Abstract
Objective:
Intensity modulated radiation therapy (IMRT) using a volumetric-modulated arc therapy technique may offer dosimetric and clinical benefits compared to the historical standard of care 3D-conformal radiotherapy (3D-CRT) in definitive treatment of bladder cancer. We hypothesized that IMRT with CBCT would reduce dose to the rectum, bowel, and bladder compared to 3D-CRT.
Methods:
We reviewed nineteen patients treated with maximal transurethral resection of bladder tumor followed by concurrent chemotherapy with IMRT. All patients received 45 Gy to the entire empty bladder followed by 19.8 Gy tumor boost treated with full bladder. 3D-CRT treatment plans were created for the same prescription. Paired t-test or Wilcoxon matched-pairs signed rank test analyzed dosimetry and bladder volumes.
Results:
The rectum and bowel V40, V45, V50, V55, and V60 were reduced by over 50% in the IMRT plans compared to 3D-CRT (p<0.0001). IMRT also reduced volume of bladder irradiated compared to 3D-CRT (p<0.01). After CBCT, patients were likely to undergo clinically significant shifts ≥ 0.5 cm before boost delivery (p=0.001). Bladder volumes were significantly lower during boost treatments compared to pre-treatment simulation (p=0.002). There were 4 (21%) grade 3 genitourinary toxicities and 1 (5%) grade 3 gastrointestinal toxicity.
Conclusion:
IMRT is superior to 3D-CRT for bladder cancer and spares dose to bowel, rectum, and bladder with improved acute toxicity compared to published clinical literature. For boost treatment, daily full bladder volume and positioning are not always reproducible, supporting the need for CBCT for optimal localization of the primary bladder tumor.
Keywords: muscle-invasive bladder cancer, bladder preservation, radiation, dosimetry, toxicity
Introduction
Since the 1980’s, most international clinical trials for muscle-invasive bladder cancer (MIBC) that use bladder-preserving radiotherapy have mandated a 3D-conformal radiation treatment (3D-CRT) technique and prohibited intensity-modulated radiation therapy (IMRT). This historical standard-of-care 3D-CRT approach uses static-positioned fields with various beam arrangements including 4-field boxes to cover the bladder and its primary tumor while using MLCs to block critical structures. A common prescription established by RTOG-8903 involves treating the entire empty bladder to 45 Gy and then boosting an additional 19.8 Gy to the partial volume containing the gross tumor within a full bladder [1].
The use of 3D-CRT has been questioned with the advent of modern treatment techniques, particularly IMRT [2–7]. IMRT can create high dose gradients using inverse planning to customize the positions of MLCs so that a target is treated while sparing surrounding structures. Dose algorithms determine the optimized MLC movement via inverse planning by fulfilling pre-determined dose constraints. This optimized delivery allows for high conformality and coverage of the target volume while reducing dose to critical nearby organs [8–11].
Nonetheless, while IMRT is gaining significant traction in clinical trials and in practice, 3D-CRT likely remains the more common approach for definitive treatment of MIBC, in part due to the complexity of treatment planning and dose delivery [12–14]. Appropriate anatomical alignment in executing daily MIBC treatment is difficult because of the variability of bladder volume and positioning. While patients are instructed to fill the bladder to ‘comfortably full’ capacity for boost treatments, genitourinary comorbidities of the MIBC patient population, in addition to the accumulating side effects of treatment, often challenge optimal reproducible filling during the boost course [15]. Such patient-dependent daily variability in the size of the bladder raises significant concerns of highly conformal techniques such as IMRT. Although cone beam computed tomography (CBCT) is a common technique for image-guided radiation treatment (IGRT), CBCT is underutilized for the management of bladder cancer, with little data in the bladder cancer literature [16–19].
Therefore, we sought to evaluate whether IMRT could reduce dose to critical organs and reduce toxicities while maintaining adequate tumor coverage. We hypothesized that IMRT using a modern volumetric-modulated arc therapy (VMAT) technique would significantly reduce the dose to nearby critical organs, including the bowel, rectum, and bladder compared to 3D-CRT. We secondarily explored the importance of using CBCT for tumor targeting in MIBC and assessed variability in daily bladder volumes during boost treatments. The goal of our study was to generate evidentiary support for recommending IMRT with the use of CBCT-based IGRT as an appropriate radiation treatment paradigm for MIBC.
Methods
After IRB approval, we retrospectively evaluated 19 consecutive patients treated at our single institution between 2016 and 2019 with maximal transurethral resection followed by curative-intent IMRT by the VMAT technique and concurrent chemotherapy for bladder cancer. Charlson comorbidity index and Eastern Cooperative Oncology Group (ECOG) performance status were scored at consultation [20]. Toxicities were characterized retrospectively according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Stage was determined by American Joint Committee on Cancer, 8th edition. Overall survival (OS), disease-free survival (DFS), and progression-free survival (PFS) were measured from the end of treatment to event or censor.
CT simulation was performed with full bladder followed by an empty bladder at a single simulation session. To fill the bladder, patients were provided with 20 oz. of water and instructed to finish drinking 40 minutes before CT simulation or treatment. A single radiation oncologist (ANK) contoured all structures following RTOG guidelines [21, 22]. Briefly, the initial phase clinical target volume was defined by a 1.5 cm expansion of the empty bladder, a 1 cm expansion around the prostate or proximal urethra, and the pelvic nodes including perivesical, obturator, external iliac, internal iliac, and common iliac regions defined by a 7 mm boundary around the presacral, internal and external iliac vessels [21, 22]. A 7 mm expansion of the clinical target volume was used to create the initial phase planning target volume, which was prescribed to 45 Gy using 1.8 Gy fractions for 25 fractions. The boost clinical target volume was defined as a 1.5 cm expansion of the primary gross tumor volume, determined based on the CT simulation scan, prior CT scans, pathology reports, and TURBT operative reports, within a full bladder. The boost planning target volume included a 5–7 mm expansion of the boost clinical target volume and was treated to 19.8 Gy using 1.8 Gy per fraction for 11 fractions. In two patients, clinically positive nodes were also treated as gross disease and boosted to 64.8 Gy. Bowel was contoured as bowel bag.
