Abstract
Recent studies demonstrate that working memory (WM) is integral to etiological models of ADHD; however, significant questions persist regarding the relation between WM performance across tasks with varying cognitive demands and ADHD symptoms. The current study incorporates an individual differences approach to WM heterogeneity (i.e., latent profile analysis) to (a) identify differential profiles of WM across the phonological and visuospatial WM subsystems; and (b) characterize differences in symptom presentation among WM profiles. Parent and teacher ratings of child behavior, obtained for boys with (n=51) and without (n=38) a diagnosis of ADHD, were compared across latent classes of visuospatial and phonological WM performance. Latent profile analysis identified three classes of WM functioning: Low WM, Moderate WM, and High WM. Membership in the Low and Moderate WM classes was associated with greater levels of parent- and teacher-rated inattentive and hyperactive symptoms. While 84% of the ADHD group were assigned to the Low and Moderate WM classes, more than a quarter of children without ADHD exhibited Moderate WM limitations. Collectively, these findings extend prior work suggesting that there is substantial heterogeneity in WM functioning in children with and without ADHD and that these differences contribute to the expression of symptoms of inattention and hyperactivity.
Keywords: ADHD, working memory, latent profile analysis, children, executive functioning
Working Memory and ADHD Symptoms in Boys: Examining the Heterogeneity of Working Memory Functioning Using Latent Profile Analysis
Attention-Deficit/Hyperactivity Disorder (ADHD) affects approximately 5% of children worldwide (Polanczyk, Willcutt, Salum, Kieling, & Rohde, 2014) and is characterized by persistent and impairing difficulties with inattention, hyperactivity, and impulsivity (American Psychiatric Association, 2013). Moreover, the disorder is associated with a number of adverse outcomes including social difficulties (e.g., Bunford et al., 2015; Wehmeier, Schacht, & Barkley, 2010), academic underachievement (e.g., Daley & Birchwood, 2010), and functional impairments at home (e.g., Barkley, 2014) that persist into adolescence and young adulthood (Barbaresi et al., 2013; Molina et al., 2009; Sibley et al., 2017). Further, the disorder is associated with substantial individual (Altszuler et al., 2016) and societal (Pelham, Foster, & Robb, 2007) financial burden. Despite well-documented cross-domain functional outcomes, significant questions remain regarding the underlying mechanisms contributing to symptom presentation.
The last two decades have given rise to substantial interest in understanding how executive functions contribute to the core symptoms and poor functional outcomes experienced by individuals with ADHD (Bunford et al., 2015; Burgess et al., 2010; Castellanos & Tannock, 2002; Nigg, 2003). Multiple studies have indicated that not all children with ADHD experience similar levels of impairment in cognition (Chhabildas, Pennington, & Willcutt, 2001; Fair et al., 2012; Kasper et al., 2012; Willcutt et al., 2005). When multiple executive functioning constructs are included, almost 90% of children with ADHD exhibit impaired performance on at least one executive function with significant variability in the specific construct and degree of impairment, as well as the extent of concurrent impairment across multiple executive functions (Kofler et al., 2019). Specifically, meta-analytic studies suggest that up to 80% of children with ADHD may have a working memory (WM) deficit (Kasper, Alderson, & Hudec, 2012) whereas approximately 50% may exhibit poor inhibitory control (Nigg, Willcutt, Doyle, & Sonuga-Barke, 2005; Willcutt et al., 2005). Collectively, this work indicates that rather than experiencing similar executive function deficits, the disorder may be better characterized by heterogeneity with respect to cognitive dysfunction. Further, these differences in specific neurocognitive impairments in children with ADHD may aid in our understanding of their contribution to heterogeneity in functional impairments (Kofler et al., 2017) as well as symptom remission (Karalunas et al., 2017).
Previous studies have attempted to leverage the consistent heterogeneity of neuropsychological impairments in ADHD (Nigg et al., 2005) into potential endophenotypes for ADHD (Biederman et al., 2004; Lambek et al., 2010; Nigg, Blaskey, Stawicki, & Sachek, 2004). Specifically, varying cognitive profiles and associations with behavioral symptoms and clinical outcomes in ADHD have given rise to theoretical models to explain the heterogeneity of cognitive abilities in ADHD (see Luo, Weibman, Halperin, & Li, 2019). The models differ in the perceived role of executive functions and motivational processes in the emergence of ADHD and their differential association with symptom domains. For example, the dual-pathway model (Lambek et al., 2018; Sonuga-Barke, 2002) posits that cognitive and motivational processes differentiate symptom domains such that inattention is closely associated with executive dysfunction while motivational deficits are more strongly associated with hyperactive symptoms. In contrast, the cognitive-energetic (Sergeant, 2000) and neurodevelopmental models (Halperin & Schulz, 2006) propose that the interplay between neurocognitive abilities results in the emergence of behavioral difficulties, which would indicate that executive functions and motivational processes have associations with both inattentive and hyperactive symptom domains. Despite these theoretical models, evidence for an association between ADHD subtype and executive function performance remains scant (Lockwood, Marcotte, & Stern, 2001). Notably, studies investigating subtype and executive function performance only include group comparisons between the inattentive and combined presentations of ADHD and typically developing children leading to a limited understanding of the relationship between cognitive performance and specific symptom domains (i.e., inattention and hyperactivity; Sonuga-Barke et al., 2008). Consequently, dimensional approaches towards investigating symptom severity associated with specific executive dysfunctions may be more appropriate for addressing specificity of the two ADHD symptom domains.
Many researchers have postulated that WM difficulties represent a core impairment in ADHD (Castellanos & Tannock, 2002; Rapport et al., 2001; Martinussen, Hayden, Hogg-Johnson, & Tannock, 2005) leading to behavioral difficulties; however, little is known about the association between WM and ADHD symptoms independent of diagnosis. Working memory is a limited capacity cognitive function essential for attending to relevant stimuli through the temporary storage, rehearsal, updating, and manipulation of internally-held modality-specific information (Martinussen et al., 2005). One particular model of WM that has been examined extensively in children with ADHD is the Baddeley (2007) WM model that conceptualizes WM as being primarily comprised of four subcomponents ─ two limited capacity domain short term memory storage/rehearsal systems (i.e., phonological and visuospatial) working in tandem with a domain-general central executive to store and maintain information in WM for the purpose of task execution. The episodic buffer has been proposed as a fourth limited capacity subsystem which binds and temporarily stores information from the two modality specific subsystems thereby forming a unitary episodic representation for information. However, among the handful of studies examining the episodic buffer in elementary school-aged children, with and without ADHD (Alderson, Kasper, Patros, Hudec, Tarle, & Lea, 2015; Gray et al., 2017; Kofler, Spiegel, Austin, Irwin, Soto, & Sarver, 2018), the studies suggest that there are no between-group differences in this component in youth with the disorder relative to those without the disorder. As a result, the current study focuses on the components of the model that have consistently been implicated as deficient in children with ADHD (i.e., phonological storage/rehearsal, visuospatial storage/rehearsal, and central executive).
While WM deficits among children and adults with ADHD are well documented in both meta-analytic and experimental work (Brocki, Randall, Bohlin, & Kerns, 2008; Martinussen, Hayden, Hogg-Johnson, & Tannock, 2005; Rapport, Alderson, Kofler, & Raiker, 2008; Willcutt et al., 2005), examinations of these deficits and their association with symptoms of the disorder are mixed. Some (Brocki, Eninger, Thorell, & Bohlin, 2010; Gathercole et al., 2008; Lui & Tannock, 2007) but not all studies (Gallego-Martínez, García-Sevilla, & Fenollar-Cortés, 2018; Sonuga-Barke, Dalen, Daley, & Remington, 2002) find substantial relations between ratings of inattentive symptoms and poor performance on assessments of WM performance. Furthermore, few studies have demonstrated an association between composite WM performance, representing simultaneous utilization of multiple WM subsystems for successful task execution (e.g., phonological working memory plus central executive), and parent and teacher rated symptoms of hyperactivity (Kofler et al., 2017; Kuntsi, Oosterlaan, & Stevenson, 2001).
Investigations providing a more granular analysis of the WM subsystems have found visuospatial and phonological storage/rehearsal to be related to a diagnosis of ADHD (Alloway, 2011; Rapport et al., 2008) as well as to subjective evaluations of inattention but not hyperactivity/impulsivity (Thorell, 2007; Tillman, Eninger, Forssman, & Bohlin, 2011). When inattention and hyperactivity/impulsivity were evaluated using objective measures, both symptom domains of ADHD have been found to be differentially associated with the WM subsystems. Specifically, Kofler and colleagues (2010) found that increasing cognitive load in both phonological and visuospatial WM tasks was associated with decreased orientation towards the task, indicating increased inattention. Studies utilizing actigraphy have found hyperactivity to be associated with visuospatial (Kofler, Sarver, & Wells, 2015; Patros, Alderson, Hudec, Tarle, & Lea, 2017), phonological (Rapport et al., 2009), and central executive processes (Kofler, Sarver, & Wells, 2015). Similarly, impulsivity, measured using computerized paradigms, was found to be associated also with visuospatial (Patros et al., 2015), phonological, and central executive processes (Raiker, Rapport, Kofler, & Sarver, 2012). The current study seeks to examine whether heterogeneity in cognitive performance in ADHD extends to modality-specific performance and whether this heterogeneity is associated with differences in severity of symptom presentation as rated by parents and teachers.
