Abstract
Neocortical sleep spindles have been shown to occur more frequently following a memory task, suggesting that a method to increase spindle activity could improve memory processing. Stimulation of the neocortex can elicit a slow oscillation (SO) and a spindle, but the feasibility of this method to boost SO and spindles over time has not been tested. In rats with implanted neocortical electrodes, stimulation during slow wave sleep significantly increased SO and spindle rates compared to control rest periods before and after the stimulation session. Coordination between hippocampal sharp-wave ripples and spindles also increased. These effects were reproducible across five consecutive days of testing, demonstrating the viability of this method to increase SO and spindles.
Neocortical sleep spindles are prominent oscillations during slow-wave sleep (SWS) (Kandel and Buzsáki 1997; Steriade and Deschenes 1984; Steriade et al. 1993) that play a role in consolidation of both declarative (Gais et al. 2002; Schabus et al. 2004; Schmidt et al. 2006) and nondeclarative memories (Nishida and Walker 2007; Peters et al. 2008; Barakat et al. 2011; Johnson et al. 2012). Spindles are preferred times of memory reactivation in the cortex during sleep, suggesting that reactivation during spindles contributes to memory performance (Peyrache et al. 2009; Ramanathan et al. 2015; Eckert et al. 2020).
Based on this evidence, a method to induce spindle oscillations may prove beneficial to memory consolidation. Recent optogenetic experiments have shown that it is possible to induce cortical spindles by activating the thalamic reticular nucleus (Halassa et al. 2011; Kim et al. 2012), and that these induced spindles improve memory performance (Latchoumane et al. 2017). Given the large gap between optogenetic manipulation and clinical practice, a more practical technique for enhancing spindles would be desirable. Electrical stimulation of the neocortex with an implanted electrode can evoke a slow oscillation (SO) and a spindle, but the reliability and longevity of this effect are unknown (Steriade and Deschenes 1984; Contreras and Steriade 1995; Vyazovskiy et al. 2009). Here we show that electrical pulse stimulation reliably evokes spindles for the duration of a 1-h stimulation session, resulting in a significant increase in SO and spindle density.
Four adult male Fisher–Brown Norway rats had recording electrodes implanted bilaterally in the motor cortex, hippocampus, and neck. The stimulating electrode was implanted in the deep motor cortex of one hemisphere adjacent to one of the cortical recording electrodes (see Supplemental Fig. S1 for details, as well as the Supplemental Material for greater detail of all methods). After recovering from surgery, the animals were habituated to a quiet, dimly lit room and recording box for 3 d. All procedures and recordings were performed during the animal's light cycle. The vivarium maintained a 12-h light cycle (7 a.m. on) and the animals were tested in the same order every day such that recordings on different days occurred at approximately the same time (±1 h). On experiment days, rats were taken to the recording room and they rested in a small cage after being connected to the recording system. The recording consisted of three consecutive 1-h sessions: a baseline recording with no stimulation, a recording with repeated single pulse electrical stimulation, and a final recording session with no stimulation. The procedure was repeated on five consecutive days to test the reliability of the method.
Recording was done on a Cheetah system (Neuralynx) and all signals were sampled at 2 kHz. Stimulation was delivered by a constant current stimulus isolation unit that was controlled by an Arduino microcontroller. Delivery of stimulation pulses was targeted to SWS using a real-time sleep state detector written in MATLAB. A 3-sec buffer of recording was used to calculate the ratio of δ (1–4 Hz) to θ (5–10 Hz) in the hippocampal LFP as well as the amount of EMG activity. SWS detection occurred when there was a high δ/θ ratio and low EMG activity. For each animal, the threshold value for the SWS detection was determined during a habituation session prior to the start of the stimulation experiment. Once SWS was detected, a single biphasic pulse (150 µS per phase, 500 µA) was delivered every 3 sec for as long as the animal remained in SWS (Fig. 1A). The real-time detection corresponded well with offline sleep structure analysis (Supplemental Fig S3).
Figure 1.
