Abstract
Fear-motivated avoidance extinction memory is prone to hippocampal brain-derived neurotrophic factor (BDNF)-dependent reconsolidation upon recall. Here, we show that extinction memory recall activates mammalian target of rapamycin (mTOR) in dorsal CA1, and that post-recall inhibition of this kinase hinders avoidance extinction memory persistence and recovers the learned aversive response. Importantly, coadministration of recombinant BDNF impedes the behavioral effect of hippocampal mTOR inhibition. Our results demonstrate that mTOR signaling is necessary for fear-motivated avoidance extinction memory reconsolidation and suggests that BDNF acts downstream mTOR in a protein synthesis-independent manner to maintain the reactivated extinction memory trace.
Repeated or prolonged nonreinforced recall may induce extinction of consolidated memories, a form of learning involving the formation of a new association that inhibits the expression of the original one (Bouton 2004). On the contrary, brief re-exposure to retrieval cues may destabilize consolidated memories, which must then be reconsolidated to persist (Przybyslawski and Sara 1997; Nader et al. 2000). Psychotherapy based on extinction enhancement or reconsolidation disruption might reduce the intrusive recollection of aversive events and help in the treatment of post-traumatic stress disorder (PTSD), a prevalent mental health condition characterized by the persistent avoidance of places, people, and objects resembling traumatic experiences (Ressler et al. 2004; Schwabe et al. 2014; Dunbar and Taylor 2017; Bryant 2019). Therefore, considerable effort has been lately dedicated to analyze the properties and potential interactions of fear memory extinction and reconsolidation. In this regard, it has been reported that these processes are mutually exclusive (Merlo et al. 2014), and that extinction training during the reconsolidation time window enhances extinction learning and prevents the recovery of fear (Monfils et al. 2009). Moreover, we have previously shown that recall renders fear-motivated avoidance extinction memory susceptible to amnesia, indicating that this memory type is prone to reconsolidation when active and suggesting that targeting extinction memory reconsolidation can be a feasible treatment strategy for PTSD (Rossato et al. 2010; Rosas-Vidal et al. 2015). However, the neurochemical basis of extinction memory reconsolidation has seldom been analyzed.
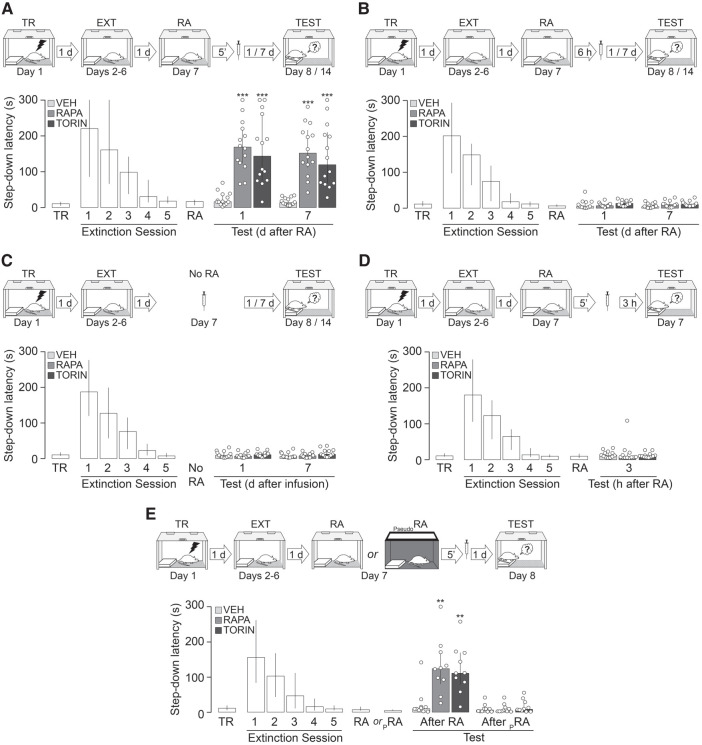
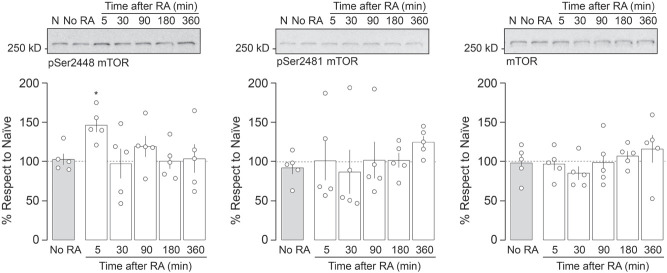
Mammalian target of rapamycin (mTOR) is a 289-kDa phospho-inositide 3-kinase (PI3K)-related serine-threonine protein kinase that functions as a key element of mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) signaling modules to regulate protein synthesis through the phosphorylation of eukaryotic initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1) and p70 ribosomal S6 kinase (p70S6K) (Hay and Sonenberg 2004). A well-known mediator of cell growth and proliferation (Hall 2008; Ryskalin et al. 2017), mTOR involvement in synaptic plasticity was first suggested by studies showing that rapamycin (RAPA), a macrolide that selectively inhibits mTORC1 signaling by interacting with the chaperone FKBP12 and binding to mTOR FKBP12–RAPA-binding domain, impairs long-term facilitation in Aplysia as well as long-term potentiation (LTP) in the rat hippocampus (Casadio et al. 1999; Tang et al. 2002). Interestingly, avoidance memory consolidation and recall need mTOR signaling in the dorsal hippocampus (Bekinschtein et al. 2007; Pereyra et al. 2018), as it also happens with the reconsolidation and extinction of several other memory types (Myskiw et al. 2008; Gafford et al. 2011; Zubedat and Akirav 2017; Jarome et al. 2018; Lee et al. 2018; Yang et al. 2019). Here, we examined whether reconsolidation of fear-motivated avoidance extinction memory requires mTOR activity in the CA1 region of the dorsal hippocampus. To