Abstract
We report the first example of enantioselective, intermolecular diarylcarbene insertion into Si–H bonds for synthesis of silicon-stereogenic silanes. Dirhodium(II) carboxylates catalyze an Si–H insertion using carbenes derived from diazo compounds where selective formation of an enantioenriched silicon center is achieved using prochiral silanes. Fourteen prochiral silanes were evaluated with symmetrical and prochiral diazo reactants to produce a total of 25 novel silanes. Adding an ortho substituent on one phenyl ring of a prochiral diazo enhances enantioselectivity up to 95:5 er with yields up to 98 %. Using in situ IR spectroscopy, the impact of the off-cycle azine formation is supported based on the structural dependence for relative rates of diazo decomposition. A catalytic cycle is proposed where the Si–H insertion step is rate-determining, supported by kinetic isotope effect experiments. Transformations of an enantioenriched silane derived from this method, including selective synthesis of a novel sila-indane, are demonstrated.
The potential utility of chiral-at-silicon compounds incorporated into more complex structures has not been fully understood due to a shortage of synthetic methods. Silicon-stereogenic molecules are rare in number and diversity of structures as compared to carbon. Selected examples to generate silicon-stereogenic silanes include dehydrocouplings,1–3 arylation,4,5 hydrosilylation,6–9 Si–C activation,10,11 and reactions controlled by chiral auxillaries.12–14 Brief explorations of the effect of silicon chirality on reaction outcome to produce more complex molecules have occurred,15–17 yet remain limited.
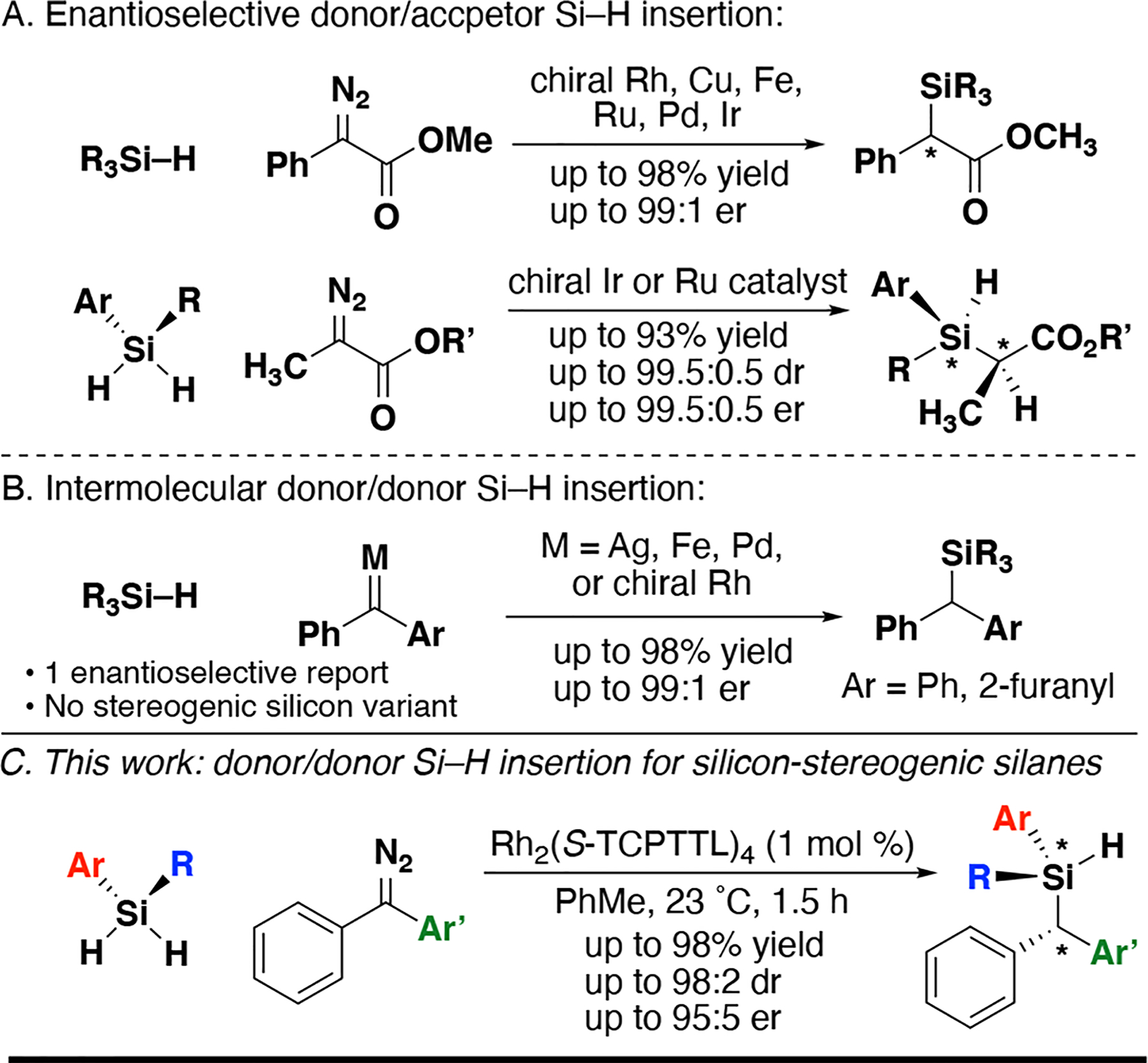
The catalytic insertion of carbenes into Si–H bonds to generate organosilicon compounds has been intermittently explored since Doyle’s original work in 1988.18,19 Methods to date have focused on generation of stereogenic carbon centers using donor/acceptor carbenes (Figure 1A).20–23 Si–H insertion to generate stereogenic silicon centers has been demonstrated by Katsuki24 and Iwasa25 using donor/acceptor carbenes (Figure 1A). Donor/donor carbenes (also referred to as diarylcarbenes) are less reactive, with few reports of intermolecular Si–H insertion, and one report of an enantioselective variant using functionalized alkynes as precursors (Figure 1B).26–29
Figure 1.
Insertion of carbenes into Si–H bonds.
Donor/donor carbenes have recently emerged as useful substrates for highly selective C–H insertion reactions.30–34 Rhodium carbene complexes demonstrate sufficient reactivity at the insertion carbon despite the presence of two aryl rings for potential stablization.35,36 The Franz group has a long-standing interest in organosilicon chemistry and expertise synthesizing prochiral dihydridosilanes with variation of steric and electronic factors.37–39 We envisioned that the additional aryl ring could accomplish an enantioselective intermolecular Si-H insertion process with prochiral silanes (Figure 1C). Herein, we communicate the first enantioselective diarylcarbene Si–H insertion to produce silicon-stereogenic organosilanes.
