Abstract
Young people and adults diagnosed with an HIV indicator condition should be offered an HIV test (NICE [National Institute of Clinical Excellence] guidance). Community-acquired pneumonia (CAP) is considered to be an HIV indicator condition as it has an undiagnosed HIV prevalence of 0.76%. We observed however, that the offer of HIV testing to patients with radiologically diagnosed CAP remained low even after a senior respiratory physician review. Our aim was to improve the percentage of patients being offered an HIV test with CAP requiring hospital admission across four acute medical wards at Royal Derby Hospital within 12 months. We identified several key steps in the process. These included the identification of CAP, the role of the medical clerking team and the respiratory infections nursing team that manage pneumonia admissions. After collecting baseline data and staff interviews, we conducted seven plan-do-study-act (PDSA) interventions. These included; iterative communication, educational interventions, system changes that involved a direct HIV test offering by our respiratory infection team and the addition of an HIV test to the electronic CAP bundle. Data collected from 177 patients were analysed over a period of one year. The main outcome measure of the project ‘Did patients with a diagnosis of CAP on admission have a documented HIV test offered?’ improved from 28% during the first cycle of data collection to 76.4% during the final cycle. Patients were more likely to be offered an HIV test if they had no comorbidity compared with those with a diagnosis of asthma or chronic obstructive pulmonary disease. Our most impactful PDSA interventions were the respiratory infection nurses directly offering an HIV test to patients and adding HIV to the electronic ordering CAP bundle. Our quality improvement programme has shown that educational, communication and system changes can help improve the uptake of HIV testing. Education on HIV testing is now part of our induction programme for new doctors and we are using a new CAP bundle to help streamline the request of HIV testing at the first clinician clerking. Our dedicated respiratory infection nursing team also ensures that patients with CAP have a documented offer of an HIV test.
Keywords: quality improvement, audit and feedback, clinical practice guidelines, hospital medicine
Problem
The 2017 NICE guidance on ‘HIV testing: encouraging uptake’ recommends that young people and adults newly diagnosed with an HIV indicator condition are offered an HIV test.1 Community-acquired pneumonia (CAP) is considered to be an HIV indicator condition as it has an undiagnosed HIV prevalence of 0.76%.2 The National UK policy for HIV testing recommends offering HIV testing universally to all medical admissions where the locally diagnosed HIV prevalence is greater than 2 per 1000 population and those with any indicator disease, such as CAP, should always be offered testing in any setting.3
Latest figures from Public Health England report the prevalence of diagnosed HIV as 2.49 per 1000 population in Derby of those aged 15–59 years, although when looking at Derby and Derbyshire as a combined population the prevalence is lower at 1.63 per 1000 population.4 Importantly of the total diagnoses of HIV, 41.7% of those in Derby and 50% of those in Derbyshire were considered to be late diagnoses. Overall the number of late diagnoses in Derby and Derbyshire area is worse compared with the Public Health England benchmark,4 suggesting there is scope for improvement in earlier diagnoses of positive cases in this area. It is widely reported that the key to successful treatment of HIV is early diagnosis.5 6 Moreover, from a public health point of view, increasing uptake of testing will allow commissioners and the NHS to reduce health inequalities, as HIV disproportionately affects minority groups.7
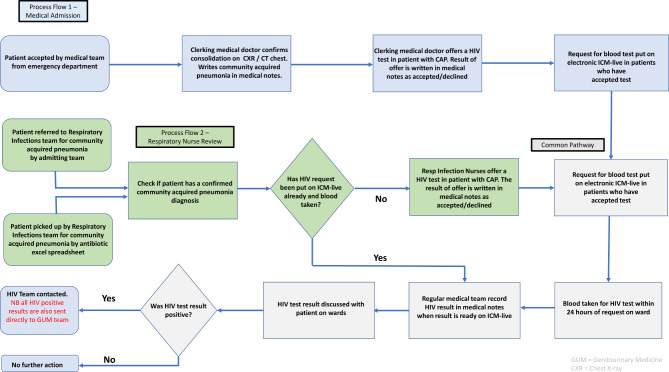
University Hospitals of Derby and Burton NHS Foundation Trust serves a population of more than one million and has two acute sites with emergency departments. There are three 29-bedded respiratory wards at the Royal Derby Hospital (RDH) and one 58-bedded medical admission unit (MAU). Patients on these wards comprise mostly respiratory and acute medical patients. The wards are staffed with training-grade doctors and nursing staff. Patient care on the respiratory wards is facilitated by a daily multidisciplinary team board round meeting and there are consultant-led ward rounds every day of the week. Medical patients arrive via the emergency department, as community referrals from general practice or are admitted to MAU from out-of-hours clinics. The pathway is summarised as follows:
Initial clerking is done by a trainee junior doctor. If a diagnosis of CAP is confirmed on radiological imaging, there is an opportunity to offer HIV testing to the patient.