The well-established heterogeneity found in symptom presentation as well as neurocognitive deficits necessitates novel person-centered approaches to examining the relationship between cognition and symptoms of the disorder. This approach has the potential to isolate meaningful differences within groups to identify homogenous groups of individuals that share similar characteristics not detected by traditional analytic approaches. To date, only one study has adopted this approach to understanding relationships between WM performance and ADHD. Specifically, Gomez and colleagues (2014) utilized latent profile analysis (LPA) to identify distinct ADHD subgroups of varying WM impairment (severely impaired, moderately impaired, and not impaired). The three ADHD groups were not significantly different from each other on inattentive symptoms and exhibited greater inattentive symptoms relative to a typically developing (TD) group. While this study by Gomez and colleagues (2014) represents a critical step in better understanding the heterogeneity in WM dysfunction in ADHD, the separate examination of these profiles in children with ADHD and typically developing children limits the conclusions that can be drawn. Specifically, recent evidence suggests that heterogeneity in executive functioning is likely nested (or overlapping) within similar variability observed in the TD population (Fair et al., 2012; Mella, Fagot, & de Ribaupierre, 2016; Mostert et al., 2015). Furthermore, WM ability has also been found to be associated with age (Gathercole, Pickering, Ambridge, & Wearing, 2004) and racial/ethnic background (Lawson, Hook, & Farah, 2014) necessitating a need to evaluate demographic variables as potential contributors to the observed heterogeneous WM ability within samples of children with ADHD.
The current study utilized latent profile analysis to characterize variation in WM through the examination of subgroups across children with and without ADHD to capture the natural variation of WM in the broader population. Additionally, the current study is the first to evaluate whether WM ability varies across modalities and cognitive load demands (i.e., manipulation of an increasingly greater number of stimuli) leading to latent profiles characterized by decrements in performance in one modality with intact performance in the other at varying levels of cognitive load. The association between children’s WM profiles and specific symptoms of ADHD are examined. Consistent with past findings, we anticipate the detection of multiple WM subgroups reflecting between group differences in WM as cognitive demand increases. Furthermore, it is hypothesized that more pervasive impairment in WM will be associated with higher parent/teacher-rated inattentive and hyperactive symptom severity. Finally, we hypothesize that each WM subgroup will be comprised of both individuals with and without ADHD consistent with previous work (Fair et al., 2012) demonstrating nested cognitive heterogeneity among groups with the weakest performing groups being mostly comprised of individuals with ADHD.
Method
Participants
The sample1 was comprised of eighty-nine English-speaking boys with and without ADHD aged 7 to 12 years old (M = 9.71 years, SD = 1.25 years) recruited or referred to a university-based Children’s Learning Clinic (CLC) through community resources (e.g., referrals from primary care physicians, community mental health clinics, self-referral) and whose parents agreed to have them participate in developmental/clinical research studies. A psychoeducational evaluation was provided pro bono to parents of all participants. Typically developing (TD) children (those without a suspected psychological disorder) generally were self-referred families interested in learning about their child’s cognitive and academic profile. The racial and ethnic make-up of the sample was consistent with the surrounding population: 69.7% Caucasian non-Hispanic, 18% Hispanic, 5.6% African-American, and 6.7% multi-racial or other race or ethnicity. All parents and children provided their informed consent/assent to participate in the study, and the university’s Institutional Review Board approved the study prior to the onset of data collection.
Procedures
Parents and children participated in a detailed, semi-structured clinical interview including all screening questions of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Aged Children (K-SADS; Kaufman et al., 1997) with supplemental questions for parent-indicated elevated symptoms. The K-SADS assesses for current and past episodes of psychopathology as reflected by symptoms and their associated onset, course, duration, severity, and degree of impairment based on criteria from the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV; American Psychiatric Association, 2003)2. Clinical interviews were supplemented with parent and teacher versions of symptoms ratings from the Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2001), Teacher Report Form (TRF; Achenbach & Rescorla, 2001), and Child Symptom Inventory (CSI-4: Gadow, Sprafkin, & Salisbury, 2004). Children were excluded from the study if they presented with a history of seizures, psychosis, or received a Full Scale IQ score less than 85. Children with gross neurological, sensory, or motor impairment were excluded as well. Full Scale IQ scores in the ADHD group (M = 105.57, SD = 11.45) and TD group (M = 109.53, SD = 10.78), were not significantly different from each other, t(87) = 1.65, p = .10.
Fifty-one children were diagnosed with ADHD and met the following criteria: (1) an independent diagnosis of any presentation of ADHD by the directing clinical psychologist based on the results of the K-SADS; (2) A score at least two standard deviations above the mean on the Attention Problems clinical syndrome scale of the CBCL or exceeding the ADHD criterion for inattentive, hyperactive, or combined subscale score for the parent version of the CSI-4; and (3) A score at least two standard deviations above the mean on the Attention Problems clinical syndrome scale of the TRF or exceeding the ADHD criterion for inattentive, hyperactive, or combined subscale score for the teacher version of the CSI-4. Nearly the entire sample diagnosed with ADHD was formally diagnosed with combined subtype/presentation (n = 44). The remaining children met criteria for inattentive subtype/presentation (n = 4), hyperactive subtype/presentation (n = 1), or not otherwise specified (n = 2). A subset of these children additionally met diagnoses for Oppositional Defiant Disorder (n = 8), a Learning Disorder (n = 6), or an Anxiety disorder (n = 6). A majority of the children in the ADHD group (60%) did not meet criteria for any comorbid disorders. Aside from stimulant medication (n = 15), families did not report any additional prescribed psychiatric medication.
Thirty-eight children were included in the TD group based on the following criteria: (1) K-SADS clinical interview reviewed by the directing clinical psychologist did not reveal evidence of clinically elevated levels of any clinical disorder; (2) typical developmental history as reported by a parent with no indication of potential developmental delays or history of clinically elevated DSM-IV symptoms; (3) ratings below 1.5 standard deviations on the externalizing clinical syndrome scales of the CBCL and TRF; and (4) ratings below the ADHD criterion on the CSI-4 subscales as rated by both parents and teachers.
Parents of children in the ADHD group were asked to withhold medication for a minimum of 24 hours prior to testing for children currently prescribed psychostimulants. The WM tasks described below were counterbalanced and administered as part of a larger battery of neurocognitive tasks requiring the child’s presence for approximately 2.5 hours during a single session. To minimize fatigue, children received brief breaks (2–3 minutes) following each task and longer breaks (10–15 minutes) following the completion of multiple tasks. All tasks were programmed using SuperLab Pro (Version 2; 2002) and administered to the child on a computer.
ADHD symptoms
The CSI-4 (Gadow, Sprafkin, & Salisbury, 2004) is a 97-item behavior rating scale completed by both the parent and teacher that assesses a variety of emotional and behavioral disorders in childhood. Parents and teachers rate whether symptoms occur never (0), sometimes (1), often (2), or very often (3), providing a severity score for each item. The CSI-4 has shown high test-retest reliability (.75 to .84) and internal consistency (.86 and .88; Sprafkin, Gadow, Salisbury, Schneider, & Loney, 2002). In the current study, the inattention, hyperactivity, and combined inattention and hyperactivity severity scores subscales were used as dependent variables reflecting the sum of the severity scores across items.
The CBCL and TRF (Achenbach & Rescorla, 2001) are broadband measures of behaviors associated with childhood psychopathology completed by parents and teachers, respectively. Parents and teachers rate the child’s behavior across 113-items within the past six months. The items contribute to subscales that assess problem behaviors and functioning across multiple domains (e.g., withdrawn/depressed, somatic complaints, attention problems; Syndrome Scales) as well as subscales consistent with DSM diagnoses (i.e., DSM Oriented Scales). These scales have well-established psychometric properties including test-retest reliability (.63 to .97) and internal consistency (.66 to .92; Achenbach & Rescorla, 2001). T-scores from the ADHD related subscales of the CBCL and TRF (Attention Problems, Inattention, Hyperactive/Impulsive, DSM-Oriented ADHD, DSM-Oriented Inattention, and DSM-Oriented Hyperactivity) were used in the analyses.
Phonological (PH) Working Memory Task
The phonological (PH) working memory task used in this study is similar to the Letter-Number Sequencing subtest of the WISC-IV (Wechsler, 2003) and is identical to the task described in Rapport et al. (2008). Specifically, the stimuli consisted of a capital letter and a series of numbers (approximately 4 cm in height) which appeared on a computer monitor for approximately 800 ms with a 200 ms interstimulus interval. The stimuli were presented in a jumbled order (e.g., 3 F 1 6) and children were instructed to verbally recall the sequence presented in numerical order, from smallest to largest, and state the letter last (e.g., 1 3 6 F). Participants were required to achieve 80% accuracy on five practice trials to ensure understanding of the task demands prior to advancing to the full task. The serial position of the letter was counterbalanced across trials and never appeared first or last in a series of letters and numbers to minimize recency and primacy effects. Four different set sizes (3, 4, 5, and 6 stimuli), each with 24 unique trials, were administered to evaluate performance across varying set size conditions. Requiring participants to remember the letter presented while maintaining presented numbers and reordering them sequentially places demands on attentional shifting abilities, mental manipulation of stimuli and modality-specific storage and rehearsal processes. Therefore, the task can be considered to tap central executive and storage rehearsal processes simultaneously. Two trained research assistants, blind to diagnosis and seated outside of the room, recorded children’s verbal responses independently of one another. Children’s responses were scored correct if the numbers were stated in the correct serial position (i.e., lowest to highest) and the letter recalled as the final stimulus in the presented list. The primary dependent variable for each set size was average stimuli correct per trial as recommended by Conway, Kane, Bunting, & Engle (2005).