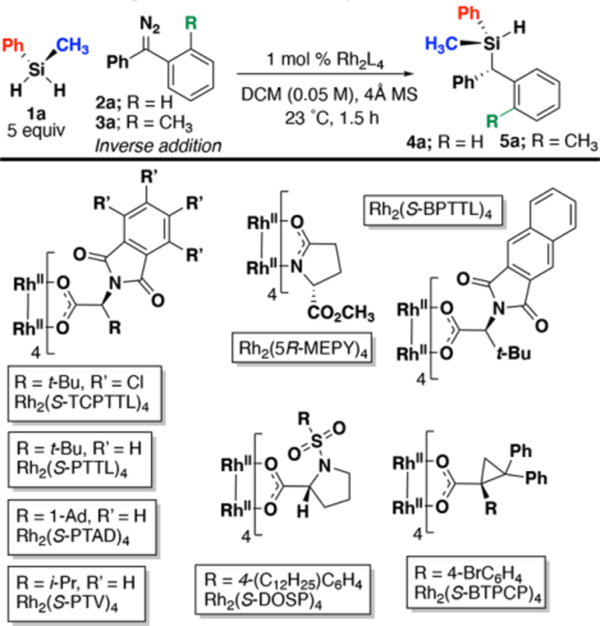
We began our studies screening metal catalysts [Ru(II), Ir(I), Fe(II), Rh(II) and Cu(II)] with diphenyldiazomethane (2a) and prochiral methylphenylsilane (1a). For all experiments, inverse addition of 2a using a syringe pump over 1 hour increased yield of 3a by preventing azine formation, as seen in previous studies with Si–H insertion methodologies.21,40,41 Insertion product 4a was only observed using dirhodium tetraacetate (Table 1, entry 1).42 Based on this lead result, we proceeded to screen chiral dirhodium(II)-based catalysts to identify an enantioselective variant.
Table 1.
Optimization of donor/donor Si–H insertion
| entry | R | Rh2L4 | % yielda | drb | erc |
|---|---|---|---|---|---|
| 1 | H | Rh2(OAc)4 | 34 | - | 50:50 |
| 2 | H | Rh2(5R-MEPY]4 | <5 | - | ND |
| 3 | H | Rh2(S-BTPCP)4 | <5 | - | ND |
| 4 | H | Rh2(S-DOSP)4 | 65 | - | 55:45 |
| 5 | H | Rh2(R-PTAD)4 | 67 | - | 61:39 |
| 6 | H | Rh2(S-PTTL)4 | 62 | - | 64:36 |
| 7 | H | Rh2(S-BPTTL)4 | 62 | - | 64:36 |
| 8 | H | Rh2(S-PTV)4 | 67 | - | 59:41 |
| 9 | H | Rh2(S-TCPTTL)4 | 76 | - | 76:24 |
| 10 | H | Rh2(S-TCPTTL)4d | 78 | - | 82:18 |
| 11 | CH3 | Rh2(OAc)4 | 45 | 55:45 | 50:50 |
| 12 | CH3 | Rh2(R-PTAD)4 | 72 | 60:40 | ND |
| 13 | CH3 | Rh2(S-DOSP)4 | 75 | 61:39 | ND |
| 14 | CH3 | Rh2(S-TCPTTL)4d | 91 | 93:7 | 93:7 |
| 15e | CH3 | Rh2(S-TCPTTL)4d | 81 | 93:7 | 93:7 |
NMR yield using Ph-TMS as an internal standard.
Determined using 1H NMR Spectroscopy.
Determined using CSP-HPLC analysis of silanol obtained from Pd/C hydrolysis; major diastereomer if relevant.
Toluene used as a solvent.
Diazo added via syringe over five minutes.
A screen of well-studied chiral dirhodium compounds highlighted the reactivity of dirhodium tetracarboxylates. Carboxylate ligands afforded higher yields compared to amido-containing ligands due to the increased electrophilicity of the metal center and resulting carbene (entry 2 vs. entries 4–9).43 Of the catalysts studied, Rh2(S-TCPTTL)4 provided the highest levels of enantioselectivity when compared to others (entries 5–8 vs 9), which improved further using toluene (entry 10). When an insertion was tested using prochiral diazo compound 3a, the enantioselectivity of silane product 5a increased from 82:18 to 93:7 er, with a notable increase in yield (76% to 91% yield, entry 10 vs. 14). Manual slow addition of 3a is to seen to form 5a in comparable yield and selectivity to use of a syringe pump (81%, entry 14 vs. 15), but the use of a syringe pump was continued for further studies. Reducing the reaction temperature below 23 °C did not increase selectivity and no insertion was observed below −30 °C. With optimized conditions in hand, we investigated the effect of substituents with both symmetrical and prochiral diazo compounds.
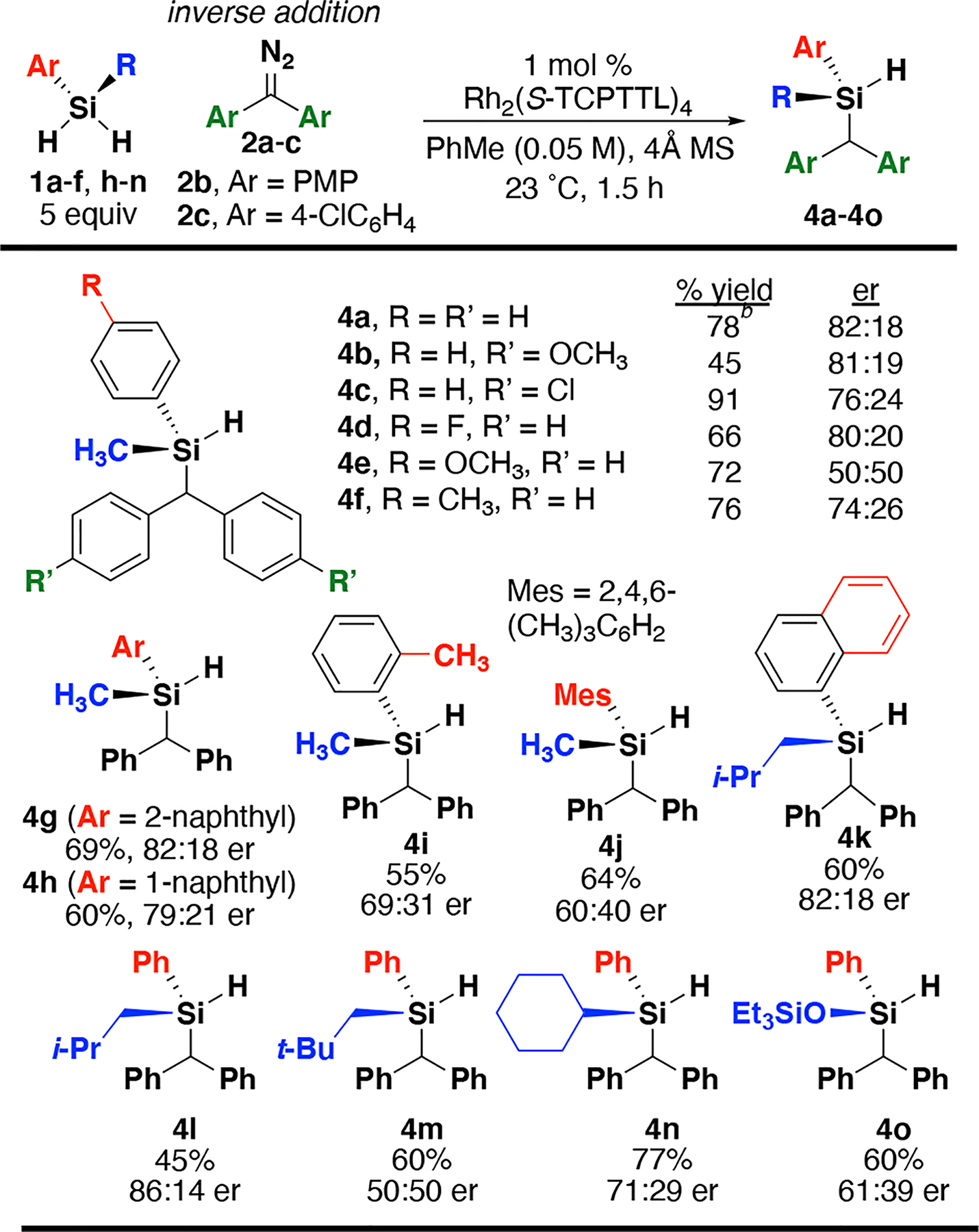