All patients were treated with IMRT utilizing a VMAT technique planned in Eclipse software (Varian Medical Systems, Palo Alto, CA). Primary IMRT plans were created either by the AAA version 11 or version 13 dose calculation algorithm. Boost plans were created using the same algorithm to treat the primary bladder tumor only within a full bladder. All IMRT plans were reviewed at a quality assurance conference consisting of radiation oncologists, medical physicists, and dosimetrists.
3D-CRT plans were retrospectively created for each patient with the same prescription of treating the empty bladder to 45 Gy followed by a boost of 19.8 Gy with a full bladder. Contoured volumes remained identical to the IMRT plans [21, 22]. Fields were designed according to RTOG-8903 and are described in the Supplement [1]. RTOG-8903 constraints were also used for organs at risk, including posterior rectum wall constrained to max dose < 55 Gy, but femoral heads were constrained to max dose < 50 Gy. 100% of the gross tumor volume was required to have a minimum coverage at 95% of the prescription. Constraints were evaluated by creating plan sums of the primary and boost treatment for both the IMRT and 3D-CRT plans.
After confirmation of plan satisfaction, 3D-CRT and IMRT primary and boost plans were exported to MIM software (MIM Software Inc., Beachwood, OH). The empty bladder CT and full bladder CT with structures were fused with a deformable registration algorithm, and dose was accumulated to create a composite plan sum. Dose volume histograms were generated from the composite plan sum, and dose-volume data were abstracted.
In the initial whole bladder phase, once weekly CBCT was obtained to ensure the empty bladder was properly targeted. In the boost phase, patients with a full bladder underwent kV orthogonal paired imaging with alignment to pelvic bony anatomy followed by daily CBCT. The patient was shifted based on CBCT alignment to the target bladder wall region and treated in that position. The difference between the initial bony alignment and the treated bladder target-aligned position by CBCT was determined by the three-dimensional shift magnitude, which was calculated by the square-root of the sum of the individual squared longitudinal, vertical, and lateral position differences. The full bladder was contoured for each daily CBCT during the boost phase.
Survival outcomes were described by fitting Kaplan-Meier curves. Based on the normality of the covariate tested by Shapiro-Wilk, paired t-test or Wilcoxon matched-pairs signed rank test was used to compare dose-volume relationships between IMRT and 3D-CRT plans; pairwise testing was utilized as each patient served as their own control. To account for tied values, Wilcoxon matched-pairs signed rank test with Pratt correction compared full bladder volumes from initial CT simulation and during each boost treatment CBCT. CBCT shifts were analyzed by Wilcoxon matched-pairs signed rank test against an a priori clinically relevant shift threshold of 0.5 cm [16–19]. All confidence intervals were reported at 95% and all p values were reported as two-sided, with an alpha level of 0.05. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC); all plots were generated using Prism version 7 (GraphPad Software, La Jolla, CA).
Results
A total of 19 patients with median follow-up of 10.3 months (IQR 3.2–20.7) were enrolled (Table 1). The most typical patient was an older Caucasian gentleman with comorbidities presenting with clinical stage II T2N0M0 high grade muscle-invasive urothelial carcinoma. Other rare presentations included one patient with limited-stage non-muscle-invasive small cell carcinoma, which by multidisciplinary genitourinary tumor board was considered aggressive enough disease to warrant treatment similar to a muscle-invasive bladder cancer. Another patient presented with metastatic disease to the para-aortic and retroperitoneal lymph nodes. This patient was treated with neoadjuvant gemcitabine and cisplatin with complete resolution of lymphadenopathy and partial response of the primary bladder tumor. PET scan post neoadjuvant chemotherapy demonstrated avidity in the external iliac nodes without uptake in the retroperitoneal or para-aortic nodes. Given this response, the patient elected for bladder-preserving IMRT to the bladder, pelvic nodes, and previously enlarged para-aortic nodes.
Table 1.
Patient clinicopathologic characteristics.
| Clinicopathologic Characteristic | Mean/Median or Incidence | IQR/95% CI or % |
|---|---|---|
| Age (years) | 78 | 74–81 |
| Male sex | 15 | 79 |
| Race | ||
| NH White | 17 | 89 |
| NH Black | 2 | 11 |
| Body Mass Index (kg/m2) | 28 | 25–31 |
| Tobacco Use | 12 | 63 |
| Charlson Comorbidity Index | 7 | 6–9 |
| ECOG Performance Status | ||
| 0 | 5 | 26 |
| 1 | 9 | 47 |
| 2 | 5 | 26 |
| Clinical Stage | ||
| I* | 1 | 3 |
| II | 16 | 84 |
| III | 1 | 3 |
| IV | 1 | 3 |
| T2 | 17 | 89 |
| N0 | 16 | 84 |
| Histology | ||
| Urothelial | 17 | 89 |
| Squamous | 1 | 5 |
| Small cell | 1 | 5 |
| Differentiation Pattern | ||
| Papillary | 5 | 26 |
| Sarcomatoid | 2 | 11 |
| Plasmacytoid | 1 | 5 |
| Squamous | 4 | 21 |
| Not otherwise specified | 8 | 42 |
| Grade | ||
| High | 16 | 84 |
| Moderate | 1 | 3 |
| MIBC | 17 | 89 |
| Tumor Location | ||
| Dome | 2 | 11 |
| Lateral Wall | 7 | 37 |
| Posterior Wall | 4 | 21 |
| Trigone/Neck | 3 | 16 |
| Multifocal | 3 | 16 |
| Maximum Tumor Diameter | 4.18 | 3.22–5.12 |
| Chemotherapy | ||
| MMC/5FU | 15 | 79 |
| Gemcitabine | 2 | 11 |
| Cisplatin | 2 | 11 |
Small cell carcinoma of the bladder
All patients underwent maximal transurethral resection of bladder tumor prior to chemoradiotherapy. Two patients had planned split-course treatments with a four week break between the primary and boost for interval cystoscopy in the manner of RTOG-8903; all others were treated without planned interruption per Rodel et al [1, 23]. Most patients (79%) were treated with mitomycin C and 5-flurouracil during radiotherapy.
