Abstract
Introduction
Dexamethasone (DEX) is administered for multiple days to prevent chemotherapy-induced nausea and vomiting for patients receiving highly emetogenic chemotherapy (HEC); however, its notorious side effects have been widely reported. Although our multicentre randomised double-blind comparative study verified non-inferiority of sparing DEX after day 2 of chemotherapy when combined with neurokinin-1 receptor antagonist (NK1-RA) and palonosetron (Palo) for patients receiving HEC regimen, DEX sparing was not non-inferior in patients receiving cisplatin (CDDP)-based HEC regimens in subgroup analysis. Recently, the efficacy of the addition of olanzapine (OLZ) to standard triple antiemetic therapy on HEC has been demonstrated by several phase III trials. This study aims to confirm non-inferiority of DEX sparing when it is combined with NK-1RA, Palo and OLZ in patients receiving CDDP-based HEC regimens.
Methods and analysis
This is a randomised, double-blind, phase III trial. Patients who are scheduled to receive CDDP ≥50 mg/m2 as initial chemotherapy are eligible. Patients are randomly assigned to receive either DEX on days 1–4 or DEX on day 1 combined with NK1-RA, Palo and OLZ (5 mg). The primary endpoint is complete response (CR) rate, defined as no emesis and no rescue medications during the delayed phase (24–120 hours post-CDDP administration). The non-inferiority margin is set at −15.0%. We assume that CR rates would be 75% in both arms. Two hundred and sixty-two patients are required for at least 80% power to confirm non-inferiority at a one-sided significance level of 2.5%. After considering the possibility of attrition, we set our final required sample size of 280.
Ethics and dissemination
The institutional review board approved the study protocol at each of the participating centres. The trial result will be presented at international conferences and published in peer-reviewed journals.
Trial registration number
UMIN000032269.
Keywords: chemotherapy, adult palliative care, clinical trials, adverse events
Strengths and limitations of this study.
This is the first trial to evaluate whether adding olanzapine to neurokinin-1 receptor antagonist, palonosetron and dexamethasone (DEX) can spare DEX administration on day 2–4 for patients receiving cisplatin-based regimens.
This study is a multicenter, placebo-controlled, double-blinded, randomised phase III study.
A limitation of this study is that it was conducted solely within the Japanese population.
Introduction
Chemotherapy-induced nausea and vomiting (CINV) is one of the most frequent adverse reactions associated with chemotherapy and considerably reduces patient quality of life (QOL). CINV has been traditionally assessed in overall (0–120 hour postchemotherapy), acute (0–24 hour postchemotherapy) or delayed (24–120 hour postchemotherapy) phases.1 Intravenously administered cytotoxic agents are categorised into four emetic risk groups (high, moderate, low and minimal).2 Highly emetogenic chemotherapy (HEC) including cisplatin (CDDP)-based regimen and anthracycline plus cyclophosphamide (AC) regimen can lead to a >90% incidence of emesis in patients without an adequate antiemetic prophylaxis.3 When patients were administered prophylactic antiemetics prior to HEC treatment, the incidence of emesis (or requiring additional antiemetics) was found to be 35% according to a recent study.4
Dexamethasone (DEX), 5-hydroxytryptamine type 3 receptor antagonists (5-HT3-RA) and neurokinin-1 receptor antagonists (NK1-RA) have been developed to inhibit CINV from HEC.5 6 DEX is typically administered for multiple days from the start of chemotherapy to care for delayed CINV,7; whereas frequent administration of corticosteroids has been associated with many adverse effects such as insomnia, hyperglycaemia and reduced bone mineral density.8–10 Therefore, corticosteroid-minimising (DEX-sparing) strategies, which administer DEX in the acute phase (day 1) and omit DEX in the delayed phase (day 2 and later), have been evaluated.11 Our multicentre randomised double-blind comparative study (DEX-1 study) showed the complete response (CR; no emesis, no use of rescue medication) rate in the overall phase of DEX on day 1 provided a non-inferior antiemetic efficacy to a treatment of DEX on days 1–3 when combined with NK1-RA and palonosetron (Palo) for patients receiving HEC including AC and CDDP-based regimen. (44.0% vs 46.9 %, p=0.007).12 In a subgroup analysis of patients receiving CDDP-based