Abstract
Background
Data on incidence of ventilator-associated pneumonia (VAP) and invasive pulmonary aspergillosis in patients with severe SARS-CoV-2 infection are limited.
Methods
We conducted a monocenter retrospective study comparing the incidence of VAP and invasive aspergillosis between patients with COVID-19-related acute respiratory distress syndrome (C-ARDS) and those with non-SARS-CoV-2 viral ARDS (NC-ARDS).
Results
We assessed 90 C-ARDS and 82 NC-ARDS patients, who were mechanically ventilated for more than 48 h. At ICU admission, there were significantly fewer bacterial coinfections documented in C-ARDS than in NC-ARDS: 14 (16%) vs 38 (48%), p < 0.01. Conversely, significantly more patients developed at least one VAP episode in C-ARDS as compared with NC-ARDS: 58 (64%) vs. 36 (44%), p = 0.007. The probability of VAP was significantly higher in C-ARDS after adjusting on death and ventilator weaning [sub-hazard ratio = 1.72 (1.14–2.52), p < 0.01]. The incidence of multi-drug-resistant bacteria (MDR)-related VAP was significantly higher in C-ARDS than in NC-ARDS: 21 (23%) vs. 9 (11%), p = 0.03. Carbapenem was more used in C-ARDS than in NC-ARDS: 48 (53%), vs 21 (26%), p < 0.01. According to AspICU algorithm, there were fewer cases of putative aspergillosis in C-ARDS than in NC-ARDS [2 (2%) vs. 12 (15%), p = 0.003], but there was no difference in Aspergillus colonization.
Conclusions
In our experience, we evidenced a higher incidence of VAP and MDR-VAP in C-ARDS than in NC-ARDS and a lower risk for invasive aspergillosis in the former group.
Keywords: COVID-19, ARDS, Nosocomial pneumonia, Ventilator-associated pneumonia, Invasive aspergillosis
Background
The pandemic of severe acute respiratory coronavirus 2 (SARS-CoV-2), responsible of coronavirus disease 2019 (COVID-19), has resulted in high rates of hospitalization and intensive care unit (ICU) admission to treat acute respiratory distress syndrome (ARDS).
There is scarce information in the literature on the rates of coinfections in COVID-19 patients. A total of 62/806 (8%) cases of bacterial/fungal coinfection were reported in nine cohort studies on COVID-19 patients [1]. These studies did not uniformly report bacterial coinfection, thus potentially underestimate the rates of respiratory coinfections. Broad-spectrum antibacterial therapy was widely used in more than 90% of COVID-19 cases receiving antibacterial therapy in ICU [2, 3]. However, there has been no robust report on ventilator-associated pneumonia (VAP) in COVID-19-associated ARDS patients (C-ARDS) to date. ARDS is a known risk factor for VAP but little is known about C-ARDS in this concern. Similar to severe influenza complications, recent reports have documented invasive pulmonary aspergillosis in COVID-19 patients but given no real incidence analysis [4, 5].
We conducted a retrospective study aimed at comparing the incidence of VAP and of invasive pulmonary aspergillosis between patients with C-ARDS and those with non-SARS-CoV-2 viral ARDS (NC-ARDS).
Methods
Setting and patients
We conducted a cohort study which retrospectively enrolled, between October 1, 2009, and April 29, 2020, all patients referred to the medical ICU of a French tertiary hospital for viral ARDS and who required mechanical ventilation for more than 48 h. ARDS was defined according to the Berlin definition [6]. C-ARDS were patients with ARDS and a positive polymerase chain reaction (PCR) test for SARS-COV2. NC-ARDS patients were those having ARDS and a positive polymerase chain reaction (PCR) test for influenza, human metapneumovirus, respiratory syncytial virus, parainfluenza virus, other endemic human coronaviruses (OC43, NL63, HK-U1, 229E), and adenovirus. RT-PCR duplex targeting influenza A/B, respiratory syncytial virus, metapneumovirus, coronavirus, adenovirus, and parainfluenza were performed using r-gene™ (Argene, Biomérieux S.A.) according to the manufacturer’s instructions. We carefully identified all patients with viral ARDS using a triple check involving the ICU medical reports, the medical information system database, and the virology department registry. Of note, respiratory viruses were systematically searched in all patients admitted in our ICU for pneumonia. This observational study was approved by the Institutional Review Board of the French intensive care medicine society (CE SRLF 20-45) and informed consent was waived.
The mechanical ventilation of ARDS patients followed a standardized protective ventilation strategy [7]. Other treatments, including neuromuscular blocking agents, inhaled nitric oxide, prone positioning, and veno-venous extra-corporeal membrane oxygenation were administered with respect to national guidelines [8]. Sedation protocol was based on the adaptation of the dose of sedative (midazolam or propofol) on the Richmond Agitation Sedation Scale by the nurse every three hours and did not significantly changed during the study period. Preventing VAP followed an educational program, regular reminders and feedback with a prevention care bundle in accordance with guidelines [9], based on hand hygiene with alcohol-based sanitizer, inclining patients in a semi-recumbent position (30°–45°), oral chlorhexidine mouth washing at least four times a day, tracheal cuff pressure maintenance between 20 and 30 cm H2O, orogastric rather than nasogastric tubes, and daily chlorhexidine body washing. No routine antibiotic prophylaxis or decontamination antibiotic regimens were prescribed and if stress ulcer prophylaxis was needed, proton pump inhibitor was preferred. All patients had a closed tracheal suction system without daily change.
Demographic, clinical, and laboratory data
Demographic characteristics, comorbidities, Charlson comorbidity index [10], clinical, biological, and imaging features at ICU admission, and consumption of alcohol-based handrub liquid (retrieved from hospital pharmacy) was collected, then analyzed on May 25, 2020, after a minimal follow-up period of 28 days for the most recent patients. Respiratory tract secretions were cultured for VAP diagnosis purposes, and susceptibility profiles of recovered microorganisms were recorded.
The primary endpoint was the difference in incidence of first VAP between C-ARDS and NC-ARDS patients. VAP was clinically suspected if any of its classical criteria happened 48 h or more after mechanical ventilation initiation: new or worsening infiltrates on chest roentgenogram, systemic signs of infection (new-onset fever, leukocytosis or leucopenia, increased need for vasopressors to maintain blood pressure), purulent secretions, and impaired oxygenation [11]. All suspected VAP were confirmed from quantitative cultures of lower respiratory tract secretions sampled before administering new antibiotics using a blinded protected telescope catheter [12] or bronchoscopy (103 and 104 colony forming units/mL for protected telescope catheter and bronchoalveolar lavage, respectively). VAP onset was defined as the day on which the lung sample tested positive. The secondary endpoints were the difference in occurrence of bacterial coinfection at ICU admission, putative invasive pulmonary aspergillosis, and multi-drug-resistant bacteria (MDR) VAP. MDR pathogens included methicillin-resistant Staphylococcus aureus, extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBL-PE), and carbapenem-resistant Enterobacteriaceae (CRE). During the study period, our management of VAP/hospital-acquired pneumonia (HAP) was derived from that recommended by the French guidelines to treat adult ICU-acquired pneumonia [13]. Bacterial coinfection at ICU admission was evidenced by the detection of bacteria in the sputum or in blood samples, in the absence of other sources of infection, or by a positive pneumococcal or L. pneumophila serotype 1 urinary antigen test.
