Abstract
The objective of this study was to evaluate the efficacy of a new Mycoplasma hyopneumoniae bacterin against a Korean M. hyopneumoniae challenge under experimental conditions. Fifteen pigs were allocated randomly into 3 groups (5 pigs per group) that were designated in 1 of 3 ways: vaccinated-challenged, unvaccinated-challenged, or unvaccinated-unchallenged. The pigs in the vaccinated-challenged group were immunized with an M. hyopneumoniae whole-cell bacterin at a 1.0 mL dose-level at 21 d old. At 42 d old (0 d post-challenge), the pigs in the vaccinated-challenged and unvaccinated-challenged groups were inoculated intranasally with a strain of Korean M. hyopneumoniae. Vaccinated-challenged pigs elicited a strong cell-mediated immunity as measured by M. hyopneumoniae-specific interferon-γ secreting cells when compared with unvaccinated-challenged pigs. Vaccination of pigs with this new M. hyopneumoniae bacterin reduced nasal shedding and lung lesions. The evaluated vaccine was therefore considered effective in controlling M. hyopneumoniae infection.
Résumé
L’objectif de cette étude était d’évaluer l’efficacité d’une nouvelle bactérine de Mycoplasma hyopneumoniae contre une infection défi avec une souche coréenne de M. hyopneumoniae dans des conditions expérimentales. Quinze porcs ont été répartis au hasard en trois groupes (5 porcs par groupe) qui ont été désignés de l’une des trois façons suivantes : vaccinés-infectés, non vaccinés-infectés, non vaccinésnon infectés. Les porcs du groupe vacciné-infectés ont été immunisés avec 1,0 mL d’une bactérine à cellules entières de M. hyopneumoniae à 21 jours d’âge. A l’âge de 42 jours (0 jour après la provocation), les porcs dans les groupes vaccinés-infectés et non vaccinés-infectés ont été inoculés par voie intranasale avec une souche coréenne de M. hyopneumoniae. Les porcs vaccinés-infectés ont manifesté une forte immunité à médiation cellulaire telle que mesurée par les cellules sécrétant l’interféron-γ spécifique à M. hyopneumoniae par rapport aux porcs non vaccinés-infectés. La vaccination des porcs avec cette nouvelle bactérine de M. hyopneumoniae a réduit l’excrétion nasale et les lésions pulmonaires. Le vaccin évalué a donc été considéré comme efficace pour maitriser l’infection à M. hyopneumoniae.
(Traduit par Docteur Serge Messier)
Mycoplasma hyopneumoniae infection alone causes relatively mild disease in the absence of environmental stressors, but when complicated by secondary bacterial invaders, may result in obvious clinical disease and severe production losses in intensively reared pigs (1). This respiratory disease is referred to as enzootic pneumonia. Mycoplasma hyopneumoniae is probably the most frequent bacterial respiratory infection in pig production and continues to be economically significant worldwide (1).
Vaccination is the most effective strategy for reducing economic losses and the clinical effects of M. hyopneumoniae infection on the Asian pork industry. A new single-dose M. hyopneumoniae whole-cell bacterin (Hyogen; CEVA Santé Animale, Libourne Cedex, France) was recently introduced into the Asian market to protect pigs against M. hyopneumoniae infection. In Europe, the same single-dose M. hyopneumoniae whole-cell bacterin provided protection against Belgian M. hyopneumoniae field isolates (2). Mycoplasma hyopneumoniae field isolates are known to be highly genetic, antigenic, and pathogenically variable between herds and geographical locations (3–5). Moreover, the genetic diversity of M. hyopneumoniae field isolates may be one of the factors that affects the efficacy of M. hyopneumoniae vaccines (6).
These results strongly suggest that protection of this bacterin against Belgian M. hyopneumoniae field isolates does not guarantee the same effective protection against Korean M. hyopneumoniae field isolates. The objective of this study was to evaluate the efficacy of the new single-dose M. hyopneumoniae whole-cell bacterin (Hyogen; CEVA Santé Animale) based on strain BA 2940–99, oil adjuvanted with paraffin and Escherichia coli J5 LPS with thiomersal as excipient, in pigs experimentally infected with M. hyopneumoniae for registration as recommended by the Republic of Korea’s Animal, Plant & Fisheries Quarantine & Inspection Agency (QIA), http://qia.go.kr
Unnecessary animal usage was eliminated in accordance with QIA guidelines by selecting and assigning the recommended 5 piglets for each treatment group. A total of 15 colostrum-fed, crossbred, conventional piglets was weaned and purchased at 18 d old from a commercial farm that was free of porcine reproductive and respiratory syndrome virus (PRRSV) and M. hyopneumoniae based on serological testing of the breeding herd and long-term clinical and slaughter history. At 21 d old, sera samples from pigs were found seronegative for porcine circovirus 2 (PCV2), PRRSV, and M. hyopneumoniae according to routine serological testing. Sera samples were negative for PCV2 and PRRSV and nasal swabs were negative for M. hyopneumoniae when tested by real-time polymerase chain reaction (RT-PCR) (7).