do that, we used 3-mo-old, 300- to 350-g, male Wistar rats (n = 320), housed in groups of five with free access to water and food in a holding room at 22°C–23°C on a normal light cycle (12 h light:12 h dark; lights on at 6.00 a.m.). Animals were implanted with 22-gauge guides aimed at the CA1 region of the dorsal hippocampus (Supplemental Fig. S1, stereotaxic coordinates in millimeters: anteroposterior, −4.2; laterolateral, ±3.0; dorsoventral, −3.0), as previously described (Radiske et al. 2015), and allowed to recover from surgery for 10 d before being handled by the experimenter once per day for 2 d. One day later, the animals were trained in a one-trial step-down inhibitory avoidance (SDIA) task, an aversive learning paradigm in which stepping down from a platform is paired with a mild footshock. Briefly, the SDIA training box (50 × 25 × 25 cm) was made of Plexiglas and fitted with a grid floor through which scrambled electric shocks could be delivered to the rat's feet. Over the left end of the grid floor there was a 5-cm-high, 8-cm-wide, 25-cm-long wooden platform. For training, the animals were individually placed on the platform facing the left rear corner of the training box and, when they stepped down and placed their four paws on the grid, received a 2-sec, 0.4-mA scrambled footshock, whereupon they were immediately withdrawn from the training box. This training protocol induces a long-lasting, hippocampus-dependent, fear-motivated avoidance memory expressed as an increase in step-down latency at test (Bernabeu et al. 1995; Paratcha et al. 2000; Katche et al. 2013). However, repeated testing in the absence of the footshock causes clear-cut extinction (Cammarota et al. 2005; Rossato et al. 2006; Bonini et al. 2011). Therefore, to extinguish the learned avoidance response, we submitted SDIA trained rats to one daily unreinforced test session for five consecutive days. To that end, we put the animals back on the training box platform until they stepped down to the grid. No footshock was given, and the animals were allowed to freely explore the training apparatus for 30 sec after stepping down. During this time, the animals stepped up onto the platform and down again several times. This procedure induces an SDIA extinction memory immune to spontaneous recovery, reinstatement and renewal that lasts for at least 14 d and requires NMDA receptor activation as well as protein synthesis and gene expression in dorsal CA1 to consolidate (Cammarota et al. 2003; Rossato et al. 2010; Radiske et al. 2015). One day after the last extinction session, extinction memory was reactivated by placing the animals on the training box platform until they stepped down from it. Five minutes or 6 h later, the animals received bilateral intradorsal CA1 infusions (1 µL/side) of vehicle (VEH; 5% DMSO in saline), RAPA (0.02 µg/side) or the selective ATP-competitive inhibitor of mTOR, TORIN2 (TORIN; 0.20 µg/side). RAPA and TORIN were dissolved in DMSO and diluted to working concentration in sterile saline (<5% DMSO). The doses used were determined based on pilot experiments and previous studies showing the behavioral and biochemical effects of each compound (Bekinschtein et al. 2007; Revest et al. 2014; Renard et al. 2016; Lee et al. 2018). Retention was evaluated at different times after extinction memory reactivation by placing the animals on the training box platform and measuring their latency to step down. Because of the 300-sec ceiling imposed on test latency, step-down data were expressed as median ± IQR and analyzed using the Kruskal–Wallis test followed by Dunn's post hoc comparisons. We found that animals that received VEH recalled SDIA extinction memory normally regardless of the time elapsed between reactivation and test sessions. Conversely, RAPA and TORIN given 5 min, but not 6 h, after SDIA extinction memory reactivation impaired retention of extinction and induced reappearance of the SDIA response 1 d and 7 d