Abstract
Objectives
Malnutrition plays a critical role in the onset and progress of the coronavirus disease 2019 (COVID-19). The aim of the present study was to explore the association of the prognostic nutritional index (PNI) score with the severity of COVID-19 and its predictive value of the severe form of COVID-19.
Methods
Clinical data were collected from 122 patients infected with COVID-19 and hospitalized at the Sixth People's Hospital of Wenzhou, China, a specialized infectious hospital affiliated with the Wenzhou Central Hospital. PNI score was calculated as serum albumin (g/L) + 5 × total lymphocyte count (/nL).
Results
The study population consisted of 105 patients (86.1%) with a common form and 17 patients (13.9%) with a severe form of COVID-19. PNI score significantly decreased from patients with common to severe forms of COVID-19 (P = .029) regardless of sex, age range, and body mass index (BMI). After adjustment for sex, age, indexes of liver and renal function, C-reactive protein, and current smoking status, PNI scores remained independently and inversely associated with the severity of COVID-19 (odd ratio: 0.797; P = .030). A receiver operating characteristic analysis showed that PNI scores had a similar accuracy to predict severe forms of COVID-19 compared with its combination with sex, age, and BMI (P = .402). PNI < 49 was defined as the cutoff value to predict the severe form of COVID-19.
Conclusions
Poorer nutritional status predisposed patients infected with COVID-19 to its severe form. Independently associated with the severity of COVID-19, PNI score could serve as a simple, fast, and effective predictor among patients with different sex, age, and BMI.
Keywords: Coronavirus disease 2019, Prognostic nutritional index, Prediction, Severity
Introduction
Coronavirus disease 2019 (COVID-19), which emerged at the end of 2019, has developed into a worldwide pandemic, posing tremendous challenges and threats to individual health and health care systems, as well as social stability and development [1]. Since the declaration of COVID-19 as a pandemic by the World Health Organization [2], the rate of diagnosed cases has been changing every day, and the number of confirmed cases reached as high as 22 179 934 on August 19,2020, with 781 932 deaths [3].
The clinical spectrum of COVID-19 ranges from asymptomatic infection and mild upper respiratory tract infection to severe pneumonia, which might progress to respiratory failure and further result in multiorgan failure [4,5]. The majority of deaths involved older and polymorbid patients, among whom malnutrition is likely common [5,6]. A previous study provided evidence that malnutrition is one of the leading predictors of mortality in viral infections [7]. With the severe acute respiratory syndrome coronavirus 2 as a pathogen, malnutrition and its related weakened immune response are suspected of playing a critical role in the onset and progress of COVID-19. The importance of assessment of nutritional status has been advocated and emphasized in expert statements and practical guidance for the nutritional management of COVID-19 endorsed by the European Society for Clinical Nutrition and Metabolism Council [8]. Therefore, a simple and effective index should be identified to evaluate the nutritional status of patients infected with COVID-19.
The prognostic nutritional index (PNI), first introduced as a nutritional assessment for nonemergency general surgical patients, was simplified as a calculation of peripheral blood lymphocyte count and serum albumin (Alb) concentration and is able to reflect the immune-nutritional status of patients [9]. Recently, evidence has been accumulating that PNI can predict the clinical outcomes of patients with certain types of cancers, including lung, gastrointestinal, breast, and gynecologic cancers [10], [11], [12], [13]. However, to date, no study has concentrated on the role of PNI in the assessment and prediction of the severity of COVID-19.
Furthermore, critically ill patients usually suffer a drastic reduction in food intake because of severe inflammation and anorexia, and are more predisposed to develop respiratory failure. Therefore, nutritional assessment and further derangements should be systematically and urgently managed in patients infected with COVID-19 [14,15]. For patients with the most severe manifestations of the infection, however, assessments of nutritional status with common tools can be quite difficult because of physical constraints and difficulties in collecting anthropometric and dietary information. Therefore, rapid screening instruments to assess nutritional status, such as PNI, should be considered. The aim of the present study was to explore the association of PNI score with the severity of COVID-19, as well as its predictive value of the severe form of COVID-19.
