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan BA, & Reed DD Essential value, Pmax, and Omax Automated Calculator [spreadsheet application] (2014). Retrieved from: http://hdl.handle.net/1808/14934 [Google Scholar]
- Kearns DN, Kim JS, Tunstall BJ, et al. Essential values of cocaine and non-drug alternatives predict the choice between them. Addiction Biology, 22: 1501–1514 (2017). 10.1111/adb.12450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, et al. Escalation of methamphetamine self-administration in rats: a dose–effect function. Psychopharmacology, 186, 48–53 (2006). 10.1007/s00213-006-0353-z [DOI] [PubMed] [Google Scholar]
- Koob GF Antireward, compulsivity, and addiction: seminal contributions of Dr. Athina Markou to motivational dysregulation in addiction. Psychopharmacology 234, 1315–1332 (2017). 10.1007/s00213-016-4484-6 [DOI] [PubMed] [Google Scholar]
- Kuczyńska K, Grzonkowski P, Kacprzak Ł, et al. Abuse of fentanyl: An emerging problem to face. Forensic Sci Int 289, 207–214 (2018). 10.1016/j.forsciint.2018.05.042 [DOI] [PubMed] [Google Scholar]
- Mohammadkhani A, James MH, Pantazis CB, et al. Persistent effects of the orexin-1 receptor antagonist SB-334867 on motivation for the fast acting opioid remifentanil. Brain Research. 1731, 146461 (2020). 10.1016/j.brainres.2019.146461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Ortiz MM, Gantz SC, Tunstall, et al. Fentanyl vapor self-administration model in mice to study opioid addiction. Science Advances. [In Press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LC, Kallupi M, Tieu L, et al. Validation of a nicotine vapor self-administration model in rats with relevance to electronic cigarette use. Neuropsychopharmacology. 45, 1909–1919 (2020). https://10.1038/s41386-020-0734-8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spragg SDS Morphine addiction in chimpanzees. Comparative Psychology Monographs, 15, 7, 132 (1940). [Google Scholar]
- Towers EB, Tunstall BJ, McCracken ML, et al. Male and female mice develop escalation of heroin intake and dependence following extended access. Neuropharmacology. 151, 189–194 (2019). 10.1016/j.neuropharm.2019.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime. World Drug Report 2019. (United Nations publication, Sales No. E.19.XI.8) [Google Scholar]
- Vendruscolo LF, Schlosburg JE, Misra KK, et al. Escalation patterns of varying periods of heroin access. Pharmacology Biochemistry and Behavior, 98, 570–574 (2011). 10.1016/j.pbb.2011.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo J, Tunstall B, Carmack S et al. Compulsive-Like Sufentanil Vapor Self-Administration in Rats. Neuropsychopharmacol 43, 801–809 (2018). 10.1038/npp.2017.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade-Galuska T, Galuska CM Winger G Effects of daily morphine administration and deprivation on choice and demand for remifentanil and cocaine in rhesus monkeys. Journal of the Experimental Analysis of Behavior, 95, 75–89 (2011). 10.1901/jeab.2011.95-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade C, Vendruscolo L, Schlosburg J et al. Compulsive-Like Responding for Opioid Analgesics in Rats with Extended Access. Neuropsychopharmacol 40, 421–428 (2015). 10.1038/npp.2014.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA (1987) Intravenous Drug Self-Administration: A Special Case of Positive Reinforcement In: Bozarth MA (eds) Methods of Assessing the Reinforcing Properties of Abused Drugs. Springer, New York, NY [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.