later (Fig. 1A, 1 d after RA: H = 24.42, P < 0.001; P < 0.001 for VEH vs. RAPA, P < 0.001 for VEH vs. TORIN; 7 d after RA: H = 26.85, P < 0.001; P < 0.001 for VEH vs. RAPA, P < 0.001 for VEH vs. TORIN in Dunn's multiple comparisons after Kruskal–Wallis test; Fig. 1B, 1 d after RA: H = 4.510, P = 0.1049; 7 d after RA: H = 4.606, P = 0.0999 in Kruskal–Wallis test). Neither RAPA nor TORIN affected SDIA extinction memory when administered 24 h after the last extinction session in the absence of extinction memory reactivation (Fig. 1C, 1 d after infusion: H = 2.141, P = 0.3428; 7 d after infusion: H = 4.086, P = 0.1296 in Kruskal–Wallis test) or when given 5 min post-reactivation but retention was evaluated 3 h thereafter (Fig. 1D, H = 1.654, P = 0.4375 in Kruskal–Wallis test). Moreover, RAPA and TORIN had no effect on extinction memory retention if injected in dorsal CA1 5 min after an extinction pseudoreactivation session carried out in an avoidance training box rendered nonaversive for SDIA-trained animals (Fig. 1E, After RA: H = 13.86, P = 0.001; P < 0.01 for VEH vs. RAPA, P < 0.01 for VEH vs. TORIN; After PseudoRA: H = 0.7503, P = 0.6872 in Dunn's multiple comparisons after Kruskal–Wallis test; Supplemental Fig. S2). mTOR activity is regulated by phosphorylation at different sites (Watanabe et al. 2011). Phosphorylation at Ser2448 is mediated by p70S6K, occurs mainly to mTOR associated with mTORC1 (Chiang and Abraham 2005; Holz and Blenis 2005; Akcakanat et al. 2007), enables mTOR binding to regulatory-associated protein of mTOR (RAPTOR), and correlates with mTORC1 activation (Rosner et al. 2010). On the contrary, Ser2481 is an autophosphorylation site insensitive to acute rapamycin treatment that is phosphorylated only when mTOR makes part of mTORC2 complexes (Peterson et al. 2000; Copp et al. 2009). To analyze mTOR phosphorylation levels, we performed immunoblotting on total homogenates from the CA1 region of the dorsal hippocampus. Samples were not pooled. Equal amounts of proteins (15 µg) were fractionated by SDS-PAGE and transferred to PVDF membranes. Blots were blocked for 1 h, incubated overnight at 4°C with anti-pSer2448 mTOR (1:10,000; RRID:AB_330970), anti-pSer2481 mTOR (1:10,000; RRID:AB_2262884), or anti-mTOR (1:10,000; RRID:AB_330978), and then incubated for 2 h at room temperature with HRP-coupled anti-IgG secondary antibody. Immunoreactivity was detected using the Amersham ECL Prime Western Blotting Detection Reagent and the Amersham Imager 600 system. Densitometric analyses were performed using the ImageQuant TL 8.1 analysis software (GE Healthcare). We found that pSer2448 mTOR levels peaked 5 min after SDIA extinction memory reactivation and returned to control values within 30 min (Fig. 2, F(5,20) = 2.805, P = 0.0446; P < 0.05 for 5 min vs. No RA in Dunnett's multiple comparison test after repeated measures ANOVA). No changes in pSer2481 mTOR or total mTOR levels were found up to 6 h post-reactivation (Fig. 2, pSer2481 mTOR: F(5,20) = 1.241, P = 0.3274; mTOR: F(5,20) = 1.208, P = 0.3411 in repeated measures ANOVA; Supplemental Fig. S3). mTORC1 activation stimulates brain-derived neurotrophic factor (BDNF) production in hippocampal neurons (Jeon et al. 2015), which in turn may induce mTOR-dependent activation of dendritic mRNA translation (Takei et al. 2004). Previously, we reported that hippocampal BDNF maintains fear-motivated avoidance extinction memory after recall (Radiske et al. 2015). In agreement with this finding, coinfusion of recombinant BDNF (0.25 µg/side) after SDIA extinction memory reactivation impeded the recovery of the avoidance response provoked by RAPA (Fig. 3, 1 d after RA: H = 27.52, P < 0.001; P < 0.001 for VEH vs. RAPA, P < 0.001 for BDNF vs. RAPA, P < 0.05 for RAPA vs. RAPA + BDNF; 7 d after RA: H = 26.76, P < 0.001; P < 0.001 for VEH vs. RAPA, P < 0.001 for BDNF vs. RAPA, P < 0.01 for RAPA vs. RAPA + BDNF in Dunn's multiple comparisons after Kruskal–Wallis test).