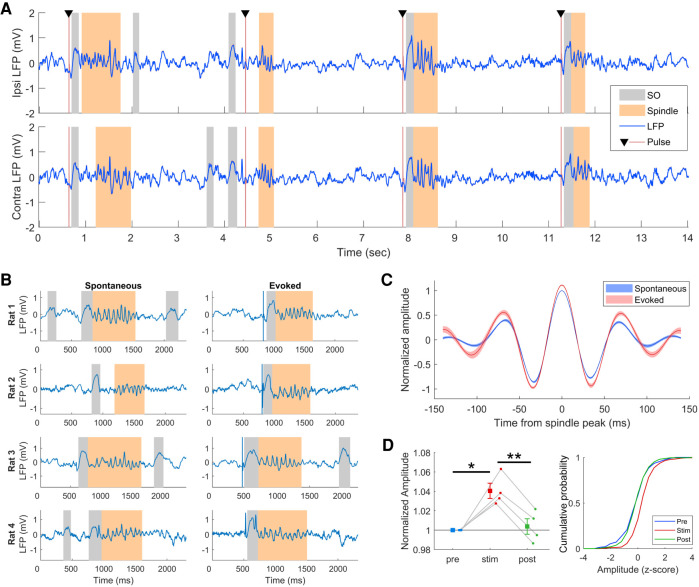
Neocortical stimulation during SWS reliably evokes spindles. (A) Segment of LFP from SWS showing SO and spindle oscillations occurring following stimulation pulses. Colored patches indicate offline detected SO and spindles. (B) Comparison of spontaneous and evoked spindles from each rat. (C) Average waveforms of spontaneous and evoked spindles showing larger amplitude of evoked spindles. Waveforms are aligned to the peak amplitude of the spindle and normalized by the amplitude in the prestim session. Shaded region is SEM of n = 4 rats. (D) Quantification of spindle amplitude across prestim and post-stim recording sessions. Amplitude is normalized to the spindle peak of the prestim session. For the distributions, prestim spindles were used to standardize the amplitude. (*) P < 0.05, t3 = 10.45; (**) P < 0.01, t3 = 5.16. Error bars are SEM of n = 4 rats.
Spindles, SO, and SWR were detected automatically based on thresholded power signals of filtered LFP recordings (SO 1–4 Hz, spindle 10–20 Hz, SWR 100–250 Hz) (see the Supplemental Material for details). Threshold values for automated detection of LFP features was done while visually inspecting the prestimulation recording from day 1, and then verifying that they were appropriate by checking the prestimulation session from days 2–5. Once set, the same thresholds were used for all recording sessions on all days. Spindles that occurred within 750 msec of a SO (measured from the peak of the hyperpolarization), SWR, or stimulation pulse were considered “coupled” to the SO/SWR or “evoked” by the stimulation pulse. SO within 500 msec of a stimulation pulse were considered “evoked” (Latchoumane et al. 2017). For triple coupling of SO, spindles, and SWR, the SO was taken as time zero and then both a spindle and SWR had to occur within 750 msec of the SO. Although SOs, spindles and SWR appear similar in mice and rats (Mölle et al. 2009; Niethard et al. 2018; Csernai et al. 2019), differences in detection methods and parameters can give rise to different event timings. Furthermore, definitions of “coupling” can vary across studies, with gaps of 2 sec between SO and spindles accepted as coupled (Kam et al. 2019). Because of these issues, we tested several gap durations where we report significant coupling to ensure the coupling is not specific to a particular gap.
As previously reported (Vyazovskiy et al. 2009), brief stimulation pulses delivered to the corpus callosum during SWS evoked SO and spindles in the motor cortex (Fig. 1A). Spindle oscillations occurred reliably following the SO throughout the 1-h stimulation session, an effect that occurred ipsilaterally as well as contralaterally to the stimulation electrode.