Visuospatial (VS) Working Memory Task
The visuospatial (VS) working memory task is identical to the one described in Rapport et al. (2008). Specifically, a series of black dots and one red dot (approximately 2.5 cm in diameter) appeared one at a time in one of nine squares arranged in three offset vertical columns (to prevent phonological encoding) with no two dots appearing in the same square on any given trial. Children were instructed to recall the order in which the dots were presented then indicate the position of the red dot last (regardless of when it was presented) on a modified keyboard number pad that corresponded to the three offset vertical columns presented on the screen. The serial position of the red dot was counterbalanced across trials and never appeared first or last in the series to minimize recency and primacy effects. All participants achieved 80% accuracy on five practice trials to ensure understanding before progressing to the full task. Four different set sizes (3, 4, 5, and 6 stimuli) of 24 trials each were administered to evaluate children’s performance across varying cognitive demand. Similar to the PH working memory task, the task places demands on attentional shifting abilities between new (i.e., black dots appearing after the red dot) and old information (i.e., black dots appearing after the red dot). Further participants have to mentally manipulate stimuli to meet task demands and rehearse VS information until all stimuli are presented. Therefore, the task can be considered to tap central executive and storage rehearsal processes simultaneously. Each button press corresponding with the order and location of the presented dots, regardless of the preceding and ensuing stimuli, was scored as correct. This included correctly identifying the red dot’s location as the last response emitted. The primary dependent variable for each set size was the average stimuli correct per trial as recommended by Conway and colleagues (2005).
Data Analytic Plan
Performance data, or the average stimuli correct per trial distinguished by set size and modality, for participants in both diagnostic groups was submitted to a latent profile analysis (LPA) in Mplus Version 8.2 (Muthén & Muthén, 2017). Latent profile analyses detect latent subgroups within a population without sacrificing statistical power due to unequal subgroup size (Lanza, Tan, & Bray, 2013). Additionally, the use of finite mixture models, such as LPA, as opposed to more traditional group-based approaches, reduces the risk of Type I errors by reducing the overall number of comparisons that must be conducted in a single analysis (Lanza & Rhoades, 2013). Because guidelines concerning the necessary sample size for LPA are unavailable, emerging literature in this area demonstrates that factors reflecting significant strengths of the current study (e.g., strong indicators of the construct, increased number of indicators, degree of class separation) are critical determinants of adequate power to detect subgroups in LPA and may attenuate limitations introduced by smaller samples (Muthén & Muthén, 2002; Tein, Coxe, & Cham, 2013; Wurpts & Geiser, 2014). Additionally, a recent simulation study conducted by Dziak, Lanza, and Tan (2014), indicated that a sample size of 100 is sufficient to detect a medium effect size. Given prior evidence of large magnitude effect size differences on these WM tasks (PH: 1.89, VS: 2.31; Rapport et al., 2008) this study was sufficiently powered to recover the latent profiles in this sample (n=89). Performance on the phonological and visuospatial WM tasks across the four set sizes (i.e., 3, 4, 5, and 6 stimuli load) were used as class indicators to identify latent classes based exclusively on WM performance.
Analyses used robust maximum likelihood estimation with 150 random starting values to avoid convergence on a local maximum (Asparouhov & Muthén, 2014). The number of latent classes was determined by evaluating fit for a one class solution and then adding additional classes in sequence until optimal model fit was accomplished. Selection criteria were as follows: (1) low Bayesian Information Criteria (BIC) and sample-size adjusted BIC (ABIC) which indicate the amount of unexplained variance remaining in the model with lower numbers indicating less unexplained variance; (2) significant bootstrap likelihood ratio test (BLRT) which reflects whether the difference in fit between K classes compared to K-1 classes is statistically significant (α<0.05); and (3) high entropy values which reflect how accurate K classes are at predicting individual class membership. For the latter index, values closer to 1 indicate fewer classification errors (i.e., greater number of individuals classified correctly).
Following the selection of the optimal class model, mean scores across ratings of ADHD symptom domains (dimensional) and diagnostic status (categorical), were evaluated across set sizes as distal outcomes. Differences in class symptom profiles were modeled using the BCH method (Bakk & Vermunt, 2016) in which equality of means in distal outcomes are multiply imputed utilizing posterior probabilities. Following estimation of means, a chi-square distribution, rather than an F-distribution, is used to compare the classes on the distal outcomes (continuous and categorical). Diagnostic status was categorized based on whether the participant belonged to the ADHD group or the typically developing group and included in the analysis utilizing the DCAT method (Asparouhov & Muthén, 2014).
Results
Model fit
To estimate the optimal number of classes, we evaluated the fit of models reflecting 1 to 5 classes. The best log-likelihood values were replicated with starting values of 100, 500, and 1000. Table 1 provides the BIC, ABIC, BLRT, and entropy values for the models. Unexplained variance (BIC, ABIC) was the lowest for the 3-, 4-, and 5-class models. Closer examination of these models revealed that the ABIC differences between the 5- and 4-class models (ΔABIC = 37.94) and the 4- and 3-class models (ΔABIC = 42.83) were negligible relative to the difference between the 3- and 2-class models (ΔABIC = 80.84). As expected, the BLRT was significant across all class models indicating better fit as the number of latent classes increased. All models generated entropy values ranging between .92 and .93—a finding that indicates a desirable level of class separation (average posterior probability at or above .90) with good classification accuracy across all models. Finally, examination of the number of participants classified across the latent classes indicated that one of the classes in the 5-class model contained only 7 participants (i.e., 8% of the sample). Due to this small class size, the additional class was judged as not providing significantly greater information relative to the 4-class model. Due to the similarity in fit between the 4- and 3-class models, we examined linear plots to determine which model was more interpretable as recommended by Bauer and Curran (2003). The linear plots indicated that the 4-class model produced two classes that were very similar to one another in terms of WM performance. Collectively, consideration of the indices and model parsimony supported a 3-class model as the optimal solution. As a result, the three classes were compared to one another in subsequent analyses.
Table 1.
Fit statistics of the latent profile analysis models.
| Model | BIC | Adjusted BIC | BLRT p value | Entropy | N assigned to each Profile (P) |
|---|---|---|---|---|---|
| 1-class | 1769.25 | 1718.76 | - | - | P1= 89 |
| 2-class | 1499.76 | 1420.86 | p<.01 | .93 | P1=42; P2=47 |
| 3-class | 1447.32 | 1340.02 | p<.01 | .92 | P1=19; P2=35; P3=35 |
| 4-class | 1432.89 | 1297.19 | p<.01 | .93 | P1=30; P2=12; P3=9; P4=38 |
| 5-class | 1423.35 | 1259.25 | p<.01 | .92 | P1=9; P2=7; P3=25; P4=32; P5=16 |
BIC= Bayesian Information Criteria, BLRT=Bootstrap likelihood ratio test.
Prior to conducting the LPA, bivariate correlations and one-way ANOVAs were conducted to investigate whether demographic variables (i.e., age and race) were significantly associated with any of the indicator variables. Results of the one-way ANOVA indicated that race was not significantly associated with PH or VS working memory performance, F(4, 84)= .54 – 1.69, ps =.16 −.71. Pearson correlations indicated age was significantly associated with PH and VS working memory performance rs(89) = .24 – .43, ps < .05. When the LPA was repeated with the inclusion of age as a covariate, the pattern of results remained unchanged, indicating that assignment to latent classes was independent of this variable. As a result, the simple three-class model with no covariates is reported below to facilitate interpretation.