We based our definition of invasive pulmonary aspergillosis on one of the following: (1) the recently published influenza‑associated pulmonary aspergillosis definition by expert panel (Influenza-Associated Pulmonary Aspergillosis—IAPA case definition) [14]; (2) the crude AspICU algorithm, as proposed by Blot et al. to distinguish putative invasive pulmonary aspergillosis from respiratory tract Aspergillus spp. colonization in critically-ill patients, relying on clinical, radiological, and mycological criteria [15]; (3) the modified AspICU algorithm of invasive pulmonary aspergillosis, which includes the positivity of serum or bronchoalveolar lavage galactomannan, as proposed by the Dutch-Belgian Mycosis study group to define invasive pulmonary aspergillosis in critically ill patients with severe influenza [16].
Statistical analysis
No statistical sample size calculation was performed a priori, and sample size was equal to the number of patients treated during the study period. The results are reported as median and interquartile range (25th–75th percentiles) or numbers with percentages. Initial bivariate statistical comparisons were conducted using χ2 or Fisher’s exact tests for categorical data and Mann–Whitney U test for continuous data.
To identify risk factors for VAP and invasive aspergillosis, we used classical multivariable logistic regression with a backward procedure, because the role of competing events like extubation and death was not relevant for this analysis. Non-redundant variables selected in bivariate analysis (p < 0.10) and considered clinically relevant were entered into the logistic regression model. Variables included in the final model for VAP were male gender, C-ARDS, congestive heart failure (NYHA 3-4), SAPS II at ICU admission, and bacterial coinfection at ICU admission. The results are expressed as crude and adjusted odd ratios (OR) with their 95% confidence intervals (CI).
Competing risks analysis
As the risk of VAP cumulatively increases over time of mechanical ventilation, death and ventilator weaning are competing risks for VAP occurrence [17]. Patients are no longer at risk for VAP after death or ventilator weaning; conversely, weaning may be prolonged because of VAP. In this context, standard survival methods (Kaplan–Meier method and Cox model) are inappropriate because they assume that censoring is non-informative [18], hence the need to consider specific competing risk methods. We therefore used a competing risk model (cumulative incidence function of the Gray model) [19, 20] to properly estimate the effect of COVID-19 on VAP development, while considering death and ventilator weaning as competing events. The strength of the association between each variable and the outcome was assessed with the sub-hazard ratio and the cumulative incidence function, estimated using cmprsk package developed by Gray in R software (http://biowww.dfci.harvard.edu/~gray/cmprsk_2.1-4.tar.gz). Because there were differences between the two groups, resulting from the particular profile of patients prone to have severe forms of COVID-19 (e.g., cardiovascular comorbidities) or non-COVID-19 viral pneumonia (e.g., immunosuppression), we further matched C-ARDS and NC-ARDS and performed a sensitivity analysis as follows. First, we screened all differences between groups. Second, we used a seminal review summarizing risk factors for VAP [21], to select among variables that were different between the two groups, those that could explain more VAP in the C-ARDS group as compared to the NC-ARDS group. Following this process, 68 C-ARDS patients could be matched 1:1 to 68 NC-ARDS for ARDS severity (mild, moderate, or severe) and diabetes mellitus. Of note, these matched pairs were comparable regarding age and gender. A sensitivity analysis with competing risk was performed in this matched cohort. Two-sided p values < 0.05 were considered significant. The other analyses were conducted using SPSS Base 21.0 statistics software package (SPSS Inc., Chicago, IL).
Results
The study
Between October 1, 2009, and April 29, 2020, 3821 consecutive patients were mechanically ventilated in our ICU. Among them, 199 had a viral positive PCR, including 172 pneumonia with ARDS criteria that were mechanically ventilated for more than 48 h. Ninety patients had C-ARDS with positive real-time reverse transcriptase PCR tests for COVID-19, while 82 patients had NC-ARDS, including 50 with severe influenza (with two respiratory syncytial virus coinfections), six with endemic human coronavirus (with one respiratory syncytial virus coinfection), 14 with respiratory syncytial virus alone, five with human metapneumovirus, five with parainfluenza, and two with adenovirus. Thus, the present study comprises 90 patients with C-ARDS ad 82 with NC-ARDS (Additional file 1: Figure S1).
Patients’ characteristics
The characteristics of C-ARDS and NC-ARDS patients are displayed in Table 1. NC-ARDS patients had worse past history (Mc Cabe classification and Charlson comorbidity index) and were often immunosuppressed, whereas C-ARDS patients were often diabetic and hypertensive males. At ICU admission almost all patients received antibiotics in both groups, but C-ARDS received less steroid had less organ failure (as assessed by Sequential Organ Failure Assessment score), and lower PaO2/FiO2 ratio than NC-ARDS patients. During ICU stay, C-ARDS patients more often required neuromuscular blockade, prone positioning, nitric oxide inhalation, extra-corporeal membrane oxygenation support, and longer duration of mechanical ventilation than NC-ARDS patients. In-ICU and day 28 mortalities were similar in both.
Table 1.
