Abstract
In many human cancers, the expression of the prostaglandin receptor EP4 (EP4R) is associated with the development of malignancy and a poor prognosis. The expression of EP4R has not yet been evaluated in canine tumors. The objective of this study was to characterize the messenger RNA (mRNA) expression of EP4R in canine osteosarcoma (OSA). Gene expression of EP4R was evaluated using RNA in-situ hybridization (RNAscope). In all canine OSA samples evaluated, strong universal positive expression of EP4R was identified. Gene expression was significantly higher in OSA tissue samples than in normal nasal turbinate bone, possibly implicating EP4R in the pathogenesis of canine OSA.
Résumé
Dans de nombreux cancers humains, l’expression du récepteur des prostaglandines EP4 (EP4R) est associée au développement d’une malignité et à un mauvais pronostic. L’expression d’EP4R n’a pas encore été évaluée dans les tumeurs canines. L’objectif de cette étude était de caractériser l’expression de l’ARN messager (ARNm) de l’EP4R dans l’ostéosarcome canin (OSA). L’expression génique de l’EP4R a été évaluée en utilisant l’hybridation in situ d’ARN (RNAscope). Dans tous les échantillons canins OSA évalués, une forte expression positive généralisée d’EP4R a été identifiée. L’expression génique était significativement plus élevée dans les échantillons de tissus OSA que dans l’os normal du cornet nasal, ce qui impliquait peut-être EP4R dans la pathogenèse de l’OSA canin.
(Traduit par Docteur Serge Messier)
Osteosarcoma (OSA) is diagnosed in 85% of canine malignant skeletal tumors, making OSA the most common canine primary bone tumor (1). Osteosarcoma is an aggressive neoplasm, with destructive local bone involvement and over 90% of dogs developing distant metastatic disease within 1 y of diagnosis (1). Standard of care includes amputation or limb-sparing procedure (surgical or radiation therapy-based), followed by adjuvant chemotherapy. Amputation alone typically results in a survival time of 5.4 mo (1). Several adjuvant chemotherapy protocols have been assessed, resulting in a large range of median survival times (7 to 18 mo) (1). Despite aggressive therapy, most dogs with OSA succumb to metastatic disease, which indicates that alternative treatment modalities are needed to improve overall outcome.
Inflammation and immune activation triggered by a carcinogenic stimulant can promote the development of cancer (2). This is typically mediated through upregulation of non-specific pro-inflammatory cytokines and enzymes, the most important of which is the cyclooxygenase enzyme 2 (COX-2). This enzyme stimulates angiogenesis, inhibits apoptosis, and promotes cell proliferation and motility through production of prostaglandin E2 (PGE2) (2). The activities of PGE2 are mediated through its 4 recognized receptors [EP1 receptor to EP4 receptor (EP1R to EP4R)] on the surface of the target cells (3).
Canine OSA has been shown to overexpress COX-2, suggesting that COX-2 is a reasonable therapeutic target (4). Blocking COX-2, however, decreases the production of complementary prostanoids that are important in homeostasis and could result in significant side effects (5). Treatment options that target the prostaglandin pathway more specifically may therefore be advantageous.
Many malignancies in humans and dogs have been shown to overexpress COX-2 and/or PGE2 (6,7). In addition, the EP receptors, EP4R in particular, are expressed and associated with the development of malignancy and poor prognosis in several human cancers (8). Although characterization of the canine EP4R has been completed (9) and expression of EP2R has been confirmed in canine OSA (4), it is unknown if EP4R is overexpressed in canine OSA.
Based on the available human and canine literature, it is reasonable to hypothesize that expression of EP4R may be present in canine OSA and may play a role in its development and progression. The objective of this study was to evaluate the gene expression of EP4R in canine OSA using the novel in-situ hybridization platform, RNAscope.
Archived biopsy and necropsy tissue specimens maintained by the Department of Veterinary Pathology at Iowa State University were searched for tissue samples of formalin-fixed, paraffin-embedded appendicular OSA and normal nasal turbinate bone. The aim was to collect 9 specimens each of appendicular OSA and normal bone with adequate RNA for analysis. Based on preliminary data, a power calculation indicated that 9 specimens in each group would be statistically adequate to identify a difference of 2 transcript copy numbers/cell between tumor and normal bone (alpha = 0.05, beta = 0.2). The Veterinary Teaching Hospital and Department of Veterinary Pathology allowed the authors to use the clinical data and samples from specimens gathered through routine clinical assessment and care.