Figure 1.
mTOR is required for fear-motivated avoidance extinction memory reconsolidation. (A) Animals were trained in SDIA (TR; 0.4 mA/2 sec) and beginning 24 h later submitted to one daily extinction session for five consecutive days (EXT). Twenty-four hours after the last session, extinction memory was reactivated (RA) and, 5 min thereafter, the animals received bilateral intradorsal CA1 infusions of vehicle (VEH; 5% DMSO in saline), rapamycin (RAPA; 0.02 µg/side) or TORIN (0.20 µg/side). Retention was assessed 1 and 7 d later (Test). (B) Animals were treated as in A except that they received intra-CA1 infusions of VEH, RAPA, or TORIN 6 h after RA. (C) Animals were treated as in A, except that they received VEH, RAPA, or TORIN in dorsal CA1 24 h after the last extinction session in the absence of RA (No RA). (D) Animals were treated as described in A, except that VEH, RAPA, or TORIN were given 5 min after RA and retention was assessed 3 h later. (E) Animals were treated as in A, except that a subgroup of animals received VEH, RAPA, or TORIN 5 min after an extinction pseudoreactivation session in an avoidance training box rendered nonaversive for SDIA-trained animals. The nonaversive box was similar in dimensions to the SDIA training box, but it was made of dark gray wood and had a Plexiglas platform. (PRA) Pseudoreactivation session. Data are expressed as median ± IQR. (**) P < 0.01, (***) P < 0.001 versus VEH in Dunn's multiple comparisons after Kruskal–Wallis test.
Figure 2.
Reactivation of fear-motivated avoidance extinction memory increases mTOR phosphorylation at Ser2448, but not at Ser2481, in the CA1 region of the dorsal hippocampus. Animals were trained in SDIA (0.4 mA/2 s) and beginning 24 h later submitted to one daily extinction session for 5 consecutive days. Twenty-four hours after the last session, extinction memory was reactivated (RA) and the animals killed by decapitation at different post-reactivation times (5–360 min). The CA1 region of the dorsal hippocampus was dissected out, homogenized, and used to determine of pS2448 mTOR, pS2481 mTOR, or mTOR levels by immunoblotting. (N) Naïve animals, (No RA) animals trained in SDIA that were submitted to five daily extinction sessions and killed 24 h after the last extinction session. Data are expressed as mean ± SEM. (*) P < 0.05 versus No RA in Dunnett's multiple comparison test after repeated measures ANOVA.
Figure 3.
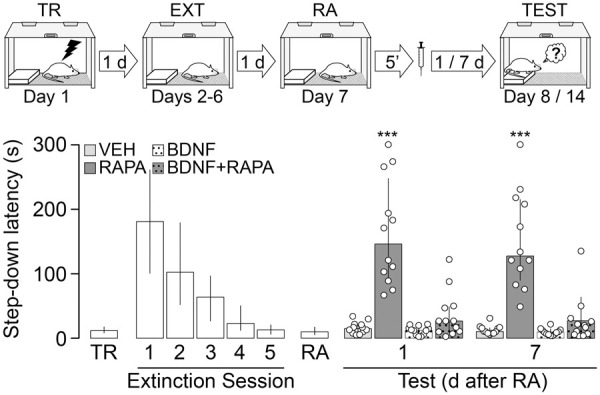
Coinfusion of recombinant BDNF reverses the effect of RAPA on fear-motivated avoidance extinction memory reconsolidation. Animals were trained in SDIA (TR; 0.4 mA/2 sec) and beginning 24 h later were submitted to one daily extinction session for five consecutive days (EXT). Twenty-four hours after the last session, extinction memory was reactivated (RA) and 5 min later the animals received bilateral intradorsal CA1 infusions of vehicle (VEH; 5% DMSO in saline), rapamycin (RAPA; 0.02 µg/side), BDNF (0.25 µg/µL), or RAPA plus BDNF (RAPA + BDNF). Retention was assessed 1 and 7 d later (Test). Data expressed as median ± IQR. (***) P < 0.001 versus VEH in Dunn's multiple comparisons after Kruskal–Wallis test.
