Abstract
The aim of the present study was to characterize the bacterial microbiota of anal sacs in healthy dogs using NGS. Swabs were used to sample the rectum and secretions from each anal sac in 15 healthy dogs. DNA was extracted from swabs and the V4 hypervariable region of the 16S rRNA gene was amplified and sequenced with Illumina MiSeq. Overall, 14 different bacterial phyla were identified in the rectum and in both anal sacs, the 5 main ones being Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, and Fusobacteria. The rectum had higher microbial diversity and richness than the left and right anal sacs. Community membership and structure significantly differed between the rectum and both anal sacs, but not between the right and the left anal sacs. This study showed that the diversity and richness of the bacterial microbiota of the anal sacs in dogs is greater than what has been reported in previous studies with culture-based methods. In conclusion, the bacterial microbiota of the anal sacs in dogs varies between individuals and differs from the rectal bacterial microbiota.
Résumé
L’objectif de la présente étude était de caractériser le microbiote bactérien des sacs anaux de chiens en santé en utilisant le séquençage d’ADN à haut débit. Des écouvillons ont été utilisés pour échantillonner le rectum et les sécrétions provenant de chaque sac anal chez 15 chiens en santé. L’ADN a été extrait des écouvillons et la région hypervariable V4 du gène codant pour l’ARN ribosomique 16S a été amplifiée et séquencée avec Illumina MiSeq. En tout, 14 phyla bactériens différents ont été identifiés dans les sacs anaux droit et gauche et le rectum, les cinq principaux étant Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria et Fusobacteria. Le microbiote bactérien du rectum avait une diversité et une richesse plus importantes que le microbiote bactérien des deux sacs anaux. L’appartenance à la communauté et sa structure différaient significativement entre le rectum et les sacs anaux, mais pas entre le sac anal droit et le sac anal gauche. Cette étude a démontré que la diversité et la richesse du microbiote bactérien des sacs anaux chez le chien sont plus importantes que ce qui a été rapportées dans les études précédentes avec des cultures. En conclusion, le microbiote bactérien des sacs anaux chez le chien varie d’un individu à l’autre et diffère de celui du rectum.
(Traduit par les auteurs)
Introduction
Dogs have a pair of anal sacs, which are skin invaginations between the muscles of the internal and external anal sphincters (1,2). They are connected to the anocutaneous junction by a duct where the secretions produced by the apocrine and sebaceous glands in the anal sacs are evacuated during defecation. The full content of the anal sacs’ secretions is a mixture of glandular secretions, desquamated keratinocytes from the stratified squamous epithelium lining the anal sacs and ducts, cellular debris, and resident microorganisms such as bacteria and yeasts (1–4). Some cytology studies also reported the presence of leucocytes and erythrocytes in normal anal sacs (2,4,5). The function of anal sacs is not clearly established, but it has been suggested that the volatile compounds of anal sac secretions may have a role in scent communication in dogs (2,6).
Dogs can be affected by diseases related to anal sacs, such as impaction, inflammation, infection, or neoplasia. Some of these conditions may require regular anal sac expression, local or systemic antibiotic treatment, dietary modification, or in more severe or refractory cases, surgery (5,7). Unfortunately, the pathophysiology and risk factors related to anal sac diseases are mostly uncertain, making the prevention of these conditions more difficult (8).
Bacterial microbiota is defined as the bacteria living in a specific environment (9). It is very important for maintenance of health: it participates in digestion and detoxification, competes with pathogens to prevent their colonization, and interacts with the immune system (10–13). Interactions between bacterial microbiota and the immune system are essential in order to have adequate innate and adaptive immune responses (13–15). Moreover, the microbiota stimulates the immune system at the surface of the skin and the digestive, respiratory, and genitourinary epithelia and mucosa to help protect these areas against pathogens (16). Thus, imbalances in bacterial microbiota have been associated with the pathogenesis of certain diseases (16–18).