Characteristics of patients with acute respiratory distress syndrome related to Coronavirus disease 19 (C-ARDS) or other viruses (NC-ARDS)
| Variables | NC-ARDS (n = 82) | C-ARDS (n = 90) | p value |
|---|---|---|---|
| Age, median [IQR] | 63 [57–71] | 59 [53–69] | 0.09 |
| Male gender | 54 (66%) | 74 (82%) | 0.01 |
| Medical history | |||
| Mc Cabe and Jackson classification | < 0.001 | ||
| No underlying disease | 47 (57%) | 76 (84%) | |
| Ultimately fatal | 24 (29%) | 12 (13%) | |
| Rapidly fatal disease | 11 (14%) | 2 (2%) | |
| Charlson comorbidity index | 2 [1–3] | 1 [0–2] | < 0.001 |
| Diabetes mellitus | 23 (28%) | 39 (43%) | 0.037 |
| Congestive heart failure (NYHA 3–4) | 6 (7%) | 7 (8%) | 0.91 |
| Supraventricular arrhythmia | 12 (15%) | 8 (9%) | 0.24 |
| Hypertension | 36 (44%) | 59 (66%) | 0.004 |
| COPD | 10 (12%) | 9 (10%) | 0.64 |
| Chronic renal failure | 11 (13%) | 14 (16%) | 0.69 |
| Dialysis | 3 (4%) | 2 (2%) | 0.67 |
| Stroke | 5 (6%) | 4 (4%) | 0.74 |
| Liver cirrhosis (Child C) | 1 (1%) | 0 | 0.48 |
| Current smoking | 22 (27%) | 25 (28%) | 0.89 |
| Immunosuppression conditions | 40 (49%) | 16 (18%) | < 0.001 |
| Solid cancer | 4 (5%) | 5 (6%) | 0.99 |
| Blood cancer | 17 (21%) | 1 (1%) | < 0.001 |
| Organ transplant | 9 (11%) | 5 (6%) | 0.19 |
| HIV infection | 4 (5%) | 3 (3%) | 0.71 |
| Sickle cell disease | 2 (2%) | 3 (3%) | 0.99 |
| Others | 5 (6%) | 2 (2%) | 0.26 |
| Clinical characteristics upon ICU admission | |||
| SAPS II | 49 [37–67] | 36 [27–45] | < 0.001 |
| Baseline SOFA—median [IQR] | 9 [5–12] | 7 [4–8] | < 0.001 |
| PaO2/FiO2 ratio (mmHg) median [IQR] | 162 [101–210] | 120 [92–163] | 0.005 |
| ARDS classification (Berlin definition) | 0.018 | ||
| Mild | 24 (29%) | 11 (12%) | |
| Moderate | 39 (48%) | 49 (54%) | |
| Severe | 19 (23%) | 30 (33%) | |
| Norepinephrine, n (%) | 43 (52%) | 42 (47%) | 0.45 |
| Serum creatinine (µmol/L) | 108 [72–195] | 83 [6–128] | 0.004 |
| White blood cell count (× 109/L) | 7.5 [0–15] | 8.2 [5–12] | 0.49 |
| Lymphocyte count (× 109/L) | 0.6 [0.3–1.1] | 0.8 [0.5–1.2] | 0.03 |
| Lymphocyte count (× 109/L) in non-immunocompromised patients | 0.8 [0.4–1.2] | 0.8 [0.5–1.2] | 0.62 |
| Documented bacterial coinfection | 38 (48%) | 14 (16%) | < 0.001 |
| Treatment during the first 24 h | |||
| Antibiotics | 81 (99%) | 90 (100%) | 0.48 |
| Antiviral treatment | 58 (71%) | 69 (76%) | 0.39 |
| Corticosteroids (any dose)* | 30/81 (37%) | 12/87 (14%) | 0.001 |
| Corticosteroids (low dose)*# | 29/81 (36%) | 10/87 (12%) | < 0.001 |
| Corticosteroids (high dose)* | 1/81 (1%) | 2/87 (2%) | 0.60 |
| ARDS treatment during ICU stay | |||
| Corticosteroids (any dose)* | 37/81 (46%) | 35 /87 (40%) | 0.48 |
| Corticosteroids (low dose)*# | 33/81 (41%) | 25/87 (29%) | 0.10 |
| Corticosteroids (high dose)* | 3/81 (4%) | 10/87 (12%) | 0.06 |
| Prone position | 34 (42%) | 75 (83%) | < 0.001 |
| Neuromuscular blockade | 53 (65%) | 83 (92%) | < 0.001 |
| Inhaled nitric oxide | 10 (12%) | 31 (34%) | 0.01 |
| Extra-corporeal membrane oxygenation | 9 (11%) | 23 (26%) | 0.014 |
| ICU-acquired infections | |||
| First VAP | 36 (44%) | 58 (64%) | 0.007 |
| Number of days of mechanical ventilation before first VAP | 7 [5–9] | 8 [5–12] | 0.89 |
| Number of VAP during ICU | 0 [0–1] | 1 [0–2] | < 0.001 |
| Recurrent VAP | 10 (12%) | 22 (25%) | 0.36 |
| MDR VAP during ICU stay | 9 (11%) | 21 (23%) | 0.03 |
| ESBL PE VAP | 9 (11%) | 18 (20%) | 0.10 |
| MRSA VAP | 0 | 1 (1%) | 0.99 |
| CRE VAP | 0 | 3 (3%) | 0.095 |
| Sampling frequency (number/day of MV) | 0.23 [0.14–0.37] | 0.32 [0.20–0.38] | 0.03 |
| Organ support and outcome during ICU stay | |||
| Subglottic secretion drainage | 26 (32%) | 42 (47%) | 0.045 |
| Renal replacement therapy during ICU stay | 28 (34%) | 30 (33%) | 0.91 |
| Norepinephrine, n (%) | 66 (81%) | 67 (74%) | 0.34 |
| ICU length of stay among survivors, days | 15 [10–20] | 30 [19–45] | < 0.001 |
| Successful mechanical ventilation weaning | 54 (66%) | 46 (51%) | 0.05 |
| Death at day 28 | 25 (31%) | 36 (40%) | 0.19 |
| Death in the ICU | 27 (33%) | 37 (41%) | 0.27 |
| Still in ICU or in weaning center (until May 28th, 2020) | 0 | 8 (9%) | 0.007 |
VAP ventilator-associated pneumonia, COPD chronic obstructive pulmonary disease, HIV human immunodeficiency virus, SAPS II Simplified Acute Physiology Score II, SOFA sequential organ failure assessment, ICU intensive care unit, MDR multi-drug resistant, ESBL-PE extended-spectrum β-lactamase-producing Enterobacteriaceae, MRSA methicillin-resistant Staphylococcus aureus, CRE carbapenem-resistant Enterobacteriaceae, MV mechanical ventilation
*Four missing values because two patients received dexamethasone or placebo in a randomized controlled trial
#Less than 1 mg/kg of prednisone or equivalent
Coinfection at ICU admission
There were significantly fewer documented bacterial coinfections in C-ARDS than in NC-ARDS (Table 1). The microorganisms involved in bacterial coinfection at ICU admission are reported in Additional file 2: Table S1. The types of isolated microorganisms differed between the groups with fewer Gram-positive cocci in C-ARDS than in NC-ARDS, 4/14 (29%) vs. 23/39 (59%), p = 0.05, but similar Gram-negative bacilli [9/14 (64%) vs. 19/39 (49%), p = 0.32].