For the study, 15 pigs were allocated into 3 groups (5 pigs per group) using the Excel random number generator function (Microsoft, Redmond, Washington, USA). At −21 d post-challenge [(dpc) 21 d old], the pigs in the vaccinated-challenged (Vac/Ch) group were administered a single, 1.0-mL dose of M. hyopneumoniae whole-cell bacterin (Hyogen, Lot No. 1405582B; CEVA Santé Animale) intramuscularly based on the manufacturer’s instructions. The pigs in unvaccinated-challenged (UnVac/Ch) and unvaccinated-unchallenged (UnVac/UnCh) groups were administered an equal volume of phosphate-buffered saline (PBS, 0.01 M, pH 7.4, 1.0 mL) at 21 d old. At 0 dpc (42 d old), the pigs in the Vac/Ch and UnVac/Ch groups were inoculated with M. hyopneumoniae (strain SNU98703). Infection of pigs with M. hyopneumoniae strain SNU98703 caused severe mycoplasmal pneumonia (8).
Pigs in the Vac/Ch and UnVac/Ch groups were anesthetized with a mixture of 2.2 mg/kg body weight (BW) xylazine hydrochloride (Rumpon; Bayer, Leverkussen, Germany), 2.2 mg/kg BW tiletamine hydrochloride, and 2.2 mg/kg BW zolazepam hydrochloride (Zoletil 50; Virbac) by intramuscular injection. Post-anesthetization, pigs were inoculated intratracheally with 7 mL of M. hyopneumoniae (strain SNU98703) culture medium containing 107 color-changing units (CCUs)/mL. Pigs in the UnVac/UnCh group were inoculated with 7 mL of PBS in the same manner. After challenge, the pigs in the Vac/Ch and UnVac/Ch groups were randomly assigned to 1 room. The rooms each contained 2 pens with 5 pigs housed per pen. Pigs in the UnVac/UnCh group were randomly placed into 1 pen in the remaining room.
Blood and nasal swabs were collected at −21, 0, 7, 14, and 21 dpc. All 15 pigs were sedated by an intravenous injection of sodium pentobarbital and then euthanized by electrocution at 21 dpc as described in a previous study (9). Tissues were collected from each pig at necropsy. Post-collection, the tissues were fixed for 24 h in 10% neutral-buffered formalin, routinely processed, and embedded in paraffin. All of the methods were previously approved by the Seoul National University Institutional Animal Care and Use Committee and Animal Experiment Ethics Committee.
After M. hyopneumoniae inoculation, the pigs were monitored daily for physical condition and scored weekly for severity of clinical respiratory disease using scores ranging from 0 (normal) to 6 (severe dyspnea and abdominal breathing) (10). The live weight of each pig was measured at 2 time points throughout the study as follows: −21 (21 d old) and 21 dpc (63 d old). On conclusion of the study, the average daily weight gain [(ADWG) grams/pig per day) was calculated over production stage from 21 to 63 d old. Data for dead or removed pigs were included in the calculation.
Genomic DNA copies of M. hyopneumoniae were quantified by real-time quantitative PCR after DNA was extracted from nasal swabs using a commercial kit (QIAamp DNA Mini Kit; QIAGEN, Valencia, California, USA) as described in a previous study (7). Serum samples were tested for antibodies against M. hyopneumoniae (M. hyo. Ab test; IDEXX Laboratories, Westbrook, Maine, USA). Serum samples were considered positive for M. hyopneumoniae antibodies if the sample-to-positive (S/P) ratio was 0.4.