Other than the presence of a stimulation artifact, which was removed with an automated algorithm (Supplemental Fig. S2), stimulation-evoked spindles appeared qualitatively similar to spontaneously occurring spindles (Fig. 1B). Examination of overlaid average waveforms of spindles from the prestim and stimulation sessions revealed that evoked spindles were larger in amplitude (Fig. 1C). Measuring the peak amplitude showed a significant increase in the amplitude of evoked spindles compared to spontaneous spindles in the prestim session (t3 = 5.16, P < 0.05) (Fig. 1D). This effect did not last beyond the stimulation session, and spindle amplitude in the post-stim session was not significantly different than prestim amplitude (t3 = 0.47, P = 1). We also compared frequency and duration of evoked and spontaneous spindles. Detected spindles were 400–600 msec in duration on average, which is in the range normally reported for spindles (Gardner et al. 2013; Latchoumane et al. 2017), and stimulation did not alter the duration (Supplemental Fig. S4). Similarly, spindle frequency was not changed by stimulation (Supplemental Fig. S4). Like evoked spindles, the amplitude of evoked SO was significantly larger compared to spontaneous SO from the prestim session (Supplemental Fig. S5). Unlike spindles, the amplitude of spontaneous SO in the post-stim session, although smaller than evoked SO, remained significantly larger than the prestim SO (Supplemental Fig. S5).
Importantly, stimulation did not affect the rats’ sleep. They spent most of the time sleeping, and the amount of sleep was similar between the stimulation session and the post-stimulation session (Supplemental Fig. S6A). The amount of sleep in the prestim session was less than the other sessions, likely because the rats were still aroused after being transported to the recording room. However, the proportion of SWS was similar between all epochs, indicating that stimulation did not significantly alter the sleep structure (Supplemental Fig. S6B).
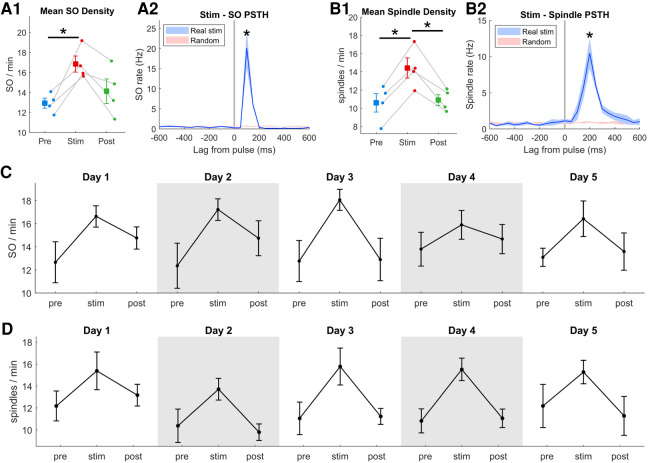
SO and spindles occurred reliably following stimulation in both hemispheres. On average, 76% of pulses were followed by a SO and 59% were followed by spindles (Supplemental Fig. S7). To quantify the increase in the number of SO and spindles events over the duration of the 1-h stimulation session, we compared the SO and spindle density (number per minute; averaged across hemispheres) of the prestim and post-stim sessions. SO and spindle density both increased significantly during the stimulation session, SO by an average of 30% (range 18%–45%) (Fig. 2A1), and spindles by an average of 37% (range 23%–54%) (Fig. 2B1; individual hemispheres in Supplemental Fig. S8). Temporal coupling of SO and spindles was previously identified as important for memory (Latchoumane et al. 2017). Using a similar measure, we classified spindles as coupled to a SO if they occurred within 750 msec. Despite the significant increase in both SO and spindles, the SO-spindle coupling was not increased by stimulation (Supplemental Fig. S9). Furthermore, stimulation did not appear to cause any lasting effect on SO or spindle density as both SO and spindle density in the post-stim session were comparable to the prestim session. We also calculated the peristimulus time histogram (PSTH) of SO and spindle occurrences and found a significant increase in SO and spindle rate following stimulation pulses (Fig. 2A2,B2). To test the reproducibility and longevity of the stimulation-induced increase in SO and spindles, we repeated the experiment on five consecutive days and observed similar increases in SO and spindle density on each day (Fig. 2C,D).