WM across latent class
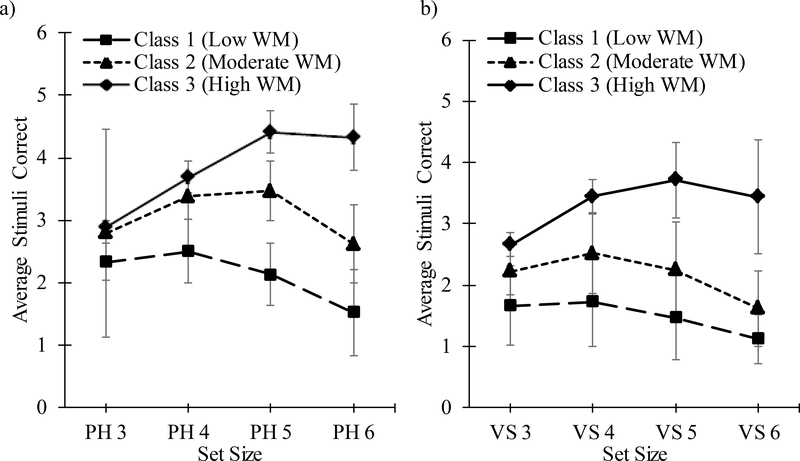
Examination of performance across set sizes revealed that the classes were representative of a pattern of differential WM performance. Overall, the difference in WM performance among classes did not differ in terms of modality (i.e. PH and VS). As such, performance is described in terms of WM performance across set sizes, regardless of modality, in which the average stimuli correctly recalled is expected to increase as the number of available stimuli (i.e., set size) increases until cognitive load capacity is exceeded for an individual. The first class consisted of a Low WM class with overall poor recall ability across all set sizes with gradual decrements in performance at higher set sizes. Specifically, performance in this group reflected the ability to recall, on average, two stimuli per trial across both modalities (M=1.80, SD=.46) even on trials with larger set sizes. Class two demonstrated stable or decreasing recall ability at moderate set sizes (i.e., four and/or five stimuli) indicating that they were able to recall, on average, three stimuli accurately (M=2.61, SD=.61), reflecting a Moderate WM class. The third class demonstrated High WM based on a pattern of stable performance across set sizes without a decrement in performance at higher set sizes. Specifically, those in the High WM class evinced accurate recall of, on average, four stimuli (M=3.57, SD=.61). As illustrated in Figure 1, the three WM classes were significantly different from each other with respect to number of stimuli accurately recalled across all set sizes and across both modalities (visuospatial and phonological). For all set sizes the Low WM class had the lowest scores, followed by the Moderate WM class with the High WM class obtaining the highest scores across both modalities (see Table 2).
Figure 1.
Mean (and standard deviation) of average total stimuli correct per trial across set sizes for the (a) phonological working memory task, and (b) visuospatial working memory task. PH=Phonological, VS=Visuospatial, WM=Working Memory.
Table 2.
Contrasts of measures across the three latent classes.
| 1. Low WM n=19 | 2. Moderate WM n=35 | 3. High WM n=35 | X2 (df=2) | Group Contrasts | |
|---|---|---|---|---|---|
| Phonological WM | |||||
| Set Size 3 | 2.23 (.30) | 2.79 (.17) | 2.89 (.11) | 90.64*** | 3>2>1 |
| Set Size 4 | 2.50 (.51) | 3.39 (.38) | 3.69 (.25) | 93.48*** | 3>2>1 |
| Set Size 5 | 2.13 (.49) | 3.46 (.48) | 4.41 (.34) | 343.58*** | 3>2>1 |
| Set Size 6 | 1.52 (.68) | 2.62 (.62) | 4.33 (.53) | 299.67*** | 3>2>1 |
| Visuospatial WM | |||||
| Set Size 3 | 1.67 (.64) | 2.23 (.39) | 2.66 (.20) | 70.71*** | 3>2>1 |
| Set Size 4 | 1.73 (.73) | 2.52 (.65) | 3.45 (.27) | 144.59*** | 3>2>1 |
| Set Size 5 | 1.47 (.69) | 2.25 (.78) | 3.72 (.62) | 165.54*** | 3>2>1 |
| Set Size 6 | 1.12 (.39) | 1.62 (.62) | 3.44 (.93) | 164.17*** | 3>2>1 |
| CSI-4 Parent | |||||
| Inattentive | 75.50 (10.91) | 69.42 (13.36) | 55.03 (13.99) | 38.13*** | 1=2>3 |
| Hyperactivity | 70.52 (15.32) | 66.45 (16.38) | 49.11 (12.07) | 41.01*** | 1=2>3 |
| Combined | 74.39 (13.27) | 69.90 (14.77) | 52.48 (13.35) | 43.23*** | 1=2>3 |
| CSI-4 Teacher | |||||
| Inattentive | 65.67 (9.87) | 61.52 (11.45) | 54.27 (11.37) | 15.78*** | 1=2>3 |
| Hyperactivity | 60.68 (13.52) | 62.12 (13.81) | 51.16 (9.86) | 17.58*** | 1=2>3 |
| Combined | 64.83 (12.14) | 63.07 (12.55) | 53.31 (11.24) | 17.03*** | 1=2>3 |
| CBCL | |||||
| Attention Problems | 75.94 (11.26) | 67.93 (11.96) | 57.93 (9.09) | 40.69*** | 1>2>3 |
| DSM-Oriented ADHD | 68.98 (9.69) | 67.96 (10.13) | 56.47 (9.33) | 32.67*** | 1=2>3 |
| TRF | |||||
| Attention Problems | 63.55 (9.88) | 62.92 (9.80) | 56.65 (7.35) | 12.70** | 1=2>3 |
| Inattention | 84.95 (14.57) | 77.27 (18.39) | 66.08 (19.49) | 16.401*** | 1=2>3 |
| Hyperactive/Impulsive | 81.68 (17.61) | 79.74 (18.91) | 66.92 (18.90) | 11.31** | 1=2>3 |
| DSM-Oriented ADHD | 64.42 (9.18) | 63.34 (9.32) | 57.32 (7.68) | 12.71** | 1=2>3 |
| DSM-Oriented Inattention | 87.80 (13.29) | 80.25 (17.84) | 67.47 (18.31) | 22.34*** | 1=2>3 |
| DSM-Oriented Hyperactivity | 82.43 (17.49) | 81.42 (18.22) | 66.77 (18.59) | 14.29** | 1=2>3 |
p<.001
p<0.01
p<0.05.
WM=Working Memory, CSI-4=Child Symptom Inventory, CBCL=Child Behavior Checklist, TRF=Teacher Report Form, DSM=Diagnostic and Statistical Manual of Mental Disorders.
Comparison of inattentive and hyperactive symptoms across classes
Parent Ratings
WM performance for all of the children, regardless of diagnostic status, were submitted to the latent profile analysis. As such, comparisons between WM class and ADHD symptoms are independent of diagnostic status (see Table 2). Comparison of the inattentive and hyperactive symptom raw scores for the parent CSI-4 and subscale T-scores for the CBCL revealed significant class differences, all χ2s (2) > 41.01, ps<.001. Specifically, the High WM class was rated as exhibiting significantly fewer symptoms (p≤.05) across the ADHD domains relative to the Low and Moderate WM classes. Effect sizes fell within the large range (d = 0.90 – 1.17; Cohen [1992]), for all symptom domains when comparing the High WM class and the Moderate WM class. Effect sizes were greatest (d = 1.24 – 1.53) when comparing the High WM and Low WM classes, indicating that parents’ perceptions of inattentive and hyperactive symptoms differed to a greater degree between the High and Low WM classes than between the High and Moderate WM classes. In contrast, the Moderate WM class was not significantly different (p>.05) from the Low WM class for the parent rating subscales (ps > .07), with one exception (i.e., CBCL Attention Problems subscale). Specifically, the Moderate WM class had a significantly lower T-score on the CBCL Attention Problems relative to the Low WM class, χ2 (2) = 5.96, p<.05, Cohen’s d = .65.
Teacher ratings
As reported in Table 2, a similar pattern was observed for teacher reported symptoms across the subscale T-scores for the CSI-4 and the TRF with significant differences emerging across all subscales (all χ2s (2) > 6.10, ps< .05). Examination of between class differences revealed that membership in the High WM class was associated with significantly lower ratings relative to the Moderate WM class on all CSI-4 and TRF subscales (all p-values ≤ .05; Cohen’s d ranged from 0.55 to 0.87). Relative to the Low WM class, the High WM class was associated with significantly lower ratings (all p-values ≤ .05) across all CSI-4 and TRF Teacher subscales. Differences among latent classes across all subscales of the TRF and CSI were associated with large effect sizes (ds = 0.80 – 1.71), with the exception of the Hyperactive/Impulsive subscale of the TRF (d = 0.74) which was associated with a medium to large magnitude effect size (Cohen, 1992). Similar to the parent ratings, no significant differences emerged (all p-values > .05) between the Low and Moderate WM classes on the CSI-4 Subscales and the TRF subscales (ds = 0.06 – 0.44). In essence, there were significant medium to large magnitude differences in parent and teacher perceptions of inattentive and hyperactive behavior between individuals in the Low and High WM classes as well as between the Moderate and High WM classes across multiple rating scales. There were small to medium differences in teachers’ perceptions between the Low and Moderate WM classes with respect to the inattentive subscales of the TRF and CSI; however, no significant teacher rated differences for the hyperactivity subscales emerged for the two worse performing WM classes. In contrast, there were small nonsignificant differences in parent perceptions between the Low and Moderate WM classes for the hyperactivity and combined subscales of the CSI.
Distribution of Diagnostic Status across Latent Classes
The distribution of individuals comprising the two diagnostic groups (ADHD and TD) across varying levels of WM was also examined. Overall, the contingency tables indicated that assignment to WM class was related to diagnosis, χ2(2)=58.05, p<.001, wherein 94.8% and 5.2% of the Low WM class was comprised of individuals from the ADHD and TD groups, respectively. In contrast, 71.5% and 28.5% of the Moderate WM class was comprised of children from the ADHD and TD groups, respectively. Finally, the High WM class was comprised primarily of TD children (78.6%) relative to the ADHD (21.4%) group. Collectively, the results reveal that the majority of children (84%) with ADHD exhibited low (35%) or moderate (49%) WM performance while nearly all children in the TD group (98%) exhibited moderate (26%) or high (72%) WM performance.