Chemoradiotherapy was well-tolerated for most patients with the expected grade 1 and grade 2 genitourinary, gastrointestinal, and hematologic toxicities (Table 2). Four patients (21%) experienced a grade 3 genitourinary toxicity, and 1 patient (5%) experienced a grade 3 gastrointestinal toxicity. No patients had radiotherapy interruptions due to toxicity; eight patients had chemotherapy modifications due to hematologic toxicity.
Table 2.
Incidence of treatment-related side effects during chemoradiotherapy according to maximal severity per the Common Terminology Criteria for Adverse Events version 5.0. Both maximal specific and system toxicities are displayed.
| Toxicity | Grade 0 n (%) | Grade 1 n (%) | Grade 2 n (%) | Grade 3 n (%) | Grade 4 n (%) |
|---|---|---|---|---|---|
| Genitourinary | 1 (5) | 10 (53) | 4 (21) | 4 (21) | 0 (0) |
| Frequency | 5 (26) | 11 (58) | 2 (11) | 0 (0) | 0 (0) |
| Cystitis Non-Infection | 6 (33) | 10 (56) | 2 (11) | 0 (0) | 0 (0) |
| Urinary Tract Infection | 14 (74) | 0 (0) | 1 (5) | 4 (21) | 0 (0) |
| Urinary Tract Pain | 15 (83) | 2 (11) | 1 (6) | 0 (0) | 0 (0) |
| Urgency | 4 (22) | 13 (72) | 1 (6) | 0 (0) | 0 (0) |
| Bladder Spasm | 14 (78) | 2 (11) | 2 (11) | 0 (0) | 0 (0) |
| Dysuria | 9 (50) | 8 (44) | 1 (6) | 0 (0) | 0 (0) |
| Gastrointestinal | 5 (26) | 9 (47) | 4 (21) | 1 (5) | 0 (0) |
| Nausea | 16 (84) | 1 (5) | 2 (11) | 0 (0) | 0 (0) |
| Diarrhea | 6 (32) | 10 (53) | 2 (11) | 1 (5) | 0 (0) |
| Proctitis | 16 (84) | 1 (5) | 1 (5) | 1 (5) | 0 (0) |
| Colitis | 17 (89) | 1 (5) | 0 (0) | 1 (5) | 0 (0) |
| Hematologic/Laboratory | 4 (21) | 2 (11) | 3 (16) | 6 (32) | 4 (21) |
| Leukopenia | 4 (27) | 5 (33) | 4 (27) | 0 (0) | 2 (13) |
| Anemia | 2 (13) | 5 (33) | 5 (33) | 3 (20) | 0 (0) |
| Thrombocytopenia | 5 (33) | 7 (47) | 1 (7) | 1 (7) | 1 (7) |
| Neutropenia | 10 (71) | 0 (0) | 3 (21) | 0 (0) | 1 (7) |
| Lymphocytopenia | 0 (0) | 1 (7) | 3 (21) | 9 (64) | 1 (7) |
| Elevated Creatinine | 8 (53) | 6 (40) | 1 (7) | 0 (0) | 0 (0) |
DFS, PFS, and OS are plotted on Kaplan-Meier curves (Supplement Figure 4). Two patients (11%) had local failure, both approximately 1 year after chemoradiotherapy. The first patient received intravesical Bacillus Calmette-Guerin and remains disease-free now three years post-chemoradiotherapy. The second patient expired due to other comorbidities (not due to bladder cancer) within a few months after recurrence. Local control rate was 89%. Two patients (11%) failed regionally; locoregional control rate was 79%. The regional failure in the first patient was treated definitively with stereotactic body radiation therapy to two para-aortic lymph nodes followed by 3-months of atezolizumab; currently this patient has no evidence of disease now 24 months after regional recurrence. The second patient with regional failure concurrently developed distant metastases and initiated pembrolizumab. In addition to this patient, three other patients failed distantly, and median PFS was 22 months. At last follow-up, 12 patients (63%) remained alive without evidence of disease.
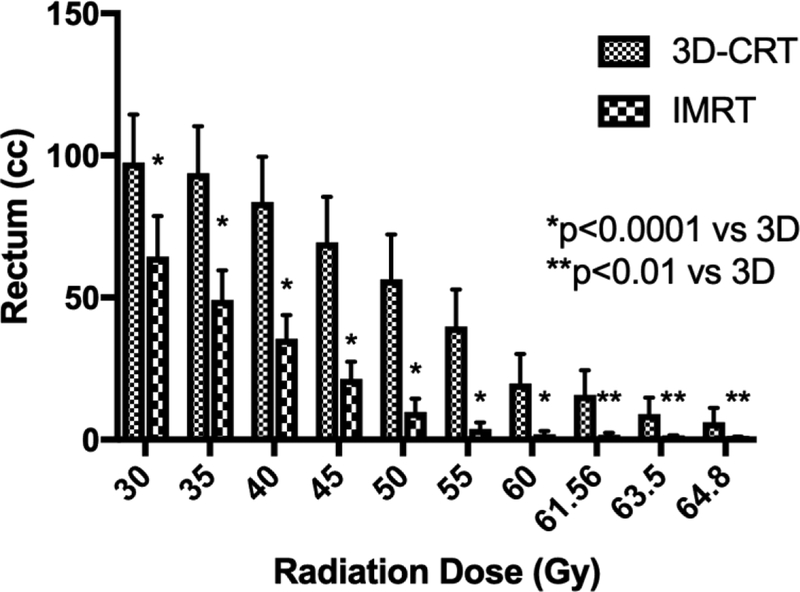
Primary target coverage for both 3D-CRT and IMRT plans was comparable and both techniques met or improved upon RTOG-8903 constraints. However, for all doses ≥ 30 Gy, IMRT significantly reduced the volume of rectum irradiated versus 3D-CRT (Figure 1a). Compared to 3D-CRT, IMRT reduced the volume of rectum receiving between 30 Gy and 60 Gy (p<0.0001) as well as the volume of rectum receiving greater than 60 Gy (p<0.05) (Supplemental Table 1). For example, rectum V50 was 43.4 cc for 3D-CRT versus 5.2 cc with IMRT (p<0.0001). Similarly, the mean percent of rectal volume receiving ≥ 45 Gy was 21.8% with IMRT versus 71.4% with 3D-CRT (p<0.0001).