Our results show that dorsal CA1 mTOR inhibition during a short post-recall time window persistently impairs retention of SDIA extinction memory and causes avoidance reappearance. This effect took time to develop, was time-dependent, concomitant with SDIA extinction memory reactivation, and occurred after the administration of mTOR inhibitors with different mechanisms of action, suggesting that it was not spontaneous or caused by nonspecific pharmacological interactions but due to bona fide impairment of an active mTOR-dependent reconsolidation process. This conclusion is further supported by findings showing that SDIA extinction memory reactivation rapidly and transiently increased mTOR phosphorylation at Ser2448, a post-translational modification customarily used as a proxy for mTOR activation (Reynolds et al. 2002; Guertin and Sabatini 2007; Rivas et al. 2009; Guo et al. 2017; Dong et al. 2018; Rosa et al. 2019). Most findings indicate that BDNF modulates protein synthesis through mTOR (Takei et al. 2001, 2004). In fact, BDNF controls hippocampal synaptic mRNA translation by regulating mTORC activation state (Briz et al. 2013; Leal et al. 2014), which seems to be necessary for SDIA memory consolidation (Slipczuk et al. 2009). However, in agreement with previous findings that BDNF is sufficient to restabilize a reactivated extinction memory trace, even when hippocampal protein synthesis and gene expression are inhibited (Radiske et al. 2015), our results show that mTOR acts upstream BDNF during the reconsolidation of extinction, and suggest not only that BDNF is a key protein synthesis product for this process but also that its actions are not mediated by mTOR-dependent mRNA translation. Indeed, mTOR signaling controls BDNF activity-dependent dendritic translation (Baj et al. 2016), and several protein synthesis-dependent plastic mechanisms, including late-LTP and memory consolidation, are rescued by BDNF when protein synthesis is impaired (Pang and Lu 2004; Moguel-González et al. 2008; Martínez-Moreno et al. 2011; Ozawa et al. 2014). Exogenous BDNF becomes quickly available for activity-dependent secretion, rapidly replacing the endogenous biosynthetic pathway after its administration (Santi et al. 2006). Thus, the rapid modulation of hippocampal high-frequency transmission produced by this neurotrophin is unaffected by protein synthesis inhibitors (Gottschalk et al. 1999; Tartaglia et al. 2001) and BDNF administration may induce the lasting structural reorganization and potentiation of hippocampal synapses in an mRNA synthesis and protein translation-independent manner (Martínez-Moreno et al. 2020), perhaps through a mechanism involving PKMζ activity regulation (Mei et al. 2011). In fact, hippocampal PKMζ acts downstream BDNF to control AMPAR synaptic insertion through a protein synthesis-independent mechanism during declarative memory reconsolidation (Rossato et al. 2019).
In conclusion, our results confirm that extinction does not erase the SDIA response but generates an inhibitory memory that coexists with it and controls its expression. The data also corroborate that avoidance extinction memory enters a labile state when reactivated by recall and needs to be reconsolidated through a mechanism involving hippocampal mTOR/BDNF signaling activation to maintain its dominance over the aversive trace. Finally, though not less important, our findings emphasize the necessity of understanding the dynamics of memory competition in order to develop better therapeutic strategies for PTSD treatment.
Supplementary Material
Acknowledgments
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil). A.R. is a postdoctoral research fellow supported by CAPES. D.A.N. holds a CAPES doctoral research fellowship through Programa de Pós-Graduação em Psicobiologia at Universidade Federal do Rio Grande do Norte (UFRN, Brazil).
Footnotes
[Supplemental material is available for this article.]
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.052068.120.
References
- Akcakanat A, Singh G, Hung MC, Meric-Bernstam F. 2007. Rapamycin regulates the phosphorylation of rictor. Biochem Biophys Res Commun 362: 330–333. 10.1016/j.bbrc.2007.07.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baj G, Pinhero V, Vaghi V, Tongiorgi E. 2016. Signaling pathways controlling activity-dependent local translation of BDNF and their localization in dendritic arbors. J Cell Sci 129: 2852–2864. 10.1242/jcs.177626 [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Igaz LM, Bevilaqua LR, Izquierdo I, Medina JH. 2007. Persistence of long-term memory storage requires a late protein synthesis- and BDNF- dependent phase in the hippocampus. Neuron 53: 261–277. 10.1016/j.neuron.2006.11.025 [DOI] [PubMed] [Google Scholar]