A medical senior review then takes place by either a consultant or specialty registrar, providing another opportunity to offer HIV testing. Then the patient is assigned to the appropriate medical specialty ward.
Once a patient arrives on a respiratory ward a further senior review by a respiratory senior (a consultant or registrar) is undertaken. HIV testing is offered here if not requested previously.
In addition, there is a dedicated respiratory infection team (RIT) established at RDH who routinely review any patient that receives CAP antibiotics. They will also review any patients on atypical antibiotics for CAP if a referral is requested by the medical team. The RIT have the same opportunity to offer HIV testing if appropriate.
At the point where a patient accepts HIV testing, it should be requested on the electronic ordering system ‘ICM-live’ (iSoft Clinical Manager, 2004). The sample should then be taken within 24 hours of the request. The medical team should then follow-up the result, document it in the notes, act if positive and inform the patient. The HIV testing process map is summarised in figure 1.
Figure 1.
Schematic process map for HIV test ordering. CAP, community-acquired pneumonia; ICM-live, iSoft Clinical Manager, 2004.
We observed however, that the offering of HIV testing to patients with radiologically diagnosed CAP remained low even after a senior respiratory physician review. As part of a trainee-led quality improvement programme, our aim was to improve the percentage of HIV testing offered to 90% patients with CAP requiring hospital admission across four acute medical wards at RDH within 12 months.
Background
Routine testing of conditions where the prevalence of HIV is >0.1% is reported to be cost-effective as well as leading to earlier diagnosis of HIV.8 A national observational cohort study in the UK found that delay in testing was a major factor that contributed to mortality in HIV-positive people, with late diagnosis being a strong predictor for death.9 Early diagnosis improves treatment outcomes and reduces the risk of transmission to others.1
Clinical indicator conditions for HIV testing fall into three categories:2
Conditions which are AIDS defining.
Conditions associated with an undiagnosed HIV prevalence of >0.1%.
Conditions where not identifying the presence of HIV infection may have significant adverse implications for the individual’s clinical management.
CAP falls into the second category with an undiagnosed HIV prevalence of 0.76%.2 Furthermore the WHO considers recurrent pneumonia, with two or more episodes in a 12-month period, to be an AIDS-defining condition.10 Therefore it is important to offer testing to those with indicator conditions to avoid the consequences of undiagnosed HIV infection, and to also improve the response to indicator conditions in the event of a positive HIV diagnosis.1
In the UK, of those estimated to have HIV, 17% are unaware they are infected.11 CAP is common among patients with coexisting illnesses and can be the initial manifestation of these comorbid diseases.12 Lower respiratory tract infections are 25 times more common among those that are HIV-positive than in the general population.13 It is also of note that the clinical presentation of CAP in HIV-positive individuals is similar to those that are HIV-negative.13
Our quality improvement programme therefore needed to incorporate multidisciplinary interventions that included all steps from counselling appropriate patients for HIV testing through to the tests being processed and patients being informed of the result (figure 1).
Measurement
Once weekly, patients on each of the four medical wards were screened to determine whether they were on treatment for CAP. Imaging was reviewed to ensure a positive diagnosis of CAP in the medical notes; this was confirmed by radiological findings. Overall 36 measurement cycles took place during our project. Data were collected by six trainee medical doctors. The measurements during the project happened over two time periods. All baseline measurements were calculated from the median value of the first eight measurement cycles. The first from 27 February 2019 to 12 July 2019 and the second from 13 November 2019 to 25 February 2020. Progress and continuation of plan-do-study-act (PDSA) planning and actions continued throughout the whole year.
Our main outcome measure was whether patients admitted with CAP were offered HIV testing. We identified four other measures that are important to facilitate HIV testing in those with a diagnosis of CAP. The measures that we collected data on are listed below:
Was the patient’s decision to accept or decline the HIV test recorded in the notes?