A series of sterically and electronically varied silanes and symmetrical diazo compounds were evaluated to study the effects on enantioselectivity (Scheme 1, 4a-o). Electron-rich diazo 2b was less reactive than 2a and provides lower yield for 4b (45%, 81:19 er). Yield improved using diazo 2c (91%) and lower enantioselectivity was observed for silane 4c (76:24 er). Electronwithdrawing groups do not strongly affect selectivity (4d, 80:20 er), while electron-donating groups on the silane proved deleterious to enantioselectivity (4e and 4f, 50:50 er and 74:26 er respectively). Additional steric bulk on the aryl ring of the silane generally eroded enantioselectivity (4g-j) but maintained fair to good yields (55–69%). Selectivity similar to 4a (82:18) was also observed using 2-naphthyl silane 1g, with the yield also higher compared to 4h (69 vs 60%). A slight recovery of enantioselectivity was also observed with 4k (52%, 82:18 er) compared to 4h (79:21 er), and comparable to 4a. Studies with varied alkyl substitution on the silicon center were conducted with diazo 2a.
Scheme 1.
Scope of enantioselective Si–H insertion with symmetrical diazo compoundsa
a isolated yields; er determined using 1H NMR spectroscopy; er Determined using CSP-HPLC analysis of silanol obtained from Pd/C hydrolysis. bReaction performed using 1.00 g of 3a and 0.05 mol % catalyst, at 0.1 M in toluene. cdr was determined using 19F NMR spectroscopy. dRelative configuration assigned by X-ray analysis.
Isobutyl-containing 4l provided the highest enantioselectivity observed using 2a (86:14 er). However, neopentyl substitution led to loss of enantioselectivity (4m, 50:50 er), and cyclohexyl substitution reduced enantioselectivity as well (4n, 70:30 er). Lastly, switching to a siloxane also deleteriously affected enantioselectivity while maintaining fair yield (40, 0 60%, 61:39 er). We next turned our focus to insertion of prochiral diazo reactants.
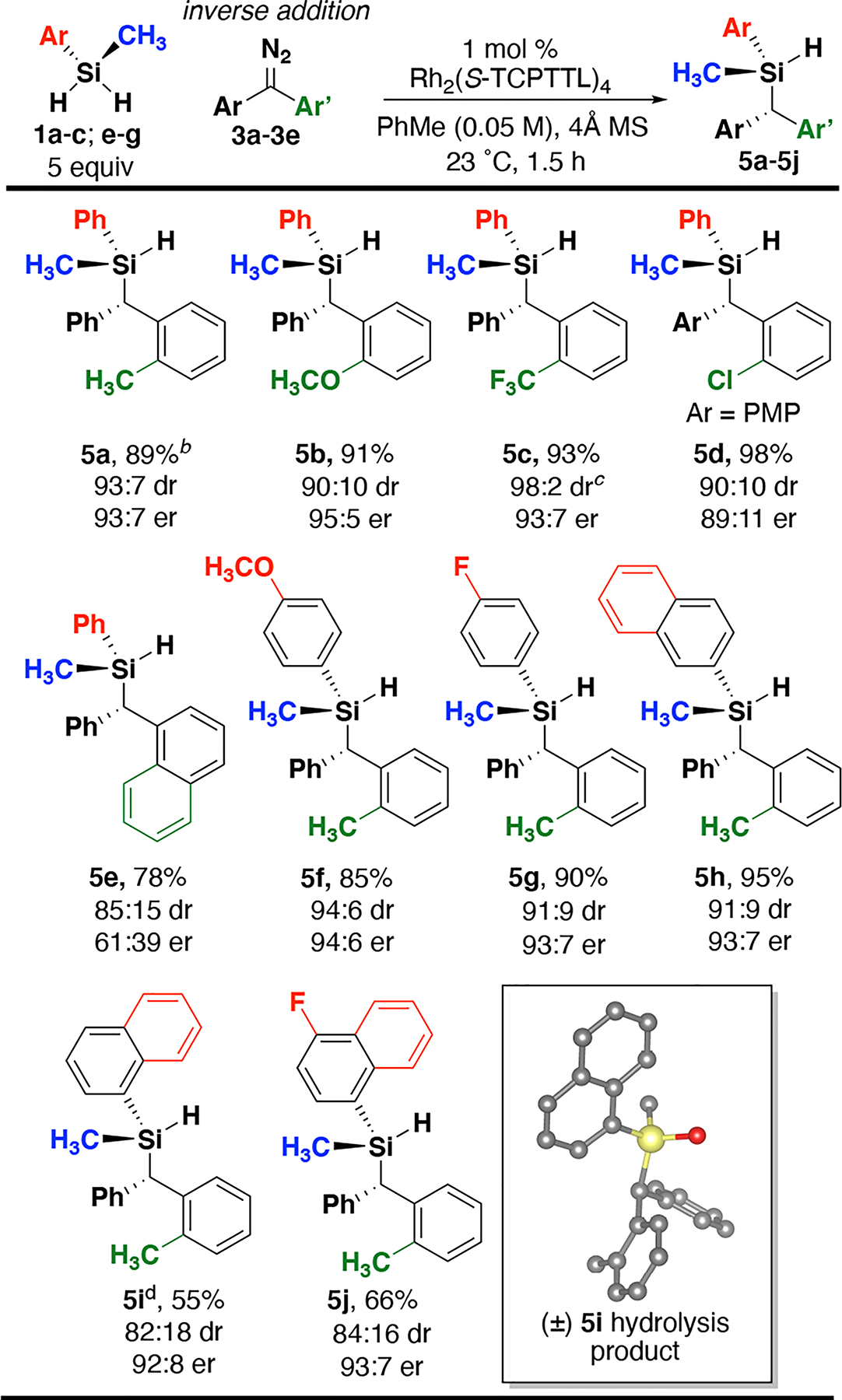
The ability of the ortho substituent on one phenyl ring of the diazo compounds to control enantioselectivity was explored (Scheme 2). Electron-donating substituents lower diastereoselectivity (5b, 90:10 dr vs 93:7 dr), but slightly improve enantioselectivity (95:5 vs 93:7 er). With an electron-withdrawing group (5c), excellent yield and enantioselectivity is observed (93%, 93:7 er) and diastereoselectivity increased (98:2 vs 93:7 dr). Recent work has noted potential synergistic effects of electronics and ortho substitution on the selectivity of donor/donor carbene chemistry.44 Substitution on both phenyl rings was able to achieve excellent yield and good selectivity in 5d (98% yield, 90:10 dr. 89:11 er), although slightly lower compared to other substitution patterns. The steric and push-pull electronic effects combined improve enantioselectivity compared to symmetrical diazo compounds. These substrates demonstrate that the presence of any ortho-substitution may promote enantioselectivity. Replacing phenyl with a 1-naphthyl group led to decreased diastereoselectivity (5e, 85:15 dr) and low enantioselectivity (61:39 er), suggesting other competitive steric effects are present. We sought to explore varied substitution