VAP occurrence and risk factors
At day 28 of ICU admission, significantly more patients developed at least one VAP episode in C-ARDS group than in NC-ARDS group: 56 (62%) vs. 35 (43%), p = 0.016. The mechanical ventilation lasted longer in C-ARDS than in NC-ARDS group: 16.5 [9.0–28.8] vs 9.0 [6.0–17.3] days, p < 0.0001. Fine and Gray model results showed that VAP probability was significantly higher in C-ARDS group after adjusting for death and ventilator weaning [sub-hazard ratio = 1.72 (1.14–2.57), p < 0.01, Fig. 1]. Conversely, the probability of successful ventilator weaning was significantly reduced in C-ARDS group after adjusting for VAP and death as competing events [sub-hazard ratio = 0.34 (0.19–0.63), p < 0.001]; the probability of death was similar in both groups [sub-hazard ratio = 1.18 (0.58–2.41), p = 0.64, Fig. 1]. These results persisted after matching for ARDS severity (mild, moderate or severe) and diabetes mellitus, with a higher VAP probability (after adjusting for death and ventilator weaning) and a lower weaning probability (after adjusting for VAP and death) in C-ARDS group [sub-hazard ratio of 1.74 (1.09–2.80), p = 0.02, and 0.37 (0.20–0.70), p < 0.01, respectively]. Risk factors for developing VAP were tested by univariate analysis in Additional file 3: Table S2. The factors associated with VAP occurrence, as shown by multivariable analysis (Additional file 4: Table S3), were C-ARDS [OR = 2.1 (1.1–4.0), p = 0.02] and male gender [OR = 2.2 (1.04–4.5), p = 0.04]. These results were similar by competing risk analysis: VAP probability was significantly higher in C-ARDS group as compared to NC-ARDS [sub-hazard ratio = 1.58 (1.05–2.39), p = 0.03] and in males as compared to females [sub-hazard ratio = 1.72 (1.03–2.88), p = 0.04], while adjusting for death and ventilator weaning.
Fig. 1.
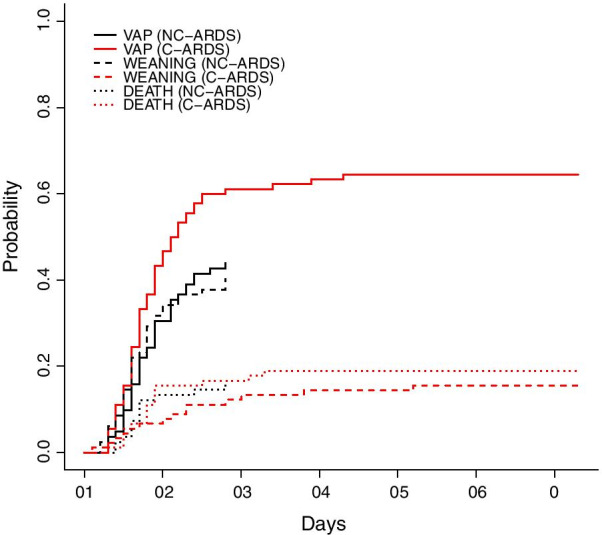
Cumulative probability of ventilator-associated pneumonia (VAP) in C-ARDS (red) and NC-ARDS (blue) patients. For analysis purpose, time from intubation to VAP (continuous line), to death (dotted line), and to weaning (dashed line) were handled as competing risks
VAP documentation and management
The most commonly isolated microorganisms in both groups with VAP were Enterobacteriaceae, which were more common in C-ARDS than in NC-ARDS group: n = 42 (72%) vs. 17 (47%), p = 0.01 at the first VAP episode (Table 2). MDR bacteria (all were ESBL-PE except one CRE New Delhi metallo-β-lactamase (NDM)) were retrieved in eleven (20%) C-ARDS and seven (19%) NC-ARDS patients during the first VAP episode (p = 0.57). However, the incidence of MDR VAP was significantly higher in C-ARDS than NC-ARDS during the entire ICU stay: 21 (23%) vs. 9 (11%), p = 0.03. Three C-ARDS patients had MDR VAP caused by CRE [two NDM and one oxacillinase-48 (OXA-48) producing Enterobacteriaceae, all without ESBL coproduction], 18 had ESBL-PE, and one had polymicrobial VAP caused by methicillin-resistant Staphylococcus aureus and ESBL-PE. In NC-ARDS patients, nine had their VAP caused by ESBL-PE. All patients received antibiotics during their ICU stay. Rate of administering Carbapenem for the first VAP was 16 (18%) in C-ARDS vs. 9 (11%) in NC-ARDS, p = 0.21. The three mostly used antibiotics were amoxicillin/clavulanic acid, third-generation cephalosporin, and piperacillin/tazobactam in NC-ARDS group, versus third-generation cephalosporin, carbapenem, and aminoglycoside in C-ARDS group. Carbapenem was more used in C-ARDS than in NC-ARDS patients: 48 (53%), vs 21 (26%), p < 0.01 (Additional file 5: Table S4). Consuming alcohol-based handrub liquid in the ICU reached 135 mL per patient-day for the NC-ARDS study period versus 522 mL per patient-day for the C-ARDS study period.
Table 2.
Microorganisms involved in first ventilator-associated pneumonia in patients with acute respiratory distress related to Coronavirus disease 19 (C-ARDS) or other viruses (NC-ARDS)
| Microorganisms | NC-ARDS (n = 36) | C-ARDS (n = 58) |
|---|---|---|
| Gram-negative bacilli | ||
| Haemophilus sp | 4 (11%) | 0 |
| Enterobacteriaceae | 17 (47%) | 42 (72%) |
| Enterobacter sp | 4 (11%) | 23 (40%) |
| Klebsiella pneumoniae | 6 (17%) | 4 (7%) |
| Citrobacter sp | 1 (3%) | 2 (4%) |
| Escherichia coli | 4 (11%) | 10 (17%) |
| Hafnia | 0 | 2 (4%) |
| Morganella morganii | 1 (3%) | 0 |
| Serratia | 2 (6%) | 1 (2%) |
| Proteus | 0 | 4 (7%) |
| Extended-spectrum beta-lactamase-producing enterobacteriaceae | 7 (19%) | 10 (18%) |
| Carbapenem-resistant enterobacteriaceae | 0 | 1 (2%) |
| Non-fermenting gram-negative bacilli | 20 (56%) | 24 (41%) |
| Acinetobacter sp | 1 (3%) | 1 (2%) |
| Pseudomonas sp | 17 (47%) | 16 (28%) |
| Burkholderia Cepacia | 0 | 1 (2%) |
| Stenotrophomonas maltophilia | 2 (6%) | 3 (5%) |
| Gram-positive bacteria | 0 | 4 (3%) |
| Streptococcus pneumoniae | 0 | 0 |
| Others Streptococcus sp | 0 | 2 (4%) |
| Methicillin-sensitive Staphylococcus aureus | 0 | 2 (4%) |
| Methicillin-resistant Staphylococcus aureus | 0 | 0 |
| Enterococcus faecalis | 0 | 1 (2%) |
| Polymicrobial | 4 (11%) | 13 (22%) |
| Diagnostic sampling techniques | ||
| Bronchoalveolar lavage | 4 (11%) | 3 (5%) |
| Blind protected telescope catheter | 32 (89%) | 55 (95%) |
The total number of microorganisms is greater than 100% because more than one microorganism may be retrieved from a given respiratory sample
Invasive aspergillosis
Diagnostic criteria for invasive pulmonary aspergillosis according to IAPA case definition, crude AspICU definition, and modified AspICU definition are shown in Table 3. There was no proven aspergillosis case in the entire study. Probable aspergillosis (as per IAPA case definition) and putative aspergillosis (as per crude and modified AspICU criteria) were less common in C-ARDS than in NC-ARDS patients (Table 3). According to AspICU algorithm, there was no difference in Aspergillus colonization between C-ARDS and NC-ARDS patients. Univariate analysis of risk factors for developing invasive pulmonary aspergillosis is reported in Additional file 6: Table S5. The factors associated with invasive pulmonary aspergillosis as tested by multivariable analysis (Additional file 7: Table S6) were immunodepression [OR = 3.6 (1.4–9.1), p = 0.01] and having influenza, which fell short of statistical significance [OR = 2.5 (0.88–6.4), p = 0.052). Invasive pulmonary aspergillosis was associated with ICU mortality (Additional file 6: Table S5).