Discussion
Heterogeneity in WM performance of children with ADHD likely reflects the contribution of multiple neuropsychological pathways to the disorder (Castellanos & Tannock, 2002; Nigg & Casey, 2005; Sonuga-Barke, 2002). Consistent evidence for heterogeneity in WM functioning in ADHD (Fair et al., 2012; Kofler et al., 2019; Willcutt et al., 2005) highlights the need to adopt novel analytic approaches such as latent profile analysis to account for individual differences in neurocognitive functioning and clarify the role of children’s WM abilities in parent- and teacher-rated symptoms of the disorder (i.e., inattention, hyperactivity, and impulsivity) as well diagnostic status. The current study demonstrates that WM performance in children with and without ADHD may be more nuanced than dichotomous conceptualizations (i.e., impaired or not impaired). Specifically, the ability to manipulate, store, rehearse, and recall increasingly greater numbers of stimuli until capacity limitations are reached, at which point performance stabilized or decreases (Cowan, 2001), may vary along a larger range of abilities. As such, increased recognition of varying levels of WM performance (e.g., low, moderate, high) may advance the field. The High WM class, on average, did not evince decrements in accuracy to recall rearranged stimuli. In contrast, the Moderate WM class evinced worse performance relative to the High WM class across both phonological and visuospatial WM tasks for all set sizes. Children in the Low WM class displayed consistently worse performance relative to the two other classes, regardless of the cognitive burden across both phonological and visuospatial tasks. Therefore, the Low and Moderate WM classes represent poor WM classes such that those in the Low WM class evinced the weakest WM performance.
The current study is the first to utilize latent profile analysis to examine subgroups of WM across distinct WM domains in children with and without ADHD, and to elucidate the association between WM and parent and teacher-rated ADHD symptoms regardless of diagnoses. Consistent with Gomez and colleagues (2014), this study replicated three classes of WM––High, Moderate, and Low, with similar distributions of children with ADHD within the Low and Moderate WM classes. The finding that poor WM performance was not exclusive to children diagnosed with ADHD were consistent with previous studies that have found impaired WM in typically developing populations (Cowan, 2014; Fair et al., 2012). Further, despite the overlap in WM performance between the ADHD and TD groups, the poorest WM performing group consisted nearly exclusively of children with a diagnosis of ADHD. Twenty-nine percent of the Moderate WM class was comprised of TD children, which is consistent with past evidence of nested heterogeneity in cognitive performance in children with and without ADHD. The class groupings revealed a subset of children without ADHD that evince poor WM performance; however, children with ADHD exhibited, overall, the weakest WM performance. Such groupings support the notion that WM occurs on a spectrum such that WM in typically developing children and children with ADHD represent extremes along the same continuum with some overlap.
The best fitting model did not indicate a difference in change in performance across set sizes between modalities, indicating that capacity limitations were not specific to modality (e.g., visual, phonological). These findings suggest that poor performance in one modality may be closely associated with poor performance in the second modality. Specifically, ADHD may be characterized by impairment in both visuospatial and phonological storage/rehearsal processes. Alternatively, given that both tasks involve central executive processes, it is also plausible that impairment in the central executive may be contributing to poor performance across both tasks. An overall WM deficiency linked to central executive impairments is consistent with investigations that suggest that poor WM performance is associated with a central executive deficit (Alderson, Kasper, Hudec, & Patros, 2013; Dovis, Van der Oord, Wiers, & Prins, 2013; Raiker, Rapport, Kofler, & Sarver, 2012; Rapport et al., 2008).
A second purpose of the current investigation was to examine the relationship between latent profiles of WM and symptoms of ADHD. Consistent with past findings (Alloway, Gathercole, Kirkwood, & Elliott, 2009; Brocki et al., 2010; Gathercole et al., 2008), the current study found that children with deficits in WM were characterized by elevated parent/teacher-rated symptoms of inattention and hyperactivity/impulsivity. The Low and Moderate WM were not significantly different from each other in terms of symptom T-scores for all subscales except for the Attention Problems of the CBCL, for which the Low WM class had significantly higher ratings. Across all of the subscales, the Low and Moderate WM classes had significantly higher ratings of inattention and hyperactivity relative to the High WM class. Collectively, these findings suggest that any impairment in WM is associated with greater perceived levels of inattentive and hyperactive behavior by parent and teachers relative to no WM impairment (Friedman & Miyake, 2004; Kofler et al., 2010; Poole & Kane, 2009; Unsworth & Engle, 2007). Moreover, there was no difference in behavioral ratings between the weakest WM classes, indicating that, while WM may be dimensional in nature, there may not be an absolute correspondence between WM and severity of behavioral symptoms. Specifically, children with moderate WM may exhibit similar behavioral symptoms as those with low WM, but there are additional factors that determine whether a child with moderate WM will meet criteria for a diagnosis of ADHD.
The higher parent- and teacher-rated symptoms of hyperactivity in the classes with worse WM performance is consistent with prior studies demonstrating a robust relation between WM and objectively measured impulsivity (Raiker et al., 2012) and hyperactivity (Kofler et al., 2016; Rapport et al., 2009; Sarver et al., 2015). While any magnitude of poor WM performance was associated with greater hyperactive and inattentive symptoms, the highest levels of WM impairment, as reflected by membership in the Low WM class, was associated with even greater levels of inattentive behavior. This finding is consistent with prior work demonstrating a stronger association of WM with inattentive symptoms than with hyperactive symptoms (Brocki et al., 2010), yet only one of the inattentive subscales across both parent and teaching ratings was significantly different between the groups, indicating that this association may be highly dependent on the rating scale utilized.
A novelty of the current study is the examination of WM distributions across individuals meeting diagnostic criteria for ADHD and TD children who do not meet criteria for ADHD simultaneously to inform understanding of potential nested heterogeneity (Fair et al., 2012). Results revealed that the ADHD group exhibited mostly low to moderate WM (84%) performance. In contrast, more than a quarter (29%) of children in the TD group exhibited low to moderate WM performance. This finding replicates previous work highlighting heterogeneity in neurocognitive dysfunction across typically developing samples (e.g., Costa Dias et al., 2015; Fair et al., 2012) and indicates that children with the most severe impairment in WM may be more readily identifiable and at greater risk for meeting diagnostic criteria for ADHD (Kane, Bleckley, Conway, & Engle, 2001). The current findings are consistent with studies that have identified heterogeneity in WM performance as well as a lack of a unique association with one symptom domain over another (e.g. Bunford et al., 2015). Specifically, WM performance was associated with both inattentive and hyperactive domains such that severity of symptoms was associated with latent WM class membership. The finding that children assigned to the Moderate and Low WM classes exhibited similar levels of ADHD symptoms suggests that it may be more difficult to differentiate among children with low to moderate WM based solely on parent/teacher symptom ratings consistent with the nonpathognomonic nature of symptoms of ADHD (e.g., inattention; Wanmaker et al., 2014).
Despite the use of a well-characterized sample, a novel analytic approach (i.e., latent profile analysis), and cognitive tasks derived from a well-established theoretical model of WM, the current study findings should be interpreted with caution. The study utilized a relatively modest sample size; however, the incorporation of multiple indicators (i.e., eight) with strong psychometric properties and construct validity as well as the identification of multiple classes with well-defined separation suggests the study was adequately powered to detect subgroups (Dziak et al., 2014; Muthén & Muthén, 2002; Wurpts & Geiser, 2014). Additionally, given that the tasks utilized in the current study did not require cross-modality binding of information, the current study did not address the potential role of the episodic buffer. Future work may benefit from the inclusion of an older sample as well as WM tasks that require the use of the episodic buffer. In terms of sample composition, the current study excluded children with diagnoses other than ADHD (e.g. anxiety, autism, and depression) without comorbid ADHD yet included children that met diagnoses for ADHD as well as additional diagnoses. Considering that symptoms of inattention and hyperactivity are nonpathognomonic to ADHD and are often reported in individuals with other clinical disorders (e.g., anxiety, autism spectrum disorder; Dawson et al., 2002; Griffith, Pennington, Wehner, & Rogers, 1999; Wanmaker, Geraerts, Franken, 2014), dimensional approaches to the evaluation of WM performance across muliple disorders may help elucidate further the relationship between WM and ADHD symptoms. Such an approach is consistent with the research-based framework outlined by the Research Domain Criteria (RDoC; Insel et al., 2010). Further, given that the average IQ score of the ADHD group did not differ significantly from that of the typically developing group, the current ADHD sample may represent a group of children with higher than average IQ compared to ADHD samples used in prior studies (Frazier, Demaree, & Youngstrom, 2004). Additionally, a minority of the children with ADHD (40%) met criteria for comorbid diagnoses, which is lower than the degree of comorbidity typical of the ADHD population (i.e., 59%; Wilens, et al., 2002). As a result, considering the high correlation between IQ and WM (Kane, Hambrick, & Conway, 2005), the sample may have limited the number of children with severely impaired WM.