regimen, CR rates of acute phase demonstrated a non-inferiority of DEX on day 1 to days 1–3 (95.6% vs 95.6 %, p=0.007); whereas CR rates of the overall and delayed phase DEX on day 1 were lower than those of DEX on days 1–3 (57.8% vs 66.7%, p=0.272; 57.8% vs 68.9%, p=0.349, respectively). However, the DEX-1 study was underpowered to evaluate whether multiple days of DEX can be spared for CDDP-based regimens because patients who received the CDDP-based regimen represented only 23% of the total sample population.13 Therefore, the plausibility of DEX sparing in CDDP remains inconclusive. Some guidelines still recommend multiple-days DEX in combination with 5-HT3-RA and NK1-RA for CDDP-based regimens.3 14–16
Olanzapine (OLZ) is classified as a multiacting receptor-targeted antipsychotic and blocks dopamine receptors (D1, D2, D4), 5-HT receptors (5-HT2A, 5-HT2C, 5-HT3), α1 adrenergic receptors, histamine receptors and multiple muscarine receptors,17 which might affect CINV. In the randomised, double-blind, phase III trial involving patients receiving HEC regimens, it was more effective to combine OLZ 10 mg than placebo with NK1-RA, 5-HT3-RA and DEX for the prevention of nausea and vomiting in acute and delayed phases4; however, OLZ (10 mg) had excessive sedation. Therefore, it is difficult to use OLZ 10 mg for all patients in practice setting. Recently, a randomised, double-blind, placebo-controlled phase III trial evaluating the significance of adding 5 mg OLZ to NK1-RA, Palo and DEX in CDDP-based regimens(J-FORCE study) showed that significantly more patients receiving OLZ achieved CR from delayed CINV compared with those who received placebo (79% vs 66%, p<0.001); moreover, no differences were found between two groups in the incidence of sedation.18 The addition of OLZ in combination with NK1-RA, Palo and DEX has greater benefit and becomes a standard antiemetic therapy in patients receiving CDDP-based regimens.
Treatment with OLZ was associated with metabolic effects, including elevated glucose concentrations manifesting as insulin resistance.19 A phase III study showed that grade 3 hyperglycaemia was observed more frequently in the OLZ versus placebo group.4 Therefore, there is a concern that the combination of OLZ and multiple-day DEX may worsen glucose intolerance. In another study, Navari et al20 also demonstrated that OLZ, combined with a single dose of DEX and Palo, was very effective at controlling acute and delayed phase CINV in patients receiving HEC; moreover, this regimen was not associated with significant hyperglycaemia.
Based on these results, we speculate that the antiemetic regimen of OLZ 5 mg, NK1-RA, Palo and a single dose of DEX could be effective and safe for delayed phase CINV in patients receiving CDDP-based regimen. We planned this randomised, double-blind, phase III trial to evaluate the non-inferiority of DEX on day 1 compared with DEX on days 1–4 when combined with NK1-RA, Palo and OLZ in patients receiving CDDP-based regimens.
Methods and analysis
Study design
The Standard Protocol Items for Randomized Trials statement and checklist were followed in preparing the protocol. This multicentre, placebo-controlled, double-blinded, randomised, non-inferiority, phase III study aims to confirm the non-inferiority of DEX on day 1 compared with DEX on days 1–4 combined with NK1-RA, Palo and OLZ 5 mg to prevent CINV in patients with solid malignant tumour receiving CDDP-based regimens.
Study setting and participants
Recruiting will be performed in 10 sites across Japan. The inclusion and exclusion criteria are summarised in box 1.
Box 1. Inclusion and exclusion criteria.
Inclusion criteria
Patients with malignant tumour, excluding those with haematologic malignancies or those receiving firstline treatment with cisplatin (CDDP) >50 mg/m2 (previous use of moderate or low emetic chemotherapy is permitted).
Age: 20–74 years at the time of enrolment.
Absence of nausea and vomiting within 24 hours prior to registration.
Eastern Cooperative Oncology Group performance status of 0–1.
-
Meeting the following standard values of general clinical tests within 2 weeks prior to enrolment:
alanine aminotransferase <100 IU/L.
Aspartate aminotransferase <100 IU/L.
Total bilirubin <2.0 mg/dL.
Serum creatinine <1.5 mg/dL.
Patients with an expected prognosis of 3 months or more.
Patients who provided written informed consent.
Exclusion criteria
Patients undergoing systemic glucocorticoid therapy.
Patients using antiemetics other than the trial drug.