The RNAscope mRNA in-situ hybridization platform (Advanced Cell Diagnostics, Newark, California, USA) was used to evaluate messenger RNA (mRNA) expression of EP4R gene ptger4 in the appendicular OSA and normal bone tissue samples. According to the manufacturer’s instructions and as previously reported (10), single-plex, manual chromogenic RNAscope analysis was conducted with an RNAscope 2.5 High Definition (HD)-RED Assay (Catalog #322350; Advance Cell Diagnostics). Briefly, 3 sections of each paraffin-embedded OSA specimen were cut to a 5-μm depth. Preparations were baked for 1 h at 60°C, deparaffinized, and protease treated to expose RNA. The 3 sections were then hybridized separately with a test probe targeting canine ptger4 (Probe CI-PTGER4, Catalog #499011; Advanced Cell Diagnostics), a positive control probe targeting canine housekeeping gene ubc (CI-UBC Positive Control, Catalog #409851; Advanced Cell Diagnostics), and a negative control probe targeting Bacillus subtilis dapB (DapB Negative Control, Catalog #310043; Advance Cell Diagnostics).
Hybridization to target mRNA was carried out by incubating the preparation with the respective probe at 40°C for 2 h in a HybEZ hybridization oven (Advanced Cell Diagnostics). Subsequent wash and signal amplification steps were taken according to the manufacturer’s protocol. Target mRNA was detected using alkaline phosphatase Fast Red chromogenic stain (Catalog #320701; Advanced Cell Diagnostics). Samples were also stained with hematoxylin (American Master Tech Scientific, Lodi, California, USA) to allow visualization of nuclei. To initially assess the performance of the ptger4 RNAscope experimental probe, ptger4 expression was assessed in 2 sections each of normal canine heart, lung, and kidney tissue. Expression of ptger4 has previously been reported in these canine tissues (9) and was identified in all 3 tissue types with the RNAscope analysis with this ptger4 probe.
For each hybridized slide, 10 400× magnified non-overlapping microscopic field views were digitally photographed. Digitized photomicrographs were then evaluated with the RNAscope image analysis software HALO with the RNAscope Modules (Indica Labs, Albuquerque, New Mexico, USA) (11). For tumor samples, the neoplastic cells were manually identified post-processing and gated for EP4R analysis. To ensure neoplastic cells were appropriately identified for analysis, a hematoxylin and eosin histological correlate of the exact tissue block was simultaneously evaluated. For normal tissue samples, the appropriate normal cell population was manually identified and gated for analysis. Samples were considered to have adequate residual RNA for ptger4 expression analysis if the corresponding positive control ubc hybridization yielded > 15 transcript dots/cell in > 90% of the target cells. Average transcript copy numbers/cell, percent probe positive expression (percent of cells positive for EP4R mRNA), and H-score (a weighted expression scale used to evaluate heterogeneity in marker expression; A Guide for RNAscope Data Analysis, Advanced Cell Diagnostics) were calculated for ptger4 expression based on the cumulative analysis results of the 10 digitized photomicrographs for the slide. These metrics allow the gene expression within a specific cell type and tissue context to be quantified and expression across tumor types to be compared.
A D’Agostino Pearson test was used to assess normality of copy number per cell, H-score, and percent probe positive expression for OSA and normal bone. A simple t-test was used to assess differences in these parameters between OSA and bone. Statistical significance was defined as P < 0.05. Quantitative parametric data are presented as mean ± 95% confidence interval (CI) of mean and nonparametric data are presented as median ± range. Statistical comparisons were done using a commercially available software package (Prism 6; GraphPad Software, San Diego, California, USA); power calculation was conducted with a separate software package (MedCalc Software, Ostend, Belgium).
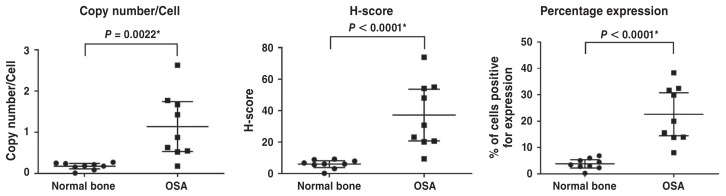
Fourteen appendicular OSA and 13 normal nasal turbinate samples were evaluated to identify 9 of each with sufficient mRNA for analysis (see Table I for details of the 9 OSA samples). These samples (all OSA and all normal turbinate) expressed EP4R mRNA. The mean copy number per cell [OSA 1.10, 95% CI: 0.53 to 1.74; nasal turbinate 0.18, 95% CI: 0.11 to 0.24; P-value 0.0022]; H-score (OSA 37.4, 95% CI: 20.9 to 53.6; nasal turbinate 6.1, 95% CI: 3.9 to 8.3; P-value 0.0001); and percent probe positive scores (OSA 22.6, 95% CI: 14.5 to 30.8; nasal turbinate 3.8, 95% CI: 2.3 to 5.4; P-value 0.0001) were statistically higher in the OSA samples (specifically the malignant OSA cells) than in the normal nasal turbinate samples (Figures 1 and 2).
Table I.