In order to determine the role of bacterial microorganisms in healthy and diseased anal sacs, we must first better define the bacterial microbiota in anal sacs of healthy dogs. Previous studies using culture-based approaches have identified staphylococci, streptococci, micrococci, Bacillus spp., Escherichia coli, Proteus spp., and Pseudomonas spp. as normal commensal bacteria in anal sacs, but to the best of the authors’ knowledge, no studies have been performed on the content of anal sacs in dogs using new methods of DNA sequencing (2,19,20).
The objectives of this study were to describe the anal sac microbiota of healthy dogs using next-generation DNA sequencing (NGS) and to investigate the influence of rectal bacteria in the composition of the anal sac microbiota.
Materials and methods
Animal selection
This study was approved by the Faculty of Veterinary Medicine of the University of Montreal’s Animal Care Committee. The MIRA Foundation, a non-profit organization that offers free guide dogs and service dogs, signed a written consent form to participate in this study.
The breeding dogs lived with foster families. Several days before giving birth, the pregnant bitches were transferred to the MIRA Foundation’s facility (MIRA Campus) where they gave birth. The puppies stayed in this facility for 9 wk. At 9 wk of age, the puppies were brought to a foster family with whom they stayed until they reached 12 to 18 mo of age. Then, the dogs came back to MIRA Campus to be trained for assistance (visual and mobility impairment, autism spectrum).
Fifteen healthy dogs from the MIRA Foundation were enrolled in this study. Because the MIRA Foundation breeds and manages their own dog colony, the dogs had a related genetic background. Thirteen Laberneses and 2 Labrador retrievers aged 1 to 11.8 y (median = 1.3 y) were enrolled in this study and fed similar diets (Table I). Dogs 2 to 6, 8, and 14 were all in contact, living together at MIRA Campus. Dogs 10, 12, and 13 were in lactation and lived in the same facility but in different pens at MIRA Campus. The remaining dogs (1, 7, 9, 11, and 15) lived in separate houses.
To be included in the study, the dogs could not be bathed and could not receive systemic or topical drugs (antibiotics, anti-inflammatories, essential fatty acids) 1 and 3 mo before the study, respectively. The dogs had to be healthy based on physical examination (no clinical signs or physical examination findings consistent with cutaneous or systemic disease, or with neoplasm).
Sample collection
Three swabs were collected on the same day on each dog: one from the rectum and one from each anal sac (left and right). All the samples were collected from November 2018 to March 2019.
The perianal area was cleaned with sterile gauzes and 4% chlorhexidine (Dermachlor 4; Dechra, Pointe-Claire, Quebec). Two minutes after cleaning the area, a sample from the rectum was obtained by inserting a sterile flocked swab (FLOQSwabs; Murrieta, California, USA) 2 cm into the rectum.
After putting on sterile gloves, sterile lubricant was applied to the index finger, which was gently inserted into the anus. With the thumb outside the dog’s anus, the left anal sac was expressed by bringing the index and thumb together so that the content of the anal sac got through the duct opening on the left side of the anus. The first drops were not collected, to decrease the risk of contamination and to be more representative of the content of the anal sac. The secretions were collected with a sterile flocked swab. The secretions of the right anal sac were collected in the same fashion. Before collecting the secretions from the right anal sac, the perianal area was cleaned again with 4% chlorhexidine and the sterile gloves were changed. All the samples were transported, refrigerated, and subsequently frozen at −80°C until DNA extraction.
DNA extraction and sequencing
The DNA extraction was performed on all samples, including 2 unused swabs (negative controls), with a commercial kit DNeasy PowerSoil (Qiagen, Hilden, Germany) as recommended by the manufacturer (21). In the first step of DNA extraction, the tip of the swab was cut off and deposited in the provided tube containing the beads. A solution provided by the commercial kit was added to allow cell lysis and the tube was vortexed for 10 min. The supernatant was then transferred to another tube and the remaining steps were followed as recommended by the manufacturer. Previously reported primers (515 forward paired with 806 reverse) were used to amplify the V4 hypervariable region of the bacterial 16S ribosomal RNA gene by polymerase chain reaction (PCR) (22). Sequencing was performed at the Genome Quebec Innovation Centre. The Illumina MiSeq IEMFile version 4 platform was used for sequencing using the V2 reagent kit (2 × 250 cycles). Sequences are available at the NCBI Sequence Read Archive under accession number PRJNA681230.