An enzyme-linked immunospot (ELISPOT) assay was conducted to measure the numbers of M. hyopneumoniae-specific interferon-γ secreting cells (IFN-γ-SCs). Mycoplasma hyopneumoniae (strain SNU98703) antigens were prepared as described in a previous study (11,12). The numbers of M. hyopneumoniae-specific IFN-γ-SCs stimulated by the aforementioned challenge M. hyopneumoniae antigen were determined in peripheral blood mononuclear cells (PBMCs) (11,12). The IFN-γ positive spots on the membranes were imaged, analyzed, and counted using an automated ELISPOT Reader (AID ELISPOT Reader; AID GmbH, Strassberg, Germany). The results were expressed as the numbers of IFN-γ-SCs per million PBMCs. The ELISPOT assay was done in duplicate.
Morphometric analysis of the macroscopic pulmonary lesion was scored on a total scale of 100 points as follows: 10 points each to the right cranial lobe, right middle lobe, left cranial lobe, and left middle lobe; 27.5 points each to the right caudal lobe and left caudal lobe; and 5 points to the accessory lobe (10). Microscopic mycoplasmal pulmonary lesions were scored (0 to 6) based on the severity of peribronchiolar and perivascular lymphoid tissue hyperplasia (13). All lung section scoring was evaluated blindly by 2 pathologists.
Prior to statistical analysis, RT-PCR data were transformed to log10 values. Data were tested for normal distribution using the Shapiro-Wilk test. One-way analysis of variance (ANOVA) was used to examine whether there were statistically significant differences at each time point within the 3 groups. A 1-way ANOVA test result with such a statistical significance was further evaluated by conducting a post-hoc test for a pairwise comparison with Tukey’s adjustment. If the normality assumption was not met, the Kruskal-Wallis test was conducted. A result from the Kruskal-Wallis test that showed statistical significance was further evaluated with the Mann-Whitney test to include Tukey’s adjustment to compare the differences among the groups. Results were reported in P-value in which a value of P < 0.05 was considered to be significant.
The mean scores for respiratory disease were significantly lower (P < 0.05) in pigs from the Vac/Ch group when compared to the UnVac/Ch group at 14 and 21 dpc. The pigs from the UnVac/UnCh group remained normal throughout the experiment. There was no significant difference in ADWG among the 3 groups from 21 to 63 d old (Table I).
Table I.
Average daily weight gain (ADWG) from 21 to 63 d old and pathology data (mean ± standard deviation) of 5 pigs in each of 3 groups at 21 d post-challenge.
| Groups | Vaccinated-challenged | Unvaccinated-challenged | Unvaccinated-unchallenged |
|---|---|---|---|
| ADWG | 295.71 ± 22.30 | 291.90 ± 26.76 | 301.90 ± 16.62 |
| Macroscopic lung lesion scores | 7.3 ± 6.53a | 22.7 ± 11.42b | 0 ± 0a |
| Microscopic lung lesion scores | 1.68 ± 0.39a | 3.64 ± 0.57b | 0 ± 0c |
Different superscripts (a, b, and c) indicate significant (P < 0.05) difference among 3 groups.
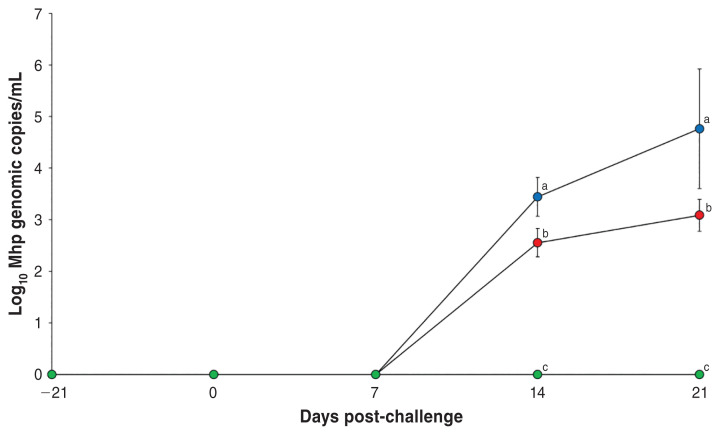
Pigs in the Vac/Ch group had significantly less (P < 0.05) M. hyopneumoniae genomic copies in their nasal swabs compared to the UnVac/Ch group at 14 and 21 dpc (Figure 1). No M. hyopneumoniae was detected in the pigs from the UnVac/UnCh group.
Figure 1.