Collectively, the current findings extend prior literature on WM deficits in ADHD (Gomez et al., 2014; Martinussen et al., 2005; Pennington & Ozonoff, 1996; Willcutt et al., 2005) by examining the heterogeneity of WM dysfunction across varying cognitive load and its association with parent/teacher rated behavioral ADHD symptoms. The results demonstrate that parent/teacher rated ADHD symptoms are more easily detectable when comparing children with strong WM ability to children with poor WM ability; however, more nuanced consideration may be necessary when considering children with more moderate WM abilities. Notably, these findings indicate that poor WM performance is not unique to ADHD but are also observed in children without the disorder, consistent with evidence that ADHD reflects a quantitative rather than qualitative developmental difference in brain maturation over the course of development (Shaw et al., 2007). Future work should evaluate the extent to which intact functioning in specific WM domains (i.e., phonological storage/rehearsal, visuospatial storage/rehearsal, and central executive) exerts a protective effect with respect to symptom presentation or across other areas of functional impairment (e.g., social functioning, academic performance). Additionally, employing similar approaches with other neurocognitive functions, as well as objective assessments, is also recommended to provide a better understanding of the relation between executive functioning and ADHD symptoms. Finally, the field may benefit significantly from studies examining the development of executive functions in tandem with fluctuations of ADHD symptoms across development. Such studies would elucidate the correspondence between changes in WM subcomponents and ADHD symptoms, as well as the utility of the integration of WM assessment into the examination of secondary deficits associated with ADHD such as impaired social, occupational, and academic functioning.
Acknowledgments
Funding: During the production of this manuscript, the corresponding author was supported in part by the Brain and Behavior Research Foundation, the Children’s Trust, NIMH, and NSF. Additionally, at least one of the other authors was supported by NIMH during production of this manuscript. None of the views expressed in this manuscript represent the views of any of these funding agencies. The authors declare they have no conflicts of interest.
Footnotes
Compliance of Ethical Standards: During the production of this manuscript, Dr. Raiker was supported in part by the Brain and Behavior Research Foundation (#66791), the Children’s Trust (#7561, #7161), NIMH (MH099030, MH112002), and NSF (CNS-1532061). Additionally, Dr. Friedman was supported by NIMH during the production of this manuscript (T32-MH018261). None of the views expressed in this manuscript represent the views of any of these funding agencies. Ms. Campez, Dr. Raiker, Dr. Sarver, Dr. Friedman, Dr. Orban, and Dr. Rapport have no further potential conflicts of interest.
Ethical Approval: This research was approved by the Institution’s Ethics Board. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed parental consent and child assent (for children under age 18 years) was obtained from all individual participants included in the study.
A subset of these children were included in previous studies (Alderson, Rapport, Hudec, Sarver, & Kofler, 2010; Bolden, Rapport, Raiker, Sarver & Kofler, 2012; Calub, Rapport, Friedman, & Eckrich, 2019; Kofler, Alderson, Raiker, Bolden, Sarver, & Rapport, 2014; Raiker, Rapport, Kofler, & Sarver, 2012; Rapport et al., 2008; Rapport, Bolden, Kofler, Sarver, Raiker, & Alderson, 2009; Sarver, Rapport, Kofler, Raiker, & Friedman, 2015) to examine conceptually distinct hypotheses. Notably, this sample has never been submitted to latent profile analysis and the association between the experimental laboratory-based tasks and the rating scales used in the current study have not been previously examined.
All participants within the ADHD group also met criteria for ADHD based on the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5; American Psychiatric Association, 2013), as determined by the KSADS (2013 update), which was published during the collection of this data.
References
- Achenbach TM, & Rescorla LA (2001). Manual for the ASEBA school-age forms & Profile. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families. [Google Scholar]
- Alderson RM, Hudec KL, Patros CH, & Kasper LJ (2013). Working memory deficits in adults with attention-deficit/hyperactivity disorder (ADHD): An examination of central executive and storage/rehearsal processes. Journal of Abnormal Psychology, 122(2), 532. [DOI] [PubMed] [Google Scholar]
- Alderson RM, Kasper LJ, Hudec KL, & Patros CHG (2013). Attention-deficit/hyperactivity disorder (ADHD) and working memory in adults: A meta-analytic review. Neuropsychology, 27(3), 287–302. [DOI] [PubMed] [Google Scholar]
- Alderson RM, Kasper LJ, Patros CH, Hudec KL, Tarle SJ, & Lea SE (2015). Working memory deficits in boys with attention deficit/hyperactivity disorder (ADHD): An examination of orthographic coding and episodic buffer processes. Child Neuropsychology, 21(4), 509–530 [DOI] [PubMed] [Google Scholar]
- Alderson RM, Rapport MD, Hudec KL, Sarver DE, & Kofler MJ (2010). Competing core processes in Attention-Deficit/Hyperactivity Disorder (ADHD): Do working memory deficiencies underlie behavioral inhibition deficits? Journal of Abnormal Child Psychology, 38(4), 497–507. [DOI] [PubMed] [Google Scholar]
- Alloway TP (2011). A comparison of working memory profiles in children with ADHD and DCD. Neuropsychology, 17(5), 483–494. [DOI] [PubMed] [Google Scholar]
- Alloway TP, Gathercole SE, Kirkwood H, & Elliott J (2009). The cognitive and behavioral characteristics of children with low working memory. Child Development, 80(2), 606–621. [DOI] [PubMed] [Google Scholar]
- Altszuler AR, Page TF, Gnagy EM, Coxe S, Arrieta A, Molina BS, & Pelham WE Jr (2016). Financial dependence of young adults with childhood ADHD. Journal of Abnormal Child Psychology, 44(6), 1217–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders: DSM-5. Washington, D. C.: American Psychiatric Association. [Google Scholar]
- Asparouhov T, & Muthén B (2014). Auxiliary variables in mixture modeling: Three-step approaches using Mplus. Structural Equation Modeling: A Multidisciplinary Journal, 21(3), 329–341. [Google Scholar]
- Baddeley A (2007). Working memory, thought, and action. Oxford: Oxford University Press. [Google Scholar]
- Bakk Z, & Vermunt JK (2016). Robustness of stepwise latent class modeling with continuous distal outcomes. Structural Equation Modeling: A Multidisciplinary Journal, 23(1), 20–31. [Google Scholar]
- Barbaresi WJ, Colligan RC, Weaver AL, Voigt RG, Killian JM, & Katusic SK (2013). Mortality, ADHD, and psychosocial adversity in adults with childhood ADHD: A prospective study. Pediatrics, 131(4), 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA (Ed.). (2014). Attention-deficit hyperactivity disorder: A handbook for diagnosis and treatment (5th ed.). New York: Guilford Publications. [Google Scholar]
- Bauer DJ, & Curran PJ (2003). Overextraction of latent trajectory classes: Much ado about nothing? Reply to Rindskopf (2003), Múthen (2003), and Cudeck and Henly (2003). Psychological Methods, 8(3), 384–393. [DOI] [PubMed] [Google Scholar]
- Biederman J, Monuteaux MC, Doyle AE, Seidman LJ, Wilens TE, Ferrero F, Morgan CL, & Faraone SV (2004). Impact of executive function deficits and attention-deficit/hyperactivity disorder (ADHD) on academic outcomes in children. Journal of Consulting and Clinical Psychology, 72(5), 757–66. [DOI] [PubMed] [Google Scholar]
- Bolden J, Rapport MD, Raiker JS, Sarver DE, & Kofler MJ (2012). Understanding phonological memory deficits in boys with Attention-Deficit/Hyperactivity Disorder (ADHD): Dissociation of short-term storage and articulatory rehearsal processes. Journal of Abnormal Child Psychology, 40(6), 999–1011. [DOI] [PubMed] [Google Scholar]
- Brocki KC, Eninger L, Thorell LB, & Bohlin G (2010). Interrelations between executive function and symptoms of hyperactivity/impulsivity and inattention in preschoolers: A two year longitudinal study. Journal of Abnormal Child Psychology, 38(2), 163–171. [DOI] [PubMed] [Google Scholar]
- Brocki KC, Randall KD, Bohlin G, & Kerns KA (2008). Working memory in school-aged children with attention-deficit/hyperactivity disorder combined type: Are deficits modality specific and are they independent of impaired inhibitory control? Journal of Clinical and Experimental Neuropsychology, 30(7), 749–759. [DOI] [PubMed] [Google Scholar]
- Bunford N, Brandt NE, Golden C, Dykstra JB, Suhr JA, Owens JS (2015). Attention-deficit/hyperactivity disorder symptoms mediate the association between deficits in executive functioning and social impairment in children. Journal of Abnormal Child Psychology, 43, 133–147. [DOI] [PubMed] [Google Scholar]
- Burgess GC, Depue BE, Ruzic L, Willcutt EG, Du YP, & Banich MT (2010). Attentional control activation relates to working memory in attention-deficit/hyperactivity disorder. Biological Psychiatry, 67, 632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calub CA, Rapport MD, Friedman LM, & Eckrich SJ (2019). IQ and academic achievement in children with ADHD: The differential effects of specific cognitive functions. Journal of Psychopathology and Behavioral Assessment, 1–13. [Google Scholar]