The software mothur was used to perform the bioinformatic analysis (23). It clustered the good quality reads in operation taxonomic units (OTUs) at the genus level (> 94% similarity). The classification of OTUs was performed using the Ribosomal Database Project databank.
Statistical analysis
The alpha diversity was evaluated with the Chao index (estimator of richness), Shannon index, and inverse Simpson index (diversity indices) (9,24). The Anderson-Darling test was performed to assess the normality of data distribution and results from the inverse Simpson index were transformed into log to obtain a normal distribution. A 2-way analysis of variance was performed with the sampling site and the dog as the independent variables, followed by a Tukey test to identify significant differences in the alpha diversity indices, with P < 0.05 considered significant.
Beta diversity, which is the comparison of similarities between samples, was assessed using the Jaccard index, which evaluates community membership (i.e., which bacteria are present or absent in a community), and by the Yue and Clayton index, which evaluates community structure (i.e., which bacteria are present based on their abundance in a community) (9,25). The analysis of molecular variance was used to compare the community membership and community structure between groups. A P < 0.05 was considered significant. Principal coordinate analysis (PCoA) plots were obtained to visualize the similarities between groups (rectum and left and right anal sacs) (9). Linear discriminant analysis effect size (LEfSe) was used to find significant associations between relative abundances across anatomical sites (26).
Results
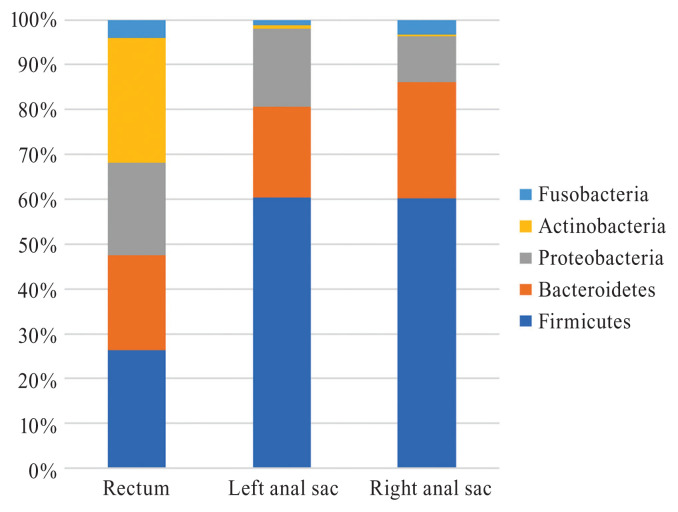
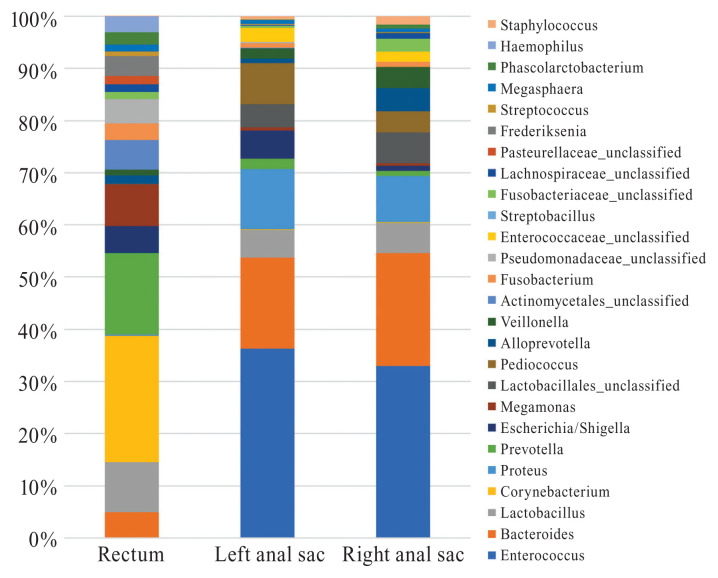
Ten and 9 bacterial phyla were identified in the left and right anal sacs, respectively, while 12 bacterial phyla were identified in the rectum. The predominant phyla in the left and right anal sacs and rectum were Firmicutes (60.4%, 60.1%, and 26.2%, respectively), Bacteroidetes (20.2%, 26.0%, and 21.3%, respectively), Proteobacteria (17.4%, 10.2%, and 20.5% respectively), Fusobacteria (1.1%, 3.3%, and 4.1%, respectively), and