Table 3.
Diagnostic criteria of invasive aspergillosis in patients with acute respiratory distress syndrome related to Coronavirus disease 19 (C-ARDS) or other viruses (NC-ARDS)
| NC-ARDS (n = 82) | C-ARDS (n = 90) | p value | |
|---|---|---|---|
| IAPA case definition | |||
| Proven invasive pulmonary aspergillosis: Biopsy or brush specimen of airway plaque, pseudomembrane, or ulcer showing hyphal elements and Aspergillus growth on culture or positive Aspergillus PCR on tissue. Lung biopsy showing invasive fungal elements and Aspergillus growth on culture or positive Aspergillus PCR on tissue | 0 | 0 | |
| Probable invasive pulmonary aspergillosis | 17 (21%) | 7 (8%) | 0.01 |
| Probable invasive pulmonary aspergillosis diagnosed at ICU admission | 9 (53%) | 3 (43%) | |
| Time from admission to diagnosis of probable pulmonary aspergillosis during ICU stay, days | 10 [7–14] | 6 [5–11] | |
| Aspergillus tracheobronchitis | 1 (1%) | 1 (1%) | 0.95 |
| Airway plaque, pseudomembrane, or ulcer | 3/49 | 1/24 | |
| IAPA in patients without documented Aspergillus tracheobronchitis | 16 (20%) | 6 (7%) | 0.01 |
| Pulmonary infiltrate | 82 | 90 | |
| Cavitating infiltrate (not attributed to another cause) | 1 | 1 | |
| Serum GM index > 0.5 | 6/40 | 5/88 | |
| BAL GM index ≥ 1.0 | 5/31 | 0/0 | |
| Positive BAL culture | 10/50 | 4/24 | |
| Crude AspICU criteria | |||
| Proven invasive pulmonary aspergillosis: | 0 | 0 | |
| Putative invasive pulmonary aspergillosis | 12 (15%) | 2 (2%) | 0.003 |
| 1. Aspergillus-positive lower respiratory tract specimen culture* | 17 | 6 | |
| 2. Compatible signs and symptoms | 16 | 6 | |
| 3. Abnormal medical imaging by portable chest X-ray or CT-scan | 14 | 5 | |
| 4a. Host risk factors | 9 | 0 | |
| 4b. Semiquantitative Aspergillus-positive culture of BAL fluid (+ or ++), without bacterial growth together with a positive cytological smear showing branching hyphae | 8 | 2 | |
| Colonization | 5 (6%) | 4 (5%) | 0.63 |
| Modified AspICU criteria | |||
| Proven invasive pulmonary aspergillosis: | 0 | 0 | |
| Putative invasive pulmonary aspergillosis | 15 (18%) | 6 (7%) | 0.02 |
| Mycological criteria | 18 | 7 | |
| Histopathology or direct microscopic evidence of dichotomous septate hyphae with positive culture for Aspergillus on tissue | 0 | 0 | |
| Serum GM index > 0.5 | 6/40 | 5/88 | |
| BAL GM index ≥ 1.0 | 5/31 | 0/0 | |
| Positive BAL culture | 10/50 | 4/24 | |
| Compatible signs and symptoms§ | 17/18 | 6/7 | |
| Abnormal medical imaging by portable chest X-ray or CT-scan§ | 16/18 | 7/7 | |
| Other diagnostic criteria | |||
| Aspergillus PCR on lower respiratory tract: positive cases / performed cases | 0 /7 (0%) | 16 /81(20%) | |
| 1,3-β-D-glucan: positive cases /performed cases | 8/23(35%) | 9 /88(10%) |
*Entry criterion for Crude AspICU criteria. Data are n (%) or n/N (%). BAL bronchoalveolar lavage
§Among patients with positive mycological criteria
Discussion
In this study of patients having viral ARDS, we have evidenced the following findings: i) Fewer documented bacterial coinfections in C-ARDS than in NC-ARDS at ICU admission; ii) a higher incidence of VAP and MDR VAP in C-ARDS than in NC-ARDS; and iii) a lower risk of putative invasive pulmonary aspergillosis in the C-ARDS group.
Coinfection at ICU admission
There were significantly fewer documented bacterial coinfections in C-ARDS than in NC-ARDS. Bacterial coinfection rate in NC-CARDS group is in accordance with previous studies on ARDS secondary to influenza. Bacterial coinfection rate at ICU admission is reported in less than 10% in C-ARDS cases [22], except in small series using multiplex PCR assay [23, 24].
Mechanical ventilation
The high incidence of VAP found in our study is in accordance with the selected population of ARDS (see flowchart), as reported in previous studies [11, 21, 25, 26]. Using ARDS as a selection criteria allowed inclusion of a relatively homogenous group of patients with reduction of potential bias, given that ARDS is a known major risk factor for VAP. In our cohort, all patients with SARS-CoV-2 pneumonia requiring mechanical ventilation fulfilled ARDS criteria. This finding may be ascribable to the virulence of SARS-CoV-2 and/or to the fact that some intermediate care units have been deployed upstream the ICU for the care of patients not requiring immediate intubation during the pandemic. Invasive mechanical ventilation is a cornerstone in the development of VAP. The duration of mechanical ventilation was twice longer in C-ARDS than in NC-ARDS patients, with more recurrent VAP episodes in the former group. Strategies aimed at avoiding intubation, such as continuous positive airway pressure [27], high-flow nasal oxygen [28], or awake prone position in spontaneously breathing patients [29] should be further explored. Sedation protocols should also be optimized to reduce the duration of mechanical ventilation. SARS-CoV-2 infection was associated with encephalopathy, agitation, and confusion [30]. Our competing risk model yielded a reduced probability of ventilator weaning and a higher probability of VAP in C-ARDS patients whenever adjusted for ventilator weaning. Other factors may influence the risk for VAP in C-ARDS, like infectious process or infection control.