- Castellanos FX, & Tannock R (2002). Neuroscience of attention-deficit/hyperactivity disorder: The search for endophenotypes. Nature Reviews Neuroscience, 3(8), 617–628. [DOI] [PubMed] [Google Scholar]
- Chhabildas N, Pennington BF, & Willcutt EG (2001). A comparison of the neuropsychological profiles of the DSM-IV subtypes of ADHD. Journal of Abnormal Child Psychology, 29(6), 529–540. [DOI] [PubMed] [Google Scholar]
- Cohen J (1992). A power primer. Psychological Bulletin, 112(1), 155. [DOI] [PubMed] [Google Scholar]
- Conway AR, Kane MJ, & Engle RW (2003). Working memory capacity and its relation to general intelligence. Trends in Cognitive Sciences, 7(12), 547–552. [DOI] [PubMed] [Google Scholar]
- Costa Dias TG, Iyer SP, Carpenter SD, Cary RP, Wilson VB, Mitchell SH, … & Fair DA (2015). Characterizing heterogeneity in children with and without ADHD based on reward system connectivity. Developmental Cognitive Neuroscience, 11, 155–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N (2001). The magical number four in short-term memory: A reconsideration of mental storage capacity. Behavioral and Brain Sciences, 24, 87–114. [DOI] [PubMed] [Google Scholar]
- Cowan N (2014). Working memory underpins cognitive development, learning, and education. Educational Psychology Review, 26(2), 197–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Munson J, Estes A, Osterling J, McPartland J, Toth K, Carver L, Abbott R (2002). Neurocognitive function and joint attention ability in young children with autism spectrum disorder versus developmental delay. Child Devevelopment, 73, 345–358. [DOI] [PubMed] [Google Scholar]
- Daley D, & Birchwood J (2010). ADHD and academic performance: Why does ADHD impact on academic performance and what can be done to support ADHD children in the classroom? Child: Care, Health and Development, 36(4), 455–464. [DOI] [PubMed] [Google Scholar]
- Dovis S, Van der Oord S, Wiers RW, & Prines PJ (2013). What part of working memory is not working in ADHD? Short-term memory, the central executive and effects of reinforcement. Journal of Abnormal Child Psychology, 41(6), 901–917. [DOI] [PubMed] [Google Scholar]
- Dziak JJ, Lanza ST, & Tan X (2014). Effect size, statistical power and sample size requirements for the bootstrap likelihood ratio test in latent class analysis. Structural Equation Modeling, 21(4), 534–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Bathula D, Nikolas MA, & Nigg JT (2012). Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proceedings of the National Academy of Sciences, 109(17), 6769–6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier TW, Demaree HA, & Youngstrom EA (2004). Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/hyperactivity disorder. Neuropsychology, 18(3), 543–55. [DOI] [PubMed] [Google Scholar]
- Friedman NP, & Miyake A (2004). The relations among inhibition and interference control functions: A latent-variable analysis. Journal of Experimental Psychology: General, 133, 101–135. [DOI] [PubMed] [Google Scholar]
- Gadow KD, Sprafkin J, & Salisbury H (2004). Further validity evidence for the teacher version of the Child Symptom Inventory-4. School Psychology Quarterly, 19, 50–71. [Google Scholar]
- Gallego-Martinez A, Garcia-Sevilla J, & Fenollar-Cortés J (2018). Implicación de la memoria visoespacial y fonológica en la heterogeneidad clínica del Trastorno por Déficit de Atención con Hiperactividad (TDAH). Anales de psicología, 34(1), 16–22. [Google Scholar]
- Gathercole SE, Pickering SJ, Ambridge B, & Wearing H (2004). The structure of working memory from 4 to 15 years of age. Developmental Psychology, 40(2), 177–190. [DOI] [PubMed] [Google Scholar]
- Gathercole SE, Alloway TP, Kirkwood HJ, Elliot JG, Holmes J, & Hilton KA (2008). Attentional and executive function behaviours in children with poor working memory. Learning and Individual Differences, 18(2), 214–223. [Google Scholar]
- Gomez R, Gomez RM, Winther J, & Vance A (2014). Latent profile analysis of working memory performance in a sample of children with ADHD. Journal of Abnormal Child Psychology, 42(8), 1367–1379. [DOI] [PubMed] [Google Scholar]
- Gray S, Green S, Alt M, Hogan T, Kuo T, Brinkley S, & Cowan N (2017). The structure of working memory in young children and its relation to intelligence. Journal of Memory and Language, 92, 183–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith EM, Pennington BF, Wehner EA, Rogers SJ (1999). Executive functions in young children with autism. Child Development, 70, 817–832. [DOI] [PubMed] [Google Scholar]
- Halperin JM, & Schulz KP (2006). Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychological Bulletin, 132(4), 560. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, … & Wang P (2010). Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. The American Journal of Psychiatry, 167, 748–751. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Bleckley MK, Conway AR, & Engle RW (2001). A controlled-attention view of working-memory capacity. Journal of Experimental Psychology: General, 130(2), 169–183. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Hambrick DZ, & Conway ARA (2005). Working memory capacity and fluid intelligence are strongly related constructs: Comment on Ackerman, Beier, and Boyle (2005). Psychological Bulletin, 131(1), 66–71. [DOI] [PubMed] [Google Scholar]
- Karalunas SL, Gustafsson HC, Dieckmann NF, Tipsord J, Mitchell SH, & Nigg JT (2017). Heterogeneity in development of aspects of working memory predicts longitudinal attention deficit hyperactivity disorder symptom change. Journal of Abnormal Psychology, 126(6), 774–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper LJ, Alderson RM, & Hudec KL (2012). Moderators of working memory deficits in children with attention-deficit/hyperactivity disorder (ADHD): A meta-analytic review. Clinical Psychology Review, 32(7), 605–617. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao UMA, Flynn C, Moreci P, … Ryan N (1997). Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry, 36(7), 980–988. [DOI] [PubMed] [Google Scholar]
- Kofler MJ, Alderson RM, Raiker JS, Bolden J, Sarver DE, & Rapport MD (2014). Working memory and intraindividual variability as neurocognitive indicators in ADHD: Examining competing model predictions. Neuropsychology, 28(3), 459–471 [DOI] [PubMed] [Google Scholar]
- Kofler MJ, Irwin LN, Soto EF, Groves NB, Harmon SL, & Sarver DE (2019). Executive functioning heterogeneity in pediatric ADHD. Journal of Abnormal Child Psychology, 47, 273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler MJ, Raiker JS, Sarver DE, Wells EL, & Soto EF (2016). Is hyperactivity ubiquitious in ADHD or dependent on environmental demands? Evidence from meta-analysis. Clinical Psychology Review, 46, 12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler MJ, Rapport MD, Bolden J, Sarver DE, & Raiker JS (2010). ADHD and working memory: The impact of central executive deficits and exceeding storage/rehearsal capacity on observed inattentive behavior. Journal of Abnormal Child Psychology, 38(2), 149–161. [DOI] [PubMed] [Google Scholar]
- Kofler MJ, Sarver DE, Spiegel JA, Day TN, Harmon SL, & Wells EL (2017). Heterogeneity in ADHD: Neurocognitive predictors of peer, family, and academic functioning. Child Neuropsychology, 23(6), 733–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler MJ, Sarver DE, & Wells EL (2015). Working memory and increased activity level (hyperactivity) in ADHD. Journal of Attention Disorders. [DOI] [PubMed] [Google Scholar]
- Kofler MJ, Spiegel JA, Austin KE, Irwin LN, Soto EF, & Sarver DE (2018). Are episodic buffer processes intact in ADHD? Experimental evidence and linkage with hyperactive behavior. Journal of Abnormal Child Psychology, 46(6), 1171–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsi J, Oosterlaan J, & Stevenson J (2001). Psychological mechanisms in hyperactivity: I response inhibition deficit, working memory impairment, delay aversion, or something else? The Journal of Child Psychology and Psychiatry and Allied Disciplines, 42(2), 199–210. [PubMed] [Google Scholar]
- Lanza ST, & Rhoades BL (2013). Latent class analysis: An alternative perspective on subgroup analysis in prevention and treatment. Prevention Science, 14(2), 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza ST, Tan X, & Bray BC (2013). Latent class analysis with distal outcomes: A flexible model-based approach. Structural Equation Modeling, 20(1), 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambek R, Sonuga-Barke E, Tannock R, Sørensen AV, Damm D, & Thomsen PH (2018). Are there distinct cognitive and motivational sub-groups of children with ADHD? Psychological Medicine, 48(10), 1722–1730 [DOI] [PubMed] [Google Scholar]
- Lambek R, Tannock R, Dalsgaard S, Trillingsgaard A, Damm D, & Thomsen PH (2010). Validating neuropsychological subtypes of ADHD: How do children with and without an executive function deficit differ? Journal of Child Psychology and Psychiatry, 51(8), 895–904. [DOI] [PubMed] [Google Scholar]
- Lawson GM, Hook CJ, farah m. J. (2014). A meta-analysis of the relationship between socioeconomic status and executive function performance among children. Developmental Science, 21(2), 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood KA, Marcotte AC, & Stern C (2001). Differentiation of attention-deficit/hyperactivity disorder subtypes: Application of a neuropsychological model of attention. Journal of Clinical and Experimental Neuropsychology, 23(3), 317–330. [DOI] [PubMed] [Google Scholar]