Was the HIV test requested on the electronic ordering system?
Was the test marked as collected on the electronic ordering system?
Was the result processed and available on the electronic ordering system?
One balancing measure was evidence of HIV testing in the last three months. This was checked on the electronic ordering system. A further balancing measure was failure to act on a positive test evaluated by examining the medical notes.
Sources of information used included the medical notes, and the electronic prescribing and result viewing software—ICM-live. We collected additional information on comorbidities and CURB-65 Score (Score for pneumonia severity which estimates mortality of community acquired pneumonia) of the patients selected for analysis.
Design
The quality improvement project (QIP) was performed using the model for improvement methodology by the Institute of Healthcare Improvement.14 The improvement team consisted of a respiratory consultant who was our mentor, six trainee medical doctors, three respiratory infection specialist nurses and an HIV specialist nurse. The team conducted several meetings to understand the issues with offering HIV testing among patients admitted with CAP. This allowed an opportunity to discuss where the potential flaws in the current testing process lay. Trainee doctors commented that they had little experience or training in counselling patients on HIV testing. The respiratory infection team noted that often an HIV test could be ordered on ICM-live, but if it is not taken within 24 hours, the order expires. This often occurred when bloods had already been taken by the phlebotomy team, which would then require ward doctors to take further bloods for testing.
These discussions informed the potential areas to target our interventions. The following primary drivers were identified:
Training and education of staff.
Communication resources for staff and patients.
System changes to the pathway and computerised order sets.
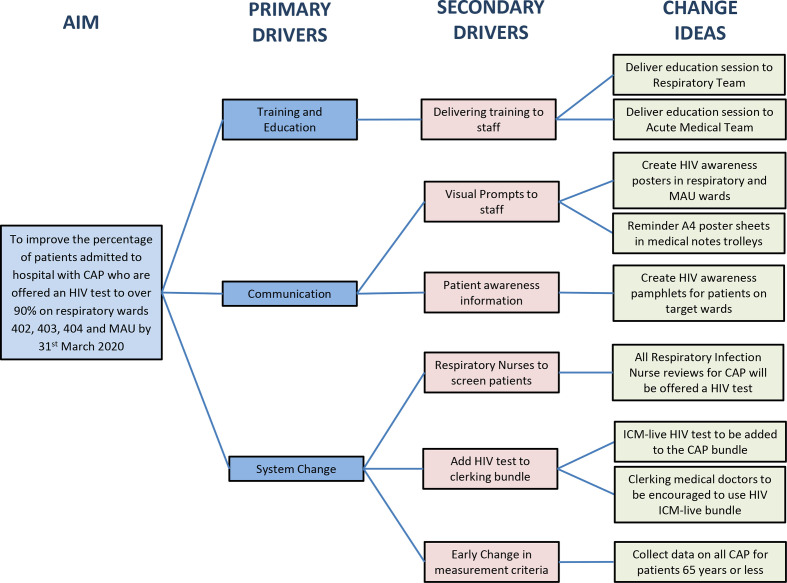
The driver diagram (figure 2) summarises the potential change ideas that were formulated during this project. A number of emergent iterative change ideas were formulated after the project started; these included:
Figure 2.
Driver diagram to improve percentage of HIV testing offered to patients with community-acquired pneumonia (CAP). ICM-live, iSoft Clinical Manager, 2004; MAU, medical admission unit.
Educational session in MAU.
A4 poster sheets in medical notes trolley.
Respiratory infection nurses to offer HIV tests to all patients with CAP 65 years and under.
Change in data collection criteria.
Strategy
We undertook seven PDSA interventions. Regular meetings were set up and facilitated by our mentor throughout the project. These meetings evaluated progress of the QIP and assessed PDSA interventions through review of measurements and planning future interventions.
PDSA cycle 1. Educational session on HIV testing to Respiratory Quality and Safety meeting (20 March 2019)
The offering of HIV tests to patients was discussed with the wider respiratory consultant and nursing group at a bimonthly Respiratory Quality and Safety meeting. The objective of the educational session was to reinforce the importance of offering HIV tests in patients with CAP. The feedback was positive with a willingness from the consultant group to consider offering HIV tests in CAP more often. We did receive feedback that there was a previous audit completed that showed little evidence of positive HIV test results in those over 65 years old in the Derby area and as such it may not be required to focus the project in this age group.