Patients receiving moderately emetogenic chemotherapy within 6 days before and after CDDP administration.
Patients who cannot be hospitalised until after 120 hours of starting CDDP administration as the study requires daily use of an electronic patient-reported outcome system.
Patients receiving radiation therapy for the abdomen or pelvis within 6 days prior to registration until 6 days after CDDP administration.
Patients with diabetes mellitus receiving treatment with insulin and/ or oral hypoglycemic agents or patients with haemoglobin A1c (National Glycohemoglobin Standardization Program) >6.5% (>6.1% in the event of JDS).
Patients with symptomatic brain metastasis, convulsive disorder requiring treatment with anticonvulsants and mental illness or psychiatric symptoms that impede activities of daily life.
Patients who are incapable of taking oral agents.
Patients with a history of allergy to study drugs or similar compounds.
Breastfeeding women, pregnant women or patients not willing to use contraception.
Patients deemed ineligible for the study by the investigator (eg, patients who are unable to maintain medication adherence or who may experience difficulty using electronic devices).
The main inclusion criterion is patients who are eligible in the study are 20–74 years old with malignant tumour, excluding haematological malignancies, receiving firstline treatment with CDDP ≥50 mg/m2 (previous use of moderately or low emetogenic chemotherapy is permitted). The main exclusion criteria are as follows: (1) presence of systemic glucocorticoid therapy, (2) patients using antiemetics other than the trial drug, (3) patients receiving moderately emetogenic chemotherapy within 6 days before and after CDDP administration (minimally to low emetogenic agents are allowed), (4) patients receiving radiation therapy to abdomen or pelvis within 6 days prior to enrolment until 6 days after CDDP, (5) patients with symptomatic brain metastasis, diabetes mellitus and convulsive disorder and (6) patients who are incapable of taking oral agents.
Recruitment, randomisation, masking and follow-up
Recruitment
Eligible patients satisfying the screening inclusion and exclusion criteria will be invited to participate in the study by site investigators.
Randomisation
Physicians will introduce the trial to patients. On enrollment and after providing informed consent, eligible patients will be randomly assigned to receive either DEX on day 1–4 or DEX on day 1 with placebo on days 2–4 as part of prophylactic antiemetic therapy. Randomisation is centrally performed by random allocation modules of electronic data captures (EDC) using the minimisation method with balancing prognostic factors for age (<60 vs≥60 years), sex, CDDP dose level (≥70 mg/m2 vs <70 mg/m2) and institution.
Masking
Patients and clinicians responsible for treatment will be blinded to administration of DEX or placebo. Only an unblinded pharmacist who prepares the study drug, but is not involved in patient care, will know the assignment and outcome. All study drugs will be prepared by this pharmacist. As a rule, no data will be disclosed until fixed. However, during the trial period, when it is considered necessary to know the details of the trial drug to ensure participant safety, such as for serious adverse events, the study representative and study secretariat will make an inquiry to and discuss the need for disclosure with the Efficacy and Safety Evaluation Committee. When disclosure is deemed necessary as a result of this consultation, the details will be communicated to the study secretariat, and the details of the trial drug will be disclosed.
Data management, central monitoring and auditing
The data centre is located in the Department of Clinical Trial Data Management, Graduate School of Medicine, Tokyo University, Tokyo, Japan. Enrolment, randomisation, data collection and monitoring will be performed using EDC system Viedoc 4 and Viedoc me (Viedoc Technologies). Data entry to the electronic case report form is performed by investigators using EDC at each site. Patient-reported outcome (PRO) data are collected electronically from patients through an electronic tablet device. No personally identifiable information is entered into the EDC, and the data centre does not collect personal information. The central monitoring will be conducted by the data centre, and monthly and semi-annually monitoring reports will be disseminated to investigators to inform about the trial progress and discuss data quality-related issues. The protocol review committee and independent Data Monitoring Committee will assess the protocol amendments, serious adverse events reports and monitoring reports and provide any necessary recommendation to investigators. Auditing will be conducted as necessary in this study.
Harms
Investigators must record all adverse events in the medical records and web systems. The Common Terminology Criteria for Adverse Events (CTCAE, V.4.0) will be used to grade each adverse event. In conjunction with the CTCAE to grade adverse events, the PRO-CTCAE will be also administered to patients for their completion to complement information about subjective symptoms. All adverse events are to be followed up continually during the course of treatment. All severe adverse events must be reported to the institutional review board (IRB) and reported to investigators in all sites and discussed through a mail. Patients who are enrolled into the study will be treated by the healthcare services that are provided by their health insurance.