Methods
Study design and participants
The present single-centered, retrospective study was conducted at the Sixth People's Hospital of Wenzhou, China, a specialized infectious hospital affiliated with the Wenzhou Central Hospital. Data were derived from 122 patients admitted to the hospital between January 17, 2020 and February 21, 2020. All patients were diagnosed with COVID-19 infection, and categorized as having a mild, common, severe, or extremely severe form during hospitalization according to the standards for the Diagnosis and Treatment Scheme of New Coronavirus Infected Pneumonia (trial version 6) [16]. The diagnostic criteria of different types of COVID-19 infection were as follows: 1) Mild form, showing mild clinical symptoms with no manifestations of pneumonia on imaging; 2) common form showing fever, respiratory, or other symptoms with manifestations of pneumonia on imaging; 3) severe form showing shortness of breath with respiratory rate ≥ 30 breaths/min, or pulse oxygen saturation in resting state < 93%, or arterial blood oxygen pressure/oxygen concentration < 300 mmHg; and 4) extremely severe form showing respiratory failure requiring mechanical ventilation, or shock, or combined with other organ failure requiring intensive care unit monitoring and treatment. Because no patient with a mild form of COVID-19 was hospitalized and the sample size of patients with an extremely severe form was relatively small (only one patient), the present study only enrolled patients with common and severe forms of COVID-19 as the study population.
This study was approved by the institutional review board of the Wenzhou Central Hospital. Due to the retrospective nature of the study, informed consent was waived.
Anthropometric and laboratory measurements
Every patient underwent a physical examination after admission, including anthropometric measurements (body height and weight) and blood pressure. Body height and weight were used to calculate body mass index (BMI) as follows: weight (kg)/height2 (m2). Overweight and obese patients were defined as BMI ≥ 24 kg/m2 and 28 kg/m2, respectively [17]. Systolic and diastolic blood pressure were assessed at the time of admission. Main arterial pressure (MAP) was calculated as (systolic blood pressure + 2 × diastolic blood pressure)/3.
Laboratory measurements were obtained in the standard manner. Venous blood samples were collected after fasting overnight. All laboratory data were obtained from the first serum collection during hospitalization. The peripheral absolute value of white blood cell, fasting plasma glucose, lipid profiles (total cholesterol, triacylglycerol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol), Alb, liver function (alanine aminotransferase, aspartate aminotransferase, gamma-glutamyltransferase, and total bilirubin), serum creatinine, and C-reactive protein (CRP) were measured using standard methods. PNI score was determined with the following formula: serum albumin (g/L) + 5 × total lymphocyte count (/nL) [9].
Statistical analysis
The statistical analyses were conducted using the statistical software package version 16.0 (SPSS Inc., Chicago, IL). A one-sample Kolmogorov-Smirnov test determined the normality of the data distribution. According to the commoner skewed distribution, continuous variables were expressed as mean ± standard deviation or median with interquartile range. Categorical variables were expressed as numbers with percentage. Intergroup comparisons of normally and skewed distributed data were carried out by the unpaired student's t and Mann-Whitney U tests, respectively. The ꭓ2 test was used for intergroup comparisons of categorical variables. Multivariate analyses of variance were applied to explore the interference of age, sex, and BMI with the association between PNI score and the severity of COVID-19. Multivariate logistic regression analyses were used to identify the independent factor associated with the severity of COVID-19. Receiver operating characteristic analyses were carried out to evaluate the predictive ability for severity of COVID-19, and determine the cutoff value of the PNI score. All reported P-values were two-tailed, and P < .05 was considered statistically significant.
Results
Clinical characteristics of study participants
A total of 122 participants with an average age of 44.0 ± 13.4 y (range, 10–85 y) were enrolled in the study, including 68 men (55.7%) and 54 women (44.3%). All participants were diagnosed with the common (n = 105; 86.1%) or severe (n = 17; 13.9%) form of COVID-19. Compared with those with the common form of COVID-19, patients with the severe form were older and showed higher levels of men-to-women ratio, BMI, fasting plasma glucose, alanine aminotransferase, aspartate aminotransferase, CRP, and proportion of current smoking status, as well as lower levels of MAP, peripheral absolute value of lymphocyte, Alb, and PNI (all P < .05). There was no difference between patients with the common and severe forms of COVID-19 with respect of other variables (Table 1 ).
Table 1.
