Abstract
Chronic cholangiohepatitis (CCH) is a common pathological condition in cats with a guarded prognosis and unknown etiology. Recently, in human medicine, there has been increased interest in enhancing liver defense mechanisms as an effective treatment strategy to control liver diseases that have a poor prognosis. Metallothionein (MT) is a ubiquitous protein, which has been widely researched for its role in liver defense through heavy metal detoxification, neutralization of reactive oxygen species, and liver regeneration. In this study, immunohistochemistry was used to evaluate the role of MT in CCH and hepatocellular regeneration in 34 cats histologically diagnosed with this condition by assessing the correlation between hepatocellular MT and Ki-67 (marker for cellular proliferation) expression with histological parameters of CCH, such as inflammation, fibrosis, and bile duct proliferation. Statistical analysis was performed using the Spearman-rank correlation test. A significant positive correlation was observed between inflammation and the number of MT-positive hepatocytes (r = 0.36, P = 0.03) and MT labelling intensity (r = 0.37, P = 0.03). In 16 of 34 cases (47%) MT labelling intensity was noted to be pronounced towards the centrilobular zone and very weak or absent towards the portal zone. The results suggest that MT is induced in the liver during chronic inflammatory conditions, which could be speculated as a host defensive mechanism to protect the liver from inflammation-mediated liver injury. Therapeutic interventions utilizing MT, therefore, may have a positive effect on cats with chronic cholangiohepatitis.
Résumé
La cholangiohépatite chronique (CCH) est une affection pathologique courante chez les chats avec un pronostic réservé et une étiologie inconnue. Récemment, en médecine humaine, il y a eu un intérêt accru pour l’amélioration des mécanismes de défense hépatique en tant que stratégie de traitement efficace pour contrôler les maladies du foie qui ont un mauvais pronostic. La métallothionéine (MT) est une protéine omniprésente, qui a été largement étudiée pour son rôle dans la défense du foie par la détoxification des métaux lourds, la neutralisation des espèces réactives de l’oxygène et la régénération du foie. Dans cette étude, l’immunohistochimie a été utilisée pour évaluer le rôle de la MT dans la CCH et la régénération hépatocellulaire chez 34 chats diagnostiqués histologiquement avec cette condition en évaluant la corrélation entre l’expression hépatocellulaire de la MT et du Ki-67 (marqueur de la prolifération cellulaire) avec les paramètres histologiques de la CCH, comme l’inflammation, la fibrose et la prolifération des voies biliaires. L’analyse statistique a été réalisée à l’aide du test de corrélation de rang de Spearman. Une corrélation positive significative a été observée entre l’inflammation et le nombre d’hépatocytes MT-positifs (r = 0,36, P = 0,03) et l’intensité de marquage MT (r = 0,37, P = 0,03). Dans 16 des 34 cas (47 %), l’intensité du marquage MT était prononcée vers la zone centrolobulaire et très faible ou absente vers la zone porte. Les résultats suggèrent que la MT est induite dans le foie pendant les états inflammatoires chroniques, ce qui pourrait être supposé comme un mécanisme de défense de l’hôte pour protéger le foie contre les lésions hépatiques induites par l’inflammation. Les interventions thérapeutiques utilisant la MT peuvent donc avoir un effet positif sur les chats atteints de cholangiohépatite chronique.
(Traduit par Docteur Serge Messier)
Introduction
Cholangiohepatitis is one of the most common pathological conditions affecting the liver of cats; second in prevalence to hepatic lipidosis (1,2). This disease condition is characterized by inflammation of bile ducts and surrounding parenchyma with infiltration of lymphocytes and plasma cells alone or in combination with neutrophils. Acute cholangiohepatitis in cats usually arises from ascending bacterial infections of the biliary tract and the inflammatory cells are mostly comprised of neutrophils (3,4). With time, occasionally, acute cholangiohepatitis progresses to a chronic non-suppurative phase, characterized by the infiltration of lymphocytes and plasma cells, proliferation of bile ducts, and peri-portal fibrosis. At a later stage, cirrhosis develops that includes bridging fibrosis, nodular regeneration, and variable degrees of chronic inflammation (4). The clinical course of progression from the acute to chronic stage of this disease is always subtle and difficult to distinguish. The pathogenesis is not well-characterized in the literature, although immune-mediated mechanisms and chronic persistent bacterial infections have been discussed in conjunction with this condition (1,4). Although acute cholangiohepatitis can be effectively controlled by prompt identification and treatment of the inciting cause (3); the chronic form of the disease has a guarded prognosis because the etiology is often unidentified (1,2,4).
Recently, in human medicine, there has been more emphasis on enhancing liver defense mechanisms as a treatment strategy for chronic liver disease, instead of targeting the initial inciting cause (5). Metallothionein (MT), an inducible intracellular heavy metal binding protein, has been proven to have hepatoprotective properties. The putative functions of MT include homeostasis, detoxification of heavy metals, and protection against oxidative stress by scavenging a wide range of oxidants and electrophiles (6–8). Metallothionein is capable of binding toxic metals such as cadmium, mercury, platinum, and silver, protecting cells and tissues from heavy metal-induced toxicity. The protein also acts as a major reserve for biologically essential metals such as zinc and copper (9). The anti-inflammatory, antifibrotic, and hepatocellular regenerative properties of MT have been explicated in previous studies in mice (5,10,11). In dogs, MT expression was found to be positively correlated with hepatocellular regeneration and negatively correlated to hepatic fibrosis (12). However, in a recent study in horses, no significant correlation was found between MT expression and Ki-67 expression with inflammation, fibrosis, and bile duct proliferation (13). These results suggest the possibility for species difference in MT expression, in relation to the histologic parameters in chronic liver inflammation.
An investigation of MT expression in the liver of cats affected by chronic cholangiohepatitis (CCH) has not been conducted previously. Hepatic inflammation in cats is primarily centered on bile ducts, with involvement of hepatic parenchyma being secondary; hence the term cholangitis or cholangiohepatitis is used to describe inflammatory conditions of the liver in cats (3,4). Clinical manifestations of CCH in cats are often subtle and provide diagnostic and therapeutic challenges to clinicians. The pathogenesis of the disease is poorly understood and the etiology is often not identified (2). Therefore, cats, similar to humans, may benefit from treatment modalities enhancing the expression of MT when CCH is diagnosed.