Figure 2.
One hour of stimulation reliably increases SO and spindle rate. (A1) Average SO density across prestim and post-stim sessions (t3 = 5.19). (A2) Peristimulus time histograms (PSTH) of SO relative to stimulation pulses or randomly distributed pulse times (t3 = 3.86). (B1) Average spindle density across sessions (prestim: t3 = 8.05; stim-post: t3 = 4.88). (B2) PSTH of spindle occurrences relative to stimulation pulses (t3 = 4.71). (C,D) Stimulation-induced increase in SO and spindle rate is consistent across five consecutive days. In all panels, (*) P < 0.05, error bars are SEM of n = 4 rats.
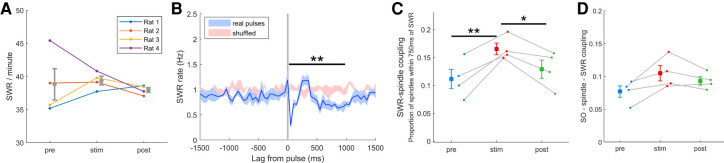
We next examined the possible effect of cortical stimulation on hippocampal sharp-wave ripples (SWR). Unlike spindles, stimulation did not affect the overall rate of SWR occurrence (Fig. 3A). However, the timing of SWR was affected by stimulation. SWR were more likely to occur within 300–400 msec following a cortical stimulation pulse, and were less likely to occur at other latencies (ANOVA F(19,114) = 6.7, P < 0.01) (Fig. 3B). The increased tendency of SWR within this time window led to increased coupling between SWR and spindles (t3 = 4.72, P < 0.01) (Fig. 3C). To verify that the increased coupling was not due to the definition of coupling (750-msec gap), we tested a range of gap durations (250–2000 msec) and found the coupling to be robust (Supplemental Fig. S10). The increased SWR-spindle coupling was only present during the stimulation session, and there was a decrease in coupling in the post-stim session such that there was no difference between the prestim and post-stim coupling. Finally, we tested for possible triple coupling of SO, spindles, and SWR. Although triple coupling increased in all four rats during the stimulation session, it failed to reach statistical significance (t3 = 2.10, P = 0.63) (Fig. 3D).
Figure 3.
Effects of cortical stimulation on hippocampal sharp-wave ripples (SWR). (A) The rate of SWR in the prestim and post-stim sessions were similar, indicating that stimulation did not affect the overall rate of SWR. (B) Stimulation altered the timing of SWR. The PSTH of SWR relative to stimulation pulses shows an overall suppression of SWR following the pulse, with a grouping of SWR occurring 300–400 msec after the pulse. (**) P < 0.01, ANOVA F(19,114) = 6.7. (C) Stimulation caused an increase SWR-spindle coupling. Compared to the prestim session, there was a significant increase in the proportion of spindles that occurred within 750 msec of a SWR during the stim session. (**) P < 0.01, t3 = 4.72. The increase in SWR-spindle coupling during the stim session did not persist into the post-stim session. (*) P < 0.05, t3 = 2.49. (D) Triple coupling of SO, spindles, and SWR increased in all four rats during the stimulation session but failed to reach statistical significance (t3 = 2.10, P = 0.063).
In summary, our results show that spindles can be evoked reliably by single-pulse electrical stimulation of the neocortex for the duration of a 1-h stimulation session, resulting in a significant increase in SO and spindle density, and an increase in SWR-spindle coupling. One potential limitation of our study is the within animal design. Because the same parameters were used to detect spindles and SO, the observed increase in spindles and SO could be due to an endogenous increase that occurs over time. While we cannot strictly rule out this possibility, it is worth noting that the increase in spindle and SO density decreased significantly in the post-stim session, so the increase cannot be due to a progressive increase in spindles and SO. Together with the very strong cross-correlation of stimulation pulses and spindles/SO, the evidence favors the view that the increase in spindle/SO density was indeed due to the stimulation. Furthermore, this increase is reproducible across five consecutive days of stimulation, suggesting that this method is viable as a deep-brain stimulation implant for increasing SO and spindles.