Characteristics of the study participants
| Variable | Total n = 122 | Normal form n = 105 | Severe form n = 17 |
|---|---|---|---|
| Sex, men/women, n | 68/54 | 54/51 | 14/3* |
| Age, y | 44.0 ± 13.4 | 42.7 ± 13.1 | 51.9 ± 13.1† |
| Body mass index, kg/m2 | 23.6 ± 3.5 | 23.3 ± 3.3 | 25.6 ± 4.5* |
| Main arterial pressure, mmHg | 98.4 ± 11.9 | 99.0 ± 12.4 | 94.7 ± 7.1* |
| White blood cell, × 109/L | 5.0 ± 1.6 | 5.0 ± 1.6 | 5.2 ± 1.8 |
| Neutrophils | 3.0 (2.3–3.9) | 2.9 (2.2–3.8) | 3.3 (2.6–5.1) |
| Lymphocyte | 1.3 (1.0–1.8) | 1.3 (1.0–1.8) | 0.9 (0.7–1.4)† |
| Fasting plasma glucose, mmol/L | 5.4 (4.9–5.9) | 5.3 (4.8–5.9) | 5.6 (5.4–6.6)* |
| Total cholesterol, mmol/L | 3.8 (3.2–4.2) | 3.8 (3.3–4.2) | 3.3 (2.9–4.2) |
| Triglyceride, mmol/L | 1.2 (0.9–1.7) | 1.2 (0.9–1.7) | 1.2 (1.0–1.8) |
| HDL-c, mmol/L | 1.3 ± 0.3 | 1.3 ± 0.4 | 1.2 ± 0.3 |
| LDL-c, mmol/L | 1.9 ± 0.7 | 1.9 ± 0.7 | 1.8 ± 0.7 |
| Albumin, g/L | 42.1 (39.6–44.9) | 42.8 (39.9–45.5) | 39.6 (36.7–41.2)† |
| Alanine aminotransferase, U/L | 22.0 (14.0–36.0) | 19.0 (13.0–31.0) | 32.0 (21.0–57.5)* |
| Aspartate aminotransferase, U/L | 24.0 (19.0–34.0) | 23.0 (18.0–31.0) | 35.0 (25.5–56.0)† |
| Gamma-glutamyltransferase, U/L | 27.0 (15.8–51.0) | 25.0 (14.5–51.0) | 30.0 (23.0–80.0) |
| Total bilirubin, μmol/L | 13.5 (10.0–18.2) | 13.3 (10.0–18.6) | 14.1 (11.2–17.5) |
| Serum creatinine, μmol/L | 69.5 (58.0–82.0) | 69.0 (57.5–82.0) | 73.0 (63.5–82.0) |
| C-reactive protein, mg/L | 12.2 (3.0–30.9) | 7.9 (2.5–24.3) | 41.7 (21.9–54.6)† |
| Prognostic nutritional index score | 49.1 ± 6.8 | 50.0 ± 6.7 | 43.4 ± 4.0† |
| Current smoking status, n (%) | 8 (6.6) | 5 (4.8) | 3 (17.6)* |
HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol
Continuous data are expressed as means ± standard deviation or median (interquartile range).
P < .05 versus normal form
P < .01 versus normal form
Interference of sex, age, and body mass index
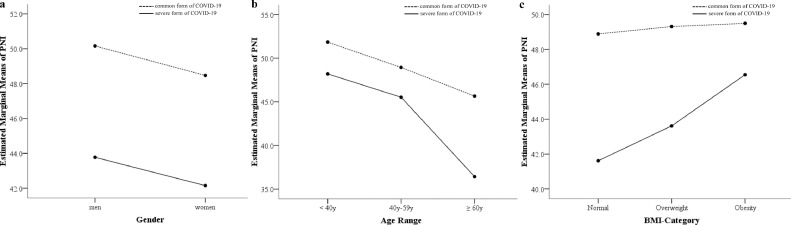
Analyses of variance uncovered that PNI score significantly decreased for patients with the common to severe forms of COVID-19 (P = .029) regardless of sex, age range (<40 y, 40–59 y, ≥60 y), and BMI category (normal, overweight, obesity). The associations of sex, age range, and BMI category with PNI did not interfere with the association between severity of COVID-19 and PNI score (Fig. 1 ).
Fig. 1.
Interference of sex, age, and body mass index with the association between the prognostic nutritional index (PNI) and severity of coronavirus disease 2019 (COVID-19), showing that the associations of (A) sex with PNI did not interfere with association between severity of COVID-19 and PNI, (B) age range with PNI did not interfere with the association between severity of COVID-19 and PNI, and (C) body mass index category with PNI did not interfere with the association between severity of COVID-19 and PNI.