Characterization of evaluated appendicular osteosarcoma samples.
| Sample | Breed of dog | Anatomic location | Histologic diagnosis |
|---|---|---|---|
| 1 | Doberman pinscher | Left distal radius | Osteoblastic osteosarcoma |
| 2 | Labrador retriever | Right proximal femur | Osteoblastic osteosarcoma |
| 3 | Labrador retriever | Left proximal tibia | Osteoblastic osteosarcoma |
| 4 | Australian shepherd | Left proximal humerus | Osteoblastic osteosarcoma |
| 5 | Mixed breed | Left distal tibia | Osteoblastic osteosarcoma |
| 6 | Rat terrier | Left distal femur | Osteoblastic osteosarcoma |
| 7 | Mixed breed | Left distal radius | Fibroblastic osteosarcoma |
| 8 | Shetland sheepdog | Right proximal humerus | Fibroblastic osteosarcoma |
| 9 | Shetland sheepdog | Left proximal humerus | Chondroblastic osteosarcoma |
Figure 1.
EP4R mRNA expression metrics in canine osteosarcoma and normal nasal turbinate bone. Data presented as mean +/− 95% confidence interval (CI) of mean and individual data points.
* Denotes differences were statistically significant.
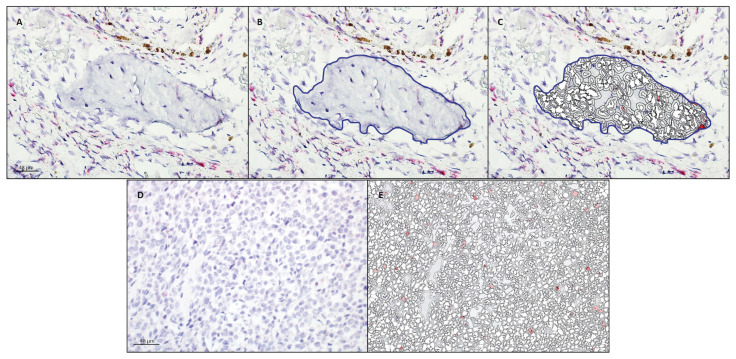
Figure 2.
Analysis of ptger4 transcription in a section of nasal turbinate bone (A, B, C) and osteosarcoma (D, E) following RNAscope mRNA in-situ hybridization with hematoxylin counterstain. A — Native photomicrograph to be analyzed for copy numbers/cell, H-score, and percentage transcript expression using HALO software with RNAscope modules. B — Nasal turbinate bone was manually gated (blue line) for analysis. C — HALO-generated probe markup from which copy numbers/cell, H-score, and percentage transcript expression are calculated. D — Native photomicrograph to be analyzed for copy numbers/cell, H-score, and percentage transcript expression using HALO software with RNAscope modules. E — HALO-generated probe markup from which copy numbers/cell, H-score, and percentage transcript expression are calculated; no blue gate line applied since entire field consisted of neoplastic cells.
It was found that the canine OSA tumor cells had significantly higher gene expression of EP4R than the normal turbinate cells. Although the true cell of origin in the development of OSA is still debatable, recent literature suggests that OSA most likely forms from mutated osteoblasts or cells that are committing to being of osteoblast lineage (12). The 2 most important EP receptors on normal osteoblasts are EP2 and EP4 (13). Notably, in a recent model of periodontal disease, the human OSA cell line Saos-2 was used to evaluate the impact of lipopolysaccharide (LPS) on the production of PGE2 and expression of EP4R. It was found that exposure to LPS significantly increased PGE2 production and subsequent gene expression of EP4R (14).
Prostaglandin signaling through EP4R stimulates monocytes to form mature osteoclasts, leading to the classic osteolysis seen in metastatic bone lesions (15). Specifically, direct cell-cell contact between the tumor cells and osteoblasts activates the NFkB/mitogen-activated protein (MAP) kinase pathway, increasing expression of COX-2. Subsequently, increased COX-2 leads to additional PGE2 secretion, which binds to EP4R on nearby osteoblasts, increasing production of receptor activator for NFkB ligand (RANKL), inducing osteoclastogenesis via receptor activator for NFkB (RANK) (15). Inherently increased EP4R expression would likely promote this process. Expression of RANKL has been identified in canine OSA (16), the presence of which could theoretically be attributed to increased EP4R, which would make EP4R an attractive therapeutic target.
The expression of EP receptors is associated with the development of malignancy and poor prognosis in several human cancers (8). Positive gene expression of EP4R mRNA in canine OSA samples suggests that EP4R expression may play a role in the pathogenesis and development of canine OSA.