PDSA cycle 2. Amendment of data measurement inclusion criteria from patients aged 65 years and younger only (01 April 2019)
Following the feedback from PDSA cycle 1, we reviewed data with age and national HIV testing. CAP is documented as having a prevalence of HIV >0.1% in adults aged 16–65 years.8 In the UK, the prevalence for new cases of HIV for those aged 65 years and over is very low.15 Our QIP measurement inclusion criteria were therefore revised to include only patients who were aged 65 years or younger. This had not affected the outcome measure as patients aged over 65 years were retrospectively removed from analysis. However, it did provide greater focus for the project and significantly reduced time for collecting data on a demographic that was unlikely to benefit from testing. More importantly, it reduced unnecessary blood tests and the potential anxiety of HIV testing in those aged above 65 years.
PDSA cycle 3. Nurses to offer HIV test for patients with CAP 65 years and under (13 April 2019)
On review of our process map (figure 1), one of the key pathways to offering an HIV test in patients with CAP was by a routine inpatient review by our respiratory infections nurses. It was agreed that as part of the standard operating protocol for the team, they would review the HIV test status for all CAP reviews of patients 65 years and under. If not requested, they would offer the test to the patient and request the test on the ICM-live electronic ordering system, if the patient was willing to have the test done. Our data suggest that this was a key step in improving the number of tests offered. Although our nurses requested the test, they did also offer advice to the medical teams with regards to HIV testing when cases for potential testing were missed.
PDSA cycle 4. Development of patient-facing educational pamphlets regarding the QIP to be available on respiratory wards (11 May 2020)
According to WHO, HIV testing and counselling form the gateway to care, treatment and support for persons in need.16 We designed a patient-facing HIV test paper pamphlet. This was to give more information regarding the importance of HIV testing, and explaining the benefits of early testing to our patients. This was created following feedback from the trainee doctor group. They stated that offering HIV testing was often rushed and the context of this being a standard test for CAP in our patients was somewhat lost. Although there was little perceived impact on our main outcome measure, doctors did report that they found the pamphlets helpful. Patients were also grateful for having the extra information as an adjunct to the verbal advice.
PDSA cycle 5. Junior doctor teaching session to MAU staff on the importance of front-door testing for HIV in patients admitted with HIV (17 May 2019)