Treatment
All patients receive Palo (0.75 mg intravenous infusion on day 1 at 30 min before the start of chemotherapy), NK1-RA (aprepitant 125 mg oral administration on day 1 and 80 mg on days 2 and 3 or fosaprepitant 150 mg intravenous infusion on day 1 at 1 hour before the start of chemotherapy) and OLZ (5 mg oral administration on days 1–4 after dinner). DEX is administered as follows: patients in both arms receive DEX 9.9 mg intravenous infusion on day 1; patients receive DEX 6.6 mg or placebo intravenous infusion on days 2–4. When using fosaprepitant, the dose level is increased on days 3 and 4 due to interaction with DEX up to day 2, therefore, patients receive an intravenous DEX 13.2 mg or placebo on days 3 and 4. Patients were allowed to take rescue medication throughout the study period for nausea or vomiting, if necessary. The choice of recommended rescue is determined by each investigator from among prochlorperazine, metoclopramide, domperidone, chlorpheniramine, alprazolam, lorazepam and haloperidol.
Study endpoints
The primary endpoint is CR rate (no emesis and no rescue medications) during the delayed phase (24–120 hours post-CDDP administration). Secondary endpoints are as follows: (1) CR rate during the acute phase (24 hours post-CDDP administration) and the overall phase (120 hours post-CDDP administration), (2) complete control (no emesis, no rescue use and no significant nausea) rate, (3) total control (no emesis, no rescue use and no nausea) rate (4) no emesis rate and no nausea rate in the overall, acute and delayed phase, (5) time to treatment failure (ie, time to first emesis or using rescue, whichever occurred first) and (6) severity of nausea during the overall phase. Adverse events are associated with antiemetic therapy (CTCAE V.4.0 Japanese Clinical Oncology Group (JCOG) version and the PRO-CTCAE V.1.0.).
Outcome assessments
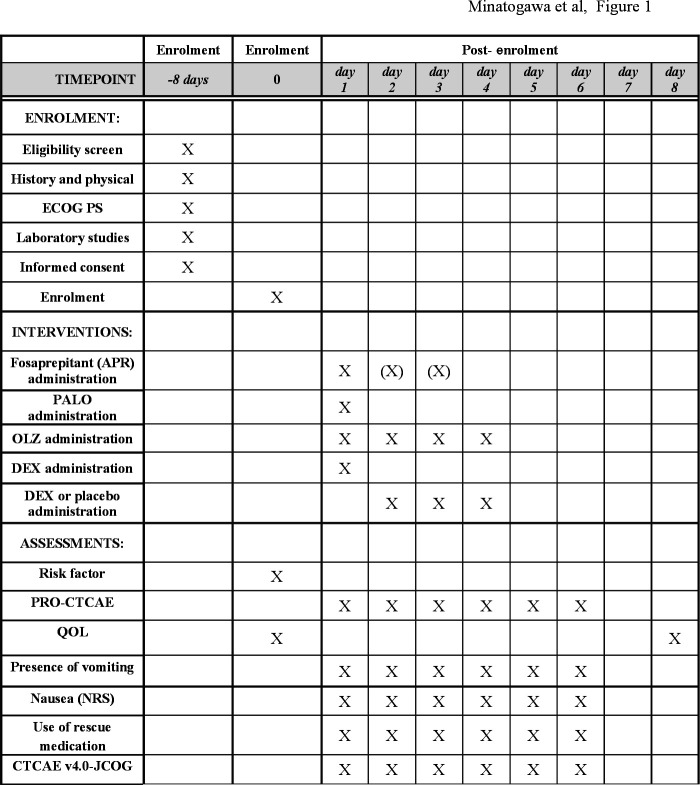
Figure 1 provides details of the schedule of enrolment, interventions and assessments.
Figure 1.
The schedule of enrolment, interventions and assessments. APR, aprepitant; CTCAE, common terminology criteria for adverse events; DEX, dexamethasone; ECOG, Eastern Cooperative Oncology Group; NRS, numerical rating scale; OLZ, olanzapine; Palo, palonosetron; QOL, quality of life.
Presence of emesis and severity of nausea will be assessed by patients using a 2-point categorical scale and 11-point numerical rating scale (NRS), respectively. Significant nausea is defined as 3 points or greater on the NRS. The use of rescue medications will be assessed by pharmacists.