Multivariate logistic regression analyses of severity of COVID-19
To define the severity of COVID-19 as a dependent variable, the multivariate logistic regression analyses took into consideration the metabolic indexes, including BMI, MAP, fasting blood glucose, triacylglycerol, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and PNI score. The unadjusted model found that a higher BMI (odd ratio [OR]: 1.260; P = .009) and lower PNI score (OR: 0.744; P < .001) were associated with the severe form of COVID-19 independently. After adjustment for sex and age, only PNI score had an independent association with the severity of COVID-19 (OR: 0.757; P = .003), which remained significant when even further adjusted for indexes of liver and renal function, CRP, and current smoking status (OR: 0.797; P = .030; Table 2 ).
Table 2.
Logistic regression analyses of severity of coronavirus disease 2019
| Model | Independent variables | Discharged from hospital in time |
||
|---|---|---|---|---|
| Hazard ratio | 95% confidence interval | P-value | ||
| Model 1 | BMI | 1.260 | 1.060–1.499 | .009 |
| PNI | 0.744 | 0.631–0.878 | < .001 | |
| Model 2 | BMI | 1.192 | 0.989–1.437 | .065 |
| PNI | 0.757 | 0.628–0.911 | .003 | |
| Model 3 | BMI | 1.153 | 0.936–1.419 | .180 |
| PNI | 0.794 | 0.645–0.978 | .030 | |
BMI, body mass index; PNI, prognostic nutritional index
Model 1: Original independent variables: BMI, main arterial pressure, fasting plasma glucose, total cholesterol, triglyceride, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and PNI; unadjusted
Model 2: Original independent variables in Model 1, adjusted for age and sex
Model 2: Original independent variables in Model 1, adjusted for age and sex, indexes of liver and renal function (alanine aminotransferase, aspartate aminotransferase, gamma-glutamyltransferase, total bilirubin; serum creatinine), C-reactive protein, and current smoking status
Receiver operating characteristic analyses to predict severe form of COVID-19
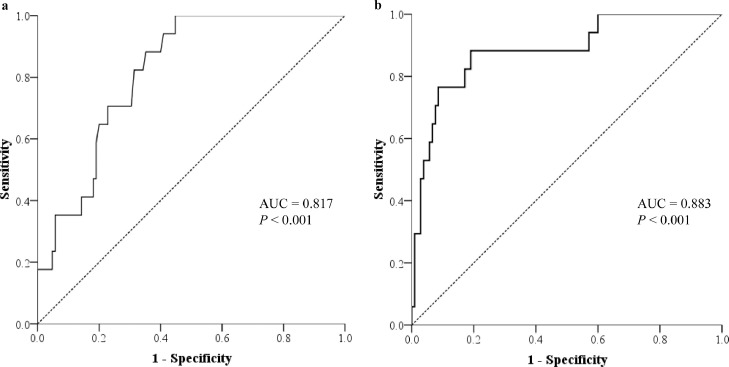
Receiver operating characteristic analyses of sex, age, BMI, PNI score, and the combination of these indexes to predict the severe form of COVID-19 had an area under the curve of 0.655 (95% confidence interval [CI], 0.526–0.784; P = .041), 0.691 (95% CI, 0.562–0.820; P = .012), 0.642 (95% CI, 0.502–0.782; P = .061), 0.817 (95% CI, 0.733–0.901; P < .001), and 0.883 (95% CI, 0.792–0.975; P < .001), respectively. Compared with the combined index, only PNI score showed a similar accuracy to predict the severe form of COVID-19 (P = .402; Fig. 2 ), and other indexes’ accuracies were significantly decreased (all P < .05). PNI < 49 was defined as a cutoff value to predict the severe form of COVID-19 with a sensitivity of 100.0% (specificity: 56.2%).
Fig. 2.
Receiver operating characteristic analyses for the prediction of the severe form of coronavirus disease 2019 of (A) prognostic nutritional index (PNI) for prediction, and (B) the combination of sex, age, body mass index, and PNI for prediction. The area under the curve of PNI is similar to that of the combination of sex, age, body mass index, and PNI (P = .042).
Discussion
The present study revealed that PNI score decreased with the progress of COVID-19 from common to severe forms. The association of PNI score with sex, age, and BMI did not interfere with its association with the severity of COVID-19. PNI score was an inverse and independent factor associated with the severe form of COVID-19. Since the accuracy of the PNI was similar to its combination with age, sex, and BMI, PNI score could serve as a predictor of the severity of COVID-19 independently, with a cutoff value of 49.