Low dose dexamethasone is reported as the first drug to improve survival in COVID-19 pneumonia [31]. In our work, 36% of NC-ARDS and 12% of C-ARDS patients were on low-dose corticosteroid at ICU admission, yet did not increase their risk for VAP. A recent randomized controlled study on dexamethasone treatment in ARDS did not show more ICU acquired infections [32]. Seven patients in the C-ARDS group received tocilizumab. Anti-inflammatory treatment may be associated with the development of bloodstream infection or late onset infections in recent studies [33, 34].
Infection control
Hand hygiene compliance rate was not evaluated by internal audits in our ICU during COVID-19 outbreak. Consumption of alcohol-based handrub liquid was 135 mL per patient-day before the outbreak versus 522 mL per patient-day during COVID 19 period. This increase is probably due to the increased number of health care workers coming to reinforce our team. We do not know if the overall increase in alcohol-based handrub solution consumption was associated with a better hand hygiene compliance. Previous studies showed that during routine clinical care of patients with MDR bacteria, health care workers often contaminate protective gowns and gloves [35]. Moreover, teams dedicated to some procedures like prone positioning were created to decrease nurse work strain, but their transversal nature may have increased the risk of cross contamination. Now more than ever, health systems should continue investing in their infection prevention programs, beyond the current pandemic.
Invasive aspergillosis
Severe influenza infection has been associated with invasive pulmonary aspergillosis. IAPA case definition and modified AspICU algorithm, specifically designed for severe influenza, were used in this study to assess the role of invasive aspergillosis in C-ARDS. A comprehensive diagnostic approach for invasive aspergillosis was implemented in C-ARDS using a systematic serum galactomannan test, PCR and culture of lower respiratory tract secretions for Aspergillus species, and 1,3-β-D-glucan. Four patients with putative aspergillosis had no previous risk factors, suggesting that C-ARDS is a host factor for invasive aspergillosis. However, the incidence of invasive aspergillosis was significantly lower in C-ARDS than in NC-ARDS and was consistent with previously reported patients with bacterial ARDS [36]. Pre-pandemic environmental air sampling found Aspergillus conidia in our ICU rooms. Laminar air flow unit and high-efficiency particulate air filters were installed during the outbreak which may have decrease invasive Aspergillus nosocomial infection [37] and explain the lower rate of invasive aspergillosis in C-ARDS in our work, but half of invasive aspergillosis cases were diagnosed at ICU admission. The lack of galactomannan in BAL performed in C-ARDS patients may underestimate invasive aspergillosis using IAPA case definition and modified AspICU algorithm, but putative aspergillosis was less common in C-ARDS using crude AspICU criteria. Bartoletti et al. [38] found a higher incidence of invasive pulmonary aspergillosis in COVID-19 patients mostly treated with high doses corticosteroids and tocilizumab. In our cohort, NC-ARDS patients were often immunosuppressed with more blood malignancies and corticosteroid, which are known risk factors for invasive aspergillosis in patients with ARDS or severe influenza [16, 36]. These underlying diseases may at least in part explain the higher risk of invasive aspergillosis in this group.
Strengths and limitations
The strengths of our study come from the detailed description of VAP and the use of competing risk models (cumulative incidence function of Gray model) to properly estimate the effect of COVID-19 on VAP risk, after adjustment on death and ventilator weaning as competing events.
Our study has some limitations. First, due to its monocentric design, our results may not be applicable on other centers. The risks of VAP may vary between centers in parallel with the variation in infection prevention measures and health crisis preparedness strategies. Clinical wards air and contact surfaces, sources of pathogenic fungi, may highly vary between ICUs, especially during a crisis. Second, the study period used to recruit the cohort was long (11 years), which is a major limitation. The lower incidence of ARDS in patients with non-COVID viral pneumonia may be ascribable to a lower virulence and transmissibility of non-SARS-CoV-2 respiratory viruses as compared to SARS-CoV-2 [39]. However, our management protocol for ARDS did not significantly change during the study period. Third, we found different baseline characteristics between groups. C-ARDS patients were mostly males [40, 41], a known risk factor of VAP; however, the latter association with C-ARDS persisted in the multivariable analysis and in matched analysis. Fourth, it might be difficult to interpret chest X-ray because of preexisting parenchymal injury in ARDS patients [42]. The microbiological investigation on lower respiratory tract samples is currently the main diagnostic tool of VAP in C-ARDS and NC-ARDS [43]. The higher respiratory sampling in C-ARDS patients may have theoretically contributed to an overestimation of the VAP frequency in this group, but VAP was first clinically suspected if any of its classical criteria happened and sampling was then performed to confirm VAP. We cannot exclude that respiratory deterioration-labeled VAP was to some extent relative to progression of COVID-19 with a bystander positive bacterial culture.
Conclusions
In this retrospective study, we have observed a higher incidence of VAP and MDR VAP in C-ARDS as compared with NC-ARDS patients. Further, probably multicenter, research work are needed to confirm this association.
Supplementary Information
Additional file 1. Figure S1 (online supplement): Flowchart of the study. ARDS denotes Acute Respiratory Distress Syndrome; C-ARDS denotes COVID-19-related ARDS; NC-ARDS denotes non-COVID-19 related ARDS.
Additional file 2. Table S1. Microorganisms involved in bacterial coinfection documented at intensive care unit admission in patients with acute respiratory disease related to Coronavirus disease 19 (C-ARDS) or other viruses (NC-ARDS).
Additional file 3. Table S2. Univariate analysis of variables associated with ventilator-associated pneumonia (VAP) in patients with acute respiratory distress syndrome related to Coronavirus disease 19 (C-ARDS) or other viruses (NC-ARDS).
Additional file 4. Table S3. Multivariable logistic regression testing factors associated with ventilator-associated pneumonia in patients with acute respiratory distress syndrome related to Coronavirus disease 19 (C-ARDS) or other viruses (NC-ARDS).
Additional file 5. Table S4. Antibiotics use during intensive care unit stay in patients with acute respiratory disease syndrome related to Coronavirus disease 19 (C-ARDS) or other viruses (NC-ARDS).