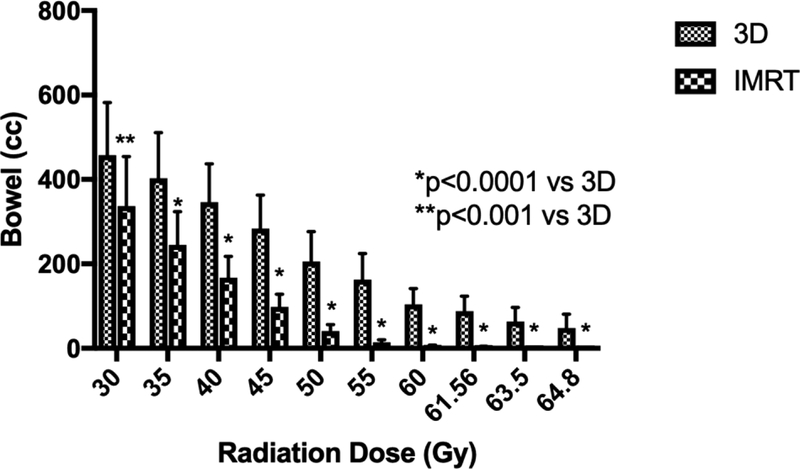
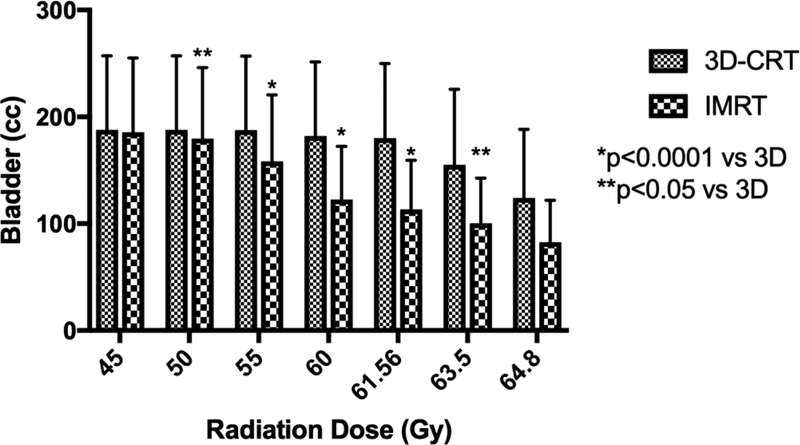
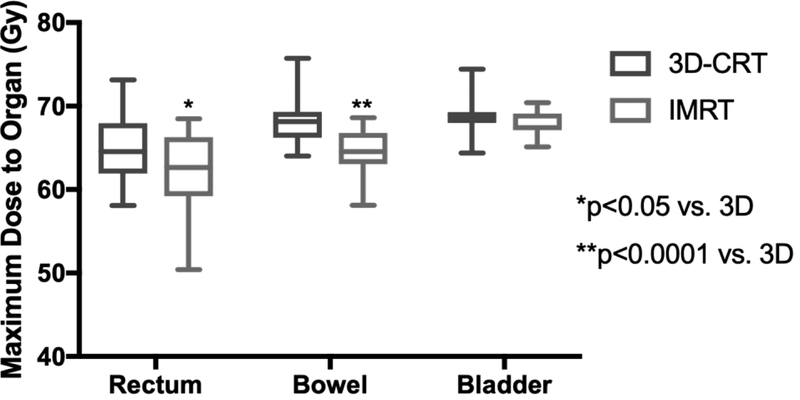
Figure 1.
3D-CRT and IMRT mean treatment volumes for organs-at-risk. Doses 30 Gy and higher are displayed for (A) rectum and (B) bowel. Doses 45 Gy and higher are shown for (C) bladder. (D) Maximum dose is displayed for each structure. Error bars represent 95% confidence intervals of the mean.
IMRT also reduced bowel irradiation at all dose levels investigated (Figure 1b). The V40, V45, V50 for IMRT were over 50% lower compared to 3D-CRT (p<0.0001) for absolute and relative bowel volumes (Supplemental Table 2). For example, the mean V45 for the 3D-CRT plan was 239 cc compared to 85.9 cc for the IMRT plan. Higher dose levels showed similar results, with IMRT dose reduced by over 50% compared to the 3D-CRT plan. For instance, less than 5% of bowel was treated to ≥ 50 Gy by IMRT, compared to 24% of bowel by 3D-CRT (p<0.0001).
Dosimetric comparison of the bladder is plotted in Figure 1c. The entire bladder received 45 Gy by prescription (Supplemental Table 3). From the 50 Gy dose level to the 63.5 Gy level, IMRT spared bladder significantly more so than 3D-CRT (p=0.002). At the 95% dose level, the V61.56 for 3D-CRT was 113.6 cc while IMRT was 83.6 cc (p<0.0001). Similarly, the percentage of bladder irradiated at and above the 98% dose level, 63.5 Gy, was 96.7% by 3D-CRT and only 50.9% by IMRT and (p=0.003), sparing nearly half of the bladder compared to 3D-CRT.