of silanes with prochiral diazo 3a, given the increase in yield and enantioselectivity compared to using 2a. Prochiral silanes were tested with diazo 3a and all demonstrated above 90:10 er for the major diastereomer (Scheme 2, 5f-5j). Additionally, the reaction performed with 1 gram of 3a using <1 mol% catalyst affords excellent yield, diastereoselectivity and enantioselectivity (Scheme 2, 5a). Overall, the data shows that diastereoselectivity is substrate controlled, while enantioselectivity is controlled by the rhodium catalyst. Notably, using a diastereoselective reaction with silane 1c promotes enantioselectivity with 5f (94:6 er) compared to 4e (50:50 er). This result highlights the benefit of using prochiral 3a to improve enantioselectivity.
Scheme 2.
Scope of enantioselective Si–H insertion with prochiral diazo reagentsa
a Isolated yields; dr determined using 1H NMR spectroscopy; er Determined using CSP-HPLC analysis of silanol obtained from Pd/C hydrolysis. bReaction performed using 1.00 g of 3a and 0.05 mol % catalyst, at 0.1 M in toluene. cdr was determined using 19F NMR spectroscopy. dRelative configuration assigned by X-ray analysis.
A catalytic cycle for the enantioselective Si–H insertion of donor/donor carbenes is proposed (Figure 2A).36 The Rh(II) carboxylate catalyst (I) reacts with the diazo compound (2a or 3a) to form complex II, which is approached by prochiral silane 1a to produce the silicon-stereogenic silane and regenerate catalyst. Kinetic isotope effect experiments support the rate-determining insertion step (kH/kD = 1.6), fitting closely with previous experiments of Si–H insertion with donoracceptor20,41,45 and donor/donor carbenes.29. Off-cycle formation of azine (6 or 7) can occur when metal carbene II reacts with another diazo reactant. Using in situ IR spectroscopy, we determined that the ortho-substituted prochiral diazo 3a has a significantly reduced rate of azine formation (vs 2a), which accounts for higher yields of the Si–H insertion products. Relative rates of azine formation (krel > 120) was observed for decomposition of diazo 2a vs 3a with Rh2(S-TCPTTL)4 (in toluene) in the absence of silane (Figure 2A).46 Increased yields of Si–H insertion products with ortho-substitution (5a-i) are attributed to steric interactions blocking the approach of diazo 3a to II, which reduces off-cycle azine formation. The addition of 4Å mol sieves reduces off-cycle processes leading to formation of siloxane 8.42 The increase in enantioselectivity observed with prochiral donor/donor diazo 3 is attributed to a twisting of the ortho substituted aryl ring, which blocks one face of the carbene in II to promote selective approach of the silane (Figure 2B).44,47,48 There has been a recent report proposing the twisting effect to have electronic contributions similar to that of a donoracceptor carbene.44 However, we hypothesize that the steric effect of an out-of-plane phenyl twist is more significant than the electronic effects, although the analogy is noted.
Figure 2.