A short presentation was given to medical staff during weekly teaching on MAU. The aim of this was to ensure that medical staff that clerked patients during initial admission were aware of the need for HIV testing. Following this intervention there was an improvement in the outcome measure. However, it is difficult to ascertain whether this was sustained over time. The potential weakness in this intervention is that the nature of staff rotas means that a large proportion of staff are not able to attend weekly teaching, particularly on MAU where there is a high turnover of different staff members on duty from day to day.
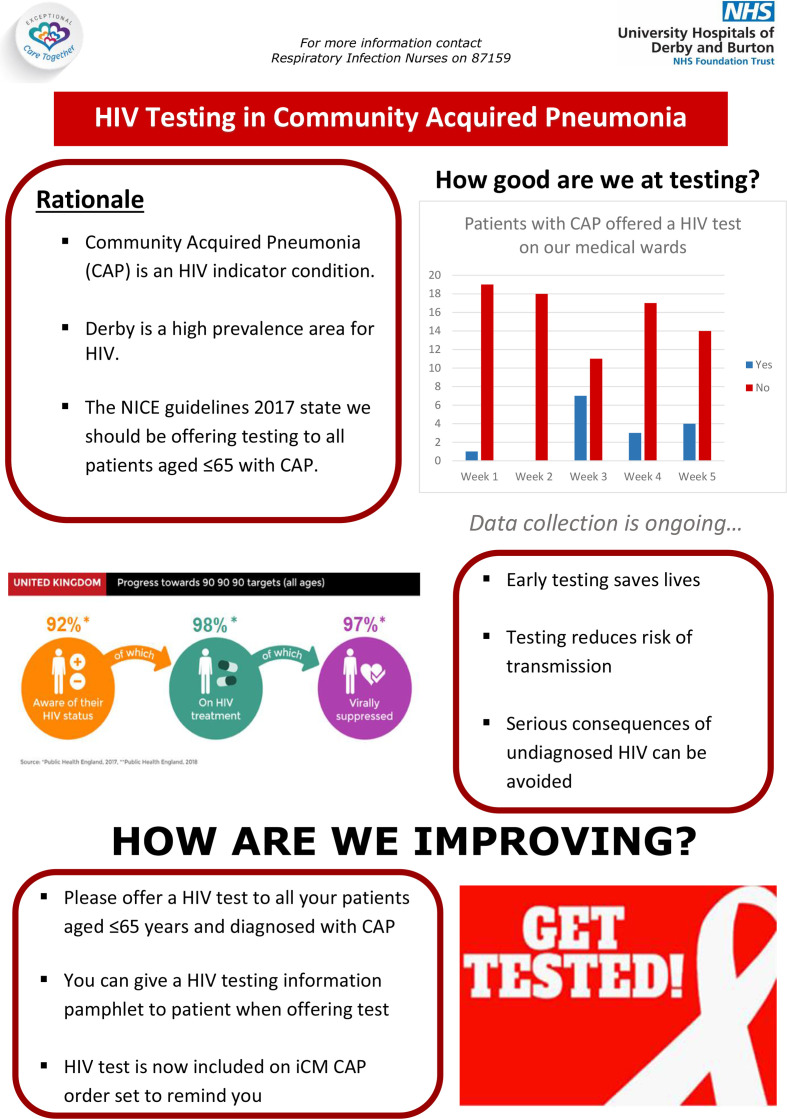
PDSA cycle 6. Development of staff-facing educational posters regarding the QIP to be available on respiratory wards and MAU (21 October 2019)
The aim was to provide a permanent visual prompt to remind staff of the importance of offering HIV testing in patients aged 65 years or younger admitted with CAP. An A1 size communication poster was developed and displayed in the doctor’s offices on MAU and in staff rooms on Respiratory Wards 402, 403 and 404. This intervention had little perceived effect on our outcome measure. The information on the posters was concise with focused messaging to staff (figure 3). However, there is no guarantee that staff were reading them. Furthermore, the doctor’s office on the MAU is often busy and crowded with multiple other competing visual messages. It is unlikely that this would have had a significant impact.
Figure 3.
A1 Communication poster for staff to educate and communicate the importance of offering HIV testing to patients displayed on respiratory wards and medical admission unit (MAU).
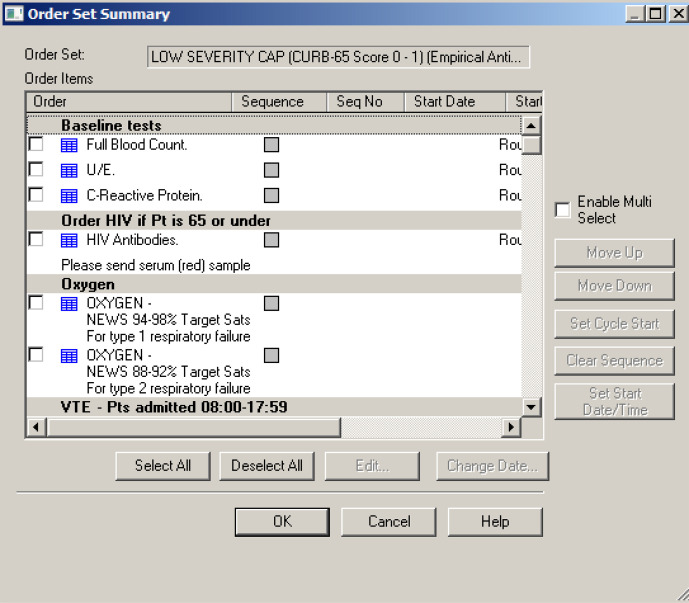
PDSA cycle 7. Adding the HIV antibody test to the CAP order set on the electronic ordering system ICM-live (13 January 2020)
This intervention incorporated the HIV test into a pre-existing order set on ICM-live for relevant investigations and management for patients with CAP (figure 4). The aim of this intervention was to improve the proportion of patients offered an HIV test by increasing awareness of the CAP order set. Email communications were sent to a junior doctor mailing list reminding them of the CAP order set and that the HIV test was now included in it. This information was communicated again before teaching sessions for junior doctors. This intervention had a positive impact on our outcome measure. Unlike many of the other measures, which simply increased awareness, this measure was accompanied by an incentive for medical staff to change their behaviour. The order set makes clerking a patient with CAP easier and therefore staff are incentivised to use it, with a beneficial effect of this being the increased percentage of patients being offered an HIV test with use of the order set.