Previous attempts to boost spindle expression during sleep have used different methods with mixed results. Playing auditory stimuli during sleep is a minimally invasive method that has shown promise in altering SWS oscillations. In the context of a TMR experiment, when the auditory stimulus was previously paired with a learning task, tones presented during sleep increased spindles whose content was related to the memory task (Cairney et al. 2018). Even when auditory tones are used in non-TMR experiment (i.e., the tone was not previously paired with a learning task), tones played during sleep increased spindle power and improved memory performance (Ngo et al. 2013). However, a recent study showed a similar increase in spindle power following tone presentation, yet there was no corresponding memory improvement (Henin et al. 2019). Furthermore, the practical use of auditory stimuli outside of a clinical setting has been questioned because it is less reliable and subject to interference from other sounds (Ngo et al. 2015).
Initial studies of transcranial electrical stimulation (TES) used mild oscillating currents during SWS and showed that slow oscillations and spindles were increased, and that memory performance was improved (Marshall et al. 2006). Subsequent studies using similar protocols yielded mixed results, and a recent study showed that currents typically used in TES studies are too weak to affect neural activity (Lafon et al. 2017). Our more invasive method of implanting a stimulating electrode in the cortex and delivering brief pulses of current was first shown to be effective at evoking spindles many years ago in anesthetized cats (Roy et al. 1984; Steriade and Deschenes 1984; Contreras and Steriade 1995, 1996). Since then, an increase in spindle power has been observed following electrical stimulation of the neocortex in sleeping rats (Vyazovskiy et al. 2009), or by transcranial magnetic stimulation in humans (Bergmann et al. 2012; Massimini et al. 2007). Despite these results, it has not been shown that repeated cortical stimulation is capable of increasing spindle rates, and our current results show that stimulation pulses through implanted electrodes can be used to evoke spindles reliably, not only during a 1-h recording session, but across multiple days.
A recent optogenetic study showed that thalamic reticular activation increased the coupling of spindles and slow oscillations and improved contextual fear memory (Latchoumane et al. 2017). Interestingly, they did not report a net increase in spindle density, so the improved memory was attributed to the increased coordination between SO, spindles, and SWR. We observed a significant increase in SWR-spindle coupling, as well as a strong trend toward increased triple coupling of SO, spindles, and SWR, although this failed to reach statistical significance. The weaker triple coupling is possibly due to the fact that our stimulation pulses occurred randomly with respect to SO, whereas the reticular stimulation in Latchoumane et al. (2017) was triggered by a SO. If our electrical stimulation was triggered by SO, then it is possible the triple coupling would be increased as well, although this remains to be tested. Another critical next step is to determine if the substantial stimulation-induced increase spindle and SO density, as well as the increased SWR-spindle coupling, is sufficient to improve memory.
Supplementary Material
Acknowledgments
This work was supported by the National Sciences and Engineering Research Council of Canada (NSERC) via Discovery grants to both M.T. and D.R.E and by funds from Alberta Innovates Health Solutions provided to M.T. and D.R.E. as part of the Polaris award, 2008–2018.
Footnotes
[Supplemental material is available for this article.]
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.052464.120.