Additional file 6. Table S5. Univariate analysis of factors associated with invasive pulmonary aspergillosis (Influenza-Associated Pulmonary Aspergillosis case definition) in patients with acute respiratory distress syndrome related to Coronavirus disease 19 (C-ARDS) or other viruses (NC-ARDS).
Additional file 7. Table S6. Multivariable logistic regression of factors associated with invasive pulmonary aspergillosis (Influenza-Associated Pulmonary Aspergillosis case definition) in patients with acute respiratory distress syndrome related to Coronavirus disease 19 (C-ARDS) or other viruses (NC-ARDS).
Acknowledgements
We thank Jean Hazebroucq, Clement Ourghanlian, Christian Brun-Buisson, and Francois Hemery for their invaluable help.
Abbreviations
- VAP
Ventilator-associated pneumonia
- C-ARDS
Coronavirus disease 19-related acute respiratory distress syndrome
- NC-ARDS:
Non-Coronavirus disease 19 viral ARDS
- MDR
Multi-drug-resistant bacteria
- ESBL-PE
Extended-spectrum β-lactamase-producing Enterobacteriaceae
Authors’ contributions
Dr. KR had full access to all of the study data and is deemed responsible for data integrity and accuracy of their analysis. Dr. KR and Dr. AMD contributed to the study initial design, analysis, interpretation of data, drafting of the initial manuscript, critical revision of intellectual content, and approval of the article final version. RA, AFM, BB, GC, JWD, SF, PLW, FB, FS, CAN, and NdP contributed to the study design and analysis, interpretation of data, drafting of initial manuscript, critical revision of intellectual content, and approval of the submitted version of the article.
Funding
No funding was received for this study.
Availability of data and materials
The datasets supporting the conclusions are included within the article and supplementary data.
Ethics approval and consent to participate
This observational study was approved by the Ethical Review Board of the French society for intensive care medicine (Société de Réanimation de Langue Française). As per the French law, no informed consent was required for this type of studies.
Consent for publication
As per the French law, no informed consent was required for this type of studies.
Competing interests
All authors report no conflict of interest relevant to this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Francoise Botterel and Nicolas de Prost contributed equally to this work
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-020-03417-0.
References
- 1.Rawson TM, Moore LSP, Zhu N, Ranganathan N, Skolimowska K, Gilchrist M, et al. Bacterial and fungal co-infection in individuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis Off Publ Infect Dis Soc Am. 2020;71(9):2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P, Liu Z, Chen Y, Xiao Y, Huang X, Fan X-G. Bacterial and fungal infections in COVID-19 patients: a matter of concern. Infect Control Hosp Epidemiol. 2020;41(9):1124–1125. doi: 10.1017/ice.2020.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Arkel ALE, Rijpstra TA, Belderbos HNA, van Wijngaarden P, Verweij PE, Bentvelsen RG. COVID-19 associated pulmonary aspergillosis. Am J Respir Crit Care Med. 2020;202(1):132–135. doi: 10.1164/rccm.202004-1038LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alanio A, Dellière S, Fodil S, Bretagne S, Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir Med. 2020;8(6):e48–e49. doi: 10.1016/S2213-2600(20)30237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–33. [DOI] [PubMed]
- 7.Mercat A, Richard J-CM, Vielle B, Jaber S, Osman D, Diehl J-L, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:646–55. doi: 10.1001/jama.299.6.646. [DOI] [PubMed] [Google Scholar]
- 8.Papazian L, Aubron C, Brochard L, Chiche J-D, Combes A, Dreyfuss D, et al. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care. 2019;9:69. doi: 10.1186/s13613-019-0540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouadma L, Mourvillier B, Deiler V, Le Corre B, Lolom I, Régnier B, et al. A multifaceted program to prevent ventilator-associated pneumonia: impact on compliance with preventive measures. Crit Care Med. 2010;38:789–796. doi: 10.1097/CCM.0b013e3181ce21af. [DOI] [PubMed] [Google Scholar]
- 10.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 11.Papazian L, Klompas M, Luyt C-E. Ventilator-associated pneumonia in adults: a narrative review. Intensive Care Med. 2020;46(5):888–906. doi: 10.1007/s00134-020-05980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brun-Buisson C, Fartoukh M, Lechapt E, Honoré S, Zahar J-R, Cerf C, et al. Contribution of blinded, protected quantitative specimens to the diagnostic and therapeutic management of ventilator-associated pneumonia. Chest. 2005;128:533–544. doi: 10.1378/chest.128.2.533. [DOI] [PubMed] [Google Scholar]
- 13.Leone M, Bouadma L, Bouhemad B, Brissaud O, Dauger S, Gibot S, et al. Brief summary of French guidelines for the prevention, diagnosis and treatment of hospital-acquired pneumonia in ICU. Ann Intensive Care. 2018;8:104. doi: 10.1186/s13613-018-0444-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verweij PE, Rijnders BJA, Brüggemann RJM, Azoulay E, Bassetti M, Blot S, et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med. 2020;46(8):1524–1535. doi: 10.1007/s00134-020-06091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blot S, Koulenti D, Dimopoulos G, Martin C, Komnos A, Krueger WA, et al. Prevalence, risk factors, and mortality for ventilator-associated pneumonia in middle-aged, old, and very old critically ill patients*. Crit Care Med. 2014;42:601–609. doi: 10.1097/01.ccm.0000435665.07446.50. [DOI] [PubMed] [Google Scholar]
- 16.Schauwvlieghe AFAD, Rijnders BJA, Philips N, Verwijs R, Vanderbeke L, Van Tienen C, et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6:782–792. doi: 10.1016/S2213-2600(18)30274-1. [DOI] [PubMed] [Google Scholar]
- 17.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(SICI)1097-0258(19990330)18:6<695::AID-SIM60>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 18.Resche-Rigon M, Azoulay E, Chevret S. Evaluating mortality in intensive care units: contribution of competing risks analyses. Crit Care Lond Engl. 2006;10:R5. doi: 10.1186/cc3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fine JP, Gray RJ. A Proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 20.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat Inst Math Stat. 1988;16:1141–1154. [Google Scholar]