- Bernabeu R, Princ F, de Stein ML, Fin C, Juknat AA, Batile A, Izquierdo I, Medina JH. 1995. Evidence for the involvement of hippocampal CO production in the acquisition and consolidation of inhibitory avoidance learning. Neuroreport 6: 516–518. 10.1097/00001756-199502000-00027 [DOI] [PubMed] [Google Scholar]
- Bonini JS, Da Silva WC, Da Silveira CK, Köhler CA, Izquierdo I, Cammarota M. 2011. Histamine facilitates consolidation of fear extinction. Int J Neuropsychopharmacol 14: 1209–1217. 10.1017/S1461145710001501 [DOI] [PubMed] [Google Scholar]
- Bouton ME. 2004. Context and behavioral processes in extinction. Learn Mem 11: 485–494. 10.1101/lm.78804 [DOI] [PubMed] [Google Scholar]
- Briz V, Hsu YT, Li Y, Lee E, Bi X, Baudry M. 2013. Calpain-2-mediated PTEN degradation contributes to BDNF-induced stimulation of dendritic protein synthesis. J Neurosci 33: 4317–4328. 10.1523/JNEUROSCI.4907-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RA. 2019. Post-traumatic stress disorder: a state-of-the-art review of evidence and challenges. World Psychiatry 18: 259–269. 10.1002/wps.20656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarota M, Bevilaqua LR, Kerr D, Medina JH, Izquierdo I. 2003. Inhibition of mRNA and protein synthesis in the CA1 region of the dorsal hippocampus blocks reinstallment of an extinguished conditioned fear response. J Neurosci 23: 737–741. 10.1523/JNEUROSCI.23-03-00737.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarota M, Bevilaqua LR, Rossato JI, Ramirez M, Medina JH, Izquierdo I. 2005. Relationship between short- and long-term memory and short- and long-term extinction. Neurobiol Learn Mem 84: 25–32. 10.1016/j.nlm.2005.03.002 [DOI] [PubMed] [Google Scholar]
- Casadio A, Martin KC, Giustetto M, Zhu H, Chen M, Bartsch D, Bailey CH, Kandel ER. 1999. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell 99: 221–237. 10.1016/S0092-8674(00)81653-0 [DOI] [PubMed] [Google Scholar]
- Chiang GG, Abraham RT. 2005. Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J Biol Chem 280: 25485–25490. 10.1074/jbc.M501707200 [DOI] [PubMed] [Google Scholar]
- Copp J, Manning G, Hunter T. 2009. TORC-specific phosphorylation of mammalian target of rapamycin (mTOR): phospho-Ser2481 is a marker for intact mTOR signaling complex 2. Cancer Res 69: 1821–1827. 10.1158/0008-5472.CAN-08-3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Zhou Q, Wei Z, Yan S, Sun F, Cai X. 2018. Protein kinase A mediates scopolamine-induced mTOR activation and an antidepressant response. J Affect Disord 227: 633–642. 10.1016/j.jad.2017.11.041 [DOI] [PubMed] [Google Scholar]
- Dunbar AB, Taylor JR. 2017. Reconsolidation and psychopathology: moving towards reconsolidation-based treatments. Neurobiol Learn Mem 142: 162–171. 10.1016/j.nlm.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafford GM, Parsons RG, Helmstetter FJ. 2011. Consolidation and reconsolidation of contextual fear memory requires mammalian target of rapamycin-dependent translation in the dorsal hippocampus. Neuroscience 182: 98–104. 10.1016/j.neuroscience.2011.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk WA, Jiang H, Tartaglia N, Feng L, Figurov A, Lu B. 1999. Signaling mechanisms mediating BDNF modulation of synaptic plasticity in the hippocampus. Learn Mem 6: 243–256. [PMC free article] [PubMed] [Google Scholar]
- Guertin DA, Sabatini DM. 2007. Defining the role of mTOR in cancer. Cancer Cell 12: 9–22. 10.1016/j.ccr.2007.05.008 [DOI] [PubMed] [Google Scholar]
- Guo JN, Tian LY, Liu WY, Mu J, Zhou D. 2017. Activation of the Akt/mTOR signaling pathway: a potential response to long-term neuronal loss in the hippocampus after sepsis. Neural Regen Res 12: 1832–1842. 10.4103/1673-5374.219044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MN. 2008. mTOR—what does it do? Transplant Proc 40: S5–S8. 10.1016/j.transproceed.2008.10.009 [DOI] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. 2004. Upstream and downstream of mTOR. Genes Dev 18: 1926–1945. 10.1101/gad.1212704 [DOI] [PubMed] [Google Scholar]
- Holz MK, Blenis J. 2005. Identification of S6 kinase 1 as a novel mammalian target of rapamycin (mTOR)-phosphorylating kinase. J Biol Chem 280: 26089–26093. 10.1074/jbc.M504045200 [DOI] [PubMed] [Google Scholar]
- Jarome TJ, Perez GA, Hauser RM, Hatch KM, Lubin FD. 2018. EZH2 methyltransferase activity controls Pten expression and mTOR signaling during fear memory reconsolidation. J Neurosci 38: 7635–7648. 10.1523/JNEUROSCI.0538-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon MT, Nam JH, Shin WH, Leem E, Jeong KH, Jung UJ, Bae YS, Jin YH, Kholodilov N, Burke RE, et al. 2015. In vivo AAV1 transduction with hRheb(S16H) protects hippocampal neurons by BDNF production. Mol. Ther 23: 445–455. 10.1038/mt.2014.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katche C, Dorman G, Gonzalez MC, Kramar CP, Slipczuk L, Rossato JI, Cammarota M, Medina JH. 2013. On the role of retrosplenial cortex in longlasting memory storage. Hippocampus 23: 295–302. 10.1002/hipo.22092 [DOI] [PubMed] [Google Scholar]