Figure 4.
A screenshot of the CAP bundle on the ICM-live electronic ordering system, now incorporating an HIV tick box. CAP, community-acquired pneumonia; ICM-live, iSoft Clinical Manager, 2004.
Results
Data were collected and analysed from 177 patients during this project. All patients had a radiologically confirmed diagnosis of CAP. The majority of patients had a low CURB-65 Pneumonia Severity Score at the time of measurement (score 0, n=86; score 1, n=51; score 2, n=22; score 3 or above, n=18).
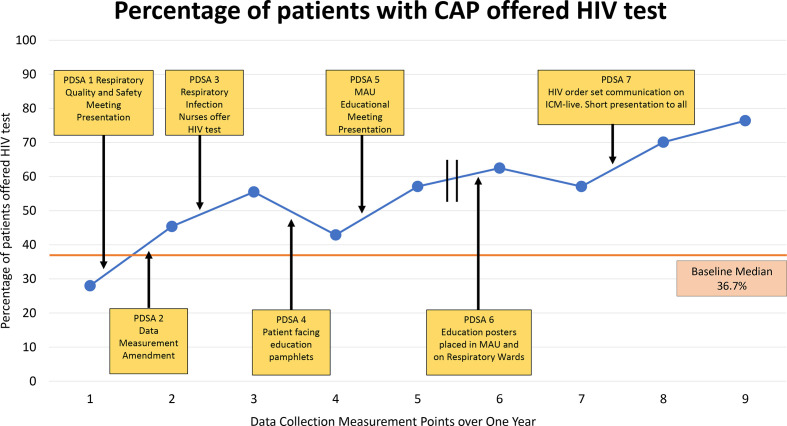
The main outcome measure of the project ‘Did patient have a HIV test offered and have a diagnosis of CAP on admission’ improved from 28%, 7/25 patients, during the first cycle of data collection to 76.4%, 13/25 patients, during the final round of data collection (figure 5).
Figure 5.
Main outcome measure annotated with seven PDSA interventions. Each measurement point represents cumulative data over 4 weeks. The first five data measurement points were collected between 27 February 2019 and 12 July 2019 and the last four data measurement points were collected between 13 November 2019 and 25 February 2020. CAP, community-acquired pneumonia; ICM-live, iSoft Clinical Manager, 2004; MAU, medical admission unit; PDSA, plan-do-study-act.
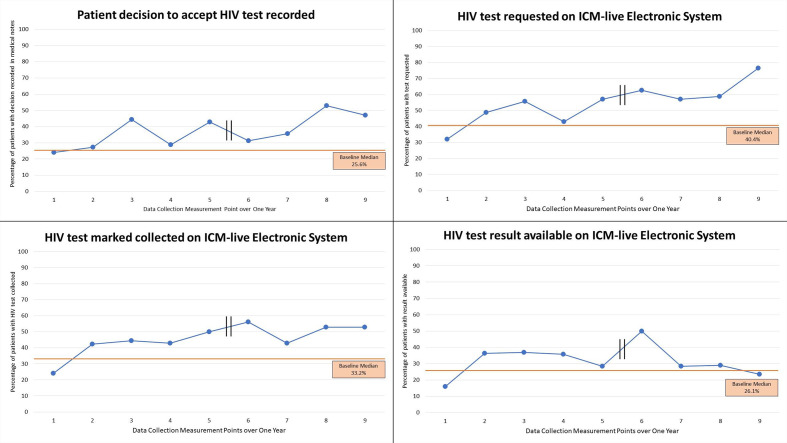
Of the other measures collected; recording the patient decision to accept or decline the HIV test (24.0% (6/25 patients) first cycle, 47.1% (8/17 patients) last cycle), HIV test requested on ICM-live (32% (8/25 patients) first cycle, 76.5% (13/17 patients) last cycle) and HIV test marked as collected on ICM-live (24% (6/25 patients) first cycle, 52.9% (9/17 patients) last cycle) all showed improvement. The measure ‘was the result processed and available on ICM-live?’ did not show improvement (16.0% (4/25 patients) first cycle, 23.5% (4/17 patients) last cycle). Graphs illustrating the data for these measures are available (figure 6).
Figure 6.