Actinobacteria (0.9%, 0.4%, and 27.7%, respectively) (Figure 1). The predominant genera found in the left and right anal sacs were Enterococcus (34.3% and 31.5%, respectively), Bacteroides (16.5% and 20.6%, respectively), and Proteus (10.9% and 8.5%, respectively), while in the rectum, the predominant genera identified were Corynebacterium (21.5%), Prevotella (13.8%), and Lactobacillus (8.5%) (Figure 2). No detectable bacterial DNA was amplified from the blank samples after PCR. The Corynebacteriaceae family was significantly associated with rectal samples, but no other discriminant markers were found with the LefSe analysis.
Figure 1.
Relative abundance of the main bacterial communities inhabiting the left and right anal sacs and rectum of dogs at the phylum level.
Figure 2.
Relative abundance of the main bacterial communities inhabiting the left and right anal sacs and rectum of dogs at the genus level.
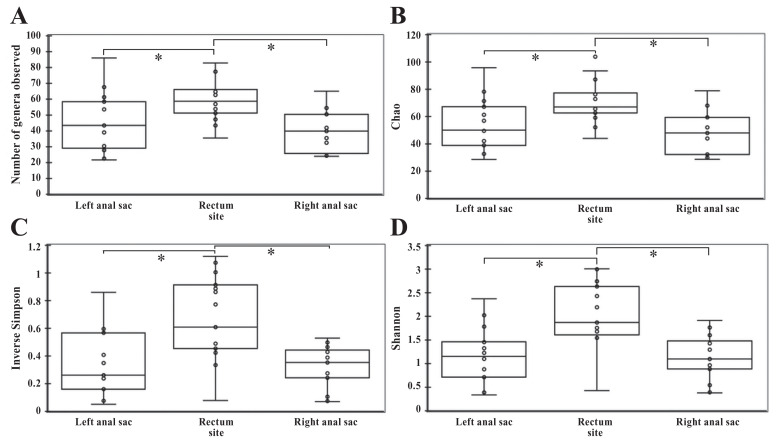
The richness (number of bacteria genera observed) was significantly lower in the left and right anal sacs compared to the rectum (P < 0.001 and P < 0.001, respectively), but there was no statistical difference between the right and left anal sacs (P = 0.161) (Figure 3). The Chao index, which is an estimator of true richness, was significantly lower in the left and right anal sacs compared to the rectum (P < 0.001 and P < 0.001, respectively), but there was no significant difference between both anal sacs (P = 0.337). The diversity was significantly lower in the left and right anal sacs compared to the rectum according to the Shannon index (P < 0.001 and P < 0.001, respectively) and inverse Simpson index (P = 0.002 and P = 0.001, respectively). There was no statistical difference in the diversity between the right and left anal sacs according to the Shannon index (P = 0.950) and inverse Simpson index (P = 0.981).
Figure 3.
Alpha diversity measurements in different sites (left and right anal sacs and rectum) in healthy dogs. A — Number of genera observed. B — Chao (estimator of richness). C — Inverse Simpson index (estimator of diversity). D — Shannon index (estimator of diversity). Error bars represent the standard deviations.
* P < 0.05.