- Leal G, Comprido D, Duarte CB. 2014. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology 76: 639–656. 10.1016/j.neuropharm.2013.04.005 [DOI] [PubMed] [Google Scholar]
- Lee B, Bang E, Yang WS, Paydar A, Ha GE, Kim S, Kim JH, Cho T, Lee SE, Lee S, et al. 2018. The possible role of neurobeachin in extinction of contextual fear memory. Sci Rep 8: 13752 10.1038/s41598-018-30589-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Moreno A, Rodríguez-Durán LF, Escobar ML. 2011. Late protein synthesis-dependent phases in CTA long-term memory: BDNF requirement. Front Behav Neurosci 5: 61 10.3389/fnbeh.2011.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Moreno A, Rivera-Olvera A, Escobar ML. 2020. BDNF induces in vivo long-lasting enhancement of synaptic transmission and structural reorganization at the hippocampal mossy fibers in a transcription and translation-independent manner. Neurobiol Learn Mem 167: 107125 10.1016/j.nlm.2019.107125 [DOI] [PubMed] [Google Scholar]
- Mei F, Nagappan G, Ke Y, Sacktor TC, Lu B. 2011. BDNF facilitates L-LTP maintenance in the absence of protein synthesis through PKMζ. PLoS One 6: e21568 10.1371/journal.pone.0021568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo E, Milton AL, Goozée ZY, Theobald DE, Everitt BJ. 2014. Reconsolidation and extinction are dissociable and mutually exclusive processes: behavioral and molecular evidence. J Neurosci 34: 2422–2431. 10.1523/JNEUROSCI.4001-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moguel-González M, Gómez-Palacio-Schjetnan A, Escobar ML. 2008. BDNF reverses the CTA memory deficits produced by inhibition of protein synthesis. Neurobiol Learn Mem 90: 584–587. 10.1016/j.nlm.2008.06.003 [DOI] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE. 2009. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science 324: 951–955. 10.1126/science.1167975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myskiw JC, Rossato JI, Bevilaqua LR, Medina JH, Izquierdo I, Cammarota M. 2008. On the participation of mTOR in recognition memory. Neurobiol Learn Mem 89: 338–351. 10.1016/j.nlm.2007.10.002 [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. 2000. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406: 722–726. 10.1038/35021052 [DOI] [PubMed] [Google Scholar]
- Ozawa T, Yamada K, Ichitani Y. 2014. Hippocampal BDNF treatment facilitates consolidation of spatial memory in spontaneous place recognition in rats. Behav Brain Res 263: 210–216. 10.1016/j.bbr.2014.01.034 [DOI] [PubMed] [Google Scholar]
- Pang PT, Lu B. 2004. Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: role of secreted proteins tPA and BDNF. Ageing Res Rev 3: 407–430. 10.1016/j.arr.2004.07.002 [DOI] [PubMed] [Google Scholar]
- Paratcha G, Furman M, Bevilaqua L, Cammarota M, Vianna M, de Stein ML, Izquierdo I, Medina JH. 2000. Involvement of hippocampal PKCβI isoform in the early phase of memory formation of an inhibitory avoidance learning. Brain Res 855: 199–205. 10.1016/S0006-8993(99)02323-9 [DOI] [PubMed] [Google Scholar]
- Pereyra M, Katche C, de Landeta AB, Medina JH. 2018. mTORC1 controls long-term memory retrieval. Sci Rep 8: 8759 10.1038/s41598-018-27053-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RT, Beal PA, Comb MJ, Schreiber SL. 2000. FKBP12-rapamycin-associated protein (FRAP) autophosphorylates at serine 2481 under translationally repressive conditions. J Biol Chem 275: 7416–7423. 10.1074/jbc.275.10.7416 [DOI] [PubMed] [Google Scholar]
- Przybyslawski J, Sara SJ. 1997. Reconsolidation of memory after its reactivation. Behav Brain Res 84: 241–246. 10.1016/S0166-4328(96)00153-2 [DOI] [PubMed] [Google Scholar]
- Radiske A, Rossato JI, Köhler CA, Gonzalez MC, Medina JH, Cammarota M. 2015. Requirement for BDNF in the reconsolidation of fear extinction. J Neurosci 35: 6570–6574. 10.1523/JNEUROSCI.4093-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard J, Loureiro M, Rosen LG, Zunder J, de Oliveira C, Schmid S, Rushlow WJ, Laviolette SR. 2016. Cannabidiol counteracts amphetamine-induced neuronal and behavioral sensitization of the mesolimbic dopamine pathway through a novel mTOR/p70S6 kinase signaling pathway. J Neurosci 36: 5160–5169. 10.1523/JNEUROSCI.3387-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. 2004. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry 61: 1136–1144. 10.1001/archpsyc.61.11.1136 [DOI] [PubMed] [Google Scholar]
- Revest JM, Le Roux A, Roullot-Lacarrière V, Kaouane N, Vallée M, Kasanetz F, Rougé-Pont F, Tronche F. 2014. BDNF-TrkB signaling through Erk1/2 MAPK phosphorylation mediates the enhancement of fear memory induced by glucocorticoids. Mol Psychiatry 19: 1001–1009. 10.1038/mp.2013.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds TH IV, Bodine SC, Lawrence JC Jr. 2002. Control of Ser2448 phosphorylation in the mammalian target of rapamycin by insulin and skeletal muscle load. J Biol Chem 277: 17657-17662. 10.1074/jbc.M201142200 [DOI] [PubMed] [Google Scholar]