References
- Barakat M, Doyon J, Debas K, Vandewalle G, Morin A, Poirier G, Martin N, Lafortune M, Karni A, Ungerleider LG, et al. 2011. Fast and slow spindle involvement in the consolidation of a new motor sequence. Behav Brain Res 217: 117–121. 10.1016/j.bbr.2010.10.019 [DOI] [PubMed] [Google Scholar]
- Bergmann TO, Mölle M, Schmidt MA, Lindner C, Marshall L, Born J, Siebner HR. 2012. EEG-guided transcranial magnetic stimulation reveals rapid shifts in motor cortical excitability during the human sleep slow oscillation. J Neurosci 32: 243–253. 10.1523/JNEUROSCI.4792-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairney SA, Guttesen AAV, El Marj N, Staresina BP. 2018. Memory consolidation is linked to spindle-mediated information processing during sleep. Curr Biol 28: 948–954.e944. 10.1016/j.cub.2018.01.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras D, Steriade M. 1995. Cellular basis of EEG slow rhythms: a study of dynamic corticothalamic relationships. J Neurosci 15: 604–622. 10.1523/JNEUROSCI.15-01-00604.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras D, Steriade M. 1996. Spindle oscillation in cats: the role of corticothalamic feedback in a thalamically generated rhythm. J Physiol 490: 159–179. 10.1113/jphysiol.1996.sp021133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernai M, Borbély S, Kocsis K, Burka D, Fekete Z, Balogh V, Káli S, Emri Z, Barthó P. 2019. Dynamics of sleep oscillations is coupled to brain temperature on multiple scales. J Physiol 597: 4069–4086. 10.1113/JP277664 [DOI] [PubMed] [Google Scholar]
- Eckert MJ, McNaughton BL, Tatsuno M. 2020. Neural ensemble reactivation in rapid eye movement and slow-wave sleep coordinate with muscle activity to promote rapid motor skill learning. Philos Trans R Soc B Biol Sci 375: 20190655 10.1098/rstb.2019.0655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gais S, Mölle M, Helms K, Born J. 2002. Learning-dependent increases in sleep spindle density. J Neurosci 22: 6830–6834. 10.1523/JNEUROSCI.22-15-06830.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RJ, Hughes SW, Jones MW. 2013. Differential spike timing and phase dynamics of reticular thalamic and prefrontal cortical neuronal populations during sleep spindles. J Neurosci 33: 18469–18480. 10.1523/JNEUROSCI.2197-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Siegle JH, Ritt JT, Ting JT, Feng G, Moore CI. 2011. Selective optical drive of thalamic reticular nucleus generates thalamic bursts and cortical spindles. Nat Neurosci 14: 1118–1120. 10.1038/nn.2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henin S, Borges H, Shankar A, Sarac C, Melloni L, Friedman D, Flinker A, Parra LC, Buzsaki G, Devinsky O, et al. 2019. Closed-loop acoustic stimulation enhances sleep oscillations but not memory performance. eNeuro 10.1523/ENEURO.0306-0319.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Blakely T, Hermes D, Hakimian S, Ramsey NF, Ojemann JG. 2012. Sleep spindles are locally modulated by training on a brain–computer interface. Proc Natl Acad Sci 109: 18583–18588. 10.1073/pnas.1207532109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam K, Pettibone WD, Shim K, Chen RK, Varga AW. 2019. Dynamics of sleep spindles and coupling to slow oscillations following motor learning in adult mice. Neurobiol Learn Mem 166: 107100 10.1016/j.nlm.2019.107100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel A, Buzsáki G. 1997. Cellular–synaptic generation of sleep spindles, spike-and-wave discharges, and evoked thalamocortical responses in the neocortex of the rat. J Neurosci 17: 6783–6797. 10.1523/JNEUROSCI.17-17-06783.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A, Latchoumane C, Lee S, Kim GB, Cheong E, Augustine GJ, Shin H-S. 2012. Optogenetically induced sleep spindle rhythms alter sleep architectures in mice. Proc Natl Acad Sci 109: 20673–20678. 10.1073/pnas.1217897109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafon B, Henin S, Huang Y, Friedman D, Melloni L, Thesen T, Doyle W, Buzsáki G, Devinsky O, Parra LC, et al. 2017. Low frequency transcranial electrical stimulation does not entrain sleep rhythms measured by human intracranial recordings. Nat Commun 8: 1199 10.1038/s41467-017-01045-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchoumane C-FV, Ngo H-VV, Born J, Shin H-S. 2017. Thalamic spindles promote memory formation during sleep through triple phase-locking of cortical, thalamic, and hippocampal rhythms. Neuron 95: 424–435.e426. 10.1016/j.neuron.2017.06.025 [DOI] [PubMed] [Google Scholar]