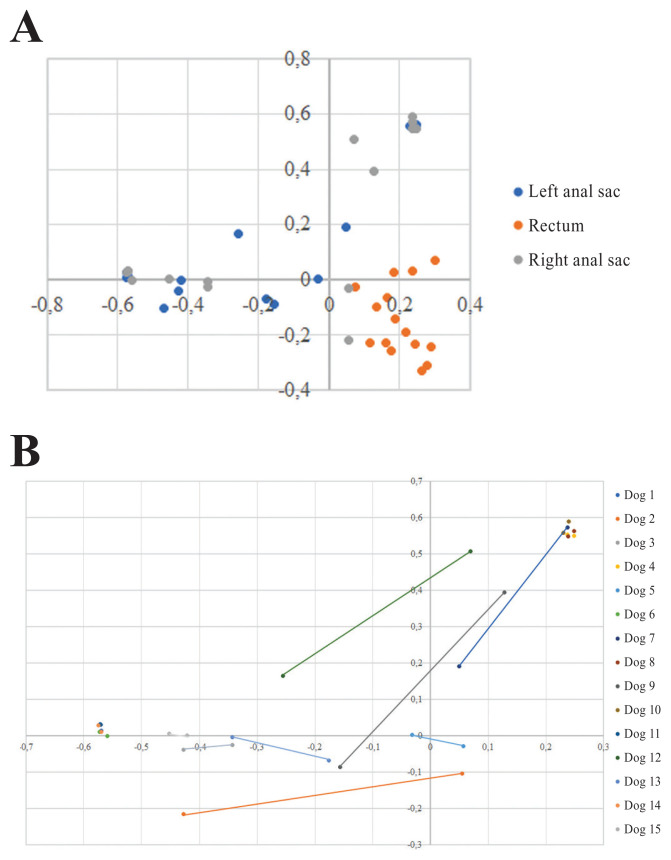
The PCoA plots revealed clear differences between anal sacs and rectum community structure, and to a lesser extent, between anal sacs and rectum community membership (Figure 4a). When considering individual factors such as diet, place of housing, and lactation, no clustering was observed. Interestingly, community structure of the left and right anal sacs clustered according to the dog in some of the samples, indicating a possible influence of environment in those dogs (Figure 4b). The statistical comparison of community beta diversity showed a significant difference in community membership of the rectum compared to the left and right anal sacs (P = 0.032 and P < 0.001, respectively), but not between both anal sacs (P = 0.972). There was also a statistical difference in community structure of the rectum compared to the left and right anal sacs (P < 0.001 and P < 0.001, respectively), but there was no statistical difference between both anal sacs (P = 0.660).
Figure 4.
Principal coordinate analysis plots of the community structure between sampling sites and dogs. A — Comparison of community structure of rectum and anal sacs. B — Community structure of both anal sacs in each dog (connected by lines mainly when they were not clustering).
Discussion
This study shows that the bacterial microbiota of anal sacs is much more diverse and richer than previously reported in studies using culture-based methods (2,19,20). The bacteria mainly identified in the healthy anal sacs with standard culture media were Micrococcus spp., Staphylococcus spp., Streptococcus faecalis, and Escherichia coli (2,19,20). Proteus mirabilis, beta-hemolytic Streptococcus spp., Bacillus spp., and Pseudomonas aeruginosa have also been reported (2). Although a major limitation of NGS is the low resolution at lower taxonomic levels (i.e., species level) (27), DNA consistent with all those bacterial genera, except Bacillus spp., could be detected in the anal sacs of the dogs in the present study. While Micrococcus spp. have been repeatedly isolated from the anal sacs of healthy dogs with culture-based methods, this genus was only detected in left anal sacs at very low relative abundances (~0.00012%) in the present study (2,19). This finding could be explained by the fact that the sequenced region used in the present study may not be ideal for selecting Micrococcus spp. (primer bias). The primer bias could also explain why Bacillus spp. was not found in the anal sacs in this study. The low number of cases in this study could also be an explanation. In addition, many other bacteria were detected by NGS, likely because these bacteria do not grow or grow poorly on standard culture media.