The objective of this study was to evaluate the expression of MT in formalin-fixed paraffin-embedded liver sections from cats affected by CCH using immunohistochemistry and to assess the correlation between its expression and hepatic regeneration, inflammation, fibrosis, and bile duct proliferation (BDP). It was hypothesized that MT expression is positively correlated with hepatic regeneration and inflammation and negatively correlated with BDP and fibrosis.
Materials and methods
Case selection and histologic scoring
Case records from 2009 to 2018, inclusive, from the archives of Prairie Diagnostic Services Inc., Saskatoon, Saskatchewan, Canada, were searched for cats with the histologic diagnosis of CCH. Hematoxylin and eosin (H&E) stained glass slides, corresponding to these cases were reviewed by a Board-certified pathologist (A.N.A) to confirm the initial diagnosis and 34 cases (Tables I, II) were selected if at least 1 cm2 of liver tissue was available for examination. Liver tissues from these selected cases were scored for inflammation, fibrosis and BDP, which were the diagnostic criteria for CCH as outlined by the World Small Animal Veterinary Association Liver Standardization Group (14).
Table I.
Demographic data for cats selected in this study from which liver samples originated. Samples were assessed for metallothionein and Ki-67 labelling, inflammation, fibrosis, and bile duct proliferation.
| Group | Sex | Age (years) | Breed | Number of cats |
|---|---|---|---|---|
| CCH | Female | 1 to 5 | Balinese | 1 |
| DLH | 3 | |||
| DSH | 1 | |||
| Siamese | 1 | |||
| 6 to 10 | DMH | 1 | ||
| DSH | 3 | |||
| Unknown breed | 1 | |||
| 11 to 15 | DLH | 1 | ||
| DSH | 3 | |||
| Siamese | 1 | |||
| Unknown breed | 1 | |||
| 16 to 20 | Unknown breed | 1 | ||
| Unknown age | DSH | 2 | ||
| Male | 1 to 5 | DLH | 1 | |
| DSH | 1 | |||
| 6 to 10 | DMH | 1 | ||
| DSH | 1 | |||
| Unknown breed | 1 | |||
| 11 to 15 | DSH | 4 | ||
| Himalayan | 1 | |||
| Manx | 1 | |||
| 16 to 20 | DLH | 1 | ||
| DSH | 1 | |||
| Unknown breed | 1 | |||
| Control | Female | 1 to 5 | DSH | 2 |
| Unknown age | Unknown breed | 1 | ||
| Male | 1 to 5 | DMH | 1 | |
| Unknown age | Unknown breed | 1 |
DLH — Domestic long hair; DMH — Domestic medium hair; DSH — Domestic short hair.
Table II.
Data summary of 34 diseased and 5 normal liver samples assessed for metallothionein and Ki-67 labelling, inflammation, fibrosis, and bile duct proliferation.
| Case numbera | Inflammatory cellsb | Fibrosisc, 0–3 | BDPd | MT-positive hepatocytese in 0.625 mm2 | MT labelling intensityf, 0–3 | Ki-67 positive hepatocytesg in 0.625 mm2 |
|---|---|---|---|---|---|---|
| 1 | 121.8 | 2 | 2.7 | 6.1 | 1.6 | 0 |
| 2 | 501.8 | 2.5 | 2.6 | 49 | 2 | 0.6 |
| 3 | 999.0 | 3 | 4 | 72.3 | 2.8 | 0 |
| 4 | 197.0 | 2 | 11.5 | 8.8 | 1.6 | 0 |
| 5 | 173.0 | 1.5 | 12.4 | 13.7 | 1.6 | 0 |
| 6 | 36.4 | 2.5 | 1.8 | 14.7 | 1.4 | 0 |
| 7 | 137.2 | 2.5 | 3 | 43.2 | 1.9 | 0.7 |
| 8 | 107.8 | 1.5 | 5.8 | 9.6 | 1.1 | 1.6 |
| 9 | 170.2 | 2 | 5.2 | 26.6 | 1.5 | 0 |
| 10 | 49.0 | 1.5 | 1.8 | 0.1 | 2 | 0.4 |
| 11 | 84.8 | 1 | 10.4 | 84.9 | 2.5 | 0 |
| 12 | 120.0 | 3 | 2.9 | 41.6 | 1.9 | 0 |
| 13 | 210.2 | 1.5 | 5.2 | 30.1 | 2.2 | 2.8 |
| 14 | 27.8 | 2.5 | 2.2 | 1 | 1.3 | 1.2 |
| 15 | 10.8 | 2 | 2.3 | 9.1 | 2 | 0.1 |
| 16 | 55.6 | 2 | 2.7 | 0.2 | 1.9 | 1.2 |
| 17 | 25.8 | 1.5 | 3 | 0 | 0.6 | 0 |
| 18 | 62.4 | 2 | 4.3 | 17.5 | 2 | 0.7 |
| 19 | 6.6 | 2 | 2 | 77.2 | 2.5 | 0 |
| 20 | 31.2 | 2 | 2.3 | 23.4 | 2 | 0 |
| 21 | 40.6 | 2 | 2.6 | 27.8 | 2.1 | 0 |
| 22 | 229.8 | 1.5 | 4.2 | 0.9 | 2.1 | 0 |
| 23 | 124.2 | 1.5 | 2.6 | 49.3 | 1.9 | 0 |
| 24 | 105.4 | 3 | 4.5 | 9 | 1.8 | 0 |
| 25 | 76.8 | 2 | 2.6 | 0 | 1.1 | 0 |
| 26 | 53.4 | 3 | 2.4 | 0.9 | 1.4 | 0 |
| 27 | 204.4 | 2.5 | 2.7 | 45.4 | 2.3 | 0 |
| 28 | 247.8 | 3 | 11.2 | 3.1 | 2.1 | 0 |
| 29 | 29.2 | 2.5 | 4.5 | 71.1 | 1.6 | 0 |
| 30 | 430.8 | 2.5 | 10.4 | 77.6 | 2 | 0.6 |
| 31 | 52.2 | 2.5 | 2.2 | 1.2 | 2 | 0 |
| 32 | 374.0 | 3 | 5 | 67.7 | 2.4 | 0 |
| 33 | 127.2 | 0.5 | 8.4 | 50.1 | 1.8 | 0 |
| 34 | 373.2 | 2 | 2.5 | 89.25 | 2.9 | 0.8 |
| 35 | 6.6 | 0 | 1.8 | 0.5 | 1.1 | 0.4 |
| 36 | 11.4 | 0 | 1.8 | 0.4 | 1.5 | 0 |
| 37 | 6.8 | 0 | 1.4 | 0.1 | 1.3 | 0 |
| 38 | 12.8 | 0 | 2 | 0.1 | 2.1 | 0 |
| 39 | 6.8 | 0 | 1.4 | 0 | 2 | 0 |
MT — metallothionein; BDP — bile duct proliferation.