Dose maxima are shown in Figure 1d. Rectum max dose for 3D-CRT was 64.54 Gy (IQR 61.92–67.95) compared to 62.64 Gy (IQR 59.21–66.28) in IMRT (p<0.05). Bowel had a max dose of 68.14 Gy (IQR 66.23–69.31) for 3D-CRT while IMRT max dose was only 64.56 Gy (63.05–66.79 Gy) (p<0.0001). Max bladder dose was 68.84 Gy (IQR 67.97–69.29) for 3D-CRT and 67.43 Gy (IQR 67.11–69.13) for IMRT (p=0.17).
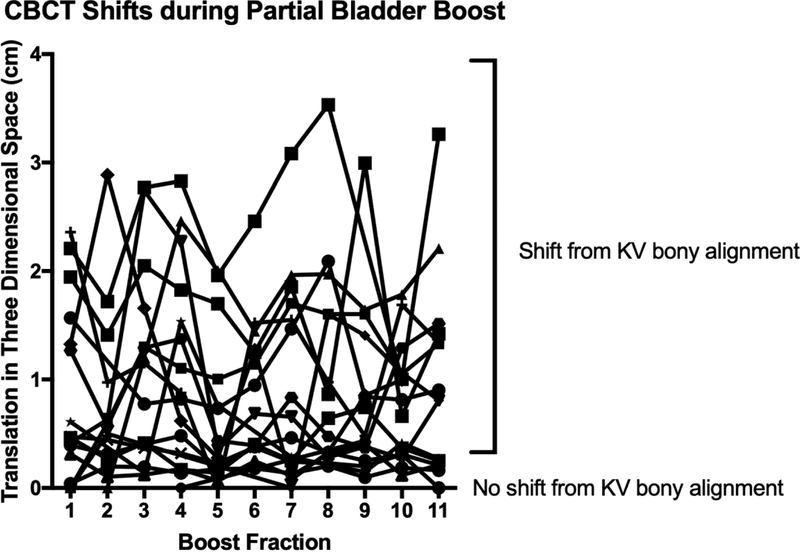
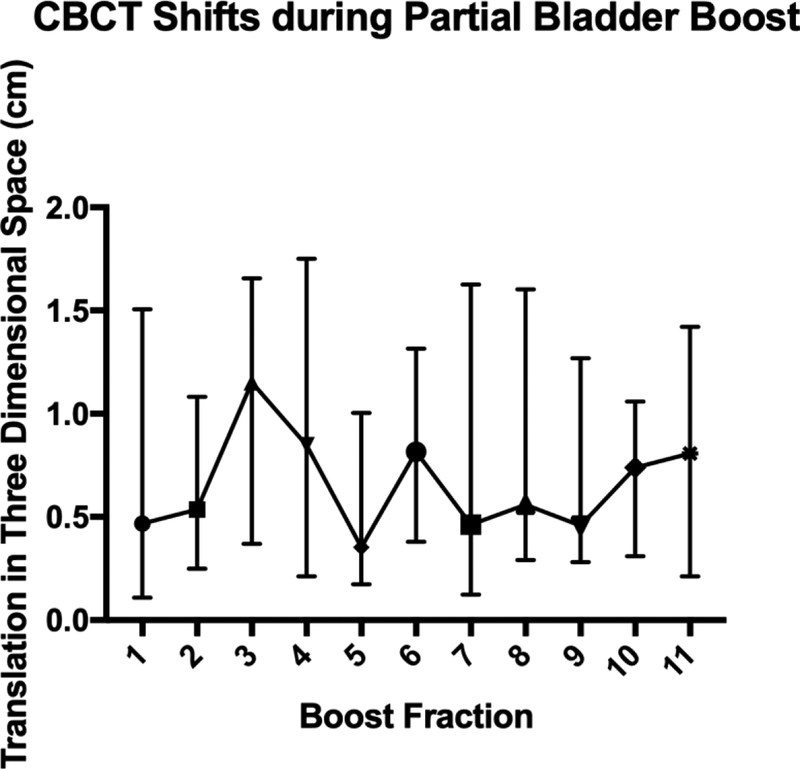
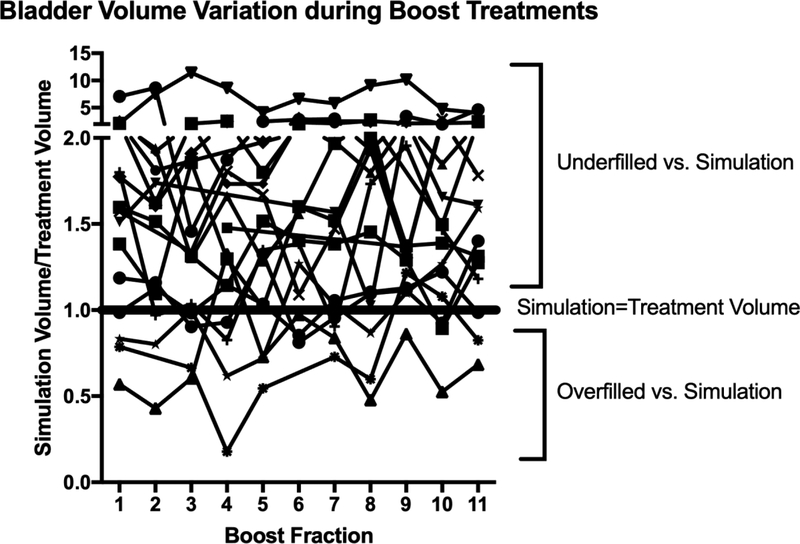
Median three-dimensional shifts determined by CBCT alignment of the target bladder wall following initial kV imaging aligned to bony anatomy are plotted in Figure 2a. For 19 patients with 170 CBCTs, the median three-dimensional shift after the CBCT was 0.62 cm (IQR 0.26–1.41). Four patients did not have CBCTs available for analysis. A wide range in shift requirements was evident and remained persistent for each fraction (Figure 2b). Patients were likely to undergo clinically relevant shifts greater than 0.5 cm at each fraction after daily CBCT (p=0.001). Patients underfilled the bladder during the boost phase compared to full bladder simulation (p=0.002). Substantial day-to-day variation in bladder volumes was evident during treatment (Figure 3).