Current documents from the European Society for Clinical Nutrition and Metabolism put emphasis on the early identification of the risk and presence of malnutrition in the general evaluation of every patient infected with COVID-19 [8]. However, rare published data have put focus on the impact of nutritional status on the severity of COVID-19. Liu et al. conducted a retrospective study among older patients with COVID-19, age >65 y, to assess their nutritional risks and their associations with clinical outcomes. Four nutritional risk screening tools were used, and the results showed that the Nutrition Risk Screening 2002, Mini Nutrition Assessment Shortcut, and Nutrition Risk Index are useful and practical tools with respect to screening for nutritional risk in patients with COVID-19 [18].
The present study extended the age range of the study population, and found that nutritional status was worse in patients with the severe form of COVID-19, which is consistent with the results of the previous study. Furthermore, the cutoff value of 49 was selected for PNI to predict the severe form of COVID-19, which is close to the cutoff value of 50 for PNI to predict pulmonary complications and long-term outcomes after the curative resection of lung cancer [19]. Therefore, with no need to fully understand the history of weight loss, nutritional intake, cognitive status, and other health conditions, PNI could be a simple, fast, and effective index to evaluate nutritional status and predict disease progression, especially in children, elderly people, and those paying less attention to their own health conditions or who cannot recall and describe their previous health conditions clearly.
Recently, a large prospective cohort study of 20 133 hospital inpatients infected with COVID-19 uncovered that advanced age, male sex, and obesity are independent risk factors of mortality from COVID-19. In terms of nutritional status, older adults are at risk of protein-energy malnutrition, of which the predictor and determinants were sex-specific [20]. Older adults are vulnerable to nutritional deficiency, and published data show that malnourishment occurs in 35% to 65% of hospitalized elderly patients and 25% to 60% of institutionalized older adults [18,21]. Obesity due to overnutrition or excess storage of fats relative to energy expenditure is another form of malnutrition [22]. The present study discovered that the different nutritional status between men and women, the young and the elderly, and the thin and the obese, did not interfere with the association of PNI with the severity of COVID-19, suggesting that as a predictor of severity of COVID-19, PNI score is suitable for both men and women of varied age and BMI.
The mechanism underlying the association of PNI score and severity of COVID-19 remains unclear. As a combination of peripheral blood lymphocyte count and Alb concentration, PNI links nutritional status to immune response for patients [9]. Nutrition is known to play a critical role in the regulation of immune responses. Protein-energy malnutrition is associated with immunodeficiency [22], manifested as an impairment of cell-mediated immunity, function of phagocyte, complement system, and cytokine production, which have been observed in patients with a severe form of COVID-19 with decreases in lymphocytes and T cell count and increases in inflammatory cytokines [23].
There are some limitations in the present study, which might bring potential bias. First, the study was a retrospective, single-center, and small sample-sized study, from which the findings should be confirmed and generalized in a larger cohort. Second, without data on weight and diet of patients before they were admitted to the hospital, the present study could not obtain other classic nutritional index scores, such as Nutrition Risk Screening 2002, Mini Nutrition Assessment Shortcut, and Nutrition Risk Index, and compare the predictive ability for the severity of COVID-19 between the PNI and these indexes. Third, although serum levels of Alb have historically been utilized as surrogates for undernourishment, other pathologic states also contribute to a decrease in serum Alb levels, such as inflammation, acute or chronic liver dysfunction, catabolism, nephrotic syndrome, and protein-losing enteropathy. The utility of preoperative serum levels of Alb might be limited to the provision of prognostic information [24]. However, PNI, incorporating serum Alb and lymphocyte levels, has been used in many patient populations to predict prognostic outcomes [[10], [11], [12], [13],25].
Conclusions
Poorer nutritional status predisposed patients infected with COVID-19 to its severe form. As an independent factor associated with the severity of COVID-19, PNI score could serve as a simple, fast, and effective predictor among patients of different sex, age, and BMI.
Acknowledgments
This research was funded by a grant from the National Natural Science Foundation of China (81900737) and the Wenzhou Science and Technology Bureau (Y20190129, Y20190126, and Y2020263).
Footnotes
Xuemei Gu and Xiaobo Wang contributed equally to this report.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . 11 March 2020. WHO Director-General's opening remarks at the media briefing on COVID-19. Available at: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020. [Google Scholar]
- 3.Johns Hopkins University. COVID-19 map. Available at: https://coronavirus.jhu.edu/map.html. Accessed June 1, 2020.