Considering that anal sacs in dogs are skin invaginations, it could have been hypothesized that bacterial communities in anal sacs and in skin microbiota are similar (1,2). In some studies reporting on skin microbiota in healthy dogs, samples were taken from the perianal area, which is the closest region to the anal sacs (28–30). The predominant bacterial phyla reported in the perineal region were similar to the ones found in the anal sacs of dogs in the present study (Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, and Fusobacteria). The main bacteria at the genus level found in the left and right anal sacs were Enterococcus (mean relative abundance of 34.3% and 31.5%, respectively), while the mean relative abundance of this genus in the perianal area was less than 2% in skin microbiota studies (28–30). This difference observed at the genus level is likely caused by different environmental conditions between the anatomical sites.
Previous studies have investigated the microbiota of the rectum or feces in dogs (31–35). Overall, the main bacterial phyla identified from feces samples taken from the rectum at necropsy or following dog defecation were the same as the ones found in this study, namely, Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, and Fusobacteria (31–35). However, the relative abundance of phyla varied across studies. The relative abundance of Actinobacteria phylum is the one that contrasted most strongly with the results of the present study. In previous studies, the mean or median relative abundance of Actinobacteria was less than 5%, whereas in the present study, it was 27.7% in dogs; this may be due to the materials and methods used in each study, such as the DNA extraction kit or the primer sets (31–35).
The present study also aimed to determine whether the microbiota of the rectum would correlate with the microbiota of the anal sacs, since these structures are very close to each other. Indeed, anal sacs in dogs are located laterally to the anus and communicate with the mucocutaneous junction of the anus via a duct (1,2). However, the present study showed that the microbiota of healthy anal sacs and rectum are different. It has been shown that there is a significant difference between the bacterial composition of the mucous membranes and the haired skin regions (30). Since the rectum is a mucous membrane and the anal sacs are an invagination of the skin, this could be a factor in explaining the difference between the bacterial microbiota of the rectum and the anal sacs (2,33). However, there are probably other more important factors that could explain this difference, such as secretions from the sebaceous and apocrine glands in the anal sacs, the presence of stool in the rectum, and environmental discrepancies (e.g., temperature, humidity, oxygen availability, etc.) (2,3). The Corynebacteriaceae family was overrepresented in the rectal samples according to the LEfSe. The lack of other statistically significant differences is probably a consequence of the interindividual variability on community structure (and relative abundances) and the low sample size used in the study.
The selection of a homogeneous population of dogs for this study was aimed to reduce confounding factors that could potentially bias results. Yet, a high interindividual variability was demonstrated. It has been shown that breed may affect the skin microbiota of dogs; therefore, further studies enrolling more diverse populations (i.e., different breeds, ages, and environments) are needed before the results of this study can be extrapolated to all dogs (29).
Some studies have shown that diet can influence fecal microbiota of dogs (34,35). The animals used in this study were fed similar diets. Although the study was not specifically designed to assess the impact of environmental factors on the microbiota of anal sacs, the PCoA plots suggest that the bacterial composition of these animals was not influenced by location, diet, or lactation. The design of the study and the small number of dogs enrolled preclude further extrapolations of results.
In conclusion, compared to previous studies using culture-based methods, a larger number of bacterial genera and a more diverse bacterial microbiota were present in the anal sacs of healthy dogs using NGS. The bacterial communities were similar between the left and right anal sacs, but significantly different between the anal sacs and the rectum. In order to better understand the role of the bacteria in anal sac diseases, future studies comparing the microbiota of healthy and diseased anal sacs are required.
Acknowledgments
This study was financially supported by the Companion Animals Health Fund of the Faculty of Veterinary Medicine of the University of Montreal, Zoetis, and the Fonds du Centenaire of the Faculty of Veterinary Medicine of the Université de Montréal. We would like to thank the MIRA Foundation for their assistance in recruiting cases and for allowing their dogs to participate to this study.