Mean values of the genomic copy number of Mycoplasma hyopneumoniae DNA in nasal swabs from vaccinated-challenged (Vac/Ch,
 ), unvaccinated-challenged (UnVac/Ch,
), unvaccinated-challenged (UnVac/Ch,
 ), and unvaccinated-unchallenged (UnVac/UnCh,
), and unvaccinated-unchallenged (UnVac/UnCh,
 ) groups. Variation is expressed as the standard deviation. Different superscripts (a, b, and c) indicate significant (P < 0.05) difference among the 3 groups.
) groups. Variation is expressed as the standard deviation. Different superscripts (a, b, and c) indicate significant (P < 0.05) difference among the 3 groups.
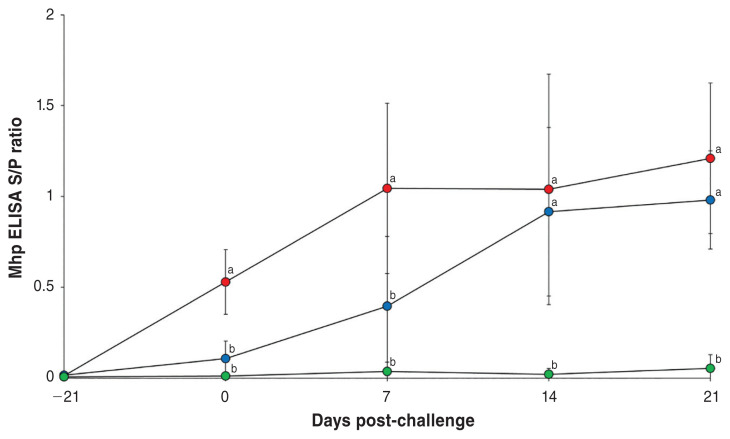
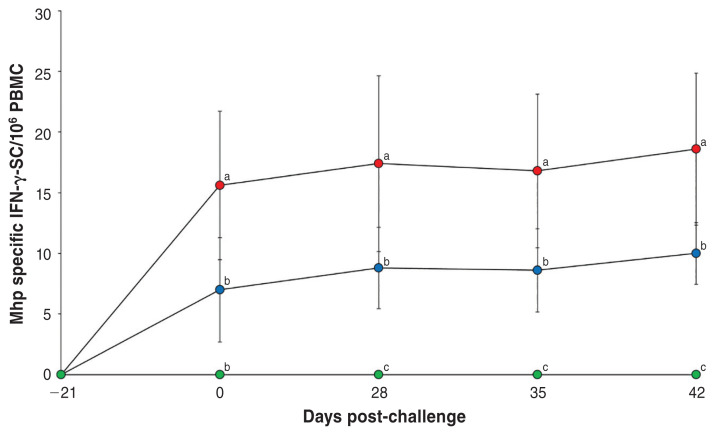
Pigs in the Vac/Ch group had a significantly higher (P < 0.05) M. hyopneumoniae enzyme-linked immunosorbent assay (ELISA) S/P ratio in their serum samples when compared with the UnVac/Ch group from 0 to 7 dpc (Figure 2), as well as a significantly higher number of M. hyopneumoniae-specific interferon-γ secreting cells (IFNγ-SCs) in their PBMCs (Figure 3) when compared with the UnVac/Ch group from 0 to 21 dpc. No M. hyopneumoniae-specific antibodies and IFN-γ-SCs were detected in pigs from the UnVac/UnCh group.
Figure 2.
Mycoplasma hyopneumoniae-specific ELISA antibody levels in serum from vaccinated-challenged (Vac/Ch,
 ), unvaccinated-challenged (UnVac/Ch,
), unvaccinated-challenged (UnVac/Ch,
 ), and unvaccinated-unchallenged (UnVac/UnCh,
), and unvaccinated-unchallenged (UnVac/UnCh,
 ) groups. Variation is expressed as the standard deviation. Different superscripts (a and b) indicate significant (P < 0.05) difference among the 3 groups.
) groups. Variation is expressed as the standard deviation. Different superscripts (a and b) indicate significant (P < 0.05) difference among the 3 groups.
Figure 3.
Frequency of Mycoplasma hyopneumoniae-specific interferon-γ secreting cells (IFN-γ-SCs) in peripheral blood mononuclear cells (PBMCs) from vaccinated-challenged (Vac/Ch,
 ), unvaccinated-challenged (UnVac/Ch,
), unvaccinated-challenged (UnVac/Ch,
 ), and unvaccinated-unchallenged (UnVac/UnCh,
), and unvaccinated-unchallenged (UnVac/UnCh,
 ) groups. Variation is expressed as the standard deviation. Different superscripts (a, b, and c) indicate significant (P < 0.05) difference among the 3 groups.