While this is exciting preliminary data, it must be interpreted in light of the small sample size and the inherent differences between appendicular bones, which are normally affected by OSA, and nasal turbinate bone, which was used as the control tissue. The impact of prolonged decalcification on the interpretation of RNAscope is not known. Anecdotally, it does not appear to affect results, but out of an abundance of caution, it was elected to use nasal turbinate bone that requires less decalcification, potentially having less of an impact on the results. In addition, this work is limited in scope and does not confirm the protein expression of EP4R. Similarly, the prognostic value of increased EP4R expression and its potential as a viable therapeutic target was not fully evaluated. As this represents a pilot and proof-of-concept study, additional tumor specimens, along with appendicular bone and reactive bone, the protein expression of EP4R, and its value as a therapeutic target will need to be evaluated to confirm the results of this study.
Acknowledgments
The authors thank Dr. Olufemi Fasina for his assistance with the histopathological characterization of the osteosarcoma (OSA) samples. This research was generously supported by Fetch-a-Cure and internal funding opportunities from Iowa State University.
Footnotes
Conflict of interest: Margaret L. Musser and Chad M. Johannes receive funding from Elanco for clinical studies investigating the use of grapiprant.
References
- 1.Withrow SJ, Vail DM, Page RL. Withrow and MacEwen’s Small Animal Clinical Oncology. 5th ed. St. Louis, Missouri: Elsevier; 2013. [Google Scholar]
- 2.O’Byrne KJ, Dalgleish AG. Chronic immune activation and inflammation as the cause of malignancy. Br J Cancer. 2001;85:473–483. doi: 10.1054/bjoc.2001.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman RA, Smith WL, Narumiya S. International Union of Pharmacology classification of prostanoid receptors: Properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- 4.Millanta F, Asproni P, Cancedda S, Vignoli M, Bacci B, Poli A. Immunohistochemical expression of COX-2, mPGES and EP2 receptor in normal and reactive canine bone and in canine osteosarcoma. J Comp Pathol. 2012;147:153–160. doi: 10.1016/j.jcpa.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Kirkby Shaw K, Rausch-Derra LC, Rhodes L. Grapiprant: An EP4 prostaglandin receptor antagonist and novel therapy for pain and inflammation. Vet Med Sci. 2016;2:3–9. doi: 10.1002/vms3.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazhar D, Gillmore R, Waxman J. COX and cancer. QJM. 2005;98:711–718. doi: 10.1093/qjmed/hci119. [DOI] [PubMed] [Google Scholar]
- 7.Doré M. Cyclooxygenase-2 expression in animal cancers. Vet Pathol. 2011;48:254–265. doi: 10.1177/0300985810379434. [DOI] [PubMed] [Google Scholar]
- 8.Yokoyama U, Iwatsubo K, Umemura M, Fujita T, Ishikawa Y. The prostanoid EP4 receptor and its signaling pathway. Pharmacol Reviews. 2013;65:1010–1052. doi: 10.1124/pr.112.007195. [DOI] [PubMed] [Google Scholar]
- 9.Castleberry TA, Lu B, Smock SL, Owen TA. Molecular cloning and functional characterization of the canine prostaglandin E2 receptor EP4 subtype. Prostaglandins Other Lipid Mediat. 2001;65:167–187. doi: 10.1016/s0090-6980(01)00129-0. [DOI] [PubMed] [Google Scholar]
- 10.Wang F, Flanagan J, Su N, et al. RNAscope: A novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14:22–29. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson CM, Zhang B, Miller M, et al. Fully automated RNAscope in situ hybridization assays for formalin-fixed paraffin-embedded cells and tissues. J Cell Biochem. 2016;117:2201–2208. doi: 10.1002/jcb.25606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mutsaers AJ, Walkley CR. Cells of origin in osteosarcoma: Mesenchymal stem cells or osteoblast committed cells? Bone. 2014;62:56–63. doi: 10.1016/j.bone.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Xu Z, Choudhary S, Voznesensky O, et al. Overexpression of COX-2 in human osteosarcoma cells decreases proliferation and increases apoptosis. Cancer Res. 2006;66:6657–6664. doi: 10.1158/0008-5472.CAN-05-3624. [DOI] [PubMed] [Google Scholar]
- 14.Shoji M, Tanabe N, Mitsui N, et al. Lipopolysaccharide stimulates the production of prostaglandin E2 and the receptor Ep4 in osteoblasts. Life Sci. 2006;78:2012–2018. doi: 10.1016/j.lfs.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Chen YC, Sosnoski DM, Mastro AM. Breast cancer metastasis to the bone: Mechanisms of bone loss. Breast Cancer Res. 2010;12:215. doi: 10.1186/bcr2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barger AM, Fan TM, de Lorimier LP, Sprandel IT, O’Dell-Anderson K. Expression of receptor activator of nuclear factor kappa-B ligand (RANKL) in neoplasms of dogs and cats. J Vet Intern Med. 2007;21:133–140. doi: 10.1892/0891-6640(2007)21[133:eoraon]2.0.co;2. [DOI] [PubMed] [Google Scholar]