References
- 1.Goldschmidt MH, Zoltowski C. Anal sac gland adenocarcinoma in the dog: 14 cases. J Small Anim Pract. 1981;22:119–128. doi: 10.1111/j.1748-5827.1981.tb00591.x. [DOI] [PubMed] [Google Scholar]
- 2.Pappalardo E, Martino PA, Noli C. Macroscopic, cytological and bacteriological evaluation of anal sac content in normal dogs and in dogs with selected dermatological diseases. Vet Dermatol. 2002;13:315–322. doi: 10.1046/j.1365-3164.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- 3.Lake AM, Scott DW, Miller WH, Jr, Erb HN. Gross and cytological characteristics of normal canine anal-sac secretions. J Vet Med A Physiol Pathol Clin Med. 2004;51:249–253. doi: 10.1111/j.1439-0442.2004.00629.x. [DOI] [PubMed] [Google Scholar]
- 4.Robson DC, Burton GG, Lorimer MF. Cytological examination and physical characteristics of the anal sacs in 17 clinically normal dogs. Aust Vet J. 2003;81:36–41. doi: 10.1111/j.1751-0813.2003.tb11418.x. [DOI] [PubMed] [Google Scholar]
- 5.James DJ, Griffin CE, Polissar NL, Neradilek MB. Comparison of anal sac cytological findings and behaviour in clinically normal dogs and those affected with anal sac disease. Vet Dermatol. 2011;22:80–87. doi: 10.1111/j.1365-3164.2010.00916.x. [DOI] [PubMed] [Google Scholar]
- 6.Miyazaki T, Nishimura T, Yamashita T, Miyazaki M. Olfactory discrimination of anal sac secretions in the domestic cat and the chemical profiles of the volatile compounds. J Ethol. 2018;36:99–105. doi: 10.1007/s10164-017-0532-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halnan CR. Therapy of anal sacculitis in the dog. J Small Anim Pract. 1976;17:685–691. doi: 10.1111/j.1748-5827.1976.tb06930.x. [DOI] [PubMed] [Google Scholar]
- 8.van Duijkeren E. Disease conditions of canine anal sacs. J Small Anim Pract. 1995;36:12–16. doi: 10.1111/j.1748-5827.1995.tb02756.x. [DOI] [PubMed] [Google Scholar]
- 9.Costa M, Weese JS. Methods and basic concepts for microbiota assessment. Vet J. 2019;249:10–15. doi: 10.1016/j.tvjl.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Bernet MF, Brassart D, Neeser JR, Servin AL. Lactobacillus acidophilus LA 1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut. 1994;35:483–489. doi: 10.1136/gut.35.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller TL, Wolin MJ. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl Environ Microbiol. 1996;62:1589–1592. doi: 10.1128/aem.62.5.1589-1592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peppercorn MA, Goldman P. The role of intestinal bacteria in the metabolism of salicylazosulfapyridine. J Pharmacol Exp Ther. 1972;181:555–562. [PubMed] [Google Scholar]
- 13.Talham GL, Jiang HQ, Bos NA, Cebra JJ. Segmented filamentous bacteria are potent stimuli of a physiologically normal state of the murine gut mucosal immune system. Infect Immun. 1999;67:1992–2000. doi: 10.1128/iai.67.4.1992-2000.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaboriau-Routhiau V, Rakotobe S, Lécuyer E, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677–689. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Tizard IR, Jones SW. The microbiota regulates immunity and immunologic diseases in dogs and cats. Vet Clin North Am Small Anim Pract. 2018;48:307–322. doi: 10.1016/j.cvsm.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Janeczko S, Atwater D, Bogel E, et al. The relationship of mucosal bacteria to duodenal histopathology, cytokine mRNA, and clinical disease activity in cats with inflammatory bowel disease. Vet Microbiol. 2008;128:178–193. doi: 10.1016/j.vetmic.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Simpson KW, Dogan B, Rishniw M, et al. Adherent and invasive Escherichia coli is associated with granulomatous colitis in boxer dogs. Infect Immun. 2006;74:4778–4792. doi: 10.1128/IAI.00067-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halnan CR. The diagnosis of anal sacculitis in the dog. J Small Anim Pract. 1976;17:527–535. doi: 10.1111/j.1748-5827.1976.tb06996.x. [DOI] [PubMed] [Google Scholar]
- 20.Harvey RG, Lloyd DH. The distribution of Staphylococcus intermedius and coagulase-negative Staphylococci on the hair, skin surface, within the hair follicles and on the mucous membranes of dogs. Vet Dermatol. 1994;5:75–81. [Google Scholar]
- 21.QIAGEN [homepage on the Internet] c2013–2020 DNeasy PowerSoil Kit Handbook. [Last accessed October 6, 2020]. Available from: https://www.qiagen.com/us/resources/resourcedetail?id=5a0517a7-711d-4085-8a28-2bb25fab828a&lang=en.