) groups. Variation is expressed as the standard deviation. Different superscripts (a, b, and c) indicate significant (P < 0.05) difference among the 3 groups.
Pigs in the Vac/Ch group had significantly lower (P < 0.05) macroscopic and microscopic lung lesion scores when compared with the UnVac/Ch group at 21 dpc. No macroscopic and microscopic lung lesions were detected in pigs from the UnVac/UnCh group (Table I).
The results of the present study demonstrate that vaccinated-challenged pigs develop fewer lung lesions and nasal route excretion than unvaccinated-challenged pigs. This variance between the 2 groups is probably due to differences in protective immunity. Protective immunity against M. hyopneumoniae is not fully understood. The fact that the pathogen is non-invasive, but can still induce pneumonia, implies that cellular immune response plays a significant role (14,15). Vaccinated-challenged pigs elicited a strong cell-mediated immunity as measured by M. hyopneumoniae-specific IFN-γ-SCs when compared with unvaccinated-challenged pigs. Induction of cell-mediated immunity by M. hyopneumoniae vaccine plays a significant role in protecting pigs against M. hyopneumoniae infection, as implied by previous studies (12).
There are 2 ways to assess the efficacy of vaccines: field clinical and experimental challenge trials. Field clinical trials are suitable for evaluating pig productivity. Vaccination against M. hyopneumoniae improved pig productivity and was reported as increased growth performance and decreased mortality under field conditions (16–20). Despite vaccination efforts, M. hyopneumoniae continues to circulate within pig herds, leading to the possibility of exposure and re-exposure to the virus by horizontal transmission under field conditions. Meanwhile, experimental challenge trials are suitable for microbiological, immunological, and pathological evaluation.
Growth performance was also evaluated in the present experimental challenge study. There was no significant difference in ADWG between vaccinated-challenged and unvaccinated-challenged groups because of the small number of pigs in each group and the short duration observed after challenge with M. hyopneumoniae. These results agree with a previous study in which the same vaccine showed no significant difference in growth performance under experimental conditions (3). Nevertheless, vaccination of pigs with this newly evaluated M. hyopneumoniae bacterin benefits the pig by eliciting cell-mediated immunity and reducing nasal shedding and lung lesions. The newly evaluated vaccine may therefore be an effective tool in controlling M. hyopneumoniae infection.
Acknowledgments
The authors’ research was supported by contract research funds (Grant no. 550-20180057) from the Research Institute for Veterinary Science (RIVS) of the College of Veterinary Medicine at Seoul National University and by the BK 21 FOUR Future Veterinary Medicine Leading Eduation and Research Center.
References
- 1.Maes D, Verdonck M, Deluyker H, de Kruif A. Enzootic pneumonia in pigs. Vet Quart. 1996;18:104–109. doi: 10.1080/01652176.1996.9694628. [DOI] [PubMed] [Google Scholar]
- 2.Michiels A, Arsenakis I, Boyen F, Krejci R, Haesebrouck F, Maes D. Efficacy of one dose vaccination against experimental infection with two Mycoplasma hyopneumoniae strains. BMC Vet Res. 2017;13:274. doi: 10.1186/s12917-017-1195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayor D, Zeeh F, Frey J, Kuhnert P. Diversity of Mycoplasma hyopneumoniae in pig farms revealed by direct molecular typing of clinical material. Vet Res. 2007;38:391–398. doi: 10.1051/vetres:2007006. [DOI] [PubMed] [Google Scholar]