- Rivas DA, Yaspelkis BB III, Hawley JA, Lessard SJ. 2009. Lipid-induced mTOR activation in rat skeletal muscle reversed by exercise and 5′-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside. J Endocrinol 202: 441-451. 10.1677/JOE-09-0202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa JM, Pazini FL, Olescowicz G, Camargo A, Moretti M, Gil-Mohapel J, Rodrigues ALS. 2019. Prophylactic effect of physical exercise on Aβ1-40-induced depressive-like behavior: role of BDNF, mTOR signaling, cell proliferation and survival in the hippocampus. Prog Neuropsychopharmacol Biol Psychiatry 94: 109646 10.1016/j.pnpbp.2019.109646 [DOI] [PubMed] [Google Scholar]
- Rosas-Vidal LE, Rodriguez-Romaguera J, Do-Monte FH, Andero R. 2015. Targeting the reconsolidation of extinction memories: a novel potential strategy to treat anxiety disorders. Mol Psychiatry 20: 1264–1265. 10.1038/mp.2015.136 [DOI] [PubMed] [Google Scholar]
- Rosner M, Siegel N, Valli A, Fuchs C, Hengstschläger M. 2010. mTOR phosphorylated at S2448 binds to raptor and rictor. Amino Acids 38: 223–228. 10.1007/s00726-008-0230-7 [DOI] [PubMed] [Google Scholar]
- Rossato JI, Bevilaqua LR, Lima RH, Medina JH, Izquierdo I, Cammarota M. 2006. On the participation of hippocampal p38 mitogen-activated protein kinase in extinction and reacquisition of inhibitory avoidance memory. Neuroscience 143: 15–23. 10.1016/j.neuroscience.2006.07.025 [DOI] [PubMed] [Google Scholar]
- Rossato JI, Bevilaqua LR, Izquierdo I, Medina JH, Cammarota M. 2010. Retrieval induces reconsolidation of fear extinction memory. Proc Natl Acad Sci 107: 21801–21805. 10.1073/pnas.1016254107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossato JI, Gonzalez MC, Radiske A, Apolinário G, Conde-Ocazionez S, Bevilaqua LR, Cammarota M. 2019. PKMζ inhibition disrupts reconsolidation and erases object recognition memory. J Neurosci 39: 1828–1841. 10.1523/JNEUROSCI.2270-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryskalin L, Lazzeri G, Flaibani M, Biagioni F, Gambardella S, Frati A, Fornai F. 2017. mTOR-dependent cell proliferation in the brain. BioMed Res Int 2017: 7082696 10.1155/2017/7082696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi S, Cappello S, Riccio M, Bergami M, Aicardi G, Schenk U, Matteoli M, Canossa M. 2006. Hippocampal neurons recycle BDNF for activity-dependent secretion and LTP maintenance. EMBO J 25: 4372–4380. 10.1038/sj.emboj.7601303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Nader K, Pruessner JC. 2014. Reconsolidation of human memory: brain mechanisms and clinical relevance. Biol Psychiatry 76: 274–280. 10.1016/j.biopsych.2014.03.008 [DOI] [PubMed] [Google Scholar]
- Slipczuk L, Bekinschtein P, Katche C, Cammarota M, Izquierdo I, Medina JH. 2009. BDNF activates mTOR to regulate GluR1 expression required for memory formation. PLoS One 4: e6007 10.1371/journal.pone.0006007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei N, Kawamura M, Hara K, Yonezawa K, Nawa H. 2001. Brain-derived neurotrophic factor enhances neuronal translation by activating multiple initiation processes: comparison with the effects of insulin. J Biol Chem 276: 42818–42825. 10.1074/jbc.M103237200 [DOI] [PubMed] [Google Scholar]
- Takei N, Inamura N, Kawamura M, Namba H, Hara K, Yonezawa K, Nawa H. 2004. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci 24: 9760–9769. 10.1523/JNEUROSCI.1427-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. 2002. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci 99: 467–472. 10.1073/pnas.012605299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia N, Du J, Tyler WJ, Neale E, Pozzo-Miller L, Lu B. 2001. Protein synthesis-dependent and -independent regulation of hippocampal synapses by brain-derived neurotrophic factor. J Biol Chem 276: 37585–37593. 10.1074/jbc.M101683200 [DOI] [PubMed] [Google Scholar]
- Watanabe R, Wei L, Huang J. 2011. mTOR signaling, function, novel inhibitors, and therapeutic targets. J Nucl Med 52: 497–500. 10.2967/jnumed.111.089623 [DOI] [PubMed] [Google Scholar]
- Yang SJ, Song ZJ, Wang XC, Zhang ZR, Wu SB, Zhu GQ. 2019. Curculigoside facilitates fear extinction and prevents depression-like behaviors in a mouse learned helplessness model through increasing hippocampal BDNF. Acta Pharmacol Sin 40: 1269–1278. 10.1038/s41401-019-0238-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubedat S, Akirav I. 2017. The involvement of cannabinoids and mTOR in the reconsolidation of an emotional memory in the hippocampal-amygdala-insular circuit. Eur Neuropsychopharmacol 27: 336–349. 10.1016/j.euroneuro.2017.01.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.