Other measures collected for HIV test ordering. Each measurement point represents cumulative data over 4 weeks. The first five data measurement points were collected between 27 February 2019 and 12 July 2019 and the last four data measurement points were collected between 13 November 2019 and 25 February 2020. ICM-live, iSoft Clinical Manager, 2004.
One balancing measure was whether the patients had a previous HIV test done in the last 3 months. This was the case for 8/177 patients. Of these eight patients, two were tested again for HIV. A further balancing measure was ‘was there failure to act on a positive HIV test?’. There were no cases of this. Only one patient had a positive HIV result throughout the duration of the QI programme. The HIV test was offered by the doctor, with the test being taken the same day and the result being explained to the patient the following day once confirmed. The patient had visits to numerous healthcare professionals in the preceding few months with symptoms of breathlessness, sore throat and oral candida. The patient was not offered an HIV test prior to the admission described above.
On review of comorbidities and HIV requesting, we found that of the total of 177 patients, 41 had a diagnosis of asthma or chronic obstructive pulmonary disease (COPD), 120 had no significant respiratory comorbidity, 14 had others and two had no data available. A significant finding was that 76/120 (63%) in the no comorbidity group had an HIV test offered. This compared with only 15/41 (37%) in the asthma/COPD group, χ2 test p<0.001.
Lessons and limitations
Our main aim was to improve the number of patients with a diagnosis of CAP who were offered an HIV test. This outcome showed improvement over the course of the data collection cycles, although it did not reach our predefined target of 90%. The majority of other measures also showed improvement.
We adhered to several key components of a quality improvement programme using the model for improvement methodology. The outcome measure and other measures were selected using SMART (Specific, Measurable, Achievable, Relevant, Time-based) criteria. Data collection was prospective, timely and measurable allowing for rapid data collection and analysis to inform further PDSAs. The development of a process flow chart and driver diagram enabled PDSAs and allowed the team to identify potential targets to improve HIV test offering. Regular meetings with our consultant mentor meant that changes could be made to the project as it progressed with many PDSAs being emergent (ie, formulated after project initiation).
Although our data collection and PDSAs were over two separate time periods, improvement was shown both within these time periods and between them. A change-over of staff in August 2019 resulted in a temporary delay in data collection, a common issue that is regularly encountered in trainee-led projects. The project mentor supported the recruitment of new trainees to continue the programme of work in September 2019. It was however, encouraging to see the improvement was sustained over a longer period of one year and suggests our improvement was upheld. In terms of ongoing sustainability, a number of the implemented changes were designed to continue. The HIV test being added to the ICM-live order set has been a permanent change, and in addition to thinking towards the future its frequency of utilisation, could easily be investigated to ensure ongoing utility. As mentioned earlier, a limitation of junior doctor projects can be the loss of trainees to other specialities when they rotate. Therefore, involving the multidisciplinary team in the project permitted ongoing sustainable change. More permanent staff members, such as the respiratory consultants, respiratory infection nurses and HIV specialist nurses can provide ongoing continuity of practice. Furthermore, HIV testing advice is now part of the induction package for medical staff joining the respiratory department including all junior doctors that rotate into the respiratory department at 4-month intervals.
An unexpected finding in our project was that patients with asthma/COPD were less likely to be offered an HIV test compared with those without a respiratory illness. This may be related to clinicians assuming that their chronic health condition was the cause of their CAP rather than HIV being a potential aetiology.
There was no improvement in the number of HIV test results available on ICM-live. This was explained by data being collected early in their hospital stay. It would have been too early to complete all the steps necessary and to have an HIV result ready on the electronic system. Therefore, this measure may represent the methodology of data collection rather than any true lack of improvement.
We did not collect data in all wards that manage CAP. Many cases are managed in the emergency department, or admitted to medicine for the elderly or other general medical wards. Future improvement projects could look to expand to these areas. Particularly with our finding of many patients having a low CURB-65 Score, so may potentially be discharged directly from the emergency department.3
One area of development for the future would be the consideration of obtaining some qualitative data from patients involved. For example, surveying patients on their acceptability of HIV testing and exploring reasons behind why those that refuse testing do so. One of the members of the team spoke with a patient who tested positive during the QIP about their thoughts on it. This patient gave consent for their anonymised story to be shared for the purpose of medical education. This patient had seen several healthcare professionals both in the community and in secondary care before being offered a test and following diagnosis reflected on why they had not been offered a test earlier. Although this is anecdotal it does highlight the utility of patient partnership in a project such as this.