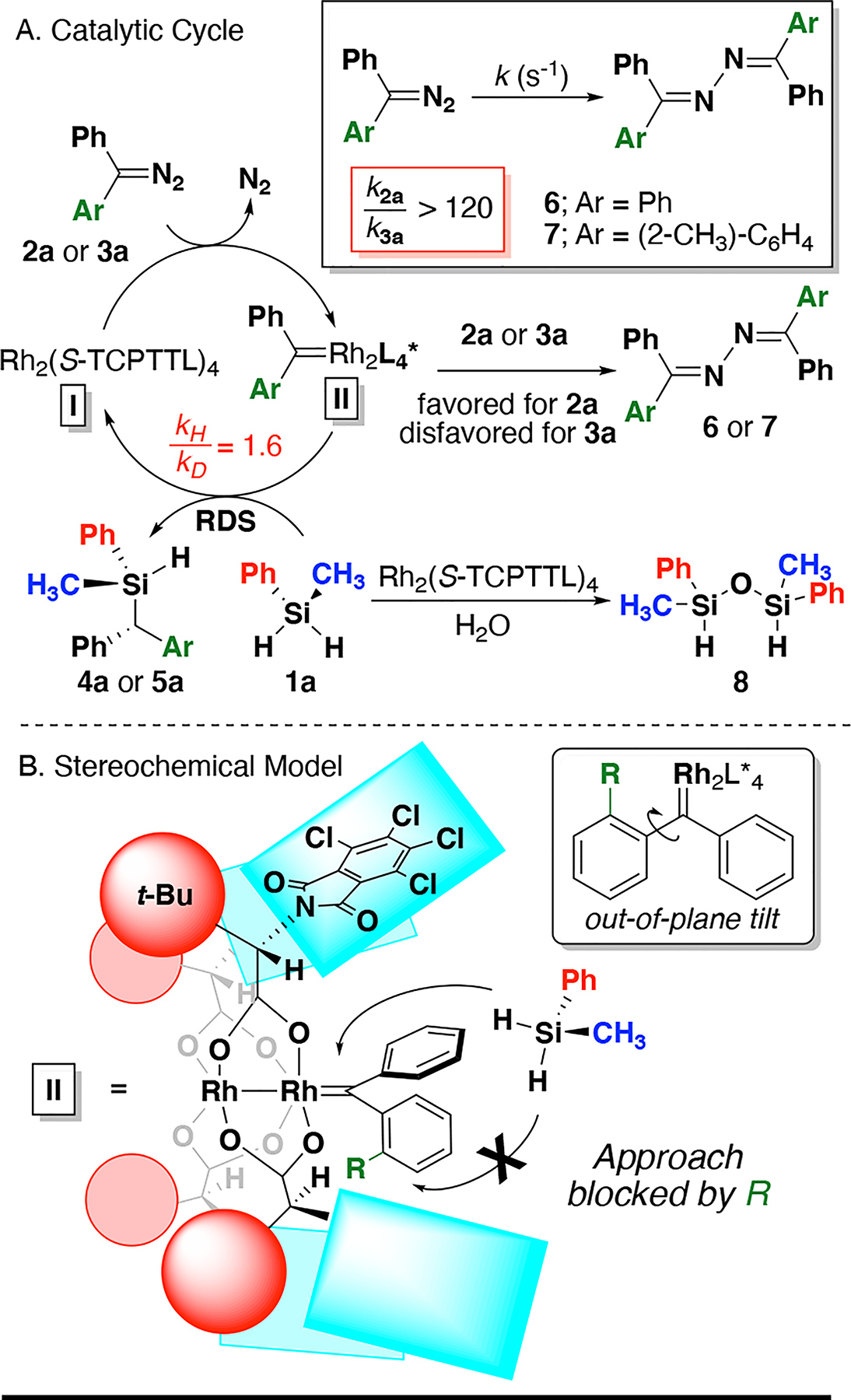
A. Proposed catalytic cycle with kinetic isotope effect; B. Diagram of proposed selectivity rationale of donor/donor insertions.
To demonstrate the utility of enantioenriched silanes, silane 5a was transformed to silanol, dehydrocoupling, and intramolecular C–H silylation products. Silanes are useful intermediates in stereoselective synthesis, and have versatile reactivity with the remaining Si–H bond.49 It is well know that transition metals are capable of oxidative insertion into Si–H bonds with retention of configuration.50,51 Pd/C-catalyzed silane hydrolysis affords silanol 9 in 90% yield with 90:10 dr and 93:7 er.38,51,52 Under attempted hydrosilylation conditions, an unexpected dehydrocoupling product 10 was isolated in good yield (62%) and 93:7 dr.53–55 Exploiting the presence of the ortho-methyl group, diasteroenriched sila-indane 11 was accessed in 90% yield with 90:10 dr using Ir-catalyzed C–H silylation methodology developed by the Hartwig group.56,57
In conclusion, the first example of enantioselective diarylcarbene insertion into Si–H bonds has been accomplished with Rh2(S-TCPTTL)4, yielding silicon-stereogenic benzhydryl silanes. While symmetrical diazo compounds demonstrated initial enantioselectivity, using a prochiral diazo reactant dramatically improved the reaction, providing yields up to 98% with 98:2 dr and 95:5 er. A catalytic cycle is proposed and the impact of the off-cycle azine formation is supported based on the structural dependence for relative rates of diazo decomposition. Transformation of the enantioenriched silane affords access to silicon-stereogenic silanol, dehydrocoupling and intramolecular C–H silylation products.
Supplementary Material
Scheme 3.
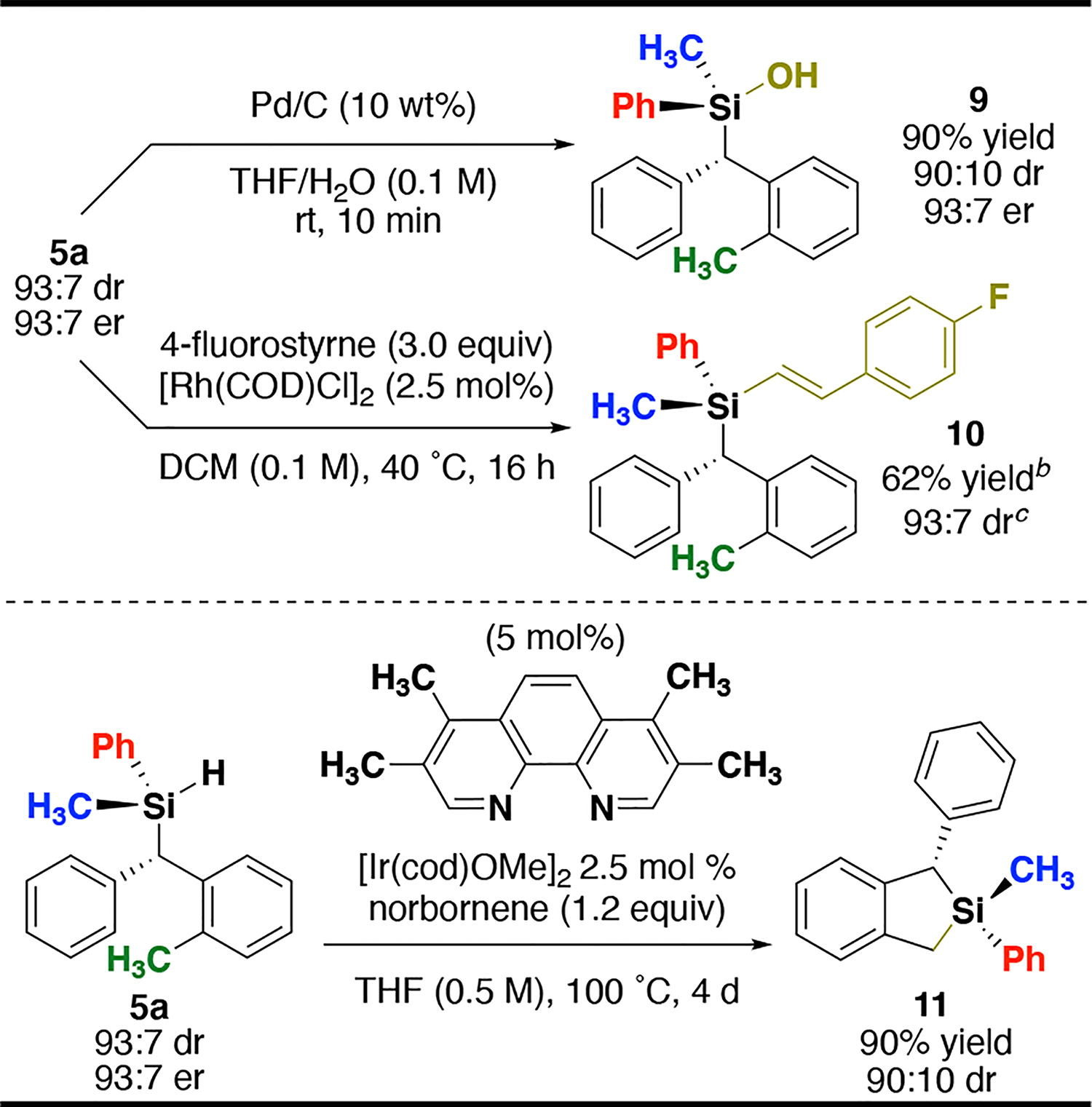
Transformations of Si–H insertion productsa
a Isolated yields; dr determined using 1H NMR spectroscopy; er determined using CSP-HPLC. bIsolated as a 85:15 (major) mixture with the hydrosilylation product. See SI for more information. cdr determined using 19F NMR spectroscopy.