- Lui M, & Tannock R (2007). Working memory and inattentive behaviour in a community sample of children. Behavioral and Brain Functions, 3(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Weibman D, Halperin J, & Li X (2019). A review of heterogeneity in Attention Deficit/Hyperactivity Disorder (ADHD). Frontiers in Human Neuroscience, 13, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinussen R, Hayden J, Hogg-Johnson S, & Tannock R (2005). A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 44(4), 377–84. [DOI] [PubMed] [Google Scholar]
- Mella N, Fagot D, & de Ribaupierre A (2016). Dispersion in cognitive functioning: Age differences over the lifespan. Journal of Clinical and Experimental Neuropsychology, 38(1), 111–126. [DOI] [PubMed] [Google Scholar]
- Molina BSG, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, … Elliott GR (2009). The MTA at 8 years: Prospective follow-up of children treated for combined-type ADHD in a multisite study. Journal of the American Academy of Child & Adolescent Psychiatry, 48(5), 484–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostert JC, Hoogman M, Onnink AMH, van Rooji D, von Rhein D, van Hulzen KJE,…, & Franke B (2015). Similar subgroups based on cognitive performance parse heterogeneity in adults with ADHD and healthy controls. Journal of Attention Disorders, 22(3), 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (2002). How to use a Monte Carlo study to decide on sample size and determine power. Structural Equation Modeling, 9, 599–620. [Google Scholar]
- Muthén LK, & Muthén BO (2017). Mplus User’s Guide. Eighth Edition. Los Angeles, CA: Muthén, & Muthén. [Google Scholar]
- Nigg JT (2003). Response inhibition and disruptive behaviors: Toward a multiprocess conception of etiological heterogeneity for ADHD combined type and conduct disorder early-onset type. Annals of the New York Academy of Science, 1008(1), 170–182. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Blaskey LG, Stawicki JA, & Sachek J (2004). Evaluating the endophenotype model of ADHD neuropsychological deficit: Results for parents and siblings of children with ADHD combined and inattentive subtypes. Journal of Abnormal Psychology, 113(4), 614. [DOI] [PubMed] [Google Scholar]
- Nigg JT, & Casey BJ (2005). An integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Development and Psychopathology, 17(3), 785–806. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Willcutt EG, Doyle AE, & Sonuga-Barke EJ (2005). Causal heterogeneity in attention-deficit/hyperactivity disorder: Do we need neuropsychologically impaired subtypes? Biological Psychiatry, 57(11), 1224–1230. [DOI] [PubMed] [Google Scholar]
- Patros CH, Alderson RM, Hudec KL, Tarle SJ, & Lea SE (2017). Hyperactivity in boys with attention-deficit/hyperactivity disorder: The influence of underlying visuospatial working memory and self-control processes. Journal of Experimental Child Psychology, 154, 1–12. [DOI] [PubMed] [Google Scholar]
- Patros CH, Alderson RM, Lea SE, Tarle SJ, Kasper LJ, & Hudec KL (2015). Visuospatial working memory underlies choice-impulsivity in boys with attention-deficit/hyperactivity disorder. Research in Developmental Disabilities, 38, 134–144. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Foster EM, & Robb JA (2007). The economic impact of attention-deficit/hyperactivity disorder in children and adolescents. Journal of Pediatric Psychology, 32(6), 711–727. [DOI] [PubMed] [Google Scholar]
- Pennington BF, & Ozonoff S (1996). Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry, 37(1), 51–87. [DOI] [PubMed] [Google Scholar]
- Poole BJ, & Kane MJ (2009). Working memory capacity predicts the executive control of visual search among distractors: The influences of sustained and selective attention. Quarterly Journal of Experimental Psychology, 62(7), 1430–1454. [DOI] [PubMed] [Google Scholar]
- Polanczyk GV, Willcutt EG, Salum GA, Kieling, & Rohde LA (2014). ADHD prevalence estimates across three decades: An updated systematic review and meta-regression analysis. International Journal of Epidemiology, 43, 434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiker JS, Rapport M, Kofler MJ, & Sarver DE (2012). Objectively-measured impulsivity and Attention-Deficit/Hyperactivity Disorder (ADHD): Testing competing predictions from the working memory and behavioral inhibition models of ADHD. Journal of Abnormal Child Psychology, 40(5), 699–713. [DOI] [PubMed] [Google Scholar]
- Rapport MD, Alderson RM, Kofler MJ, & Raiker JS (2008). Working memory deficits in boys with ADHD: The contribtion of central executive and subsystem processes. Journal of Abnormal Child Psychology, 36(6), 825–837. [DOI] [PubMed] [Google Scholar]
- Rapport MD, Bolden J, Kofler MJ, Sarver DE, Raiker JS, & Alderson RM (2009). Hyperactivity in boys with Attention-Deficit/Hyperactivity Disorder (ADHD): A ubiquitous core symptom or manifestation of working memory deficits? Journal of Abnormal Child Psychology, 37(4), 521–534. [DOI] [PubMed] [Google Scholar]
- Rapport MD, Chung KM, Shore G, Issacs P (2001). A conceptual model of child psychopathology: Implications for understanding attention-deficit/hyperactivity disorder (ADHD) and treatment efficacy. Journal of Clinical Child Psychology, 30, 48–58. [DOI] [PubMed] [Google Scholar]
- Sarver DE, Rapport MD, Kofler MJ, Raiker JS, & Friedman LM (2015). Hyperactivity in Attention-Deficit/Hyperactivity Disorder (ADHD): Impairing deficit or compensatory behavior? Journal of Abnormal Child Psychology, 43(7), 1219–1232. [DOI] [PubMed] [Google Scholar]
- Sergeant J (2000). The cognitive-energetic model: An empirical approach to attention-deficit hyperactivity disorder. Neuroscience & Biobehavioral Reviews, 24(1), 7–12. [DOI] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch J, Greenstein DEEA, … Rapoport JL (2007). Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proceedings of the National Academy of Sciences, 104(49), 19649–19654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley MH, Swanson JM, Arnold LE, Hetchman LT, Owens EB, Stehli A,…, & Pelham WE (2017). Defining ADHD symptom persistence in adulthood: Optimizing sensitivity and specificity. The Journal of Child Psychology and Psychiatry, 58(6), 655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJ (2002). Psychological heterogeneity in AD/HD—a dual pathway model of behaviour and cognition. Behavioural Brain Research, 130(1), 29–36. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Dalen L, Daley D, & Remington B (2002). Are planning, working memory, and inhibition associated with individual differences in preschool ADHD symptoms? Developmental Neuropsychology, 21(3), 255–272. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Sergeant JA, Nigg J, & Willcutt E (2008). Executive dysfunction and delay aversion in attention deficit hyperactivity disorder: Nosologic and diagnostic implications. Child and Adolescent Psychiatric Clinics of North America, 17(2), 367–384. [DOI] [PubMed] [Google Scholar]
- Sprafkin J, Gadow KD, Salisbury H, Schneider J, & Loney J (2002). Further evidence of reliability and validity of the Child Symptom Inventory-4: Parent checklist in clinically referred boys. Journal of Clinical Child and Adolescent Psychology, 31(4), 513–524. [DOI] [PubMed] [Google Scholar]
- SuperLab Pro (Version 2). (2002). [Computer Software]. San Pedro, CA: Cedrus Corporation. [Google Scholar]
- Tein J, Coxe S, & Cham H (2013). Statistical power to detect the correct number of classes in latent profile analysis. Structural Equation Modeling: A Multidisciplinary Journal, 20(4), 640–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorell LB (2007). Do delay aversion and executive function deficits make distinct contributions to the functional impact of ADHD symptoms? A study of early academic skill deficits. Journal of Child Psychology and Psychiatry, 48(11), 1061–1070. [DOI] [PubMed] [Google Scholar]
- Tillman C, Eninger L, Forssman L, & Bohlin G (2011). The relation between working memory components and ADHD symptoms from a developmental perspective. Developmental Neuropsychology, 36(2), 181–198. [DOI] [PubMed] [Google Scholar]
- Unsworth N, & Engle RW (2007). The nature of individual differences in working memory capacity: Active maintenance in primary memory and controlled search from secondary memory. Psychological Review, 114(1), 104–132. [DOI] [PubMed] [Google Scholar]
- Wanmaker S, Hopstaken JF, Asselbergs J, Geraerts E, & Franken IHA (2014). Decreasing dysphoric thoughts by a working memory training: A randomized double-blind placebo-controlled trial. Journal of Depression and Anxiety, 3(165), 2167–1044. [Google Scholar]
- Wechsler D (2003). Wechsler intelligence scale for children. San Antonio: Psychological Corporation. [Google Scholar]
- Wehmeier PM, Schacht A, & Barkley RA (2010). Social and emotional impairment in children and adolescents with ADHD and the impact on quality of life. Journal of Adolescent Health, 46(3), 209–217. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, & Pennington BF (2005). Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biological Psychiatry, 57(11), 1336–1346. [DOI] [PubMed] [Google Scholar]
- Wurpts IC & Geiser C (2014). Is adding more indicators to a latent class analysis beneficial or detrimental? Results of a Monte-Carlo study. Frontiers in Psychology, 5, 920. [DOI] [PMC free article] [PubMed] [Google Scholar]