Figure 2.
Daily CBCT translational shifts plotted for each fraction of partial bladder irradiation (A) individually and (B) summarized with median and interquartile range.
Figure 3.
Bladder volumes for each patient plotted for each fraction of partial bladder irradiation. Bladder volumes are displayed as normalized ratios to the simulation full bladder volume.
Discussion
In this study, we demonstrate that IMRT utilizing a modern VMAT technique is dosimetrically superior to 3D-CRT by sparing substantial volumes of the rectum and bowel above 30 Gy while maintaining appropriate tumor target coverage. IMRT also spares bladder at 50 Gy and higher versus 3D-CRT. In fact, bladder volumes receiving 63.5 Gy for IMRT are nearly half of 3D-CRT. These data illustrate the versatility of IMRT in sparing tissues adjacent to a target is remarkable even though the uninvolved bladder is abutting the primary target. Overall, the ability of IMRT to spare rectum, bowel, and bladder from large volumes receiving high doses is robust and demands attention in bladder-preservation clinical trials.
The dosimetric advantage afforded by IMRT over 3D-CRT is promising for the reduction of acute treatment-related toxicities. For instance, we found that mean bowel volume treated to ≥ 45 Gy was 239 cc for the 3D-CRT plan and only 85.9 cc for the IMRT plan. This mean 3D-CRT bowel volume exceeds the QUANTEC value of 195 cc, suggesting that the risk of grade 3 toxicity in the 3D-CRT exceeds 10% [24]. Given the direct relationship between bowel toxicity and treated bowel volume shown by McDonald and colleagues, the dosimetric advantage demonstrated by our study is highly encouraging for clinical translation into reduced toxicity [25]. This is corroborated by other authors who reported a significant decrease in grade 2+ diarrhea in patients treated with IMRT vs. 3D-CRT [2]. In our study, only 1 patient (5%) experienced a grade 3 gastrointestinal toxicity. In contrast, the historical 3D-CRT trial RTOG 0712 reported high grade gastrointestinal toxicity in 9% of its study cohort receiving daily radiation and gemcitabine [14]. Similarly, BC2001 reported rates of 9.6% high grade gastrointestinal toxicity during 3D-CRT with mitomycin C and 5-fluorouracil, even with 40% of those patients randomized to hypofractionated dose of 55 Gy [26]. The likelihood of rectal toxicity also appears to be significantly reduced with IMRT, as the entire dose distribution to the rectum appears markedly improved compared to 3D-CRT [27]. Furthermore, the probability of genitourinary toxicity also appears substantially reduced given known dose-volume effects of the bladder [15]. BC2001 observed high grade genitourinary toxicity in 21% of chemoradiotherapy patients, which appears equivalent to our study’s genitourinary toxicity rate of 21% [26]. However, it may be possible that, given the dosimetric advantages demonstrated for IMRT in our study, higher patient numbers or a comparison 3D-CRT cohort may have yielded more obvious benefits in clinical genitourinary toxicity [15]. For instance, one study reported a significant decrease in acute urinary toxicity for patients treated with IMRT compared to patients treated with 3D-CRT [5]. Finally, while our study did not evaluate late effects, long-term pelvic toxicity after 3D-CRT is uncommon [28]. Most likely, IMRT affords comparable, if not improved, rates of long-term toxicity.
To capitalize on the dosimetric and clinical utility of IMRT, we advocate for daily precision imaging with CBCT-based IGRT during the boost phase due to variability of bladder size and positioning. After receiving 45 Gy to the bladder, patients often have difficulty reproducibly maintaining a full bladder for the subsequent boost phase. Here, we show that patients underfill the bladder in approximately 80% of boost fractions compared to simulation. Bladder volumes significantly differed from simulation, and this disparity in volume may create significant alignment error for primary tumor, and consequently underdose to tumor and/or overdose to surrounding organs. Additionally, although our approach is to obtain daily CBCT during the boost phase, the use of daily CBCT during both the whole bladder phase and boost phase, as some authors suggest, may furnish further dosimetric and clinical benefit [29]. With this alternate strategy, target volumes in the whole bladder phase can be further decreased via elimination of the CTV margin, although the merits of such an approach, as well as drawbacks, require further investigation at this time.
Additionally, we find that patients were shifted on average over 0.6 cm even after alignment of bony anatomy with orthogonal kV imaging. The extent of shift was highly variable each day. The probability of missing tumor during boost treatments is likely high with conformal techniques such as IMRT in the absence of 3D-image guidance, leading to justifiable concerns [15]. Although 3D-CRT utilizes larger field sizes, the shifts observed in our study were often more than 2 cm, and standard 3D-CRT boost field margins may be insufficient for adequate tumor coverage. For remedy, in this study we demonstrate that even though bladder position and size vary considerably during each day of partial bladder irradiation, the risk of improper tumor coverage or overdosing of native organs can be mitigated by the application of daily CBCT. Efforts to improve target localization even further with the use of intravesical tumor bed fiducial markers including hydrogels (NCT03125226) are under active investigation [30]. At minimum, we strongly recommend daily CBCT-based IGRT during partial bladder irradiation.
Tumor control may be improved by IMRT with CBCT due to improved target accuracy. With CBCT, primary target bladder wall of a full bladder can be visualized and aligned for precise IMRT targeting. Other techniques for defining the boost target volumes, such as MRI, CT-urogram, and/or cystoscopic mapping, may further improve targeting. In our IMRT-treated cohort utilizing a CBCT-based approach, local control was 89%, and locoregional control was 79% at last follow-up. In the context of BC2001’s locoregional control rate of 67% at two years, the potential for IMRT to improve disease control rates over 3D-CRT appears promising [26]. IMRT with chemotherapy may even offer locoregional control comparable to neoadjuvant chemotherapy and radical cystectomy [31]. However, it is unlikely that a head-to-head trial of trimodality therapy versus radical cystectomy will be conducted in the near future [32]. Nonetheless, prospective studies evaluating locoregional efficacy of IMRT are certainly merited.