Adverse events will be evaluated according to the CTCAE V.4.0 (JCOG) version, and the PRO-CTCAE V.1.0. The Japanese version of PRO-CTCAE is linguistically and psychometrically validated.21 22 QOL will be assessed by the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30) V.3 that is also validated in the Japanese version.23
Patients are asked to assess QOL before CDDP administration and on day 8, with emesis and nausea assessed the PRO-CTCAE every 24 hours until 120 hours after CDDP administration. The data from the PRO-CTCAE are assessed electronically using a tablet device in the hospital setting, except for QOL on day 8, which is assessed on paper-based questionnaire at home. The PRO-CTCAE data will be not reviewed by the site investigators during the protocol treatment.
Statistical analysis
Sample size calculation is based on an analysis of the primary endpoint. In previous studies with OLZ added to conventional antiemetic treatment for CDDP,20 24 25 the delayed phase CR rate ranged from 75% to 85%, therefore we expect that CR rate in the delayed phase would be 75% in both arms. The non-inferiority margin is set at −15.0%. Two hundred sixty-two patients are required for at least 80% power to confirm non-inferiority at a one-sided significance level of 2.5%. After considering the possibility of attrition, we set our final required sample size of 280. Point estimates and CIs for the CR rate will be calculated and will be compared between groups by using the Mantel-Haenszel test with adjustment for allocation factors. Interim analysis is not planned. We will use a full analysis set. It consists of the registered participant population who received at least a part of the protocol treatment; however, participants who were deemed as ineligible for the study after registration and those who were not administered CDDP-based regimens will be excluded from the analysis set. For the primary analysis, we will impute non-CR for missing primary endpoints.
Patient and public involvement
Patients and/or public were not involved in the design of this study.
Ethics and dissemination
All patients will be required to provide written informed consent (see online supplemental file 1). The study will be performed in accordance with the Declaration of Helsinki and Ethical Guidelines for Medical and Health Research Involving Human Subjects published by Japan’s Ministry of Education, Science and Technology and the Ministry of Health, Labour, and Welfare and the modified Act on the Protection of Personal Information. This protocol was approved by the Ethics Committee (approval ID 4035) of St. Marianna University School of Medicine on July 27, 2018. The protocol was approved by the IRB at each study site (Showa University Northern Yokohama Hospital, Yokohama Rosai Hospital, Nippon Medical School Musashi Kosugi Hospital, Aichi Cancer Center, Gifu University, Kitasato University, and Shizuoka Cancer Center). This trial has been registered at the University Hospital Medical Information Network (UMIN) Clinical Trials Registry. Modifications in the study protocol will be communicated to the IRB at each study site as well as to the protocol review committee. Each Ethics Committee or IRB will revise informed consent materials given to participants and adapt the informed consent according to their own institution’s guidelines. The main result will be presented at an international conference and published in an English journal. Authorship will be ascribed in accordance with the International Committee of Medical Journal Editors guidance.
bmjopen-2020-041737supp001.pdf (55.2KB, pdf)
Access to data
Only clinical data managers at the central data centre have access to collected data through the EDC system during the study. Site investigators have access to case data within their institutions. After study closure, final data set and related materials will be archived in UMIN Individual Case Data Repository.
Participating institutions
St. Marianna University School of Medicine Hospital, St. Marianna University Kawasakishi Municipal Tama Hospital, St. Marianna University Yokohama City Seibu Hospital, Showa University Northern Yokohama Hospital, Yokohama Rosai Hospital, Nippon Medical School Musashi Kosugi Hospital, Aichi Cancer Center Hospital, Gifu University Hospital, Kitasato University Hospital, Shizuoka Cancer Center.
Trial status
The trial started in October 2018 and 183 subjects were randomised by May 2020. The recruitment is scheduled to be completed in March 2021.
Confidentiality
Data will be retained in accordance with the Japanese ethical guidelines for clinical research. Participants will be allocated a unique identification (ID) number at entry. The master list linking participant personal information and ID number will be managed at each institution. Data will be analysed by ID number only. Records will be retained for 5 years after study completion and then will be destroyed at each institution.
Supplementary Material
Acknowledgments
We are grateful for support from the Japan Agency for Medical Research and Development, Grant-in-Aid for Scientific Research. The authors thank in advance all the patients, investigators and institutions involved in this study.