ACKNOWLEDGMENT
Will Jewell is acknowledged for assistance with acquiring mass spectrometry data. Benjamin Bergstrom, Sarah Dishman and Lucas Souza are acknowledged for helpful discussion.
Funding Sources
We acknowledge the National Science Foundation for support of this research (CHE-1900300, CHE-1363375; AKF) and the dual source diffractometer (CHE-1531193) used in this study. The National Institutes of Health (R01 GM124234; JTS) is also acknowledged for support of this research.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Schmidt DR; O’Malle SJ; Leighton JL Catalytic Asymmetric Silane Alcoholysis: Practical Access to Chiral Silanes. J. Am. Chem. Soc 2003, 125 (5), 1190–1191. [DOI] [PubMed] [Google Scholar]; See ref. 2 and 3 for more examples of dehydrocoupling methodology to access stereogenic silanes.
- (2).Li N; Guan B Yttrium–Benzyl Complexes Bearing Chiral Iminophosphonamide Ligands: Synthesis and Application in Catalytic Asymmetric Amine-Silane Dehydrocoupling Reactions. Adv. Synth. Catal 2017, 359 (20), 3526–3531. [Google Scholar]
- (3).Corriu RJP; Moreau JJE Asymmetric Synthesis at Silicon. J. Organomet. Chem 1976, 120 (3), 337–346. [Google Scholar]
- (4).Koga S; Ueki S; Shimada M; Ishii R; Kurihara Y; Yamanoi Y; Yuasa J; Kawai T; Uchida TA; Iwamura M; Nozaki K; Nishihara H Access to Chiral Silicon Centers for Application to Circularly Polarized Luminescence Materials. J. Org. Chem 2017, 82 (12), 6108–6117. [DOI] [PubMed] [Google Scholar]; See ref. 5 for more examples of arylation methodology to access stereogenic silanes.
- (5).Kurihara Y; Nishikawa M; Yamanoi Y; Nishihara H Synthesis of Optically Active Tertiary Silanes via Pd-Catalyzed Enantioselective Arylation of Secondary Silanes. Chem. Commun 2012, 48 (94), 11564–11566. [DOI] [PubMed] [Google Scholar]
- (6).Igawa K; Yoshihiro D; Ichikawa N; Kokan N; Tomooka K Catalytic Enantioselective Synthesis of Alkenylhydrosilanes. Angew. Chemie Int. Ed 2012, 51 (51), 12745–12748. [DOI] [PubMed] [Google Scholar]; See ref. 7–9 for more examples of hydrosilylation methodology to access stereogenic silanes.
- (7).Hayashi T; Yamamoto K; Kumada M Asymmetric Synthesis of Bifunctional Organosilicon Compounds via Hydrosilylation. Tetrahedron Lett 1974, 15 (4), 331–334. [Google Scholar]
- (8).Ohta T; Ito M; Tsuneto A; Takaya H Asymmetric Synthesis of Silanes with a Stereogenic Centre at Silicon via Hydrosilylation of Symmetric Ketones with Prochiral Diaryl Silanes Catalysed by Binap-Rh Complexes. J. Chem. Soc. Chem. Commun 1994, No. 21, 2525–2526. [Google Scholar]
- (9).Zhao Z-Y; Nie Y-X; Tang R-H; Yin G-W; Cao J; Xu Z; Cui Y-M; Zheng Z-J; Xu L-W Enantioselective Rhodium-Catalyzed Desymmetric Hydrosilylation of Cyclopropenes. ACS Catal. 2019, 9 (10), 9110–9116. [Google Scholar]
- (10).Shintani R Recent Advances in the Transition-Metal-Catalyzed Enantioselective Synthesis of Silicon-Stereogenic Organosilanes. Asian J. Org. Chem 2015, 4, 510–514. [Google Scholar]; See ref. 11 for more examples of Si–C activation to access stereogenic silanes.
- (11).Shintani R Recent Progress in Catalytic Enantioselective Desymmetrization of Prochiral Organosilanes for the Synthesis of Silicon-Stereogenic Compounds. Synlett 2018, 29 (4), 388–396. [Google Scholar]
- (12).Bauer JO; Strohmann C Stereocontrol in Nucleophilic Substitution Reactions at Silicon: The Role of Permutation in Generating Silicon-Centered Chirality. J. Am. Chem. Soc 2015, 137 (13), 4304–4307. [DOI] [PubMed] [Google Scholar]; See ref. 13 and 14 for more examples of using chiral auxillaries to access stereogenic silanes.
- (13).Bauer JO; Strohmann C Stereoselective Synthesis of Silicon-Stereogenic Aminomethoxysilanes: Easy Access to Highly Enantiomerically Enriched Siloxanes. Angew. Chemie Int. Ed 2014, 53 (3), 720–724. [DOI] [PubMed] [Google Scholar]
- (14).Kimiko K; Takayuki K; Masafumi U; Shinji M Asymmetric Synthesis of Organosilicon Compounds Using a C2 Chiral Auxiliary. Bull. Chem. Soc. Jpn 1997, 70 (6), 1393–1401. [Google Scholar]
- (15).Xu L-W; Li L; Lai G-Q; Jiang J-X The Recent Synthesis and Application of Silicon-Stereogenic Silanes: A Renewed and Significant Challenge in Asymmetric Synthesis. Chem. Soc. Rev 2011, 40 (3), 1777–1790. [DOI] [PubMed] [Google Scholar]; See ref. 16 and 17 for more examples of transformations using silicon-stereogenic silanes.