Case number: 1–34, liver samples with chronic cholangiohepatitis; 35–39, normal liver samples.
Mean number of inflammatory cells surrounding 5 random portal areas.
Mean fibrosis score by 2 independent pathologists in 5 random HPF (40×).
Mean number of bile ducts counted in 5 random portal areas by 2 independent pathologists.
Mean number of hepatocytes with positive MT labelling in 10 random HPF (40×).
Mean MT labelling intensity in 10 random HPF (40×).
Mean number of hepatocytes with positive Ki-67 staining in 10 random HPF (40×).
High resolution microscopic images were captured using an Olympus DP70 digital camera and an Olympus microscope at 40× magnification to score inflammation. The number of neutrophils, lymphocytes, and plasma cells infiltrating the hepatic portal tracts were counted in 5 random high-power fields (40×) using Image Pro 9.2 software (Media Cybernetics, Rockville, Maryland, USA), by individually tagging the inflammatory cells; a mean was then calculated. For fibrosis scoring, all slides were stained with Masson’s Trichrome and scored 0, 1, 2, or 3, where 0 indicated absence of fibrosis; 1 indicated presence of fibrosis around portal tracts only; 2 represented the presence of nonbridging fibrosis extending beyond portal or centrilobular areas into the hepatic parenchyma; and 3 was associated with the presence of bridging fibrosis. Bile duct proliferation was assessed by counting the number of bile ducts in 5 random high-power field (40×) locations that contained at least 1 bile duct and a mean was calculated. Fibrosis and BDP were independently assessed by 2 experienced pathologists (A.N.A and A.L.A) and the means of their scores were taken.
Immunohistochemistry and scoring for MT and Ki-67 expression
The slides for MT and Ki-67 expression were stained using a slightly modified version of a staining protocol previously adopted by Verhoef et al (13). Briefly, consecutive 4-μm sections from formalin-fixed, paraffin-embedded tissue blocks were mounted on ProbeOn Plus (Fischer Scientific, Waltham, Massachusetts, USA) charged glass slides. The slides were oven-baked at 60°C for 1 h, then deparaffinized in xylene for 5 min, followed by rehydration in graded concentrations of alcohol (100%, 95%, and 70%) for 5 min each. The slides were then rinsed in distilled water. Endogenous peroxide activity was blocked with 3% hydrogen peroxide in methanol for 10 min followed by a 5-minute rinse in phosphate-buffered saline with Tween 20 (PBST). The slides were incubated for 20 min in Tris ethylenediamine tetra-acetic acid (EDTA) buffer (pH 9) in a Dako PT Link water bath set at 97°C.
The primary antibodies for MT (clone E9, mouse anti-horse, monoclonal IgG1; Dako, Burlington, Ontario) and Ki-67 (clone MIB-1, mouse anti-human, monoclonal IgG1; Dako) were diluted to 1:1000 and 1:50, respectively, using antibody diluent (Dako) and incubated at room temperature for 30 min followed by a 5-minute rinse in PBST. Color development was performed using diaminobenzidine buffer (DAB) and DAB chromogen buffer. The slides were counterstained with hematoxylin for 4 min and mounted with cover slips. Antibody specificity was assessed both by exclusion of primary antibody (omission) and substitution with an irrelevant antibody. Histologically normal cat kidney and lymph node tissues were used as positive controls for MT and Ki-67, respectively. Five histologically normal liver tissues were also stained with MT and Ki-67, for comparison.
Hepatocellular MT expression was evaluated by the following 2 methods: i) The number of hepatocytes with positive MT staining in 10 random high-power fields (40×) was counted using an intraocular square grid measuring 0.25 mm × 0.25 mm and a mean was calculated; and ii) MT labelling intensity was scored subjectively from 0 to 3 in 10 random high-power fields (40×) and a mean was calculated. A score of 0 indicated no labelling; 1 indicated the presence of weak labelling intensity; 2 represented the presence of moderate labelling intensity; and 3 was associated with the presence of strong labelling intensity. Liver regeneration was assessed by counting the number of hepatocytes with positive Ki-67 staining (Ki-67 expression) in 10 random high-power fields (40×) using the same grid and a mean was calculated.
Statistical analysis
Statistical data were analyzed using SAS 9.4 (SAS Institute, Cary, North Carolina, USA). Correlation between MT expression and Ki-67 expression, hepatocellular inflammation, fibrosis, and BDP were analyzed using Spearman’s rank correlation test. Inter-observer variations in fibrosis and BDP scoring were also assessed using Spearman’s rank correlation test. Differences were considered statistically significant when P < 0.05.
Results
Numerical values for all the evaluated parameters are presented in Table II. A variable degree of inflammation was present in all liver sections affected by CCH with lymphocytes, plasma cells, and neutrophils being primarily seen (Figure 1). Masson’s trichrome staining highlighted increased collagen deposits (blue) with increased severity of fibrosis (Figures 2A–C).
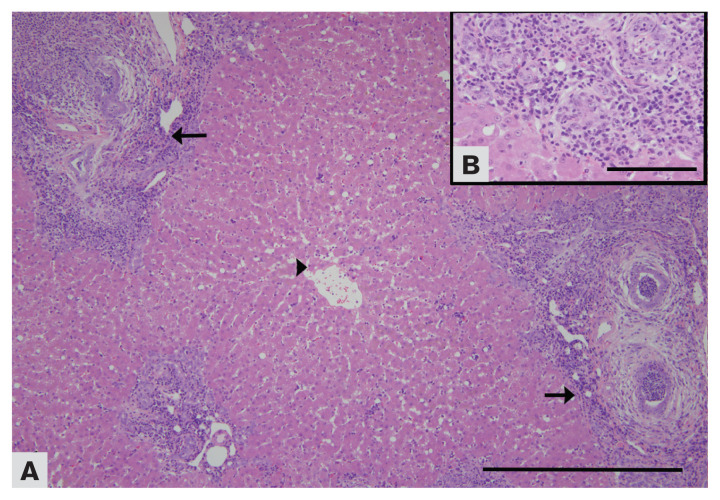
Figure 1.