- 4.Mayor D, Jores J, Korczak BM, Kuhnert P. Multilocus sequence typing (MLST) of Mycoplasma hyopneumoniae: A diverse pathogen with limited clonality. Vet Microbiol. 2008;127:63–72. doi: 10.1016/j.vetmic.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Vicca J, Stakenborg T, Maes D, et al. Evaluation of virulence of Mycoplasma hyopneumoniae field isolates. Vet Microbiol. 2003;97:177–190. doi: 10.1016/j.vetmic.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Strait EL, Rapp-Gabrielson VJ, Erickson BZ, et al. Efficacy of a Mycoplasma hyopneumoniae bacterin in pigs challenged with two contemporary pathogenic isolates of M. hyopneumoniae. J Swine Health Prod. 2008;16:200–206. [Google Scholar]
- 7.Dubosson CR, Conzelmann C, Miserez R, et al. Development of two real-time PCR assays for the detection of Mycoplasma hyopneumoniae in clinical samples. Vet Microbiol. 2004;102:55–65. doi: 10.1016/j.vetmic.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Kwon D, Choi C, Chae C. Chronologic localization of Mycoplasma hyopneumoniae in experimentally infected pigs. Vet Pathol. 2002;39:584–587. doi: 10.1354/vp.39-5-584. [DOI] [PubMed] [Google Scholar]
- 9.Beaver BV, Reed W, Leary S, et al. 2000 Report of the AVMA panel on euthanasia. J Am Vet Med Assoc. 2001;218:669–696. doi: 10.2460/javma.2001.218.669. [DOI] [PubMed] [Google Scholar]
- 10.Halbur PG, Paul PS, Frey ML, et al. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol. 1995;32:648–660. doi: 10.1177/030098589503200606. [DOI] [PubMed] [Google Scholar]
- 11.Bandrick M, Pieters M, Pijoan C, Molitor TW. Passive transfer of maternal Mycoplasma hyopneumoniae-specific cellular immunity to piglets. Clin Vaccine Immunol. 2008;15:540–543. doi: 10.1128/CVI.00466-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park C, Jeong J, Choi K, Chae C. Efficacy of a new bivalent vaccine of porcine circovirus type 2 and Mycoplasma hyopneumoniae (FosteraTM PCV MH) under experimental conditions. Vaccine. 2016;34:270–275. doi: 10.1016/j.vaccine.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 13.Thacker EL, Halbur PG, Ross RF, Thanawongnuwech R, Thacker BJ. Mycoplasma hyopneumoniae potentiation of porcine reproductive and respiratory syndrome virus-induced pneumonia. J Clin Microbiol. 1999;37:620–627. doi: 10.1128/jcm.37.3.620-627.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Djordjevic SP, Eamens GJ, Romalis LF, Nicholls PJ, Taylor V, Chin J. Serum and mucosal antibody responses and protection in pigs vaccinated against Mycoplasma hyopneumoniae with vaccines containing a denatured membrane antigen pool and adjuvant. Aust Vet J. 1997;75:504–511. doi: 10.1111/j.1751-0813.1997.tb14383.x. [DOI] [PubMed] [Google Scholar]
- 15.Thacker EL, Thacker BJ, Kuhn M, Hawkins PA, Waters WR. Evaluation of local and systemic immune responses induced by intramuscular injection of a Mycoplasma hyopneumoniae bacterin to pigs. Am J Vet Res. 2000;61:1384–1389. doi: 10.2460/ajvr.2000.61.1384. [DOI] [PubMed] [Google Scholar]
- 16.Del Pozo Sacristán R, Sierens A, Marchioro SB, et al. Efficacy of early Mycoplasma hyopneumoniae vaccination against mixed respiratory disease in older fattening pigs. Vet Rec. 2014;174:197. doi: 10.1136/vr.101597. [DOI] [PubMed] [Google Scholar]
- 17.Jensen CS, Ersbøll AK, Nielsen JP. A meta-analysis comparing the effect of vaccines against Mycoplasma hyopneumoniae on daily weight gain in pigs. Prev Vet Med. 2002;54:265–278. doi: 10.1016/s0167-5877(02)00005-3. [DOI] [PubMed] [Google Scholar]
- 18.Maes D, Deluyker H, Verdonck M, et al. The effect of vaccination against Mycoplasma hyopneumoniae in pig herds with a continuous production system. Zoonoses Public Health. 1998;45:495–505. doi: 10.1111/j.1439-0450.1998.tb00820.x. [DOI] [PubMed] [Google Scholar]
- 19.Maes D, Deluyker H, Verdonck M, et al. Effect of vaccination against Mycoplasma hyopneumoniae in pig herds with an all-in/all-out production system. Vaccine. 1999;17:1024–1034. doi: 10.1016/s0264-410x(98)00254-0. [DOI] [PubMed] [Google Scholar]
- 20.Wilson S, Van Brussel L, Saunders G, et al. Vaccination of piglets at 1 week of age with an inactivated Mycoplasma hyopneumoniae vaccine reduces lung lesions and improves average daily gain in body weight. Vaccine. 2012;30:7625–7629. doi: 10.1016/j.vaccine.2012.10.028. [DOI] [PubMed] [Google Scholar]