Footnotes
Contributors: HM, NI and TEN contributed to the trial conception and are the principal investigators. HM, NI, TK, TM, TY and TEN participated in the design of the study. TY played a primary role in designing statistical analysis. TK and TM played a primary role in designing the data management approach. HM, NI, KS, KH, HI, YO, YI, HA, HM, NH, MS, CK, SN, HI, AT and TT have carried out recruitment and collected the data. Data analysis and interpretation will be conducted by HM, NI, TK, TM, TY and TEN. HM and NI wrote the first draft of the manuscript. All authors have read, approved the paper and meet the criteria for authorship as established by the International Committee of Medical Journals Editors.
Funding: This study is supported by Japan Agency for Medical Research and Development (AMED) grant number 19ck0106501h0001. AMED had and will have no involvement in the design and conduct of the study, collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript.
Competing interests: NI has received honoraria from Takeda Pharma CO., Ltd, Eli Lilly, Japan, Ono Pharma CO., Ltd, and Daiichi Sankyo Company. Author HA has received grant from Taiho, Chugai and Nippon Kayaku; personal fees from Novartis, Sanofi, Ono, Kyowa Kirin and Takeda. TY has received grant and personal fees from Ono Pharmaceutical Co., Ltd. TEN has received grant and personal fee from Taiho Pharmaceutical Co., Chugai Pharmaceutical Co., Takeda Pharmaceutical Co., Sanofi K.K., Daiichi Sankyo Co., Eli Lilly Japan K.K., Nippon Kayaku Co., Ono Pharmaceutical Co. and MSD K.K.; personal fees from Mochida Pharmaceutical, Celltrion Healthcare Japan, Merck Serono Co., Sawai Pharmaceutical Co., Bayer Yakuhin, Bristol-Myers Squibb, Teijin Pharma, Pfizer Japan Inc., Novartis Japan, Yakult Honsha Co. and Nipro Co; grant from Astellas Pharma Inc., Sumitomo Dainippon Pharma Co., Eisai Co and Solasia Pharma K.K. The other authors have declared no conflicts of interest.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Navari RM, Aapro M. Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J Med 2016;374:1356–67. 10.1056/NEJMra1515442 [DOI] [PubMed] [Google Scholar]
- 2.Hesketh PJ, Kris MG, Grunberg SM, et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol 1997;15:103–9. 10.1200/JCO.1997.15.1.103 [DOI] [PubMed] [Google Scholar]
- 3.Aogi K, Takeuchi H, Saeki T, et al. Optimizing antiemetic treatment for chemotherapy-induced nausea and vomiting in Japan: Update summary of the 2015 Japan Society of Clinical Oncology Clinical Practice Guidelines for Antiemesis. Int J Clin Oncol 2020. 10.1007/s10147-020-01818-3. [Epub ahead of print: 08 Nov 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med 2016;375:134–42. 10.1056/NEJMoa1515725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hesketh PJ, Grunberg SM, Gralla RJ, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin-the Aprepitant Protocol 052 Study Group. J Clin Oncol 2003;21:4112–9. 10.1200/JCO.2003.01.095 [DOI] [PubMed] [Google Scholar]
- 6.Gralla RJ, de Wit R, Herrstedt J, et al. Antiemetic efficacy of the neurokinin-1 antagonist, aprepitant, plus a 5HT3 antagonist and a corticosteroid in patients receiving anthracyclines or cyclophosphamide in addition to high-dose cisplatin: analysis of combined data from two phase III randomized clinical trials. Cancer 2005;104:864–8. 10.1002/cncr.21222 [DOI] [PubMed] [Google Scholar]
- 7.Grunberg SM. Antiemetic activity of corticosteroids in patients receiving cancer chemotherapy: dosing, efficacy, and tolerability analysis. Ann Oncol 2007;18:233–40. 10.1093/annonc/mdl347 [DOI] [PubMed] [Google Scholar]