A — Photomicrograph of a liver from a cat affected by chronic cholangiohepatitis (10×). Note the centrilobular zones (arrowhead) devoid of inflammatory cells and the portal zones surrounded by abundant infiltration of lymphocytes and plasma cells, together with fibrosis and bile duct proliferation (arrows). B — Portal zones contain multiple bile ducts surrounded by abundant infiltration of lymphocytes and plasma cells. Case no. 2. H & E staining.
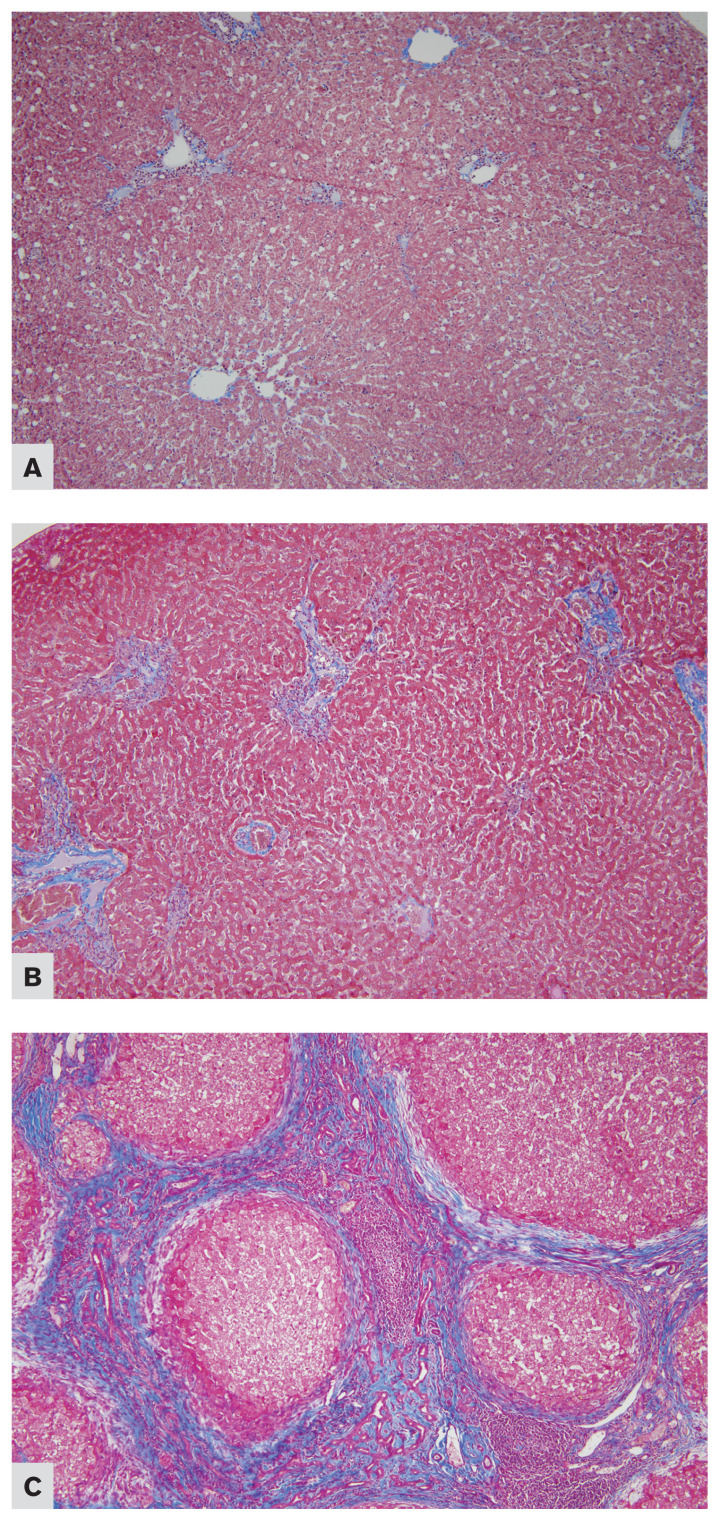
Figure 2.
Photomicrographs of the liver from cats affected by chronic cholangiohepatitis, stained with Masson’s Trichrome (10×). A — Score ‘1,’ presence of fibrosis around portal tracts only. Case no. 11. B — Score ‘2,’ presence of nonbridging fibrosis extending beyond portal or centrilobular areas into the hepatic parenchyma. Case no. 20. C — Score ‘3,’ presence of bridging fibrosis. Case no. 32.
Metallothionein expression was present in 94.1% of cases (32/34). The expression was mostly cytoplasmic within hepatocytes, which rarely exhibited variable nuclear staining (Figure 3). The mean number of hepatocytes with positive MT staining ranged from 0 to 89 cells in 10 random high-power fields (40×). Mean MT labelling intensity ranged from 0.6 to 2.9 in 10 random high power (40×) fields (Tables II, III; Figures 4, 5).
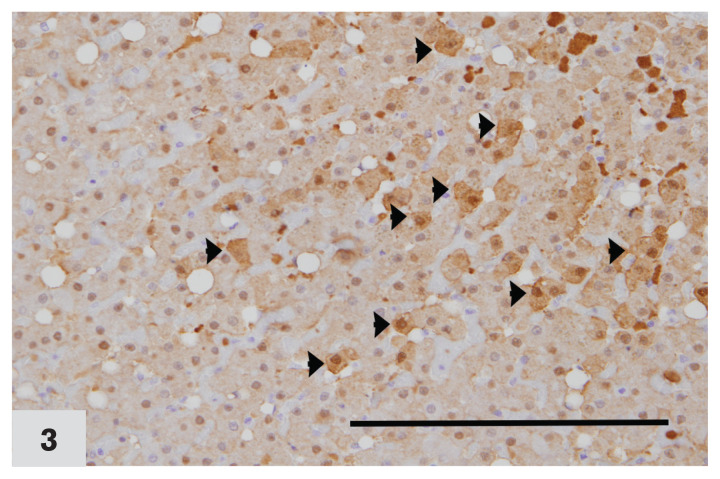
Figure 3.