- 22.Walters W, Hyde ER, Berg-Lyons D, et al. Improved bacterial 16S rRNA gene (V4 and V4–5) and fungal internal transcribed spacer marker gene primers for microbial community surveys. mSystems. 2015;1:e00009–15. doi: 10.1128/mSystems.00009-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol. 2013;79:5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lozupone CA, Knight R. Species divergence and the measurement of microbial diversity. FEMS Microbiol Rev. 2008;32:557–578. doi: 10.1111/j.1574-6976.2008.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yue JC, Clayton MK. A similarity measure based on species proportions. Commun Stat Theory Methods. 2005;34:2123–2131. [Google Scholar]
- 26.Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boers SA, Jansen R, Hays JP. Understanding and overcoming the pitfalls and biases of next-generation sequencing (NGS) methods for use in the routine clinical microbiological diagnostic laboratory. Eur J Clin Microbiol Infec Dis. 2019;38:1059–1070. doi: 10.1007/s10096-019-03520-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuscó A, Belanger JM, Gershony L, et al. Individual signatures and environmental factors shape skin microbiota in healthy dogs. Microbiome. 2017;5:139. doi: 10.1186/s40168-017-0355-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuscó A, Sánchez A, Altet L, Ferrer L, Francino O. Individual signatures define canine skin microbiota composition and variability. Front Vet Sci. 2017;4:6. doi: 10.3389/fvets.2017.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffmann AR, Patterson AP, Diesel A, et al. The skin microbiome in healthy and allergic dogs. PLoS One. 2014;9:e83197. doi: 10.1371/journal.pone.0083197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Mazcorro JF, Dowd SE, Poulsen J, Steiner JM, Suchodolski JS. Abundance and short-term temporal variability of fecal microbiota in healthy dogs. Microbiologyopen. 2012;1:340–347. doi: 10.1002/mbo3.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Handl S, Dowd SE, Garcia-Mazcorro JF, Steiner JM, Suchodolski JS. Massive parallel 16S rRNA gene pyrosequencing reveals highly diverse fecal bacterial and fungal communities in healthy dogs and cats. FEMS Microbiol Ecol. 2011;76:301–310. doi: 10.1111/j.1574-6941.2011.01058.x. [DOI] [PubMed] [Google Scholar]
- 33.Honneffer JB, Steiner JM, Lidbury JA, Suchodolski JS. Variation of the microbiota and metabolome along the canine gastrointestinal tract. Metabolomics. 2017;13:26. [Google Scholar]
- 34.Middelbos IS, Vester Boler BM, Qu A, White BA, Swanson KS, Fahey GC., Jr Phylogenetic characterization of fecal microbial communities of dogs fed diets with or without supplemental dietary fiber using 454 pyrosequencing. PLoS One. 2010;5:e9768. doi: 10.1371/journal.pone.0009768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swanson KS, Dowd SE, Suchodolski JS, et al. Phylogenetic and gene-centric metagenomics of the canine intestinal microbiome reveals similarities with humans and mice. ISME J. 2011;5:639–649. doi: 10.1038/ismej.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]