- (16).Bauer JO; Strohmann C Recent Progress in Asymmetric Synthesis and Application of Difunctionalized Silicon-Stereogenic Silanes. Eur. J. Inorg. Chem 2016, 2016 (18), 2868–2881. [Google Scholar]
- (17).Shintani R Catalytic Asymmetric Synthesis of Siliconstereogenic Compounds by Enantioselective Desymmetrization of Prochiral Tetraorganosilanes. Yuki Gosei Kagaku Kyokaishi/Journal Synth. Org. Chem 2018, 76 (11), 1163–1169. [Google Scholar]
- (18).Keipour H; Carreras V; Ollevier T Recent Progress in the Catalytic Carbene Insertion Reactions into the Silicon-Hydrogen Bond. Org. Biomol. Chem 2017, 15 (26), 5441–5456. [DOI] [PubMed] [Google Scholar]; See ref 19–25 for work on enantioselective donor/acceptor carbene insertions into Si–H bonds.
- (19).Bagheri V; Doyle MP; Taunton J; Claxton EE A New and General Synthesis of .Alpha.-Silyl Carbonyl Compounds by Silicon-Hydrogen Insertion from Transition Metal-Catalyzed Reactions of Diazo Esters and Diazo Ketones. J. Org. Chem 1988, 53 (26), 6158–6160. [Google Scholar]
- (20).Chen D; Zhu D-X; Xu M-H Rhodium(I)-Catalyzed Highly Enantioselective Insertion of Carbenoid into Si–H: Efficient Access to Functional Chiral Silanes. J. Am. Chem. Soc 2016, 138 (5), 1498–1501. [DOI] [PubMed] [Google Scholar]
- (21).Gu H; Han Z; Xie H; Lin X Iron-Catalyzed Enantioselective Si–H Bond Insertions. Org. Lett 2018, 20 (20), 6544–6549. [DOI] [PubMed] [Google Scholar]
- (22).Dakin LA; Ong PC; Panek JS; Staples RJ; Stavropoulos P Speciation and Mechanistic Studies of Chiral Copper(I) Schiff Base Precursors Mediating Asymmetric Carbenoid Insertion Reactions of Diazoacetates into the Si–H Bond of Silanes. Organometallics 2000, 19 (15), 2896–2908. [Google Scholar]
- (23).Kan SBJ; Lewis RD; Chen K; Arnold FH Directed Evolution of Cytochrome c for Carbon-Silicon Bond Formation: Bringing Silicon to Life. Science 2016, 354 (6315), 1048–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Yasutomi Y; Suematsu H; Katsuki T Iridium(III)-Catalyzed Enantioselective Si–H Bond Insertion and Formation of an Enantioenriched Silicon Center. J. Am. Chem. Soc 2010, 132 (13), 4510–4511. [DOI] [PubMed] [Google Scholar]
- (25).Nakagawa Y; Chanthamath S; Fujisawa I; Shibatomi K; Iwasa S Ru(II)-Pheox-Catalyzed Si–H Insertion Reaction: Construction of Enantioenriched Carbon and Silicon Centers. Chem. Commun 2017, 53 (26), 3753–3756. [DOI] [PubMed] [Google Scholar]
- (26).Liu Z; Li Q; Yang Y; Bi X Silver(i)-Promoted Insertion into X–H (X = Si, Sn, and Ge) Bonds with N-Nosylhydrazones. Chem. Commun 2017, 53 (16), 2503–2506. [DOI] [PubMed] [Google Scholar]; See ref. 27–29 for previous work on donor/donor carbene insertion into Si–H bonds.
- (27).Liu Z; Huo J; Fu T; Tan H; Ye F; Hossain ML; Wang J Palladium(0)-Catalyzed C(Sp3)–Si Bond Formation via Formal Carbene Insertion into a Si–H Bond. Chem. Commun 2018, 54 (81), 11419–11422. [DOI] [PubMed] [Google Scholar]
- (28).Wang EH; Ping YJ; Li ZR; Qin H; Xu ZJ; Che CM Iron Porphyrin Catalyzed Insertion Reaction of N-Tosylhydrazone-Derived Carbenes into X-H (X = Si, Sn, Ge) Bonds. Org. Lett 2018, 20 (15), 4641–4644. [DOI] [PubMed] [Google Scholar]
- (29).Huang M-Y; Yang J-M; Zhao Y-T; Zhu S-F Rhodium-Catalyzed Si–H Bond Insertion Reactions Using Functionalized Alkynes as Carbene Precursors. ACS Catal. 2019, 9 (6), 5353–5357. [Google Scholar]
- (30).Soldi C; Lamb KN; Squitieri RA; González-López M; Di Maso MJ; Shaw JT Enantioselective Intramolecular C–H Insertion Reactions of Donor–Donor Metal Carbenoids. J. Am. Chem. Soc 2014, 136 (43), 15142–15145. [DOI] [PMC free article] [PubMed] [Google Scholar]; See ref. 31–34 on recent work with donor/donor carbenes.
- (31).Souza LW; Squitieri RA; Dimirjian CA; Hodur BM; Nickerson LA; Penrod CN; Cordova J; Fettinger JC; Shaw JT Enantioselective Synthesis of Indolines, Benzodihydrothiophenes, and Indanes by C–H Insertion of Donor/Donor Carbenes. Angew. Chemie 2018, 130 (46), 15433–15436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Lamb KN; Squitieri RA; Chintala SR; Kwong AJ; Balmond EI; Soldi C; Dmitrenko O; Castiñeira Reis M; Chung R; Addison JB; Fettinger JC; Hein JE; Tantillo DJ; Fox JM; Shaw JT Synthesis of Benzodihydrofurans by Asymmetric C–H Insertion Reactions of Donor/Donor Rhodium Carbenes. Chem. – A Eur. J 2017, 23 (49), 11843–11855. [DOI] [PubMed] [Google Scholar]
- (33).Zhu D; Chen L; Fan H; Yao Q; Zhu S Recent Progress on Donor and Donor–Donor Carbenes. Chem. Soc. Rev 2020, 49 (3), 908–950. [DOI] [PubMed] [Google Scholar]
- (34).Nickerson LA; Bergstrom BD; Gao M; Shiue YS; Laconsay CJ; Culberson MR; Knauss WA; Fettinger JC; Tantillo DJ; Shaw JT Enantioselective Synthesis of Isochromans and Tetrahydroisoquinolines by C-H Insertion of Donor/Donor Carbenes. Chem. Sci 2020, 11(2), 494–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Werlé C; Goddard R; Philipps P; Farès C; Fürstner A Structures of Reactive Donor/Acceptor and Donor/Donor Rhodium Carbenes in the Solid State and Their Implications for Catalysis. J. Am. Chem. Soc 2016, 138 (11), 3797–3805. [DOI] [PubMed] [Google Scholar]
- (36).Davis PJ; Harris L; Karim A; Thompson AL; Gilpin M; Moloney MG; Pound MJ; Thompson C Substituted Diaryldiazomethanes and Diazofluorenes: Structure, Reactivity and Stability. Tetrahedron Lett. 2011, 52 (14), 1553–1556. [Google Scholar]
- (37).Tran NT; Wilson SO; Franz AK Cooperative Hydrogen-Bonding Effects in Silanediol Catalysis. Org. Lett 2012, 14 (1), 186–189. [DOI] [PubMed] [Google Scholar]; See ref. 38 amd 39 for our previous work on the synthesis of silanes.