Limitations of this study include retrospective nature and sample size. While most patients received mitomycin C and 5-fluoruracil, chemotherapy heterogeneity may have introduced bias. Additionally, toxicities were retrospectively graded. However, 89% of patients were seen during treatment by a single genitourinary radiation oncologist (ANK) who provided standardized descriptions of side effects in the medical record. To that extent, all structures were contoured by ANK, and all plans were reviewed and approved by ANK. Therefore, the application of these techniques may not yet be generalizable outside of tertiary centers. Additionally, retrospectively generated plans for research purposes may be biased towards less optimal design compared to plans used in patient care, because research-generated plans do not undergo departmental peer review. While substantial efforts were made to construct 3D-CRT plans that would otherwise be suitable for treatment, inherent bias may inadvertently favor IMRT plans. Finally, our single-arm study was not designed to analyze tumor control rates. SWOG/NRG S1806 (NCT03775265) is a prospective phase III study randomizing patients to chemoradiotherapy with or without atezolizumab. This is the first large-scale clinical trial to allow physicians to treat MIBC with IMRT at physician discretion. S1806 provides considerable flexibility toward treatment volumes (full or partial bladder), elective nodal coverage, and chemotherapy regimen; the treatment style reported in this manuscript is included as an option in S1806.
While maintaining coverage of the tumor target, IMRT utilizing a modern VMAT technique significantly reduces dose to the surrounding rectum, bowel, and bladder as compared to 3D-CRT for the treatment of bladder cancer. Daily CBCT for partial bladder irradiation during the boost phase is essential to account for significant inter-fraction variations in bladder volume and positioning. IMRT dose-sparing may reduce toxicities compared to 3D-CRT. These findings merit validation in well-powered prospective trials.
Supplementary Material
Acknowledgements:
ANK is supported by the National Institutes of Health grant, No. 5K12CA090625-18 from the Vanderbilt Clinical Oncology Research Career Development Program.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Disclosure of potential conflicts of interest: Alexander Sherry, Adam Stewart, Guozhen Luo, and Austin Kirschner declare no conflicts of interest.
Ethical approval: All human and animal studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. This article does not contain any studies with human or animal subjects performed by any of the authors.
Informed consent: Informed consent was waived by the IRB due to the determination of minimal risk of the study.
References
- 1.Shipley WU, Winter KA, Kaufman DS, et al. (1998) Phase III trial of neoadjuvant chemotherapy in patients with invasive bladder cancer treated with selective bladder preservation by combined radiation therapy and chemotherapy: Initial results of Radiation Therapy Oncology Group 89–03. J Clin Oncol 16:3576–3583. 10.1200/JCO.1998.16.11.3576 [DOI] [PubMed] [Google Scholar]
- 2.Sondergaard J, Holmberg M, Jakobsen AR, et al. (2014) A comparison of morbidity following conformal versus intensity-modulated radiotherapy for urinary bladder cancer. Acta Oncol (Madr) 53:1321–1328. 10.3109/0284186X.2014.928418 [DOI] [PubMed] [Google Scholar]
- 3.Vestergaard A, Søndergaard J, Petersen JB, et al. (2010) A comparison of three different adaptive strategies in image-guided radiotherapy of bladder cancer. Acta Oncol (Madr) 49:1069–1076. 10.3109/0284186X.2010.501813 [DOI] [PubMed] [Google Scholar]
- 4.Baumann BC, Noa K, Wileyto EP, et al. (2015) Adjuvant radiation therapy for bladder cancer: A dosimetric comparison of techniques. Med Dosim 40:372–377. 10.1016/j.meddos.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 5.Lutkenhaus LJ, van Os RM, Bel A, Hulshof MCCM (2016) Clinical results of conformal versus intensity-modulated radiotherapy using a focal simultaneous boost for muscle-invasive bladder cancer in elderly or medically unfit patients. Radiat Oncol 11:45 10.1186/s13014-016-0618-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Søndergaard J, Høyer M, Petersen JB, et al. (2009) The normal tissue sparing obtained with simultaneous treatment of pelvic lymph nodes and bladder using intensity-modulated radiotherapy. Acta Oncol (Madr) 48:238–244. 10.1080/02841860802251575 [DOI] [PubMed] [Google Scholar]
- 7.Lee CY, Yang KL, Ko HL, et al. (2014) Trimodality bladder-sparing approach without neoadjuvant chemotherapy for node-negative localized muscle-invasive urinary bladder cancer resulted in comparable cystectomy-free survival. Radiat Oncol 9:213 10.1186/1748-717X-9-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizowaki T (2015) Intensity-modulated radiation therapy for locally advanced prostate cancer In: Nishimura Y, Komaki R (eds) Intensity-Modulated Radiation Therapy: Clinical Evidence and Techniques. Springer, pp 379–402 [Google Scholar]
- 9.Bauman G, Rumble RB, Chen J, et al. (2012) Intensity-modulated Radiotherapy in the Treatment of Prostate Cancer. Clin Oncol 24:461–473. 10.1016/j.clon.2012.05.002 [DOI] [PubMed] [Google Scholar]