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal E., Miller M., Yaxley A., Isenring E. Malnutrition in the elderly: A narrative review. Maturitas. 2013;76:296–302. doi: 10.1016/j.maturitas.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Reyes L., Arvelo W., Estevez A., Gray J., Moir J.C., Gordillo B. Population based surveillance for 2009 pandemic influenza A (H1N1) virus in Guatemala. Influenza Other Respir Viruses. 2009;4:129–140. doi: 10.1111/j.1750-2659.2010.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barazzoni R., Bischoff S.C., Breda J., Wickramasinghe K., Krznaric Z., Nitzan D. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. 2020;39:1631–1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Onodera T., Goseki N., Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85:1001–1005. [PubMed] [Google Scholar]
- 10.Wang Z., Wang Y., Zhang X., Zhang T. Pretreatment prognostic nutritional index as a prognostic factor in lung cancer: Review and meta-analysis. Clin Chim Acta. 2018;486:303–310. doi: 10.1016/j.cca.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 11.Shi W.K., Zhang X.H., Zhang J., Yu M., Yuan Y.J., Xiong W. Predictive ability of Prognostic Nutritional Index in surgically resected gastrointestinal stromal tumors: A propensity score matching analysis. Jpn J Clin Oncol. 2019;49:823–831. doi: 10.1093/jjco/hyz078. [DOI] [PubMed] [Google Scholar]
- 12.Mohri T., Mohri Y., Shigemori T., Takeuchi K., Itoh Y., Kato T. Impact of Prognostic Nutritional Index on long-term outcomes in patients with breast cancer. World J Surg Oncol. 2016;14:170. doi: 10.1186/s12957-016-0920-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X., Wang Y. The Prognostic Nutritional Index is prognostic factor of gynecological cancer: A systematic review and meta-analysis. Int J Surg. 2019;67:79–86. doi: 10.1016/j.ijsu.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 14.Caccialanza R., Laviano A., Lobascio F., Montagna E., Bruno R., Serena Ludovisi S. Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): Rationale and feasibility of a shared pragmatic protocol. Nutrition. 2020;74 doi: 10.1016/j.nut.2020.110835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azzolino D., Saporiti E., Proietti M., Cesari M. Nutritional considerations in frail older patients with COVID-19. J Nutr Health Aging. 2020;24:696–698. doi: 10.1007/s12603-020-1400-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Health Commission of the People's Republic of China. Diagnosis and treatment scheme of new coronavirus infected pneumonia. Available at: http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2.shtml?security_session_verify=ee9c2d723cdbcd6a4b2c0d881dcfcb2c. Accessed February 1, 2020.
- 17.Obesity Group of Chinese Society of Endocrinology Expert consensus on prevention and treatment of obesity in Chinese adults. Chin J Endocrinol Metab. 2011;27:711–717. [Google Scholar]
- 18.Liu G., Zhang S., Mao Z., Wang W., Hu H. Clinical significance of nutritional risk screening for older adult patients with COVID-19. Eur J Clin Nutr. 2020;74:876–883. doi: 10.1038/s41430-020-0659-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park S., Ahn H.J., Yang M., Kim J.A., Kim J.K., Park S.J. The Prognostic Nutritional Index and postoperative complications after curative lung cancer resection: A retrospective cohort study. J Thorac Cardiovasc Surg. 2020;160:276–285. doi: 10.1016/j.jtcvs.2019.10.105. [DOI] [PubMed] [Google Scholar]
- 20.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corish C.A., Bardon L.A. Malnutrition in older adults: Screening and determinants. Proc Nutr Soc. 2019;78:372–379. doi: 10.1017/S0029665118002628. [DOI] [PubMed] [Google Scholar]
- 22.Chandra R.K. Nutrition and the immune system: An introduction. Am J Clin Nutr. 1997;66:460S. doi: 10.1093/ajcn/66.2.460S. 3. [DOI] [PubMed] [Google Scholar]
- 23.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman A.N., Fadem S.Z. Reassessment of albumin as a nutritional marker in kidney disease. J Am Soc Nephrol. 2010;21:223–230. doi: 10.1681/ASN.2009020213. [DOI] [PubMed] [Google Scholar]
- 25.Loftus T.J., Brown M.P., Slish J.H., Rosenthal M.D. Serum levels of prealbumin and albumin for preoperative risk stratification. Nutr Clin Pract. 2019;34:340–348. doi: 10.1002/ncp.10271. [DOI] [PubMed] [Google Scholar]