- (38).Diemoz KM; Wilson SO; Franz AK Synthesis of Structurally Varied 1,3-Disiloxanediols and Their Activity as Anion-Binding Catalysts. Chemistry (Easton). 2016, 22 (51), 18349–18353. [DOI] [PubMed] [Google Scholar]
- (39).Diemoz KM; Hein JE; Wilson SO; Fettinger JC; Franz AK Reaction Progress Kinetics Analysis of 1,3-Disiloxanediols as Hydrogen-Bonding Catalysts. J. Org. Chem 2017, 82 (13), 6738–6747. [DOI] [PubMed] [Google Scholar]
- (40).Keipour H; Jalba A; Delage-Laurin L; Ollevier T Copper-Catalyzed Carbenoid Insertion Reactions of α-Diazoesters and α-Diazoketones into Si–H and S–H Bonds. J. Org. Chem 2017, 82 (6), 3000–3010. [DOI] [PubMed] [Google Scholar]
- (41).Landais Y; Parra-Rapado L; Planchenault D; Weber V Mechanism of Metal-Carbenoid Insertion into the Si–H Bond. Tetrahedron Lett. 1997, 38 (2), 229–232. [Google Scholar]
- (42).Hydrolysis of Si–H bonds using diRhodium(II) compounds has been previous observed:; Doyle MP; High KG; Bagheri V; Pieters R; Lewis PJ; Pearson MM Rhodium(II) Perfluorobutyrate Catalyzed Silane Alcoholysis. A Highly Selective Route to Silyl Ethers. J. Org. Chem 1990, 55 (25), 6082–6086. [Google Scholar]
- (43).Lloret J; Carbó JJ; Bo C; Lledós A; Pérez-Prieto J Influence of the Nature of the Ligand on Dirhodium(II) Carbene Species: A Theoretical Analysis. Organometallics 2008, 27 (12), 2873–2876. [Google Scholar]
- (44).Previous work supports the twisting of the phenyl ring for carbene II caused by ortho substitution, see:; Lee M; Ren Z; Musaev DG; Davies HML Rhodium-Stabilized Diarylcarbenes Behaving as Donor/Acceptor Carbenes. ACS Catal. 2020, 6240–6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Yasutomi Y; Suematsu H; Katsuki T Iridium(III)-Catalyzed Enantioselective Si–H Bond Insertion and Formation of an Enantioenriched Silicon Center. J. Am. Chem. Soc 2010, 132 (13), 4510–4511. [DOI] [PubMed] [Google Scholar]
- (46).Azines 6 and 7 Were Formed >90 % yield during kinetic exeperiments as determined using 1H NMR spectroscopy with Ph-TMS as an internal standard; thereore the rates of azine formation were assumed to correlate directly with the consumption of diazo compounds 2a and 3a.
- (47).The rationale for the “all up” conformation of ligands is based on:; Lindsay VNG; Charette AB Design and Synthesis of Chiral Heteroleptic Rhodium(II) Carboxylate Catalysts: Experimental Investigation of Halogen Bond Rigidification Effects in Asymmetric Cyclopropanation. ACS Catal. 2012, 2 (6), 1221–1225. [Google Scholar]
- (48).The rationale for a twisted phenyl ring in the structure of carbene II is based of X-ray data from: Werlé C; Goddard R; Fürstner A The First Crystal Structure of a Reactive Dirhodium Carbene Complex and a Versatile Method for the Preparation of Gold Carbenes by Rhodiumto-Gold Transmetalation. Angew. Chemie Int. Ed 2015, 54 (51), 15452–15456. [DOI] [PMC free article] [PubMed] [Google Scholar]; Refer to ref. 44 and 32 for computational support for this structure.
- (49).Fleming I; Barbero A; Walter D Stereochemical Control in Organic Synthesis Using Silicon-Containing Compounds. Chem. Rev 1997, 97 (6), 2063–2192. [DOI] [PubMed] [Google Scholar]
- (50).The insertion of Rh(I) and Ir(I) is known to occur with retention of stereochemistry: Oestreich M. Chirality Transfer from Silicon to Carbon. Chem. – A Eur. J 2006, 12 (1), 30–37. [DOI] [PubMed] [Google Scholar]; See ref. 51 for more information.
- (51).Corriu RJP; Guérin C; Moreau JJE Stereochemistry at Silicon. Topics in Stereochemistry. January 1, 1984, pp 43–198. [Google Scholar]
- (52). Hydrolysis of the silane to the silanol using Pd/C/H2O conditions occurs with inversion of stereochemistry, see ref. 15 and 51 for more information. Hydrolysis of the silane to the silanol under these conditons was performed on all substrates to ensure separation of enantiomers for CSP-HPLC analysis.
- (53).Onopchenko A; Sabourin ET; Beach DL Vinyl- and Allylsilanes from the Rhodium(I)-Catalyzed Hydrosilylation of 1-Alkenes with Trialkylsilanes. J. Org. Chem 1984, 49 (18), 3389–3392. [Google Scholar]
- (54).Kakiuchi F; Nogami K; Chatani N; Seki Y; Murai S Dehydrogenative Silylation of 1,5-Dienes with Hydrosilanes Catalyzed by RhCl(PPh3)3. Organometallics 1993, 12 (12), 4748–4750. [Google Scholar]
- (55).Adams C; Riviere P; Riviere-Baudet M; Morales-Verdejo C; Dahrouch M; Morales V; Castel A; Delpech F; Manríquez JM; Chávez I Catalytic Study of Heterobimetallic Rhodium Complexes Derived from Partially Alkylated S-Indacene in Dehydrogenative Silylation of Olefins. J. Organomet. Chem 2014, 749, 266–274. [Google Scholar]
- (56).Cheng C; Hartwig JF Catalytic Silylation of Unactivated C–H Bonds. Chem. Rev 2015, 115 (17), 8946–8975. [DOI] [PubMed] [Google Scholar]
- (57).Karmel C; Chen Z; Hartwig JF Iridium-Catalyzed Silylation of C–H Bonds in Unactivated Arenes: A Sterically Encumbered Phenanthroline Ligand Accelerates Catalysis. J. Am. Chem. Soc 2019, 141 (17), 7063–7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.