- 8.Vardy J, Chiew KS, Galica J, et al. Side effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapy. Br J Cancer 2006;94:1011–5. 10.1038/sj.bjc.6603048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeong Y, Han HS, Lee HD, et al. A pilot study evaluating steroid-induced diabetes after antiemetic dexamethasone therapy in chemotherapy-treated cancer patients. Cancer Res Treat 2016;48:1429–37. 10.4143/crt.2015.464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura M, Ishiguro A, Muranaka T, et al. A prospective observational study on effect of short-term periodic steroid premedication on bone metabolism in gastrointestinal cancer (ESPRESSO-01). Oncologist 2017;22:592–600. 10.1634/theoncologist.2016-0308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Celio L, Bonizzoni E, Zattarin E, et al. Impact of dexamethasone-sparing regimens on delayed nausea caused by moderately or highly emetogenic chemotherapy: a meta-analysis of randomised evidence. BMC Cancer 2019;19:1268. 10.1186/s12885-019-6454-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito Y, Tsuda T, Minatogawa H, et al. Placebo-controlled, double-blinded phase III study comparing dexamethasone on day 1 with dexamethasone on days 1 to 3 with combined neurokinin-1 receptor antagonist and palonosetron in high-emetogenic chemotherapy. J Clin Oncol 2018;36:1000–6. 10.1200/JCO.2017.74.4375 [DOI] [PubMed] [Google Scholar]
- 13.Celio L, Bonizzoni E, Aapro M. Is the dexamethasone-sparing strategy ready for cisplatin? too early for an answer. J Clin Oncol 2018;36:2741–2. 10.1200/JCO.2018.78.8109 [DOI] [PubMed] [Google Scholar]
- 14.MASCC/ESMO antiemetic guideline 2016 with updates in 2019, 2019. Available: https://www.mascc.org/assets/Guidelines-Tools/mascc_antiemetic_guidelines_english_v.1.5SEPT29.2019.pdf
- 15.NCCN clinical practice guidelines in oncology: antiemesis Version2. 2020. Available: https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf
- 16.Hesketh PJ, Bohlke K, Kris MG, et al. Antiemetics: American Society of clinical oncology clinical practice guideline update summary. J Oncol Pract 2017;13:825–30. 10.1200/JOP.2017.026351 [DOI] [PubMed] [Google Scholar]
- 17.Bymaster FP, Calligaro DO, Falcone JF, et al. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology 1996;14:87–96. 10.1016/0893-133X(94)00129-N [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto H, Abe M, Tokuyama O, et al. Olanzapine 5 Mg plus standard antiemetic therapy for the prevention of chemotherapy-induced nausea and vomiting (J-FORCE): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2020;21:242–9. 10.1016/S1470-2045(19)30678-3 [DOI] [PubMed] [Google Scholar]
- 19.Watts LM, Manchem VP, Leedom TA, et al. Reduction of hepatic and adipose tissue glucocorticoid receptor expression with antisense oligonucleotides improves hyperglycemia and hyperlipidemia in diabetic rodents without causing systemic glucocorticoid antagonism. Diabetes 2005;54:1846–53. 10.2337/diabetes.54.6.1846 [DOI] [PubMed] [Google Scholar]
- 20.Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol 2011;9:188–95. 10.1016/j.suponc.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 21.Miyaji T, Iioka Y, Kuroda Y, et al. Japanese translation and linguistic validation of the US National cancer Institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Patient Rep Outcomes 2017;1:8. 10.1186/s41687-017-0012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawaguchi T, Azuma K, Sano M, et al. The Japanese version of the National cancer Institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE): psychometric validation and discordance between clinician and patient assessments of adverse events. J Patient Rep Outcomes 2017;2:2. 10.1186/s41687-017-0022-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi K, Takeda F, Teramukai S, et al. A cross-validation of the European organization for research and treatment of cancer QLQ-C30 (EORTC QLQ-C30) for Japanese with lung cancer. Eur J Cancer 1998;34:810–5. 10.1016/S0959-8049(97)00395-X [DOI] [PubMed] [Google Scholar]
- 24.Yanai T, Iwasa S, Hashimoto H, et al. A double-blind randomized phase II dose-finding study of olanzapine 10 mg or 5 mg for the prophylaxis of emesis induced by highly emetogenic cisplatin-based chemotherapy. Int J Clin Oncol 2018;23:382–8. 10.1007/s10147-017-1200-4 [DOI] [PubMed] [Google Scholar]
- 25.Nakashima K, Murakami H, Yokoyama K, et al. A phase II study of palonosetron, aprepitant, dexamethasone and olanzapine for the prevention of cisplatin-based chemotherapy-induced nausea and vomiting in patients with thoracic malignancy. Jpn J Clin Oncol 2017;47:840–3. 10.1093/jjco/hyx084 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-041737supp001.pdf (55.2KB, pdf)