Photomicrograph of a liver from a cat affected by chronic cholangiohepatitis, immunohistochemically stained for MT expression (40×). Arrowheads indicate hepatocytes with positive MT staining. Note diffuse dark brown cytoplasmic staining and variable nuclear staining in MT positive cells. Labelling intensity score ‘2.’ Case no. 24. DAB staining & hematoxylin counterstaining.
Table III.
Mean, standard deviation, minimum and maximum values of variables assessed in 34 liver samples with histologic diagnosis of cholangiohepatitis.
| Mean | SD | Minimum | Maximum | |
|---|---|---|---|---|
| MT positive hepatocytes | 30.1 | 29.3 | 0 | 89.3 |
| MT labelling intensity | 1.9 | 0.5 | 0.6 | 2.9 |
| Ki-67 positive hepatocytes | 0.3 | 0.6 | 0 | 2.8 |
| Inflammation | 164.7 | 193 | 6.6 | 999 |
| Fibrosis | 2.1 | 0.6 | 0.5 | 3 |
| BDP | 4.5 | 3.1 | 1.8 | 12.4 |
SD — Standard deviation; MT — Metallothionein; BDP — Bile duct proliferation.
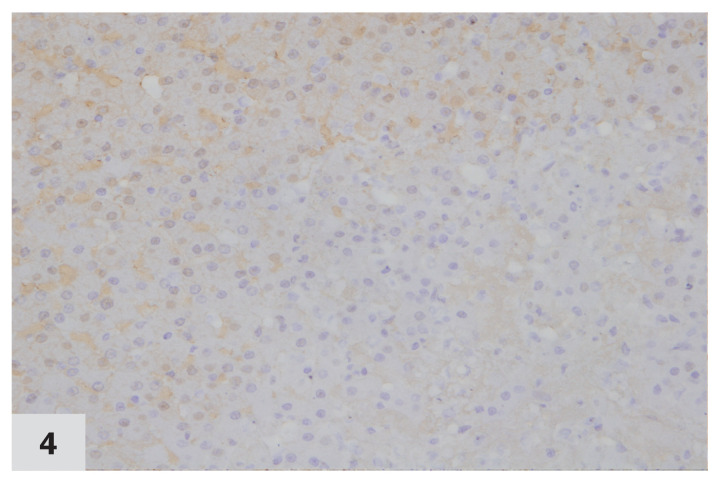
Figure 4.
Photomicrograph of a normal liver from a cat, immunohistochemically stained for MT expression (40×). None of the cells stain positive for MT. Labelling intensity score ‘0.’ Case no. 37. DAB staining & hematoxylin counterstaining.
Figure 5.
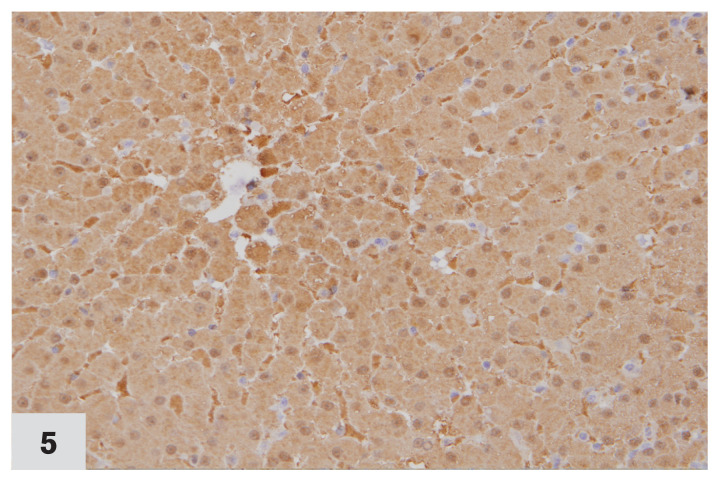
Photomicrograph of a liver from a cat affected by chronic cholangiohepatitis, immunohistochemically stained for MT expression (40×). All the cells in the section express MT. Labelling intensity score ‘3.’ Case no. 1. DAB staining & hematoxylin counterstaining.
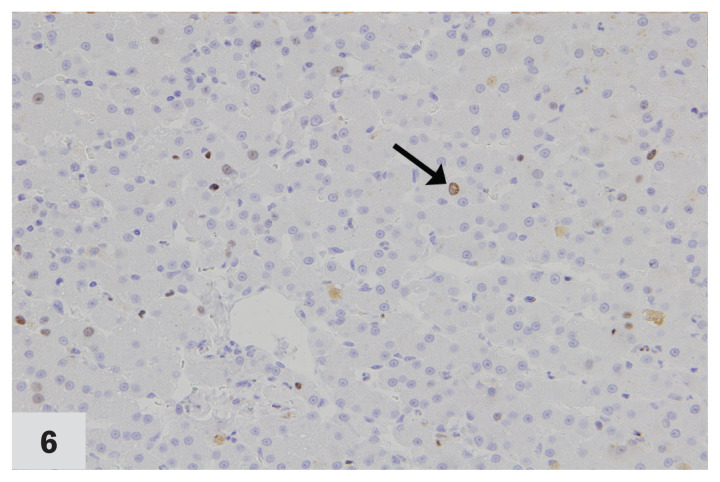
Positive Ki-67 expression was present in both hepatocytes and Kupffer cells in 35% of the liver samples (Figure 6). Ki-67 expression was absent in 15% of samples and present only in Kupffer cells in 50% of samples. In liver samples with positive Ki-67 expression, the mean number of positive hepatocytes ranged from 0 to 2.8 in 0.625 mm2 (Figure 7; Table III).
Figure 6.
Photomicrograph of a liver from a cat affected by chronic cholangiohepatitis, immunohistochemically stained for Ki-67 expression (40×). Arrow points to the dark brown intranuclear staining in a hepatocyte with positive Ki-67 expression. Case no. 12. DAB staining & hematoxylin counterstaining.
Figure 7.