- Marshall L, Helgadóttir H, Mölle M, Born J. 2006. Boosting slow oscillations during sleep potentiates memory. Nature 444: 610 10.1038/nature05278 [DOI] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Esser SK, Riedner BA, Huber R, Murphy M, Peterson MJ, Tononi G. 2007. Triggering sleep slow waves by transcranial magnetic stimulation. Proc Natl Acad Sci 104: 8496–8501. 10.1073/pnas.0702495104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölle M, Eschenko O, Gais S, Sara SJ, Born J. 2009. The influence of learning on sleep slow oscillations and associated spindles and ripples in humans and rats. Eur J Neurosci 29: 1071–1081. 10.1111/j.1460-9568.2009.06654.x [DOI] [PubMed] [Google Scholar]
- Ngo H-VV, Martinetz T, Born J, Mölle M. 2013. Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron 78: 545–553. 10.1016/j.neuron.2013.03.006 [DOI] [PubMed] [Google Scholar]
- Ngo H-VV, Miedema A, Faude I, Martinetz T, Mölle M, Born J. 2015. Driving sleep slow oscillations by auditory closed-loop stimulation—a self-limiting process. J Neurosci 35: 6630–6638. 10.1523/JNEUROSCI.3133-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethard N, Ngo H-VV, Ehrlich I, Born J. 2018. Cortical circuit activity underlying sleep slow oscillations and spindles. Proc Natl Acad Sci 115: E9220–E9229. 10.1073/pnas.1805517115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M, Walker MP. 2007. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS ONE 2: e341 10.1371/journal.pone.0000341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters KR, Ray L, Smith V, Smith C. 2008. Changes in the density of stage 2 sleep spindles following motor learning in young and older adults. J Sleep Res 17: 23–33. 10.1111/j.1365-2869.2008.00634.x [DOI] [PubMed] [Google Scholar]
- Peyrache A, Khamassi M, Benchenane K, Wiener SI, Battaglia FP. 2009. Replay of rule-learning related neural patterns in the prefrontal cortex during sleep. Nat Neurosci 12: 919 10.1038/nn.2337 [DOI] [PubMed] [Google Scholar]
- Ramanathan DS, Gulati T, Ganguly K. 2015. Sleep-dependent reactivation of ensembles in motor cortex promotes skill consolidation. PLoS Biol 13: e1002263 10.1371/journal.pbio.1002263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy JP, Clercq M, Steriade M, Deschenes M. 1984. Electrophysiology of neurons of lateral thalamic nuclei in cat: mechanisms of long-lasting hyperpolarizations. J Neurophysiol 51: 1220–1235. 10.1152/jn.1984.51.6.1220 [DOI] [PubMed] [Google Scholar]
- Schabus M, Gruber G, Parapatics S, Sauter C, Klosch G, Anderer P, Klimesch W, Saletu B, Zeitlhofer J. 2004. Sleep spindles and their significance for declarative memory consolidation. Sleep 27: 1479–1485. 10.1093/sleep/27.7.1479 [DOI] [PubMed] [Google Scholar]
- Schmidt C, Peigneux P, Muto V, Schenkel M, Knoblauch V, Münch M, de Quervain DJ-F, Wirz-Justice A, Cajochen C. 2006. Encoding difficulty promotes postlearning changes in sleep spindle activity during napping. J Neurosci 26: 8976–8982. 10.1523/JNEUROSCI.2464-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Deschenes M. 1984. The thalamus as a neuronal oscillator. Brain Res Rev 8: 1–63. 10.1016/0165-0173(84)90017-1 [DOI] [PubMed] [Google Scholar]
- Steriade M, McCormick D, Sejnowski T. 1993. Thalamocortical oscillations in the sleeping and aroused brain. Science 262: 679–685. 10.1126/science.8235588 [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Faraguna U, Cirelli C, Tononi G. 2009. Triggering slow waves during NREM sleep in the rat by intracortical electrical stimulation: effects of sleep/wake history and background activity. J Neurophysiol 101: 1921–1931. 10.1152/jn.91157.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.