Conclusion
We showed an improvement in the proportion of patients aged 65 years or under with a diagnosis of CAP for whom an HIV test was offered. The PDSA interventions with the most positive impact on this measure were that of respiratory infection nurses offering HIV tests to patients and the introduction of the CAP HIV ICM-live bundle, both of which were system changes. The lack of improvement in other measurements may reflect the data collection methodology of the study.
Trust-wide scaling would also enable projects which offer HIV tests to patients 65 years and under with CAP (CURB 65 Score 0/1) who are discharged from the emergency department.
Footnotes
Twitter: @kcundy5
Contributors: RM proposed the idea. AC, RM and HK drafted the manuscript. All authors made critical revision and approved the final manuscript. RM, HK, TKL, RB, AS and WC performed the data collection. AS, VP, LH, SA, KC, HK, RM and RB helped with development of specific PDSA interventions. All authors analysed and studied the data. AC acted as mentor for quality improvement programme.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.NICE Hiv testing: encouraging uptake. NICE guidance, 2017. Available: https://www.nice.org.uk/guidance/qs157/resources/hiv-testing-encouraging-uptake-pdf-75545545013701 [Accessed 17 Jul 2019].
- 2.HIV in Europe HIV Indicator Conditions: Guidance for implementing HIV testing in adults in health care settings [Internet]. Available: http://www.eurotest.org/Portals/0/Documents/Guidance.pdf.pdf?ver=2014-01-29-113626-000
- 3.BHIVA UK National Guidelines for HIV Testing [Internet], 2008. Available: www.bhiva.org
- 4.Public Health England Public Health Profiles [Internet], 2017. Available: https://fingertips.phe.org.uk/search/HIV#page/0/gid/1/pat/6/par/E12000004/ati/102/are/E06000015 [Accessed 17 Jul 2019].
- 5.May MT. Better to know: the importance of early HIV diagnosis, 2017. Available: www.thelancet.com/public-health [DOI] [PubMed]
- 6.Clifton DC, Clement ME, Holland TL, et al. Suboptimal HIV testing among patients admitted with pneumonia: a missed opportunity. AIDS Educ Prev 2017;29:377–88. 10.1521/aeap.2017.29.4.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Public Health England guidance Health matters: increasing the update of HIV testing [Internet], 2016. Available: https://www.gov.uk/government/publications/health-matters-increasing-the-uptake-of-hiv-testing/health-matters-increasing-the-uptake-of-hiv-testing
- 8.CHIP Definitions of indicator conditions and recommendations for HIV testing [Internet]. Available: http://www.eurotest.org/Portals/0/Indicatordiseases/CHIP_Guidanceinshort_UK_updatedJUN2016_mlj.pdf
- 9.Croxford S, Kitching A, Desai S, et al. Mortality and causes of death in people diagnosed with HIV in the era of highly active antiretroviral therapy compared with the general population: an analysis of a national observational cohort. Lancet Public Health 2017;2:e35–46. 10.1016/S2468-2667(16)30020-2 [DOI] [PubMed] [Google Scholar]
- 10.World Health Organisation WHO Case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children [Internet], 2006. Available: https://www.who.int/hiv/pub/guidelines/WHOHIVStaging.pdf
- 11.NICE HIV testing: increasing uptake among people who may have undiagnosed HIV [Internet], 2019. Available: https://www.nice.org.uk/terms-and-
- 12.Falguera M, Martín M, Ruiz-González A, et al. Community-Acquired pneumonia as the initial manifestation of serious underlying diseases. Am J Med 2005;118:378–83. 10.1016/j.amjmed.2005.01.011 [DOI] [PubMed] [Google Scholar]
- 13.Feldman C, Anderson R. Hiv-Associated bacterial pneumonia. Clin Chest Med 2013;34:205–16. 10.1016/j.ccm.2013.01.006 [DOI] [PubMed] [Google Scholar]
- 14.Institute of Health Improvement Quality improvement essential toolkit, 2017. Available: http://www.ihi.org/resources/Pages/Tools/Quality-Improvement-Essentials-Toolkit.aspx
- 15.Public Health England National HIV surveillance data tables [Internet]. Public Health England, 2018. Available: https://www.gov.uk/government/statistics/hiv-annual-data-tables
- 16.Who | HIV testing and counselling: the gateway to treatment, care and support. who 2010.