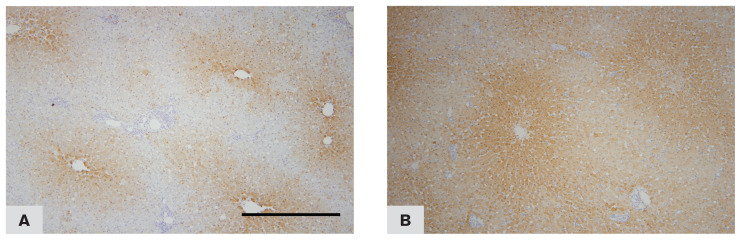
Photomicrograph of a liver from a cat affected by chronic cholangiohepatitis, immunohistochemically stained for MT expression (10×). Note the labelling intensity is strongest in cells near the centrilobular zone and weak in cells near the portal zone. A — Case no. 9. B — Case no. 11. DAB staining & hematoxylin counterstaining.
In 37% of cases (16/43) MT labelling intensity was noted to be pronounced towards the centrilobular zone and was very weak or absent towards the portal zone (Figure 7A, B). A significant correlation (Table IV) was observed between the number of hepatocytes with positive MT staining and inflammation (r = 0.36, P = 0.03) and also between MT labelling intensity and inflammation (r = 0.37, P = 0.03). No correlation was found between the number of hepatocytes with positive MT staining, MT labelling intensity, or Ki-67 expression and fibrosis or BDP.
Table IV.
Spearman rank correlation analysis of MT, Ki-67 expression and selected histologic parameters in 34 liver samples with histologic diagnosis of cholangiohepatitis.
| Variable | MT-positive cells in 0.625 mm2 | MT labelling intensity | Ki-67 positive cells 0.625 mm2 | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| P-value | r | P-value | r | P-value | r | |
| Inflammationa | 0.03* | 0.366 | 0.03* | 0.370 | 0.20 | −0.225 |
| Fibrosisb | 0.66 | 0.078 | 0.70 | 0.070 | 0.87 | −0.028 |
| BDPc | 0.23 | 0.211 | 0.92 | −0.016 | 0.90 | −0.021 |
| Ki-67d | 0.95 | 0.011 | 0.65 | 0.079 | — | — |
MT — Metallothionein; BDP — Bile duct proliferation.
Mean number of inflammatory cells surrounding 5 random portal areas.
Mean fibrosis score by 2 independent pathologists examining 5 random HPF (40×).
Mean number of bile ducts counted in 5 random portal areas by 2 independent pathologists.
Mean number of hepatocytes with positive Ki-67 staining in 10 random HPF (40×).
Statistically significant.
Assessment of interobserver variation revealed significant correlation among the histologic scores of the 2 pathologists for BDP (r = 0.73, P < 0.01) and fibrosis (r = 0.53, P < 0.01). MT and Ki-67 expression in hepatocytes in normal liver samples were very weak or absent.
Discussion
Metallothionein is a family of small proteins, which play an essential role in metal homeostasis, inflammation, and regeneration. Four major isoforms of MT have been identified (MT-I — IV) (7,9,13). MT-I and MT-II are closely related, and considered ubiquitous in mammalian cells and are induced by stress (7,9,15). MT-III is mainly expressed in the central nervous system where it is thought to inhibit neuronal growth. The levels of MT-III have been found reduced in the brain of patients affected by Alzheimer’s disease (16). MT-IV expression is restricted to squamous epithelium where it plays a role in zinc and copper homeostasis (17).
Studies in MT-knockout and wild type mice have demonstrated that the induction of MT is critical at times of acute stress (18). In the liver, MT gene expression is rapidly upregulated by various stressors including hot and cold environmental conditions, heat burn, strenuous exercise, and endotoxin-induced systemic inflammation resulting from bacterial infections. Metallothionein is, therefore, considered an acute phase protein (19). The role of MT in inflammation is imparted by its ability to scavenge a wide range of reactive oxygen species (ROS), including superoxide, hydrogen peroxide, hydroxyl radicals, and nitric oxide (7). Metallothionein also acts as a zinc chaperone for the activation of matrix metalloproteinases (MMPs) (15), which are zinc-dependent enzymes, involved in degradation of collagen and extracellular matrix components, thus promoting tissue repair and regeneration during chronic inflammation. MMP’s are also regulators of cytokines and chemokines and thus thought to play a role in inflammation and immunity (20).
The objective of our study was to examine the expression of MT in feline liver tissues affected by CCH and to correlate its expression with liver regeneration, inflammation, fibrosis, and BDP. “MT expression” will be used hereafter to refer to both the number of hepatocytes with positive MT staining and MT labelling intensity as they are discussed together. A significant positive correlation between MT expression and inflammation was observed, which supports the idea that MT plays a role in inflammation in CCH in cats. A similar positive correlation between MT expression and inflammation has been documented previously by Sridharan et al (12), in a study conducted in dogs affected by chronic liver disease. Previous studies in mice have investigated the protective role of MT in liver inflammation and toxicity conditions (7,8,21). Increased pulmonary MT expression was observed following chemical induced oxidative stress, with cytoprotective effects (22). Increased hepatic MT concentrations are reported to occur during the early phase of inflammation in bacterial infections and endotoxemia (23). A few studies utilized wild type and MT knockout mice to demonstrate the regulatory effect of MT in inflammation and toxicity conditions. Histopathological and ultrastructural examination of lung tissue following intratracheal administration of lipopolysaccharide, demonstrated significant increase in pulmonary edema and inflammation in wild type mice compared to MT knockout mice (8). In another such study, wild type mice demonstrated a rise in MT protein concentrations, with significantly lower levels of oxidative damage (lipid peroxidation) and tissue injury (necrosis), when compared to knockout mice, following induction of acute hepatotoxicity by intraperitoneal injection of thioacetamide (21). Studies have shown that MT knockout mice have an increased sensitivity to harmful metals such as cadmium, mercury, and arsenic, oxidative stress, chemical carcinogenesis, and neurodegenerative diseases compared to wild type mice, which supports the idea that MT plays an essential role in heavy metal-induced toxicity (24). In human patients with chronic hepatitis C, MT expressed in hepatocytes is shown to play a defensive role in oxidative stress by limiting inflammation and viral replication (11). Our findings are in line with the previous research investigating the role MT in inflammatory conditions (12,25) and likely represents a protective response of MT to the increased oxidative stress occurring during inflammation. Being an acute phase protein upregulated during acute inflammation, the finding of a correlation between MT expression and chronic inflammation in our study may indicate that tissue injury is still occurring.