- 21.Chastre J, Fagon J-Y. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 22.Azoulay E, Fartoukh M, Darmon M, Géri G, Voiriot G, Dupont T, et al. Increased mortality in patients with severe SARS-CoV-2 infection admitted within seven days of disease onset. Intensive Care Med. 2020;46(9):1714–1722. doi: 10.1007/s00134-020-06202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kreitmann L, Monard C, Dauwalder O, Simon M, Argaud L. Early bacterial co-infection in ARDS related to COVID-19. Intensive Care Med. 2020;46(9):1787–1789. doi: 10.1007/s00134-020-06165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verroken A, Scohy A, Gérard L, Wittebole X, Collienne C, Laterre P-F. Co-infections in COVID-19 critically ill and antibiotic management: a prospective cohort analysis. Crit Care [Internet]. 2020 [cited 2020 Aug 31];24. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7347259/. [DOI] [PMC free article] [PubMed]
- 25.Delclaux C, Roupie E, Blot F, Brochard L, Lemaire F, Brun-Buisson C. Lower respiratory tract colonization and infection during severe acute respiratory distress syndrome: incidence and diagnosis. Am J Respir Crit Care Med. 1997;156:1092–1098. doi: 10.1164/ajrccm.156.4.9701065. [DOI] [PubMed] [Google Scholar]
- 26.Chastre J, Trouillet JL, Vuagnat A, Joly-Guillou ML, Clavier H, Dombret MC, et al. Nosocomial pneumonia in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1998;157:1165–1172. doi: 10.1164/ajrccm.157.4.9708057. [DOI] [PubMed] [Google Scholar]
- 27.Oranger M, Gonzalez-Bermejo J, Dacosta-Noble P, Llontop C, Guerder A, Trosini-Desert V, et al. Continuous positive airway pressure to avoid intubation in SARS-CoV-2 pneumonia: a two-period retrospective case-control study. Eur Respir J. 2020 doi: 10.1183/13993003.01692-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang K, Zhao W, Li J, Shu W, Duan J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020;10:37. doi: 10.1186/s13613-020-00653-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elharrar X, Trigui Y, Dols A-M, Touchon F, Martinez S, Prud’homme E, et al. Use of prone positioning in nonintubated patients with COVID-19 and hypoxemic acute respiratory failure. JAMA [Internet]. 2020 [cited 2020 May 16]. https://jamanetwork.com/journals/jama/fullarticle/2766292. [DOI] [PMC free article] [PubMed]
- 30.Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 33.Pettit NN, Nguyen CT, Mutlu GM, Wu D, Kimmig L, Pitrak D, et al. Late onset infectious complications and safety of tocilizumab in the management of COVID-19. J Med Virol. 2020 doi: 10.1002/jmv.26429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giacobbe DR, Battaglini D, Ball L, Brunetti I, Bruzzone B, Codda G, et al. Bloodstream infections in critically ill patients with COVID-19. Eur J Clin Invest. 2020;50(10):e13319. doi: 10.1111/eci.13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mulvey D, Mayer J, Visnovsky L, Samore M, Drews F. Frequent and unexpected deviations from personal protective equipment guidelines increase contamination risks. Am J Infect Control. 2019;47:1146–1147. doi: 10.1016/j.ajic.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 36.Contou D, Dorison M, Rosman J, Schlemmer F, Gibelin A, Foulet F, et al. Aspergillus-positive lower respiratory tract samples in patients with the acute respiratory distress syndrome: a 10-year retrospective study. Ann Intensive Care. 2016;6:52. doi: 10.1186/s13613-016-0156-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hahn T, Cummings KM, Michalek AM, Lipman BJ, Segal BH, McCarthy PL. Efficacy of high-efficiency particulate air filtration in preventing aspergillosis in immunocompromised patients with hematologic malignancies. Infect Control Hosp Epidemiol. 2002;23:525–531. doi: 10.1086/502101. [DOI] [PubMed] [Google Scholar]
- 38.Bartoletti M, Pascale R, Cricca M, Rinaldi M, Maccaro A, Bussini L, et al. Epidemiology of invasive pulmonary aspergillosis among COVID-19 intubated patients: a prospective study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang C, Yao X, Zhao Y, Wu J, Huang P, Pan C, et al. Comparative review of respiratory diseases caused by coronaviruses and influenza A viruses during epidemic season. Microbes Infect. 2020;22:236–244. doi: 10.1016/j.micinf.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forel J-M, Voillet F, Pulina D, Gacouin A, Perrin G, Barrau K, et al. Ventilator-associated pneumonia and ICU mortality in severe ARDS patients ventilated according to a lung-protective strategy. Crit Care Lond Engl. 2012;16:R65. doi: 10.1186/cc11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ayzac L, Girard R, Baboi L, Beuret P, Rabilloud M, Richard JC, et al. Ventilator-associated pneumonia in ARDS patients: the impact of prone positioning. A secondary analysis of the PROSEVA trial. Intensive Care Med. 2016;42:871–8. doi: 10.1007/s00134-015-4167-5. [DOI] [PubMed] [Google Scholar]
- 42.Klompas M. Does this patient have ventilator-associated pneumonia? JAMA. 2007;297:1583–1593. doi: 10.1001/jama.297.14.1583. [DOI] [PubMed] [Google Scholar]
- 43.François B, Laterre P-F, Luyt C-E, Chastre J. The challenge of ventilator-associated pneumonia diagnosis in COVID-19 patients. Crit Care Lond Engl. 2020;24:289. doi: 10.1186/s13054-020-03013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Figure S1 (online supplement): Flowchart of the study. ARDS denotes Acute Respiratory Distress Syndrome; C-ARDS denotes COVID-19-related ARDS; NC-ARDS denotes non-COVID-19 related ARDS.
Additional file 2. Table S1. Microorganisms involved in bacterial coinfection documented at intensive care unit admission in patients with acute respiratory disease related to Coronavirus disease 19 (C-ARDS) or other viruses (NC-ARDS).
Additional file 3. Table S2. Univariate analysis of variables associated with ventilator-associated pneumonia (VAP) in patients with acute respiratory distress syndrome related to Coronavirus disease 19 (C-ARDS) or other viruses (NC-ARDS).
Additional file 4. Table S3. Multivariable logistic regression testing factors associated with ventilator-associated pneumonia in patients with acute respiratory distress syndrome related to Coronavirus disease 19 (C-ARDS) or other viruses (NC-ARDS).
Additional file 5. Table S4. Antibiotics use during intensive care unit stay in patients with acute respiratory disease syndrome related to Coronavirus disease 19 (C-ARDS) or other viruses (NC-ARDS).
Additional file 6. Table S5. Univariate analysis of factors associated with invasive pulmonary aspergillosis (Influenza-Associated Pulmonary Aspergillosis case definition) in patients with acute respiratory distress syndrome related to Coronavirus disease 19 (C-ARDS) or other viruses (NC-ARDS).
Additional file 7. Table S6. Multivariable logistic regression of factors associated with invasive pulmonary aspergillosis (Influenza-Associated Pulmonary Aspergillosis case definition) in patients with acute respiratory distress syndrome related to Coronavirus disease 19 (C-ARDS) or other viruses (NC-ARDS).
Data Availability Statement
The datasets supporting the conclusions are included within the article and supplementary data.