- 10.Xu D, Li G, Li H, Jia F (2017) Comparison of IMRT versus 3D-CRT in the treatment of esophagus cancer: A systematic review and meta-analysis. Med 96:e7685 10.1097/MD.0000000000007685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemanski C, Thariat J, Ampil FL, et al. (2014) Image-Guided Radiotherapy for Cardiac Sparing in Patients with Left-Sided Breast Cancer. Front Oncol 4:257 10.3389/fonc.2014.00257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Block AM, Korpics M, Martin B, Solanki AA (2017) Intensity Modulated Radiation Therapy in Muscle-Invasive Bladder Cancer: Predictors of Utilization and Survival Outcomes. Int J Radiat Oncol 99:E215–E216. 10.1016/j.ijrobp.2017.06.1119 [DOI] [Google Scholar]
- 13.Solanki AA, Martin B, Korpics M, et al. (2017) Bladder-Preserving Therapy Patterns of Care: A Survey of US Radiation Oncologists. Int J Radiat Oncol Biol Phys 99:383–387. 10.1016/j.ijrobp.2017.04.009 [DOI] [PubMed] [Google Scholar]
- 14.Coen JJ, Zhang P, Saylor PJ, et al. (2019) Bladder preservation with twice-a-day radiation plus fluorouracil/cisplatin or once daily radiation plus gemcitabine for muscle-invasive bladder cancer: NRG/RTOG 0712—a randomized phase II trial. J Clin Oncol 37:44–51. 10.1200/JCO.18.00537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viswanathan AN, Yorke ED, Marks LB, et al. (2010) Radiation Dose-Volume Effects of the Urinary Bladder. Int J Radiat Oncol Biol Phys 76:S116–S122. 10.1016/j.ijrobp.2009.02.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meijer GJ, Van Der Toorn PP, Bal M, et al. (2012) High precision bladder cancer irradiation by integrating a library planning procedure of 6 prospectively generated SIB IMRT plans with image guidance using lipiodol markers. Radiother Oncol 105:174–179. 10.1016/j.radonc.2012.08.011 [DOI] [PubMed] [Google Scholar]
- 17.Murthy V, Master Z, Adurkar P, et al. (2011) “Plan of the day” adaptive radiotherapy for bladder cancer using helical tomotherapy. Radiother Oncol 99:55–60. 10.1016/j.radonc.2011.01.027 [DOI] [PubMed] [Google Scholar]
- 18.Whalley D, Caine H, McCloud P, et al. (2015) Promising results with image guided intensity modulated radiotherapy for muscle invasive bladder cancer. Radiat Oncol 10:205 10.1186/s13014-015-0499-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Rooijen DC, Van de Kamer JB, Pool R, et al. (2009) The effect of on-line position correction on the dose distribution in focal radiotherapy for bladder cancer. Radiat Oncol 4:38 10.1186/1748-717X-4-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40:373–383. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 21.Baumann BC, Bosch WR, Bahl A, et al. (2016) Development and Validation of Consensus Contouring Guidelines for Adjuvant Radiation Therapy for Bladder Cancer After Radical Cystectomy. Int J Radiat Oncol Biol Phys 96:78–86. 10.1016/j.ijrobp.2016.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawton CAF, Michalski J, El-Naqa I, et al. (2009) RTOG GU Radiation Oncology Specialists Reach Consensus on Pelvic Lymph Node Volumes for High-Risk Prostate Cancer. Int J Radiat Oncol Biol Phys 74:383–387. 10.1016/j.ijrobp.2008.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rödel C, Grabenbauer GG, Kühn R, et al. (2002) Combined-modality treatment and selective organ preservation in invasive bladder cancer: Long-term results. J Clin Oncol 20:3061–3071. 10.1200/JCO.2002.11.027 [DOI] [PubMed] [Google Scholar]
- 24.Kavanagh BD, Pan CC, Dawson LA, et al. (2010) Radiation Dose-Volume Effects in the Stomach and Small Bowel. Int J Radiat Oncol Biol Phys 76:S101–S107. 10.1016/j.ijrobp.2009.05.071 [DOI] [PubMed] [Google Scholar]
- 25.McDonald F, Waters R, Gulliford S, et al. (2015) Defining bowel dose volume constraints for bladder radiotherapy treatment planning. Clin Oncol 27:22–29. 10.1016/j.clon.2014.09.016 [DOI] [PubMed] [Google Scholar]
- 26.James N, Hussain S, Hall E, et al. (2012) Radiotherapy with or without Chemotherapy in Muscle-Invasive Bladder Cancer. N Engl J Med 366:1477–1488. 10.1056/NEJMoa1106106 [DOI] [PubMed] [Google Scholar]
- 27.Gulliford SL, Foo K, Morgan RC, et al. (2010) Dose-Volume Constraints to Reduce Rectal Side Effects From Prostate Radiotherapy: Evidence From MRC RT01 Trial ISRCTN 47772397. Int J Radiat Oncol Biol Phys 76:747–754. 10.1016/j.ijrobp.2009.02.025 [DOI] [PubMed] [Google Scholar]
- 28.Efstathiou JA, Bae K, Shipley WU, et al. (2009) Late pelvic toxicity after bladder-sparing therapy in patients with invasive bladder cancer: RTOG 89–03, 95–06, 97–06, 99–06. J Clin Oncol 27:4055–4061. 10.1200/JCO.2008.19.5776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams B, Diaz D, Pollack A, Abramowitz M (2014) Bladder Carcinoma In: Lee N, Riaz N, Lu J (eds) Target Volume Delineation for Conformal and Intensity-Modulated Radiation Therapy. Springer International Publishing, Cham, Switzerland, pp 377–386 [Google Scholar]
- 30.Biancia C Della, Yorke E, Kollmeier MA (2014) Image guided radiation therapy for bladder cancer: Assessment of bladder motion using implanted fiducial markers. Pract Radiat Oncol 4:108–115. 10.1016/j.prro.2013.07.008 [DOI] [PubMed] [Google Scholar]
- 31.Grossman HB, Natale RB, Tangen CM, et al. (2003) Neoadjuvant Chemotherapy plus Cystectomy Compared with Cystectomy Alone for Locally Advanced Bladder Cancer. N Engl J Med 349:859–866. 10.1056/NEJMoa022148 [DOI] [PubMed] [Google Scholar]
- 32.Huddart RA, Birtle A, Maynard L, et al. (2017) Clinical and patient-reported outcomes of SPARE – a randomised feasibility study of selective bladder preservation versus radical cystectomy. BJU Int 120:639–650. 10.1111/bju.13900 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.