A gradual increase in the MT labelling intensity towards the centrilobular areas was noticed in 47% of tissue samples, while inflammation was concentrated around the portal areas. In the remaining 53% samples, MT labelling intensity was homogeneous, without a zonal predilection. Yin et al (25), reported that immune cells migrate chemotactically in the presence of a gradient of MT. Nagamine and Nakajima (11) observed that MT proteins were mostly observed around portal tracts together with inflammation and fibrosis in liver biopsies from human patients with chronic hepatitis C virus infection. The increased MT labelling intensity in hepatocytes close to the centrilobular zones observed in less than half of the samples in our study seems intriguing, considering the fact that the hepatocytes adjacent to the site of inflammation are more prone to oxidative stress compared to those away from these sites. A higher MT expression in those cells close to inflammation would be expected, which is just the opposite of what we found. In previous studies investigating MT expression in chronic liver disease of dogs (12) and horses (13), no zonal variation in MT labelling intensity was reported. It is plausible that there may be factors, yet unknown to us, that may influence MT expression in cats, and further studies may be necessary to unravel the mechanism behind this.
No significant correlation was observed between MT expression and other parameters associated with chronic inflammatory conditions, such as liver fibrosis and BDP. Sridharan et al (12) reported a significant negative correlation between the percentage of hepatocytes with positive MT staining and liver fibrosis in dogs. These results were in concordance with previous studies conducted in mice. Wild type mice exposed to long-term treatment of carbon tetrachloride developed irreversible liver fibrosis. Fibrosis was reversed when MT was given intravenously through adenoviral gene delivery (10). These results provide evidence that MT plays a role against fibrosis. Similar to our findings, Verhoef et al (13) did not find any significant correlation between liver fibrosis and MT expression in horses. Metallothionein expression was not related to BDP, which is consistent with the findings of Sridharan et al (12) and Verhoef et al (13), who also failed to observe a correlation between MT expression and BDP in studies conducted in dogs and horses, respectively. Overall, the absence of correlation between MT expression and liver fibrosis or BDP in our study may suggest that MT expression is not directly related to liver fibrosis or BDP in cats. It could be speculated that different species vary in their MT expression in relation to these parameters, as previously proposed by Verhoef et al (13). The absence of a correlation could also be related to the small sample size used in our study.
Previous reports have shown that MT plays an essential role in hepatic regeneration (26,27). Following liver injury, MT production is induced to meet the increased demands of zinc in regenerating cells (26). In a study comparing hepatic regeneration following partial hepatectomy in wild type and MT knockout mice, wild type mice demonstrated a significant hepatic regeneration compared to MT knockout mice (27). Following exposure to the hepatotoxin, thioacetamide, MT knockout mice showed a significant reduction in their potential for hepatic regeneration (21). A significant but weak correlation between Ki-67 and MT expression was also noticed in dogs by Sridharan et al (12). However, we did not observe a significant correlation between MT expression and Ki-67 expression in our study. Similar observations were made by Verhoef et al (13) in horses. The authors also identified a positive correlation between Ki-67 expression in Kupffer cells and MT expression in hepatocytes of equine livers with chronic hepatitis, but such a correlation was not present in our samples. Hepatocytes with positive Ki-67 expression in our samples ranged from 0 to 2.8. This range is much lower than the values obtained by Sridharan et al (12) in dogs, but comparable with the values of the horse study by Verhoef et al (13). The low Ki-67 expression in the liver of cats with CCH may indicate a low regenerative capacity in these livers and may explain the guarded prognosis in cats affected by this disease.
A few disadvantages in our approach was the small sample size and use of a single marker (Ki-67) to assess hepatic regeneration. However, this is the first study that examined MT expression in the liver of cats and future studies may consider experiments with large cohorts incorporating age, sex, and breed of animals in the study design, to identify if any of these factors can influence MT expression and liver regeneration in cats affected with CCH. The use of multiple markers (28) instead of a single marker (Ki-67) could also be attempted, for a better estimation of regenerating hepatocytes.
Inflammation is pivotal in the body’s defense against infectious agents; however, at high levels, inflammation-induced oxidative stress, can cause deleterious effects to cell membranes, nucleic acids, and proteins, and may lead to tissue necrosis and loss of function. Metallothioneins are considered part of the innate and adaptive immune defense mechanism of the body, and thought to have protective effects against inflammation-induced tissue injury (18). Apart from being primarily an intracellular protein, MT is also present in extracellular compartments such as liver sinusoids, serum, urine, and bronchoalveolar spaces. Immunomodulatory effects of extracellular MT are demonstrated by its ability to direct chemotaxis of leucocytes (25). The beneficial effects of MT as an anti-inflammatory molecule and it’s role in tissue repair and regeneration indicate that MT plays a protective role during acute and chronic inflammation. The positive correlation between MT expression and inflammation in CCH in cats in our study substantiates this argument and suggests that chronic inflammatory conditions can augment the production of this protein in the liver to improve host defense. It may be feasible, therefore, to explore the possibility of using MT as a therapeutic agent for the manipulation of inflammation in vivo.
Acknowledgments
We thank Dr. Sarah Parker for assistance with the statistical analysis; Melissa Koehnlein and Jolanda Verhoef for assistance with immunohistochemistry. This study was funded by a grant from the Western College of Veterinary Medicine Companion Animal Health Fund, Saskatoon, Saskatchewan, Canada.
References
- 1.Callahan Clark JE, Haddad JL, Brown DC, Morgan MJ, Van Winkle TJ, Rondeau MP. Feline cholangitis: A necropsy study of 44 cats (1986–2008) J Feline Med Surg. 2011;13:570–576. doi: 10.1016/j.jfms.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Day DG. Feline cholangiohepatitis complex. Vet Clin North Am Small Anim Pract. 1995;25:375–385. doi: 10.1016/s0195-5616(95)50032-4. [DOI] [PubMed] [Google Scholar]
- 3.Brain PH, Barrs VR, Martin P, Baral R, White JD, Beatty JA. Feline cholecystitis and acute neutrophilic cholangitis: Clinical findings, bacterial isolates and response to treatment in six cases. J Feline Med Surg. 2006;8:91–103. doi: 10.1016/j.jfms.2005.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gagne JM, Weiss DJ, Armstrong PJ. Histopathologic evaluation of feline inflammatory liver disease. Vet Pathol. 1996;33:521–526. doi: 10.1177/030098589603300506. [DOI] [PubMed] [Google Scholar]
- 5.Solís Herruzo JA, Solís-Muñoz P, Muñoz-Yagüe T, García-Ruiz I. Molecular targets in the design of antifibrotic therapy in chronic liver disease. Rev Esp Enfermedades Dig. 2011;103:310–323. [PubMed] [Google Scholar]
- 6.Coyle P, Philcox JC, Carey LC, Rofe AM. Metallothionein: The multipurpose protein. Cell Mol Life Sci. 2002;59:627–647. doi: 10.1007/s00018-002-8454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue K, Takano H, Shimada A, Satoh M. Metallothionein as an anti-inflammatory mediator. Mediators Inflamm. 2009;2009 doi: 10.1155/2009/101659. 101659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takano H, Inoue K, Yanagisawa R, et al. Protective role of metallothionein in acute lung injury induced by bacterial endotoxin. Thorax. 2004;59:1057–1062. doi: 10.1136/thx.2004.024232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thirumoorthy N, Manisenthil Kumar K-T, Shyam Sundar A, Panayappan L, Chatterjee M. Metallothionein: An overview. World J Gastroenterol. 2007;13:993–996. doi: 10.3748/wjg.v13.i7.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang Y, Kang YJ. Metallothionein gene therapy for chemicalinduced liver fibrosis in mice. Mol Ther. 2004;10:1130–1139. doi: 10.1016/j.ymthe.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Nagamine T, Nakajima K. Significance of metallothionein expression in liver disease. Curr Pharm Biotechnol. 2013;14:420–426. doi: 10.2174/1389201011314040006. [DOI] [PubMed] [Google Scholar]
- 12.Sridharan S, Allen AL, Kidney B, Al-Dissi AN. Metallothionein expression in dogs with chronic hepatitis and its correlation with hepatic fibrosis, inflammation, and Ki-67 expression. Vet Pathol. 2015;52:1127–1133. doi: 10.1177/0300985815588607. [DOI] [PubMed] [Google Scholar]
- 13.Verhoef JNC, Allen AL, Harding JCS, Al-Dissi AN. Metallothionein expression in horses with chronic liver disease and its correlation with Ki-67 immunoreactivity. Vet Pathol. 2018;55:703–710. doi: 10.1177/0300985818777802. [DOI] [PubMed] [Google Scholar]
- 14.van den Ingh T, Cullen J, Twedt D, Thomas VW, Valeer D, Jan R. In: WSAVA Standards for Clinical and Histological Diagnosis of Canine and Feline Liver Diseases. Rothuizen J, Susan B, Jenny C, et al., editors. Philadelphia, Pennsylvania: Saunders, Elsevier; 2006. pp. 61–101. [Google Scholar]
- 15.Ruttkay-Nedecky B, Nejdl L, Gumulec J, et al. The role of metallothionein in oxidative stress. Int J Mol Sci. 2013;14:6044–6066. doi: 10.3390/ijms14036044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vašák M, Meloni G. Mammalian metallothionein-3: New functional and structural insights. Int J Mol Sci. 2017;18:1117. doi: 10.3390/ijms18061117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meloni G, Zovo K, Kazantseva J, Palumaa P, Vasák M. Organization and assembly of metal-thiolate clusters in epithelium-specific metallothionein-4. J Biol Chem. 2006;281:14588–595. doi: 10.1074/jbc.M601724200. [DOI] [PubMed] [Google Scholar]
- 18.Subramanian Vignesh K, Deepe SG., Jr Metallothioneins: Emerging modulators in immunity and infection. Int J Mol Sci. 2017;18:2197–2222. doi: 10.3390/ijms18102197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh SH, Deagen JT, Whanger PD, Weswig PH. Biological function of metallothionein. V. Its induction in rats by various stresses. Am J Physiol Endocrinol Metab Gastrointest Physiol. 1978;3:282–285. doi: 10.1152/ajpendo.1978.234.3.E282. [DOI] [PubMed] [Google Scholar]
- 20.Duarte S, Baber J, Fujii T, Coito AJ. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol. 2015;44–46:147–56. doi: 10.1016/j.matbio.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliver JR. Augmented hepatic injury followed by impaired regeneration in metallothionein-I/II knockout mice after treatment with thioacetamide. Toxicol Appl Pharmacol. 2006;210:190–199. doi: 10.1016/j.taap.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Inoue K, Takano H. Metallothionein as a negative regulator of pulmonary inflammation. Curr Pharm Biotechnol. 2013;14:414–419. doi: 10.2174/1389201011314040005. [DOI] [PubMed] [Google Scholar]
- 23.Sobocinski PZ, Canterbury WJ. Hepatic metallothionein induction in inflammation. Ann NY Acad Sci. 1982;389:354–367. doi: 10.1111/j.1749-6632.1982.tb22149.x. [DOI] [PubMed] [Google Scholar]
- 24.Satoh M. Analysis of toxicity using metallothionein knockout mice. Yakugaku Zasshi. 2007;127:709–717. doi: 10.1248/yakushi.127.709. [DOI] [PubMed] [Google Scholar]
- 25.Yin X, Knecht D, Lynes M. Metallothionein mediates leukocyte chemotaxis. BMC Immunol. 2005;6:21. doi: 10.1186/1471-2172-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cherian MG, Kang YJ. Metallothionein and liver cell regeneration. Exp Biol Med. 2006;231:138–144. doi: 10.1177/153537020623100203. [DOI] [PubMed] [Google Scholar]
- 27.Oliver JR, Mara TW, Cherian MG. Impaired hepatic regeneration in metallothionein-I/II knockout mice after partial hepatectomy. Exp Biol Med. 2005;230:61–67. doi: 10.1177/153537020523000108. [DOI] [PubMed] [Google Scholar]
- 28.Assy N, Minuk GY. Liver regeneration: Methods for monitoring and their applications. J Hepatol. 1997;26:945–952. doi: 10.1016/s0168-8278(97)80266-8. [DOI] [PubMed] [Google Scholar]